Abstract
Algarroba flour is used to supplement lysine-limiting systems such as wheat flour due to its amino acidic composition. The effects of adding up to 30% of this flour to wheat flour (W-A30) on dough characteristics and breadmaking performance were studied. Dough rheology was tested by farinograph, oscillatory rheometry and texture profile analyses. Molecular mobility was evaluated by nuclear magnetic resonance, and thermal properties were analyzed by differential scanning calorimetry and viscoamylograph studies. Besides, different bread quality parameters were evaluated. Incorporation of algarroba flour resulted into increase in water absorption, development time and degree of softening, and decrease in stability of wheat flour, leading to softer, less adhesive and elastic dough, although at intermediate replacement levels cohesiveness improved. At the molecular level, a reduction of water activity and limited proton motion were observed in W-A30 samples, suggesting that protons were highly bound to the dough matrix. Dough samples with algarroba flour showed lower G′ and G″ values than the control, although with the formation of a more elastic structure for W-A30. In addition, algarroba flour produced a protective effect on starch granule disruption and interfered with amylose–amylose association during cooling. The specific volume of breads decreased with the increase in algarroba level, W-A30 reaching the highest decrease (15%). Bread crumbs with algarroba flour exhibited higher values of hardness and resilience. The use of algarroba flour resulted in lower quality when compared to the control. However, algarroba flour at 20% level can be added to wheat flour to obtain bakery products of similar technological quality and with improved nutritional components.
Keywords: Algarroba flour, Wheat flour, Breadmaking, Rheology, Thermal properties
Introduction
Leguminous trees of the genus Prosopis are native to semiarid regions. Forty-four species from different parts of the world have been identified (Burkart 1976). Prosopis spp. are nitrogen-fixing trees that present drought and heat tolerance and thus are able to grow in more severe environmental conditions than common annual legumes (Felker et al. 2013). Although Prosopis species were the major food staple for indigenous people in North and South America before the arrival of Europeans (Beresford-Jones et al. 2009), pods have increasingly become less important as human food and more important as livestock feed during the last centuries (Pasiecznik et al. 2001). Prosopis pods contain a high amount of carbohydrates, mainly fiber and soluble sugars, and a moderate quantity of proteins (Sciammaro et al. 2016). The flour obtained from those pods is brown, sweet and has a taste and aroma with hints of cafe, cocoa, coconut, and hazelnut due to the presence of volatile compounds (Felker et al. 2013). The flour obtained from Prosopis pods is named as “mesquite flour” in the United States and Mexico and as “algarroba flour” in South American countries. Prosopis spp. proteins contain a relatively high level of lysine, and the limiting amino acids are methionine and cysteine (Marangoni and Alli 1988). Thus, algarroba flour is useful for supplementing lysine-limiting systems such as wheat flour (Shewry 2004), and furthermore wheat flour can provide sulfur amino acids that algarroba flour lacks (Duodu and Minnaar 2011). In addition, Prosopis flour contains a high level of potassium, calcium and iron (Sciammaro et al. 2016), thus, it could also contribute to increasing the level of minerals that were eliminated from wheat flour during milling. Therefore, Prosopis flour constitutes an ingredient of high nutritional value. Commercial algarroba flour is usually obtained in markets of nutraingredients, and is commonly used to supplement wheat flour in various types of sweet baked products due to its high nutritive value. In spite of the promising use of Prosopis flour in foods, it is not widely used in breadmaking due to its composition. First we studied the quality aspects of wheat breads complemented with Prosopis alba flour, including the staling phenomenon (Bigne et al. 2016); nevertheless, in this study a commercial flour widely sold in dietary groceries was utilized. The aim of this work was to study changes in the rheological, physicochemical and thermal properties of wheat dough after incorporation of commercial algarroba flour, and to evaluate breadmaking performance and technological quality of composite breads as well.
Materials
Commercial wheat flour Type 000 (Argentinean Alimentarius Codex 2016) and commercial algarroba flour were used (Todo Dieta SRL, Mendoza, Argentina). Wheat flour for breadmaking (Type 000) presented typical alveographic characteristics, high extensibility (L = 93 mm), tenacity (P = 96 mm H2O) and alveographic work (W = 326 10−4 J). Distilled water and commercial salt (NaCl) were used to prepare the dough.
Methods
Proximate composition
Protein (method 46-12.01), lipid (method 30-25.01), moisture (44-19), ash (method 08-01) and total dietary fiber (method 32-05.01) contents of commercial algarroba and wheat flour were determined according to AACC methods (AACC 2000). The protein content for algarroba flour was calculated as nitrogen content X 6.25; in the case of wheat flour the Kjeldahl factor used was 5.7. Total carbohydrate content was estimated as: 100 − (%protein − %lipids − %moisture − %ash − %total dietary fiber). Reducing and nonreducing carbohydrates (mainly glucose and sucrose) previously inverted by a treatment with HCl for 30 min, were determined using Somogyi–Nelson method (Sadasivam and Manickam 1992).
Farinographic assays
Wheat flour-algarroba flour-NaCl (2%) blends were prepared. The farinographic assay was conducted according to the standard method 54-21 (AACC 2000) in 300-g Brabender equipment (Duisburg, Germany), and the algarroba flour levels used were: 0% (control), 10% (W-A10), 15% (W-A15), 20% (W-A20) and 30% (W-A30) on a wheat flour basis. The farinographic parameters determined were: percentage of water to yield a consistency of 500 BU (water absorption), time to reach up to 500 BU (development time), time that dough remains at a consistency of 500 BU (stability) and softening degree, which is the difference in BU after 12 min from leaving 500 BU.
Dough preparation
The dough for rheological and physicochemical assays was formulated without using fresh yeast, but using NaCl 2% and the amount of water corresponding to farinographic absorption of the control sample (wheat flour). Dough was prepared in a small-scale kneader with planetary mixing action (Kenwood, Italy). The farinograph development time of wheat flour was used as mixing time for all blends (0–30% algarroba flour). Dough was then sheeted in equipment (Pastafacil, Argentina) where rods were manually adjusted and turned, and laminated four times to improve gluten development, turning dough 90° after each passage by the rods. After this procedure, the dough was covered with a plastic film to avoid dehydration and left to rest for 15 min at room temperature. For texture assays, the dough was sheeted to 1 cm height and cut into small cylindrical pieces (3 cm diameter). For rheometric assays, cylindrical pieces of 3 cm diameter and 2 mm height were obtained from laminated dough. Doughs were prepared in duplicate.
Physicochemical properties of dough
Moisture content Dough moisture was indirectly determined by air-drying in an oven at 135 °C for 2 h (method 44-19) (AACC 2000). Determinations were carried out in triplicate.
Water activity Measurements were performed at 25 °C in an AQUALAB (Decagon Devices, Inc., Pullman, USA). Determinations were carried out in triplicate.
