Abstract
Large amounts of waste are generated by the minimally processed vegetables industry, such as those from beetroot processing. The aim of this study was to determine the best method to obtain flour from minimally processed beetroot waste dried at different temperatures, besides producing a colorant from such waste and assessing its stability along 45 days. Beetroot waste dried at 70 °C originates flour with significant antioxidant activity and higher betalain content than flour produced from waste dried at 60 and 80 °C, while chlorination had no impact on the process since microbiological results were consistent for its application. The colorant obtained from beetroot waste showed color stability for 20 days and potential antioxidant activity over the analysis period, thus it can be used as a functional additive to improve nutritional characteristics and appearance of food products. These results are promising since minimally processed beetroot waste can be used as an alternative source of natural and functional ingredients with high antioxidant activity and betalain content.
Keywords: Functional ingredients, Betalain, Natural colorant, Vegetable flour
Introduction
The minimally processed food industry produces a large amount of plant food waste daily, which causes economic and nutritional losses and great environmental impact. This waste material is increasingly recognized as a natural source of bioactive compounds and it can be used to obtain ingredients with high nutritional and commercial value such as fibers and pigments. Thus, this waste becomes an interesting material as it is available in large quantities and is usually discarded, which ensures its low cost.
The demand for healthy foods has contributed to the interest in the development of natural ingredients. Natural pigments, for example, present health benefits that turn them into attractive alternatives to replace synthetic colorants. In combination with consumer demand, there is growing interest in the use of these colorants obtained from natural sources, such as food processing waste.
In this context, researchers have focused on the use of this waste for the development of new food products and ingredients. For example, orange peel, grape seeds, and tomato pomace have been used to produce an extruded snack food (Yagci and Gogus 2008). Crizel et al. (2015) evaluated the incorporation of orange juice waste (fibers) to produce fresh fettuccini pasta. Among these vegetal foods that produce a lot of waste, some stand out for their high pigment potential, such as beetroot.
Beetroot (Beta vulgaris L.) is a popular plant food with diverse consumption that bears nutritional benefits. Because of the demand for healthy foods and for its practicality, beetroot stands out among minimally processed vegetables. This vegetable has functional characteristics due to its significant fiber content, both soluble and insoluble, which can provide interesting technological properties for the food industry, such as enrichment of pasta, cakes, and cookies.
Another beetroot attribute is its betalain content, which is a natural water-soluble pigment of the class of nitrogen compounds that consists of betacyanins, responsible for red-violet color, and betaxanthins, accounting for yellow-orange color. Furthermore, the antioxidant activity of betalains, which was associated with protection against degenerative diseases (Azeredo 2009), has been demonstrated.
Considering that synthetic pigments are increasingly avoided by consumers, beetroot waste may be an alternative source to obtain a natural colorant, since these colorants are provided by renewable sources, generally plant material. Natural colorants are formulated to be appropriate for application in a variety of foods and drinks and to improve their stability (Mortenesen 2006), being safe to use, nutritious, less polluting, and creating soft and subtle effects. Betalain pigment, for example, can be applied as an additive for food, cosmetics, and drugs in beetroot juice or powder form. Furthermore, its advantage is that, even though anthocyanins are the most widespread and commonly used natural pigments to reach color ranging from red to purple, betalains are more stable in face of variations in pH and temperature (Ravichandran et al. 2013).
In addition to betalain, beetroot is rich in other functional compounds. Previous studies have shown that beetroot extract has high inhibitory effect on the proliferation of cancer cells, especially those affecting prostate and stomach tissues. Besides this anti-proliferative effect, betalain also has other biological activities, including anti-inflammatory and hepatoprotective actions (Boivin et al. 2009; Georgiev et al. 2010). Beetroot is one of the most powerful vegetables concerning antioxidant activity, which is attributed to its phenolic compound content, and it causes free-radical stabilization activity that prevents the oxidation of biological molecules (Pedreño and Escribano 2001; Vulic et al. 2012.).
In this context, beetroot waste may represent a good alternative to obtain functional ingredients, including plant food flour rich in fibers and natural colorants. Thus, the aim of this study was to determine the best method to obtain flour from minimally processed beetroot waste and to produce a colorant and assess its stability along 45 days.
Materials and methods
Beetroot waste flour
The minimally processed beetroot waste was provided by Degasperi Atacadista, located in Estrela (RS, Brazil), and consisted of peels, parings, and stalks. The waste (30 kg) was homogenized and washed with water for 5 min for further use.
The material was subjected to two different treatments. In the first one, the waste was soaked in 200 ppm chlorine solution for 15 min and then washed in running water for 5 min to remove residual chlorine. The second portion was immersed in water for 15 min with no chlorination. Excess humidity of all samples was removed by centrifugation at 100×g (equipment developed at the Institute of Agronomy at UFRGS). Next, samples were dried in an oven with air circulation (DeLeo, model B5AFD, Brazil) at three different temperatures (60, 70, and 80 °C) to obtain flour with moisture between 10 and 12%. A drying curve was constructed by removing samples (5 g) every hour and placing them into the oven at 105 °C (DeLeo, TLK model 48, Brazil) for 24 h. After the optimal time/temperature binomial to reach the desired moisture was obtained, beetroot waste was dried under the ideal conditions. The dry material was ground (Arbelmill, model MCF55, Brazil) and sieved to separate particles smaller than 125 mm (115 mesh) (Bertel, Brazil). The flours obtained were vacuum packed (Fastvac, model F200, Brazil) and stored in the dark at room temperature (25 °C).
