Abstract
Successful gene therapy depends on the development of efficient, non-toxic gene delivery systems. To accomplish this objective, our laboratory has focused on solid-phase synthesized peptide carriers, in which the amino acid sequence can be varied precisely to augment intracellular DNA transport. We previously determined that linear and branched co-polymers of histidine and lysine in combination with liposomes enhanced the efficiency of gene transfection. In this study, we have modified two branched histidine-lysine (HK) peptides by adding a histidine-rich tail. In a variety of cell lines, this histidine-rich tail markedly improved transfection efficiency, presumably by increasing the buffering capacity of the polymer. One polymer with a histidine-rich tail, H2K4bT, compared favorably with the commonly used transfection agents. Together with modification of our transfection protocol, these improved HK peptides alone, without liposomes, are the effective carriers of plasmids into a variety of cells. We anticipate that branched HK peptides will continue to be developed as carriers of nucleic acids for in vitro and in vivo applications.
INTRODUCTION
Non-viral carriers offer the potential of delivering genes to targets in a highly efficient, safe and specific manner. Among the non-viral candidates for gene delivery, which include liposomes, peptides, proteins and synthetic non-peptide polymers (1–6), our laboratory has focused primarily on solid-phase synthesized peptides that mediate gene transfer. These transfection peptides have not gained widespread use as sole carriers of nucleic acids nor have they been commercially viable, partly because of their low-transfection efficiency compared with that of other non-viral methods. Nevertheless, there have been a few promising examples using these peptides as transfection carriers, including (LARL6)-α-helical, MPG and KALA peptides (7–9). In contrast to these infrequently used peptide carriers, the related dendrimeric polymers [polyethylinimine (10,11), SuperFect (3) and polyamidoamine (12,13)] have been more commonly used and in general have higher transfection efficiency. Peptides synthesized by solid-phase offer potential advantages, however, in that the amino acid sequence can be varied considerably to augment transfection yet the amino acid sequence can be carefully controlled within the peptide.
We previously demonstrated that branched peptides, when combined with cationic liposomes, were efficient carriers of plasmids (14,15). The branched co-peptide is comprised of lysine and histidine; the lysine component forms a complex with and partially neutralizes the negative charge of the plasmid DNA, and the histidine component with a pKa of ∼6.0, buffers and aids in the release of plasmid DNA from endosomal vesicles. Without liposomes, however, the branched histidine–lysine (HK) peptides were not efficient carriers of nucleic acids in many cell lines.
Our goals in this study were 2-fold. First, we wanted to identify a branched polymer that was a more effective in vitro carrier of DNA into cells without the aid of liposomes. We believe that such a sole peptide carrier has intrinsic advantages over the more complicated bipartite carrier. To accomplish this, we designed several peptides that were enriched with histidines. Although HK peptides already have histidines within their structure, we reasoned that further increases in histidine content within the polymer would increase transfection. Nevertheless as we determined, the location of this histidine-rich domain within the peptide was critical for enhancing transfection.
In addition to discovering better peptide carriers, we developed a simplified protocol, which took advantage of some of the properties of HK peptides. As such peptides in complex with DNA are stable to the effects of serum, we thought that this might permit prolonged exposure of the intact complex with the cells and thereby increase transfection. In this study, we report a simple and effective protocol that enables the HK peptide to be an efficient carrier of plasmid DNA without the aid of liposomes.
MATERIALS AND METHODS
Cell culture and media
MDA-MB-435, MDA-MB-231 and MCF7 (three human cancer cell lines), as well as SVR-bag4 (SV-40-transformed endothelial cells) were maintained in the DMEM containing 10% fetal calf serum (FCS) and 20 mM glutamine at 37°C in an atmosphere of 95% humidified air and 5% CO2.
HK peptides
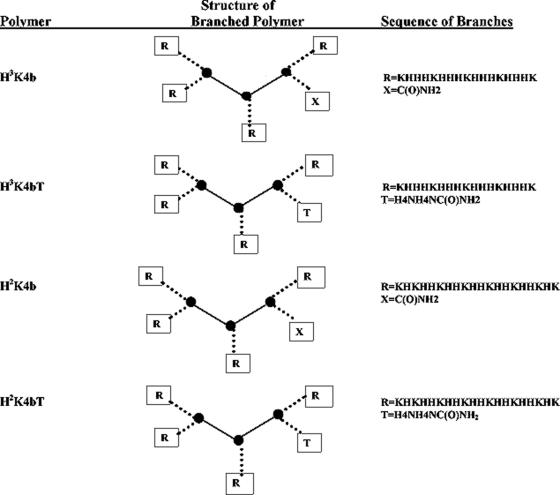
The biopolymer core facility at the University of Maryland synthesized the peptides on a Ranin Voyager synthesizer (PTI, Tucson, AZ) as described previously (16). The following peptides (Figure 1 and Table 2) were synthesized: (i) H3K4b, (ii) H3K4bT, (iii) H2K4b, (iv) H2K4bT, (v) H2K4bNLS1, (vi) H2K4bNLS2 and (vii) H2K4bRGD. H3K4b and H3K4bT were similar except that the latter has a histidine-rich tail; similarly, H2K4b and H2K4bT have the same structure except that H2K4bT has a histidine-rich tail. Polymers (v)–(vii) were similar in structure to H2K4bT except that the histidine-rich tail has been replaced with other transfection augmenting groups (Table 2). The peptides were purified on a high-performance liquid chromatography (Beckman, Fullerton, CA) and analyzed using mass spectroscopy (Perseptive Biosystems, Foster City, CA) to verify the predicted molecular mass.
