Abstract
Protein mimotopes, or blocking peptides, are small therapeutic peptides that prevent protein-protein interactions by selectively mimicking a native binding domain. Inexpensive technology facilitates straightforward design and production of blocking peptides in sufficient quantities to allow preventive and therapeutic trials in both in vitro and in vivo experimental disease models. The kidney is an ideal peptide target, since small molecules undergo rapid filtration and efficient bulk absorption by tubular epithelial cells. Because the half-life of peptides is markedly prolonged in the kidneys compared with the bloodstream, blocking peptides are an attractive tool for treating diverse renal diseases, including ischemia, proteinuric states, such as membranous nephropathy and focal and segmental glomerulosclerosis, and renal cell carcinoma. Therapeutic peptides represent one of the fastest-growing reagent classes for novel drug development in human disease, partly because of their ease of administration, high binding affinity, and minimal off-target effects. This review introduces the concepts of blocking peptide design, production, and administration and highlights the potential use of therapeutic peptides to prevent or treat specific renal diseases.
Keywords: acute kidney injury, Bax, nucleophosmin, ischemia, membranous nephropathy, phospholipase A2 receptor, podocyte, foot process, slit diaphragm, renal cell carcinoma, Jade-1
What Is a Blocking Peptide?
A blocking peptide competitively inhibits protein-protein interaction by mimicking one of their binding domains, or “binding epitopes.” The term mimicking epitope, or “mimotope,” is credited to Mario Geysen and initially referred to a peptide macromolecule that reproduced, or “mimicked,” the epitope structure that elicits the same specific antibody response as the intact macromolecule from which it was derived (21). Subsequently, the term mimotope was expanded to include peptides that competitively interrupt protein-protein interactions by binding to one of the partner’s binding domains, including those that interfere with protein-protein interactions (7, 32), of both extra- and intracellular proteins (7, 17, 35). Blocking peptides have also been referred to as “peptide aptamers” (7). The term “aptamers” is derived from the Latin, meaning “fitting,” and includes small nucleic acid and peptide ligands designed to interfere with RNA function. By design, small peptide aptamers, or peptide mimotopes, possess many of the attractive features of monoclonal antibodies, including high specificity and ligand binding affinity [with IC50 as low as the picomolar range (35)]. Peptides are much smaller and and require less time to synthesize than monoclonal antibodies and can be targeted to the extra- and intracellular compartments (35). In this review we use the term mimotope to refer to small peptides that interfere with protein-protein interactions.
Small peptides are surprisingly amenable to diverse applications, including mapping interacting epitopes (55), identifying novel drug targets (34, 39), confirming the functional significance of protein-protein interactions in complex biological systems (58, 61), and generating vaccines (38) and novel diagnostics (7, 32, 58, 61). Since the surface that modulates protein-protein interaction is relatively small, competitive disruption by a blocking peptide is both feasible and therapeutically attractive (63). Importantly, small blocking peptides replicate the amino acid sequence of native proteins and, therefore, are unlikely to induce an antibody response or lose efficacy over time (“tachyphylaxis”) (14, 35, 42). Technical improvements have overcome most limitations of small peptides: instability, poor water solubility, the propensity to form aggregates, a short serum half-life, and limited cell entry. Partly as a result of these innovations, therapeutic peptides have been called the “sweet spot” between pharmaceutical agents and small molecules (17).
Small Blocking Peptides as Therapeutic Tools
Over 7,000 naturally occurring peptides have been identified: ≥500 peptide reagents are in preclinical trials, 140 are in active clinical trials, and 60 are currently approved for use as medications by the US Food and Drug Administration (17). Peptide aptamers have been primarily directed against extracellular targets and cell receptors. These targets include patients with type 2 diabetes mellitus (glucagon-like peptide 1), macular degeneration (anti-VEGF peptide), various cancers, Alzheimer's disease, prions that cause Creutzfeld-Jakob disease, Mycobacterium tuberculosis, hepatitis C, human immunodeficiency virus, and other viral infections (35). Peptide aptamers have also been used to treat disease states associated with anti-platelet-derived growth factor, von Willebrand factor, and anemia (14, 35, 42). In general, native peptides fail to cross cell membranes. The coupling of small blocking peptides to cell-penetrating peptides (CPP, e.g., penetratin and others) has opened the therapeutic door for intracellular peptides. Furthermore, amino acid substitutions at potential cleavage sites, chemical modifications (e.g., acylation), or conjugation (e.g., to albumin or polyethylene glycol) can increase water solubility, extend serum half-life, and minimize peptide aggregation (17). Additional modifications also permit oral (e.g., cyclosporine and desmopressin), transdermal, or nasal (vasopressin) peptide administration. This review focuses on the use of blocking peptides (aptamers, or mimotopes) to disrupt selected protein-protein interactions for the purpose of preventing and treating specific renal diseases.
Designing a Blocking Peptide
When the target sequence that mediates protein-protein interaction is known.
If the binding domain of two interacting proteins has been identified, the pathway to effective blocking peptide design is clear. The features of an ideal blocking peptide are outlined in Table 1. In theory, a single protein-protein interaction will be disrupted by a competitive blocking peptide directed against either of their binding domains. In more complex cases, multiple domains mediate protein-protein interaction. If the sequence of each binding domain is known, a cocktail of peptide mimotopes can be mixed and administered. This multiple-peptide approach has been successfully used to develop effective vaccines (28) and can be readily employed to disrupt the interaction between two proteins with multiple binding domains (see below). An ideal blocking peptide competitively interferes with protein-protein interaction with an affinity in the nano- or micromolar range (34), similar to the affinity of monoclonal antibodies (35) If the blocking peptide is effective at low dose (i.e., binding affinity is high), fewer off-target side effects are likely to be encountered. A variety of assays have been used to estimate binding affinity between the blocking peptide and its intended target domain in cell-free systems (10, 34). However, the ability of these assays to approximate the effectiveness of the same peptide in vivo is less clear.
Table 1.
Ideal blocking peptide characteristics
| Shortest amino acid sequence disrupting single protein-protein interaction |
| Adequate uptake by target cell/tissue |
| Short serum half-life |
| Prolonged biological half-life |
| Minimally antigenic (HLA haplotype matching might reduce antigenicity, especially if repeated peptide doses are administered) |
HLA, human leukocyte antigen.
When the target sequence that mediates protein-protein interaction is not known.
The domain(s) responsible for protein-protein interaction may be unknown. In this case, alternative approaches can be used to identify an effective blocking peptide. An increasing number of peptides that bind to individual protein targets are readily available in large online libraries (29). Usually, the substance used to screen a peptide library is referred to as the “target.” In contrast, the “template” represents the target’s binding partner. Peptide databases, composed of a large number of known peptides with therapeutic potential, are based on hundreds of published manuscripts using phage-displayed random peptide libraries. These libraries include combinatorial peptide catalogs that cast a “wide scientific net” or “biopanning,” a process akin to searching for “gold nuggets.” MimoDB, released in 2010, contains ≥15,000 peptides collected from >800 manuscripts using phage-displayed random peptide libraries that are grouped into 1,818 sets based on verified sequences and are organized by mimotope sets, target, template, library, and complex structure (29). This free MimoDB database (http://immunet.cn/mimodb) offers tools for simple and advanced peptide searches, supports the visualization of mimotope peptide structure, interfaces with BLAST for peptide sequence recognition, and provides alignment information. This bioinformatics approach allows investigators to anticipate potential off-target peptide effects. The primary goal of using these tools is to attain broad background information on the target, template, library, and predicted structure of each therapeutic blocking peptide. These databases can also provide the “denominator” for high-throughput screening of combinatorial peptide libraries by rapidly identifying candidate peptide sequences with biological activity.