To determine the optimal conditions to obtain beetroot waste flour, the effects of chlorinated water were evaluated through microbiological analysis and the effects of temperature treatments on proximate composition, betalain content, and antioxidant activity were assessed.
Microbiological analysis
The beetroot waste and the flours obtained were microbiologically tested to verify safety and availability to use.
Total aerobic mesophilics
Beetroot waste and flour were diluted in distilled water to 10−1, 10−2, and 10−3. An aliquot (1 mL) of each dilution was transferred to sterile Petri dishes and 20 mL Plate Count Agar (PCA) were added. The dishes were incubated at 35 °C for 48 h and the number of colonies was counted (Phoenix counter EC–589). The results were expressed as CFU/g (FDA 1995).
Fecal coliforms at 45 °C by MPN (most probable number)
Presumptive test
Lauryl sulfate tryptose medium (LST) (20 mL) was used to inoculate samples (10 g), which were incubated at 35 °C for 48 h. The procedure was performed in a series of three tubes containing inverted Durham tube and the ones with gas formation and acid production (as evidenced by the formation of yellow color) were considered positive (FDA 1995).
Total coliform confirmation test
The LST-positive tubes were replicated in EC (Escherichia coli) broth and incubated at 45 °C for 24 h in a water bath with agitation. The MPN was calculated from the number of positive tubes, based on a specific table, and the result was expressed as MPN/g (FDA 1995).
Beetroot waste colorant
The colorant was obtained from 5 g of beetroot waste with 25 mL of ethanol solution at 70% (in triplicate). This mixture was homogenized in an Ultra-Turrax (IKA, T25, China) disperser, centrifuged at 25,400×g (CR GIII, Hitachi, Japan) for 15 min, and the supernatant was recovered. This process was repeated four times to totally remove the beetroot waste coloration. The extracts were stored in amber flasks at room temperature (25 °C).
Waste ingredient characterization
Proximate analysis of beetroot waste flours
Beetroot waste flour analyses were performed according to the AOAC (2002). Total protein content was determined by the Kjeldahl method (N × 5.7%); lipid content was determined using a Soxhlet extractor (Foss Soxtec, model 2055TM, Denmark); ash content was measured in a muffle (Linn ElektroTherm, 312.6 OS LM 1729, Germany) at 550 °C; moisture was assessed by oven-drying at 105 °C (DeLeo, TLK model 48, Brazil) for 24 h; total dietary fibers (soluble and insoluble) were determined by the enzymatic–gravimetric method; and carbohydrate content was obtained by difference. All analyses were performed in triplicate. The results were expressed as grams per 100 g of dry matter (DM).
Water and oil holding capacity of beetroot waste flour
Water holding capacity (WHC) analysis was performed according to Fernández-López et al. (2009) with minor modifications. A suspension of distilled water (30 mL) and beetroot waste flour (1 g) was homogenized with a vortex (Quimis, model Q920-A2, Brazil) for 1 min and left at room temperature for 24 h. After centrifugation (3,000×g for 20 min, Sigma, model 4K15, England), the supernatant was removed and the residue was weighed. WHC was expressed as grams of water per gram of beetroot waste flour. Oil holding capacity (OHC) was determined in the same way as WHC, but distilled water was replaced by sunflower oil and the results were expressed as grams of oil per gram of beetroot waste flour.
Color
Color analysis of beetroot waste flour and colorant was performed with a colorimeter (Minolta®, CR400, Japan) using the CIELab system, D65 illuminant. Parameter L* corresponds to brightness, ranging from black to white (0–100), a* represents color varying from green to red (−60 to +60), and b*, blue to yellow (−60 to +60).
Betalain content
Betalain contents in both ingredients obtained from beetroot waste, flour, and colorant were determined using a well-established method. For flour analysis, samples (2 g) were homogenized with distilled water (5 mL) in an Ultra-Turrax (T25, IKA, China) disperser and the supernatant was obtained after centrifugation at 25,400×g (CR GIII, Hitachi, Japan) for 15 min. A fraction (400 µL) of this extract was dissolved in 4 mL of distilled water so the betalain content could be assessed. For colorant analysis, 400 µL of beetroot waste colorant was directly homogenized with 4 mL of distilled water every 5 days over 45 days. The measurements were taken at absorbances of 476, 538 and 600 nm in a spectrophotometer (Shimadzu, UV-1800, Japan). The results were calculated according to Vitti et al. (2005) using the following equations:
where a = absorbance reading at 538 nm; b = absorbance reading at 476 nm; c = absorbance reading at 600 nm; x = betacyanin absorbance; y = betaxanthin absorbance; z = impurity absorbance.
The results were expressed as mg of betalain per 100 g dry matter (DM).
Determination of antioxidant potential
Antioxidant activity was evaluated by DPPH radical scavenging and the reducing capacity, by the spectrophotometric method with Folin–Ciocalteu reagent.
DPPH analysis
Antioxidant activity was determined based on capturing radical 2,2-diphenyl-1-picrylhydrazyl (DPPH) according to Brand-Wiliams et al. (1995).
Beetroot waste flour (5 g) was diluted in 50% methanol (30 mL), 70% acetone was added (30 mL), and the mixture was homogenized for 5 min in an Ultra-Turrax (T25, IKA, China) disperser. The extracts were centrifuged at 25,400×g (CR-GIII, Hitachi, Japan) for 15 min and the supernatant was recovered, from which an aliquot (0.1 mL) was taken to react with DPPH radical (3.9 mL) for 30 min and absorbance was measured at 515 nm in a spectrophotometer (Shimadzu, UV-1800, Japan). The antioxidant activity of beetroot waste colorant was measured in the same way: The reaction was prepared from 0.1 mL of colorant with DPPH (3.9 mL) and antioxidant activity was evaluated every five days for 45 days. For the control, an analysis was performed with 50% methanol: 70% acetone (1:1) (0.1 mL) for flour samples and 70% ethanol (0.1 mL) for the colorant, both homogenized with DPPH radical (3.9 mL). The results were expressed as inhibition percentage.
