Abstract
The shelf life of rambutan is often limited due to rapid water loss from the spinterns and browning of the pericarp. An integrated approach, which combined hot water treatment (HWT) (56 °C for 1 min), oxalic acid (OA) dip (10% for 10 min) and modified atmosphere packaging (MAP), was used to study their effectiveness on the quality of rambutan during storage (10 °C, 90–95% relative humidity). Significant differences were observed in rambutan quality with the combination of MAP + HWT + OA after 20 days of storage. This treatment combination resulted into better retention of firmness and colour (L and a* values) than in the control. Change in the total soluble solid content was significantly delayed however the titratable acidity showed no significant change in comparison to the control at the end of storage.
Keywords: Rambutan, Oxalic acid, Firmness, Colour, Weight loss
Introduction
Rambutan (Nephelium lappaceum L.) is a non-climacteric tropical fruit in the family Sapindaceae which includes litchi and longan (Sun et al. 2010). It was domesticated in Southeast Asia with West Malaysia and Sumatra thought to be the place of origin. In 2000, approximately 6960 tonnes of fresh rambutan, worth about 4.8 million ringgit, were exported from Malaysia (Sarip 2002). The fruit is well appreciated globally due to the attractive colour of its peel and spinterns as well as the distinctive flavour of the arils.
The marketability of rambutan is often limited due to rapid skin desiccation and browning during storage (O’Hare 1995). The spinterns (hair-like protuberances) can facilitate water loss from the peel which makes the fruit highly perishable (Yingsanga et al. 2008). Landrigan et al. (1996) reported that browning of the pericarp and spinterns was positively correlated. They suggested that the marketability of rambutan following storage was unacceptable when 25–40% of water was lost and there was rapid development of browning of the pericarp. Pericarp browning of rambutan postharvest is due to the oxidation of two main phenolic substrates, epicatechin and proanthocyanin, by the enzyme polyphenol oxidase (PPO) to quinones, which further polymerise to melanin (Sun et al. 2010).
Fumigation with sulphur dioxide (SO2) and dipping in hydrochloric acid have been applied commercially to inhibit pericarp browning and extend the shelf life of longan, litchi (Saengnil et al. 2006) and rambutan (Mohamed et al. 1988). However, Mohamed et al. (1988) reported that the organoleptic properties of rambutan fumigated with SO2 were adversely affected as the skin was bleached, due to the formation of colourless anthocyanin-SO3 complex, and a strong sulphurous odour was developed in the flesh. Similarly, Latifah et al. (2009) reported that rambutan packed in polyethylene bags resulted in better retention of red colour, higher relative humidity and significantly less weight loss when compared to that of control fruit which showed accelerated senescence at the end of storage. However, the method applied can only extend the shelf life of rambutan for 6 days. Also, Nurhuda et al. (2013) reported that water blanching was effective to inhibit the enzymatic activity of PPO and polyphenol peroxidase (POD), but the peel colour was bleached after 5 min. To overcome the problems inherent with these treatments, an alternative needs to be developed to deliver safe and high quality fresh commodities to consumers.
Recently, oxalic acid (OA) has received much attention due to its potential application in the pre- or post-harvest preservation of fresh commodities. Being a natural organic acid which has some antioxidant activity, OA may confer significant benefits in relation to environmental stress, systemic resistance and anti-senescence in harvested fruits (Huang et al. 2013; Jin et al. 2014). For example, OA delayed ripening and enhanced resistance against pathogen invasion in banana (Huang et al. 2013), (Wang et al. 2009), plum (Wu et al. 2011) and mango (Zheng et al. 2007). The application of OA alone or in combination with other treatments to inhibit pericarp browning has been demonstrated in studies on litchi (Saengnil et al. 2006; Zheng and Tian 2006) and longan (Whangchai et al. 2006).
To the best of our knowledge, no study has been conducted on the combination of OA, HWT and MAP on the storage life of rambutan. Therefore, the aim of this study was to investigate the effects of this integrated approach on the physicochemical properties of rambutan postharvest.