Figure 1.
Schematic structure of branched HK peptides. H3K4b and H3K4bT: these peptides have a similar DNA-binding domain (R), but H3K4b and H3K4bT differ in that H3K4bT has a histidine-rich tail. H2K4b and H2K4bT: similarly, the difference between H2K4b and H2K4bT is that the latter has a histidine-rich tail.
Table 2.
Comparison of gene delivery carriers in which the histidine-rich tail of H2K4bT has been replaced
| Carrier | Groups | % Transfection |
|---|---|---|
| H2K4bT | -HHHHNHHHHN | 100 |
| H2K4bNLS1 | -GNYNNQSSNFGPMKGGN | 33 |
| H2K4bNLS2 | -PKKKRKV | 8 |
| H2K4bRGD | -GRGD | 25 |
All carriers have the same DNA-binding branches but they have different tails. H2K4bT had the highest transfection of a luciferase plasmid and other polymers transfection abilities were based on H2K4bT (expressed as % transfection of H2K4bT).
Titration of HK peptides
Titrations of HK peptides were performed as described previously for dextran-grafted polyethylenimine (17) with minor modifications. In brief, HK peptides were diluted to a concentration of 0.1 N NaCl and the pH was adjusted to 4.5 before titration. An aliquot of 10 μl (10−3 mEq) of 0.1 N NaOH was sequentially added to 12 ml of a 0.1 mg/ml polymer solution, and pH changes were recorded.
Preparation of liposomes
Preparation of the liposome–plasmid complexes have been described previously (18). In brief, DH5α bacteria (Invitrogen, Carlsbad, CA) containing the plasmids were grown in Superbroth to mid-log phase. The plasmids, PCI-Luc or PCI-βgal, were then purified with Qiagen columns (Valencia, CA). An analytical gel of each plasmid (cut and uncut) was performed to ensure that there was no contamination with other nucleic acids. Liposomes were comprised of 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP) (Avanti, Birmingham, AL). After hydration of the lipids, the liposomes were sonicated until clear with a Branson 1210 bath sonicator in the presence of argon. The liposomes were then extruded through 50 nm polycarbonate membranes with LipsoFast-Basic (Avestin Inc., Ottawa, ON). The final concentration of the liposomes was 1 μg/μl.
Transfection protocol
Initially, 1 × 105 cells were plated in 24-well plates in the presence of 500 μl of DMEM with 10% serum; after 24 h, the cells reached ∼70% confluency. Unless otherwise indicated, 4 μg of the polymer (in 8 μl) were mixed with 1.0 μg of the plasmid (PCI-Luc or PCI-βgal in 42 μl of Opti-MEM) and the mixture was allowed to stand for 30 min. Similarly, DOTAP (4 μg), Lipofectamine (2, 4 or 8 μg) (Invitrogen) and SuperFect (5 μg) (Qiagen) were mixed with 1 μg of PCI-Luc, and the mixture was allowed to stand for 30 min. To prepare the combination carrier, the HK co-polymer (4 μg) was first incubated with PCI-Luc (1.0 μg) for 30 min in Opti-MEM, and then cationic liposomes (1, 1.5 or 2 μg) were then added, gently mixed and allowed to stand for an additional 30 min. The standard or one-step protocol was then performed.
The standard protocol was performed as described previously with a few modifications (14). In brief, 50 μl of the transfection complex were mixed with 200 μl of Opti-MEM and this was then added to the cells for 4 h. The cells were washed once with phosphate-buffered saline (PBS), DMEM + 10% serum was then added and incubation was continued for a further 24 h. The reporter assay was then performed.
With the one-step method, the transfection complex (50 μl of the carrier–DNA complex) was added to the well containing the cells and 500 μl of DMEM containing 10% serum. After 24 h, the appropriate reporter assay was then performed.
Luciferase reporter assay
After the cells were washed once with 1× PBS, they were lysed with 100 μl of 1× passive lysis buffer (Promega Corp., Madison, WI). Protein concentration was measured by using the BCA protein assay kit (Pierce). Luciferase activity was measured by the direct current Turner 20/20 luminometer (Turner Design, Sunnyvale, CA) as described previously (14,19), and relative light units were converted into picograms (pg) of luciferase by using recombinant luciferase (Promega) as a standard. Duplicate measurements were performed for each concentration and three separate experiments were conducted; the data from the luciferase activity was expressed as the mean ± SEM.
