Abstract
Volatile organic compounds (VOCs) emitted by plants are secondary metabolites that mediate the plant interaction with pathogens and herbivores. These compounds may perform direct defensive functions, i.e., acting as antioxidant, antibacterial, or antifungal agents, or indirectly by signaling the activation of the plant’s defensive responses. Using a non-targeted GC-MS metabolomics approach, we identified the profile of the VOCs associated with the differential immune response of the Rio Grande tomato leaves infected with either virulent or avirulent strains of Pseudomonas syringae DC3000 pv. tomato. The VOC profile of the tomato leaves infected with avirulent bacteria is characterized by esters of (Z)-3-hexenol with acetic, propionic, isobutyric or butyric acids, and several hydroxylated monoterpenes, e.g., linalool, α-terpineol, and 4-terpineol, which defines the profile of an immunized plant response. In contrast, the same tomato cultivar infected with the virulent bacteria strain produced a VOC profile characterized by monoterpenes and SA derivatives. Interestingly, the differential VOCs emission correlated statistically with the induction of the genes involved in their biosynthetic pathway. Our results extend plant defense system knowledge and suggest the possibility for generating plants engineered to over-produce these VOCs as a complementary strategy for resistance.
Keywords: metabolomics, tomato, bacteria, VOCs, defense
Introduction
Plants have developed multiple defense mechanisms to protect themselves against biotic and abiotic stresses. Accumulation of secondary metabolites, which display numerous biological properties, constitutes a major component of stress responses (Dixon, 2001; Pusztahelyi et al., 2015; Qian et al., 2015; Kalaivani et al., 2016). Among them, volatile organic compounds (VOCs) are a relevant group involved in plant protection against pathogens and herbivores, and also in attracting pollinators and seed dispersers (Dudareva et al., 2013). Plant VOCs include a wide range of chemical structures, such as terpenoids and phenylpropanoids-benzenoids, as well as fatty and amino acid derivatives (Holopainen and Gershenzon, 2010; Granell and Rambla, 2013). They all have a low molecular weight and polarity, high vapor pressures, and possess the ability to both cross membranes freely and be released into the surrounding atmosphere (Dudareva et al., 2006).
The bacterial speck of tomato, caused by Pseudomonas syringae pv. tomato (Pst), is a major problem in the agricultural industry (Willis and Kinscherf, 2009). Tomato cultivar Rio Grande, which contains the Pto gene (RG-Pto), is resistant to Pst, which expresses effectors or avirulence genes avrPto/avrPtoB. Such “gene-for-gene” recognition (Pto-avrPto/avrPtoB) elicits Effector-Triggered Immunity (ETI) establishment in plants, which allows the control of bacterial spread, and results in an incompatible interaction (Jia et al., 1997; Dangl and Jones, 2001; Lin and Martin, 2005). In contrast, Pst, which bears a deletion of avrPto genes (ΔavrPto/ΔavrPtoB), becomes virulent to RG-Pto plants by causing disease in plants and the development of a compatible interaction (Salmeron et al., 1994). Therefore, this tomato pathosystem represents an excellent model to study both kinds of plant–pathogen interactions.
Different signal molecules, such as salicylic acid (SA), gentisic acid (GA), ethylene (ET), or jasmonic acid (JA), have been described to accumulate upon pathogen attacks in tomato plants. SA accumulation has been associated with avirulent infections (Block et al., 2005), while high levels of GA and ET have been found in compatible interactions in tomato (Bellés et al., 1999; Zacarés et al., 2007). JA has been associated with plant responses to herbivores or necrotrophic pathogens (Zhang et al., 2017). However, the role of these defensive molecules has not been characterized in tomato Rio Grande plants infected with virulent or avirulent Pst strains.
More and more studies are being conducted to understand the participation of small metabolites in plant–pathogen interactions (Allwood et al., 2008, 2012). However, the interest in VOCs has focused mainly on the plant response to herbivores and fruit quality, and studies of VOCs in the plant response to pathogens are scarce (Niinemets et al., 2013). Specifically, differential volatile emission has been described for tobacco and pepper leaves in response to both avirulent and virulent strains of Pseudomonas (Huang et al., 2003, 2005) and Xanthomonas (Cardoza and Tumlinson, 2006), respectively, but the biological meaning of this phenomenon is not well understood.
In this paper, we applied an untargeted GC-MS metabolomics approach to analyze the volatiles differentially emitted in RG-Pto tomato plants infected with either avirulent strain Pst DC3000 or virulent strain Pst DC3000 ΔavrPto/ΔavrPtoB. Besides, levels of classical defense hormones, such as SA, GA, ET, and JA, were characterized in both tomato interactions. Finally, the activation of a set of genes involved in the corresponding VOC biosynthesis pathways was also studied. Our results will unravel the VOC network that underlies the tomato immune response against Pseudomonas syringae.
Materials and Methods
Bacterial Strains, Growth Conditions, and Inoculum Preparation
The bacterial strains used in this study were Pseudomonas syringae pv. tomato DC3000 (Pst DC3000), and Pst DC3000 that contains deletions in genes avrPto and avrPtoB (Pst DC3000 ΔavrPto/ΔavrPtoB) (Ntoukakis et al., 2009). Bacteria were grown overnight at 28°C in 20 mL Petri dishes with King’s B agar medium supplemented with different antibiotic doses: rifampicin (10 mg/mL) and kanamicin (0.5 mg/mL) for Pst DC3000, and rifampicin (10 mg/mL), kanamycin (0.25 mg/mL) and spectinomycin (2.5 mg/mL) for Pst DC3000 ΔavrPto/ΔavrPtoB. Then bacterial colonies were transferred to 15 mL of King’s B medium supplemented with rifampicin (10 mg/mL), and were grown overnight at 28°C with stirring. Bacteria were then pelleted by centrifugation and resuspended in 10 mM of MgCl2, which contained 0.05% (v/v) Silwet L-77, to an optical density of 0.1 at 600 nm. Dilution plating was used to determine the final inoculum concentration, which averaged at 1 × 107 CFU/mL.
Plant Material and Bacterial Inoculation
Tomato seeds from the cultivar Rio Grande that contained resistance (R) gene Pto (RG-Pto) were grown under greenhouse conditions with a 16/8-h (26/30°C) light/dark photoperiod (300 μmol/m2/s) and 65% relative humidity in 12 cm-diameter pots that contained a 1:1 mixture of peat and vermiculite.
Inoculations with compatible and incompatible bacteria were produced by immersing 28-day-old RG-Pto plants in Pst DC3000 or Pst DC3000 ΔavrPto/ΔavrPtoB suspension, respectively, as previously described (Martin et al., 1993). For mock inoculations, plants were dipped in 10 mM of MgCl2 solution that contained Silwet L-77 (0.05%) without the bacterial inoculum. The third and fourth leaves, from bottom to top, were harvested and frozen in liquid nitrogen at the indicated times. The fifth leaf was placed freshly in a 10-mL screw cap vial and was kept for 5 h to take ET measurements. Six biological replicates were analyzed for each time and tomato–bacteria interaction.
Extraction and the HPLC Analysis of Salicylic and Gentisic Acids
Extraction of free and total SA and GA from tomato leaflets was performed according to our previously published protocol (Bellés et al., 2008). Aliquots of 30 μL were injected through a Waters 717 autosampler into a reverse-phase Sun Fire 5-μm C18 column (4.6 mm ×150 mm) equilibrated in 1% (v/v) acetic acid at room temperature. A 20-min linear gradient of 1% acetic acid to 100% methanol was applied using a 1525 Waters Binary HPLC pump at a flow rate of 1 mL/min. SA and GA were detected with a 2475 Waters Multi-λ Fluorescence detector (λ excitation 313 nm; λ emission 405 nm), and were quantified with the Waters Empower Pro software using authentic standard compounds (SA sodium salt and GA, Sigma–Aldrich, Madrid, Spain). Standard curves were performed for each compound using similar concentration ranges to those detected in the samples. Data were corrected for losses in the extraction procedure, and recovery of metabolites ranged between 50 and 80%.
Jasmonic Acid and Ethylene Measurements
Ethylene production was measured as described by Lieberherr et al. (2003) with some modifications. Approximately 0.5 g of fresh tissue from the fifth tomato leaf, harvested at the indicated time points after bacterial inoculation, was quickly enclosed in gas-tight 10-mL glass vials fitted with a septum. After 5 h, 400 μL of the gas phase from the vial were analyzed by gas chromatography in a 4890A Hewlett Packard gas chromatograph fitted with a flame ionization detector (FID) using a Teknokroma capillary column (2 m × 1/6″ OD × 1 mm ID, Alumina F1 80/100). The carrier gas was helium, used at a pre-column pressure of 140 kPa. The injector and detector temperatures were set at 200°C, while the oven temperature was 80°C. The retention time of the ET peak under these conditions was 2.5 min. For each time point, six replicates were analyzed and the amount of ET was calculated from the data recorded and analyzed with the MassLynx Waters software by constructing a standard ET curve.
For JA quantification, 250 mg of frozen tissue from the third and fourth tomato leaves were added to 80% methanol–1% acetic acid that contained the internal standard dihydrojasmonate (OlChemIm, Czechia), and were mixed by shaking for 1 h at 4°C. The extract was kept at -20°C overnight and was then centrifuged. The supernatant was dried in a vacuum evaporator. The dry residue was dissolved in 1% acetic acid and passed through a reverse phase Oasis HLB column (Seo et al., 2011). The dried eluate was dissolved in 5% acetonitrile–1% acetic acid, and the hormone was identified using a reverse phase Ultra Performance Liquid Chromatography (UPLC) system coupled to a Q-Exactive mass spectrometer (Orbitrap detector; Thermo Fisher Scientific) by targeted Selected Ion Monitoring (SIM). Separation was performed in a 2.6 μm Accucore RP-MS column, 50 mm long × 2.1 mm i.d.; (Thermo Fisher Scientific) using a 5–50% acetonitrile gradient that contained 0.05% acetic acid as the solvent system at 400 μL/min for 14 min. The JA concentration in the extracts was determined using embedded calibration curves with the authentic standard (OlChemIm, Czechia) and the Xcalibur 2.2 SP1 build 48 and TraceFinder software.
HS-SPME Extraction and the GC-MS Analysis of Volatile Compounds
For the volatile analysis, 100 mg of frozen tomato leaf powder were weighed in a 10-mL headspace screw-cap vial. One milliliter of a saturated CaCl2 solution and 100 μL of 750 mM EDTA (adjusted to 7.5 pH with NaOH) were added, mixed gently and sonicated for 5 min. Extraction of volatile compounds was performed by headspace solid-phase micro-extraction (HS-SPME) (Rambla et al., 2015). The pre-incubation and extraction periods, both at 50°C, were 10 and 20 min, respectively. Adsorption was performed by means of a 65 μm DVB/PDMS fiber (Supelco, Bellefonte, PA, United States). Desorption was done in the injection port of the gas chromatograph for 1 min at 250°C in the splitless mode. Volatile extraction and injection were performed automatically with a CombiPAL autosampler (CTC Analytics, Zwingen, Switzerland).