Materials and methods
Plant material
Fresh rambutan fruits (N. lappaceum cv. Anak Sekolah) of colour index 4 with light red peel and green spinterns were purchased from Pasar Borong Selayang, Selangor during the commercial harvest in September, 2013. Fruits of uniform size, shape, free from any mechanical damage and insect or pathogenic infection were selected for this study. They were washed with 0.05% (v/v) sodium hypochlorite and air dried at ambient temperature (25 °C) for 30 min.
Experimental design
Hot water treatment of fruit was carried out in a water bath (dimensions 590 × 350 × 220 mm) at 56 °C. Polyethylene (PE) bags (thickness 0.08 mm) were purchased from a local company. Based on a preliminary study (results not shown), 10% (w/v) of OA was selected as the optimum concentration for the experiment. Fruits were divided into eight treatments with three replicates of four fruits per treatment. The following treatments were applied:
C: Fruits not subjected to any treatment served as control
MAP: Fruits packaged in PE bag
OA: Fruits dipped in 10% OA solution for 10 min
HWT: Fruits immersed in hot water bath at 56 °C for 1 min
HWT + OA: Fruits immersed in hot water bath at 56 °C for 1 min and dipped in 10% OA solution for 10 min
MAP + OA: Fruits dipped in 10% OA solution for 10 min and packaged in PE bag
MAP + HWT: Fruits immersed in hot water bath at 56 °C for 1 min and packaged in PE bag
MAP + HWT + OA: Fruits immersed in hot water bath at 56 °C for 1 min, dipped in 10% OA solution for 10 min and packaged in PE bag.
After treatment, fruits were stored at 10 °C and 90–95% relative humidity (RH). Data were collected on day 0 and at intervals of 5 for 20 days.
Firmness
Fruit firmness was measured using an Instron Universal Testing Machine (Model 5540, USA) in the compression mode. Four fruits in each replication were assessed using a 2 mm diameter probe at a speed of 300 mm/min and a load range of 100 N. The maximum force (N) to penetrate the fruit was expressed as the mean of readings taken at three positions selected randomly on the equator of the fruit.
Colour
Peel colour was determined using a HunterLab MiniScan Xe Plus (Minolta Corp., Japan). Two specific points along the equator of the fruit were used to determine the colour. The data were expressed as mean ± SE for L and a* values.
Weight loss
Four fruits in each replicate for each treatment were marked before storage and weighed on a digital balance at the beginning of the experiment and at the end of each storage interval. The results were expressed as the percentage loss of initial weight.
Total soluble solid content (TSS) and titratable acidity (TA)
The TSS (°Brix) and TA were done by method given by Ali et al. (2011). Results were expressed as (°Brix) reading and percentage of citric acid per 100 g fresh weight, respectively.
Statistical analysis
The experiment was arranged in a completely randomised design (CRD) with three replicates in each treatment. The data were subjected to analysis of variance (ANOVA) using SAS 9.1 software and means were separated using Duncan’s Multiple Range Test at P < 0.05.
Results and discussion
Firmness
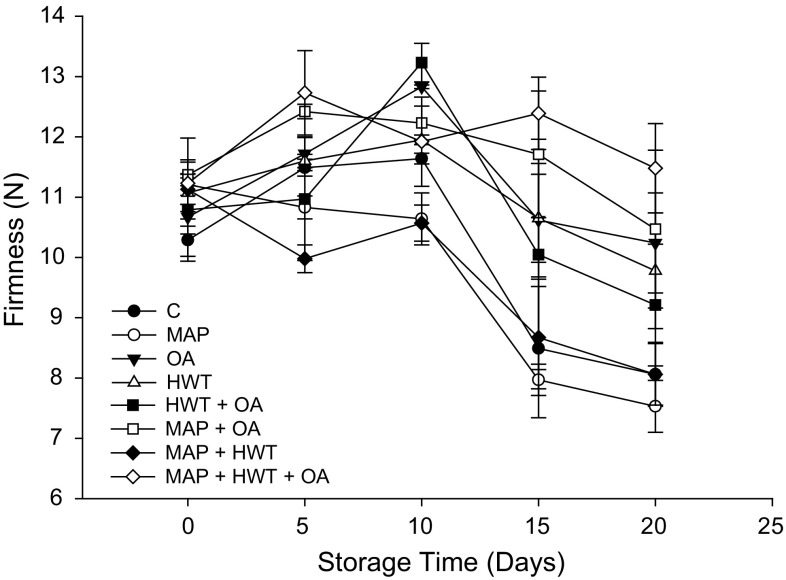
Fruit treated with MAP + HWT + OA showed significantly (P < 0.05) better retention of firmness when compared with the control (Fig. 1). Regardless of treatment, there were no differences in the firmness of treated and untreated rambutan on days 0, 5 and 10. On day 20, MAP alone was insufficient to retain firmness of the fruit. The highest firmness (11.48 N) was recorded at the end of the storage in fruit treated with MAP + HWT + OA, but was not significantly different from that of HWT or OA treated fruit. Significantly higher retention of firmness was observed in fruits treated with MAP + OA and OA on day 20 when compared to the control.