β-Galactosidase staining
MDA-MB-435 cells, at ∼60% confluency, were stained with the β-galactosidase staining kit in accordance with the manufacturer's instructions (Invitrogen). In brief, the growth medium was removed from the transfected cells (transfected by PCI-Luc) and the cells were rinsed once with PBS. The cells were fixed for 10 min at room temperature, rinsed twice with 1× PBS, and then X-Gal staining solution was added to the cells for 6 h at 37°C. At least 200 cells were counted in four different fields (200×) and those cells that stained blue were counted as expressing β-galactosidase; in MDA-MB-435 cells transfected with a control plasmid (PCI-Luc), no background staining was observed.
Flow cytometry
To determine cellular toxicity after transfection with the peptide and DNA complexes, we followed the manufacturer's instructions for the Vybrant Apoptosis Assay #4 (Molecular Probes). Twenty-four hours before transfection, 2 × 105 MDA- MB-435 cells were plated in each well of 12-well plates. The cells were transfected with a luciferase-expressing plasmid (2 μg) in complex with the carrier (peptides H3K4bT or H2K4bT; 8 μg). The cells were then harvested after 24 h, washed in ice-cold 1× PBS, and the cells were suspended in 1 ml of PBS. To this suspension, 1 μl of the green fluorescent dye stock solution (YO-PRO-1) and 1 μl of the red fluorescent stock solution (propidium iodide) were added. After incubation on ice for 30 min, the number of cells that were necrotic and/or apoptotic was analyzed by the FACScan using 488 nm excitation (Becton Dickinson, San Jose, CA).
Measurement of particle size of peptide–DNA complexes
The branched peptide (4 μg in 8 μl of Opti-MEM) was mixed with the plasmid (PCI-Luc, 1 μg in 42 μl of Opti-MEM); after standing for 30 min at room temperature, another 450 μl of Opti-MEM was added to the complex. The peptide–DNA complex particles were then measured using a N4 Submicron Particle Sizer (Coulter, Miami, FL).
RESULTS AND DISCUSSION
Comparison of the 4 and 24 h transfection efficiency in MDA-MB-435 cell line
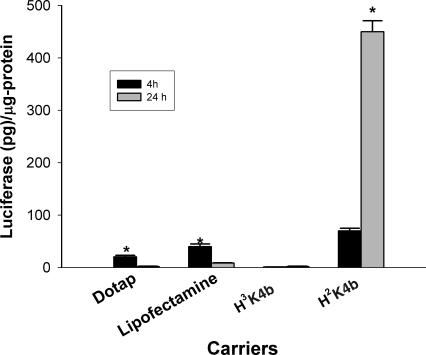
In the previous studies, after preparing the transfection complex, we incubated the carrier–DNA complex with the cells in the absence of serum for 4 h, after which we washed the cells, added growth medium with 10% serum, and measured reporter activity 24 h later. We wanted to compare this method with the one-step method in which the transfection complex was added directly to the well containing the cells and growth medium with 10% serum. After 24 h, we measured the luciferase activity. To compare these two transfection protocols, we used four carriers: DOTAP liposomes, Lipofectamine, H3K4b and H2K4b. Compared with the one-step method, the DOTAP and Lipofectamine carriers were considered to be the more effective carriers in the standard transfection method. H2K4b was the best carrier of the luciferase-containing reporter plasmid for both the standard and the one-step method; however, the one-step method using the H2K4b carrier was 7-fold more effective than the standard method (Figure 2). In addition to simplifying the transfection protocol, results from this one-step method indicate that the H2K4b polymer in complex with plasmid DNA was stable in DMEM containing 10% serum for prolonged periods of time.
Figure 2.
Comparison of 4 and 24 h transfection efficiency in MDA-MB-435 cells. MDA-MB-435 cells were transfected with DOTAP (2:1), Lipofectamine (4:1), H3K4b (4:1) and H2K4b (4:1) in complex with the PCI-Luc. For the 4 h transfection, the complex was incubated in Opti-MEM without serum for 4 h, the cells were washed, and DMEM containing 10% FCS was added; after 24 h, luciferase activity was measured. For the 24 h transfection experiments, the complex was added directly to the wells containing the 10% FCS DMEM and cells; after 24 h, luciferase activity was measured as described in Materials and Methods. *, P < 0.001; H2K4b (24 h) versus H2K4b (4 h); DOTAP (4 h) versus DOTAP (24 h); Lipofectamine (4 h) versus Lipofectamine (24 h); t-test.
