Abstract
The aim of this study was to evaluate the presence and possibility of extracting compounds with antioxidant properties of soybean cake to extend the storage stability of soybean oil. Results showed that the highest DPPH radical scavenging activity was observed for sample to solvent ratio 1:25 while extracting by 70% ethanol for 3 h). The most phenolic compounds equivalents (Gallic acid) was observed for sample to solvent ratio 1:25 while extracting by 70% methanol for 14 h. In addition, the soybean cake extract at concentrations of 50, 100, 150 and 200 ppm in soybean oil could significantly lower the peroxide, diene and p-anisidine values of soy oil during storage at 65 °C.
Keywords: Antioxidant, Soybean cake extracts, Oil protection
Introduction
Vegetable oils with higher contents of unsaturated fatty acids are more susceptible to oxidation. The oxidative changes decrease the nutritional quality and safety of oil due to degradation products, resulting in harmful effects on human health (Rehman et al. 2004; Mohdaly et al. 2011). Antioxidants are added to foods to delay oxidation process through terminating radical chain reactions (Wanasundara and Shahidi 2005; Bashash et al.2014). There is great concern about synthetic antioxidants such as butylated hydroxytoluene (BHT), butylated hydroxyanisole (BHA) and tert-butyl hydroquinone (TBHQ) due to their toxic effects on human health (Rehman et al. 2004). Fruits, leaves, seeds, and oils are natural resources of antioxidants such as flavonoids, tannins, phenolic compounds and terpenoids (Mohdaly et al. 2011). The importance of exploiting natural antioxidants from by-products obtained during food processing has increased in recent years. Replacing synthetic antioxidants by natural ones has advantages like health implications solubility in both oil and water, for food emulsions. However, there are diverse restrictions in utilizing herbs and spices due to the fact that they cause herb flavor in foods and deodorization steps may be needed (Moure et al. 2001). Extract production is a key step to obtain antioxidants with an acceptable yield. Solvent extraction is frequently used for isolation of antioxidant and extraction yield depends on the solvent and extraction method, due to the different antioxidant extraction potentials of compounds with different polarity (Moure et al. 2001; Terpinc et al. 2012). Some of the antioxidants which are not extracted in oil very well or occur in conjugation with sugar molecules, remain in the meal after oil extraction of oil seeds. This meal, also referred to as oil cake, is a good source of nutraceuticals and functional ingredients (Terpinc et al. 2012). Soybean (Glycine max L.) is one of the most commonly consumed legumes worldwide, with 200 million metric tons produced per year with each ton of crude soybean oil, approximately 4.5 tons of soybean oil meal with a protein content of about 44% are produced (FAO Web 2006). It has phytochemicals with antioxidant activity (Prakash et al. 2007).
Soybean cake was evaluated as a source of natural antioxidant in this study. The objective of this work was to compare extraction of phenolic compounds of soybean cake with two solvents ethanol 70% (v/v) and methanol 70% (v/v), two sample to solvent ratios (1:25 and 1:100) and two extraction times (3 and 14 h) by determining the total phenolic content (TPC) of extracts according to the Folin–Ciocalteu method and their ability for DPPH radical scavenging.
Materials and methods
Materials and reagents
Soybean cake, soybean oil without antioxidant and TBHQ was obtained from Naz oil company (Isfahan, Iran) in December 2013. Methanol and Folin–Ciocalteu were purchased from Merck, Germany. Ethanol was purchased from Scharlou, Spain and 1,1-Diphenyl-2-picryl hydrazyl (DPPH), diadzein (98%) and genistein (98%) were purchased from Sigma Chemical Co., Aldrich. Gallic acid was purchased from UCB, Belgium.
Composition analysis of soybean cake
The major chemical constituents of soybean cake including moisture, ash, crude fat, crude fiber and crude protein were determined in triplicate. Water contents of the sample was measured by drying in a vacuum oven (Fine Tech, model: SSVO-502, South Korea) at 50 °C for 48 h (AOAC 1990). The protein contents were measured based on Kjeldahl method (AOAC 1990). The fat contents were measured based on Soxhlet extraction according to the method of Aa 4–38 (AOCS 1993). Total dietary fiber was determined based on the method of 991.43 (AOAC 1997). Ash contents were measured according to AOAC official methods of analysis (AOAC 1990).
Sample preparation
Commercial soybean cake was ground in a home style coffee grinder. The ground material was then passed through a standard 40-mesh sieve (particle size <0.420 mm). Soybean cake powders were extracted by 70% (v/v) ethanol and methanol (Tyug et al. 2010: Terpinc et al. 2012) in two ratios of 1:25 and 1:100 (w/v) (Hubert et al. 2008;Tyug et al. 2010) and were incubated in an orbital shaker set at 150 rpm for 3 and 14 h (Lafka et al. 2007; Mohdaly et al. 2011) at room temperature. Then extraction mixtures were filtered by paper and obtained filtrates were kept at −20 °C prior to analysis.
