Abstract
We previously reported a sex-specific effect of antenatal treatment with betamethasone (Beta) on sodium (Na+) excretion in adult sheep whereby treated males but not females had an attenuated natriuretic response to angiotensin-(1–7) [Ang-(1–7)]. The present study determined the Na+ uptake and nitric oxide (NO) response to low-dose Ang-(1–7) (1 pM) in renal proximal tubule cells (RPTC) from adult male and female sheep antenatally exposed to Beta or vehicle. Data were expressed as percentage of basal uptake or area under the curve for Na+ or percentage of control for NO. Male Beta RPTC exhibited greater Na+ uptake than male vehicle cells (433 ± 28 vs. 330 ± 26%; P < 0.05); however, Beta exposure had no effect on Na+ uptake in the female cells (255 ± 16 vs. 255 ± 14%; P > 0.05). Ang-(1–7) significantly inhibited Na+ uptake in RPTC from vehicle male (214 ± 11%) and from both vehicle (190 ± 14%) and Beta (209 ± 11%) females but failed to attenuate Na+ uptake in Beta male cells. Beta exposure also abolished stimulation of NO by Ang-(1–7) in male but not female RPTC. Both the Na+ and NO responses to Ang-(1–7) were blocked by Mas receptor antagonist d-Ala7-Ang-(1–7). We conclude that the tubular Ang-(1–7)-Mas-NO pathway is attenuated in males and not females by antenatal Beta exposure. Moreover, since primary cultures of RPTC retain both the sex and Beta-induced phenotype of the adult kidney in vivo they appear to be an appropriate cell model to examine the effects of fetal programming on Na+ handling by the renal tubules.
Keywords: fetal programming, renin-angiotensin system, kidney, sex differences
the fetal programming or Developmental Origins of Health and Disease hypothesis proposes that an adverse intrauterine environment produces permanent alterations in development that predispose the individual to cardiovascular and/or metabolic disease in adult life (1–6). Diverse experimental approaches reveal that a major initiator of programming in utero is exposure of the embryo or fetus to inappropriate elevations in glucocorticoids (29, 30, 37, 38), and prenatal glucocorticoid administration in a variety of animal models produces hypertension in the offspring (24–26, 28, 37, 46, 47, 53).
There is now general agreement on the central role the kidney plays in the development of hypertension through balancing salt and water intake with excretion that serves to regulate blood pressure (13, 22, 32, 33). The renin-angiotensin system (RAS), especially the intrarenal RAS, is a major contributor to the disease in many experimental models (22, 45). Indeed, the expression of angiotensin type 1 (AT1) receptors on the proximal tubule epithelium is required for angiotensin II (ANG II)-induced hypertension (22, 23, 31, 41). Interestingly, one final common pathway by which prenatal glucocorticoid exposure programs the development of hypertension involves the kidney and the intrarenal RAS. Many investigators, ourselves included, have shown involvement of the ANG II-AT1 receptor axis in programmed hypertension (9, 15, 21, 34, 35, 39, 51, 52, 57, 58). Moreover, some of the programming effects of prenatal glucocorticoid exposure on the ANG II-AT1 receptor axis are at the level of the proximal tubule. In this regard, we have also shown that the enhanced ANG II responses (cellular uptake of sodium) evident in the whole animal are maintained in isolated renal proximal tubule cells (RPTC) from the glucocorticoid-exposed animals (21, 52).
Recently, the classic view of the RAS has been expanded to include a battery of new enzymes, peptides, and receptors (11, 18, 19). One of these peptides, Ang-(1–7), counterbalances some of the effects of ANG II by activation of the Mas receptor as well as exhibits actions such as stimulation of nitric oxide (NO) and inhibition of sodium (Na+) uptake that are independent of ANG II or its receptors (16–18, 49). Programming-induced changes in the Ang-(1–7)-Mas receptor path by antenatal betamethasone (Beta) exposure may influence renal function and the development of hypertension. For example, we have shown that prenatal Beta exposure attenuates the ability of Ang-(1–7) to enhance excretion of a Na+ load in adult male sheep (54). In contrast, adult females similarly exposed before birth maintain robust natriuretic responses to Ang-(1–7) and rapidly excrete a Na+ load (54).
Therefore, considering that the proximal tubule reabsorbs ~60% of filtered Na+ (59) and the ANG II responses are enhanced by prenatal Beta exposure in RPTC (52), we asked whether the ability of Ang-(1–7) to inhibit Na+ uptake differs in RPTC from male and female vehicle- and Beta-exposed offspring. We found a greatly attenuated Ang-(1–7) response in RPTC from Beta-exposed males but not females exposed to Beta. Moreover, the attenuated response in the male RPTC may reflect both an impairment of Ang-(1–7) to stimulate nitric oxide (NO) as well as a reduced response to the downstream NO-dependent messenger cGMP.
MATERIALS AND METHODS
All of the procedures for housing, handling, management, and euthanasia of sheep were approved by Wake Forest University’s Institutional Animal Care and Use Committee. A total of 45 adult, sexually mature animals (1 yr old) were used: 28 male (12 vehicle-treated and 16 Beta-treated) and 17 female (8 vehicle-treated and 9 Beta-treated). All methods for animal treatment with betamethasone, animal care, and tissue collection have been previously described in detail by us (52).
Preparation of primary RPTC.
Briefly, kidneys were digested in collagenase (272 U/ml) for 90 min at 37°C. Proximal tubules were isolated by Percoll gradient centrifugation and mixed with Dulbecco’s modified Eagle’s medium and Ham’s nutrient mixture (DMEM-F-12), pH 7.40. Proximal tubules cells were plated in 96-well plates. All studies were done after cells reached confluence between 6 and 9 days of culture at 37°C in a 5% CO2-humidified environment.
Cellular sodium uptake studies.
Sodium uptake by RPTC was determined by measuring the percentage of change in fluorescence emission of the sodium dye Sodium Green (Molecular Probes, Eugene, OR), which reflects changes in intracellular Na+ concentrations (50). Briefly, confluent monolayers were grown for a further 24 h in serum-free medium. Cells were incubated at 37°C for 30 min in loading medium (5 µM Sodium Green in culture medium), and the basal fluorescence signal (excitation 507 nm, emission 532 nm) of each well was measured. RPTC were exposed to different Na+ in the presence of the Na+-K+-ATPase inhibitor ouabain (50 µM), and the fluorescence signal was measured. Solutions with Na+ of 0, 32, 62, 92, and 142 mM were prepared using NaCl and Na+ substitute, equimolar N-methyl-d-glucamine (NMDG) mixed in different proportions to maintain osmotic pressure (pH 7.4). For the studies using Ang-(1–7), (±)-S-nitroso-N-acetylpenicillamine (SNAP), 8-bromoguanosine 3′,5′-cyclic monophosphate (8-Br-cGMP) stimulation, or blockade of Ang-(1–7) receptor, immediately before Na+ uptake experiments RPTC were incubated with either medium alone (basal) or Ang-(1–7) (1 pM), SNAP (100 µM), or 8-Br-cGMP (1 µM) in the presence or absence of the selective antagonist of Ang-(1–7) receptor, d-Ala (10 µM), for 1 h. The data from all experiments were normalized to the cellular protein content in each well and expressed as percentage change from basal fluorescence.