Reducing capacity
The reducing capacity of beetroot waste flour and colorant was determined by the spectrophotometric method with Folin–Ciocalteu. The flour (5 g) was homogenized with methanol (20 mL) in an Ultra-Turrax (T25, IKA, China) disperser for 2 min and centrifuged for 20 min at 25,400×g (CR-GIII, Hitachi, Japan). The supernatant (0.25 mL) was diluted in distilled water (4 mL), 1 N Folin–Ciocalteu reagent (0.125 mL) was added and, after 3 min of reaction, 1 N Na2CO3 solution (0.625 mL) was added. The mixture reacted for 2 h at room temperature in the dark and then absorbance was assessed at 725 nm in a spectrophotometer (Shimadzu, UV-1800, Japan). The colorant’s reducing capacity was analyzed every 5 days over 45 days in the same conditions by diluting the colorant directly in distilled water and adding Folin–Ciocalteu, followed by addition of 1 N Na2CO3. The results were expressed as mg of gallic acid equivalents (GAE) per gram of dry matter (DM).
Statistical analysis
The results were analyzed by ANOVA with Tukey’s comparison test at 5% significance using the software Statistica v. 10.0 (STATSOFT Inc., Tulsa, OK, USA).
Results and discussion
Drying curves of beetroot waste flour
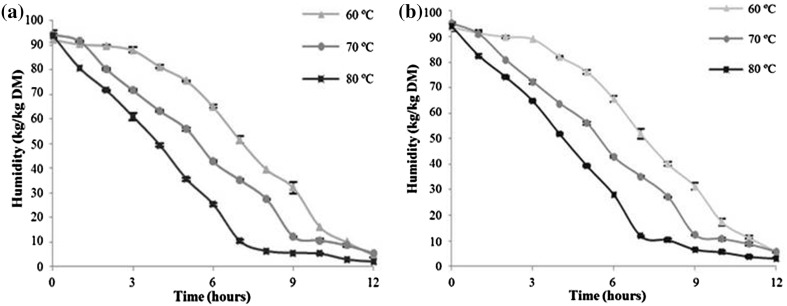
The moisture content of beetroot waste before drying was 93% and the chlorination process did not alter this initial content. Figures 1 and 2 show the drying curves of chlorinated and non-chlorinated waste, both subjected to drying at 60, 70, and 80 °C with air circulation.
Fig. 1.
Curves of chlorinated (a) and non-chlorinated (b) beetroot waste dried at 60, 70, and 80 °C for 12 h to obtain the waste flour
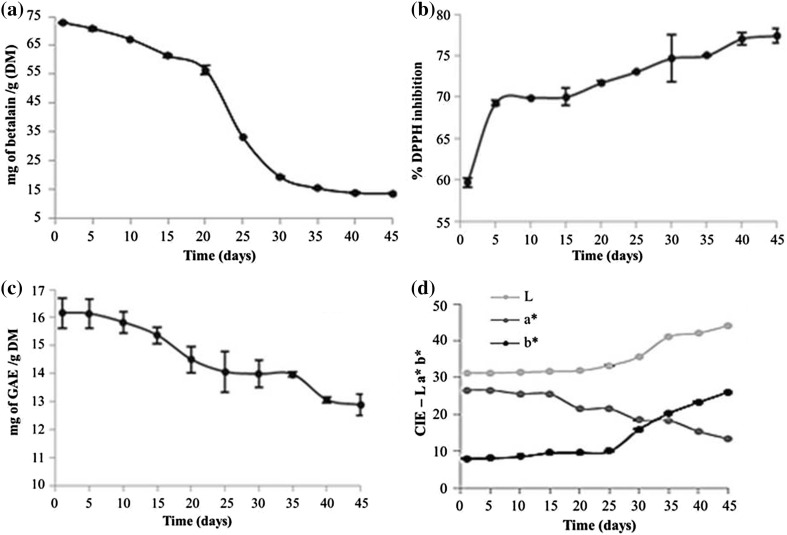
Fig. 2.
Stability of beetroot waste colorant over 45 days, with analyses carried out every 5 days. a Betalain; b DPPH inhibition; c reducing capacity and d color parameters
The drying process is applied to reduce water activity and, subsequently, microbial activity, besides to minimize reactions involved in biochemical degradation processes, hence, it can extend the shelf life of dried fruit (Vega-Mercado et al. 2001). Thus, in addition to reducing weight and volume to result in lower costs for transport and storage, the drying process usually allows obtaining products with higher concentrations of nutrients, depending on applied conditions (Park et al. 2001). According to Damodaran et al. (2010), beetroot pigments are stable with reduced water content, thus low moisture promotes a decrease in color degradation rate over time.
It was observed, as expected, that the drying process at high temperatures took less time to reach moisture content close to 10%. Required moisture values were reached after drying for 11 h at 60 °C, 9 h at 70 °C, and 7 h at 80 °C, and no difference was found between chlorinated and non-chlorinated samples. Reque et al. (2015) demonstrated that the dehydration of blueberry fruit at 80 or 90 °C led to moisture values below the limit for pathogenic microorganism contamination. On the other hand, they found that longer heat processing times caused a decrease in the bioactive and antioxidant properties of dried blueberry fruit. These results showed that more studies are fundamental to understanding the factors involved in the degradation of these compounds, including time and temperature of the drying process, to highlight the nutritive qualities of dried fruit.