Matrix molecular mobility Spin–spin relaxation times (T2) were measured using a nuclear magnetic resonance (NMR) spectrometer (Minispec, Bruker, Germany). Measurements were performed at room temperature by applying the Carr-Purcell-Meiboom-Gill (CPMG) pulse sequence with an interpulse spacing of 200 μs. The signal decay was fitted with an exponential equation (Eq. 1).
| 1 |
where I represents proton signal intensity, proportional to the amount of water in the sample, T2i corresponds to the relaxation time of protons i present in dough, and yi is the signal intensity of protons in T2i state. To obtain the parameters that gave the best fit to Eq. 1, a nonlinear regression using OriginPro8 Software was made. Determinations were carried out in triplicate.
Texture profile analysis (TPA) of dough
Cylindrical samples (n = 20) of diameter 3 cm and height 1 cm were obtained from dough. Dough texture parameters were evaluated using a TA.XT2i Texture Analyzer (Stable Micro Systems, UK) with software Texture Expert for Windows, version 1.2. Dough was subjected to two cycles of compression up to 40% of the original height with a cylindrical probe (diameter = 7.5 cm). Force time curves were obtained at a crosshead speed of 0.5 mm/s. Dough hardness, adhesiveness, cohesiveness, springiness, gumminess, and resilience were determined. Hardness is defined as the maximum force during the first compression. Adhesiveness is the negative area in the first cycle. Cohesiveness is determined as the ratio between the positive areas of the second cycle and the first cycle. Springiness is calculated as the distance ratio between the beginning and the maximum force of the second and first peaks. Gumminess is calculated as the product of hardness × cohesiveness. Resilience is calculated as the ratio between the area during the withdrawal of the first compression and the area up to the maximum of the first peak (Bourne 2002).
Oscillatory dynamic rheometry of dough
For oscillatory assays, samples were maintained refrigerated till 10 min before testing. Dynamic oscillatory tests were performed in a Haake RS600 controlled stress oscillatory rheometer (Haake, Germany) at 25 ± 0.1 °C, using a plate–plate sensor system with a 1.0 mm gap between plates. Serrated plates were used and semisolid Vaseline oil was applied to prevent sample drying during testing. All samples were allowed to rest for 15 min between plates before measurements to allow dough relaxation. In order to determine the linear viscoelastic range of the sample, deformation sweep tests were performed at a constant frequency (1 Hz). Frequency sweep tests (from 0.005 to 100 Hz) at a constant stress (5 Pa) within the linear viscoelastic range were performed at 25 °C. The dynamic moduli G′, G″ and tan δ (G″/G′) were obtained as a function of frequency. G′ is the dynamic elastic or storage modulus, related to the material response as a solid, while G″ is the viscous dynamic or loss modulus, related to the material response as a fluid. The overall viscoelastic behavior is represented by tan δ: low values of this parameter indicate a more elastic sample. Assays were performed in triplicate.
Rapid viscoamilograms
A visco analyzer (RVA-4 Newport Scientific Pty, LTD., Warriewood, Australia) was used to study the pasting properties of all commercial algarroba flour–wheat flour blends. The assay was performed following the AACC 76-21 method (AACC 2000). The sample was maintained at 50 °C for 1 min, heating from 50 to 95 °C in 4 min 42 s, held at 95 °C for 2 min 30 s, cooled from 95 to 50 °C in 3 min 48 s, and held at 50 °C for 2 min. The paddle speed was 960 rpm for the first 10 s and then 160 rpm for the rest of the cycle. The following parameters were determined using the Thermocline software for Windows (version 2.4 b 31) provided with the instrument: peak viscosity (ηp), minimum viscosity or trough (ηmin), final viscosity (ηf), breakdown or difference between peak and minimum viscosity (ηp − ηmin), setback from peak or the difference between final and peak viscosity (ηf − ηp) and setback from trough or the difference between final and minimum viscosity (ηf − ηmin). Each assay was performed in triplicate.
Differential scanning calorimetry (DSC)
Calorimetric assays were performed to evaluate starch gelatinization in control dough (wheat dough) and in dough with commercial algarroba flour. A TA Q100 calorimeter (TA Instruments, USA) was used to obtain the thermograms. Small portions of dough were weighed (10 mg) and placed in aluminum-coated pans that were then hermetically sealed. Samples were heated from 10 to 130 °C at 10 °C/min. Onset (To), peak (Tp) and final temperatures (Tf) and gelatinization enthalpies (ΔH) were measured. Determinations were carried out in duplicate.
Formulation and breadmaking process
Dough formulation on 100 g wheat flour basis was: compressed yeast, 3.0% (Calza, Argentina); salt, 2.0%; and commercial algarroba flour, 10, 15, 20 and 30% (wheat flour basis). Water and mixing time were fixed according to the values obtained for wheat flour. Dough prepared as described in 3.3 was divided into 60 g portions and put into an individual aluminum cone mold (upper width = 65 mm, bottom width = 40 mm, height = 50 mm). These pieces were proofed at 30 °C for 80 min and baked in a convection oven for 22 min at 220 °C.
Bread quality
Specific volume Bread pieces were weighed, and the volume was determined by rapeseed displacement. Specific volume was determined as volume/piece weight. Five pieces of bread were taken for measurements.
Crumb texture The texture profile analysis (TPA) of bread slices (n = 8) was performed on fresh bread. From the middle part of each bread piece, slices of 2 cm height were obtained. A texture analyzer TA.XT2i (Stable Micro Systems, UK) equipped with a 25-kg load cell was used to perform the TPA of crumb. The slices were subjected to a double compression cycle up to 40% of the original height using a 2.5 cm diameter cylindrical probe and 0.5 mm s−1 crosshead speed. The parameters determined were: hardness, cohesiveness, springiness, and resilience.
Crumb moisture It was determined on fresh bread by air-drying in an oven at 135 °C for 2 h (44-19 method) (AACC 2000). Determinations were carried out in triplicate.
Crumb color It was measured using a colorimeter (Chroma Meter CR-300C, Minolta, Japan). The Hunter scale parameters were determined: L (lightness, ranging from 0 to 100, from black to white), a (positive values indicate redness and negative values, greenness) and b (positive values indicate yellowness and negative values, blueness). Also, the Browning Index (BI) was calculated (Eq. 2). This parameter is a measure of brown color (Buera et al. 1985) and it correlates linearly with a brown pigment concentration. Particularly, in breadmaking it has been used to evaluate color changes due to different formulations (Shittu et al. 2007, 2008; Komlenić et al. 2010; Salinas et al. 2016)
| 2 |
where X is defined by Eq. 3
| 3 |
Statistical analysis
To discriminate among means, Bonferroni’s multiple comparison procedure was applied at 95% confidence level. Statgraphics Plus software was used.