Measurement of NO levels.
Cultured RPTC were preincubated with the fluorescence dye 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate (DAF; 2.5 µM; Molecular Probes, Invitrogen) in Krebs-Ringer phosphate buffer (KRP buffer) containing, in mM, 140 NaCl, 14 glucose, 4.7 KCl, 2.5 CaCl2, 1.8 MgSO4, and 1.8 KH2PO4 (pH 7.4) for 30 min at 37°C. RPTC were washed twice in KRP buffer to remove any excess probe and then incubated with KRP buffer for another 20 min. Cells were then treated with Ang-(1–7) (1 pM) alone or with d-Ala (10 µM) or the NO synthase inhibitor Nω-nitro-l-arginine methyl ester hydrochloride (l-NAME; 1 mM). Background fluorescence was obtained immediately after the addition of peptides or/and inhibitors. The end-point fluorescence was taken after 90 min of incubation. Increases in DAF that are indicative of nitric oxide (NO) production were measured using a SpectraMax M2e microplate reader (Molecular Devices, Sunnyvale, CA) at wavelengths of 495 nm (excitation) and 515 nm (emission) as described in the manufacturer’s instructions. Data are presented as percentages of control. All samples were corrected for background fluorescence.
Western blotting for Ang-(1–7)-Mas receptor protein expression.
Kidney cortex from adult sheep was collected and homogenized in lysis buffer. Fifty micrograms of protein from each sample were separated by electrophoresis on a 12% polyacrylamide gel and transferred to polyvinylidene difluoride (PVDF) membranes. Nonspecific binding was blocked by incubation in 5% milk and 0.1% Tween 20 in Tris-buffered saline. Membranes were probed with specific primary antibodies: Ang-(1–7)-Mas receptor (AAR-013; Alomone) or anti-β-actin (Abcam, Cambridge, MA) followed by incubation with enzyme-labeled secondary antibodies. The membrane was incubated in chemiluminescent substrate and then exposed to film. Immunoreactive bands were quantified by scanning densitometry, and the results are reported in arbitrary optical density units using β-actin signals as loading control.
Statistical analysis.
All data analyses were performed using the GraphPad Prism v6 statistical analysis package (GraphPad Software, La Jolla, CA). Only paired data were used to assess the effects of peptides or blockers. Data were analyzed using a two-way ANOVA followed by Tukey post hoc analysis and expressed as means ± SE. Area under the curve (AUC) data were analyzed by one-way ANOVA with additional paired analyses made by Student’s t-test. For all of the tests, statistical significance was set at P < 0.05.
RESULTS
Ang-(1–7)-induced inhibition of Na+ uptake is abolished in antenatal Beta-treated male RPTC.
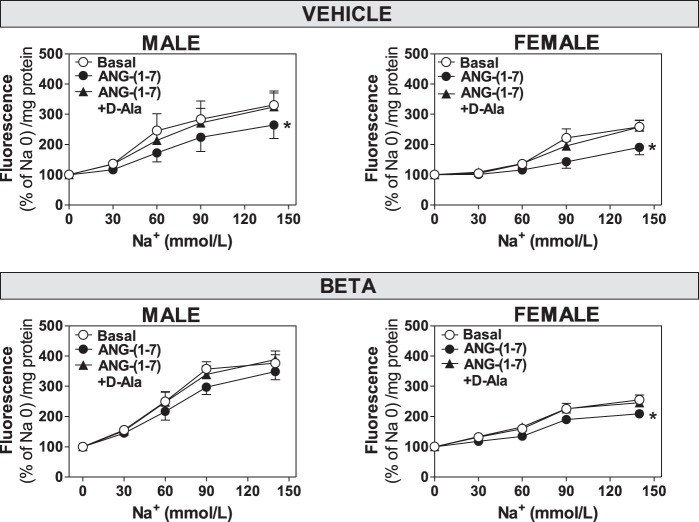
The Na+ response to 1 pM Ang-(1–7) was assessed in cultured RPTC that were isolated from male and female sheep. We (52) previously characterized the purity of the RPTC (>95%) based on the positive staining for SGLT2, a specific marker of the proximal tubule epithelium. Treatment with 1 pM Ang-(1–7) significantly decreased Na+ uptake in male vehicle (F = 3.42, P = 0.037; Figs. 1 and 2), female vehicle (F = 7.55, P = 0.0015; Figs. 1 and 2), and female Beta-treated (F = 8.52, P = 0.0003; Figs. 1 and 2) RPTC compared with basal uptake. In contrast, the same dose of Ang-(1–7) failed to reduce Na+ uptake significantly in male Beta RPTC (F = 2.10, P = 0.128; Fig. 1). The effects of Ang-(1–7) on Na+ uptake were reversed by preincubation with the AT7-Mas receptor antagonist d-Ala7-Ang-(1–7) (d-Ala; 10 µM) as indicated. In all comparisons of equivalent groups [vehicle male vs. female (basal and +Ang-(1–7); Beta male vs. female (basal and +Ang-(1–7)], the magnitude of change in Na+ uptake by RPTC from males exceeded the uptake of Na+ in the female cells with P values ≤0.001.
Fig. 1.
Antenatal Beta abolishes the inhibitory effect of Ang-(1–7) on sodium uptake in male but not female RPTC. Na+ uptake in RPTC from male (vehicle-treated, n = 6; Beta, n = 8) and female (vehicle-treated, n = 4; Beta, n = 9) animals was expressed as increase in fluorescence relative to added Na+. RPTC were untreated (Basal; ○) or preincubated with 1 pM Ang-(1–7) (●) or 1 pM Ang-(1–7) and 10 µM d-Ala (▲) as indicated. Values are means ± SE. *P < 0.05 vs. untreated cells. Na 0, Na+ concentration = 0 mM.