As an alternative to the “do-it-yourself” approach, several commercial enterprises use their own large libraries to identify and synthesize effective peptides. These proprietary peptide libraries are powerful tools for screening large numbers of peptides in the search for a few blocking peptides with selective bioactivity. Several online companies also offer design tools that structure the investigative steps required to identify minimum-length active peptide sequences and critical amino acid residues needed to design blocking peptides. Once identified, the blocking peptide of interest can be designed using the strategy outlined below and then tested in virtually any experimental model system. High-performance liquid chromatography has been routinely used to confirm that the blocking peptide is ≥80% pure (70).
assessing protein-protein interaction.
Several approaches are available for assessing the inhibitory effect of a blocking peptide on protein-protein interaction. These include immunoprecipitation in cell lysates, colocalization in intact cells or tissue, fluorescence resonance energy transfer, yeast two-hybrid screening assays, and competitive binding assays (34, 72). Each approach has distinct advantages and limitations as outlined in Table 2. Once protein-protein interactions are confirmed, the domain that mediates this interaction with its binding partner can be determined. Although a technical description exceeds the scope of this review, a detailed approach for identifying interacting domains is described elsewhere (74).
Table 2.
Techniques confirming protein-protein interaction
| Immunoprecipitation |
| Advantages |
| 1. Uses commercially available antibodies (two) to detect both proteins in a single complex |
| 2. Amenable to chemical cross-linkers that stabilize protein-protein interaction |
| 3. Applicable to both baseline and stress conditions that initiate target protein-protein interaction |
| Disadvantages |
| 1. Dependent on antibody availability and specificity |
| 2. Protein isolation disrupts low-affinity protein-protein interactions |
| 3. Protein-protein interactions may be transient, limiting detection |
| 4. Cell fractionation potentially generates nonphysiological protein-protein interactions |
| 5. Site/location of protein-protein interaction not known |
| 6. Time-consuming (~1.5 days) |
| Colocalization by Immunohistochemistry |
| Advantages |
| 1. Uses commercially available antibodies (two) to colocalize potentially interacting proteins |
| 2. Potential interaction assessed in intact cell with intact structural compartments |
| 3. Site(s) of colocalization identified |
| 4. Applicable to both baseline and stress conditions that initiate target protein-protein interaction |
| 5. Cell fixation enhances compartmentation |
| 6. Inexpensive and relatively fast |
| Disadvantages |
| 1. Colocalization potentially overestimates physical protein-protein interaction |
| 2. Less sensitive than other techniques for low-affinity interactions |
| 3. May be inadequate for transient interactions |
| FRET |
| Advantages |
| 1. Uses commercially available fluorescent antibody pairs (donor:acceptor) with overlapping emission and excitation wavelengths to indicate close (~10–100 Å) physical proximity |
| 2. Assesses protein-protein interaction in intact cells |
| 3. Site(s) of colocalization identified |
| 4. Applicable to both baseline and stress conditions that initiate target protein-protein interaction |
| 5. Measures changes in distance between 2 moving proteins |
| 6. Inexpensive |
| Disadvantages |
| 1. False-positive result if proteins are proximate but fail to physically interact |
| 2. False-negative results possible |
| 3. Fluorescence may be pH-sensitive |
| Yeast 2-Hybrid Screening |
| Advantages |
| 1. Uses commercially available reagents termed “bait” and prey” to generate a fully functional galactosidase reporter enzyme only when 2 target proteins form a complex |
| 2. Detects in situ interactions in the native cellular environment |
| 3. Highly sensitive |
| 4. Amenable to high-throughput screening |
| Disadvantages |
| 1. Detects basal interactions, not amenable to selective stimuli or stressors that initiate de novo interactions |
| 2. Can be expensive and time-consuming |
| 3. Risk of false-positives |
| 4. Detects only binary (i.e., single) protein-protein interactions unless a 3-hybrid system or >1 “bait” is used |
FRET, fluorescence resonance energy transfer.
blocking peptide delivery and uptake in vitro.
Although some cell lines lack peptide receptors and are relatively resistant to peptide uptake (65), renal epithelial cells in general and proximal tubule epithelial cells in particular readily take up small peptides. Renal epithelial cells possess endocytic “machinery” and, in some cases, an apical brush border with a large surface area that facilitates the rapid uptake of filtered peptides from the tubular lumen (65).
blocking peptide administration in vivo.
Blocking peptides are typically much smaller than 30,000 Da and, therefore, readily undergo glomerular filtration (53). Once filtered, small peptides are rapidly and effectively absorbed from the lumen by tubular epithelial cells. In proximal tubule cells, the bulk of peptide uptake was attributed to the multifunctional megalin/tubulin endocytic or “protein scavenger” receptor (8, 65), which is also present in podocytes (52). However, megalin receptor knockout in mice decreases peptide uptake only by 20–65% (65), suggesting an additional mechanism for peptide uptake in these cells. In fact, proximal tubule cells also absorb labeled peptides via a pH-dependent H+-peptide symport pathway (23). Regardless of the uptake pathway(s), at least one study has shown that as much as 90% of a single intravenous or intraperitoneal dose of a radiolabeled peptide is detected in the kidneys (Table 3) (50). Although peptides are more easily administered to animals via the intraperitoneal route (66), many investigators prefer the intravenous route (50), which may be more desirable for administration of blocking peptides to humans.
Table 3.
Tissue-specific radiolabeled peptide uptake
| Tissue Type/CPP | ||||
|---|---|---|---|---|
| Time After 111InCl3 Injection | Kidney/NLS | Liver/R9 | Heart/Tat | Brain/Penetratin |
| 10 min | 81 | 51 | 7 | 0.9 |
| 1 h | 94 | 50 | 0.9 | 0.1 |
| 4 h | 88 | 54 | 0.8 | 0.1 |
Values are expressed as percent uptake. CPP, cell-penetrating peptide; NLS, nuclear-localizing sequence. [Adapted from Sarko et al. (50).]
Blocking Peptides in Select Renal Diseases
Peptide therapy for acute kidney injury.
Although acute tubular necrosis is classically regarded as the hallmark of acute ischemic renal injury in humans, necrosis severity in renal biopsy specimens fails to predict glomerular filtration rate, the need for renal replacement therapy, or organ recovery in native or transplanted kidneys (46). This suggests that renal epithelial cells die by alternative (i.e., nonnecrotic) pathways. Mitochondria have emerged as the “cellular CPU” with regard to processing death signals after stress (36). Outer mitochondrial membrane injury resulting in membrane pore transition is required to initiate apoptosis (40, 57), membrane pore transition-regulated necrosis (41) and may be crucial in other forms of regulated cell death (25, 31, 60). The balance between prolife and prodeath members of the B cell lymphoma 2 (Bcl-2) family determines the fate of renal epithelial cells subjected to metabolic stress in an in vitro model that resembles ischemia (47, 48) and likely contributes to organ dysfunction in renal ischemia in vivo (66–68). In the kidney, proapoptotic Bcl-2 proteins, including Bax, Bak, and Bid, have been implicated in ischemic injury (68, 69). Of these proapoptotic proteins, Bax has been most well characterized (36, 43, 57, 66–68), and its active form has been consistently detected after renal ischemia in vivo (43, 66–68). Even without a known mitochondrial localizing sequence, the bulk of cytosolic Bax translocates to mitochondrial membranes after a proapoptotic insult (43). This prompted a search for a potential “Bax chaperone.” Nearly simultaneously, two laboratories reported that nucleophosmin (NPM), a transcription regulatory protein that resides in the nucleolar region, bound to conformationally active Bax detected with a 6A7 epitope-specific antibody (33, 59). Our laboratory reported that in vitro and in vivo ischemia resulted in rapid, but transient, cytosolic NPM accumulation, Bax-NPM interaction, and accumulation of both proteins in isolated mitochondria (66). Distinct protein domains, rather than nonselective nuclear membrane injury, tightly regulate nuclear NPM exit and reentry after cell stress (45, 66). Cytosolic (but not nuclear) NPM content positively correlates with mitochondrial Bax accumulation and cell death in response to proapoptotic insults. This observation suggests that the protein-protein interaction in the cytosolic compartment of the renal epithelial cell contributes to its death (45, 66).