Determination of total phenolic compounds
The content of total phenolic compounds in extracts was determined according to the Folin–Ciocalteu procedure (Bamdad et al. 2006). Extracts (1 ml) were poured into the test tubes; 5 ml of Folin–Ciocalteu reagent (1:10 diluted) and 4 ml of sodium carbonate (7.5%) were added. The tubes were mixed and allowed to stand for 30 min. Absorption was measured at 760 nm (S 2100 Double Beam UV–Visible Spectrophotometer). Gallic acid was used as a standard for calibration curve.
Determination of DPPH radical scavenging capacity
1,1-diphenyl-2-picrylhydrazyl (DPPH) procedure described by Mohdaly et al. (2011) was performed. Dissolving 22 mg of DPPH in 50 mL methanol, the stock reagent solution (1 × 10−3 M) was prepared and stored at −20 °C until use. 6 mL of the stock solution was mixed with 100 mL methanol to obtain an absorbance value of 0.8 at 515 nm for working solution (6 × 10−5 M). The soybean extracts were mixed with 3.9 mL of the DPPH solution and vortexed during 30 s and then the absorbance was measured at 515 nm after 30 min. A control sample without extract was also analyzed and the scavenging percentage was calculated according to the Eq. (1):
| 1 |
Chromatographic determination of phenolic compounds
Two forms of isoflavones, genistein and daidzein, have been found to be the major isoflavones in soybean seeds with the ability to inhibit breast cancer (Kim et al. 2006), so the sample with the highest amount of DPPH radical scavenging capacity was analyzed by HPLC using method of IOOC (International Olive Council 2009; Tyug et al. 2010) to detect the amount of genistein and daidzein presence. For the separation a reversed-phase Lichrospher C18 column (250–4 mm, I.D. 5 lm, Merck, Darmstadt, Germany) was applied. A constant flow rate of 0.6 ml/min and gradient elution with 0.5% (v/v) acetic acid (solvent A) and 100% methanol (solvent B) was performed. The gradient profile was: 100% A and 0% B at the beginning, 10% A and 90% B at 20–25 min and then back to 100% A and 0% B at 30 min. The column was set at room temperature (25 °C) and the sample extract (20 µl) was injected into the column. Monitoring the UV–Vis absorption spectra by DAD at 200–600 nm the phenolic compounds were detected at the wavelength of 280 nm.
Oil storage studies
Refined, bleached and deodorized soybean oil—without any added synthetic antioxidant such as TBHQ supplied by Naz Oil Company—was used for storage studies. Experiments were carried out with a synthetic antioxidant such as TBHQ at 200 ppm level, pure genistein and pure diadzein at 50, 100 and 150 ppm level. The extract of soybean cake by sample to solvent ratio 1:25 extracting by 70% ethanol for 3 h was completely concentrated at 50 °C and this concentrated extract was used for other treatments of oil storage experiments at 50, 100, 150, 200 ppm level. Oil samples and control—oil without added antioxidants—were stored in uniform glass containers at 65 °C for a definite period in an incubator. Samples were analyzed after 0, 1, 5, 9 and 13 days for peroxide value, diene value [conjugated di ene (CD) or conjugated tri ene (CT)], p-anisidine value and totox value (AOAC 1990; AOCS 1993; AOAC 1997) to follow the oxidative changes. The inhibition of oil oxidation (IO) percentage is given by:
| 2 |
| 3 |
The absorbance was measured at 350 nm for p-anisidine value.
| 4 |
| 5 |
Where cCD stands for concentration of congugated di ene, cCT stands for concentration of congugated tri ene and W is the weight of oil sample. All experiments were conducted with duplicate sets, and analyses of samples were run in triplicate and averaged.
Statistical analysis
The experiment was conducted in factorial form, using a completely randomized design with three replications.
Statistical analyses were conducted using SAS version 9.1.2 from SAS Institute, Inc., Cray, NC, USA. Data were reported as mean ± standard deviation (SD). Data were subjected to analysis of variance (ANOVA) and Duncan’s multiple range method was used for comparisons of means. The confidence interval used in this study was at 95% (P < 0.05).
Results and discussion
Composition of soybean cake
Soybean cake consisted of 11.3% moisture, 0.55% fat, 46% protein, 4.4% crude fiber and 7% ash respectively. Results indicated if the anti-nutritional compounds such as trypsin inhibitor (Hwei-Ming et al. 1997) is ignored or removed the soybean cake can be used as a source of protein.