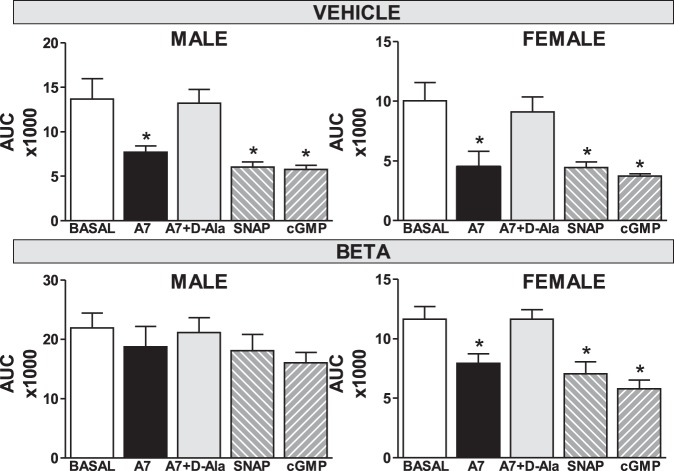
Fig. 2.
Antenatal Beta abolishes the inhibition of sodium uptake by the nitric oxide donor SNAP and cGMP analog 8-Br-cGMP in male but not female RPTC. Na+ uptake in RPTC from male (vehicle-treated, n = 6, top left; Beta, n = 8, bottom left) and female (vehicle-treated, n = 4, top right; Beta, n = 9, bottom right) animals was expressed as area under the curve (AUC), plotted as those shown in Fig. 1. For these experiments, cells were incubated with Ang-(1–7) (A7; 1 pM), the NO donor (±)-S-nitroso-N-acetylpenicillamine (SNAP; 100 µM), or the cGMP stable analog 8-Br-cGMP (1 µM). Values are means ± SE. *P < 0.05 vs. untreated cells.
NO- and cGMP-induced inhibition of Na+ uptake is abolished in antenatal Beta-treated male RPTC.
Since Ang-(1–7) has been shown to signal through the NO pathway and the tubular NO system is generally considered natriuretic, we compared the extent of Na+ uptake (expressed as area under the curve, AUC) to the NO donor SNAP and the stable cGMP analog 8-Br-cGMP with Ang-(1–7). The addition of Ang-(1–7) (1 pM), SNAP (100 µM), or 8-Br-cGMP (1 µM) significantly decreased Na+ uptake in RPTC from male and female vehicle animals as well as the Beta female cells (P < 0.05; Fig. 2). In contrast, Ang-(1–7), SNAP, and 8-Br-cGMP failed to attenuate Na+ uptake significantly in Beta-treated male RPTC (Fig. 2). Finally, the AT7-Mas receptor antagonist d-Ala (10 µM) abolished the Ang-(1–7)-dependent reduction in Na+ uptake in male and female vehicle as well as the female Beta cells (Fig. 2).
Ang-(1–7)-mediated increase in NO production is abolished in antenatal Beta-treated male RPTC.
In the next series of experiments, we directly determined whether Ang-(1–7) treatment increased endogenous NO production in RPTC from vehicle and Beta-treated animals as detected by the change (in percentage) in DAF fluorescence. Ang-(1–7) (1 pM) increased DAF fluorescence approximately twofold in both male and female vehicle RPTC (Fig. 3). Ang-(1–7) also significantly increased DAF in the Beta-treated female cells (P < 0.05; Fig. 3A) but failed to stimulate DAF in the Beta male cells. The stimulatory effects of Ang-(1–7) on NO in the RPTC were abolished by Ang-(1–7) antagonist d-Ala (10 µM) and the general nitric oxide synthase (NOS) inhibitor l-NAME (1 mM). Both d-Ala and l-NAME reduced the DAF levels in all four groups below the control values (Fig. 3, A and B).
Fig. 3.
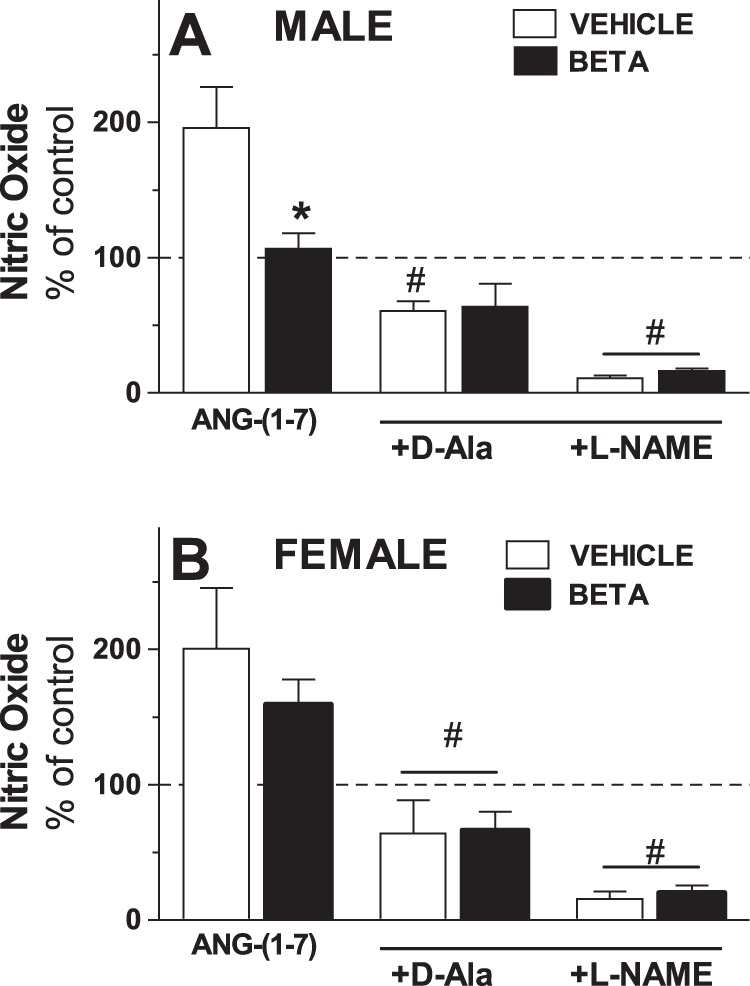
Antenatal Beta attenuates nitric oxide production to Ang-(1–7) in male but not female RPTC. Nitric oxide production was expressed as percentage of basal levels (% of control) in RPTC from male (A; vehicle, □, n = 5; Beta, ■, n = 5) and female (B; control, vehicle, □, n = 5; glucocorticoid-treated, Beta, ■, n = 5) animals treated with 1 pM Ang-(1–7) alone, with d-Ala (+D-Ala; 10 µM), or with l-NAME (+L-NAME; 1 mM). Values are means ± SE. *P < 0.05 vs. vehicle; #P < 0.05 vs. cells treated with Ang-(1–7) alone.
Ang-(1–7)-Mas receptor protein expression in renal cortex is suppressed by Beta in males but not in females.