The Bax domain responsible for binding NPM has been identified (59). Thompson and colleges reported that a small blocking peptide exhibited similar potency in disrupting the Bax-NPM interaction and in affording cytoprotection as an antibody directed against the Bax site that mediates NPM binding (59). This observation suggests that disruption of the Bax-NPM interaction, in which Bax-mediated mitochondrial membrane injury and cell death occur, has potential therapeutic implications. We subsequently generated a small peptide that mimics the Bax binding domain for NPM fused to a small peptide leader that facilitates renal uptake by an unknown mechanism (a nuclear localizing sequence) and an acetyl group to slow peptide degradation (66). This Bax blocking peptide protected against apoptosis caused by metabolic stress in vitro and ischemic kidney injury in vivo (66). Both serum blood urea nitrogen and creatinine, markers of glomerular filtration rate, were 40% lower in mice treated with the Bax blocking peptide than in control mice (66). Importantly, survival was significantly improved in animals exposed to only a single dose of Bax blocking peptide (66). This therapeutic peptide decreases the immunoprecipitation of conformationally active Bax with NPM in renal cortical homogenates, showing “proof of principal” that this blocking peptide inhibits the Bax-NPM interaction in vivo (unpublished observations) (Fig. 1). Since apoptosis is stochastic and occurs over a prolonged time period (9, 26), we predict that the blocking peptide will be effective even if its administration is delayed until after the ischemic stress. With the assumption that an insult results in both cytosolic NPM translocation and conformational Bax activation (the 2 key steps required for NPM-Bax complex formation), we predict that the same blocking peptide would likely be effective in nonischemic forms of renal cell injury.
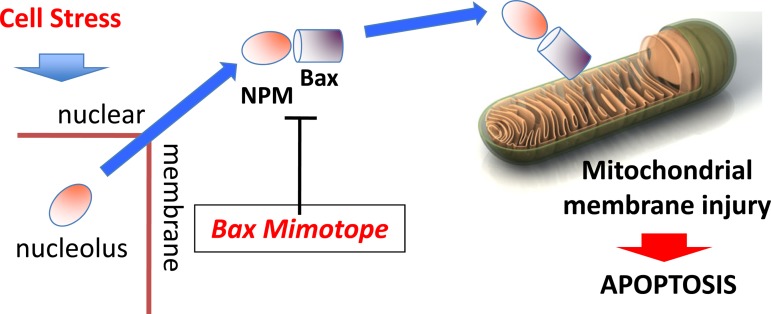
Fig. 1.
Bax-nucleophosmin (NPM) blocking peptide. In proximal tubule cells, proapoptotic stressors cause NPM to translocate from the nucleolar region into the cytosol. In the cytosolic compartment, NPM complexes with conformationally activated Bax (i.e., the NH2-terminal 6A7 epitope exposed); this complex accumulates in mitochondria, facilitating Bax delivery and outer membrane permeabilization, which promote cell death. Delivered to the kidney and absorbed by the renal epithelial cells, a small blocking peptide that mimics the Bax binding domain disrupts the Bax-NPM interaction, reducing mitochondrial Bax accumulation and organelle injury after an ischemic insult.
Blocking peptide therapy for membranous nephropathy.
Idiopathic membranous nephropathy (MN), a common cause of adult nephrotic syndrome, long represented an organ-specific autoimmune disease of unknown origin. Despite extensive investigation, identification of the target antigen that mediates this disease has been elusive. In a ground-breaking study, ~70% of patients with biopsy-proven idiopathic MN had detectable IgG antibodies, mostly of the IgG4 subclass, directed against the phospholipase A2 receptor-1 (PLA2R1) protein, in contrast to secondary forms of MN or other proteinuric diseases that lacked such autoantibodies (5). Thus, identification of PLA2R1 as the major autoimmune target in primary adult MN revolutionized this once idiopathic renal disease by suggesting that specific autoantibodies are most likely the cause, not a consequence, of podocyte injury. Specifically, interaction between PLA2R1 and the autoantibody induces podocyte injury, which causes proteinuria, a key hallmark of this renal disease (5).
PLA2R1 is a member of the mannose receptor (MR) family, a transmembrane glycoprotein with extracellular regions consisting of an NH2-terminal cysteine-rich (CysR) domain, a fibronectin II-type domain, and 8–10 C-type lectin-like domains (4). Recently, two laboratories characterized the PLA2R1 epitope domain responsible for binding the PLA2R1 autoantibody. Kao et al. showed that the key antibody-binding epitope is located within the three most NH2-terminal domains of the receptor and shares the reduction-sensitive properties initially reported in the intact molecule (30). Fresquet et al. subsequently localized the PLA2R1 antibody-binding epitope within the CysR domain (19). It had been assumed that a single interfering peptide might be sufficient to disrupt the interaction between PLA2R1 and its autoantibody. In contrast, Behnert et al. identified several linear blocking peptides (6). However, these blocking mimotopes only partially inhibited autoantibody binding to the nondenatured PLA2R1 molecule, even when used at a relatively high concentration (6). Fresquet et al. showed that a nine-amino acid blocking peptide inhibited only 47% of binding to a bacterial enzyme that shares homology with PLA2R1 (19). However, when the same blocking peptide was used, 90% of the human subjects included in the original cohort showed effective inhibition of the PLA2R1-autoantibody interaction in the in vitro assay (19). In 10% of MN patients, this same blocking peptide was less effective, suggesting that an additional epitope(s), perhaps in the COOH-terminal portion of the molecule, also mediates the PLA2R1-autoantibody interaction. If indeed the PLA2R1-autoantibody interaction is mediated by both major and minor domains, as recently suggested (54), then a “cocktail” of blocking peptides that interferes with multiple binding domains might enhance its efficacy in blocking the PLA2R1-autoantibody interaction (Fig. 2). Such a cocktail might also address epitope spreading, which occurs in some patients with idiopathic MN (54). Together, these preliminary observations support molecular mimicry as a cause of induced autoimmunity in MN and clearly suggest that therapeutic blocking peptides that disrupt the extracellular PLA2R1-autoantibody interaction may be a novel therapeutic intervention.
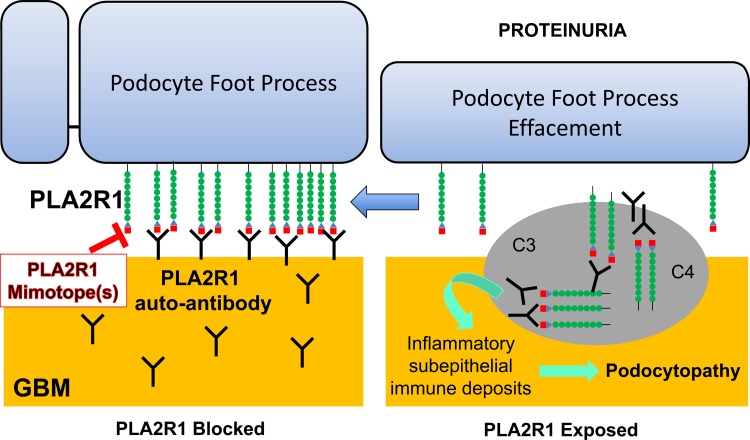
Fig. 2.
Phospholipase A2 (PLA2) receptor (PLA2R) blocking peptide. Podocytes express the receptor for PLA2 (PLA2R1), a transmembrane protein. Although the function of PLA2R1 is unknown, autoantibodies directed against one or more of its domains cause membranous nephropathy (MN), partly as a result of deposition of inflammatory, subepithelial immune deposits that result in complement-mediated podocyte injury with foot process effacement and proteinuria. Small blocking peptides, or “mimotopes,” that interfere with the interaction between PLA2R1 and its autoantibody could prevent immune complex formation to prevent or treat adult MN. GBM, glomerular basement membrane.`
Blocking peptide therapy for renal cell carcinoma.