Results and discussion
Proximate composition
Commercial wheat and algarroba flour proximate composition is shown in Table 1. Comparing both types of flours, algarroba flour has lower protein content and a higher amount of ash and lipids. Starch is the major carbohydrate in wheat flour, while in algarroba flour carbohydrates are composed mainly of fiber and sucrose. Commercial algarroba flour contains 9.2% of total dietary fiber, 2.7 ± 0.9% of reducing sugars and 16.5 ± 0.9% of sucrose. For Prosopis alba flour from whole pod, Sciammaro et al. (2016) found high (25%) and low (5.8%) contents of total dietary fiber and protein, respectively.
Table 1.
Proximate composition of wheat flour and algarroba flour
| Wheat flour | Algarroba flour | |
|---|---|---|
| Protein (%) | 11.4 ± 0.1 | 9.1 ± 0.1 |
| Ash (%) | 0.68 ± 0.01 | 2.33 ± 0.05 |
| Moisture (%) | 14.2 ± 0.1 | 8.02 ± 0.03 |
| Lipids (%) | 1.40 ± 0.07 | 2.22 ± 0.06 |
| Total dietary fiber (%) | 3.1 ± 0.1 | 9.2 ± 0.2 |
| Total carbohydrate (%)a | 69.2 ± 0.2 | 69.1 ± 0.2 |
aTotal carbohydrate content was estimated as: 100 − (%protein − %lipids − %moisture − %ash − %total dietary fibre). Values are expressed as mean ± SD
Farinographic properties of composite flours
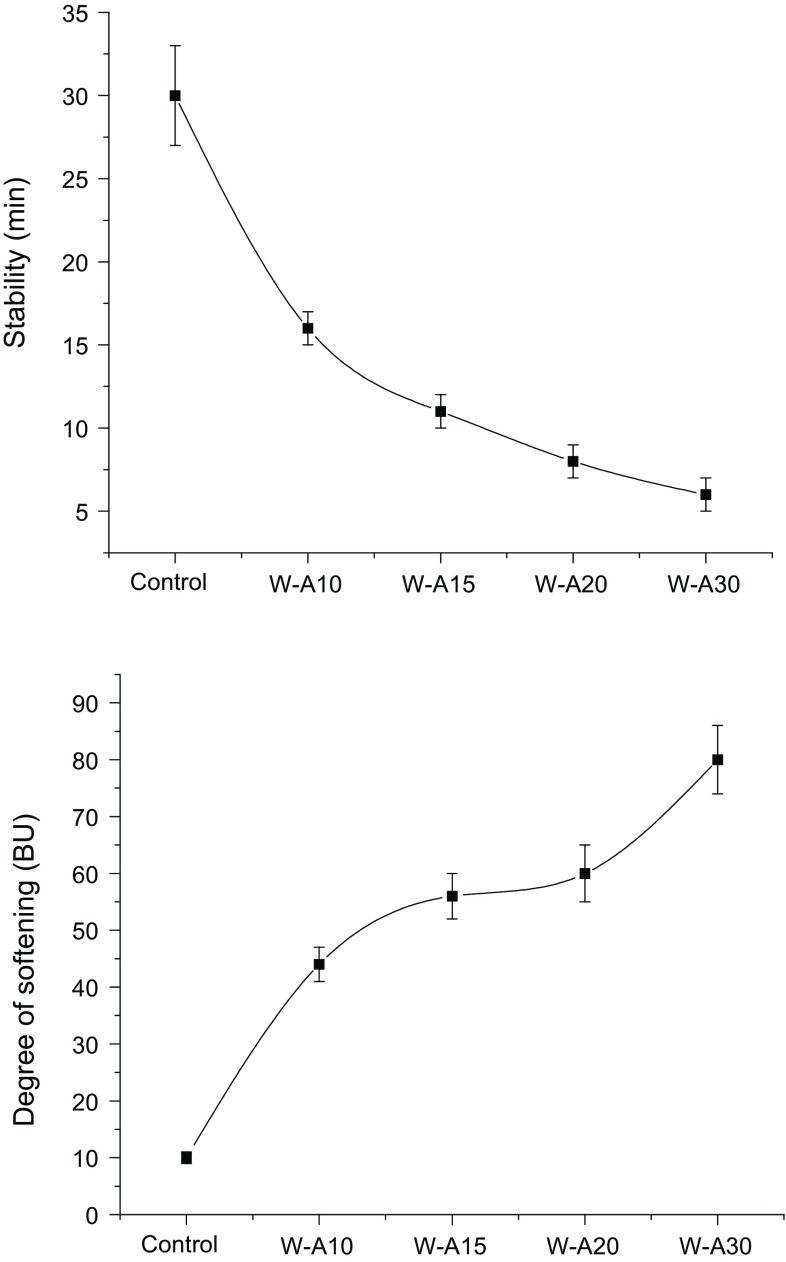
The farinograms of algarroba–wheat flour blends showed that incorporation of algarroba led to an increase in farinographic water absorption and developing time (data not shown). However, these parameters were not modified with the increase of algarroba flour level. The increase in water absorption value was lower than 5% for all samples (55.1% for wheat flour) and the maximum increase in development time with respect to the control (11 min) was caused by the incorporation of 20% of algarroba flour (W-A20) (19 min). In Fig. 1 the effect of algarroba flour on the farinographic parameters, stability and degree of softening, are shown. Composite flours exhibited lower stability and higher degree of softening than wheat flour. Dough stability showed an exponential decay; two regions could be observed, the first more pronounced up to 15% of algarroba flour and the second one with a lower slope at higher levels of algarroba flour. In the same sense, the degree of softening deeply increased up to the incorporation of 15% of algarroba flour, a behavior that could be attributed to gluten dilution. The addition of 20% of algarroba flour did not change the degree of softening (intermediate plateau), probably related to the presence of certain components of this flour, such as gums and proteins. Prosopis gums are composed of galactomannans (Ibañez and Ferrero 2003), and proteins are mainly of globular nature (Gallão et al. 2007; Sciammaro et al. 2016). Both components are capable of absorbing water and form a gel-like structure. This composed matrix (gluten + gellified structure) can resist over-kneading. However, a subsequent increment of algarroba flour (W-A30) produces a greater increase in dough softening due to the high dilution of gluten. This detrimental effect has been reported when other leguminous flours were used to prepare composite flours jointly with wheat flour (Paraskevopoulou et al. 2010; Duodu and Minnaar 2011). In these cases, the lower flour quality has been mainly attributed to the gluten content decrease. However, lupine, soya and chickpea flours used at 10% level have contributed to an increase in the stability and the tolerance index of dough (Doxastakis et al. 2002; Mohammed et al. 2012).
Fig. 1.