Microbiological analysis
Beetroot waste and its flour had counts of aerobic microorganisms of 2.15 × 104 and 1.96 × 102 CFU/g, respectively, and both materials exhibited less than 0.3 MPN/g of fecal coliforms at 45 °C. These results support the viability of this waste, as required by RDC 12/2001 (Brazil 2001). The chlorination process did not significantly alter the presence or counts of microorganisms. These results are probably a consequence of the processes applied by the minimally processed food industry before peeling vegetables, such as cleaning steps with water and immersion in chlorinated water, which generate safe waste with low microorganism content.
Characteristics of beetroot waste flour
Proximate composition, WHC, OHC, and color analysis
The chlorination process and different drying temperatures did not affect the results of proximate composition, WHC, OHC, and color analysis of beetroot waste flour, as shown in Table 1.
Table 1.
Proximate composition, water holding capacity (WHC), oil holding capacity (OHC), and color analysis of beetroot waste flour dried at different temperatures (60, 70, and 80 °C) with and without chlorination process
| 60 °C | 70 °C | 80 °C | ||||
|---|---|---|---|---|---|---|
| Non-chlorinated | Chlorinated | Non-chlorinated | Chlorinated | Non-chlorinated | Chlorinated | |
| Moisture | 10.11 ± 0.01a | 11.01 ± 0.02a | 11.05 ± 0.01a | 11.00 ± 0.01a | 10.20 ± 0.03a | 11.08 ± 0.02a |
| Carbohydratesa | 20.83 ± 0.33a | 20.72 ± 0.32a | 20.74 ± 0.31a | 20.72 ± 0.35a | 20.75 ± 0.27a | 20.75 ± 0.26a |
| Protein | 12.64 ± 0.11a | 12.69 ± 0.09a | 12.68 ± 0.10a | 12.66 ± 0.11a | 12.66 ± 0.09a | 12.63 ± 0.06a |
| Lipids | 1.31 ± 0.05a | 1.32 ± 0.05a | 1.32 ± 0.04a | 1.33 ± 0.06a | 1.31 ± 0.07a | 1.33 ± 0.07a |
| Ash | 5.62 ± 0.11a | 5.63 ± 0.11a | 5.63 ± 0.08a | 5.66 ± 0.07a | 5.63 ± 0.07a | 5.63 ± 0.09a |
| TDF | 65.22 ± 0.83a | 65.27 ± 0.83a | 65.26 ± 0.78a | 65.29 ± 0.87a | 65.28 ± 0.66a | 65.29 ± 0.67a |
| IDF | 45.08 ± 0.31a | 45.01 ± 0.31a | 45.09 ± 0.17a | 45.13 ± 0.27a | 45.03 ± 0.27a | 45.01 ± 0.18a |
| SDF | 20.14 ± 0.72a | 20.26 ± 0.61a | 20.17 ± 0.63a | 20.16 ± 0.37a | 20.25 ± 0.37a | 20.28 ± 0.18a |
| WHC | 10.17 ± 0.20a | 10.16 ± 0.18a | 10.15 ± 0.08a | 10.11 ± 0.07a | 10.15 ± 0.05a | 10.10 ± 0.06a |
| OHC | 3.35 ± 0.06a | 3.31 ± 0.04a | 3.36 ± 0.07a | 3.34 ± 0.06a | 3.34 ± 0.04a | 3.31 ± 0.09a |
| L* | 37.35 ± 0.39a | 37.52 ± 0.45a | 35.49 ± 0.35b | 35.30 ± 0.26b | 34.96 ± 0.02b | 33.50 ± 0.11c |
| a* | 12.36 ± 0.21d | 12.37 ± 0.41d | 13.52 ± 0.27c | 13.61 ± 0.07c | 14.57 ± 0.37b | 15.50 ± 0.17a |
| b* | 1.94 ± 0.03b | 1.96 ± 0.02b | 1.96 ± 0.01b | 1.95 ± 0.01b | 2.16 ± 0.04a | 2.17 ± 0.02a |
TDF total dietary fiber, IDF insoluble dietary fiber, SDF soluble dietary fiber, WHC water holding capacity—g water/g flour, OHC oil holding capacity—g oil/g flour
aDetermined by difference. Results obtained from the mean of three determinations ± standard deviation. Different letters on the same row are significantly different by Tukey’s test (p ≤ 0.05)
It was found that beetroot waste flour exhibited low levels of lipids, which provides its protection against oxidation. Protein (12.63–12.68%) and ash (5.62–5.66%) contents were not influenced by treatments (drying temperatures and chlorination) and their values were higher than those found by Yagci and Gogus (2008) for flour produced from a mixture of fruit waste (orange peel, grape seed, and tomato pomace) at 4.99% for protein and 3.64% for ash. These results show the nutritional quality of beetroot waste flour due to its protein and mineral content.
Regarding fiber content, high values found in the beetroot waste flour (around 65%) can be explained by the composition of the waste material that includes peel, parings, and stalks, which are known for their high fiber content compared to the pulp.
The TDF in beetroot waste flour showed values around 65.26%, regardless of the treatment, and these values were higher than those found by Padalino et al. (2013), who studied flours from 12 different vegetables (artichokes; asparagus; pumpkin; zucchini; tomatoes; yellow, red and green paprika; carrots; broccoli; spinach; eggplant; and fennel) dried at 65 °C for 7.5 h, and found TDF contents ranging from 19% for yellow paprika to 52% for asparagus.