In the United States, renal cell carcinoma is among the 10 most common causes of cancer in both women and men (1). A common, pathogenic cell-signaling pathway is likely, since ~90% of renal cancers are due to carcinomas (1) that arise from proximal tubule cells in ~75% of cases (20). Mutations in the von Hippel Lindau (VHL) gene are present in 90% of sporadic clear cell renal cancers (44). The VHL gene is well suited to be involved in renal tumorigenesis, since it regulates cell proliferation, survival, and angiogenesis. Loss of VHL function would predictably facilitate tumor growth by increasing blood supply, enhancing cancer cell proliferation, and increasing apoptosis. Increased apoptosis associated with VHL loss may promote genetic selection of more dangerous surviving clones of cells. In the past decade, a novel protein that interacts with the protein product of the VHL gene (pVHL) was identified and termed “gene for apoptosis and differentiation in epithelia” (Jade-1) (77). Jade-1 is a short-lived transcription factor and ubiquitin ligase with several downstream targets that regulate both cell proliferation and survival (Fig. 3). Normally, pVHL binds Jade-1, stabilizing and markedly increasing Jade-1 half-life (77). However, pVHL mutations (common in renal cell cancers) disrupt Jade-1 binding, resulting in Jade-1 destabilization (76). Failure of Jade-1 to bind mutant pVHL may facilitate greater interaction of Jade-1 with its major ubiquitin ligase Siah-1, a protein that marks Jade-1 for proteasomal degradation (18). Although Jade-1 is abundant in healthy proximal tubule cells, it is virtually undetectable in all renal cell cancer cell lines tested. These observations suggest a causal relationship between Jade-1 deficiency and renal cell cancer by one or more mechanisms. For example, Jade-1 decreases the level of Bcl-2 protein, a potent apoptosis inhibitor, resulting in a 40–50% increase in renal cell apoptosis (75). Jade-1 also prevents cell proliferation by inhibiting canonical Wnt signaling, a potent stimulus for cell proliferation. Jade-1 normally acts as a direct ubiquitin ligase for β-catenin, a primary driver of cell proliferation (11). Finally, the NH2-terminal end of Jade-1 binds to both the catalytic domain and the COOH-terminal regulatory tail of AKT, a prosurvival kinase, suggesting another mechanism by which Jade-1 normally prevents renal cell proliferation and promotes apoptosis (73). The importance of this pathway is supported by the observation that low JADE1 gene expression in clear cell renal carcinoma correlates with the activation of an AKT1 target gene signature and carries a poor prognosis (73). Thus unopposed growth of renal cell carcinoma is partly driven by the failure of mutant pVHL to stabilize Jade-1 and the direct loss of Jade-1-mediated antiproliferative effects in these cancerous epithelial cells.
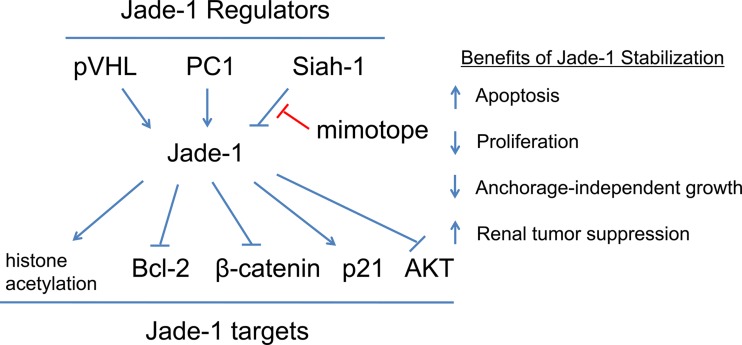
Fig. 3.
Jade-1 blocking peptide: schematic of regulators, targets, and functions of renal tumor suppressor protein Jade-1. Jade-1 is stabilized by the von Hippel-Lindau (VHL) protein (pVHL) and by polycystin-1 (PC1) and is marked for degradation by Siah-1, an E3 ubiquitin ligase. Jade-1 normally promotes apoptosis via its effect on Bcl-2, limits proliferation via β-catenin and p21, and inhibits AKT, a prosurvival kinase. A Jade-1 blocking peptide (“mimotope”) that prevents interaction between Jade-1 protein and its ubiquitin ligase Siah-1 would increase Jade-1 abundance, thereby facilitating its tumor suppressor function in renal cell carcinomas.
Although the precise Jade-1 amino acid sequence responsible for the binding ubiquitin ligase Siah-1 has not been identified, it resides in the Jade-1 NH2 terminus (18). The conceptual door is now sufficiently open to consider a therapeutic peptide that interferes with the interaction between Jade-1 and Siah-1. When available, this blocking peptide could prevent the ubiquitin ligase from binding to and promoting the degradation of Jade-1. Stabilization of Jade-1 in potentially cancerous proximal tubule cells might reverse unopposed cell growth in patients with VHL mutations at risk for renal cell cancer. Such a therapeutic Jade-1 blocking peptide might restore its multifactorial tumor suppressor effects by limiting both Wnt and AKT signaling, as well as by enhancing cancer cell apoptosis.
Curiously, the renal cysts present in VHL disease have pathogenetic features reminiscent of those in autosomal-dominant polycystic kidney disease. Recently, Jade-1 has been directly linked to polycystin-1, the protein encoded by the polycystic kidney disease (PKD1) gene (18). Similar to its interaction with pVHL, binding to polycystin-1 stabilizes Jade-1 by inhibiting its ubiquitination and degradation (18). It is therefore possible that a peptide that blocks the Jade-1-Siah-1 interaction could also be a useful tool for inhibiting cyst formation in this common monogenetic disorder that affects >12 million people worldwide (62).
Blocking Peptide Therapy for Proteinuric Renal Diseases
The glomerular epithelial cell, or podocyte, is viewed as “center of the universe” in proteinuric glomerular diseases, including focal and segmental glomerulosclerosis (FSGS) (1a, 22). Under normal circumstances, the triple barrier of the fenestrated endothelium, the glomerular basement membrane, and the podocyte form a selective filtration barrier that prevents pathological urinary protein loss (53). Biochemical and physical disruption of podocyte function usually impairs its foot processes, as well as the slit diaphragm, the final filtration barrier, or “protein gatekeeper” (53). Podocytopathy has been detected in both congenital and acquired forms of proteinuria. For example, mutations of nephrotic syndrome types 1 or 2 (NPHS1 or NPHS2) causes congenital forms of podocyte dysfunction, resulting in congenital nephrotic syndrome of the Finnish type and corticosteroid-resistant nephrotic syndrome in children as well as some genetic forms of FSGS (53, 64). Podocyte-specific proteins, including nephrin, podocin, WT1, CD2AP, ACTN4, INF2, PLCE1, ITGA3, MYO1E, and TRPC6, that encode for slit diaphragm components or ion channels or regulate podocyte differentiation, cytoskeletal dynamics, or cell survival have also been associated with proteinuric diseases in animals (13, 51, 53, 64). Despite these diverse biochemical etiologies of podocyte dysfunction, foot process disruption and “effacement” may represent the “final common pathway leading to proteinuric diseases” (49). This is likely due to the critical role of the semipermeable slit diaphragm in excluding albumin, based on both its size and charge selectivity (53). An intricate network of uniquely intertwined actin filaments forms elegant, “zipper-like” foot processes and also participates in transmitting signals from the extracellular slit diaphragm to the intracellular milieu. The morphology of a normal podocyte and its foot processes is shown in Fig. 4.
Fig. 4.