Farinographic stability and softening of composite algarroba–wheat flours. Algarroba flour levels were 0% (control), 10% (W-A10), 15% (W-A15), 20% (W-A20) and 30% (W-A30). Error bars represent the standard deviation
Physicochemical properties of dough
Dough moisture values did not exhibit significant differences among samples; this was expected since all dough samples were prepared with the control (wheat flour) farinographic water absorption value. Moisture values ranged between 42.69 and 43.23%. However, water activity did not follow the same trend. When commercial algarroba flour was added at 10% (W-A10) and 15% (W-A15) significant differences with respect to the control sample were not observed (from 0.971 to 0.966). Higher quantities of commercial algarroba flour (higher than 20%) led to a significant reduction in water activity (0.964), indicating that less water is available in dough. The reduction of water activity could be attributed to the presence of galactomannan gum and/or sucrose in algarroba flour. In spite of this reduction, in all cases water activity values were high enough to describe dough as a system that presents a high energy state of water.
The proton relaxation time (T2) determined by pulsed nuclear magnetic resonance is an indication of the molecular mobility of water in dough. From curves of proton signal decay, T2 values were obtained. The signal intensity was fitted with an exponential equation with one term, suggesting that only one population of protons is present in the dough matrix. Similar results were previously found in wheat dough systems (Salinas et al. 2012; Correa et al. 2014) and in composite flours of Ceratonia Siliqua L.-wheat flour (Salinas et al. 2015) and Prosopis alba-wheat flour (Bigne et al. 2015). However, in wheat dough matrix other authors have found two proton populations with different mobility. A less mobile fraction that has relaxation times of about 20 ms and a more mobile fraction with T2 about 60 ms (Leung et al. 1979). They could be related to water in different physical environments. Therefore, water molecules away from macromolecules relax slowly (larger T2 values) since the rapid rotation of molecules limits dephasing of spins. Furthermore, water molecules located near macromolecules present restricted motions and thus exchange energy mainly through spin–spin processes, so they exhibit shorter T2 values (Kerr and Wicker 2000). Higher T2 values indicate higher molecular mobility, where water is more loosely linked to other molecules and consequently, in a high energy state. Values of T2 were 11.3 ± 0.2 ms for the control sample, and a tendency to lower relaxation times was observed in composite doughs, with no significant differences except for W-A30 (11.0 ± 0.1 ms). This slight decrease of T2 value is in accordance with the reduction of water activity observed at the higher substitution levels. However, it should be noted that the T2 values of these doughs (around 11 ms) were small enough and similar to those obtained for Prosopis alba-wheat flour blends (Bigne et al. 2015), suggesting that water molecules present limited motion (low energy state) or are strongly bound to the dough matrix.
Dough rheology
Texture profile analysis and dynamic oscillatory measurements
Table 2 shows the textural parameters of dough. The addition of commercial algarroba flour to wheat flour led to softer and less consistent dough. Since all dough samples were prepared with the same amount of water (farinographic water absorption of wheat flour), and there were no differences in moisture values among samples, this effect may be attributed to a weaker gluten network formed during mixing. Therefore, the decrease in gluten content or the presence of certain algarroba flour components that could modify the interaction among gluten proteins would be responsible for the weakness of the network. Particularly, the galactomannan gum present in algarroba flour could interact with gluten proteins. It has been found that other hydrocolloids could interact with gluten proteins in dough, and this interaction depends highly on the gum chemical structure (Correa et al. 2014).
Table 2.
Dough textural parameters and bread quality
| Sample | Hardness (N) | Adhesiveness (N.s) | Cohesiveness (−) | Springiness (−) | Gumminess (N) |
|---|---|---|---|---|---|
| Textural parameters of dough | |||||
| Control | 2.5 ± 0.3c | −8.01 ± 0.1b | 0.72 ± 0.2a | 0.911 ± 0.009b | 1.8 ± 0.2c |
| W-A10 | 1.7 ± 0.2b | −6.6 ± 0.3a | 0.74 ± 0.2b | 0.89 ± 0.01a | 1.3 ± 0.1b |
| W-A15 | 1.4 ± 0.2a | −6.7 ± 0.5a | 0.74 ± 0.2b | 0.89 ± 0.01a | 1.1 ± 0.1a |
| W-A20 | 1.3 ± 0.2a | −6.3 ± 0.5a | 0.74 ± 0.2b | 0.90 ± 0.01a | 1.0 ± 0.1a |
| W-A30 | 1.4 ± 0.1a | −6.1 ± 0.8a | 0.72 ± 0.3ab | 0.894 ± 0.006ª | 1.0 ± 0.1a |
| Sample | Specific volume (cm3/g) | L | a | b | BI |
|---|---|---|---|---|---|
| Specific volume and crumb color of bread | |||||
| Control | 2.69 ± 0.14c | 69.6 ± 1.9d | −0.78 ± 0.11a | 15.9 ± 0.7a | 24.2 ± 1.5a |
| W-A10 | 2.51 ± 0.11b | 45.02 ± 1.9c | 11.6 ± 0.5b | 25.0 ± 0.8bc | 96.0 ± 5.8b |
| W-A15 | 2.47 ± 0.14ab | 45.0 ± 1.5c | 12.8 ± 0.5c | 25.4 ± 0.7cd | 99.97 ± 5.2b |
| W-A20 | 2.62 ± 0.10bc | 39.4 ± 1.3b | 13.8 ± 0.4d | 25.7 ± 0.9d | 122.9 ± 5.0c |
| W-A30 | 2.30 ± 0.17a | 36.6 ± 1.1a | 14.2 ± 0.5e | 24.4 ± 0.5b | 129.1 ± 5.0d |
Within each column different letters indicate significant differences among samples and/or respect to control (p < 0.05). Values are expressed as mean ± SD
As a result of hardness decrease, dough gumminess was also reduced since gumminess is defined as hardness x cohesiveness. Besides, dough springiness showed a slight decrease. Dough adhesiveness, related to the work required to overcome the attractive forces between dough and the surface of the materials with which it could take contact, was lower in composite doughs. On the other hand, the cohesiveness of doughs with algarroba flour was equal to (W-A30) or higher than that of the control, showing that matrix integrity was not negatively affected or was even improved. Recently, under a restricted water system, dough cohesiveness was observed to decrease with the increase in Prosopis alba flour (Bigne et al. 2015). Nevertheless, the composite doughs studied in this paper showed good association among dough components since cohesiveness was not negatively affected; yet, this parameter increased at levels of algarroba flour between 10 and 20%. However, these matrices were different from the control probably due to a distinct interaction between all flour components (starch, gums and proteins) and water, as is reflected by hardness and degree of softening.