HK peptides with a histidine-rich tail enhance gene transfection efficiency in the MDA-MB-435 cell line
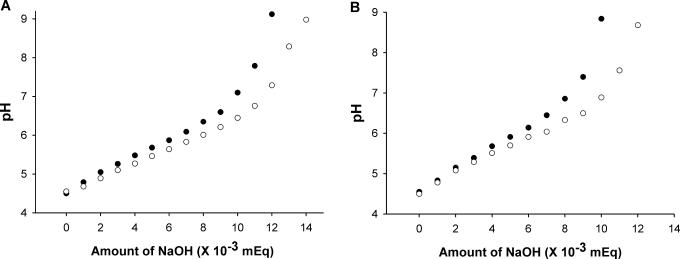
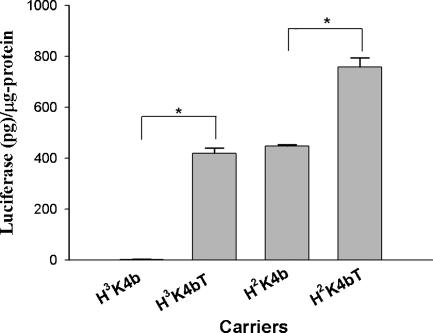
Although branched peptides are effective carriers of luciferase-containing plasmids with the modified protocol, we reasoned that these peptides could be improved by increasing their buffering capacity. Peptides with buffering capacity augment transfection by inducing endosomal swelling and lysis. Nevertheless, the location where the histidine-rich domain was added to the polymer was critical. For example, when the histidine-rich domain was moved to each of the four DNA-binding arms of the HK branched polymer (Figure 1), transfection efficiency decreased significantly (data not shown). As a result, we added the histidine-rich domain to a region of the HK peptides, which did not affect its ability to bind DNA (Figure 1). The addition of the histidine-rich domain increased the buffering capacity of the HK polymers, which is evident from the greater resistance to pH changes (Figure 3; H3K4b versus H3K4bT and H2K4b versus H2K4bT). We then tested if addition of a histidine-rich domain to the carrier affected transfection activity. The difference in luciferase activity of transfected cells between H3K4bT (with a histidine-rich domain) and H3K4b (without a histidine-rich domain) was >100-fold; the average luciferase activity for the H3K4b and H3K4bT carriers of PCI-Luc was 2.7 and 420 pg/μg-protein, respectively (Figure 4). Although the magnitude of the difference between H2K4b and H2K4bT was not as great (1.8-fold) as in MDA-MB-435 cell line (Figure 4), H2K4bT was the most effective branched HK carrier in transporting plasmids in this and all other tested cell lines. Notably, the size of these complexes could not explain the transfection differences mediated by these four HK peptides (Table 1).
Figure 3.
Titration of HK peptides. After the pH of the HK peptide solutions (0.1 mg/ml polymer solution in 0.1 N NaCl) was adjusted to 4.5, 10 μl (10−3 mEq) aliquots of 0.1 N NaOH was then sequentially added to 12 ml, and pH changes were recorded. The data represent the mean of duplicate experiments. (A) H3K4b and H3K4bT; and (B) H2K4b and H2K4bT. Open circles represent HK peptides with a histidine-rich tail.
Figure 4.
Modified branched peptide with a histidine-rich tail enhances gene transfection in MDA-MB-435 cells. MDA-MB-435 cells were transfected with PCI-Luc in complex with one of the four HK branched peptides: (i) H3K4b, (ii) H3K4bT, (iii) H2K4b and (iv) H2K4bT. In transfecting these cells, the peptides with a histidine-rich tail (H3K4bT and H2K4bT) were more effective than their counterparts without the tail. *, P < 0.001; H3K4bT versus H3K4b; H2K4bT versus H2K4b; t-test.
Table 1.
The polyplexes were prepared by mixing PCI-Luc plasmids (2 μg) with the above polymers (8 μg) in Opti-MEM for 30 min
| Polymer | Polyplex size (nm) |
|---|---|
| H3K4b | 490.3 ± 180.5 |
| H3K4bt | 654.3 ± 204.2 |
| H2K4b | 869.9 ± 341.1 |
| H2K4bT | 760.9 ± 92.4 |
The polyplexes were then diluted in Opti-MEM and the size of polyplexes was measured on N4 plus Submicron Particle Sizer.
Comparison of lacZ transfection efficiency in MDA-MB-435 with the four different HK polymers
We then determined the transfection efficiency of the HK peptides as carriers, by transfecting the plasmid lacZ with different peptide carriers into the MDA-MB-435 cell line and then staining for β-galactosidase activity within the cells (Figure 5). In general, the results obtained by staining the cells with β-galactosidase correlated closely with the luciferase activity results. H2K4bT had the highest transfection efficiency followed by H2K4b. The one interesting exception was H3K4bT. Luciferase activity after transfection with H3K4bT and H2K4b were similar in MDA-MB-435 cells, but H3K4bT had more than a 2-fold reduction in β-galactosidase staining compared with H2K4b. One explanation for this is that the histidine-rich tail may augment lysing the endosomal compartment, which is known to increase transfection. Although fewer cells were transfected with the histidine-rich tail polymer, H3K4bT, compared with H2K4b, the histidine-rich polymer apparently is more potent at augmenting the degree of transfection per cell. Notably also, the transfection efficiency differs between H3K4b, where there is a lysine for every three histidines, and H2K4b, where the lysines are separated on average by 1.8 histidines; the lower transfection efficiency by H3K4b is probably due to the reduced ability of the polymer to bind DNA.