In the final series of experiments, we assessed the protein expression of the AT7-Mas receptor in the sheep renal cortex. We (18) previously used the Alomone antibody to the Mas receptor to identify Mas predominantly on the tubular elements of the kidney. Analysis of the immunoblots showed a sex difference in effects of Beta treatment on the expression of the Mas receptor in the kidney cortex. Beta decreased the expression of the Mas receptor in males (Fig. 4A; P < 0.05 by t-test) but not in females (Fig. 4B; P > 0.05).
Fig. 4.
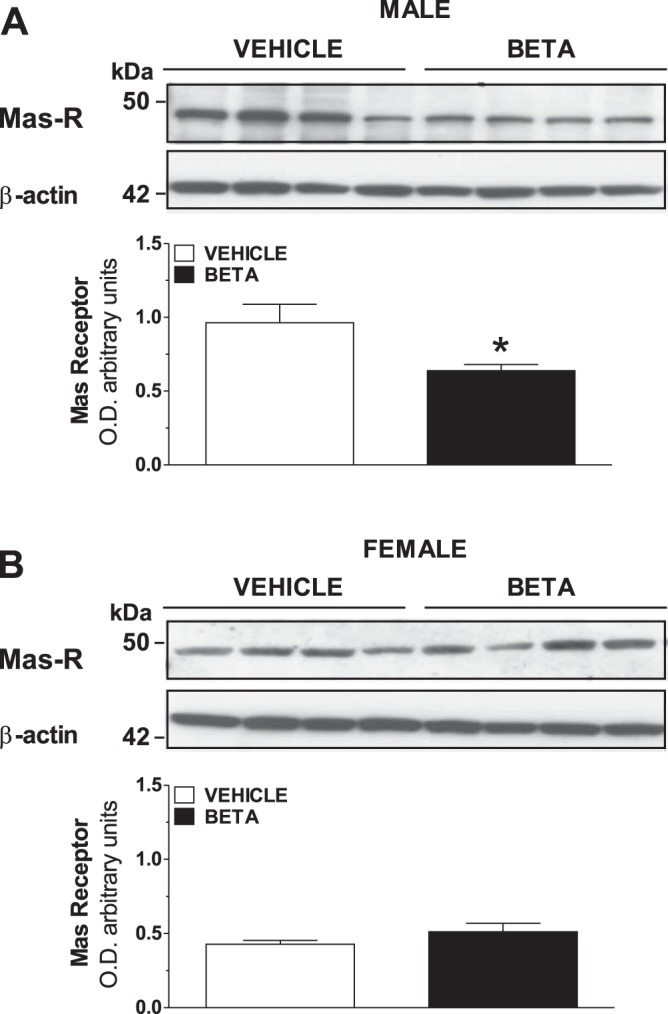
Expression levels of Ang-(1–7)-Mas receptor (Mas-R) in kidney from male and female vehicle and Beta-treated animals. Protein expression of the Ang-(1–7)-Mas receptor was measured by Western blot in kidney cortex isolated from male (A) and female (B) vehicle and Beta-exposed sheep. Signals for actin were obtained by reblotting of the same membrane; all samples were derived at the same time and processed in parallel. Densitometric analysis of gels is also shown for males (A; vehicle, □, n = 4; Beta, ■, n = 4) and females (B; vehicle, □, n = 4; Beta, ■, n = 4). Values are means ± SE. *P < 0.05 vs. vehicle. O.D., optical density.
DISCUSSION
In the present study, we sought to determine whether antenatal Beta exposure impairs the ability of Ang-(1–7) to reduce directly the Na+ uptake by proximal tubule cells in vitro. We found that antenatal Beta exposure essentially abolished both the inhibition of Na+ uptake and the stimulation of NO production by Ang-(1–7) in RPTC from adult male offspring when compared with cells from vehicle-exposed males. Moreover, the Na+ responses to the NO donor SNAP and the cGMP analog were attenuated to a similar extent as Ang-(1–7) in the Beta-treated male RPTC. In contrast, antenatal Beta exposure had no significant effect on the ability of Ang-(1–7), SNAP, or cGMP to inhibit Na+ uptake as well as no significant effect on Ang-(1–7)-induced stimulation of NO in female RPTC. Thus the sex-specific effects of antenatal Beta exposure on Ang-(1–7)-induced Na+ excretion may reflect, in part, programming effects on the AT7-Mas receptor-NO pathway to regulate Na+ uptake in male RPTC.
Over the last decade, there has been increasing acceptance of the idea, originally advocated by Barker and colleagues (2–4), that events at critical windows in early development may convey profound effects on health later in life. Among the organs influenced by this fetal programming is the kidney, and multiple reviews document the extensive evidence supporting the impact of various programming stimuli on kidney development and function (42, 55, 56). One of the hallmarks of the impact of fetal programming on the kidney is alterations in the intrarenal RAS, and these alterations tend to favor the ANG II-AT1 receptor axis (34, 44, 51). The upregulation of this axis can, among other things, lead to Na+ retention, and the evidence that ANG II acts directly on the proximal tubule epithelium to promote Na+ uptake and increase blood pressure is compelling (13, 22). Relative to fetal programming, we (52) previously showed that the RPTC from male offspring exposed to Beta before birth exhibit both greater basal and ANG II-stimulated Na+ uptake than cells of vehicle-treated animals. We (52) also reported no effect of Beta exposure on either basal or ANG II-stimulated Na+ uptake in the RPTC from the Beta-exposed female offspring. We now show a markedly attenuated ability of Ang-(1–7) to stimulate NO and to reduce Na+ uptake in male Beta cells. The combination of an increased responsiveness to ANG II (52) and the lack of Ang-(1–7) effect in RPTC from Beta male cells when compared with Beta female cells provides at least a partial explanation for the inability of the male Beta-exposed sheep to excrete a Na+ load when infused with Ang-(1–7) compared with the robust excretion of Na+ observed in females under identical experimental conditions (54).