Fig. 1.
Effect of various treatments on firmness of rambutan during 20 days of storage at 10 °C and 90–95% relative humidity (RH). Values are mean ± SE
In this study, MAP alone was insufficient to retain the firmness of rambutan at the end of storage suggesting that the retention of firmness of MAP + HWT + OA treated rambutan is mainly due to the synergistic effect of OA and HWT. Martínez-Esplá et al. (2014) suggested that less softening in OA treated fruits may be due to the formation of oxalate insoluble pectin which slows down the polymerization of pectin. It has been shown that optimizing the concentration of OA and the conditions for HWT can inhibit the activity of cell wall degrading enzymes, such as polygalacturonase (PG) and pectin methyl esterase (PME), which in turn maintains the firmness of fruits (Amnuaysin et al. 2012; Wu et al. 2011). Similar results were also obtained in OA treated fruits of banana (Huang et al. 2013), mango (Li et al. 2014) and sweet cherry (Martínez-Esplá et al. 2014).
Weight loss
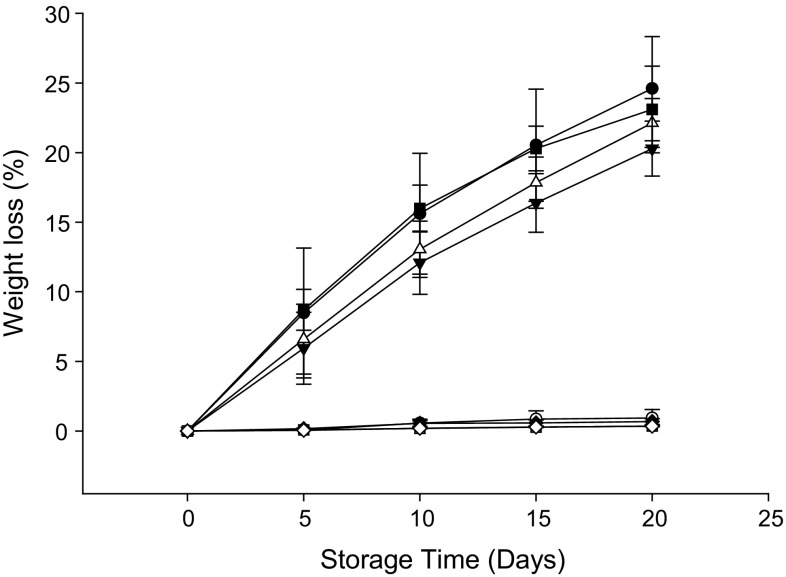
Weight loss of rambutan increased progressively with storage (Fig. 2). Minimum weight loss (0.35%) was recorded in fruit treated with MAP + HWT + OA, but there was no significant difference when compared to fruit treated with MAP, MAP + OA and MAP + HWT. The highest weight loss was recorded in control (24.6%), followed by HWT + OA (23.1%), HWT (22.1%) and OA (20.3%) treated rambutan, respectively.
Fig. 2.