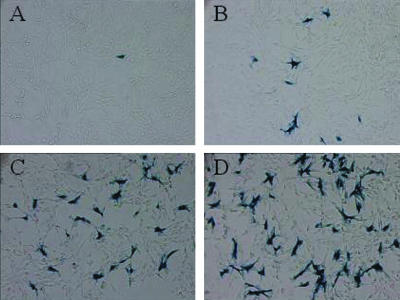
Figure 5.
Transfection efficiency of HK branched peptides. Twenty-four hours after plating, MDA-MB-435 cells were transfected with 1 μg of the lacZ plasmid in complex with one of following carriers: (A) H3K4b (4:1), (B) H3K4bT (4:1), (C) H2K4b (4:1) and (D) H2K4bT (4:1) [numbers in parentheses refer to the ratio of carrier to DNA (w/w)]. One day after the complexes were added to the cells, staining for β-galactosidase activity was performed to determine transfection efficiency. With the different carriers, the transfection efficiency with H3K4b was 2%, with H3K4bT was 19.2%, with H2K4b was 36.4% and with H2K4bT was 61.3%.
The H2K4bT transfection agent without liposomes was more effective
We wanted to identify a branched polymer that was an effective carrier of plasmid DNA into cells without the aid of liposomes. We thus compared the most effective HK polymer, H2K4bT, with and without liposomes for their ability to transfect MDA-MB-435 cells. H2K4bT was more effective as a sole carrier of a luciferase expressing plasmids compared with when it was combined with liposomes (Figure 6). Similarly, H2K4bT alone was more effective as a carrier compared with the liposome and polymer combination in other cell lines tested (MDA-MB-231, MCF-7 and SVR-bag4) (data not shown).
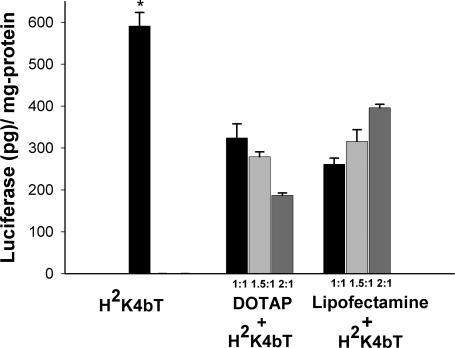
Figure 6.
Evaluation of H2K4bT co-polymer alone or in combination with liposomes in their effects on transfection. The co-polymer (4 μg) was mixed with DNA (1 μg of PCI-Luc) and in some treatment groups, liposomes (1, 1.5 or 2 μg) were then added; the mixture was then added to DMEM and 10% serum containing the MDA-MB-435 cells. Twenty-four hours after transfection, the cells were lysed to measure luciferase activity. The data represent the mean ± SEM of luciferase of three experiments. *, P < 0.01; H2K4bT versus H2K4bT and liposome treatment groups (one-way ANOVA with bonferroni-multiple comparisons).
Comparison of transfection with modified branched peptides in several cell lines
We then compared the ability of these HK peptides in transfecting three additional different cell lines (MDA-MB-231, MCF-7 and SVR-bag4). In these three cell lines, H2K4bT was the optimal polymer for transfecting PCI-Luc. Compared with its parent polymer (H2K4b), H2K4bT had an 8-fold higher transfection in MDA-MB-231 cells, a 1.4-fold higher transfection in MCF-7 cells and a 2-fold higher transfection in SVR-bag4 cells (Figure 7).
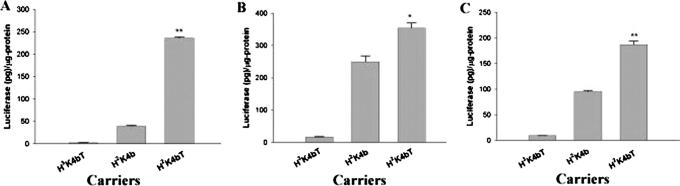
Figure 7.
Ability of the branched HK peptides to transfect various cell lines. After plating the cells, PCI-Luc in complex with the carrier (H3K4bT, H2K4b and H2K4bT) transfected the different cell lines [MDA-MB-231 (A), MCF-7 (B) and SVR-bag4 (C)] as described in Material and Methods. *, P < 0.05; **, P < 0.01; H2K4bT versus H2K4b and H3K4bT (one-way ANOVA with bonferroni-multiple comparisons).