The chromatographic separation of compounds was performed in an Agilent 6890N gas chromatograph (Santa Clara, CA, United States) equipped with a DB-5 ms fused silica capillary column (60 m long, 0.25 mm i.d., 1 μm film thickness). The oven temperature conditions were 40°C for 2 min, 5°C/min ramp until 250°C and then held isothermally at 250°C for 5 min. Helium was used as the carrier gas at 1.2 mL/min at a constant flow. Detection was performed in an Agilent 5975B mass spectrometer (Santa Clara, CA, United States), which operated in the EI mode (ionization energy, 70 eV; source temperature 230°C). Data acquisition was performed in the scan mode (m/z range 35–250; six scans per second). Chromatograms and mass spectra were recorded and processed by the Enhanced ChemStation software (Agilent).
The unequivocal compound identification of the 70 volatile compounds was carried out by comparing both mass spectra and retention times with those of pure standards. All the commercial standards were purchased from Sigma–Aldrich (Madrid, Spain). Three other compounds were tentatively identified by comparing their mass spectra with those in the NIST 05 Mass Spectral library. Such tentatively identified compounds are marked with an asterisk.
RNA Extraction and the Quantitative RT-PCR Analysis
The total RNA of tomato leaves was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, United States), following the manufacturer’s protocol. RNA was then precipitated by adding one volume of 6 M LiCl and keeping it on ice for 4 h. Afterward the pellet was washed using 3 M LiCl and was dissolved in RNase-free water. Finally, in order to remove any contaminating genomic DNA, 2 U of TURBO DNase (Ambion, Austin, TX, United States) were added per microliter of RNA.
For the quantitative RT-PCR (qRT-PCR) analysis, one microgram of total RNA was employed to obtain the corresponding cDNA target sequences using an oligo(dT)18 primer and the PrimeScript RT reagent kit (Perfect Real Time, Takara Bio Inc., Otsu, Shiga, Japan), following the manufacturer’s directions. Quantitative PCR was carried out as previously described (Campos et al., 2014). A housekeeping gene transcript, Elongation Factor 1 alpha (eEF1α), was used as the endogenous reference. The PCR primers were designed using the pcr Efficiency software (Mallona et al., 2011) and are listed in Supplementary Table S1. The primers used to amplify TomLOXF have been previously described (Shen et al., 2014).
Statistical Analysis
The statistical analyses of the signal compounds levels (GA, SA, ET, and JA) and the qRT-PCR of the selected genes were done by an analysis of variance (multifactor ANOVA) using Statgraphics Centurion XVI.
For the untargeted analysis of the volatile profile, the GC-MS data were processed with the MetAlign software (Wageningen, Netherlands) for the alignment of the chromatograms and the quantitation of each MS feature. The resulting dataset was submitted to a Partial Least Square (PLS) study by the SIMCA-P software (v. 11.0, Umetrics, Umeå, Sweden) using unit variance (UV) scaling.
For the Hierarchical Cluster Analysis (HCA), the ratios for each VOC were calculated and log2-transformed for normalization. HCA was performed with the Acuity 4.0 software (Axon Instruments) with the distance metrics based on the Pearson correlation. The normalized data were represented as a heat map using the same software. The Pearson correlations between gene expression and the VOCs concentrations were performed with the SPSS 16.0 software by considering data at 10 and 18 hpi (hour post-inoculation).
Results
RG-Pto Tomato Plants Infected Either with the Avirulent or Virulent Bacterial Strain Displayed Noticeable Symptom Differences
Symptoms development of the tomato plants infected with either avirulent strain Pst DC3000 or virulent Pst DC3000 ΔavrPto/ΔavrPtoB is shown in Figure 1. These symptoms ranged on a 0–4 scale as follows: symptomless (0), weak (1), moderate (2), severe (3), and very severe (4). Inoculation of the RG-Pto tomato plants with the virulent strain resulted in chlorotic lesions appearing by 18 hpi, which displayed a symptom degree from (0) to (1). These initial lesions increased in intensity and size, and caused significant leaf damage that ranged from (2) to (3) at 24 and 36 hpi, respectively. By 48 hpi, necrotic lesions extended to the total area, and leaves lost their firmness and completely collapsed, which was the maximum level of symptomatology (4). Strong epinasty was observed in the leaves of these symptomatic tomato plants. In contrast, no symptoms (0) were observed at any time on the RG-Pto tomato plants inoculated with avirulent strain Pst DC3000 due to Pto-avrPto/avrPtoB recognition and ETI establishment. Therefore, these immunized tomato leaves were similar to the mock-inoculated plants.
FIGURE 1.
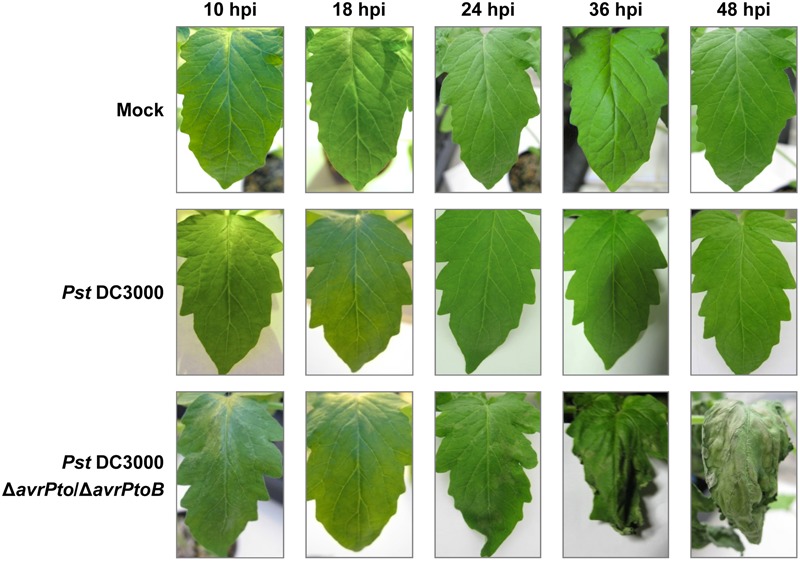
Symptom evolution in RG-Pto plants at 10, 18, 34, 36, and 48 h post-inoculation (hpi) with 10 mM of MgCl2 solution (mock), or either Pst DC3000 or Pst DC3000 ΔavrPto/ΔavrPtoB at 107 CFU/mL.
Levels of Salicylic Acid, Gentisic Acid, and Ethylene Were Enhanced in the RG-Pto Tomato Plants Infected with the Virulent Bacterial Strain, While Jasmonic Acid Drastically Lowered
The levels of the signaling defense molecules, SA, GA, ET, and JA were analyzed in the plants infected with both virulent and avirulent strains in a time-course study. Figure 2 shows the significant GA induction that occurred in all the inoculated plants that bore both interactions. The increased amount of GA was already evident in tomato plants at 10 hpi. Leaf GA accumulation increased as bacterial infection progressed, and the highest levels peaked at 48 hpi (45 nmol/g FW) in the symptomatic tomato plant leaves. By 24 hpi, a significantly higher GA value was obtained in the compatible interaction compared to the incompatible infection. No remarkable increments in SA were observed in the Pseudomonas-infected tomato plants at any time point, although differences in the SA levels started to become significant in the compatible interaction by 24 hpi.
FIGURE 2.
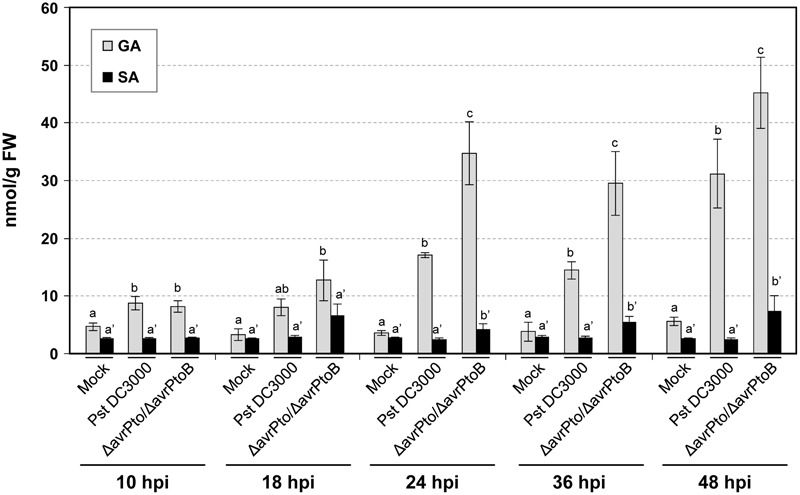
Levels of the free and total salicylic (SA) and gentisic (GA) acids, measured in an HPLC-fluorescence detector in the mock-inoculated RG-Pto tomato plants (mock) and upon infection with Pst DC3000 or Pst DC3000 ΔavrPto/ΔavrPtoB at 10, 18, 24, 36, and 48 hpi. Two multifactor ANOVA analyses were performed using GA (x) or SA (x’) data. Different letters indicate the statistical significances with a p-value < 0.05 compared with the mock-inoculated plants.
The evolution of ET from the leaves of both the compatible and incompatible tomato interactions was also measured. A dramatic increase in the production of this stress hormone was detected in the compatible interaction, which paralleled GA accumulation and appearance of symptoms (Figure 3A). Maximum ET production (188 nL/gFW/h) was reached at 18 hpi, which coincided with the onset of symptom development. These high levels of ET (up to 10-fold) could explain the strong epinasty observed in the tomato plants infected with the virulent bacteria. Remarkably during this compatible infection, ET biosynthesis was higher than that observed in the incompatible interaction, and was significantly elevated at any time point. Regarding infection with the avirulent bacteria, the differences in ET emission between the infected and mock-inoculated plants was only significant at 24, 36, and 48 hpi.
FIGURE 3.