Podocyte and foot processes: key components of the slit diaphragm. The healthy glomerular epithelial cell, or “podocyte,” surrounds the glomerular capillaries and faces the urinary space; the podocyte has numerous branching projections that terminate in numerous, interdigitating foot processes maintained by a dynamic actin cytoskeleton; the interface between individual foot processes forms the slit diaphragm, which acts as a selective barrier through which glomerular filtrate passes but albumin is normally excluded. Loss of foot process architecture is associated with albuminuria. CB, cell body; MP, major (or primary) process; SP, secondary process; FP, foot process.
Although adult podocytes are terminally differentiated, nondividing epithelial cells, their foot processes undergo constant remodeling (3), which requires balanced polymerization and depolymerization of filamentous actin (15, 53). The status of the actin cytoskeleton is partly regulated by several key proteins, including nephrin, neph1, podocin, FAT1, p-cadherin, and other transmembrane proteins that serve as key scaffolding or cytoskeletal building blocks (1a, 53). Disruption of one or more of these structural components causes foot process effacement, a distinctive morphological change that positively correlates with proteinuria (12, 53).
Recent attention has focused on imbalance between actin assembly and disassembly as a primary cause of foot process effacement in podocytopathy (1a, 16, 22, 27). The foot process is maintained by short, branching actin filaments with parallel contractile filament bundles that contribute to an effective filtration barrier that prevents proteinuria (1a). In turn, actin cytoskeleton organization is regulated by GTPases of the Rho small G protein family that determine cell morphology, substrate adhesion, and motility (27). Rho family GTPases, such as RhoA, Cdc42, and Rac1, cycle between active GTP-bound and inactive GDP-bound forms. The absence or dysfunction of any one of these GTPases causes podocytopathy and proteinuria (22, 27), suggesting that Rho signaling contributes to foot process architecture under normal, as well as pathological, conditions.
Although the actin cytoskeleton has many structural and regulatory components, synaptopodin is a relatively highly expressed, actin-binding podocyte protein intimately related to proteinuria (16). Synaptopodin-deficient mice are highly susceptible to induced proteinuria and exhibit FSGS-like glomerular injury (16). Synaptopodin interacts with α-actinin to elongate actin filaments, suggesting that it is critical for podocyte cytoskeletal reorganization (2). Furthermore, synaptopodin expression in mature podocytes coincides with the appearance of actin bundles and foot process formation. In contrast, synaptopodin knockout impairs in vitro actin filament formation, likely due to the loss of its stabilizing effect on RhoA (2). Synaptopodin deficiency shifts the podocyte phenotype from a motile to a contractile form. This biochemical change and the accompanying phenotypic shift may contribute to chronic kidney disease progression (71).
Synaptopodin expression correlates with the severity of proteinuria. For example, preserved synaptopodin expression is detected in benign forms of proteinuria such as minimal change disease, whereas synaptopodin expression is relatively reduced in more serious conditions such as FSGS (27). L-cathepsin normally degrades synaptopodin at two small, but distinct, cleavage sites at amino acids 422–426 and 612–617 (16). The synaptopodin mutant most resistant to L-cathepsin-mediated degradation in vitro contains mutations at both sites, at least one of which is highly conserved in diverse species, including humans (16). This suggests that a blocking peptide, acting as a “decoy ligand,” could increase cell synaptopodin content by interfering with the synaptopodin-L-cathepsin interaction. On the basis of prior experimental observations, this increment in podocyte synaptopodin content would be predicted to stabilize the actin cytoskeleton, restore foot process architecture, and reduce proteinuria. Ideally, and on the basis of the elegant studies by Faul and colleagues (16), a cocktail composed of blocking peptides directed at both synaptopodin-binding domains would achieve the greatest resistance to L-cathepsin-mediated proteasomal degradation and result in the least proteinuria in models in which podocyte foot process effacement and slit diaphragm dysfunction are present. The known structural components of the glomerular filtration barrier, including synaptopodin, are summarized in Fig. 5.
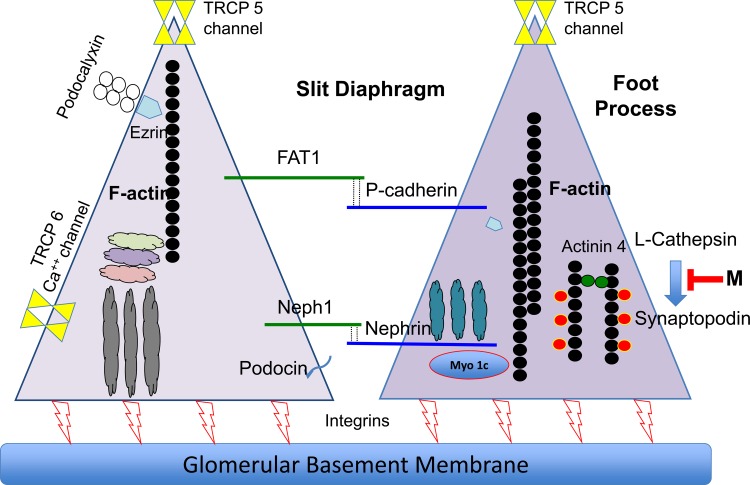
Fig. 5.
Synaptopodin-L-cathepsin blocking peptide: simplified schematic diagram of adjacent podocyte foot processes that comprise the slit diaphragm responsible for creating an albumin filtration barrier. A small peptide-blocking mimotope (“M”) that interferes with L-cathepsin-mediated synaptopodin degradation would be expected to increase podocyte synaptopodin, thereby stabilizing the actin cytoskeleton, which maintains normal foot process and slit diaphragm structure and extracellular signaling and, potentially, reduces proteinuria. Ideally, two blocking peptides would be required to maximally inhibit the synaptopodin-L-cathepsin interaction to effectively support dynamic actin cytoskeletal remodeling.
Importantly, foot process effacement is detected in acute and chronic glomerulopathies. Unfortunately, podocyte proliferation, similar to neuronal proliferation, is highly limited in the mature kidney (3). As a result, podocyte dropout may be a pathological hallmark in the progression from acute to chronic kidney disease (1a). Therefore, maneuvers that improve the structure and function of damaged podocytes are an attractive strategy for treating glomerular diseases associated with pathological albuminuria. Administration of targeted blocking peptides that restore the podocyte actin cytoskeleton may also have therapeutic potential in this common clinical setting. In addition, “therapeutic cross talk” (i.e., paracrine communication) between the podocyte and the glomerular endothelial cell is also likely, since proangiogenic factors secreted by podocytes determine the state of development, proliferation, and survival of local vascular endothelial cells that perfuse local glomeruli and tubules (24). Thus a reduction in podocytopathy could exert direct benefits on the filtration barrier that limit pathological proteinuria and indirectly limit vascular changes that contribute to the progression of chronic kidney disease.
Summary
Blocking peptide/mimotope therapy of renal disease is a biological reality: a number of blocking peptides are already in use by practicing nephrologists (17, 32). The rapid acceleration of proteomics with robust databases and search engines enables identification of specific protein-protein interactions of pathogenic significance to human kidney disease. Also, the kidney is an ideal mimotope target site, since its ample blood flow and the small size of these blocking peptides permit filtration and marked accumulation in renal epithelial cells. With fusion to structure- and cell-specific intrarenal targets, blocking peptides can be even more precisely and effectively delivered to the kidney, with the benefit of a relatively short serum half-life and minimal extrarenal peptide accumulation expected to minimize adverse or off-target effects. By testing this novel small-molecule approach in diverse renal diseases, the promise of new blocking peptides will soon be realized.
GRANTS
This work is supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-53387 (S. C. Borkan), DK-090143 (A. Havasi), and DK-078226 (W. Lu).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.H., W.L., H.T.C., L.H.B., C.I., and S.C.B. edited and revised the manuscript; W.L., H.T.C., L.H.B., and S.C.B. prepared the figures; W.L., H.T.C., L.H.B., and S.C.B. drafted the manuscript; W.L., H.T.C., L.H.B., and S.C.B. approved the final version of the manuscript.