Comparison of transfection ability of H2K4bT with other transfection agents in four cell lines
We compared the transfection efficiency of H2K4bT with two commonly used transfection reagents, Lipofectamine and SuperFect, in four cell lines (Figure 8). In the cell lines, H2K4bT was clearly most effective than Lipofectamine with the one-step protocol; the polymer was at least 4-fold most effective than Lipofectamine. In comparison with SuperFect, H2K4bT was most effective as a transfection agent in three of the four cell lines. When the one-step protocol using H2K4bT was compared with the standard method using Lipofectamine, the polymer was significantly better as a transfection agent in three of the four cell lines (data not shown). Although H2K4bT compared favorably with these transfection agents under the conditions employed, our primary goal remains the continued improvement of the HK polymer as a transfection agent.
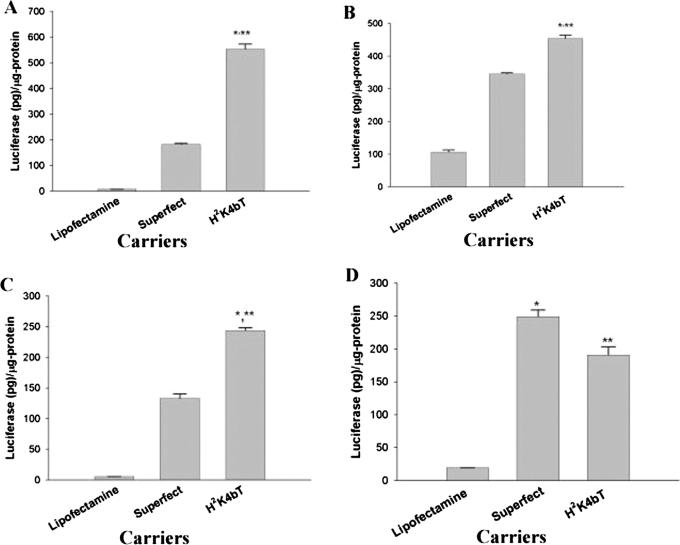
Figure 8.
Comparison of H2K4bT transfection efficiency with other transfection agents in different cell lines. The ability of H2K4bT as a carrier of PCI-Luc was compared with two commonly used transfection carriers (Lipofectamine and SuperFect) with the one-step method in different cell lines. The following four cell lines were transfected: MDA-MB-435 (A), MCF-7 (B), MDA-MB-231 (C) and SVR-bag4 (D) (for details of transfection see Materials and Methods). *, P < 0.05; H2K4bT versus SuperFect; **, P < 0.001; H2K4bT versus Lipofectamine.
The histidine-rich side chain is most effective
To determine whether other side groups might be more effective than the histidine-rich tail, we examined the effect of side groups, which, as part of transport mechanisms, have been reported to augment transfection (Table 2) (2,20–22). We selected two nuclear localization signals (see peptides H2K4bNLS1 and H2K4bNLS2 in Table 2) to aid transport of the polymer and presumably its DNA cargo into the nucleus, and an integrin-specific ligand (see H2K4bRGD peptide), which augments the initial attachment and internalization of the complex in MDA-MB-435 cells that express αv integrins (23). However, these modifications were significantly less effective than the histidine-rich tail suggesting that endosomal lysis is a critical step in achieving high transfection.
Minimal toxicity observed with HK polymers with a histidine-rich tail
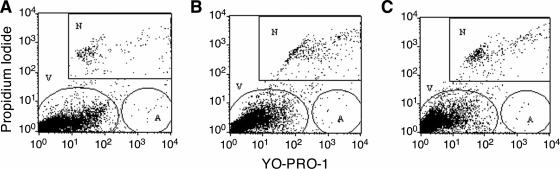
After staining the transfected MDA-MB-435 cell population with the YO-PRO-1 and PI dyes, apoptotic cells showed green fluorescence, dead cells showed red and green fluorescence, and the viable cells showed little or no fluorescence. Peptides H3K4bT and H2K4bT as transfection carriers of plasmid DNA (PCI-Luc) showed minimal toxicity compared with the untreated cells (Figure 9). In duplicate transfection experiments, the cell populations were classified into viable (V), apoptotic (A) and necrotic (N) groups. For untreated cells, the proportion of viable, apoptotic and necrotic cells was 96.6, 0.33 and 3.04, respectively; for H3K4bT-transfected cells, the proportion was 95.4 (V), 0.27 (A) and 4.33 (N); and for H2K4bT-transfected cells, the proportion was 96.0 (V), 0.27 (A) and 3.6 (N) (Figure 9). Similarly, a second cytotoxic assay based on the release of lactate dehydrogenase from cells confirmed that the polymers, H3K4bT and H2K4bT, had minimal toxicity following transfection of MDA-MB-435 and MDA-MB-231 (data not shown).
Figure 9.