Effect of various treatments on weight loss rambutan during 20 days of storage at 10 °C and 90–95% relative humidity (RH). Values are mean ± SE
It should be noted that water loss from rambutan occurs mainly through the spinterns in which the stomatal density is at least five times higher than in the main body of the fruit (Latifah et al. 2009). Consequently, the highest percentage weight loss was observed in the control fruit. MAP can control the respiration and transpiration of fruit as well as the relative humidity of the packaged fruit and hence result in lower weight loss when compared to the control.
Colour
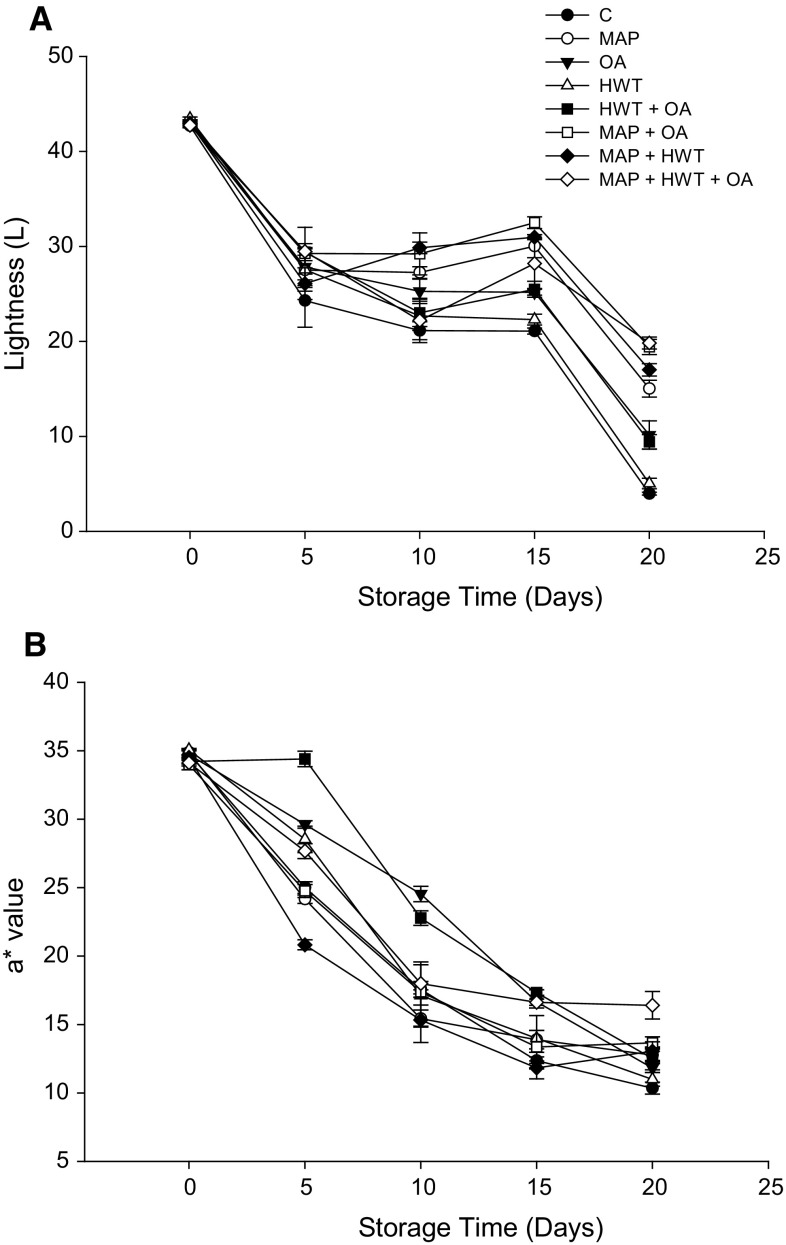
Regardless of treatment applied, the L and a* values of rambutan decreased gradually throughout the storage period (Fig. 3a, b). After 20 days, the L value of control fruit was reduced from 42.70 to 3.97, i.e. retained only 9.3% of its initial value. The highest retention of the L value (59.4%) was observed in fruit treated with MAP + HWT + OA. Similarly, a* value of rambutan treated with MAP + HWT + OA was significantly (P < 0.05) higher at the end of storage when compared with the control and other treatments. The results showed that the combination of MAP + HWT + OA can retain the bright red colour of rambutan throughout storage. In contrast, untreated rambutan became unacceptable at the end of storage as the pericarp colour changed progressively from red to dark brown. Fruits treated with MAP, HWT or OA alone showed no differences with the control at the end of storage suggesting that the treatments applied alone are ineffective to maintain the colour of rambutan.