Beetroot waste flour showed insoluble fiber content twofold higher than soluble ones. Fibers can improve lipid profile, modify glycemic response, alter intestinal function, and, thus, provide many health benefits (Borderías et al. 2005). The effects of insoluble fibers obtained from carrot waste in lowering lipids of hamsters were evaluated by Hsu et al. (2006) and the results were satisfactory, since insoluble fibers from carrot pomace reduced levels of serum triglycerides, total cholesterol, hepatic cholesterol and, on the other hand, increased HDL cholesterol levels compared to the control diet (with cellulose as fiber source). These results showed the applicability of fiber from plant food waste, which can improve consumer’s health.
Regarding technological applicability, fibers may be used as ingredients since their properties allow for various applications including fat substitute, stabilizers, thickeners, and emulsifiers in high-consumption products, such as beverages, soups, sauces, desserts, dairy products, cookies, pasta, and breads (Thebaudin et al. 1997; Crizel et al. 2013). In this context, beetroot waste flour stands out for its pectin functional quality, which demonstrated technological properties such as stabilizing oil/water emulsions, which suggests this flour is a promising food additive (Fissore et al. 2013).
Beetroot waste flour showed high WHC values (mean 10.14 g water/g flour) and there was no difference between the treatments. This result is similar to the values found by Sudha et al. (2007) for apple waste flour (8.39 g water/g flour), and Crizel et al. (2013) for orange waste flour (8.71 g water/g flour). Fibers with high WHC can be applied to increase viscosity, prevent syneresis, and modify the product’s final texture (Figuerola et al. 2005).
The values obtained for OHC did not differ among treatments (mean 3.34 g oil/g flour) and were higher than those reported by Martinez et al. (2012) for mango (1.6 g oil/g flour), passion fruit (0.9 g oil/g flour), and guava (0.7 g oil/g flour) waste. The high OHC values found in the present study may be associated with high IDF content since these fibers have the ability to retain fivefold its mass in oil. According to Chau et al. (2004), high values of WHC and OHC suggest that these flours may be applied as functional ingredients in a reduced-calorie product, for example.
The drying process affected the color parameters compared to the values assessed in waste before processing. The beetroot waste had, for non-chlorinated and chlorinated samples, respectively, values of L* 22.49 ± 0.39 and 23.54 ± 0.21; a* 5.14 ± 0.23 and 5.74 ± 0.43; b* 3.54 ± 0.13 and 3.15 ± 0.09. Comparing color parameters of dried samples with these initial values of beetroot waste allowed identifying changes caused by the drying process such as brightness, redness, and yellowness. On the other hand, among drying processes, raising the temperature affected the color parameters of waste subjected to 80 °C, especially the chlorinated samples, with higher L* (luminosity) and a* (red–green) parameters than other samples, which indicates this flour was brighter and redder. These results suggest that chlorine accelerates the oxidative reaction at high temperatures. These changes in color parameters of waste dried at different temperatures are similar to the subtle differences shown by Gokhale and Lele (2011), who investigated beetroot dried from 50 to 120 °C using color as quality parameter (L*, a*, and b*). They found a minimum variation in these parameters at all temperatures (L* 20.10–16.77, a* 12.47–9.17, and b* 5.57–2.25 for temperatures at 50–120 °C), which they attributed to the physical process of bound moisture release during drying.
Moreover, regarding the influence of temperature on betalain stability, it is well known that the heating process causes oxidative browning, which may mask the real pigment content since the colorimeter only measures surface color (Gokhale and Lele 2011). Thus, both quantification of betalain content and color measurements should be carried out to verify pigment alterations occurring during drying (Stintzing and Carle 2007).
Antioxidant capacity and betalain content in beetroot waste flour
The treatments showed significant difference in antioxidant activity (by the DPPH method) and total betalain content, but did not differ regarding reducing capacity (Folin–Ciocalteu method) (Table 2).
Table 2.
Antioxidant capacity and betalain content in flour obtained from chlorinated and non-chlorinated beetroot waste dried at 60, 70, and 80 °C
| 60 °C | 70 °C | 80 °C | ||||
|---|---|---|---|---|---|---|
| Non-chlorinated | Chlorinated | Non-chlorinated | Chlorinated | Non-chlorinated | Chlorinated | |
| Reducing capacity (mg EAG/g MS) | 24.66 ± 0.11a | 24.98 ± 0.76a | 25.05 ± 0.64a | 24.74 ± 0.11a | 24.71 ± 0.57a | 24.44 ± 0.72a |
| DPPH (% inhibition) | 65.08 ± 0.09b | 65.89 ± 0.67b | 71.34 ± 0.94a | 70.20 ± 0.19a | 61.14 ± 0.56c | 58.11 ± 0.13d |
| Total betalain content (mg/g DM) | 75.05 ± 0.18b | 75.85 ± 0.73b | 81.31 ± 0.69a | 80.16 ± 0.21a | 71.17 ± 0.52c | 68.08 ± 0.20d |
The results are the mean of three determinations ± standard deviation. Different letters on the same row are significantly different by Tukey’s test (p ≤ 0.05)
Different drying temperatures and the chlorination process did not interfere on the reduction capacity of the flours analyzed and the values were slightly higher than those reported by Stratil et al. (2006) for beetroot (20.7 mg GAE/g DM). They also analyzed 31 other vegetables and reported that higher reducing capacity values are commonly found in intensely colored plants such as beetroots, red cabbage, and red onion.