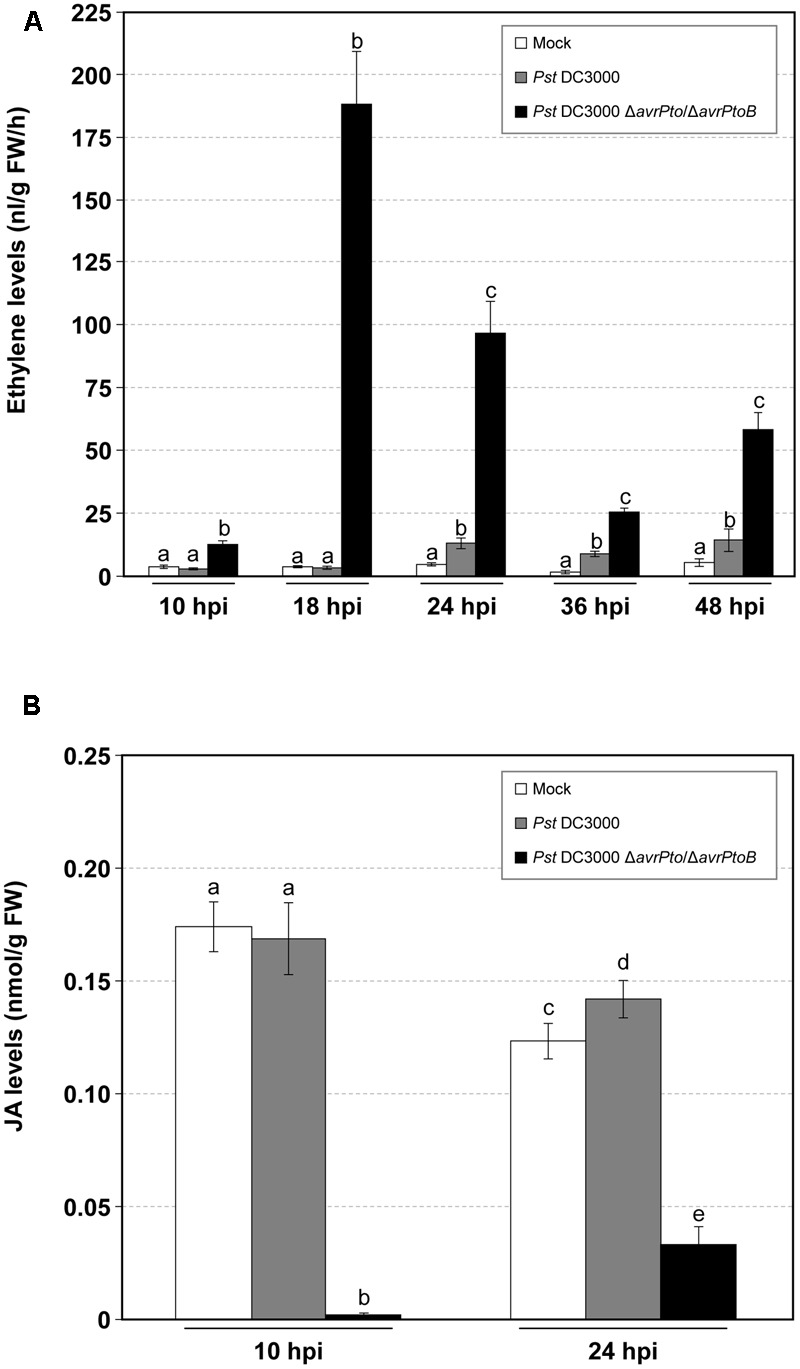
Time-course analysis of the mock-inoculated RG-Pto tomato plants (mock) and upon infection with Pst DC3000 or Pst DC3000 ΔavrPto/ΔavrPtoB at 10, 18, 24, 36, and 48 hpi for ethylene production (A), and at 10 and 24 hpi for jasmonic acid (JA) accumulation (B). Different letters indicate the statistical significances with a p-value < 0.05 compared with the mock-inoculated plants.
In contrast, the JA levels drastically lowered during virulent infection, and almost non-detectable values were displayed at 10 hpi. Regarding avirulent infection, JA production remained unaltered compared to the mock-inoculated tomato plants. A similar tendency was observed at 24 hpi (Figure 3B).
The Volatile Profile of Tomato Leaves Altered Differentially upon Infection with Both Pseudomonas syringae Strains
In order to examine the VOCs involved in the tomato–pathogen interactions, changes in the levels of these metabolites in the RG-Pto tomato plants, which were either mock-inoculated or infected with avirulent strain Pst DC3000 or virulent Pst DC3000 ΔavrPto/ΔavrPtoB, were analyzed by GC-MS in the leaves infected between 10 and 48 hpi (see Materials and Methods). Table 1 lists the VOCs detected on our HS-SPME/GC-MS platform for the mock and infected RG-Pto tomato plants. In all, 73 compounds were identified: 11 esters (9 aliphatic and 2 aromatic), 20 aldehydes (16 aliphatic, 3 aromatic and 1 norcarotenoid), 13 alcohols (6 aliphatic, 1 norcarotenoid, 5 monoterpenic, and 1 sesquiterpenic), 9 monoterpene hydrocarbons, 8 ketones (5 aliphatic, 1 aromatic, and 2 norcarotenoid), 3 sesquiterpene hydrocarbons, 2 furans, 2 nitriles, 4 aliphatic acids, and 1 aromatic hydrocarbon. They were all unequivocally confirmed by using pure standards, except for three of them, which were tentatively identified based on their mass spectra similarity (match > 900).
Table 1.
List of the VOCs identified in tomato leaves by GC-MS.
| Volatile Organic | Family | Retention | Specific | |
|---|---|---|---|---|
| Code | Compound | Code/Number | time (min) | ion (m/z) |
| 1 | Ethanol | Alc/1 | 4.95 | 45 |
| 2 | Acetone | Ket/1 | 5.68 | 58 |
| 3 | Butanol | Alc/2 | 10.41 | 56 |
| 4 | 1-Penten-3-ol | Alc/3 | 11.17 | 57 |
| 5 | 1-Penten-3-one | Ket/2 | 11.29 | 55 |
| 6 | 2-Pentanone | Ket/3 | 11.31 | 86 |
| 7 | 3-Pentanone | Ket/4 | 11.68 | 86 |
| 8 | Pentanal | Ald/1 | 11.79 | 44 |
| 9 | 2-Ethylfuran | Fur/1 | 11.85 | 81 |
| 10 | 3-Methylbutanenitrile | Nit/1 | 13.38 | 43 |
| 11 | (E)-2-Methyl-2-butenal | Ald/2 | 13.62 | 84 |
| 12 | (E)-2-Pentenal | Ald/3 | 14.07 | 83 |
| 13 | 1-Pentanol | Alc/4 | 14.36 | 42 |
| 14 | (Z)-2-Penten-1-ol | Alc/5 | 14.48 | 68 |
| 15 | (Z)-3-Hexenal | Ald/4 | 15.75 | 69 |
| 16 | Hexanal | Ald/5 | 15.84 | 72 |
| 17 | Butyl acetate | Est/1 | 16.18 | 43 |
| 18 | Methyl pentanoate | Est/2 | 16.65 | 85 |
| 19 | (Z)-3-Hexen-1-ol | Alc/6 | 18.01 | 82 |
| 20 | (E)-2-Hexenal | Ald/6 | 18.03 | 83 |
| 21 | Pentanoic acid | Acid/1 | 18.29 | 60 |
| 22 | 2-Heptanone | Ket/5 | 19.30 | 58 |
| 23 | Heptanal | Ald/7 | 19.85 | 70 |
| 24 | Methyl hexanoate | Est/3 | 20.47 | 74 |
| 25 | (E,E)-2,4-Hexadienal | Ald/8 | 20.35 | 81 |
| 26 | α-Pinene | Mt hd/1 | 21.51 | 93 |
| 27 | Hexanoic acid | Acid/2 | 21.95 | 60 |
| 28 | (E)-2-Heptenal | Ald/9 | 22.02 | 68 |
| 29 | Benzaldehyde | Ald/10 | 22.66 | 106 |
| 30 | o-Cymene∗ | Mt hd/2 | 22.96 | 119 |
| 31 | Myrcene | Mt hd/3 | 23.11 | 93 |
| 32 | Pseudocumene | Ar/1 | 23.11 | 105 |
| 33 | 2-Pentylfuran | Fur/2 | 23.22 | 81 |
| 34 | (E,Z)-2,4-Heptadienal | Ald/11 | 23.52 | 81 |
| 35 | (Z)-3-Hexenyl acetate | Est/4 | 23.53 | 43 |
| 36 | Octanal | Ald/12 | 23.64 | 84 |
| 37 | 2-Carene | Mt hd/4 | 23.89 | 93 |
| 38 | (E,E)-2,4-Heptadienal | Ald/13 | 24.10 | 81 |
| 39 | α-Phellandrene | Mt hd/5 | 24.15 | 93 |
| 40 | α-Terpinene | Mt hd/6 | 24.50 | 121 |
| 41 | p-Cymene | Mt hd/7 | 24.77 | 119 |
| 42 | Limonene | Mt hd/8 | 24.96 | 68 |
| 43 | β-Phellandrene∗ | Mt hd/9 | 25.15 | 93 |
| 44 | Phenylacetaldehyde | Ald/14 | 25.16 | 91 |
| 45 | (E)-2-Octenal | Ald/15 | 25.48 | 83 |
| 46 | Salicylaldehyde | Ald/16 | 25.77 | 122 |
| 47 | (Z)-Linalool oxidea,b | Alc/7 | 26.34 | 59 |
| 48 | Acetophenone | Ket/6 | 26.38 | 105 |
| 49 | (Z)-3-Hexenyl propionate | Est/5 | 26.77 | 67 |
| 50 | (E)-Linalool oxidea,b | Alc/8 | 26.90 | 111 |
| 51 | 2-Ethyl hexanoic acid | Acid/3 | 26.91 | 88 |
| 52 | Linaloola | Alc/9 | 27.04 | 93 |
| 53 | Nonanal | Ald/17 | 27.18 | 57 |
| 54 | (Z)-3-Hexenyl isobutyrate | Est/6 | 28.25 | 82 |
| 55 | Octanoic acid | Acid/4 | 28.84 | 60 |
| 56 | Benzonitrile | Nit/2 | 28.78 | 117 |
| 57 | (E)-2-Nonenal | Ald/18 | 28.92 | 70 |
| 58 | Benzyl acetate | Est/7 | 29.30 | 108 |
| 59 | (Z)-3-Hexenyl butyrate | Est/8 | 29.67 | 67 |
| 60 | 4 Terpineola | Alc/10 | 30.27 | 71 |
| 61 | α-Terpineola | Alc/11 | 30.65 | 59 |
| 62 | Methyl salicylate | Est/9 | 30.67 | 65 |
| 63 | Decanal | Ald/19 | 31.18 | 70 |
| 64 | β-Cyclocitralc | Ald/20 | 31.53 | 137 |
| 65 | δ-Elemene∗ | Sqt/1 | 34.88 | 121 |
| 66 | Eugenolc | Alc/12 | 35.32 | 164 |
| 67 | Ethyl decanoate | Est/10 | 35.76 | 88 |
| 68 | α-Iononec | Ket/7 | 37.25 | 121 |
| 69 | β-Caryophyllene | Sqt/2 | 37.76 | 133 |
| 70 | α-Humulene | Sqt/3 | 38.73 | 121 |
| 71 | β-Iononec | Ket/8 | 38.80 | 177 |
| 72 | Methyl dodecanoate | Est/11 | 39.30 | 74 |
| 73 | Nerolidold | Alc/13 | 40.55 | 93 |
Family Code: Alc, alcohol; Ald, aldehyde; Ar, aromatic hydrocarbon; Est, ester; Fur, furane; Ket, ketone; Mt hd, monoterpene hydrocarbon; Nit, nitrile; Sqt, sesquiterpene. ∗Tentative identification based on mass spectrum. aMonoterpene-derived compound. bIn addition to the alcohol group, it has a tetrahydrofuran group. cNorcarotenoid compound. dSesquiterpene-derived compound.
To manage the large amount of mass data, a multivariate data analysis was performed that consisted in a PLS analysis, where compound abundance was assigned to the X variable, and harvesting time (10, 18, 24, 36, and 48 hpi) and type of infection (mock, compatible, and incompatible interaction) were defined as stepwise Y variables. The PLS analysis (Figure 4) showed that the first component (PC1) explained changes in the chemical composition during the experiment (harvesting time), while the metabolic alteration due to bacterial infection was clearly characterized by the second component (PC2). Figure 4 also displays the over-emitted metabolites in both infected plants, which were identified by the loading plot analysis. As expected, the VOCs from the non-infected plants were chemically similar within 48 h of the experiment. However, the plants infected with both bacterial strains showed an evident variation in their metabolic profile compared to the mock plants. This indicated that VOCs emission was independent of symptomatology. However, no clear separation between the virulent and avirulent infections was observed in this PLS score plot.
FIGURE 4.