The mechanisms by which Ang-(1–7) affects Na+ handling in the kidney have not been firmly established. In the male RPTC that are devoid of any hemodynamic effects, our data suggest but do not prove that NO is directly linked to the attenuation of Na+ uptake. Ang-(1–7), the NO donor SNAP, and the cGMP analog exhibited equivalent effects to reduce Na+ uptake in the RPTC of vehicle males and females as well as Beta females. Moreover, the effects of all three treatments to reduce Na+ were essentially absent in the male Beta cells. These data are consistent with other reports regarding the ability of Ang-(1–7) to stimulate NO synthase and NO release as well as the effects of the peptides to reduce sodium hydrogen exchanger (NHE3) activity in the proximal tubule (14, 25, 47, 48, 55). Although some controversy exists, the majority of evidence indicates that NO tone is a natriuretic pathway in the proximal tubule epithelium (59). For example, fluid reabsorption in the proximal tubule and activity of NHE3, a primary Na+ transporter in the proximal tubule, are reduced by NO donors, whereas inhibition of NO synthesis reduces Na+ excretion (59). In addition, the natriuresis resulting from stimulation of the AT2 receptor is via a NO/cGMP mechanism in the proximal tubule (12, 13, 36), and the NO donor SNAP reduces Na+ uptake by cultured human RPTC (50). We propose that the Ang-(1–7)-stimulated increase in NO may reduce Na+ uptake by inhibiting NHE3 in RPTC but that antenatal Beta exposure attenuates the inhibition of the Na+ transporter by the Ang-(1–7)-NO axis (Refs. 14, 45, 52; Fig. 5, A and B, respectively). Furthermore, it is certainly plausible to suggest that the differential effects of Beta exposure in the kidney of the males on both the ANG II-AT1 receptor and the Ang-(1–7)-Mas receptor-NO axis may contribute to the elevated arterial blood pressure observed in the adult sheep (28, 51, 53). We certainly acknowledge that additional studies are necessary to demonstrate directly that Ang-(1–7)-derived NO accounts for the inhibitory effects on Na+ uptake in the cells from males and females as well as the possibility that other cellular mediators may contribute to the Na+ effects by Ang-(1–7).
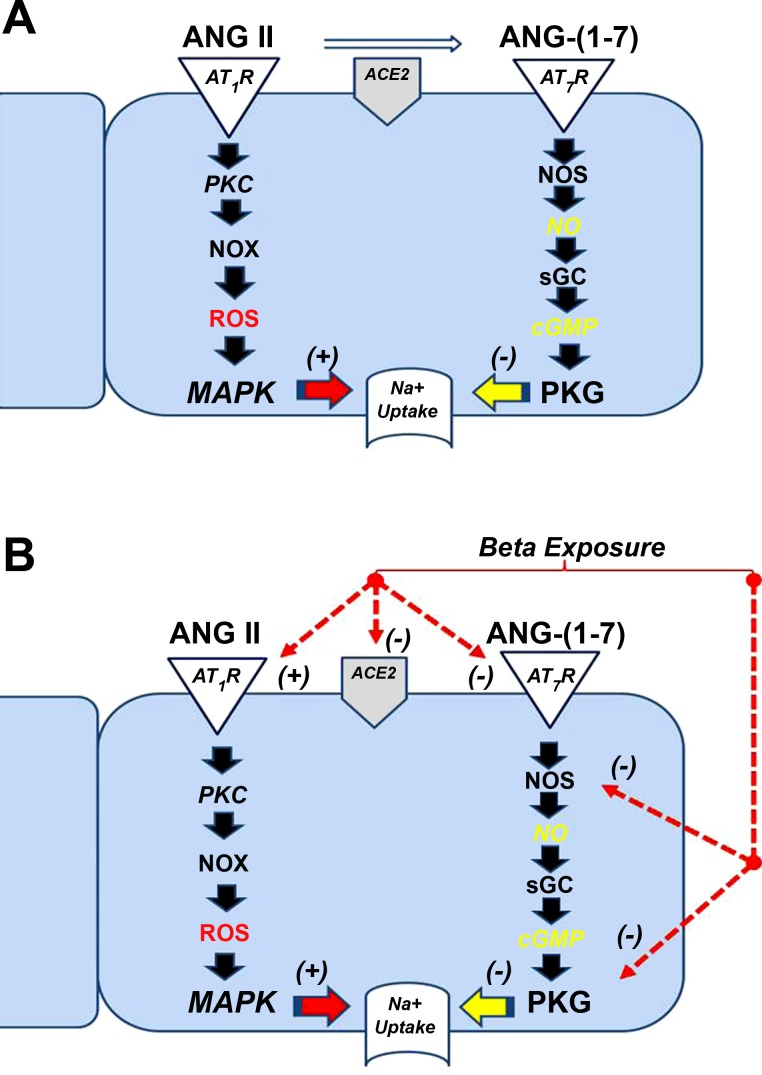
Fig. 5.
Scheme for the potential effects of betamethasone exposure on the ANG II and Ang-(1–7) pathways to influence Na+ transport in the tubule cells. A: ANG II binds to the AT1 receptor (AT1R) and may stimulate protein kinase C (PKC) and NADPH oxidase (NOX) to generate reactive oxygen species (ROS) that activates the mitogen-activated pathway (MAPK) to stimulate Na+ uptake in the tubules (40). In contrast, Ang-(1–7) binds to the AT7-Mas-R to stimulate nitric oxide synthase (NOS) to generate NO and increase cellular levels of cGMP by soluble guanylate cyclase (sGC) to stimulate protein kinase G to inhibit Na+ uptake. Angiotensin-converting enzyme 2 (ACE2), expressed on the apical face of the tubules, metabolizes ANG II to Ang-(1–7) and may influence both ANG II and Ang-(1–7) tone. In the Beta-exposed males, expression of the AT1R is higher (52) and may promote greater Na+ uptake. In contrast, both ACE2 (51) and the AT7-Mas-R expression are lower in the tubules that would attenuate the Ang-(1–7) tone and reduce Na+ uptake. Betamethasone exposure may also attenuate NO generation and PKG responsiveness that may further influence Na+ handling in the male tubules.
The expression of the AT7-Mas receptor was lower in Beta males than in controls with no differences in females. We (34) previously reported that antenatal Beta exposure decreased the component of 125I-labeled sarthran binding sensitive to d-Ala blockade in the plasma membrane and nuclear fractions of the sheep renal cortex as well as the NO response to Ang-(1–7) in isolated nuclei. Thus one contributing factor for the difference in responses to Ang-(1–7) induced by Beta in RPTC from males and females may reflect expression of the Mas receptor (16, 17, 49). Reduced expression of the Mas receptor in RPTC would be expected to reduce responses to both endogenous and exogenous Ang-(1–7), which may contribute to the effect of d-Ala and l-NAME to attenuate NO below the control values in the RPTC (Fig. 5).
The current data also reveal the possibility of sites downstream from the AT7-Mas receptor targeted by Beta exposure. The Na+ response to the NO donor SNAP and the stable cGMP analog 8-Br-cGMP were significantly blunted in the RPTC from male Beta cells. The attenuated SNAP response suggests that the effects of programming do not solely reflect the downregulation of NOS and may point to an inability of guanylate cyclase to generate cGMP. However, the fact that the natriuretic response to 8-Br-cGMP was also reduced suggests a defect distal to NOS and guanylate cyclase. cGMP is a cofactor for protein kinase G (PKG), and PKG substrates include NHE3, one of the primary Na+ transporters in the proximal tubule (59). Indeed, PKG-dependent phosphorylation of NHE3 attenuates the activity and trafficking of this transporter to the cell membrane, and chronic PKG activation may reduce NHE3 protein expression (20, 59). We (52) showed that NHE3 expression is higher in the renal cortex of the Beta-treated sheep, and preliminary studies suggest that the PKG inhibitor KT 5823 blocks the Ang-(1–7)-Na+ response in RPTC (data not shown).