DPPH radical scavenging activity
Results showed DPPH free radical assay depends on the solvent, the sample to solvent ratio and extraction time (Table 1). The highest DPPH radical scavenging activity was reported for sample to solvent ratio 1:25 while extracting by 70% ethanol for 3 h (P < 0.05). The present study has made the use of DPPH free radical-scavenging method as the way to evaluate the antioxidant capacity of the three sample extracts. DPPH is a very stable organic free radical with deep violet color which gives maximum absorption within 515–528 nm range. Upon receiving proton from any hydrogen donor, mainly from phenolic compounds, it loses its chromophore and becomes yellow. As the concentration of phenolic compounds or degree of hydroxylation of the phenolic compounds increases DPPH radical scavenging activity also increases and it can be defined as antioxidant activity (Khan et al. 2015; Sabir et al. 2015). Since these radicals are very sensitive to the presence of hydrogen donors, the whole system operates at a very low concentration which allows a large number of samples to be tested in a short time (Bursal and Köksal 2010; Mohdaly et al. 2011). As concluding remarks, extract of soybean cake by sample to solvent ratio 1:25 extracting by 70% ethanol for 3 h, exhibited high DPPH value which could be interpreted as the highest antioxidant capacity among the three studied samples. Results were in agreement with earlier observations in other studies (Malenčić et al. 2007; Kumar et al. 2010; Lafka et al. 2011).
Table 1.
Effect of the solvent, the ratio of sample to solvent and extraction time on the DPPH radical scavenging activity (%)
| Time (h) | The ratio of sample (gr) to solvent (ml) | |||
|---|---|---|---|---|
| 1:25 | 1:100 | |||
| Et. | Met. | Et. | Met. | |
| DPPH radical scavenging activity (%) | ||||
| 13 | 36.55a | 23.20b | 11.63cd | 6.79d |
| 14 | 23.60b | 15.08c | 10.59cd | 6.97d |
Values are mean ± SD. Values with the same superscript letter for each parameter are not statistically significant at the 5% level
Total phenol contents
Total phenolic compounds expressed as gallic acid equivalent (GAE) was calculated using Eq. (6) based on calibration curve:
| 6 |
where A is the absorbance and C is the concentration of phenolic compounds (mg GAE kg−1 DW).
The concentration of phenolic compounds in the extracts, expressed as mg GAE/kg sample, was dependent on the solvent, the ratio of sample to solvent and extraction time. The most phenolic compounds equivalents (Gallic acid) was reported for sample to solvent ratio 1:25 while extracting by 70% methanol for 14 h (P < 0.05) (Table 2). Phenolic compounds are widely distributed in the plants. These compounds serve as important antioxidants because of their ability to donate a hydrogen atom or an electron in order to form stable radical intermediates. Hence, they prevent the oxidation of various biological molecules (Mohdaly et al. 2011). The seeds of oil crops, particularly those containing a high percentage of polyunsaturated fatty acids are thought to be rich in antioxidants. Oil extraction process influences the partitioning behavior of the phenols and their distribution between the oil and cake. Obviously, a higher amount of phenolic compounds is released from the seeds when the oil is extracted at higher temperatures and higher pressure (Prakash et al. 2007). The highest TPC value was observed for extract of soybean cake while extracting by 70% methanol for 14 h by sample to solvent ratio of 1:25 (1042.1 mg GAE/kg), whereas TPC values of ethanolic extract of soybean cake and whole soybean seeds by sample to solvent ratio of 1:25 extracting for 3 h, were 457.7 mg/kg and 845.1 mg GAE/kg, respectively. The results were in agreement with earlier observations in other studies (Peschel et al. 2006; Kumar et al. 2010; Tyug et al. 2010).
Table 2.
Effect of the solvent, the ratio of sample to solvent and extraction time on the total phenolic content (ppm)
| Time (h) | The ratio of sample (gr) to solvent (ml) | |||
|---|---|---|---|---|
| 1:25 | 1:100 | |||
| Et. | Met. | Et. | Met. | |
| Total phenolic content expressed as gallic acid equivalent (ppm) | ||||
| 3 | 457.3b | 990.6a | 357.3b | 372.4b |
| 14 | 396.7b | 1042.1a | 569.4b | 293.6b |
Values are mean ± SD. Values with the same superscript letter for each parameter are not statistically significant at the 5% level
Solvents such as methanol, ethanol, acetone, propanol, ethyl acetate and dimethylformamide were commonly used for the extraction of phenolics from fresh products at different concentrations. The solubility of the phenolic compounds in the solvent used for the extraction process affects the recovery of polyphenols from plant materials. Solvent polarity also has a key role in increasing phenolic solubility (Alothman et al. 2008). TPC values in methanolic extracts were greater than ethanolic extract as the same as research of Lafka et al. (2011) on the olive oil mill waste, but TPC values in winery wastes in mixture of ethanol:water (1:1) extract were greater than methanolic extract (Lafka et al. 2007). Peschel et al. (2006) reported that the methanolic and ethanolic extracts of 11 fruit and vegetable by-products exhibited higher phenolic contents than the other conventional solvent extracts, which is in agreement with the data reported in present study. Although high yield of TPC was achieved using methanol for the extraction of phenols from soybean cake waste, methanol is not a food grade solvent because of its high toxicity (Peschel et al. 2006). So ethanol was selected as the most appropriate solvent for the extraction of phenolic compounds from soybean residues for the production of extracts with high phenol content and high antioxidant activity.