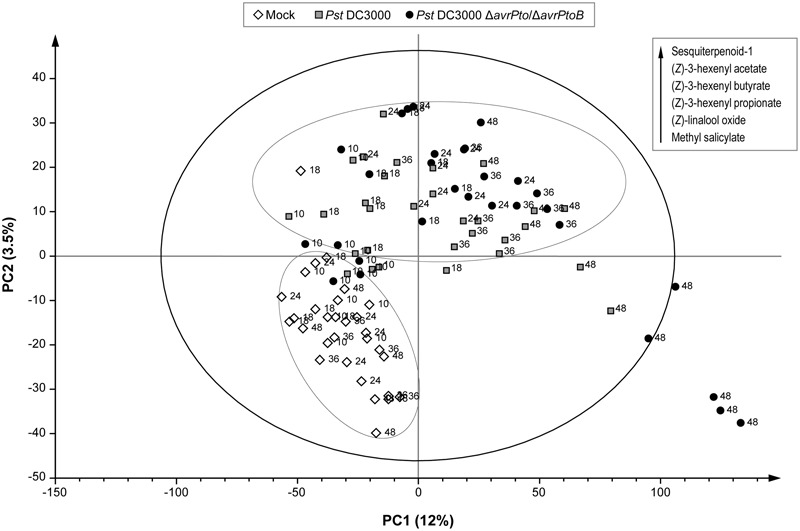
Score plot of the PLS based on the whole array of the mass spectra within a m/z range from 35 to 250. (◇) Leaves of the mock-inoculated RG-Pto plants, ( ) leaves of the RG-Pto plants upon infection with Pst DC3000, (●) leaves of the RG-Pto plants infected with Pst DC3000 ΔavrPto/ΔavrPtoB, at 10, 18, 24, 36, and 48 hpi. Metabolites displayed in the box were identified by loading plot analysis as the responsible for the observed separation.
) leaves of the RG-Pto plants upon infection with Pst DC3000, (●) leaves of the RG-Pto plants infected with Pst DC3000 ΔavrPto/ΔavrPtoB, at 10, 18, 24, 36, and 48 hpi. Metabolites displayed in the box were identified by loading plot analysis as the responsible for the observed separation.
In order to distinguish the defense metabolites associated with each plant–pathogen interaction, the two infections were independently analyzed. The volatile content of the mock-inoculated RG-Pto tomato plants was compared with that of the RG-Pto-infected one with either avirulent strain Pst DC3000 (Supplementary Figure S1) or virulent strain Pst DC3000 ΔavrPto/ΔavrPtoB (Supplementary Figure S2). In these PLS analyses, a marked separation between the infected and mock plants was clearly observed by PC2. In both cases, the loading plot revealed a specific set of VOCs that strongly contributed to the separation of samples according to the specific plant–bacterial infection. Figure 5 shows a hierarchically clustered heat map, including the most discriminative compounds by comparing the volatile profile of the tomato plants infected with the avirulent or virulent Pst strains with the control plants (column 1 and column 2, respectively).
FIGURE 5.
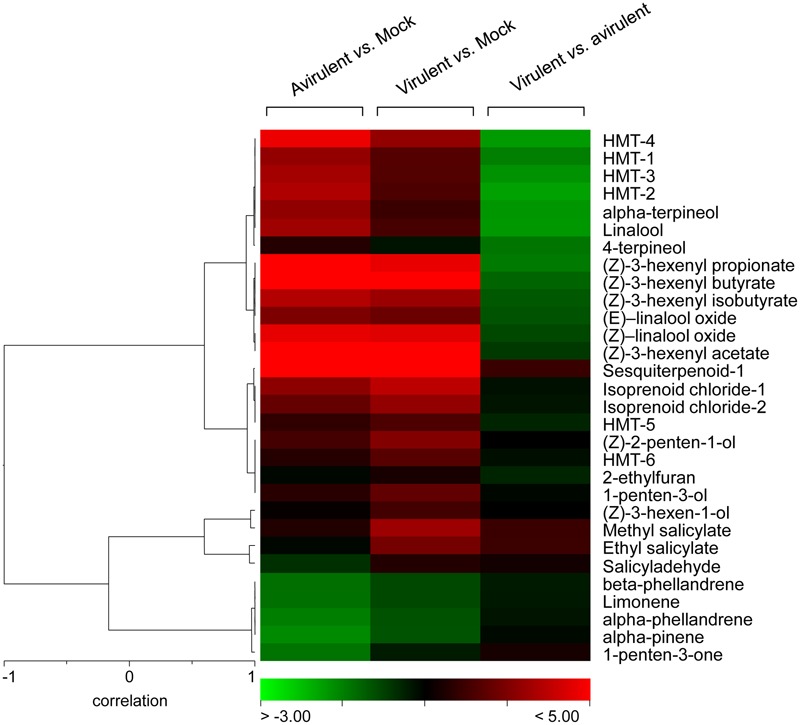
Hierarchical cluster of the volatile compounds from infected plants. Log2-transformed ratios are represented as a heat map according to the scale below. Red corresponds to higher values; green denotes lower values. Column 1 represents the ratios of the VOCs emitted by the tomato plants infected with avirulent strain Pst DC3000 versus the mock-inoculated tomato plants. Column 2 represents the ratios of the VOCs emitted by the tomato plants infected with virulent strain Pst DC3000 ΔavrPto/ΔavrPtoB versus the mock-inoculated tomato plants. Column 3 represents the ratios of the VOCs emitted by the tomato plants infected with virulent strain Pst DC3000 ΔavrPto/ΔavrPtoB versus the tomato plants infected with avirulent strain Pst DC3000.
These defense compounds, induced by both infection types, derive from three important plant metabolic pathways: fatty acids, terpenoids and benzenoids. Among them, the most prominent VOCs produced upon both bacterial infections (red in columns 1 and 2) were some esters of (Z)-3-hexenol, such as (Z)-3-hexenyl acetate, (Z)-3-hexenyl propionate, (Z)-3-hexenyl isobutyrate, (Z)-3-hexenyl butyrate, some hydroxylated monoterpenes (HMT), such as linalool, α-terpineol, both (Z)- and (E)- isomers of linalool oxide, and an unidentified sesquiterpene. The statistical analyses showed that their differential induction was significant (Supplementary Table S2). Regarding benzenoids emission, an increase in the production of methyl salicylate (MeSA), salicylaldehyde, and ethyl salicylate was also observed in both infections. The accumulation of the VOCs that derived from salicylate was consistent with the SA accumulation detected in the compatible interaction since levels of these phenolic derivatives were also higher in this virulent infection (Figure 2).
Comparison of the VOCs Profiles of Tomato Leaves upon Infection with Virulent and Avirulent Pseudomonas syringae Strains Unraveled a Specific Volatile Response for ETI
In order to identify whether a specific set of volatile metabolites was involved in the establishment of effective defense such as ETI, another PLS analysis was performed by comparing the VOCs emitted from the tomato plants infected with avirulent strain Pst DC3000 and with virulent strain Pst DC3000 ΔavrPto/ΔavrPtoB (Figure 6). Once again, the second component clearly showed the different set of volatile compounds emitted by the plant that underwent either a compatible or an incompatible interaction during the 48 hpi period. This metabolomic approach allowed us to identify the differentially induced VOCs in each infection type, by using the loading plot analysis. The hierarchically clustered heat map shows the VOCs that were differentially emitted by the tomato plants infected with virulent Pst strains compared with the avirulent infection (Figure 5, column 3). Most VOCs, which were over-emitted by tomato plants during the establishment of the ETI triggered by the avirulent strain (green-colored), showed significant differences compared with the symptomatic infection (Supplementary Table S2).
FIGURE 6.
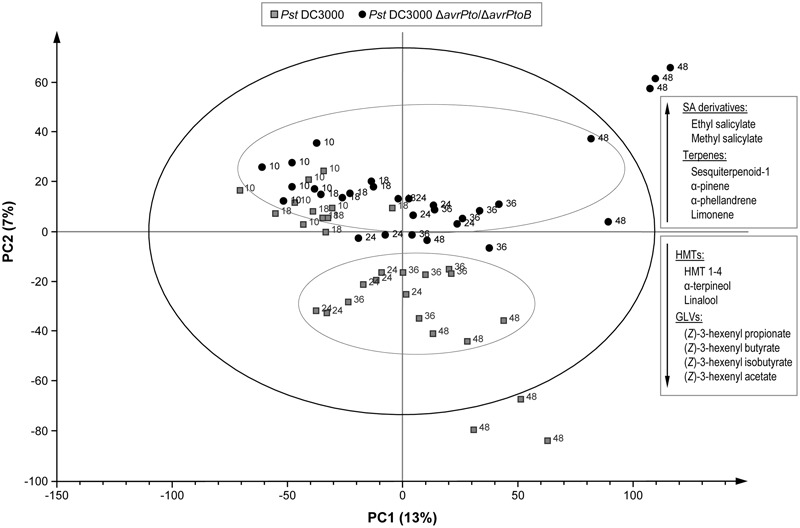
Score plot of the PLS based on the whole array of the mass spectra within a m/z range from 35 to 250. ( ) Leaves of the RG-Pto plants upon infection Pst DC3000, (●) leaves of the RG-Pto plants infected with Pst DC3000 ΔavrPto/ΔavrPto at 10, 18, 24, 36, and 48 hpi. Metabolites displayed in the boxes were identified by loading plot analysis as the responsible for the observed separation.
) Leaves of the RG-Pto plants upon infection Pst DC3000, (●) leaves of the RG-Pto plants infected with Pst DC3000 ΔavrPto/ΔavrPto at 10, 18, 24, 36, and 48 hpi. Metabolites displayed in the boxes were identified by loading plot analysis as the responsible for the observed separation.
The specific VOCs differentially released from the symptomatic tomato plants (virulent/avirulent ratio > 1; Supplementary Table S2) were an unidentified sesquiterpene, SA derivatives methyl salicylate and salicylaldehyde, monoterpenes α-pinene, α-phellandrene, β-phellandrene and limonene, as well as two isoprenoid chlorides. The strong induction of the SA derivatives was previously observed when comparing the volatile profiles of these susceptible plants to their corresponding mock-inoculated plants (Supplementary Figure S2). This study also revealed enhanced emission of monoterpenes after infection with virulent strain Pst DC3000 ΔavrPto/ΔavrPtoB.
Interestingly, we identified a set of volatiles that were significantly over-emitted by tomato plants when effectively resisting disease (green-colored in column 3 of Figure 5). Among them, several HMT, such as linalool, α-terpineol, 4-terpineol, (Z) and (E)-linalool oxides, HMT-1, HMT-2, HMT-3, and HMT-4, as well as the esters (Z)-3-hexenyl propionate, (Z)-3-hexenyl butyrate and (Z)-3-hexenyl isobutyrate, were found. The induction of these compounds is mentioned above (when comparing the volatile profiles of the resistant plants with their corresponding mock-inoculated plants; Supplementary Figure S1). The specific over-emission of these green leaf volatiles (GLVs) esters and HMTs during ETI suggests that these VOCs could participate in the defense response.
As a result of these untargeted metabolomic analyses, we conclude that the infected tomato plants emitted quantitatively different volatiles depending on each type of bacterial strain used in this experiment. Monoterpenes and SA derivatives were released at higher rates by the symptomatic plants upon successful bacterial infection, while HMT and hexenyl esters were differentially over-emitted during ETI establishment, which led to resistance.