Dynamic oscillatory assays describe dough behavior when it is subjected to small deformations and reveal changes in the matrix structure. In all cases, dough samples showed a predominant solid viscoelastic behavior since G′ > G″ in the whole frequency range studied (from 0.005 to 100 Hz). These parameters also exhibited parallel curves in this range (not shown). The commercial algarroba flour used led to a reduction of storage and viscous moduli in dough. However, these parameters did not decrease steadily with increasing algarroba flour levels. The storage modulus ranged from 11.8 ± 0.9 (W-A10) to 15.1 ± 1.4 kPa (W-A20) for composite doughs and it was 17.3 ± 1.9 kPa for control dough. Nevertheless, composite doughs showed lower tan δ values [from 0.28 (W-A10) to 0.32 (W-A30)] than control dough (0.33). These results demonstrate that the reduction of G″ is higher than that of G′, suggesting that dough is more elastic, mainly at 30% substitution. Besides, variations of tan δ indicate that structural changes have occurred in the gluten network matrix due to algarroba flour addition, leading to a more elastic network.
Thermal properties
The thermal properties of algarroba–wheat blends were studied since they are directly related to dough behavior during breadmaking.
Pasting properties
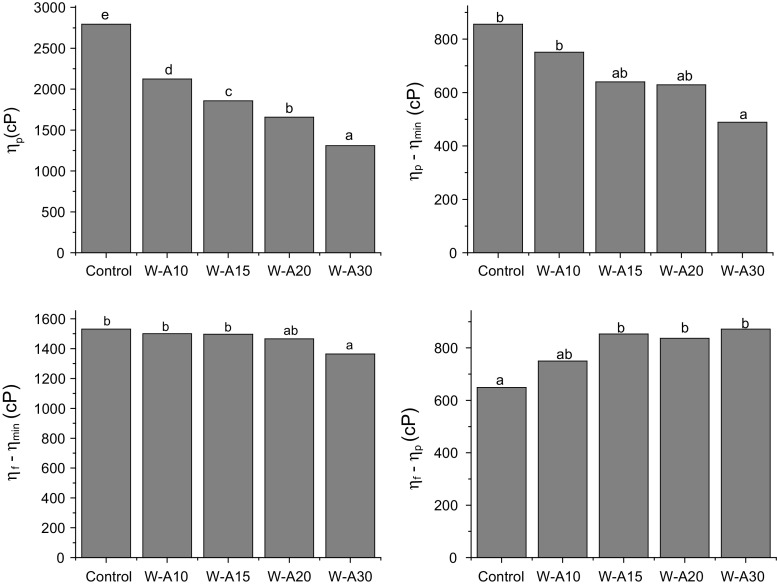
The rapid viscoamylograms show the behavior of a sample when it is subjected to a heating program in excess of water. Figure 2 shows the parameters obtained from viscoamylograms: peak viscosity, breakdown and setback from peak and from minimum. The addition of commercial algarroba flour to wheat flour led to a significant decrease of peak (ηp), minimum (ηmin) (not shown) and final viscosity (ηf) (not shown). These parameters are related to the degree of water absorption by the starch granule and the viscosity of the hot and cold paste, respectively. The decrease in viscosity along the whole RVA profile as algarroba flour level increases could be attributed to the smaller amount of starch present in the samples and to a lower starch swelling. Algarroba flour provides proteins and gums that are able to interact with water reducing the amount of water that is available to be bonded by starch. The protective effect of proteins on starch granule integrity during heating has been described by several authors. A negative correlation between protein level and peak viscosity was found in flour obtained from different durum wheat varieties (Kaur et al. 2016). In another study, it was reported that during corn starch wet milling as protein level decreases peak viscosity increases after each successive purification stage (Singh et al. 2014). These results were also in agreement with the findings of Balasubramanian et al. (2012) who reported a reduction of all viscosity parameters with increased legume levels in blends with rice extrudates, and they related this effect to a decrease of gelatinization degree with the increase in legume level. Khatkar (2005) found that the elastic character (G′) decreased drastically as the gluten content was increased. Thus, the effect caused by algarroba flour proteins could be added to the effect of water absorption of gluten proteins on the rheological behavior of wheat flour. On the other hand, the addition of high levels of insoluble fiber to wheat flour can contribute to the reduction of peak viscosity (Yadav et al. 2010). The breakdown (ηp − ηmin) reflects the hot paste stability and it is related to starch granule disintegration. The addition of commercial algarroba flour significantly decreased the breakdown value in W-A30 sample. This behavior also suggests a protective effect of algarroba flour on starch granule disruption during heating. The parameters setback from peak (ηf − ηp) and the setback from minimum (ηf − ηmin) are related to starch tendency to retrograde. Both parameters were affected by algarroba flour addition. In W-A30 sample setback from minimum showed a decrease with respect to the control sample, while setback from peak was significantly increased by algarroba flour in W-A15, W-A20, and W-A30 samples. These results demonstrate that commercial algarroba flour would be interfering with amylose–amylose association during the cooling step, leading to lower final viscosity values. Similar results were found by other authors in chickpea–wheat flour blends (Mohammed et al. 2014). However, in our case this effect could be mediated not only through the structuring algarroba proteins but also by the galactomannan chains.
Fig. 2.
Parameters obtained from rapid visco analyzer curves: peak viscosity (ηp), breakdown (ηp-min), setback from minimum (ηf-min), setback from peak (ηf-p). Algarroba flour levels were 0% (control), 10% (W-A10), 15% (W-A15), 20% (W-A20) and 30% (W-A30). Different letters indicate significant differences among samples (p < 0.05)
In conclusion, the effect caused by algarroba flour on the pasting properties of wheat flour could be related to the presence of globular proteins, galactomannan gums or the presence of fiber from algarroba flour.