Apoptosis and necrosis induced by H2K4bT and H3K4bT polymers. MDA-MB-435 cells were transfected with polymers H3K4bT and H2K4bT in complex with a luciferase-expressing plasmid as detailed in Material and Methods; the treatment groups are untreated (A), H3K4bT (B) and H2K4bT (C). The cells were then treated with YO-PRO-1 and PI fluorescence dyes and analyzed by flow cytometry using 488 nm excitation. V, viable cells; A, apoptotic cells; and N, necrotic cells.
In this study, we describe development of an in vitro agent that compares favorably with commonly used transfection agents. Previously, we found that branched HK peptides as sole DNA carriers were approximately one-third as effective as cationic liposomes in many cell lines. With the change of protocol and the addition of the histidine-rich tail to the branched HK peptides, these peptides are as efficient as many commercial cationic carriers with respect to transfection. There are two explanations for the success of these peptides as carriers of plasmid with the one-step protocol. First, with the addition of the histidine-rich domain, these peptides enhance the escape of DNA from the endosomal pathway to a greater degree thereby avoiding the degradation of DNA by lysosomes. Similar to other pH-sensitive functional groups, the histidine-rich domain, by virtue of its buffering the acidic endosomes, causes lysis of the endosomes. Second, the success of this protocol may be due to the ability of HK peptides to form relatively stable interactions with DNA in the presence of serum, unlike many transfection carriers. When HK peptides are compared with polylysine, the HK peptides in complex with DNA are relatively inert to the destabilizing effects of serum (16); we hypothesize that non-ionic interactions are occurring between the histidine component of the peptide and the DNA. This tolerance to serum allows longer exposure of the complex to the cells with reduced toxicity.
Our long-term objectives were to develop effective carriers for in vitro and in vivo applications. Preliminary data suggest that these HK peptides can be efficient carriers to the lungs and to extrapulmonary tumors. The advantages of peptide-based therapy are that the amino acid building blocks can be easily manipulated to develop improved carriers and the specific patterns of these peptides are infinite. Currently, these peptides are expensive to synthesize and hence the future challenge is to lower the cost of their synthesis. We believe this can be performed, at least in part, through assembling the different parts of the branched polymer together rather than the current en bloc synthesis that we now use.
Acknowledgments
We are grateful to Drs Pamela Talalay and Amy Fulton for their careful reading and useful comments concerning the manuscript. We thank Dr Nicholas Ambulos of the Maryland Biopolymer laboratory for synthesizing the peptides in this study. This study was supported by the National Cancer Institute grants (CA70394 and CA96984). Funding to pay the Open Access publication charges for this article was provided by National Institutes of Health.
REFERENCES
- 1.Li S., Huang L. Nonviral gene therapy: promises and challenges. Gene Ther. 2000;7:31–34. doi: 10.1038/sj.gt.3301110. [DOI] [PubMed] [Google Scholar]
- 2.Subramanian A., Ranganathan P., Diamond S.L. Nuclear targeting peptide scaffolds for lipofection of nondividing mammalian cells. Nat. Biotechnol. 1999;17:873–877. doi: 10.1038/12860. [DOI] [PubMed] [Google Scholar]
- 3.Uchida E., Mizuguchi H., Ishii-Watabe A., Hayakawa T. Comparison of the efficiency and safety of non-viral vector-mediated gene transfer into a wide range of human cells. Biol. Pharm. Bull. 2002;25:891–897. doi: 10.1248/bpb.25.891. [DOI] [PubMed] [Google Scholar]
- 4.Chen Q.R., Zhang L., Stass S.A., Mixson A.J. Co-polymer of histidine and lysine markedly enhances transfection of liposomes. Gene Ther. 2000;7:698–704. doi: 10.1038/sj.gt.3301294. [DOI] [PubMed] [Google Scholar]
- 5.Midoux P., Monsigny M. Efficient gene transfer by histidylated polylysine/pDNA complexes. Bioconjug. Chem. 1999;10:406–411. doi: 10.1021/bc9801070. [DOI] [PubMed] [Google Scholar]