It has been shown that a diet rich in fiber and phenolic compounds is associated with health benefits. Phenolic compounds, due to their electron reducing capacity, are related to antiallergenic, antiatherogenic, anti-inflammatory, antimicrobial, and antithrombotic properties, in addition to cardioprotective effects (Balasundram et al. 2006). Beetroot waste flours showed high levels of bioactive compounds, which demonstrates their potential for application as a functional ingredient.
Regarding DPPH analysis, inhibition capacity increased by 8% when temperature rose from 60 to 70 °C. This result is in agreement with the best betalain extraction observed at 70 °C (Boivin et al. 2009; Georgiev et al. 2010). However, at higher temperature (80 °C), especially with the chlorination process, a reduction in this activity was observed, probably due to the oxidizing effect of chlorine on betalain compound. Furthermore, it is important to highlight that the rate of oxidation reactions increases as temperature rises.
Similar results were found by Ravichandran et al. (2013), who evaluated the use of different thermal treatments for beetroot and found that slight heat application (microwave at 450 W for 30 s) increased by 7% the initial betalain content. On the other hand, this content decreased when more intensive treatment was applied (toasting at 180 °C and heating at 80 °C).
Thus, in order to obtain higher betalain concentration, the optimum beetroot waste drying condition was at 70 °C, regardless of the chlorination process, which yielded around 80 mg/g DM. This value is 20-fold higher than the betalain content found by Vulic et al. (2012) in different beetroot pomaces (0.75–3.75 mg/g DM). It is important to point out that betalain content should be variety-specific, as previously reported by Lee et al. (2014). Furthermore, better antioxidant activity results were also observed in flour dried at 70 °C, probably due to betalain’s antioxidant capacity. According to Gokhale and Lele (2014), betalain content of beetroot powder depends on drying temperature; when heated at high temperatures, they are degraded into brown compounds. However, with moderate temperatures, decreasing water activity leads to an increase in half-life of these pigments in dry products (Bonozzi and Dumoulin 2011). Furthermore, it has been shown that betacyanin decreased whereas betaxanthin increased when drying temperature rose from 50 to 120 °C, which was explained by the higher betaxanthin availability for extraction and betacyanin’s temperature sensitivity (Gokhale and Lele 2014).
Since the initial waste did not have significant microbial contamination, the chlorination process proved to be unnecessary in this study.
Beetroot waste colorant
Colorant stability was assessed over 45 days and an increase in antioxidant activity (DPPH method) was observed, while betalain content and reducing capability decreased (Fig. 2).
The colorant obtained from minimally processed beetroot waste showed high stability, considering that total betalain content, in the first 10 days, decreased by only 9%. It is well known that the optimal stability pH for betacyanin is from 5.5 to 5.8 and, for betaxanthin, it is from 5.0 to 6.0 (Saguy et al. 1984). In the present study, colorant pH was 6.7, which did not vary during storage, thus it could have influenced this partial reduction in betalain.
Color analysis of beetroot waste colorant showed a decrease in a* (which indicates red color) during storage, which can be related to the degradation of the betacyanins that confer red color to the extract. Consequently, it contributes to the reduction in total betalain content. Furthermore, parameter a* showed a positive correlation (0.93%) with reducing capacity, which can suggest that such reduction activity is caused by betacyanins present in the colorant, which also decreased.
On the other hand, parameter b* indicates the presence of yellow color and gradually increased over storage at the same rate as DPPH inhibition, showing a positive correlation (0.74%), which suggests that betaxanthin, which provides yellow color, has electron donor action.
The decrease in total betalain content could be directly related to phenolic compound content, which showed high antioxidant activity over 20 days of storage, dropping by only 11%. Thus, this colorant showed a highly positive correlation (0.90%) between reducing capability and betalain content.
Betalain colorants extracted from beetroot provide a natural alternative to synthetic colorants and are associated with antioxidant and antimicrobial activities, besides featuring nutritional value with non-toxic nature. Thus, betalains can be used as food additives that prevent food discoloration and also enrich food products and beverages. Considering that, in the present research, the colorant was obtained from beetroot waste, it is important to point out its ecofriendly feature, posing no environmental problems, unlike synthetic colorants (Zvitov and Nussinovitch 2005; Nayak et al. 2006; Sivakumar et al. 2009; Ravichandran et al. 2013).
It was observed that non-edible beetroot parings have greater reducing capacity than the pulp. Results found in the present study (16 GAE/g DM on the 1st day and 12 mg GAE/g DM on day 45) were similar to those reported by Kujala et al. (2000), who studied beetroot waste aqueous extract and found values of 15.5 mg GAE/g DM in the peel and 11.4 mg GAE/g DM g in the stalk. These values were higher than the reducing capacity of pulp (4.2 mg GAE/g DM), which indicates that beetroot portions not usually consumed can have higher functional capacity than the commonly consumed pulp.
Conclusion
The process to obtain beetroot waste flour is simple and generates a product with high fiber content, both soluble and insoluble, and high oil and water holding capacities, while flour obtained by drying at 70 °C exhibits higher antioxidant activity and betalain content. These results suggest that this flour has wide applicability, functionality, and high market potential as a food ingredient. The colorant obtained from beetroot waste showed color stability up until 20 days of storage, which allows applying this colorant in short- and medium-shelf-life food products. Furthermore, the colorant showed good technical quality, antioxidant activity, and presence of phenolic compounds, which suggests its use as a functional additive to improve the food nutritional characteristics. Therefore, the results presented suggest the exploitation of minimally processed beetroot waste, an abundant low-cost source that can provide natural ingredients with functional appeal.
Acknowledgements
The authors thank CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazilian Government) for the financial support.