Differential scanning calorimetry
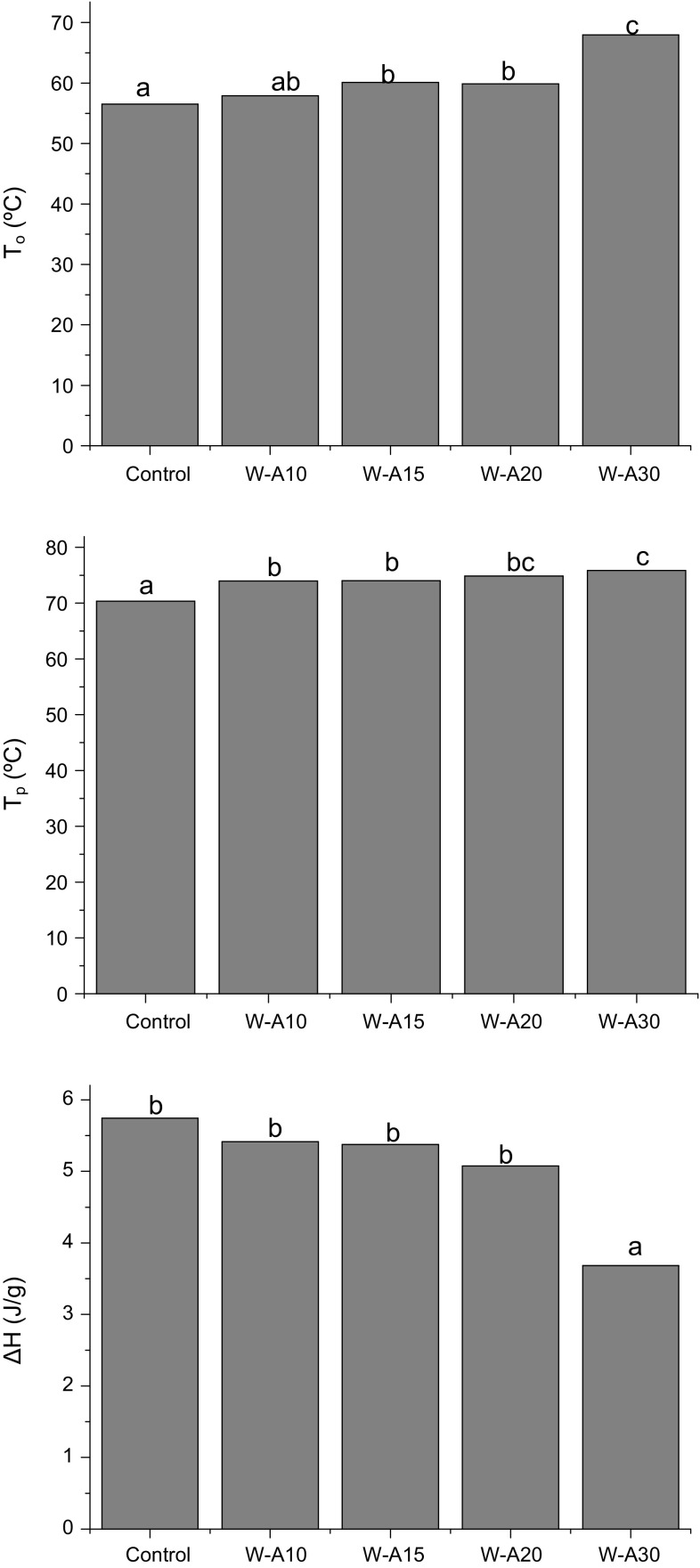
The thermal behavior of dough samples was studied by DSC to evaluate the effect of algarroba flour on starch gelatinization. Besides, suspensions of algarroba flour in distilled water at 20 and 40% (w/v) were run. However, no transitions were found in the suspensions since algarroba pods were toasted before the milling process.
All dough samples exhibited three endotherms, two of them related to starch gelatinization and a third endotherm at higher temperatures that is related to amylose-lipid dissociation. In general, as algarroba flour level increases a shift of endotherms towards higher temperatures was observed. In Fig. 3 gelatinization enthalpy (ΔH), onset (To) and peak 1 (Tp) temperatures are shown. A significant increase of To and Tp with respect to control dough was observed as algarroba flour level increased. However, there were no significant differences in both peak 2 temperature and in the final temperature. Thus, the decrease in water activity caused by algarroba flour addition could restrict the amount of water available for starch gelatinization, leading to a shift towards higher temperatures. On the other hand, a lower enthalpy value was observed in W-A30 sample. Since enthalpy is expressed as the energy necessary for starch gelatinization per gram of dry dough, the decrease of gelatinization enthalpy would be related to the decrease in the starch amount in samples as algarroba flour level increases. This behavior could also be due to the presence of algarroba flour components that could decrease water availability for starch gelatinization (Bigne et al. 2016). From a technological point of view, this is a positive aspect since a smaller amount of heat is needed to bake this product. Finally, the dissociation of amylose–lipid complex (peak 3) was not affected by the use of algarroba flour.
Fig. 3.
Thermal transitions of dough measured by DSC: onset temperature (To), peak temperature (Tp) and gelatinization enthalpies (ΔH). Algarroba flour levels were 0% (control), 10% (W-A10), 15% (W-A15), 20% (W-A20) and 30% (W-A30). Different letters indicate significant differences among samples (p < 0.05)
Breadmaking quality
The specific volume for control and composite breads is shown in Table 2. Specific volume is a parameter that reflects gluten network quality and matrix capability to retain the gases formed during the fermentation process. A significant decrease in this parameter was observed when algarroba flour was added, although at the highest level used (W-A30) the reduction of specific volume was only 15%. An exception to this behavior was W-A20 sample since it presented no significant differences with respect to the control sample. This demonstrates that the addition of 20% of algarroba flour would lead to a matrix with better breadmaking performance, as is reflected by higher gas retention.
When a change in a formulation is introduced, the characterization of color is important since it could affect consumer’s acceptability. Color parameters of the baked loaf crumbs were determined. Since algarroba flour was brown, its addition to white wheat flour influenced bread color (Table 2). It was found that the addition of increasing levels of algarroba flour led to a progressive increase in the parameters a and b and to a decrease of lightness ranging from 69.6 for the control to 36.6 for W-A30. The browning index (BI) was also calculated to better understand color changes due to algarroba flour addition. BI represents the purity of brown color and it is an important parameter in processes where enzymatic and nonenzymatic browning occur (Buera et al. 1985; Palou et al. 1999). Higher BI values are related to darker bread color, which has been successfully used by several authors to study changes in bread formulation (Shittu et al. 2007, 2008; Zhu et al. 2010). As expected, composite breads exhibited BI values significantly higher than the control. The addition of algarroba flour caused a drastic color change even at the lowest level (W-A10), and an increase of five hundred percent was observed for W-A30. Browning could be attributed to Maillard reactions mediated by reducing sugars present in commercial algarroba four.
The moisture value for control bread was 44.7%, and for breads with algarroba flour values ranged from 44.4 (W-A10) to 43.6% (W-A30). Although a significant steady decrease with increasing algarroba flour concentration was observed, these values fell in a narrow range, thus no considerable change in crumb moisture was observed.