Finally, we noted the reduction in Na+ for Ang-(1–7), SNAP, and cGMP in the female vehicle and Beta cells but the lack of an effect in the male Beta cells (Fig. 3). The protective actions evident in the female Beta cells parallel studies in the intact animal. It is possible that female sex chromosomes convey a protective effect on programming events that attenuate the AT7-Mas receptor-NO-cGMP pathway in males or that these genes may themselves be insensitive to programming events. The AT2 receptor is located on the X chromosome, and an intact AT2 receptor-NO axis is considered to convey cardiovascular protection in females (36). We (52) previously reported that the ANG II-AT2-receptor-dependent increase in NO was similar in both female and male Beta RPTC and that the ANG II effects on Na+ were comparable in the presence or absence of an AT2 receptor antagonist. These data may argue against a role for the ANG II-AT2 receptor-NO axis to account for the sex differences in Na+ handing by the RPTC; however, elucidation of the protective mechanisms in females awaits further study.
Perspectives.
Considering that the kidney plays a prominent role in regulating blood pressure by maintaining body fluid balance (22, 32, 33), it is not surprising that many of the same stimuli that program the kidney also program hypertension in the offspring through a variety of effects, including alterations of the intrarenal RAS (7, 8, 18, 44). Fetal programming of this system can lead to hypertension by influencing both antinatriuretic and natriuretic actions of angiotensin peptides. In this report, we have shown that antenatal Beta exposure to a clinically relevant dose of the steroid impairs the ability of Ang-(1–7) to reduce Na+ uptake by cells from male but not female offspring. These data suggest that there is a sex-specific effect of antenatal Beta on RPTC that favors retention of Na+ by the exposed males. There is no doubt that the remarkably beneficial effects of glucocorticoid administration to women threatening to deliver prematurely far outweigh potential side effects; thus they are widely used with ~80% of pregnant women at risk for premature delivery receiving treatment in the United States today. Consequently, ~100,000 or more individuals are born in the United States each year after exposure to glucocorticoids such as Beta (10, 27, 43, 48). As these people reach middle age, it is possible they will be at risk for developing renal dysfunction and hypertension. Thus it is important now to identify the mechanisms affecting Na+ handling by the kidney that are changed by Beta exposure to develop approaches to lessen any pathophysiological impact on renal function or blood pressure regulation that result from antenatal glucocorticoid therapy.
GRANTS
This work was supported by National Institute of Child Health and Human Development Grants P01-HD-047584, R01-HD-017644, and R21-HD-084227.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.C.C. and J.C.R. conceived and designed research; Y.S. and J.B. performed experiments; Y.S., J.B., V.M.P., M.C.C., and J.C.R. analyzed data; Y.S., J.B., V.M.P., M.C.C., and J.C.R. interpreted results of experiments; Y.S., J.B., V.M.P., and M.C.C. prepared figures; Y.S., J.B., V.M.P., and J.C.R. drafted manuscript; Y.S., J.B., V.M.P., M.C.C., and J.C.R. edited and revised manuscript; Y.S., J.B., V.M.P., M.C.C., and J.C.R. approved final version of manuscript.
REFERENCES
- 1.Barker DJ. Childhood causes of adult diseases. Arch Dis Child 63: 867–869, 1988. doi: 10.1136/adc.63.7.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barker DJ. Human growth and chronic disease: a memorial to Jim Tanner. Ann Hum Biol 39: 335–341, 2012. doi: 10.3109/03014460.2012.712717. [DOI] [PubMed] [Google Scholar]
- 3.Barker DJ, Bagby SP. Developmental antecedents of cardiovascular disease: a historical perspective. J Am Soc Nephrol 16: 2537–2544, 2005. doi: 10.1681/ASN.2005020160. [DOI] [PubMed] [Google Scholar]
- 4.Barker DJ, Bagby SP, Hanson MA. Mechanisms of disease: in utero programming in the pathogenesis of hypertension. Nat Clin Pract Nephrol 2: 700–707, 2006. doi: 10.1038/ncpneph0344. [DOI] [PubMed] [Google Scholar]
- 5.Barker DJ, Osmond C. Low birth weight and hypertension. BMJ 297: 134–135, 1988. doi: 10.1136/bmj.297.6641.134-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barker DJ. The origins of the developmental origins theory. J Intern Med 261: 412–417, 2007. doi: 10.1111/j.1365-2796.2007.01809.x. [DOI] [PubMed] [Google Scholar]
- 7.Baum M. Role of renal sympathetic nerve activity in prenatal programming of hypertension. Pediatr Nephrol. First published March 21, 2016; doi: 10.1007/s00467-016-3359-8. [DOI] [PubMed] [Google Scholar]
- 8.Baum M. Role of the kidney in the prenatal and early postnatal programming of hypertension. Am J Physiol Renal Physiol 298: F235–F247, 2010. doi: 10.1152/ajprenal.00288.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bi J, Contag SA, Chen K, Su Y, Figueroa JP, Chappell MC, Rose JC. Sex-specific effect of antenatal betamethasone exposure on renal oxidative stress induced by angiotensins in adult sheep. Am J Physiol Renal Physiol 307: F1013–F1022, 2014. doi: 10.1152/ajprenal.00354.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonanno C, Wapner RJ. Antenatal corticosteroids in the management of preterm birth: are we back where we started? Obstet Gynecol Clin North Am 39: 47–63, 2012. doi: 10.1016/j.ogc.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carey RM. Newly discovered components and actions of the renin-angiotensin system. Hypertension 62: 818–822, 2013. doi: 10.1161/HYPERTENSIONAHA.113.01111. [DOI] [PubMed] [Google Scholar]