HPLC analysis of phenolic compounds
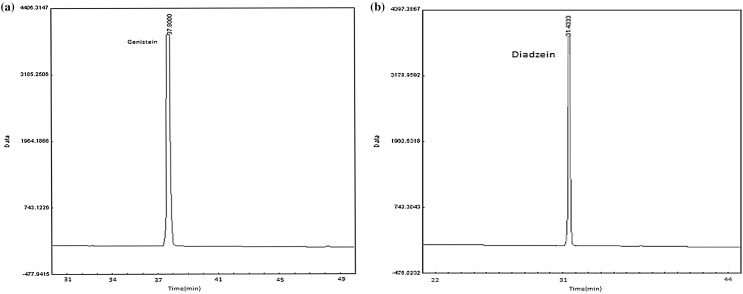
A typical HPLC chromatogram of the genistein and diadzein is shown in Fig. 1. Genistein and diadzein percent in soy cake extract were 8.44 and 10.14 respectively, according to peak area measurement of sample chromatogram. Accumulation of phenolic compounds is dependent on soil, cultivar and environmental conditions during the seed formation. Depending on the conditions imposed by soil, season, climate, plant component and other parameters, the phenolic profiles of the plants may be changed (Kim et al. 2006; Prakash et al. 2007). The appreciable concentrations of flavonoids along with phenolic acids and other antioxidant phytochemicals present in different soybean and their agri-wastes under study might be responsible for their efficient free radical-scavenging activity.
Fig. 1.
Peaks of genistein and diadzein identified by HPLC in a standard solution of genistein; b standard solution of diadzein
Storage stability analysis
Table 3 shows the peroxide value (PV) developments during the 13 days storage of soybean oil at 65 °C with various concentrations of diadzein (D), genistein (G), extracts (E), TBHQ and control containing no antioxidants. Control and soybean oil containing 50 ppm genistein (G50) reached a maximum PV after 9 days of storage. The sample with 150 ppm genistein had lower peroxide value than the control sample (P < 0.05). These results indicated that genistein extract inhibited soybean oil oxidation. Further, the peroxide value of the samples containing genistein extract at 150 ppm showed significant difference with control sample at P < 0.05 after 9 days. The protective effect offered at 150 ppm had no significant difference with control sample after 13 days (P < 0.05). The analysis showed that on the 13th day of the storage the soy oil containing diverse concentrations of soy cake ethanolic extracts had lower peroxide value in comparison to all treatments at P < 0.05. Furthermore, comparing the peroxide value of the samples including E50, E100, E150, and E200 revealed that they did not have significant difference (P < 0.05). However, the E150 concentration showed lower peroxide value comparing to other concentrations of extract. Totally, on the 13th day the samples with diadzein extract at all levels had lower PV than the samples with genistein at all levels (P < 0.05).
Table 3.
Peroxide value (mequiv. O2/kg) of soybean oil containing genistein, diadzein, TBHQ and extracts at different concentrations
| Antioxidant | Time (day) | ||||
|---|---|---|---|---|---|
| 0 | 1 | 5 | 9 | 13 | |
| Blank | 0.79 ± 0.13r | 0.82 ± 0.06r | 71.54 ± 1. 3fg | 218.1 ± 2.13a | 181.18 ± 2.48b |
| G50 | 0.79 ± 0.13r | 1.99 ± 0.8r | 45.25 ± 1.78h–k | 111.48 ± 2.39d | 45.15 ± 2.95h–k |
| G100 | 0.79 ± 0.13r | 0.76 ± 0.15r | 38.34 ± 1.85j–m | 143.95 ± 2.42c | 207.02 ± 2.04a |
| G150 | 0.79 ± 0.13r | 0.59 ± 0.1r | 21.86 ± 1.34n–q | 29.91 ± 1.71k–n | 180.96 ± 2.05b |
| D50 | 0.79 ± 0.13r | 0.95 ± 0.06r | 31.09 ± 1.2j–n | 55.64 ± 1.6hi | 90.43 ± 1.97e |
| D100 | 0.79 ± 0.13r | 0.86 ± 0.11r | 22.89 ± 1.2m–p | 56.93 ± 1.6gh | 91.61 ± 2.21e |
| D150 | 0.79 ± 0.13r | 0.72 ± 0.11r | 18.43 ± 1.96n–q | 41 ± 1.28i–l | 82.7 ± 1.42ef |
| T200 | 0.79 ± 0.13r | 0.83 ± 0.55r | 26.87 ± 2.61I–o | 80.5 ± 1.7ef | 89.7 ± 1.55e |
| E50 | 0.79 ± 0.13r | 0.49 ± 0.0r | 25.76 ± 2.15I–P | 33.57 ± 1.91j–n | 30.32 ± 1.1k–n |
| E100 | 0.79 ± 0.13r | 0.56 ± 0.06r | 13.69 ± 1.08o–r | 26.93 ± 1.5I–o | 32.64 ± 1.29j–n |
| E150 | 0.79 ± 0.13r | 0.42 ± 0.05r | 7.3 ± 0.13qr | 21.02 ± 1.42n–q | 26.52 ± 1.32I–o |
| E200 | 0.79 ± 0.13r | 0.7 ± 0.01r | 10.52 ± 0.5pqr | 46.3 ± 1.32hij | 48.37 ± 1.59h–k |