Bacterial Infection Induces the Specific Expression of the Genes Involved in VOCs Biosynthesis
To study whether differential volatile production was due to transcriptional activation, we analyzed the expression levels of several key genes involved in the VOC biosynthesis by qRT-PCR. The results of the mock-inoculated RG-Pto tomato plants and the plants infected with Pst DC3000 or Pst DC3000 ΔavrPto/ΔavrPtoB at 10, 18, 24, 36, and 48 hpi are shown in Figure 7. We used the induction of the tomato Pathogenesis-Related PR1 gene as a positive control of bacterial infection, and observed a correlation of the expression of this gene and symptom development (Figure 7A). GLVs esters are known to be synthesized by 13-lipoxygenases (13-LOX) via 13-hydroperoxides, which are later cleaved by 13-hydroperoxide lyases (13-HPL) into (Z)-3-hexenal. This last compound is reduced by alcohol dehydrogenase (ADH). Finally, ester formation is catalyzed by alcohol acyl transferases (AAT) (Scala et al., 2013a).
FIGURE 7.
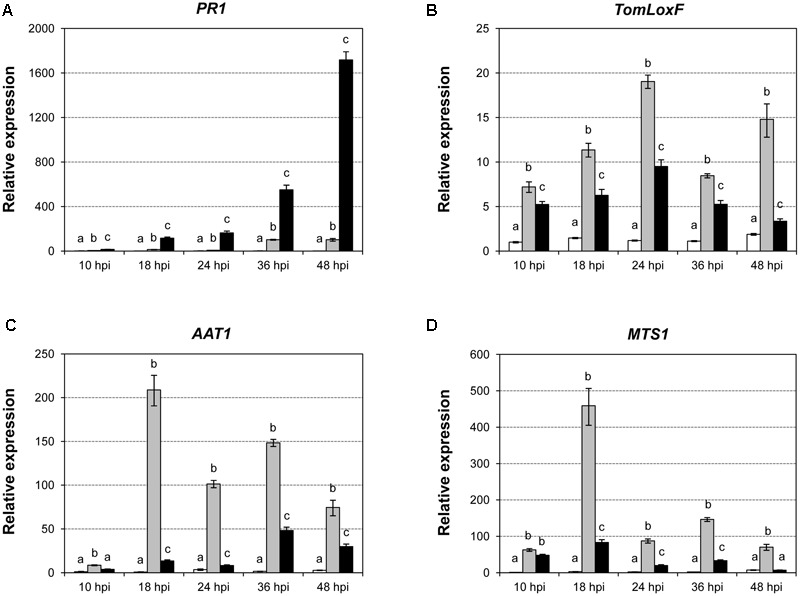
Expression levels of the tomato PR1 (A), TomLOXF (B), AAT (C), and MTS1 (D) genes in the mock-inoculated RG-Pto tomato plants (white bars) and upon infection with Pst DC3000 (light gray bars) or Pst DC3000 ΔavrPto/ΔavrPtoB (dark gray bars) at 10, 18, 24, 36, and 48 hpi, determined by a real-time qRT-PCR analysis. Values were first normalized to the Elongation Factor 1 alpha (eEF1α) expression level. Expression levels are represented as mean ± standard error of three biological repetitions. An ANOVA analysis was performed at each time point. Different letters indicate the statistical significance differences with p-value < 0.05.
In tomato, six genes that encode various types of lipoxygenases (TomloxA-F) have been described (Mariutto et al., 2011). TomloxC, TomloxD, and TomloxF encode 13-LOX lipoxygenases, and are involved in the synthesis of oxylipins, which play an important role in the response to biotic stress. Tomlox D lipoxygenase participates in the synthesis of JA, while Tomlox C and Tomlox F are involved in the biosynthesis of GLVs. As Figure 7B shows, a significant induction of TomloxF was detected upon bacterial infection with both strains at all the studied time points, and this induction was greater when ETI had been established. Regarding alcohol acyltransferases, five AAT genes (SlAAT1 - 5) have been identified in tomato (Goulet et al., 2015). We observed that the induction of AAT1 followed a similar pattern to that of TomloxF (Figure 7C). These induction patterns statistically correlated with the emission of the GLV esters (Supplementary Table S3A) in both infections, and became higher during ETI establishment. Therefore, these data are consistent with the VOCs metabolomic analysis, and suggest a possible role of the biosynthesis of GLV esters in plant defense against bacteria.
Terpene synthases (TPS) catalyze the synthesis of mono-, sesqui- and diterpenes, and are responsible for the diversity of the isoprene compounds found in nature (Degenhardt et al., 2009). There are 44 TPS genes in Solanum lycopersicum, 29 of which are potentially functional (Falara et al., 2011). By qRT-PCR, we observed a significant induction of TPS5, also known as MTS1 (Figure 7D), in the immunized tomato plants, which peaked at 18 hpi. This gene induction correlated with the production of several HMTs, such as linalool or α-terpineol (Supplementary Table S3B).
Discussion
Tomato VOCs have been associated mainly with either improved fruit quality (Rambla et al., 2014) or the response against herbivores (Wei et al., 2013). Here, we extend this knowledge to the volatile differential emission of tomato leaves infected with virulent or avirulent bacteria. We were particularly interested in studying the contribution of VOCs to the resistance mechanisms presented by tomato leaves, which display no symptoms upon bacterial infection (Figure 1).
To better characterize both types of compatible and incompatible interactions, the levels of the different signal molecules, e.g., SA, GA, ET, or JA, were measured at several time points (Figures 2, 3). Simple natural phenolics SA and GA are fundamental components of the signal transduction pathway, which triggers defense responses against different invading pathogens in many plant species. However, the biological role of these signals depends on the plant–pathogen system (Métraux and Raskin, 1993; Bellés et al., 1999; Dempsey et al., 1999). SA accumulation is associated with incompatible interactions (Métraux et al., 1990; Rasmussen et al., 1991; Malamy et al., 1992; Silverman et al., 1993; Uknes et al., 1993; Shirasu et al., 1997), while GA is associated mainly with compatible plant–pathogen systems (Bellés et al., 2006; López-Gresa et al., 2010). GA could also constitute a signal molecule that is complementary to SA since exogenous treatments with this compound are able to induce different defense responses to those triggered by SA (Bellés et al., 1999; Campos et al., 2014). Plants also produce ET in response to most biotic and abiotic stresses (Abeles et al., 1992; Merchante et al., 2013). ET has been particularly involved in the response of Rutgers tomato plants to bacterial pathogen Pseudomonas syringae when infiltrated into leaves (Bellés et al., 1989; Zacarés et al., 2007). Finally, lipid-derived hormone JA has been described as being associated with wounding response and necrotrophic infections, and produces an antagonistic effect on SA-mediated signaling (Zhang et al., 2017). Indeed JA-insensitive tomato jai1 mutants are more resistant to virulent Pseudomonas syringae DC3000 (Zhao et al., 2003). However, jasmonate-deficient def1 tomato mutants have been described as being more susceptible to Pseudomonas syringae and Xanthomonas campestris (Thaler et al., 2004), which thus indicates that the role of JA in tomato bacterial infections is still unclear.
We generally observed that SA, GA, and ET accumulated at higher levels in the tomato plants infected with the virulent bacterial strain compared to those infected with avirulent bacteria. These higher levels correlated with symptom severity. SA accumulation has been described to occur at 4 or 10 dpi in tomato plants inoculated with either avirulent or virulent Xanthomonas, respectively (O’Donnell et al., 2001; Block et al., 2005). Besides in these tomato interactions, an earlier increase in ET has been described in avirulent infection, while delayed ET synthesis happens in the tomato plants infected with virulent Xanthomonas (Ciardi et al., 2000; Block et al., 2005). These results contrast with those observed herein, where the highest ET and SA levels were detected in symptomatic bacterial infection. Several factors could be behind the different SA and ET levels described in each pathosystem, such as timing during infection progress, pathogen dose, greenhouse conditions, or the plant growth stage. Yet in all the cases, the accumulation of either SA or ET correlated with PR1 induction (Figure 7A), a classical marker gene that is useful for assessing disease development. The higher level of both ET and GA, detected in the RG tomato plants infected with the virulent Pst strain, was associated mainly with symptom development, and agrees with those previously described (Lund et al., 1998; Bellés et al., 2006). Regarding ET, the mutants and tomato genotypes impaired in ET perception or ET synthesis exhibit a significant reduction in disease symptoms versus the wild type upon infection with different pathogens (Lund et al., 1998). These previous results present compelling evidence that both biosynthesis and ET perception are critical for symptoms development in tomato leaves.
Unlike the accumulations of SA, GA, and ET, we observed that JA levels drastically lowered in the virulent infection in accordance with the well-known SA–JA antagonism (Zhang et al., 2017). However, in the plants infected with avirulent bacteria, JA accumulation remained comparable to the mock-inoculated plants, similarly to the detected SA levels. Very few reported studies have monitored JA levels in bacteria-infected plants. In Arabidopsis plants, no differences in JA levels between mock-inoculated plants and those infected with a virulent bacteria have been described at 2 and 24 hpi (Scala et al., 2013b). Unlike our dipping infection method, these authors performed inoculation with a syringe, which thus caused mechanical damage to both mock and infected leaves. This difference in the inoculation procedure could explain the divergence of our results with those previously published.
All these data indicate that the different accumulation patterns of these four signal molecules depend on the diversity of pathogens with a range of lifestyles. To our knowledge, this is the first study in which the levels of all these signal molecules have been measured in RG tomato plants infected with both Pst strains.
Interestingly, we observed that changes in VOCs emission were mostly independent of macroscopic symptoms since the tomato leaves infected with the avirulent strain overproduced some specific VOCs. We identified 73 emitted VOCs by the GC-MS technology. Although more than 300 VOCs have been reported in tomato fruit (Tikunov et al., 2005), very little information is available on the detailed volatile profile in tomato leaves (Buttery et al., 1987; Wang et al., 2001; Thelen et al., 2005; Zhang et al., 2008; Zhang and Chen, 2009; Proffit et al., 2011), thus our data contribute to this knowledge. The comparison of the VOCs profiles of tomato leaves upon infection with virulent and avirulent Pseudomonas syringae strains allowed us to identify the differentially emitted compounds associated with each interaction. The VOCs emission of a diseased leaf is enriched in monoterpenes and SA derivatives, while that of a resisting leaf is characterized mainly by esters of hexenyl GLVs and HMTs.
For the VOCs emitted by symptomatic tomato leaves, the induction of monoterpenes in tomato plants upon Botrytis infection has been described, where α-phellandrene and β-phellandrene, 2-carene, limonene, and α-pinene contributed to more than 95% of volatile emissions (Jansen et al., 2009). Nevertheless, the specific release of monoterpenes has been described in pepper plants exposed to the incompatible Xanthomonas pathogen, but not in compatible interaction (Cardoza and Tumlinson, 2006). MeSA is generally induced upon pathogen infection (Loake and Grant, 2007). In pepper plants infected with avirulent and virulent Xanthomonas strains, MeSA is also emitted at higher levels in the compatible interaction (Cardoza and Tumlinson, 2006). However, in tobacco plants infected with several P. syringae strains, MeSA levels become higher upon avirulent inoculation (Huang et al., 2003).
