Abstract
Kidney repair following injury involves the reconstitution of a structurally and functionally intact tubular epithelium. Growth factors and their receptors, such as EGFR, are important in the repair of renal tubules. Exosomes are cell-produced small (~100 nm in diameter) vesicles that contain and transfer proteins, lipids, RNAs, and DNAs between cells. In this study, we examined the relationship between exosome production and EGFR activation and the potential role of exosome in wound healing. EGFR activation occurred shortly after scratch wounding in renal tubular cells. Wound repair after scratching was significantly promoted by EGF and suppressed by EGFR inhibitor gefitinib. Interestingly, scratch wounding induced a significant increase of exosome production. The exosome production was decreased by EGF and increased by gefitinib, suggesting a suppressive role of EGFR signaling in exosome production. Conversely, inhibition of exosome release by GW4869 and manumycin A markedly increased EGFR activation and promoted wound healing. Moreover, exosomes derived from scratch-wounding cells could inhibit wound healing. Collectively, the results indicate that wound healing in renal tubular cells is associated with EGFR activation and exosome production. Although EGFR activation promotes wound healing, released exosomes may antagonize EGFR activation and wound healing.
Keywords: exosomes, EGFR, wound healing, kidney
reepithelialization is an essential process of wound healing (24). Epidermal growth factors (EGF) and their receptors, EGFR, are important in a variety of cellular processes, including cell migration and reepithelialization during wound healing (18). EGFR signaling contributes to tubular repair following kidney injury (36). Exosomes from kidney are 30- to 100-nm extracellular membrane vesicles secreted by all segments of the nephron, including proximal tubule, distal tubule, and the collecting duct (26). Exosome production occurs via an endosomal sorting complex required for transport (ESCRT)-dependent or -independent mechanism in different types of cells (29). The release of exosomes is associated with the activation of specific cell surface receptors and a number of enzymes (5, 6, 9). These small vesicles have been reported to carry proteins, lipids, mRNA, and microRNAs and to facilitate transfer of genetic information between cells (27). As such, changes and dysfunction in exosomes may be associated with the development of diseases (7, 13, 20, 30).
A role of exosomes has been suggested in kidney disease models (35). However, diverse and sometimes discordant biological effects of exosomes have been described in these models (3, 22). Therefore, it remains obscure whether these vesicles are in fact beneficial or detrimental to kidney tissues. In previous studies, exosomes derived from mesenchymal stromal cells of different sources have been shown to facilitate kidney repair from acute injury (4, 28). However, the effect of exosomes derived from parenchymal kidney cells, especially renal tubular cells, on repair is still unclear. In this study, we characterized exosome production in a scratch-wound healing model of renal tubular cells, analyzed the relationship between exosome production and EGFR activation, and investigated the effect of exosomes derived from wounded renal tubular cells on the healing processes.
MATERIALS AND METHODS
Antibodies and special reagents.
Antibodies and reagents were purchased from the following sources: monoclonal anti-EGFR and phospho-EGFR from Cell Signaling Technology (Danvers, MA); monoclonal anti-CD63 and TSG101 from System Biosciences (Palo Alto, CA); secondary antibodies for immunoblot analysis from Jackson ImmunoResearch (West Grove, PA); GW4869 from Sigma (St. Louis, MO); manumycin A from Cayman Chemical; exosome-depleted FBS from GIBCO; EGF from Sigma; and gefitinib from Cell Signaling Technology.
Cell culture.
The Boston University mouse proximal tubular (BUMPT) cell line was originally obtained from Drs. Lieberthal and Schwartz and grown in DMEM with 10% FBS or 2% exosome-depleted FBS. The cultured cells were maintained at 37°C in an atmosphere of 5% CO2. BUMPT cells were seeded in 60-mm dishes and cultured in DMEM with 10% FBS and then treated with 20 ng/ml EGF or 1 μM gefitinib with 2% exosome-depleted FBS. In some experiments, BUMPT cells were pretreated with 10 μM GW4869 and 1 μM manumycin A with 2% exosome-depleted FBS for 2 h. The culture medium was collected for exosome isolation.
Scratch-wound healing assay.