Values are mean ± SD. Values with the same superscript letter for each parameter are not statistically significant at the 5% level
Changes in p-anisidine value which represent the secondary oxidation products produced during the oxidative degradation of oil are shown in Table 4. The formation of secondary oxidation products also increased during storage. A significant difference was noted between the p-anisidine values of control sample, the sample with genistein extract at 50 ppm concentration (G50) and TBHQ at 200 ppm concentration (T200) on the 9th and on the 13th days (P < 0.05). Furthermore, the p-anisidine values of G50, T200 and control in 9th day were higher than the 13th day. To put it more clear, on the 9th day the value reached to its maximum extent and then decreased. According to Table 4, the p-anisidine values of the samples of other treatments increased during 13 days of storage. In addition, comparing the mean values of the samples on the 13th day showed that the samples with various concentrations of diadzein extract (D50, D100, D150) and soy cake ethanolic extract (E50, E100, E150, E200) had lower p-anisidine value than samples with various concentrations of genistein extract (G50, G100, G150, G200) and control (P < 0.05). It is evident that the samples with different levels of soy cake ethanolic extract (E50, E100, E150, E200) showed lower p-anisidine value (P < 0.05) comparing to other treatments. Comparing the samples with different levels of soy cake ethanolic extract (E50, E100, E150, and E200) showed the least p-anisidine value for E150 but the difference between E150 and other levels of soy cake ethanolic extract was not significant (P < 0.05).
Table 4.
p-anisidine value of soybean oil containing genistin, diadzein, TBHQ and extracts at different concentrations
| Antioxidant | Time (day) | |||
|---|---|---|---|---|
| 1 | 5 | 9 | 13 | |
| Blank | 1.58 ± 0.27tu | 18.71 ± 1.38hi | 53.39 ± 1.03b | 30.55 ± 1.4de |
| G50 | 4.68 ± 0.22m–u | 8.3 ± 0.91I–o | 30.68 ± 0.58de | 25.34 ± 1.3fg |
| G100 | 3.4 ± 0.9p–u | 8.75 ± 1.02Imn | 26.9 ± 0.98ef | 63.98 ± 1.14a |
| G150 | 2.86 ± 0.46q–u | 7.76 ± 0.92I–q | 4.84 ± 0.65m–u | 33.03 ± 1.9d |
| D50 | 2.22 ± 0.67stu | 7.8 ± 1.25I–q | 16.54 ± 0.43ij | 21.6 ± 1.94gh |
| D100 | 2.25 ± 0.81stu | 6.13 ± 0.98I–u | 13.52 ± 0.64jk | 19.26 ± 1.45hi |
| D150 | 2.75 ± 0.75r–u | 4.05 ± 1.44n–u | 4.36 ± 0.59n–u | 18.95 ± 1.00hi |
| T200 | 2.91 ± 0.43p–u | 9.74 ± 1.27KI | 37.61 ± 0.51c | 19.03 ± 1.3hi |
| E50 | 4.11 ± 0.96n–u | 7.61 ± 1.6I–r | 7.85 ± 0.66I–t | 10.75 ± 0.26kI |
| E100 | 2.83 ± 0.51q–u | 3.46 ± 0.53o–u | 6.43 ± 0.41klm | 7.49 ± 2.29I–r |
| E150 | 2.72 ± 0.71r–u | 2.09 ± 0.62tu | 7.19 ± 0.34I–s | 7.1 ± 1.03I–s |
| E200 | 2.73 ± 0.42r–u | 7.89 ± 0.65I–p | 7.75 ± 0.69I–q | 16.6 ± 3.8ij |
Values are mean ± SD. Values with the same superscript letter for each parameter are not statistically significant at the 5% level
Diene values also represent the formation of hydroperoxides which are shown in Table 5. Antioxidants are mainly used in lipids to delay the accumulation of primary oxidation products and thus to improve the oxidative stability. The primary products of lipid peroxidation are hydroperoxides which are generally referred to as peroxides. Therefore the results of peroxide value estimation give a clear indication of lipid autoxidation. Other oxidation parameters such as diene value and p-anisidine values were also measured, to confirm more on these results. Thus PV, diene value and p-anisidine value of soybean oil that contained the extract were significantly lower than that of the control (P < 0.05) which clearly showed the marked antioxidant effect of the soybean cake extract in soybean oil protection.