- 12.Carey RM. The intrarenal renin-angiotensin and dopaminergic systems: control of renal sodium excretion and blood pressure. Hypertension 61: 673–680, 2013. doi: 10.1161/HYPERTENSIONAHA.111.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carey RM. The intrarenal renin-angiotensin system in hypertension. Adv Chronic Kidney Dis 22: 204–210, 2015. doi: 10.1053/j.ackd.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Castelo-Branco RC, Leite-Delova DC, de Mello-Aires M. Dose-dependent effects of angiotensin-(1-7) on the NHE3 exchanger and [Ca(2+)](i) in in vivo proximal tubules. Am J Physiol Renal Physiol 304: F1258–F1265, 2013. doi: 10.1152/ajprenal.00401.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Celsi G, Kistner A, Aizman R, Eklöf AC, Ceccatelli S, de Santiago A, Jacobson SH. Prenatal dexamethasone causes oligonephronia, sodium retention, and higher blood pressure in the offspring. Pediatr Res 44: 317–322, 1998. doi: 10.1203/00006450-199809000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Chappell MC. Emerging evidence for a functional angiotensin-converting enzyme 2-angiotensin-(1-7)-MAS receptor axis: more than regulation of blood pressure? Hypertension 50: 596–599, 2007. doi: 10.1161/HYPERTENSIONAHA.106.076216. [DOI] [PubMed] [Google Scholar]
- 17.Chappell MC. Nonclassical renin-angiotensin system and renal function. Compr Physiol 2: 2733–2752, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chappell MC, Marshall AC, Alzayadneh EM, Shaltout HA, Diz DI. Update on the angiotensin converting enzyme 2-angiotensin (1–7)-Mas receptor axis: fetal programing, sex differences, and intracellular pathways. Front Endocrinol (Lausanne) 4: 201, 2014. doi: 10.3389/fendo.2013.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chappell MC, Modrall JG, Diz DI, Ferrario CM. Novel aspects of the renal renin-angiotensin system: angiotensin-(1-7), ACE2 and blood pressure regulation. Contrib Nephrol 143: 77–89, 2004. doi: 10.1159/000078713. [DOI] [PubMed] [Google Scholar]
- 20.Chen T, Kocinsky HS, Cha B, Murtazina R, Yang J, Tse CM, Singh V, Cole R, Aronson PS, de Jonge H, Sarker R, Donowitz M. Cyclic GMP kinase II (cGKII) inhibits NHE3 by altering its trafficking and phosphorylating NHE3 at three required sites: identification of a multifunctional phosphorylation site. J Biol Chem 290: 1952–1965, 2015. doi: 10.1074/jbc.M114.590174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Contag SA, Bi J, Chappell MC, Rose JC. Developmental effect of antenatal exposure to betamethasone on renal angiotensin II activity in the young adult sheep. Am J Physiol Renal Physiol 298: F847–F856, 2010. doi: 10.1152/ajprenal.00497.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crowley SD, Coffman TM. The inextricable role of the kidney in hypertension. J Clin Invest 124: 2341–2347, 2014. doi: 10.1172/JCI72274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crowley SD, Gurley SB, Herrera MJ, Ruiz P, Griffiths R, Kumar AP, Kim HS, Smithies O, Le TH, Coffman TM. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci USA 103: 17985–17990, 2006. doi: 10.1073/pnas.0605545103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dodic M, Abouantoun T, O’Connor A, Wintour EM, Moritz KM. Programming effects of short prenatal exposure to dexamethasone in sheep. Hypertension 40: 729–734, 2002. doi: 10.1161/01.HYP.0000036455.62159.7E. [DOI] [PubMed] [Google Scholar]
- 25.Dodic M, Hantzis V, Duncan J, Rees S, Koukoulas I, Johnson K, Wintour EM, Moritz K. Programming effects of short prenatal exposure to cortisol. FASEB J 16: 1017–1026, 2002. doi: 10.1096/fj.01-1045com. [DOI] [PubMed] [Google Scholar]
- 26.Dodic M, May CN, Wintour EM, Coghlan JP. An early prenatal exposure to excess glucocorticoid leads to hypertensive offspring in sheep. Clin Sci (Lond) 94: 149–155, 1998. doi: 10.1042/cs0940149. [DOI] [PubMed] [Google Scholar]
- 27.Fanaroff AA, Stoll BJ, Wright LL, Carlo WA, Ehrenkranz RA, Stark AR, Bauer CR, Donovan EF, Korones SB, Laptook AR, Lemons JA, Oh W, Papile LA, Shankaran S, Stevenson DK, Tyson JE, Poole WK; NICHD Neonatal Research Network . Trends in neonatal morbidity and mortality for very low birthweight infants. Am J Obstet Gynecol 196: 147.e1–147.e8, 2007. doi: 10.1016/j.ajog.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 28.Figueroa JP, Rose JC, Massmann GA, Zhang J, Acuña G. Alterations in fetal kidney development and elevations in arterial blood pressure in young adult sheep after clinical doses of antenatal glucocorticoids. Pediatr Res 58: 510–515, 2005. doi: 10.1203/01.PDR.0000179410.57947.88. [DOI] [PubMed] [Google Scholar]
- 29.Gardner DS, Jackson AA, Langley-Evans SC. The effect of prenatal diet and glucocorticoids on growth and systolic blood pressure in the rat. Proc Nutr Soc 57: 235–240, 1998. doi: 10.1079/PNS19980037. [DOI] [PubMed] [Google Scholar]
- 30.Gardner DS, Jackson AA, Langley-Evans SC. Maintenance of maternal diet-induced hypertension in the rat is dependent on glucocorticoids. Hypertension 30: 1525–1530, 1997. doi: 10.1161/01.HYP.30.6.1525. [DOI] [PubMed] [Google Scholar]
- 31.Gurley SB, Riquier-Brison AD, Schnermann J, Sparks MA, Allen AM, Haase VH, Snouwaert JN, Le TH, McDonough AA, Koller BH, Coffman TM. AT1A angiotensin receptors in the renal proximal tubule regulate blood pressure. Cell Metab 13: 469–475, 2011. doi: 10.1016/j.cmet.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guyton AC. Kidneys and fluids in pressure regulation. Small volume but large pressure changes. Hypertension 19, Suppl: I2–I8, 1992. doi: 10.1161/01.HYP.19.1_Suppl.I2. [DOI] [PubMed] [Google Scholar]
- 33.Guyton AC, Coleman TG, Cowley AV Jr, Scheel KW, Manning RD Jr, Norman RA Jr. Arterial pressure regulation. Overriding dominance of the kidneys in long-term regulation and in hypertension. Am J Med 52: 584–594, 1972. doi: 10.1016/0002-9343(72)90050-2. [DOI] [PubMed] [Google Scholar]
- 34.Gwathmey TM, Shaltout HA, Rose JC, Diz DI, Chappell MC. Glucocorticoid-induced fetal programming alters the functional complement of angiotensin receptor subtypes within the kidney. Hypertension 57: 620–626, 2011. doi: 10.1161/HYPERTENSIONAHA.110.164970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ingelfinger JR, Woods LL. Perinatal programming, renal development, and adult renal function. Am J Hypertens 15: 46S–49S, 2002. doi: 10.1016/S0895-7061(01)02302-0. [DOI] [PubMed] [Google Scholar]