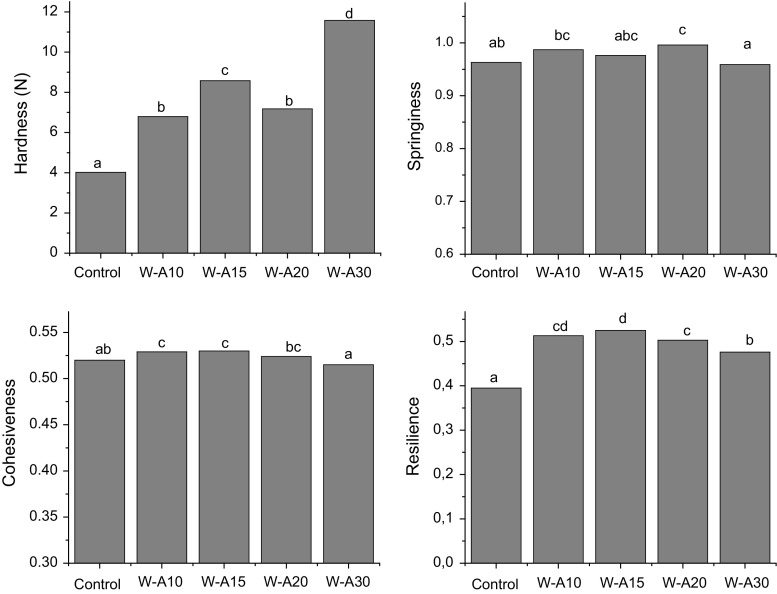
In Fig. 4 texture parameters of bread crumbs, hardness, cohesiveness, springiness and resilience, namely are shown. Crumb texture is related to other product characteristics such as moisture and crumb density. In general, crumb hardness is higher in products that show lower moisture and lower specific volume (Cauvain 2004, 2012). The addition of algarroba flour increased hardness and chewiness (not shown) mainly in a higher proportion in W-A30. This could be partly related to the low specific volume and/or the contribution of gums and proteins to form more structured cell walls in crumbs. Cohesiveness, a parameter that reflects crumb integrity, increased with 10 and 15% substitution or was not affected in the case of W-A20 and W-A30. This is a positive effect on crumb characteristics since more cohesive crumbs are less susceptible to disintegration. Finally, the parameters related to crumb recovery after a compression, springiness and resilience, improved or were not modified with the addition of algarroba flour: all the composite breads exhibited higher values of resilience with respect to the control. Elastic crumb is an attribute expected by consumers. This indicates that algarroba flour can contribute to improving the recovery after a deformation, which could be related to the presence of the galactomannan gum in algarroba flour that confers elastic properties.
Fig. 4.
Textural parameters of bread crumbs: hardness, springiness, cohesiveness and resilience. Algarroba flour levels were 0% (control), 10% (W-A10), 15% (W-A15), 20% (W-A20) and 30% (W-A30). Different letters indicate significant differences among samples (p < 0.05)
Conclusion
The use of commercial algarroba flour modified wheat dough and bread quality. This effect seems to be mediated through two major counterbalancing phenomena. Firstly, the “dilution” effect of gluten proteins and, secondly, the contribution of algarroba flour components, mainly galactomannan gum and globular proteins that would favor matrix arrangement. Dilution of gluten proteins is evidenced even at the lowest level of addition (W-A10), and this weaker network is characterized by lower stability, higher degree of softening and lower hardness. The contribution of algarroba components seems to be clear in W-A20 sample since this matrix supports over-kneading and presents similar textural characteristics to those of W-A15 sample. When a higher level of algarroba flour (W-A30) is used, this effect is enough to recover the matrix structure, and a detrimental effect is perceived. This hypothesis is also supported by the results obtained in breadmaking. Although a reduction of specific volume was obtained when algarroba flour was used at 10, 15 and 30%, this effect was countered in W-A20 sample, reaching values similar to the control bread. Besides, W-A20 crumb exhibited textural parameters similar to those obtained by W-A10 sample, showing that, at least in part, at this level the negative effect caused by algarroba addition is counterbalanced. This behavior could be attributed to galactomannan gums and globular proteins that contribute to dough matrix and balance gluten dilution. Therefore, breads of almost the same technological quality are obtained when 20% commercial algarroba flour is added to wheat flour. In addition, these breads present a higher amount of fiber and proteins of high biological value with respect to wheat bread.
Acknowledgements
Authors would like to acknowledge the UNLP, CONICET and MINCYT (Argentina) and FCT (Portugal) for the financial support (MINCYT-FCT PO-0928-2009). Authors also want to thank the kind cooperation of Elena Golovushkina and Christopher Young for the English revision of the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest
References
- AACC . Approved methods of the American Association of Cereal Chemists. Washington: AACC; 2000. [Google Scholar]
- Argentinean Alimentarius Codex (2016) Chapter IX “Alimentos farináceos – Cereales, Harinas Y Derivados”
- Balasubramanian S, Borah A, Singh K, Patil R. Rheological and nutritional quality of selected dehulled legumes blended rice extrudates. J Food Sci Technol. 2012;49(5):632–637. doi: 10.1007/s13197-010-0206-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beresford-Jones D, Arce S, Whaley O, Chepstow-Lusty AJ. The role of Prosopis in ecological and landscape change in the Samaca basin, lower Ica Valley, south coast Peru from the Earlier Horizon to the Late Intermediate period. Lat Am Antiq. 2009;20:303–332. doi: 10.1017/S1045663500002650. [DOI] [Google Scholar]
- Bigne F, Puppo MC, Ferrero C. Rheological and microstructure characterization of composite dough with wheat and mesquite (Prosopis spp) flours. Int J Food Prop. 2015;19(2):242–256. [Google Scholar]
- Bigne F, Puppo MC, Ferrero C. Fibre enrichment of wheat flour with mesquite (Prosopis spp.): effect on breadmaking performance and staling. LWT Food Sci Technol. 2016;65:1008–1016. doi: 10.1016/j.lwt.2015.09.028. [DOI] [Google Scholar]
- Bourne MC (2002) Principles of objective texture measurement. In: Bourne MC (ed) Food texture and viscosity: concept and measurement. Academic Press, California
- Buera MP, Retriella C, Lozano RD. Definition of colour in the nonenzymatic browning. Farbe. 1985;33:316–326. [Google Scholar]
- Burkart A. A monograph of the genus Prosopis (Leguminosae subfam. Mimosoideae) J Arnold Arbor. 1976;57(217–249):450–525. [Google Scholar]
- Cauvain SP. Improving the texture of bread. In: Kilcast D, editor. Food texture vol 2: solid foods. Cambridge: Woodhead Publishing Limited; 2004. [Google Scholar]
- Cauvain SP. Breadmaking: improving quality. Amsterdam: Elsevier; 2012. [Google Scholar]
- Correa MJ, Ferrer E, Añón MC, Ferrero C. Interaction of modified celluloses and pectins with gluten proteins. Food Hydrocoll. 2014;35:91–99. doi: 10.1016/j.foodhyd.2013.04.020. [DOI] [Google Scholar]
- Doxastakis G, Zafiriadis I, Irakli M, et al. Lupin, soya and triticale addition to wheat flour doughs and their effect on rheological properties. Food Chem. 2002;77:219–227. doi: 10.1016/S0308-8146(01)00362-4. [DOI] [Google Scholar]