Fig. 3.
Effect of various treatments on L (a) and a* (b) value of rambutan during 20 days of storage at 10 °C and 90–95% relative humidity (RH). Values are mean ± SE
It should be noted that the chelating property of oxalic may promote the binding of PPO with copper and POD with iron to form inactive complexes which in turn inhibit the activity of the enzymes (Saengnil et al. 2006; Zheng and Tian 2006). Saengnil et al. (2006) reported that HWT followed by oxalic acid inhibited pericarp browning in litchi fruit and suggested that HWT can reduce the high surface tension of the epidermis of the pericarp and hence enhance the permeability of oxalic acid into the pericarp of fruits. Whangchai et al. (2006) also reported that the combination of ozone and oxalic acid resulted in better colour retention of longan fruit.
TSS and TA
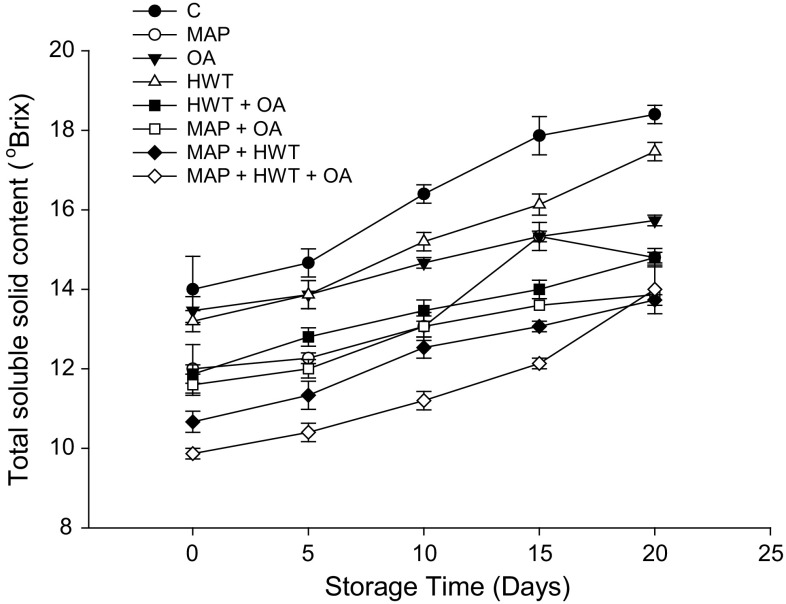
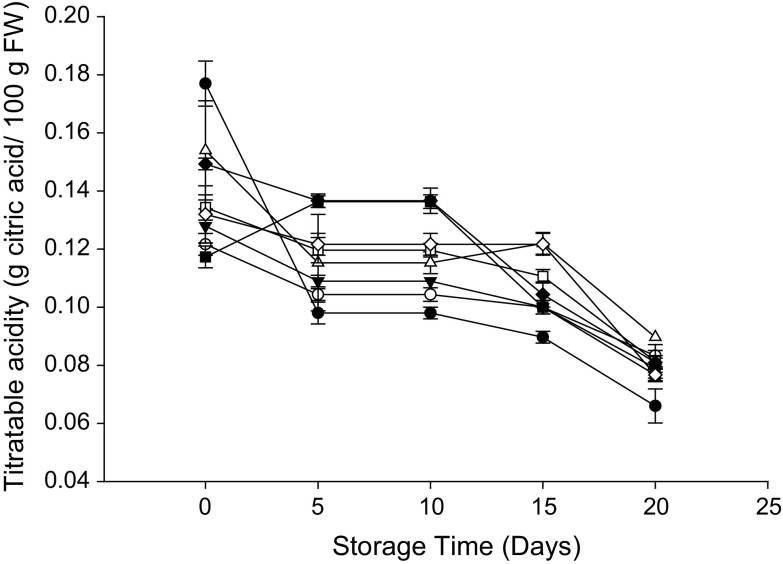
TSS increased more (P < 0.05) in the control as compared to other treatments (Fig. 4) with the highest SSC (18.4 °Brix) after storage for 20 days. In contrast, TSS in fruit treated with MAP + HWT + OA was 78.4% lower than that of the control. Regardless of treatment applied, TA of rambutan decreased throughout storage (Fig. 5). There were no differences (P > 0.05) between control and treated samples at the end of storage.