- 6.Pichon C., Roufai M.B., Monsigny M., Midoux P. Histidylated oligolysines increase the transmembrane passage and the biological activity of antisense oligonucleotides. Nucleic Acids Res. 2000;28:504–512. doi: 10.1093/nar/28.2.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niidome T., Ohmori N., Ichinose A., Wada A., Mihara H., Hirayama T., Aoyagi H. Binding of cationic alpha-helical peptides to plasmid DNA and their gene transfer abilities into cells. J. Biol. Chem. 1997;272:15307–15312. doi: 10.1074/jbc.272.24.15307. [DOI] [PubMed] [Google Scholar]
- 8.Morris M.C., Chaloin L., Mery J., Heitz F., Divita G. A novel potent strategy for gene delivery using a single peptide vector as a carrier. Nucleic Acids Res. 1999;27:3510–3517. doi: 10.1093/nar/27.17.3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wyman T.B., Nicol F., Zelphati O., Scaria P.V., Plank C., Szoka F.C., Jr Design, synthesis, and characterization of a cationic peptide that binds to nucleic acids and permeabilizes bilayers. Biochemistry. 1997;36:3008–3017. doi: 10.1021/bi9618474. [DOI] [PubMed] [Google Scholar]
- 10.Boussif O., Lezouac'h F., Zanta M.A., Mergny M.D., Scherman D., Demeneix B., Behr J.P. A versatile vector for gene and oligonucleotide transfer into cell in culture and in vivo: polyethylenimine. Proc. Natl Acad. Sci. USA. 1995;92:7297–7300. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrari S., Moro E., Pettenazzo A., Behr J.P., Zacchello F., Scarpa M. ExGen 500 is an efficient vector for gene delivery to lung epithelial cells in vitro and in vivo. Gene Ther. 1997;4:1100–1106. doi: 10.1038/sj.gt.3300503. [DOI] [PubMed] [Google Scholar]
- 12.Haensler J., Szoka F.C. Polyamidoamine cascade polymers mediate efficient transfection of cells in culture. Bioconjug. Chem. 1993;4:372–379. doi: 10.1021/bc00023a012. [DOI] [PubMed] [Google Scholar]
- 13.Kukowska-Latallo J.F., Bielinska A.U., Johnson J., Spindler R., Tomalia D.A., Baker J.R., Jr Efficient transfer of genetic material into mammalian cells using Starburst polyamidoamine dendrimers. Proc. Natl Acad. Sci. USA. 1996;93:4897–4902. doi: 10.1073/pnas.93.10.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Q.R., Zhang L., Stass S.A., Mixson A.J. Branched co-polymers of histidine and lysine are efficient carriers of plasmids. Nucleic Acids Res. 2001;29:1334–1340. doi: 10.1093/nar/29.6.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Q.R., Zhang L., Luther P.W., Mixson A.J. Optimal transfection with the HK polymer depends on its degree of branching and the pH of endocytic vesicles. Nucleic Acids Res. 2002;30:1338–1345. doi: 10.1093/nar/30.6.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang L., Ambulos N., Mixson A.J. DNA delivery to cells in culture using peptides. Methods Mol. Biol. 2004;245:33–52. doi: 10.1385/1-59259-649-5:33. [DOI] [PubMed] [Google Scholar]
- 17.Tseng W.C., Tang C.H., Fang T.Y. The role of dextran conjugation in transfection mediated by dextran-grafted polyethylenimine. J. Gene Med. 2004;6:895–905. doi: 10.1002/jgm.572. [DOI] [PubMed] [Google Scholar]
- 18.Xu M., Kumar D., Srinivas S., Detolla L.J., Yu S.F., Stass S.A., Mixson A.J. Parenteral gene therapy with p53 inhibits human breast tumors in vivo through a bystander mechanism without evidence of toxicity. Hum. Gene Ther. 1997;8:177–185. doi: 10.1089/hum.1997.8.2-177. [DOI] [PubMed] [Google Scholar]
- 19.Tang M.X., Redemann C.T., Szoka F.C., Jr In vitro gene delivery by degraded polyamidoamine dendrimers. Bioconjug. Chem. 1996;7:703–714. doi: 10.1021/bc9600630. [DOI] [PubMed] [Google Scholar]
- 20.Makkerh J.P., Dingwall C., Laskey R.A. Comparative mutagenesis of nuclear localization signals reveals the importance of neutral and acidic amino acids. Curr. Biol. 1996;6:1025–1027. doi: 10.1016/s0960-9822(02)00648-6. [DOI] [PubMed] [Google Scholar]
- 21.Ciolina C., Byk G., Blanche F., Thuillier V., Scherman D., Wils P. Coupling of nuclear localization signals to plasmid DNA and specific interaction of the conjugates with importin alpha. Bioconjug. Chem. 1999;10:49–55. doi: 10.1021/bc980061a. [DOI] [PubMed] [Google Scholar]
- 22.Harbottle R.P., Cooper R.G., Hart S.L., Ladhoff A., McKay T., Knight A.M., Wagner E., Miller A.D., Coutelle C. An RGD-oligolysine peptide: a prototype construct for integrin-mediated gene delivery. Hum. Gene Ther. 1998;9:1037–1047. doi: 10.1089/hum.1998.9.7-1037. [DOI] [PubMed] [Google Scholar]
- 23.Chen X., Park R., Tohme M., Shahinian A.H., Bading J.R., Conti P.S. MicroPET and autoradiographic imaging of breast cancer alpha v-integrin expression using 18F- and 64Cu-labeled RGD peptide. Bioconjug. Chem. 2004;15:41–49. doi: 10.1021/bc0300403. [DOI] [PubMed] [Google Scholar]