References
- AOAC (2002) Official methods of analysis of the Association of Official Analytical Chemists International 17th edn. Gaithersburgh
- Azeredo HMC. Betalains: properties, sources, applications, and stability—a review. Int J Food Sci Technol. 2009;44:2365–2376. doi: 10.1111/j.1365-2621.2007.01668.x. [DOI] [Google Scholar]
- Balasundram N, Sundram K, Samman S. Phenolic compounds in plants and agri-industrial by-products: antioxidant activity, occurrence, and potential uses. Food Chem. 2006;99:191–203. doi: 10.1016/j.foodchem.2005.07.042. [DOI] [Google Scholar]
- Boivin D, Lamy S, Lord-Dufour S, Jackson J, Beaulieu E, Côté M, Moghrabi A, Barrette S, Gingras D, Béliveau R. Antiproliferative and antioxidant activities of common vegetables: a comparative study. Food Chem. 2009;112:374–380. doi: 10.1016/j.foodchem.2008.05.084. [DOI] [Google Scholar]
- Bonozzi C, Dumoulin E (2011) Modern drying technology. In: Tsotsas E, Mujumdar AS (eds) Product quality and formulation, vol 3. Wiley-VCH, Weinheim, Germany
- Borderías AJ, Sánchez-Alonso I, Pérez-Mateos M. New applications of fibers in foods: addition to fishery products. Trend Food Sci Technol. 2005;16:458–465. doi: 10.1016/j.tifs.2005.03.011. [DOI] [Google Scholar]
- Brand-Wiliams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. Food Sci Technol. 1995;28(1):25–30. [Google Scholar]
- Brazil Ministry of Health (2001) National health surveillance agency. Resolution—RDC no. 12, 2 January. Accessed Jan 2015. http://bvsms.saude.gov.br/bvs/saudelegis/anvisa/2001/res0012_02_01_2001.html
- Chau C, Chen C, Lee M. Comparison of the characteristics, functional properties, and in vitro hypoglycemic effects of various carrot insoluble fiber-rich fractions. LWT Food Sci Technol. 2004;37:155–160. doi: 10.1016/j.lwt.2003.08.001. [DOI] [Google Scholar]
- Crizel TM, Jablonski A, Rios AO, Rech R, Flôres SH. Dietary fiber from orange byproducts as a potential fat replacer. LWT Food Sci Technol. 2013;53:9–14. doi: 10.1016/j.lwt.2013.02.002. [DOI] [Google Scholar]
- Crizel TM, Rios AO, Thys RCS, Flôres SH. Effects of orange by-product fiber incorporation on the functional and technological properties of pasta. Food Sci Technol. 2015;35(3):546–551. [Google Scholar]
- Damodaran S, Parkin KL, Fennema OR. Química de alimentos. 4. Editora: Artmed; 2010. [Google Scholar]
- FDA—Food and Drug Administration (1995) Bacteriological analytical manual, 8th edn. Association of Official Analytical Chemists International, Gaithersburg
- Fernández-López J, Sendra-Nadal E, Navarro C, Sayas E, Viuda-Martos M, Alvarez JAP. Storage stability of a high dietary fibre powder from orange by-products. Int J Food Sci Technol. 2009;44:748–756. doi: 10.1111/j.1365-2621.2008.01892.x. [DOI] [Google Scholar]
- Figuerola F, Hurtado ML, Estévez AM, Chiffelle I, Asenjo F. Fiber concentrates from apple pomace and citrus peel as potential fiber sources for food enrichment. Food Chem. 2005;91:395–401. doi: 10.1016/j.foodchem.2004.04.036. [DOI] [Google Scholar]
- Fissore EN, Rojas AM, Gerschenson LN, Willians PA. Butternut and beet root pectins: characterization and functional properties. Food Hydrocolloid. 2013;31:172–182. doi: 10.1016/j.foodhyd.2012.10.012. [DOI] [Google Scholar]
- Georgiev VG, Weber J, Kneschke EM, Denev PN, Bley T, Pavlov AI. Antioxidant activity and phenolic content of betalainextracts from intact plants and hairy root cultures of the red beet root Beta vulgaris cv. Detroit dark red. Plant Food Hum Nutr. 2010;65(2):105–111. doi: 10.1007/s11130-010-0156-6. [DOI] [PubMed] [Google Scholar]
- Gokhale SV, Lele SS. Dehydration of red beet root (Beta vulgaris) by hot air drying: process optimization and mathematical modeling. Food Sci Biotechnol. 2011;20:955–964. doi: 10.1007/s10068-011-0132-4. [DOI] [Google Scholar]
- Gokhale SV, Lele SS. Betalain content and antioxidant activity of Beta vulgaris: effect of hot air convective drying and storage. J Food Process Preserv. 2014;38:585–590. doi: 10.1111/jfpp.12006. [DOI] [Google Scholar]
- Hsu P, Chien P, Chen C, Chau C. Carrot insoluble fiber-rich fraction lowers lipid and cholesterol absorption in hamsters. LWT Food Sci Technol. 2006;39:337–342. doi: 10.1016/j.lwt.2005.02.009. [DOI] [Google Scholar]
- Kujala TS, Loponen JM, Klika KD, Pihlaja K. Phenolics and betacyanins in red beet root (Beta vulgaris) root: distribution and effect of cold storage on the content of total phenolics and three individual compounds. J Agric Food Chem. 2000;48:5338–5342. doi: 10.1021/jf000523q. [DOI] [PubMed] [Google Scholar]