Biotic stresses have been described to trigger emissions of volatile molecules, which are products of the lipoxygenase (LOX) pathway, such as C6 aldehydes, alcohols and derivatives, generally referred to as GLVs (Niinemets et al., 2013). For example, Pst infection provokes the emission of (Z)-3-hexenol and (E)-2-hexenal in bean and tobacco leaves, respectively (Croft et al., 1993; Heiden et al., 2003). GLVs are also emitted after fungal and virus infections (Scala et al., 2013a). In tomato plants, (Z)-3-hexenol, (Z)-3-hexenal, and (Z)-3-hexenyl acetate are the dominant LOX products in the volatile emission after Botrytis cinerea inoculation (Jansen et al., 2009). Volatile esters not only contribute to the aroma of many fruits and flowers, but are also related to plant defense and plant-to-plant signaling (Goulet et al., 2015). The ester (Z)-3-hexenyl acetate is one of the most abundant volatiles to be emitted from mechanically or herbivore-damaged Arabidopsis thaliana plants (D’Auria et al., 2007), and can prime a defense response in nearby plants (Engelberth et al., 2004; Frost et al., 2008). The emission of other (Z)-3-hexenyl esters has also been described in pepper plants upon Xanthomonas infection (Cardoza and Tumlinson, 2006). The induction of (Z)-3-hexenol and some of its derived esters upon both bacterial infection types in the tomato plants reported herein extend GLVs emission to other plant–pathogen interactions. Our results, together with those in which (Z)-3-hexenol induces defense genes in Arabidopsis and maize plants (Bate and Rothstein, 1998; Farag et al., 2005), suggest that this alcohol can act as a signaling molecule involved in plant response. Another short-chain alcohol, 3-pentanol, has been found to trigger induced resistance in Arabidopsis against Pseudomonas syringae (Song et al., 2015), and also in pepper against Xanthomonas axonopodis pv. vesicatoria (Choi et al., 2014), by priming the SA and JA signaling pathways. Besides, several reports have shown the antifungal (Vaughn et al., 1993) and antibacterial properties (Croft et al., 1993; Deng et al., 1993) of GLVs, which thus reinforces the role of these VOCs in plant defense. Our results also reveal the possible defensive role of GLV esterification since these GLV esters were overproduced during ETI establishment.
Some of these GLVs, including hexanal, (Z)-3-hexenal or (E)-2-hexenal, have also been described as major compounds emitted by tomato fruit (Rambla et al., 2014), where Pseudomonas syringae can cause damage (Xin and He, 2013). Although esterase activity in fruit is enhanced in the red-fruited species of the tomato clade (Goulet et al., 2012), it would be interesting to study whether infection with virulent or avirulent bacteria could produce the esterification of these aldehydes in fruit, which would thus extend the defensive role of these GLVs esters to other organs.
Other VOCs involved in the defense response of plants are terpenoids, which are emitted after wounding or egg deposition by insects. Terpenoids induced by herbivores act in plant defense by attracting insect predators, and by acting as repellents or toxic compounds (Turlings and Tumlinson, 1992; Wegener et al., 2001). Besides, they have been associated to resistance against downy mildew in grapevine (Alarcón et al., 2015). However, the role of terpenoids in plant-bacteria interactions is not well studied. Here, we detected different HMTs, such as (Z)- and (E)-linalool oxides, linalool, α-terpineol, 4-terpineol, and six putative HMTs emitted from both infected plant types, where induction was greater in avirulent infection. The release of linalool and β-ocimene has also been described in tobacco plants infected by an avirulent strain of Pseudomonas (Huang et al., 2003). Since monoterpenes are emitted mainly by symptomatic plants, and HMTs are differentially released by those displaying the immune response, terpene hydroxylation appears to be a key process in the plant defense response.
In order to study whether the increase in VOC was associated with the induction of the VOC biosynthesis machinery, the expression of several genes, e.g., Tomlox and AAT, involved in the biosynthetic pathway of the esters of GLVs, and TPS, implicated in the biosynthesis of terpenoids, was studied by qRT-PCR. We observed a positive correlation in the induction of TomloxF, AAT1, and MTS1 with the emission of the corresponding VOCs, which were differentially released in the tomato plants that displayed ETI.
Among the six described Tomlox isoforms, the induction of the TomloxF gene has also been described to result from the infection caused by Pseudomonas putida (Mariutto et al., 2011). Our results reinforce the defensive role of this tomato LOX isoform and validate the metabolomic analysis. Regarding the different isoforms of the tomato alcohol acyltransferases, AAT1 has been correlated to the production of GLV esters in tomato fruits (Goulet et al., 2015). Accordingly, we observed the induction of the AAT1 gene upon bacterial infection, and the corresponding GLV esters accumulation, being this induction greater in the tomato plants that exhibited ETI.
Volatile isoprenoids represent the most abundant group of volatile compounds in plants, and are common components of both their aroma and defensive response induced by herbivores and pathogens (Aharoni et al., 2005). The observed induction of MTS1 upon bacterial infection correlates with the detected emission of monoterpenes and HMTs, and agrees with the previously described role of TPS in plant defense. Transgenic plants of Arabidopsis thaliana, which overexpress the TPS (E)-β-caryophyllene synthase gene, emit larger amounts of this sesquiterpene and are more resistant to bacterial infection, which confirms the role of this gene in defense (Huang et al., 2012). MTS1 expression in tomato leaves has been described to be induced by spider mite-infestation, wounding and JA treatment (van Schie et al., 2007).
Terpene synthases reaction products are subsequently modified by hydroxylation, methylation, acylation, reduction, oxidation, isomerization, or glycosylation, which gives rise to more complex terpene compounds. Hydroxylation of monoterpenes, which would lead to compounds such as α-terpineol, is performed by Cytochrome P450 enzymes (CYP450) (Höfer et al., 2014). There are approximately 250 CYPs in tomato1, which means that clarifying which CYP450 might be responsible for terpene hydroxylation and is, therefore, involved in defense through this signaling pathway, is a complex task. However, approximately 25 CYP proteins have been reported to be involved in the immune response of tomato plants to bacteria Pst, which helps limit the search of the CYP450 responsible for the hydroxylation of terpenes and confirms the importance of hydroxylation in plant defense response (Pombo et al., 2014).
Our results suggested that the esters of GLVs and HMTs could play a defensive role in the tomato plant response. Unlike SA accumulation, which is the classical signal molecule in incompatible interactions that accumulates at higher levels in virulent infections, these VOCs were differentially emitted at higher levels when plants efficiently resisted bacterial infection, which indicates that they could play a defensive role. Further studies, such as pharmacological or genetic approaches, could be conducted to test this possibility. These volatile compounds could also display interesting biological properties, such as antioxidant, antimicrobial, insecticide or resistance inducers, and could be good candidates for agrochemical and pharmaceutical industries. Besides, the generation of tomato transgenic plants over-expressing enzymes involved in the biosynthesis of these volatiles could result in a new biotechnological strategy to obtain resistance.
Author Contributions
MPL-G and JMB conceived and designed the study. MPL-G, PL, and LC carried out the experiments. IR prepared the figures. MPL-G, JLR, and AG performed the GC-MS-based metabolomics approach. MPL-G and PL did the data processing and statistical analysis. MPL-G, PL, IR, VC, and JMB interpreted the results. MPL-G and PL wrote the manuscript. JMB handled the literature.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Dr. Selena Giménez-Sánchez (Centro Nacional de Biotecnología, Madrid) for kindly providing us with both strains of Pseudomonas syringae pv. tomato DC3000. We thank Dr. Isabel López-Díaz and Dr. Esther Carrera for the hormone quantification carried out at the Plant Hormone Quantification Service, IBMCP, Valencia, Spain. We are also very grateful to Teresa Caballero and Ana Ruiz for their excellent technical support.
Funding. This work was funded by Grant BIO2012-33419 from the Spanish Ministry of Economy and Competitiveness.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2017.01188/full#supplementary-material
References
- Abeles F. B., Morgan P. W., Saltveit M. E., Jr. (1992). “Regulation of ethylene production by internal, environmental, and stress factors,” in Ethylene in Plant Biology eds Abeles F., Morgan P., Saltveit M., Jr. (San Diego, CA: Academic Press Inc.) 56–119. [Google Scholar]
- Aharoni A., Jongsma M. A., Bouwmeester H. J. (2005). Volatile science? Metabolic engineering of terpenoids in plants. Trends Plant Sci. 10 594–602. 10.1016/j.tplants.2005.10.005 [DOI] [PubMed] [Google Scholar]
- Alarcón A., Lazazzara V., Cappellin L., Bianchedi P. L., Schuhmacher R., Wohlfahrt G., et al. (2015). Emission of volatile sesquiterpenes and monoterpenes in grapevine genotypes following Plasmopara viticola inoculation in vitro. Mass Spectrom. 50 1013–1022. 10.1002/jms.3615 [DOI] [PubMed] [Google Scholar]
- Allwood J. W., Ellis D. I., Goodacre R. (2008). Metabolomic technologies and their application to the study of plants and plant–host interactions. Physiol. Plant. 132 117–135. 10.1111/j.1399-3054.2007.01001.x [DOI] [PubMed] [Google Scholar]
- Allwood J. W., Heald J., Lloyd A. J., Goodacre R., Mur L. A. J. (2012). Separating the inseparable: the metabolomic analysis of plant-pathogen interactions. Methods Mol. Biol. 860 31–49. 10.1007/978-1-61779-594-7_3 [DOI] [PubMed] [Google Scholar]
- Bate N. J., Rothstein S. J. (1998). C6-volatiles derived from the lipoxygenase pathway induce a subset of defense-related genes. Plant J. 16 561–569. 10.1046/j.1365-313x.1998.00324.x [DOI] [PubMed] [Google Scholar]
- Bellés J. M., Garro R., Fayos J., Navarro P., Primo J., Conejero V. (1999). Gentisic acid as a pathogen-inducible signal, additional to salicylic acid for activation of plant defenses in tomato. Mol. Plant Microbe Interact. 12 227–235. 10.1094/MPMI.1999.12.3.227 [DOI] [Google Scholar]