REFERENCES
- 1.American Cancer Society Kidney Cancer (Adult)—Renal Cell Carcinoma (Overview). Atlanta, GA: Am. Cancer Soc, 2015. [Google Scholar]
- 1a.Arif E, Nihalani D. Podocytes as a therapeutic target. Ann Clin Exp Hypertens 1: 1004, 2013. [Google Scholar]
- 2.Asanuma K, Kim K, Oh J, Giardino L, Chabanis S, Faul C, Reiser J, Mundel P. Synaptopodin regulates the actin-bundling activity of α-actinin in an isoform-specific manner. J Clin Invest 115: 1188–1198, 2005. doi: 10.1172/JCI200523371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asanuma K, Mundel P. The role of podocytes in glomerular pathobiology. Clin Exp Nephrol 7: 255–259, 2003. doi: 10.1007/s10157-003-0259-6. [DOI] [PubMed] [Google Scholar]
- 4.Beck LH., Jr The dominant humoral epitope in phospholipase A2 receptor-1: presentation matters when serving up a slice of π. J Am Soc Nephrol 26: 237–239, 2015. doi: 10.1681/ASN.2014090877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beck LH Jr, Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, Klein JB, Salant DJ. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 361: 11–21, 2009. doi: 10.1056/NEJMoa0810457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Behnert A, Fritzler MJ, Teng B, Zhang M, Bollig F, Haller H, Skoberne A, Mahler M, Schiffer M. An anti-phospholipase A2 receptor quantitative immunoassay and epitope analysis in membranous nephropathy reveals different antigenic domains of the receptor. PLoS One 8: e61669, 2013v 10.1371/journal.pone.0061669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bunka DH, Stockley PG. Aptamers come of age - at last. Nat Rev Microbiol 4: 588–596, 2006. doi: 10.1038/nrmicro1458. [DOI] [PubMed] [Google Scholar]
- 8.Carone FA, Peterson DR. Hydrolysis and transport of small peptides by the proximal tubule. Am J Physiol Renal Physiol 238: F151–F158, 1980. [DOI] [PubMed] [Google Scholar]
- 9.Castaneda MP, Swiatecka-Urban A, Mitsnefes MM, Feuerstein D, Kaskel FJ, Tellis V, Devarajan P. Activation of mitochondrial apoptotic pathways in human renal allografts after ischemiareperfusion injury. Transplantation 76: 50–54, 2003. doi: 10.1097/01.TP.0000069835.95442.9F. [DOI] [PubMed] [Google Scholar]
- 10.Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, Colman PM, Day CL, Adams JM, Huang DC. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell 17: 393–403, 2005. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 11.Chitalia VC, Foy RL, Bachschmid MM, Zeng L, Panchenko MV, Zhou MI, Bharti A, Seldin DC, Lecker SH, Dominguez I, Cohen HT. Jade-1 inhibits Wnt signalling by ubiquitylating β-catenin and mediates Wnt pathway inhibition by pVHL. Nat Cell Biol 10: 1208–1216, 2008. doi: 10.1038/ncb1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chugh SS, Kaw B, Kanwar YS. Molecular structure-function relationship in the slit diaphragm. Semin Nephrol 23: 544–555, 2003. doi: 10.1053/S0270-9295(03)00137-2. [DOI] [PubMed] [Google Scholar]
- 13.Dryer SE, Reiser J. TRPC6 channels and their binding partners in podocytes: role in glomerular filtration and pathophysiology. Am J Physiol Renal Physiol 299: F689–F701, 2010. doi: 10.1152/ajprenal.00298.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan Q, Leuther KK, Holmes CP, Fong KL, Zhang J, Velkovska S, Chen MJ, Mortensen RB, Leu K, Green JM, Schatz PJ, Woodburn KW. Preclinical evaluation of Hematide, a novel erythropoiesis stimulating agent, for the treatment of anemia. Exp Hematol 34: 1303–1311, 2006. doi: 10.1016/j.exphem.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 15.Fan X, Li Q, Pisarek-Horowitz A, Rasouly HM, Wang X, Bonegio RG, Wang H, McLaughlin M, Mangos S, Kalluri R, Holzman LB, Drummond IA, Brown D, Salant DJ, Lu W. Inhibitory effects of Robo2 on nephrin: a crosstalk between positive and negative signals regulating podocyte structure. Cell Rep 2: 52–61, 2012. doi: 10.1016/j.celrep.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faul C, Donnelly M, Merscher-Gomez S, Chang YH, Franz S, Delfgaauw J, Chang JM, Choi HY, Campbell KN, Kim K, Reiser J, Mundel P. The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat Med 14: 931–938, 2008. doi: 10.1038/nm.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fosgerau K, Hoffmann T. Peptide therapeutics: current status and future directions. Drug Discov Today 20: 122–128, 2015. doi: 10.1016/j.drudis.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Foy RL, Chitalia VC, Panchenko MV, Zeng L, Lopez D, Lee JW, Rana SV, Boletta A, Qian F, Tsiokas L, Piontek KB, Germino GG, Zhou MI, Cohen HT. Polycystin-1 regulates the stability and ubiquitination of transcription factor Jade-1. Hum Mol Genet 21: 5456–5471, 2012. doi: 10.1093/hmg/dds391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fresquet M, Jowitt TA, Gummadova J, Collins R, O’Cualain R, McKenzie EA, Lennon R, Brenchley PE. Identification of a major epitope recognized by PLA2R autoantibodies in primary membranous nephropathy. J Am Soc Nephrol 26: 302–313, 2015. doi: 10.1681/ASN.2014050502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fu L, Wang G, Shevchuk MM, Nanus DM, Gudas LJ. Activation of HIF2α in kidney proximal tubule cells causes abnormal glycogen deposition but not tumorigenesis. Cancer Res 73: 2916–2925, 2013. doi: 10.1158/0008-5472.CAN-12-3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geysen HM, Rodda SJ, Mason TJ. A priori delineation of a peptide which mimics a discontinuous antigenic determinant. Mol Immunol 23: 709–715, 1986. doi: 10.1016/0161-5890(86)90081-7. [DOI] [PubMed] [Google Scholar]
- 22.Greka A, Mundel P. Cell biology and pathology of podocytes. Annu Rev Physiol 74: 299–323, 2012. doi: 10.1146/annurev-physiol-020911-153238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Groneberg DA, Döring F, Nickolaus M, Daniel H, Fischer A. Renal assimilation of short chain peptides: visualization of tubular peptide uptake. Pharm Res 19: 1209–1214, 2002. doi: 10.1023/A:1019810512519. [DOI] [PubMed] [Google Scholar]
- 24.Haege S, Einer C, Thiele S, Mueller W, Nietzsche S, Lupp A, Mackay F, Schulz S, Stumm R. CXC chemokine receptor 7 (CXCR7) regulates CXCR4 protein expression and capillary tuft development in mouse kidney. PLoS One 7: e42814, 2012. doi: 10.1371/journal.pone.0042814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Handke W, Krause E, Brune W. Live or let die: manipulation of cellular suicide programs by murine cytomegalovirus. Med Microbiol Immunol 201: 475–486, 2012. doi: 10.1007/s00430-012-0264-z. [DOI] [PubMed] [Google Scholar]