Table 5.
Diene value (µmol/g) of soybean oil containing genistin, diadzein, TBHQ and extracts at different concentrations
| Antioxidant | Time (day) | ||||
|---|---|---|---|---|---|
| 0 | 1 | 5 | 9 | 13 | |
| Blank | 0.45 ± 0.04n | 25.34 ± 0.66g–m | 53.61 ± 0.63ef | 102.5 ± 1.13c | 143.23 ± 1.48a |
| G50 | 0.45 ± 0.04n | 11.92 ± 0.8mn | 35.57 ± 0.78f–I | 63.48 ± 1.39de | 78.54 ± 1.95d |
| G100 | 0.45 ± 0.04n | 13.43 ± 1.62mn | 24.22 ± 0.85h–m | 50.33 ± 1.42ef | 146.43 ± 1.04a |
| G150 | 0.45 ± 0.04n | 20.43 ± 1.16j–n | 36.92 ± 1.34f–I | 39.61 ± 1.71f–k | 134.3 ± 1.35ab |
| D50 | 0.45 ± 0.04n | 18.49 ± 1.69j–n | 19.56 ± 1.2j–n | 47.26 ± 1.6ef | 117.22 ± 1.97bc |
| D100 | 0.45 ± 0.04n | 12.85 ± 1.57mn | 25.06 ± 1.2g–m | 42.58 ± 1.63fi | 109.9 ± 1.21c |
| D150 | 0.45 ± 0.04n | 17.68 ± 1.49Imn | 19.86 ± 1.96j–n | 53.47 ± 1.28ef | 143.43 ± 1.42a |
| T200 | 0.45 ± 0.04n | 16.98 ± 1.6Imn | 25.72 ± 1.61g–m | 54.08 ± 1.7ef | 111.55 ± 1.33c |
| E50 | 0.45 ± 0.04n | 15.42 ± 1.77Imn | 33.57 ± 1.15f–m | 44.61 ± 1.91e–h | 45.96 ± 1.36efg |
| E100 | 0.45 ± 0.04n | 15.60 ± 1.9Imn | 21.54 ± 1.08i–n | 35.93 ± 1.5f–I | 41.05 ± 1.28f–j |
| E150 | 0.45 ± 0.04n | 16.1 ± 1.5Imn | 17.82 ± 1.13Imn | 36.67 ± 1.42f–I | 39.86 ± 1.36f–k |
| E200 | 0.45 ± 0.04n | 16.18 ± 1.51Imn | 16.47 ± 1.5Imn | 17.08 ± 0.63Imn | 19.84 ± 1.11j–n |
Values are mean ± SD. Values with the same superscript letter for each parameter are not statistically significant at the 5% level
The diene values of the samples increased during 13 days of storage. The samples containing diverse concentrations of soy cake ethanolic extract (E50, E100, E150, E200) showed lower diene value (P < 0.05). The extracts (E50, E100, E150, E200) had not significant difference at different concentrations on the 13th day of storage but the diene value of the sample at E200 concentration was lower than other concentrations of extract (P < 0.05).
The averaged changes of the totox values in samples are represented in Table 6. The analysis showed that the totox value of the samples increased during 13 days of storage at 65 °C (P < 0.05). Furthermore, comparing the totox value of the samples containing 50 ppm genistein on the 9th day and on the 13th day revealed that the value reached to its maximum extent on the 9th day and then decreased (P < 0.05). In addition, the samples at different concentrations soy cake ethanolic extract (E50, E100, E150, E200) showed lower value in comparison to all other treatments on the 13th day (P < 0.05). The extracts (E50, E100, E150, E200) showed no significant differences at different concentrations on the 13th day of storage but the totox value of the sample at E150 concentration was lower than other concentrations of extract (P < 0.05). The samples at all levels of diadzein showed lower totox value than the samples with genistin on the 13th day (Table 6).
Table 6.