- Duodu KG, Minnaar A. Chapter 18—legume composite flours and baked goods: nutritional, functional, sensory, and phytochemical qualities. In: Patel VRPRWB, editor. Flour and breads and their fortification in health and disease prevention. San Diego: Academic Press; 2011. pp. 193–203. [Google Scholar]
- Felker P, Takeoka G, Dao L. Pod mesocarp flour of North and South American species of leguminous tree Prosopis (Mesquite): composition and food applications. Food Rev Int. 2013;29:49–66. doi: 10.1080/87559129.2012.692139. [DOI] [Google Scholar]
- Gallão MI, Vieira ÍG, Mendes FN, et al. Reserve mobilisation in mesquite (Prosopis juliflora) seed (Leguminosae) J Sci Food Agric. 2007;87:2012–2018. doi: 10.1002/jsfa.2936. [DOI] [Google Scholar]
- Ibañez MC, Ferrero C. Extraction and characterization of the hydrocolloid from Prosopis flexuosa DC seeds. Food Res Int. 2003;36:455–460. doi: 10.1016/S0963-9969(02)00192-8. [DOI] [Google Scholar]
- Kaur A, Shevkani K, Katyal M, et al. Physicochemical and rheological properties of starch and flour from different durum wheat varieties and their relationships with noodle quality. J Food Sci Technol. 2016;53(4):2127–2138. doi: 10.1007/s13197-016-2202-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr WL, Wicker L. NMR proton relaxation measurements of water associated with high methoxy and low methoxy pectins. Carbohydr Polym. 2000;42:133–141. doi: 10.1016/S0144-8617(99)00169-1. [DOI] [Google Scholar]
- Khatkar BS. Effect of protein contents and water absorption values on dynamic rheological properties of wheat flour dough. J Food Sci Technol. 2005;42(4):358–366. [Google Scholar]
- Komlenić DK, Ugarčić-Hardi Ž, Jukić M, et al. Wheat dough rheology and bread quality effected by Lactobacillus brevis preferment, dry sourdough and lactic acid addition. Int J Food Sci Technol. 2010;45:1417–1425. doi: 10.1111/j.1365-2621.2010.02282.x. [DOI] [Google Scholar]
- Leung HK, Magnuson JA, Bruinsma BL. Pulsed nuclear magnetic resonance study of water mobility in flour doughs. J Food Sci. 1979;44:1408–1411. doi: 10.1111/j.1365-2621.1979.tb06449.x. [DOI] [Google Scholar]
- Marangoni A, Alli I. Composition and properties of seeds and pods of the tree legume Prosopis juliflora (DC) J Sci Food Agric. 1988;44:99–110. doi: 10.1002/jsfa.2740440202. [DOI] [Google Scholar]
- Mohammed I, Ahmed AR, Senge B. Dough rheology and bread quality of wheat–chickpea flour blends. Ind Crops Prod. 2012;36:196–202. doi: 10.1016/j.indcrop.2011.09.006. [DOI] [Google Scholar]
- Mohammed I, Ahmed AR, Senge B. Effects of chickpea flour on wheat pasting properties and bread making quality. Ind Crops Prod. 2014;51(9):1902–1910. doi: 10.1007/s13197-012-0733-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palou E, López-Malo A, Barbosa-Cánovas GV, et al. Polyphenoloxidase activity and color of blanched and high hydrostatic pressure treated banana puree. J Food Sci. 1999;64:42–45. doi: 10.1111/j.1365-2621.1999.tb09857.x. [DOI] [Google Scholar]
- Paraskevopoulou A, Provatidou E, Tsotsiou D, Kiosseoglou V. Dough rheology and baking performance of wheat flour–lupin protein isolate blends. Food Res Int. 2010;43:1009–1016. doi: 10.1016/j.foodres.2010.01.010. [DOI] [Google Scholar]
- Pasiecznik NM, Felker P, Harris PJC, et al. The Prosopis juliflora–Prosopis pallida complex: a monograph. London: Henry Doubleday Res Assoc Coventry UK; 2001. [Google Scholar]
- Sadasivam S, Manickam A. Biochemical methods for agricultural sciences. New Yok: Wiley Eastern Limited; 1992. [Google Scholar]
- Salinas MV, Zuleta A, Ronayne P, Puppo M. Wheat flour enriched with calcium and inulin: a study of hydration and rheological properties of dough. Food Bioprocess Technol. 2012;5:3129–3141. doi: 10.1007/s11947-011-0691-7. [DOI] [Google Scholar]
- Salinas M, Carbas B, Brites C, Puppo M. Influence of different carob fruit flours (Ceratonia siliqua L.) on wheat dough performance and bread quality. Food Bioprocess Technol. 2015;8:1561–1570. doi: 10.1007/s11947-015-1527-7. [DOI] [Google Scholar]
- Salinas MV, Zuleta A, Ronayne P, Puppo MC. Wheat bread enriched with organic calcium salts and inulin. A bread quality study. J Food Sci Technol. 2016;53:491–500. doi: 10.1007/s13197-015-2008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciammaro L, Ferrero C, Puppo M. Chemical and nutritional properties of different fractions of Prosopis alba pods and seeds. J Food Meas Charact. 2016;10:103–112. doi: 10.1007/s11694-015-9282-z. [DOI] [Google Scholar]
- Shewry PR. Improving the protein content and quality of temperate cereals: wheat, barley and rye. In: Welch RM, Çakmak I, editors. Impacts of agriculture on human health and nutrition, Ross M. Welch, and Ismail Çakmak. Oxford: UNESCO, Eolss Publishers; 2004. pp. 118–137. [Google Scholar]
- Shittu TA, Raji AO, Sanni LO. Bread from composite cassava–wheat flour: I. Effect of baking time and temperature on some physical properties of bread loaf. Starch Funct III. 2007;40:280–290. [Google Scholar]
- Shittu TA, Dixon A, Awonorin SO, et al. Bread from composite cassava–wheat flour. II: effect of cassava genotype and nitrogen fertilizer on bread quality. Food Res Int. 2008;41:569–578. doi: 10.1016/j.foodres.2008.03.008. [DOI] [Google Scholar]
- Singh N, Shevkani K, Kaur A, Thakur S, Parmar N, Virdi AS. Characteristics of starch obtained at different stages of purification during commercial wet milling of maize. Starch. 2014;66:668–677. doi: 10.1002/star.201300261. [DOI] [Google Scholar]
- Yadav DN, Rajan A, Sharma GK, Bawa AS. Effect of fiber incorporation on rheological and chapati making quality of wheat flour. J Food Sci Technol. 2010;47:166–173. doi: 10.1007/s13197-010-0036-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Wang F, Huang W, et al. Rheofermentometer fermentation and breadmaking characteristics of dough containing xylo-oligosaccharide hydrolyzate from wheat bran. J Agric Food Chem. 2010;58:1878–1883. doi: 10.1021/jf902131r. [DOI] [PubMed] [Google Scholar]