- 26.Havasi A, Borkan SC. Apoptosis and acute kidney injury. Kidney Int 80: 29–40, 2011. doi: 10.1038/ki.2011.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He FF, Chen S, Su H, Meng XF, Zhang C. Actin-associated proteins in the pathogenesis of podocyte injury. Curr Genomics 14: 477–484, 2013. doi: 10.2174/13892029113146660014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsu SC, Chargelegue D, Obeid OE, Steward MW. Synergistic effect of immunization with a peptide cocktail inducing antibody, helper and cytotoxic T-cell responses on protection against respiratory syncytial virus. J Gen Virol 80: 1401–1405, 1999. doi: 10.1099/0022-1317-80-6-1401. [DOI] [PubMed] [Google Scholar]
- 29.Huang J, Ru B, Zhu P, Nie F, Yang J, Wang X, Dai P, Lin H, Guo FB, Rao N. MimoDB 2.0: a mimotope database and beyond. Nucleic Acids Res 40: D271–D277, 2012. doi: 10.1093/nar/gkr922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kao L, Lam V, Waldman M, Glassock RJ, Zhu Q. Identification of the immunodominant epitope region in phospholipase A2 receptor-mediating autoantibody binding in idiopathic membranous nephropathy. J Am Soc Nephrol 26: 291–301, 2015. doi: 10.1681/ASN.2013121315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karch J, Kanisicak O, Brody MJ, Sargent MA, Michael DM, Molkentin JD. Necroptosis Interfaces with MOMP and the MPTP in mediating cell death. PLoS One 10: e0130520, 2015. doi: 10.1371/journal.pone.0130520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaspar AA, Reichert JM. Future directions for peptide therapeutics development. Drug Discov Today 18: 807–817, 2013. doi: 10.1016/j.drudis.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 33.Kerr LE, Birse-Archbold JL, Short DM, McGregor AL, Heron I, Macdonald DC, Thompson J, Carlson GJ, Kelly JS, McCulloch J, Sharkey J. Nucleophosmin is a novel Bax chaperone that regulates apoptotic cell death. Oncogene 26: 2554–2562, 2007. doi: 10.1038/sj.onc.1210044. [DOI] [PubMed] [Google Scholar]
- 34.Kessel C, Kreuz W, Klich K, Becker-Peters K, Vorpahl F, Dietrich U, Klingebiel T, Königs C. Multimerization of peptide mimotopes for blocking of factor VIII neutralizing antibodies. ChemMedChem 4: 1364–1370, 2009. doi: 10.1002/cmdc.200900023. [DOI] [PubMed] [Google Scholar]
- 35.Khati M. The future of aptamers in medicine. J Clin Pathol 63: 480–487, 2010. doi: 10.1136/jcp.2008.062786. [DOI] [PubMed] [Google Scholar]
- 36.Kim R, Emi M, Tanabe K. Role of mitochondria as the gardens of cell death. Cancer Chemother Pharmacol 57: 545–553, 2006. doi: 10.1007/s00280-005-0111-7. [DOI] [PubMed] [Google Scholar]
- 37.Kim S, Chin HJ, Na KY, Kim S, Oh J, Chung W, Noh JW, Lee YK, Cho JT, Lee EK, Chae DW; Progressive Renal Disease and Medical Informatics and Genomics Research (PREMIER) members . Single nucleotide polymorphisms in the phospholipase A2 receptor gene are associated with genetic susceptibility to idiopathic membranous nephropathy. Nephron Clin Pract 117: c253–c258, 2011. doi: 10.1159/000320194. [DOI] [PubMed] [Google Scholar]
- 38.Knittelfelder R, Riemer AB, Jensen-Jarolim E. Mimotope vaccination--from allergy to cancer. Expert Opin Biol Ther 9: 493–506, 2009. doi: 10.1517/14712590902870386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Königs C, Pustowka A, Irving J, Kessel C, Klich K, Wegner V, Rowley MJ, Mackay IR, Kreuz W, Griesinger C, Dietrich U. Peptide mimotopes selected with HIV-1-blocking monoclonal antibodies against CCR5 represent motifs specific for HIV-1 entry. Immunol Cell Biol 85: 511–517, 2007. doi: 10.1038/sj.icb.7100077. [DOI] [PubMed] [Google Scholar]
- 40.Li F, Mao HP, Ruchalski KL, Wang YH, Choy W, Schwartz JH, Borkan SC. Heat stress prevents mitochondrial injury in ATP-depleted renal epithelial cells. Am J Physiol Cell Physiol 283: C917–C926, 2002. doi: 10.1152/ajpcell.00517.2001. [DOI] [PubMed] [Google Scholar]
- 41.Linkermann A, Chen G, Dong G, Kunzendorf U, Krautwald S, Dong Z. Regulated cell death in AKI. J Am Soc Nephrol 25: 2689–2701, 2014. doi: 10.1681/ASN.2014030262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Macdougall IC, Rossert J, Casadevall N, Stead RB, Duliege AM, Froissart M, Eckardt KU. A peptide-based erythropoietin-receptor agonist for pure red-cell aplasia. N Engl J Med 361: 1848–1855, 2009. doi: 10.1056/NEJMoa074037. [DOI] [PubMed] [Google Scholar]
- 43.Mikhailov V, Mikhailova M, Pulkrabek DJ, Dong Z, Venkatachalam MA, Saikumar P. Bcl-2 prevents Bax oligomerization in the mitochondrial outer membrane. J Biol Chem 276: 18361–18374, 2001. doi: 10.1074/jbc.M100655200. [DOI] [PubMed] [Google Scholar]
- 44.Nickerson ML, Jaeger E, Shi Y, Durocher JA, Mahurkar S, Zaridze D, Matveev V, Janout V, Kollarova H, Bencko V, Navratilova M, Szeszenia-Dabrowska N, Mates D, Mukeria A, Holcatova I, Schmidt LS, Toro JR, Karami S, Hung R, Gerard GF, Linehan WM, Merino M, Zbar B, Boffetta P, Brennan P, Rothman N, Chow WH, Waldman FM, Moore LE. Improved identification of von Hippel-Lindau gene alterations in clear cell renal tumors. Clin Cancer Res 14: 4726–4734, 2008. doi: 10.1158/1078-0432.CCR-07-4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rau R, Brown P. Nucleophosmin (NPM1) mutations in adult and childhood acute myeloid leukaemia: towards definition of a new leukaemia entity. Hematol Oncol 27: 171–181, 2009. doi: 10.1002/hon.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosen S, Heyman SN. Difficulties in understanding human “acute tubular necrosis”: limited data and flawed animal models. Kidney Int 60: 1220–1224, 2001. doi: 10.1046/j.1523-1755.2001.00930.x. [DOI] [PubMed] [Google Scholar]
- 47.Ruchalski K, Mao H, Li Z, Wang Z, Gillers S, Wang Y, Mosser DD, Gabai V, Schwartz JH, Borkan SC. Distinct hsp70 domains mediate apoptosis-inducing factor release and nuclear accumulation. J Biol Chem 281: 7873–7880, 2006. doi: 10.1074/jbc.M513728200. [DOI] [PubMed] [Google Scholar]
- 48.Ruchalski K, Mao H, Singh SK, Wang Y, Mosser DD, Li F, Schwartz JH, Borkan SC. HSP72 inhibits apoptosis-inducing factor release in ATP-depleted renal epithelial cells. Am J Physiol Cell Physiol 285: C1483–C1493, 2003. doi: 10.1152/ajpcell.00049.2003. [DOI] [PubMed] [Google Scholar]
- 49.Saleem MA, Ni L, Witherden I, Tryggvason K, Ruotsalainen V, Mundel P, Mathieson PW. Co-localization of nephrin, podocin, and the actin cytoskeleton: evidence for a role in podocyte foot process formation. Am J Pathol 161: 1459–1466, 2002. doi: 10.1016/S0002-9440(10)64421-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sarko D, Beijer B, Garcia Boy R, Nothelfer EM, Leotta K, Eisenhut M, Altmann A, Haberkorn U, Mier W. The pharmacokinetics of cell-penetrating peptides. Mol Pharm 7: 2224–2231, 2010. doi: 10.1021/mp100223d. [DOI] [PubMed] [Google Scholar]