Totox value of soybean oil containing genistin, diadzein, TBHQ and extracts at different concentrations
| Antioxidant | Time (day) | ||||
|---|---|---|---|---|---|
| 0 | 1 | 5 | 9 | 13 | |
| Blank | 4.8 ± 0.21p | 4.88 ± 0.71p | 161.78 ± 0.89fg | 489.6 ± 1.15a | 392.9 ± 1.52b |
| G50 | 4.8 ± 0.21p | 8.66 ± 0.58p | 98.8 ± 0.46hij | 253.64 ± 1.95d | 115.64 ± 1.56hi |
| G100 | 4.8 ± 0.21p | 4.92 ± 0.31p | 85.44 ± 0.87ijk | 314.75 ± 1.16c | 478.01 ± 1.33a |
| G150 | 4.8 ± 0.21p | 4.04 ± 0.64p | 51.47 ± 0.86k–o | 64.66 ± 1.63j–n | 394.94 ± 1.82b |
| D50 | 4.8 ± 0.21p | 4.13 ± 0.87p | 69.97 ± 0.17j–n | 127.82 ± 1.45gh | 202.46 ± 1.22e |
| D100 | 4.8 ± 0.21p | 3.97 ± 0.33p | 51.9 ± 0.77k–o | 127.37 ± 1.35gh | 202.48 ± 1.12e |
| D150 | 4.8 ± 0.21p | 4.19 ± 0.68p | 40.91 ± 0.47I–p | 86.35 ± 1.48ijk | 184.35 ± 1.83ef |
| T200 | 4.8 ± 0.21p | 4.57 ± 0.52p | 63.47 ± 0.61 m–o | 198.63 ± 1.42e–h | 198.44 ± 1.80ef |
| E50 | 4.8 ± 0.21p | 5.09 ± 0.96p | 59.12 ± 0.68j–n | 73.98 ± 1.23j–m | 71.52 ± 1.28j–n |
| E100 | 4.8 ± 0.21p | 3.95 ± 0.56p | 30.84 ± 0.63m–p | 63.29 ± 1.61j–n | 72.77 ± 1.37j–n |
| E150 | 4.8 ± 0.21p | 3.57 ± 0.19p | 16.69 ± 0.71op | 49.23 ± 1.74k–p | 60.14 ± 1.54j–n |
| E200 | 4.8 ± 0.21p | 4.12 ± 0.42p | 28.93 ± 0.41nop | 100.34 ± 1.69hi | 103.33 ± 1.85i–L |
Values are mean ± SD. Values with the same superscript letter for each parameter are not statistically significant at the 5% level
Conclusion
The purpose of the current study was to determine the effect of soybean cake extracts in protection of soybean oil. Results showed the highest DPPH radical scavenging activity for sample to solvent ratio 1:25 while extracting by 70% ethanol for 3 h (P < 0.05). The most phenolic compounds equivalents (Gallic acid) was reported for sample to solvent ratio 1:25 while extracting by 70% methanol for 14 h. Extraction for 3 h was better than 14 h extraction time and using 1:25 sample to solvent ratio was better than 1:100 sample to solvent ratio for extraction of polyphenols and antioxidants (P < 0.05). The geistein extract at diverse concentrations had no protection effects until the 13th day. In preventing soy oil oxidation, diadzein at different concentrations was more effective than geistein. Moreover, all levels of soy cake ethanolic extract (E50, E100, E150, and E200) showed protection effects until the 13th day and E150 was the best treatment for this purpose.
References
- Alothman M, Bhat R, Karim AA. Antioxidant capacity and phenolic content of selected tropical fruits from Malaysia, extracted with different solvents. Food Chem. 2008;115:785–788. doi: 10.1016/j.foodchem.2008.12.005. [DOI] [Google Scholar]
- AOAC . Official methods of analysis. 15. Washington: Association of Official Analytical Chemists; 1990. [Google Scholar]
- AOAC . Official methods of analysis. 16. Gaithersburg: AOAC; 1997. [Google Scholar]
- AOCS . Official methods and recommended practices of the American Oil Chemists Society. Washington: The American Oil Chemists Society; 1993. [Google Scholar]
- Bamdad F, Kadivar M, Keramat J. Evaluation of phenolic content and antioxidant activity of Iranian caraway in comparison with clove and BHT using model systems and vegetable oil. Int J Food Sci Technol. 2006;41:20–27. doi: 10.1111/j.1365-2621.2006.01238.x. [DOI] [Google Scholar]
- Bashash M, Zamindar N, Bolandi M. Evaluation of antioxidant activities of Iranian sumac (R. coriaria L.) fruit and spice extracts with different solvents. Food Meas. 2014 [Google Scholar]
- Bursal E, Köksal E. Evaluation of reducing power and radical scavenging activities of water and ethanol extracts from sumac (Rhus coriaria L.) Food Res Int. 2010;44:2217–2221. doi: 10.1016/j.foodres.2010.11.001. [DOI] [Google Scholar]
- International Olive Council (2009) Determination of biophenols in olive oils by HPLC. International olive council, COI/T.20/Doc No 29
- Food and Agriculture Organization of the United Nations (FAO Web) (2006). http://faostat.fao.org/. Accessed 2006