Multiple uniformed wounds were created in 60-mm dishes with a wounding device (17). Phase-contrast images were recorded after scratching. The width of the wound was measured at various time points to determine the healed distance. Whole cell lysate was collected after wounding for immunoblot analysis. To study the effect of exosomes, a modified scratch-wound healing model was done according to the previous study (37). In brief, BUMPT cells grown in 12-well plates with 2% exosome-depleted FBS were linearly scratched with a sterile 200-μl pipette tip. Twenty micrograms of exosomes was added in a plate. The width of the wound was measured after scratching to determine the healed distance.
Exosome isolation and characterization.
Exosomes were isolated from culture media using traditional serial centrifugation (800 g, 10 min; 2,000 g, 20 min; 10,000 g, 30 min). Subsequently, the supernatants were centrifuged at 100,000 g for 70 min (Optima L-80 XP Ultracentrifuge with SW 55 Ti Rotor; Beckman Coulter) followed by washing with phosphate-buffered saline (PBS) and purification by centrifugation at 100,000 g for 70 min. Exosomes were resuspended in 1× PBS and stored at −80°C. Nanoparticle-tracking analysis (NTA), transmission electron microscopy (TEM), and Western blots were used to identify exosomes.
Nanoparticle-tracking analysis (NTA).
Using nanoparticle-tracking analysis (NTA) with ZetaView (Particle Metrix), we analyzed the size distribution and measured the concentration of isolated exosomes. Isolated exosomes were diluted 200 times in particle-free PBS and then injected into chamber. The size distributions and vesicle concentrations were assessed with NTA software as previously described (7, 12, 32).
Transmission electron microscopy (TEM).
For the TEM morphology investigation, 15 μl of resuspended exosomes was placed on a carbon/Formvar-coated, 200-mesh, copper grid, incubated for 1 min at room temperature, and then subjected to standard uranyl acetate staining. Grids were allowed to air dry before being examined in a JEM-1230 transmission electron microscope (JEOL USA, Peabody, MA) at 110 kV and imaged with an UltraScan 4000 charge-coupled device (CCD) camera and First Light digital camera controller (Gatan, Pleasanton, CA). TEM sample preparation and imaging were performed at the Electron Microscopy and Histology Core laboratory at Augusta University (http://www.augusta.edu/mcg/cba/emhisto/).
Western blotting analysis.
Cell and exosomal protein was extracted with 2% SDS buffer. The total protein concentration was quantified with a BCA protein assay kit (Thermo Scientific). Protein was separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride (PVDF) membrane. The membrane was blocked for 1 h at room temperature with 5% bovine serum albumin and then immunoblotted with primary antibody. The blot membrane was washed three times and subsequently incubated with horseradish peroxidase-conjugated secondary antibodies. The blot signal was revealed with a chemiluminescence kit (Thermo Scientific).
Statistical analysis.
All values are expressed as means ± SD. Statistical analysis was conducted by the GraphPad Prism software (San Diego, CA). Comparison between two groups were performed by Student's t-test. For multiple comparisons, ANOVA was used. P < 0.05 was considered significant.
RESULTS
EGFR activation during wound healing in BUMPT cells.
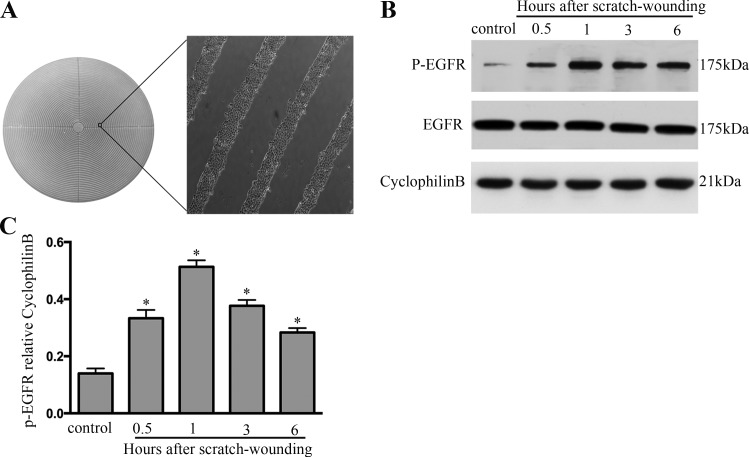
To understand the mechanism of wound healing, we initially focused on EGFR, which is involved in the repair after injury. Multiple wounds were created in BUMPT with a “stamp-wounding” device (17), which generated multiple uniformed wounds in the cell layer (Fig. 1A). EGFR activation shown by its phosphorylation was induced during wound healing in BUMPT (Fig. 1B). Marked EGFR activation was detected immediately after wounding, and the induction reached the highest level at 1 h. Thereafter, EGFR decreased, but it remained higher than the control at 6 h. The changes in EGFR expression during scratch-wound healing were further confirmed by quantification via densitometry (Fig. 1C).