- 51.Schaldecker T, Kim S, Tarabanis C, Tian D, Hakroush S, Castonguay P, Ahn W, Wallentin H, Heid H, Hopkins CR, Lindsley CW, Riccio A, Buvall L, Weins A, Greka A. Inhibition of the TRPC5 ion channel protects the kidney filter. J Clin Invest 123: 5298–5309, 2013. doi: 10.1172/JCI71165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schießl IM, Hammer A, Kattler V, Gess B, Theilig F, Witzgall R, Castrop H. Intravital imaging reveals angiotensin II-induced transcytosis of albumin by podocytes. J Am Soc Nephrol 27: 731–744, 2016. doi: 10.1681/ASN.2014111125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scott RP, Quaggin SE. Review series: The cell biology of renal filtration. J Cell Biol 209: 199–210, 2015. doi: 10.1083/jcb.201410017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seitz-Polski B, Dolla G, Payré C, Girard CA, Polidori J, Zorzi K, Birgy-Barelli E, Jullien P, Courivaud C, Krummel T, Benzaken S, Bernard G, Burtey S, Mariat C, Esnault VL, Lambeau G. Epitope spreading of autoantibody response to PLA2R associates with poor prognosis in membranous nephropathy. J Am Soc Nephrol 27: 1517–1533, 2016. doi: 10.1681/ASN.2014111061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith GP, Petrenko VA. Phage Display. Chem Rev 97: 391–410, 1997. doi: 10.1021/cr960065d. [DOI] [PubMed] [Google Scholar]
- 57.Szeto HH, Liu S, Soong Y, Wu D, Darrah SF, Cheng FY, Zhao Z, Ganger M, Tow CY, Seshan SV. Mitochondria-targeted peptide accelerates ATP recovery and reduces ischemic kidney injury. J Am Soc Nephrol 22: 1041–1052, 2011. doi: 10.1681/ASN.2010080808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thom G, Cockroft AC, Buchanan AG, Candotti CJ, Cohen ES, Lowne D, Monk P, Shorrock-Hart CP, Jermutus L, Minter RR. Probing a protein-protein interaction by in vitro evolution. Proc Natl Acad Sci U S A 103: 7619–7624, 2006. doi: 10.1073/pnas.0602341103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thompson J, Finlayson K, Salvo-Chirnside E, MacDonald D, McCulloch J, Kerr L, Sharkey J. Characterisation of the Bax-nucleophosmin interaction: the importance of the Bax C-terminus. Apoptosis 13: 394–403, 2008. doi: 10.1007/s10495-007-0177-2. [DOI] [PubMed] [Google Scholar]
- 60.Thornton C, Hagberg H. Role of mitochondria in apoptotic and necroptotic cell death in the developing brain. Clin Chim Acta 451, Pt A: 35–38, 2015. doi: 10.1016/j.cca.2015.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tong AH, Drees B, Nardelli G, Bader GD, Brannetti B, Castagnoli L, Evangelista M, Ferracuti S, Nelson B, Paoluzi S, Quondam M, Zucconi A, Hogue CW, Fields S, Boone C, Cesareni G. A combined experimental and computational strategy to define protein interaction networks for peptide recognition modules. Science 295: 321–324, 2002. doi: 10.1126/science.1064987. [DOI] [PubMed] [Google Scholar]
- 62.Torres VE, Harris PC, Pirson Y. Autosomal dominant polycystic kidney disease. Lancet 369: 1287–1301, 2007. doi: 10.1016/S0140-6736(07)60601-1. [DOI] [PubMed] [Google Scholar]
- 63.Trabuco LG, Lise S, Petsalaki E, Russell RB. PepSite: prediction of peptide-binding sites from protein surfaces. Nucleic Acids Res 40: W423–W427, 2012. doi: 10.1093/nar/gks398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tryggvason K, Patrakka J, Wartiovaara J. Hereditary proteinuria syndromes and mechanisms of proteinuria. N Engl J Med 354: 1387–1401, 2006. doi: 10.1056/NEJMra052131. [DOI] [PubMed] [Google Scholar]
- 65.Vegt E, Melis M, Eek A, de Visser M, Brom M, Oyen WJ, Gotthardt M, de Jong M, Boerman OC. Renal uptake of different radiolabelled peptides is mediated by megalin: SPECT and biodistribution studies in megalin-deficient mice. Eur J Nucl Med Mol Imaging 38: 623–632, 2011. doi: 10.1007/s00259-010-1685-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Z, Gall JM, Bonegio R, Havasi A, Illanes K, Schwartz JH, Borkan SC. Nucleophosmin, a critical Bax cofactor in ischemia-induced cell death. Mol Cell Biol 33: 1916–1924, 2013. doi: 10.1128/MCB.00015-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang Z, Gall JM, Bonegio RG, Havasi A, Hunt CR, Sherman MY, Schwartz JH, Borkan SC. Induction of heat shock protein 70 inhibits ischemic renal injury. Kidney Int, 2011. doi: 10.1038/ki.2010.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wei Q, Dong G, Chen JK, Ramesh G, Dong Z. Bax and Bak have critical roles in ischemic acute kidney injury in global and proximal tubule-specific knockout mouse models. Kidney Int 84: 138–148, 2013. doi: 10.1038/ki.2013.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wei Q, Yin XM, Wang MH, Dong Z. Bid deficiency ameliorates ischemic renal failure and delays animal death in C57BL/6 mice. Am J Physiol Renal Physiol 290: F35–F42, 2006. doi: 10.1152/ajprenal.00184.2005. [DOI] [PubMed] [Google Scholar]
- 70.Xu S-Z. Assessing TRPC channel function using pore-blocking antibodies. In: TRP Channels, edited by Zhu M. Boca Raton, FL: CRC/Taylor & Francis, 2011. [PubMed] [Google Scholar]
- 71.Yanagida-Asanuma E, Asanuma K, Kim K, Donnelly M, Young Choi H, Hyung Chang J, Suetsugu S, Tomino Y, Takenawa T, Faul C, Mundel P. Synaptopodin protects against proteinuria by disrupting Cdc42:IRSp53:Mena signaling complexes in kidney podocytes. Am J Pathol 171: 415–427, 2007. doi: 10.2353/ajpath.2007.070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Young KH. Yeast two-hybrid: so many interactions, (in) so little time. . . . Biol Reprod 58: 302–311, 1998. doi: 10.1095/biolreprod58.2.302. [DOI] [PubMed] [Google Scholar]
- 73.Zeng L, Bai M, Mittal AK, El-Jouni W, Zhou J, Cohen DM, Zhou MI, Cohen HT. Candidate tumor suppressor and pVHL partner Jade-1 binds and inhibits AKT in renal cell carcinoma. Cancer Res 73: 5371–5380, 2013. doi: 10.1158/0008-5472.CAN-12-4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhao XM, Chen L, Aihara K. A discriminative approach for identifying domain-domain interactions from protein-protein interactions. Proteins 78: 1243–1253, 2010. doi: 10.1002/prot.22643. [DOI] [PubMed] [Google Scholar]
- 75.Zhou MI, Foy RL, Chitalia VC, Zhao J, Panchenko MV, Wang H, Cohen HT. Jade-1, a candidate renal tumor suppressor that promotes apoptosis. Proc Natl Acad Sci U S A 102: 11035–11040, 2005. doi: 10.1073/pnas.0500757102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou MI, Wang H, Foy RL, Ross JJ, Cohen HT. Tumor suppressor von Hippel-Lindau (VHL) stabilization of Jade-1 protein occurs through plant homeodomains and is VHL mutation dependent. Cancer Res 64: 1278–1286, 2004. doi: 10.1158/0008-5472.CAN-03-0884. [DOI] [PubMed] [Google Scholar]
- 77.Zhou MI, Wang H, Ross JJ, Kuzmin I, Xu C, Cohen HT. The von Hippel-Lindau tumor suppressor stabilizes novel plant homeodomain protein Jade-1. J Biol Chem 277: 39887–39898, 2002. doi: 10.1074/jbc.M205040200. [DOI] [PubMed] [Google Scholar]