- Lee EJ, An D, Nguyen CT, Patil BS, Kim J, Yoo KS. Betalain and betaine composition of greenhouse- or field-produced beetroot (Beta vulgaris L.) and inhibition of HepG2 cell proliferation. J Agric Food Chem. 2014;62:1324–1331. doi: 10.1021/jf404648u. [DOI] [PubMed] [Google Scholar]
- Martinez R, Torres P, Meneses MA, Figueroa JG, Pérez-Álvarez JA, Viuda-Martos M. Chemical, technological and in vitro antioxidant properties of mango, guava, pineapple and passion fruit dietary fiber concentrate. Food Chem. 2012;135:1520–1526. doi: 10.1016/j.foodchem.2012.05.057. [DOI] [PubMed] [Google Scholar]
- Mortenesen A. Carotenoids and other pigments as natural colorants. Pure Appl Chem. 2006;78:1477–1491. [Google Scholar]
- Nayak AC, Chethana S, Rastogi NK, Raghavarao KSMS. Enhanced mass transfer during solid–liquid extraction of gamma-irradiated red beetroot. Radiat Phys Chem. 2006;75:173–178. doi: 10.1016/j.radphyschem.2005.03.015. [DOI] [Google Scholar]
- Padalino L, Mastromatteo M, Lecce L, Cozzolino F, Del Nobile MA. Manufacture and characterization of gluten-free spaghetti enriched with vegetable flour. J Cereal Sci. 2013;57:333–342. doi: 10.1016/j.jcs.2012.12.010. [DOI] [Google Scholar]
- Park KJ, Yado MKM, Brod FPR. Estudo de secagem de pêra bartlett (Pyrus sp.) em fatias. Food Sci Technol. 2001;21(3):288–292. [Google Scholar]
- Pedreño MA, Escribano J. Correlation between antiradical activity and stability of betanine from Beta vulgaris L roots under different pH, temperature and light conditions. J Sci Food Agric. 2001;81:627–631. doi: 10.1002/jsfa.851. [DOI] [Google Scholar]
- Ravichandran K, Saw NMMT, Mohdaly AAA, Gabr AMM, Kastell A, Riedel H, Cai Z, Knorr D, Smetanska I. Impact of processing of red beet on betalain content and antioxidant activity. Food Res Int. 2013;50:670–675. doi: 10.1016/j.foodres.2011.07.002. [DOI] [Google Scholar]
- Reque PM, Steckert EV, Santos FT, Danelli D, Jablonski A, Flôres SH, Rech R, Rios AO, Jong EV. Heat processing of blueberries and its effect on their physicochemical and bioactive properties. J Food Process Eng. 2015 [Google Scholar]
- Saguy I, Goldman M, Bord A, Cohen E. Effect of oxygen retained on beet powder on the stability of betanine and vulgaxanthine-I. J Food Sci. 1984;49:99–101. doi: 10.1111/j.1365-2621.1984.tb13679.x. [DOI] [Google Scholar]
- Sivakumar V, Anna JL, Vijayeeswarri J, Swaminathan G. Ultrasound assisted enhancement in natural dye extraction from beetroot for industrial applications and natural dyeing of leather. Ultrason Sonochem. 2009;16:782–789. doi: 10.1016/j.ultsonch.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Stintzing FC, Carle R. Betalains e emerging prospects for food scientists. Trends Food Sci Technol. 2007;18:514–525. doi: 10.1016/j.tifs.2007.04.012. [DOI] [Google Scholar]
- Stratil P, Klejdus B, Kuban V. Determination of total content of phenolic compounds and their antioxidant activity in vegetables evaluation of spectrophotometric methods. J Agric Food Chem. 2006;54:607–616. doi: 10.1021/jf052334j. [DOI] [PubMed] [Google Scholar]
- Sudha ML, Baskaran V, Leelavathi K. Apple pomace as a source of dietary fiber and polyphenols and its effect on the rheological characteristics and cake making. Food Chem. 2007;104:686–692. doi: 10.1016/j.foodchem.2006.12.016. [DOI] [Google Scholar]
- Thebaudin JY, Lefebvre AC, Harrington M, Bourgeois CM. Dietary fibres: nutritional and technological interest. Trend Food Sci Technol. 1997;8:41–48. doi: 10.1016/S0924-2244(97)01007-8. [DOI] [Google Scholar]
- Vega-Mercado H, Góngora-Neto MM, Barbosa-Cánovas GV. Advances in dehydration of food. J Food Eng. 2001;49:271–289. doi: 10.1016/S0260-8774(00)00224-7. [DOI] [Google Scholar]
- Vitti MCD, Yamamoto LK, Sasaki FF, Aguila JS, Kluge RA, Jacomino AP. Quality of minimally processed beet roots stored in different temperatures. Braz Arch Biol Technol. 2005;48(4):503–510. doi: 10.1590/S1516-89132005000500001. [DOI] [Google Scholar]
- Vulic J, Canaddanovic-Brunet J, Cetkovic G, Tumbas V, Djilas S, Cetojevic-Simin D, Canadanovic V. Antioxidant and cell growth activities of beet root pomace extracts. J Funct Foods. 2012;4(3):670–678. doi: 10.1016/j.jff.2012.04.008. [DOI] [Google Scholar]
- Yagci S, Gogus F. Response surface methodology for evaluation of physical and functional properties of extruded snack foods developed from food-by-products. J Food Eng. 2008;86:122–132. doi: 10.1016/j.jfoodeng.2007.09.018. [DOI] [Google Scholar]
- Zvitov R, Nussinovitch A. Low DC electrification of gel-plant tissue ‘sandwiches’ facilitates extraction and separation of substances from Beta vulgaris beet roots. Food Hydrocolloid. 2005;19(6):997–1004. doi: 10.1016/j.foodhyd.2004.12.009. [DOI] [Google Scholar]