Fig. 4.
Effect of various treatments on TSS of rambutan during 20 days of storage at 10 °C and 90–95% relative humidity (RH). Values are mean ± SE
Fig. 5.
Effect of various treatments on TA of rambutan during 20 days of storage at 10 °C and 90–95% relative humidity (RH). Values are mean ± SE
The rate of increase in TSS or decrease in TA has been reported to be dependent on the metabolic activity of fruits (Mustafa et al. 2014). The accumulation of reducing sugars and utilization of organic acids was slower in MAP treated rambutan possibly due to the reduced oxygen content in the MAP system which slows down the senescence of rambutan. Similarly, Latifah et al. (2009) reported that the increase of TSS was delayed in PE packed rambutan when compared with control fruit after 6 days of storage.
Conclusion
All the treatments tested alone or in combination for different physiochemical parameters under study showed significant differences when compared to control, with different efficacies. However, treatments when used alone (MAP, OA or HWT) were insufficient to maintain firmness, water retention, colour and TSS of rambutan fruits during storage. Amongst all the combination of MAP + HWT + OA was effective in maintaining the most of the physicochemical properties of rambutan for up to 20 days in storage. The combination treatment effect is most likely due to synergistic effect. However, more in depth studies, especially on the anthocyanin content, browning enzymes such as phenylalanine ammonia-lyase (PAL), phenol peroxidase (PPO) and peroxidase (POD) and respiration of treated and untreated rambutan, should be carried out.
References
- Ali A, Muhammad MTM, Sijam K, Siddiqui Y. Effect of chitosan coatings on the physicochemical characteristics of Eksotika II papaya (Carica papaya L.) fruit during cold storage. Food Chem. 2011;124:620–626. doi: 10.1016/j.foodchem.2010.06.085. [DOI] [Google Scholar]
- Amnuaysin N, Jones ML, Seraypheap K. Changes in activities and gene expression of enzymes associated with cell wall modification in peels of hot water treated bananas. Sci Hortic. 2012;142:98–104. doi: 10.1016/j.scienta.2012.05.006. [DOI] [Google Scholar]
- Huang H, Jing G, Guo L, Zhang D, Yang B, Duan X, Jiang Y. Effect of oxalic acid on ripening attributes of banana fruit during storage. Postharvest Biol Technol. 2013;84:22–27. doi: 10.1016/j.postharvbio.2013.04.002. [DOI] [Google Scholar]
- Jin P, Zhu H, Wang L, Shan T, Zheng Y. Oxalic acid alleviates chilling injury in peach fruit by regulating energy metabolism and fatty acid contents. Food Chem. 2014;161:87–93. doi: 10.1016/j.foodchem.2014.03.103. [DOI] [PubMed] [Google Scholar]
- Landrigan M, Morris SC, Eamus D, McGlasson WB. Postharvest water relationships and tissue browning of rambutan fruit. Sci Hortic. 1996;66:201–208. doi: 10.1016/S0304-4238(96)00915-6. [DOI] [Google Scholar]
- Latifah MN, Abdullah H, Aziz I, Fauziah O, Talib Y. Quality changes of rambutan fruit in different packaging system. J Trop Agric Food Sci. 2009;37:143–151. [Google Scholar]
- Li P, Zheng X, Liu Y, Zhu Y. Pre-storage application of oxalic acid alleviates chilling injury in mango fruit by modulating proline metabolism and energy status under chilling stress. Food Chem. 2014;142:72–78. doi: 10.1016/j.foodchem.2013.06.132. [DOI] [PubMed] [Google Scholar]