- 36.Kemp BA, Bell JF, Rottkamp DM, Howell NL, Shao W, Navar LG, Padia SH, Carey RM. Intrarenal angiotensin III is the predominant agonist for proximal tubule angiotensin type 2 receptors. Hypertension 60: 387–395, 2012. doi: 10.1161/HYPERTENSIONAHA.112.191403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Langley-Evans SC. Intrauterine programming of hypertension by glucocorticoids. Life Sci 60: 1213–1221, 1997. doi: 10.1016/S0024-3205(96)00611-X. [DOI] [PubMed] [Google Scholar]
- 38.Langley-Evans SC, Gardner DS, Welham SJ. Intrauterine programming of cardiovascular disease by maternal nutritional status. Nutrition 14: 39–47, 1998. doi: 10.1016/S0899-9007(97)00391-2. [DOI] [PubMed] [Google Scholar]
- 39.Langley-Evans SC, Welham SJ, Jackson AA. Fetal exposure to a maternal low protein diet impairs nephrogenesis and promotes hypertension in the rat. Life Sci 64: 965–974, 1999. doi: 10.1016/S0024-3205(99)00022-3. [DOI] [PubMed] [Google Scholar]
- 40.Li XC, Hopfer U, Zhuo JL. Novel signaling mechanisms of intracellular angiotensin II-induced NHE3 expression and activation in mouse proximal tubule cells. Am J Physiol Renal Physiol 303: F1617–F1628, 2012. doi: 10.1152/ajprenal.00219.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li XC, Zhuo JL. Proximal tubule-dominant transfer of AT(1a) receptors induces blood pressure responses to intracellular angiotensin II in AT(1a) receptor-deficient mice. Am J Physiol Regul Integr Comp Physiol 304: R588–R598, 2013. doi: 10.1152/ajpregu.00338.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luyckx VA, Bertram JF, Brenner BM, Fall C, Hoy WE, Ozanne SE, Vikse BE. Effect of fetal and child health on kidney development and long-term risk of hypertension and kidney disease. Lancet 382: 273–283, 2013. doi: 10.1016/S0140-6736(13)60311-6. [DOI] [PubMed] [Google Scholar]
- 43.Martin JA, Hamilton BE, Osterman MJ, Curtin SC, Matthews TJ. Births: final data for 2013. Natl Vital Stat Rep 64: 1–65, 2015. [PubMed] [Google Scholar]
- 44.Moritz KM, Cuffe JS, Wilson LB, Dickinson H, Wlodek ME, Simmons DG, Denton KM. Review: Sex specific programming: a critical role for the renal renin-angiotensin system. Placenta 31, Suppl: S40–S46, 2010. doi: 10.1016/j.placenta.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 45.Navar LG, Kobori H, Prieto MC, Gonzalez-Villalobos RA. Intratubular renin-angiotensin system in hypertension. Hypertension 57: 355–362, 2011. doi: 10.1161/HYPERTENSIONAHA.110.163519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ortiz LA, Quan A, Weinberg A, Baum M. Effect of prenatal dexamethasone on rat renal development. Kidney Int 59: 1663–1669, 2001. doi: 10.1046/j.1523-1755.2001.0590051663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ortiz LA, Quan A, Zarzar F, Weinberg A, Baum M. Prenatal dexamethasone programs hypertension and renal injury in the rat. Hypertension 41: 328–334, 2003. doi: 10.1161/01.HYP.0000049763.51269.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Polyakov A, Cohen S, Baum M, Trickey D, Jolley D, Wallace EM. Patterns of antenatal corticosteroid prescribing 1998-2004. Aust N Z J Obstet Gynaecol 47: 42–45, 2007. doi: 10.1111/j.1479-828X.2006.00677.x. [DOI] [PubMed] [Google Scholar]
- 49.Santos RA, Simoes e Silva AC, Maric C, Silva DM, Machado RP, de Buhr I, Heringer-Walther S, Pinheiro SV, Lopes MT, Bader M, Mendes EP, Lemos VS, Campagnole-Santos MJ, Schultheiss HP, Speth R, Walther T. Angiotensin-(1-7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci USA 100: 8258–8263, 2003. doi: 10.1073/pnas.1432869100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sasaki S, Siragy HM, Gildea JJ, Felder RA, Carey RM. Production and role of extracellular guanosine cyclic 3′, 5′ monophosphate in sodium uptake in human proximal tubule cells. Hypertension 43: 286–291, 2004. doi: 10.1161/01.HYP.0000112421.18551.1e. [DOI] [PubMed] [Google Scholar]
- 51.Shaltout HA, Figueroa JP, Rose JC, Diz DI, Chappell MC. Alterations in circulatory and renal angiotensin-converting enzyme and angiotensin-converting enzyme 2 in fetal programmed hypertension. Hypertension 53: 404–408, 2009. doi: 10.1161/HYPERTENSIONAHA.108.124339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Su Y, Bi J, Pulgar VM, Figueroa J, Chappell M, Rose JC. Antenatal glucocorticoid treatment alters Na+ uptake in renal proximal tubule cells from adult offspring in a sex-specific manner. Am J Physiol Renal Physiol 308: F1268–F1275, 2015. doi: 10.1152/ajprenal.00047.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tang L, Bi J, Valego N, Carey L, Figueroa J, Chappell M, Rose JC. Prenatal betamethasone exposure alters renal function in immature sheep: sex differences in effects. Am J Physiol Regul Integr Comp Physiol 299: R793–R803, 2010. doi: 10.1152/ajpregu.00590.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tang L, Carey LC, Bi J, Valego N, Sun X, Deibel P, Perrott J, Figueroa JP, Chappell MC, Rose JC. Gender differences in the effects of antenatal betamethasone exposure on renal function in adult sheep. Am J Physiol Regul Integr Comp Physiol 296: R309–R317, 2009. doi: 10.1152/ajpregu.90645.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vehaskari VM. Prenatal programming of kidney disease. Curr Opin Pediatr 22: 176–182, 2010. doi: 10.1097/MOP.0b013e328336ebc9. [DOI] [PubMed] [Google Scholar]
- 56.Vehaskari VM, Woods LL. Prenatal programming of hypertension: lessons from experimental models. J Am Soc Nephrol 16: 2545–2556, 2005. doi: 10.1681/ASN.2005030300. [DOI] [PubMed] [Google Scholar]
- 57.Woods LL. Fetal origins of adult hypertension: a renal mechanism? Curr Opin Nephrol Hypertens 9: 419–425, 2000. doi: 10.1097/00041552-200007000-00014. [DOI] [PubMed] [Google Scholar]
- 58.Woods LL, Ingelfinger JR, Nyengaard JR, Rasch R. Maternal protein restriction suppresses the newborn renin-angiotensin system and programs adult hypertension in rats. Pediatr Res 49: 460–467, 2001. doi: 10.1203/00006450-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 59.Zhuo JL, Li XC. Proximal nephron. Compr Physiol 3: 1079–1123, 2013. doi: 10.1002/cphy.c110061. [DOI] [PMC free article] [PubMed] [Google Scholar]