- Bellés J. M., Garro R., Pallás V., Fayos J., Rodrigo I., Conejero V. (2006). Accumulation of gentisic acid as associated with systemic infections but not with the hypersensitive response in plant-pathogen interactions. Planta 223 500–511. 10.1007/s00425-005-0109-8 [DOI] [PubMed] [Google Scholar]
- Bellés J. M., Granell A., Durán-Vila N., Conejero V. (1989). ACC synthesis as the activated step responsible for the rise of ethylene production accompanying Citrus Exocortis Viroid infection in tomato plants. J. Phytopathol. 125 198–208. 10.1111/j.1439-0434.1989.tb01061.x [DOI] [Google Scholar]
- Bellés J. M., López-Gresa M. P., Fayos J., Pallás V., Rodrigo I., Conejero V. (2008). Induction of cinnamate 4-hydroxylase and phenylpropanoids in virus-infected cucumber and melon plants. Plant Sci. 174 524–533. 10.1016/j.plantsci.2008.02.008 [DOI] [Google Scholar]
- Block A., Schmelz E., O’Donnell P. J., Jones J. B., Klee H. J. (2005). Systemic acquired tolerance to virulent bacterial pathogens in tomato. Plant Physiol. 138 1481–1490. 10.1104/pp.105.059246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttery R. G., Ling L. C., Light D. M. (1987). Tomato leaf volatile aroma components. J. Agric. Food Chem. 35 1039–1042. 10.1021/jf00078a043 [DOI] [Google Scholar]
- Campos L., Granell P., Tárraga S., López-Gresa P., Conejero V., Bellés J. M., et al. (2014). Salicylic acid and gentisic acid induce RNA silencing-related genes and plant resistance to RNA pathogens. Plant Physiol. Biochem. 77 35–43. 10.1016/j.plaphy.2014.01.016 [DOI] [PubMed] [Google Scholar]
- Cardoza Y. J., Tumlinson J. H. (2006). Compatible and incompatible Xanthomonas infections differentially affect herbivore-induced volatile emission by pepper plants. J. Chem. Ecol. 32 1755–1768. 10.1007/s10886-006-9107-y [DOI] [PubMed] [Google Scholar]
- Choi H. K., Song G. C., Yi H. S., Ryu C. M. (2014). Field evaluation of the bacterial volatile derivative 3-pentanol in priming for induced resistance in pepper. J. Chem. Ecol. 40 882–892. 10.1007/s10886-014-0488-z [DOI] [PubMed] [Google Scholar]
- Ciardi J. A., Tieman D. M., Lund S. T., Jones J. B., Stall R. E., Klee H. J. (2000). Response to Xanthomonas campestris pv. vesicatoria in tomato involves regulation of ethylene receptor gene expression. Plant Physiol. 123 81–92. 10.1104/pp.123.1.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft K. P. C., Juttner F., Slusarenko A. J. (1993). Volatile products of the lipoxygenase pathway evolved from Phaseolus vulgaris (L.) leaves inoculated with Pseudomonas syringae pv phaseolicola. Plant Physiol. 101 13–24. 10.1104/pp.101.1.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangl J. L., Jones J. D. G. (2001). Plant pathogens and integrated defence responses to infection. Nature 411 826–833. 10.1038/35081161 [DOI] [PubMed] [Google Scholar]
- D’Auria J. C., Pichersky E., Schaub A., Hansel A., Gershenzon J. (2007). Characterization of a BAHD acyltransferase responsible for producing the green leaf volatile (Z)-3-hexen-1-yl acetate in Arabidopsis thaliana. Plant J. 49 194–207. 10.1111/j.1365-313X.2006.02946.x [DOI] [PubMed] [Google Scholar]
- Degenhardt J., Koellner T. G., Gershenzon J. (2009). Monoterpene and sesquiterpene synthases and the origin of terpene skeletal diversity in plants. Phytochemistry 70 1621–1637. 10.1016/j.phytochem.2009.07.030 [DOI] [PubMed] [Google Scholar]
- Dempsey D. M. A., Shah J., Klessig D. F. (1999). Salicylic acid and disease resistance in plants. Crit. Rev. Plant Sci. 18 547–575. 10.1080/07352689991309397 [DOI] [Google Scholar]
- Deng W., Hamilton-Kemp T. R., Nielsen M. T., Andersen R. A., Collins G. B., Hildebrand D. F. (1993). Effects of six-carbon aldehydes and alcohols on bacterial proliferation. J. Agric. Food Chem. 41 506–510. 10.1021/jf00027a030 [DOI] [Google Scholar]
- Dixon R. A. (2001). Natural products and plant disease resistance. Nature 411 843–847. 10.1038/35081178 [DOI] [PubMed] [Google Scholar]
- Dudareva N., Klempien A., Muhlemann J. K., Kaplan I. (2013). Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 198 16–32. 10.1111/nph.12145 [DOI] [PubMed] [Google Scholar]
- Dudareva N., Negre F., Nagegowda D. A., Orlova I. (2006). Plant volatiles: recent advances and future perspectives. Crit. Rev. Plant Sci. 25 417–440. 10.1080/07352680600899973 [DOI] [Google Scholar]
- Engelberth J., Alborn H. T., Schmelz E. A., Tumlinson J. H. (2004). Airborne signals prime plants against insect herbivore attack. Proc. Natl. Acad. Sci. U.S.A 101 1781–1785. 10.1073/pnas.0308037100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falara V., Akhtar T. A., Nguyen T. T. H., Spyropoulou E. A., Bleeker P. M., Schauvinhold I., et al. (2011). The tomato terpene synthase gene family. Plant Physiol. 157 770–789. 10.1104/pp.111.179648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farag M. A., Fokar M., Abd H., Zhang H., Allen R. D., Pare P. W. (2005). (Z)-3-Hexenol induces defense genes and downstream metabolites in maize. Planta 220 900–909. 10.1007/s00425-004-1404-5 [DOI] [PubMed] [Google Scholar]
- Frost C. J., Mescher M. C., Dervinis C., Davis J. M., Carlson J. E., De Moraes C. M. (2008). Priming defense genes and metabolites in hybrid poplar by the green leaf volatile cis-3-hexenyl acetate. New Phytol. 180 722–734. 10.1111/j.1469-8137.2008.02599.x [DOI] [PubMed] [Google Scholar]
- Goulet C., Kamiyoshihara Y., Lam N. B., Richard T., Taylor M. G., Tieman D. M., et al. (2015). Divergence in the enzymatic activities of a tomato and Solanum pennellii alcohol acyltransferase impacts fruit volatile ester composition. Mol. Plant 8 153–162. 10.1016/j.molp.2014.11.007 [DOI] [PubMed] [Google Scholar]
- Goulet C., Mageroy M. H., Lam N. B., Floystad A., Tieman D. M., Klee H. J. (2012). Role of an esterase in flavor volatile variation within the tomato clade. Proc. Natl. Acad. Sci. U.S.A. 109 19009–19014. 10.1073/pnas.1216515109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granell A., Rambla J. L. (2013). “Biosynthesis of Volatile Compounds,” in The Molecular Biology and Biochemistry of Fruit Ripening eds Seymour G. B., Poole M., Giovannoni J. J., Tucker G. A. (Hoboken, NJ: Blackwell Publishing Ltd.) 135–161. 10.1002/9781118593714.ch6 [DOI] [Google Scholar]
- Heiden A. C., Kobel K., Langebartels C., Schuh-Thomas G., Wildt J. (2003). Emissions of oxygenated volatile organic compounds from plants. Part I: emissions from lipoxygenase activity. J. Atmos. Chem. 45 143–172. 10.1023/A:1024069605420 [DOI] [Google Scholar]
- Höfer R., Boachon B., Renault H., Gavira C., Miesch L., Iglesias J., et al. (2014). Dual function of the cytochrome P450 CYP76 family from Arabidopsis thaliana in the metabolism of monoterpenols and phenylurea herbicides. Plant Physiol. 166 1149–1161. 10.1104/pp.114.244814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holopainen J. K., Gershenzon J. (2010). Multiple stress factors and the emission of plant VOCs. Trends Plant Sci. 15 176–184. 10.1016/j.tplants.2010.01.006 [DOI] [PubMed] [Google Scholar]
- Huang J., Cardoza Y. J., Schmelz E. A., Raina R., Engelberth J., Tumlinson J. H. (2003). Differential volatile emissions and salicylic acid levels from tobacco plants in response to different strains of Pseudomonas syringae. Planta 217 767–775. 10.1007/s00425-003-1039-y [DOI] [PubMed] [Google Scholar]
- Huang J., Schmelz E. A., Alborn H., Engelberth J., Tumlinson J. H. (2005). Phytohormones mediate volatile emissions during the interaction of compatible and incompatible pathogens: the role of ethylene in Pseudomonas syringae infected tobacco. J. Chem. Ecol. 31 439–459. 10.1007/s10886-005-2018-5 [DOI] [PubMed] [Google Scholar]
- Huang M., Sanchez-Moreiras A. M., Abel C., Sohrabi R., Lee S., Gershenzon J., et al. (2012). The major volatile organic compound emitted from Arabidopsis thaliana flowers, the sesquiterpene (E)-β-caryophyllene, is a defense against a bacterial pathogen. New Phytol. 193 997–1008. 10.1111/j.1469-8137.2011.04001.x [DOI] [PubMed] [Google Scholar]
- Jansen R. M. C., Miebach M., Kleist E., Van Henten E. J., Wildt J. (2009). Release of lipoxygenase products and monoterpenes by tomato plants as an indicator of Botrytis cinerea-induced stress. Plant Biol. 11 859–868. 10.1111/j.1438-8677.2008.00183.x [DOI] [PubMed] [Google Scholar]
- Jia Y. L., Loh Y. T., Zhou J. M., Martin G. B. (1997). Alleles of Pto and Fen occur in bacterial speck-susceptible and fenthion-insensitive tomato cultivars and encode active protein kinases. Plant Cell 9 61–73. 10.1105/tpc.9.1.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaivani K., Kalaiselvi M. M., Senthil-Nathan S. (2016). Effect of methyl salicylate (MeSA), an elicitor on growth, physiology and pathology of resistant and susceptible rice varieties. Sci. Rep. 6:34498 10.1038/srep34498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberherr D., Wagner U., Dubuis P.-H., Métraux J.-P., Mauch F. (2003). The rapid induction of glutathione S-transferases AtGSTF2 and AtGSTF6 by avirulent Pseudomonas syringae is the result of combined salicylic acid and ethylene signaling. Plant Cell Physiol. 44 750–757. 10.1093/pcp/pcg093 [DOI] [PubMed] [Google Scholar]
- Lin N. C., Martin G. B. (2005). An avrPto/avrPtoB mutant of Pseudomonas syringae pv. tomato DC3000 does not elicit Pto-mediated resistance and is less virulent on tomato. Mol. Plant Microbe Interact. 18 43–51. 10.1094/MPMI-18-0043 [DOI] [PubMed] [Google Scholar]
- Loake G., Grant M. (2007). Salicylic acid in plant defence—the players and protagonists. Curr. Opin. Plant Biol. 10 466–472. 10.1016/j.pbi.2007.08.008 [DOI] [PubMed] [Google Scholar]
- López-Gresa M. P., Maltese F., Bellés J. M., Conejero V., Kim H. K., Choi Y. H., et al. (2010). Metabolic response of tomato leaves upon different plant–pathogen interactions. Phytochem. Anal. 21 89–94. 10.1002/pca.1179 [DOI] [PubMed] [Google Scholar]
- Lund S. T., Stall R. E., Klee H. J. (1998). Ethylene regulates the susceptible response to pathogen infection in tomato. Plant Cell 10 371–382. 10.1105/tpc.10.3.371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamy J., Hennig J., Klessig D. F. (1992). Temperature dependent induction of salicylic acid and its conjugates during the resistance response to tobacco mosaic virus infection. Plant Cell 4 359–366. 10.1105/tpc.4.3.359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallona I., Weiss J., Egea-Cortines M. (2011). pcrEfficiency: a Web tool for PCR amplification efficiency prediction. BMC Bioinformatics 12:404 10.1186/1471-2105-12-404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariutto M., Duby F., Adam A., Bureau C., Fauconnier M.-L., Ongena M., et al. (2011). The elicitation of a systemic resistance by Pseudomonas putida BTP1 in tomato involves the stimulation of two lipoxygenase isoforms. BMC Plant Biol. 11:29 10.1186/1471-2229-11-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G. B., Brommonschenkel S. H., Chunwongse J., Frary A., Ganal M. W., Spivey R., et al. (1993). Map-based cloning of a protein kinase gene conferring disease resistance in tomato. Science 262 1432–1436. 10.1126/science.7902614 [DOI] [PubMed] [Google Scholar]