- Martínez-Esplá A, Zapata PJ, Valero D, García-Viguera C, Castillo S, Serrano M. Preharvest application of oxalic acid increased fruit size, bioactive compounds, and antioxidant capacity in sweet cherry cultivars (Prunus avium L.) J Agric Food Chem. 2014;62:3432–3437. doi: 10.1021/jf500224g. [DOI] [PubMed] [Google Scholar]
- Mohamed S, Othman E, Abdullah F. Effect of chemical treatments on the shelf life of rambutans (Nephelium lappaceum)—II. Pertanika. 1988;11:407–417. [Google Scholar]
- Mustafa MA, Ali A, Manickam S, Siddiqui Y. Ultrasound-assisted chitosan–surfactant nanostructure assemblies: towards maintaining postharvest quality of tomatoes. Food Bioprocess Technol. 2014;7:2102–2111. doi: 10.1007/s11947-013-1173-x. [DOI] [Google Scholar]
- Nurhuda HH, Maskat MY, Mamot S, Afiq J, Aminah A. Effect of blanching on enzyme and antioxidant activities of rambutan (Nephelium lappaceum) peel. Int Food Res J. 2013;20:1725–1730. [Google Scholar]
- O’Hare TJ. Postharvest physiology and storage of rambutan. Postharvest Biol Technol. 1995;61:189–199. doi: 10.1016/0925-5214(95)00022-X. [DOI] [Google Scholar]
- Saengnil K, Lueangprasert K, Uthaibutra J. Control of enzymatic browning of harvested “Hong Huay” litchi fruit with hot water and oxalic acid dips. ScienceAsia. 2006;32:345–350. doi: 10.2306/scienceasia1513-1874.2006.32.345. [DOI] [Google Scholar]
- Sarip J (2002) Performance of Rambutan Genebank in Kemaman. http://www.persatuangenetikmalaysia.com/files/congress05/04Poster41.pdf. Accessed 15 Nov 2014
- Sun J, Su W, Peng H, Zhu J, Xu L, Bruñá NM. Two endogenous substrates for polyphenoloxidase in pericarp tissues of postharvest rambutan fruit. J Food Sci. 2010;75:C473–C477. doi: 10.1111/j.1750-3841.2010.01660.x. [DOI] [PubMed] [Google Scholar]
- Wang Q, Lai T, Qin G, Tian S. Response of jujube fruits to exogenous oxalic acid treatment based on proteomic analysis. Plant Cell Physiol. 2009;50:230–242. doi: 10.1093/pcp/pcn191. [DOI] [PubMed] [Google Scholar]
- Whangchai K, Saengnil K, Uthaibutra J. Effect of ozone in combination with some organic acids on the control of postharvest decay and pericarp browning of longan fruit. Crop Prot. 2006;25:821–825. doi: 10.1016/j.cropro.2005.11.003. [DOI] [Google Scholar]
- Wu F, Zhang D, Zhang H, Jiang G, Su X, Qu H, Duan X. Physiological and biochemical response of harvested plum fruit to oxalic acid during ripening or shelf-life. Food Res Int. 2011;44:1299–1305. doi: 10.1016/j.foodres.2010.12.027. [DOI] [Google Scholar]
- Yingsanga P, Srilaong V, Kanlayanarat S, Noichinda S, McGlasson WB. Relationship between browning and related enzymes (PAL, PPO and POD) in rambutan fruit (Nephelium lappaceum Linn.) cvs. Rongrien and See-Chompoo. Postharvest Biol Technol. 2008;50:164–168. doi: 10.1016/j.postharvbio.2008.05.004. [DOI] [Google Scholar]
- Zheng X, Tian S. Effect of oxalic acid on control of postharvest browning of litchi fruit. Food Chem. 2006;96:519–523. doi: 10.1016/j.foodchem.2005.02.049. [DOI] [Google Scholar]
- Zheng X, Tian S, Gidley MJ, Yue H, Li B. Effects of exogenous oxalic acid on ripening and decay incidence in mango fruit during storage at room temperature. Postharvest Biol Technol. 2007;45:281–284. doi: 10.1016/j.postharvbio.2007.01.016. [DOI] [Google Scholar]