- Hubert J, Berger M, Nepveu F, Paul F, Daydé J. Effects of fermentation on the phytochemical composition and antioxidant properties of soy germ. Food Chem. 2008;109:709–721. doi: 10.1016/j.foodchem.2007.12.081. [DOI] [PubMed] [Google Scholar]
- Hwei-Ming B, Christian V, Jean-Pierre N, Luc M. Effect of germination on chemical composition, biochemical constituents and antinutritional factors of soy bean (Glycine max) seeds. J Sci Food Agric. 1997;73:1–9. doi: 10.1002/(SICI)1097-0010(199701)73:1<1::AID-JSFA694>3.0.CO;2-B. [DOI] [Google Scholar]
- Khan H, Jan SA, Javed M, Shaheen R, Khan Z, Ahmad A, Safi SZ, Imran M. Nutritional composition, antioxidant and antimicrobial activities of selected wild edible plants. J Food Biochem. 2015 [Google Scholar]
- Kim EH, Kim SH, Chung JI, Chi HY, Kim JA, Chung IM. Analysis of phenolic compounds and isoflavones in soybean seeds (Glycine max (L.) Merill) and sprouts grown under different conditions. Eur Food Res Technol. 2006;222:201–208. doi: 10.1007/s00217-005-0153-4. [DOI] [Google Scholar]
- Kumar V, Rani A, Dixit AK, Pratap D, Bhatnagar D. A comparative assessment of total phenolic content, ferric reducing-anti-oxidative power, free radical-scavenging activity, vitamin C and isoflavones content in soybean with varying seed coat colour. Food Res Int. 2010;43:323–328. doi: 10.1016/j.foodres.2009.10.019. [DOI] [Google Scholar]
- Lafka TI, Sinanoglou V, Lazos ES. On the extraction and antioxidant activity of phenolic compounds from winery wastes. Food Chem. 2007;104:1206–1214. doi: 10.1016/j.foodchem.2007.01.068. [DOI] [Google Scholar]
- Lafka TI, Lazou AE, Sinanoglou VJ, Lazos ES. Phenolic and antioxidant potential of olive oil mill wastes. Food Chem. 2011;125:92–98. doi: 10.1016/j.foodchem.2010.08.041. [DOI] [Google Scholar]
- Malenčić D, Popović M, Miladinović J. Phenolic content and antioxidant properties of soybean (Glycine max (L.) Merr) seeds. J Mol. 2007;12:576–581. doi: 10.3390/12030576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohdaly AA, Smetanska I, Ramadan MF, Sarhan MA, Mahmoud A. Antioxidant potential of sesame (Sesamum Indicum) cake extract in stabilization of sunflower and soybean oils. Ind Crops Prod. 2011;34:952–959. doi: 10.1016/j.indcrop.2011.02.018. [DOI] [Google Scholar]
- Moure A, Cruz JM, Franco D, Domínguez JM, Sineiro J, Domínguez H, José Núñez M, Parajó JC. Natural antioxidants from residual sources. Food Chem. 2001;72:145–171. doi: 10.1016/S0308-8146(00)00223-5. [DOI] [Google Scholar]
- Peschel W, Sánchez-Rabaneda F, Diekmann W, Plescher A, Gartzía I, Jiménez D, Lamuela-Raventos R, Buxaderas S, Codina C. An industrial approach in the search of natural antioxidants from vegetable and fruit wastes. Food Chem. 2006;97:137–150. doi: 10.1016/j.foodchem.2005.03.033. [DOI] [Google Scholar]
- Prakash D, Upadhyay G, Singh BN, Singh H. Antioxidant and free radical-scavenging activities of seeds and agri-wastes of some varieties of soybean (Glycine max) Food Chem. 2007;104:783–790. doi: 10.1016/j.foodchem.2006.12.029. [DOI] [Google Scholar]
- Rehman Z, Habib F, Shah WH. Utilization of potato peels extract as a natural antioxidant in soy bean oil. Food Chem. 2004;85:215–220. doi: 10.1016/j.foodchem.2003.06.015. [DOI] [Google Scholar]
- Sabir SM, Khan MF, Rocha JBT, Boligun AA, Athyde ML. Phenolic profile, antioxidant activities and genotoxic evaluations of calendula officinalis. J Food Biochem. 2015;39:316–324. doi: 10.1111/jfbc.12132. [DOI] [Google Scholar]
- Terpinc P, Čeh B, Ulrih NP, Abramovič H. Studies of the correlation between antioxidant properties and the total phenolic content of different oil cake extracts. Ind Crops Prod. 2012;39:210–217. doi: 10.1016/j.indcrop.2012.02.023. [DOI] [Google Scholar]
- Tyug TS, Prasad KN, Ismail A. Antioxidant capacity, phenolics and isoflavones in soybean by-products. Food Chem. 2010;123:583–589. doi: 10.1016/j.foodchem.2010.04.074. [DOI] [Google Scholar]
- Wanasundara PKJPD, Shahidi F. Antioxidants: science, technology, and applications. In: Shahidi F, editor. Bailey’s industrial oil and fat products. 6. New York: Wiley; 2005. pp. 431–489. [Google Scholar]