- Merchante C., Alonso J. M., Stepanova A. N. (2013). Ethylene signaling: simple ligand, complex regulation. Curr. Opin. Plant Biol. 16 554–560. 10.1016/j.pbi.2013.08.001 [DOI] [PubMed] [Google Scholar]
- Métraux J. P., Raskin I. (1993). “Role of phenolics in plant disease resistance,” in Application on Biotechnology in Plant Pathology ed. Che I. (New York, NY: Willey; ) 191–209. [Google Scholar]
- Métraux J. P., Signer H., Ryals J., Ward E., Wyssbenz M., Gaudin J., et al. (1990). Increase in salicylic-acid at the onset of systemic acquired-resistance in cucumber. Science 250 1004–1006. 10.1126/science.250.4983.1004 [DOI] [PubMed] [Google Scholar]
- Niinemets U., Kaennaste A., Copolovici L. (2013). Quantitative patterns between plant volatile emissions induced by biotic stresses and the degree of damage. Front. Plant Sci. 4:262 10.3389/fpls.2013.00262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ntoukakis V., Mucyn T. S., Gimenez-lbanez S., Chapman H. C., Gutierrez J. R., Balmuth A. L., et al. (2009). Host inhibition of a bacterial virulence effector triggers immunity to infection. Science 324 784–787. 10.1126/science.1169430 [DOI] [PubMed] [Google Scholar]
- O’Donnell P. J., Jones J. B., Antoine F. R., Ciardi J., Klee H. J. (2001). Ethylene-dependent salicylic acid regulates an expanded cell death response to a plant pathogen. Plant J. 25 315–323. 10.1046/j.1365-313x.2001.00968.x [DOI] [PubMed] [Google Scholar]
- Pombo M. A., Zheng Y., Fernández-Pozo N., Dunham D. M., Fei Z., Martin G. B. (2014). Transcriptomic analysis reveals tomato genes whose expression is induced specifically during effector-triggered immunity and identifies the Epk1 protein kinase which is required for the host response to three bacterial effector proteins. Genome Biol. 15:492 10.1186/s13059-014-0492-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proffit M., Birgersson G., Bengtsson M., Reis R., Jr., Witzgall P., Lima E. (2011). Attraction and oviposition of Tuta absoluta females in response to tomato leaf volatiles. J. Chem. Ecol. 37 565–574. 10.1007/s10886-011-9961-0 [DOI] [PubMed] [Google Scholar]
- Pusztahelyi T., Holb I., Pócsi I. (2015). Secondary metabolites in fungus-plant interactions. Front. Plant Sci. 6:573 10.3389/fpls.2015.00573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y., Tan D.-X., Reiter R. J., Shi H. (2015). Comparative metabolomic analysis highlights the involvement of sugars and glycerol in melatonin-mediated innate immunity against bacterial pathogen in Arabidopsis. Sci. Rep. 5:15815 10.1038/srep15815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambla J. L., López-Gresa M. P., Bellés J. M., Granell A. (2015). “Metabolomic profiling of plant tissues,” in Plant Functional Genomics. Methods and Protocols eds Alonso J. M., Stepanova N. A. (New York, NY: Humana Press; ) 221–235. [DOI] [PubMed] [Google Scholar]
- Rambla J. L., Tikunov Y. M., Monforte A. J., Bovy A. G., Granell A. (2014). The expanded tomato fruit volatile landscape. J. Exp. Bot. 65 4613–4623. 10.1093/jxb/eru128 [DOI] [PubMed] [Google Scholar]
- Rasmussen J. B., Hammerschmidt R., Zook M. N. (1991). Systemic induction of salicylic acid accumulation in cucumber after inoculation with Pseudomonas syringae pv syringae. Plant Physiol. 97 1342–1347. 10.1104/pp.97.4.1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmeron J. M., Barker S. J., Carland F. M., Mehta A. Y., Staskawicz B. J. (1994). Tomato mutants altered in bacterial disease resistance provide evidence for a new locus controlling pathogen recognition. Plant Cell 6 511–520. 10.1105/tpc.6.4.511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scala A., Allmann S., Mirabella R., Haring A. M., Schuurink C. R. (2013a). Green leaf volatiles: a plant’s multifunctional weapon against herbivores and pathogens. Int. J. Mol. Sci. 14 17781–17811. 10.3390/ijms140917781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scala A., Mirabella R., Mugo C., Matsui K., Haring M., Schuurink R. (2013b). E-2-hexenal promotes susceptibility to Pseudomonas syringae by activating jasmonic acid pathways in Arabidopsis. Front. Plant Sci. 4:74 10.3389/fpls.2013.00074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo M., Jikumaru Y., Kamiya Y. (2011). “Profiling of hormones and related metabolites in seed dormancy and germination studies,” in Seed Dormancy. Methods and Protocols ed. Kermode A. R. (Totowa, NJ: Humana Press; ) 99–111. [DOI] [PubMed] [Google Scholar]
- Shen J., Tieman D., Jones J. B., Taylor M. G., Schmelz E., Huffaker A., et al. (2014). A 13-lipoxygenase, TomloxC, is essential for synthesis of C5 flavour volatiles in tomato. J. Exp. Bot. 65 419–428. 10.1093/jxb/ert382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasu K., Nakajima H., Rajasekhar V. K., Dixon R. A., Lamb C. (1997). Salicylic acid potentiates an agonist-dependent gain control that amplifies pathogen signals in the activation of defense mechanisms. Plant Cell 9 261–270. 10.1105/tpc.9.2.261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman P., Nuckles E., Ye X. S., Kuc J., Raskin I. (1993). Salicylic acid, ethylene, and pathogen resistance in tobacco. Mol. Plant Microbe Interact. 6 775–781. 10.1094/MPMI-6-775 [DOI] [Google Scholar]
- Song G. C., Choi H. K., Ryu C. M. (2015). Gaseous 3-pentanol primes plant immunity against a bacterial speck pathogen, Pseudomonas syringae pv. tomato via salicylic acid and jasmonic acid-dependent signaling pathways in Arabidopsis. Front. Plant Sci. 6:821 10.3389/fpls.2015.00821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler J. S., Owen B., Higgins V. J. (2004). The role of the jasmonate response in plant susceptibility to diverse pathogens with a range of lifestyles. Plant Physiol. 135 530–538. 10.1104/pp.104.041566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelen J., Harbinson J., Jansen R., Van Straten G., Posthumus M. A., Woltering E. J., et al. (2005). The sesquiterpene α-copaene is induced in tomato leaves infected by Botrytis cinerea. J. Plant Interact. 1 163–170. 10.1080/17429140600968177 [DOI] [Google Scholar]
- Tikunov Y., Lommen A., de Vos C. H. R., Verhoeven H. A., Bino R. J., Hall R. D., et al. (2005). A novel approach for nontargeted data analysis for metabolomics. Large-scale profiling of tomato fruit volatiles. Plant Physiol. 139 1125–1137. 10.1104/pp.105.068130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turlings T. C., Tumlinson J. H. (1992). Systemic release of chemical signals by herbivore-injured corn. Proc. Natl. Acad. Sci. U.S.A. 89 8399–8402. 10.1073/pnas.89.17.8399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uknes S., Winter A. M., Delaney T., Vernooij B., Morse A., Friedrich L., et al. (1993). Biological induction of systemic acquired resistance in Arabidopsis. Mol. Plant Microbe Interact. 6 692–698. 10.1094/MPMI-6-692 [DOI] [PubMed] [Google Scholar]
- van Schie C. C., Haring M. A., Schuurink R. C. (2007). Tomato linalool synthase is induced in trichomes by jasmonic acid. Plant Mol. Biol. 64 251–263. 10.1007/s11103-007-9149-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn S. F., Spencer G. F., Shasha B. S. (1993). Volatile compounds from raspberry and strawberry fruit inhibit postharvest decay fungi. J. Food Sci. 58 793–796. 10.1111/j.1365-2621.1993.tb09360.x [DOI] [Google Scholar]
- Wang C., Xing J., Chin C. K., Ho C. T., Martin C. E. (2001). Modification of fatty acids changes the flavor volatiles in tomato leaves. Phytochemistry 58 227–232. 10.1016/S0031-9422(01)00233-3 [DOI] [PubMed] [Google Scholar]
- Wegener R., Schulz S., Meiners T., Hadwich K., Hilker M. (2001). Analysis of volatiles induced by oviposition of elm leaf beetle Xanthogaleruca luteola on Ulmus minor. J. Chem. Ecol. 27 499–515. 10.1023/A:1010397107740 [DOI] [PubMed] [Google Scholar]
- Wei J., Yan L., Ren Q., Li C., Ge F., Kang L. (2013). Antagonism between herbivore-induced plant volatiles and trichomes affects tritrophic interactions. Plant Cell Environ. 36 315–327. 10.1111/j.1365-3040.2012.02575.x [DOI] [PubMed] [Google Scholar]
- Willis D. K., Kinscherf T. G. (2009). Population dynamics of Pseudomonas syringae pv. tomato strains on tomato cultivars Rio Grande and Rio Grande-Pto under field conditions. J. Phytopathol. 157 219–227. 10.1111/j.1439-0434.2008.01481.x [DOI] [Google Scholar]
- Xin X. F., He S. Y. (2013). Pseudomonas syringae pv. tomato DC3000: a model pathogen for probing disease susceptibility and hormone signaling in plants. Annu. Rev. Phytopathol. 51 473–498. 10.1146/annurev-phyto-082712-102321 [DOI] [PubMed] [Google Scholar]
- Zacarés L., López-Gresa M. P., Fayos J., Primo J., Bellés J. M., Conejero V. (2007). Induction of p-coumaroyldopamine and feruloyldopamine, two novel metabolites, in tomato by the bacterial pathogen Pseudomonas syringae. Mol. Plant Microbe Interact. 20 1439–1448. 10.1094/MPMI-20-11-1439 [DOI] [PubMed] [Google Scholar]
- Zhang L., Zhang F., Melotto M., Yao J., He S. Y. (2017). Jasmonate signaling and manipulation by pathogens and insects. J. Exp. Bot. 68 1371–1385. 10.1093/jxb/erw478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Chen K. (2009). Age-dependent variations of volatile emissions and inhibitory activity toward Botrytis cinerea and Fusarium oxysporum in tomato leaves treated with chitosan oligosaccharide. J. Plant Biol. 52 332–339. 10.1007/s12374-009-9043-9 [DOI] [Google Scholar]
- Zhang P.-Y., Chen K.-S., He P.-Q., Liu S.-H., Jiang W.-F. (2008). Effects of crop development on the emission of volatiles in leaves of Lycopersicon esculentum and its inhibitory activity to Botrytis cinerea and Fusarium oxysporum. J. Integr. Plant Biol. 50 84–91. 10.1111/j.1744-7909.2007.00597.x [DOI] [PubMed] [Google Scholar]
- Zhao Y., Thilmony R., Bender C. L., Schaller A., He S. Y., Howe G. A. (2003). Virulence systems of Pseudomonas syringae pv. tomato promote bacterial speck disease in tomato by targeting the jasmonate signaling pathway. Plant J. 36 485–499. 10.1046/j.1365-313X.2003.01895.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