Fig. 1.
EGFR induction during wound healing. A: multiple uniform wounds made with a wounding device. BUMPT grown in 60-mm dishes were pressed with a multiple-wounding device to induce circular cell bands. B: EGFR induction at different time points during wound healing. BUMPT in 60-mm dishes were wounded by the multiple-wounding device and then to collect whole cell lysates for immunoblot analysis of EGFR and phosphorylated (P-) EGFR. C: densitometric analysis of P-EGFR expression during scratch-wound healing. *P < 0.05 vs. control group.
Exosome induction during wound healing in BUMPT cells.
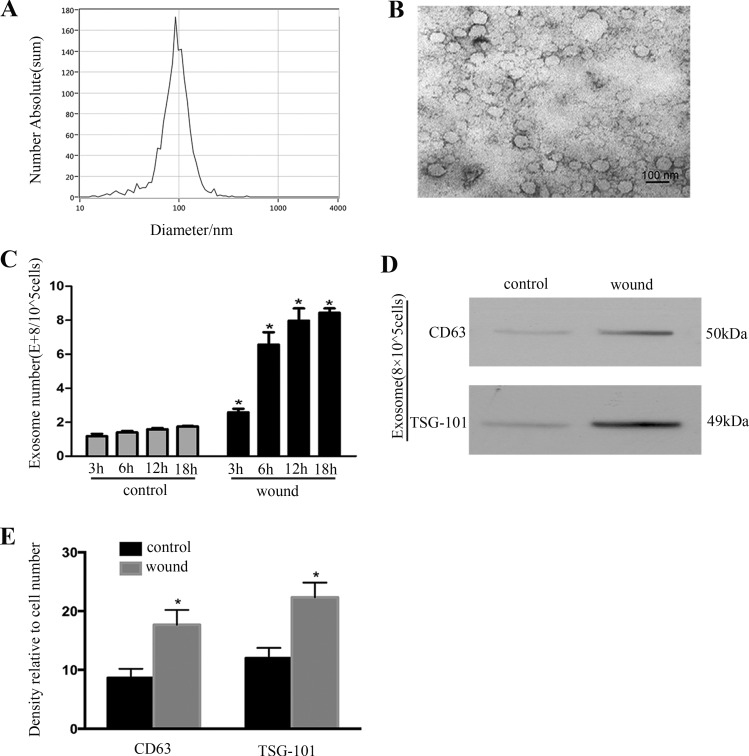
We examined exosome release by NTA, TEM, and immunoblot analysis of exosome markers (CD63 and TSG101) after scratch wounding in culture medium from BUMPT cells. Exosomes were isolated from the same number of cells. As shown in Fig. 2, A and B, the size of exosomes was ~100 nm. The number of released exosomes was dramatically increased after wounding in a time-dependent manner (Fig. 2C). The result was confirmed by evaluating the CD63 and TSG101 of immunoblots (Fig. 2D) and was further confirmed by quantification via densitometry (Fig. 2E).
Fig. 2.
Exosome production during wound healing. A: nanoparticle tracking analysis of isolated exosomes. The calculated size distribution is shown as a mean, peak at 100 nm. B: representative electron microscopic image of exosomes isolated from BUMPT showing the typical cup-shaped morphology. C: nanoparticle tracking analysis of isolated exosomes. The calculated number is shown. *P < 0.05 vs. control group at the same time point. D: representative Western blot analysis of exosomal markers (CD63 and TSG101). The expression of CD63 and TSG101 from the same number of cells in wound group is much higher than control group. E: densitometric analysis of CD63 and TSG101 expression. *P < 0.05 vs. control group.
EGF increases EGFR activation and decreases exosome production accompanied by promotion of wound healing.
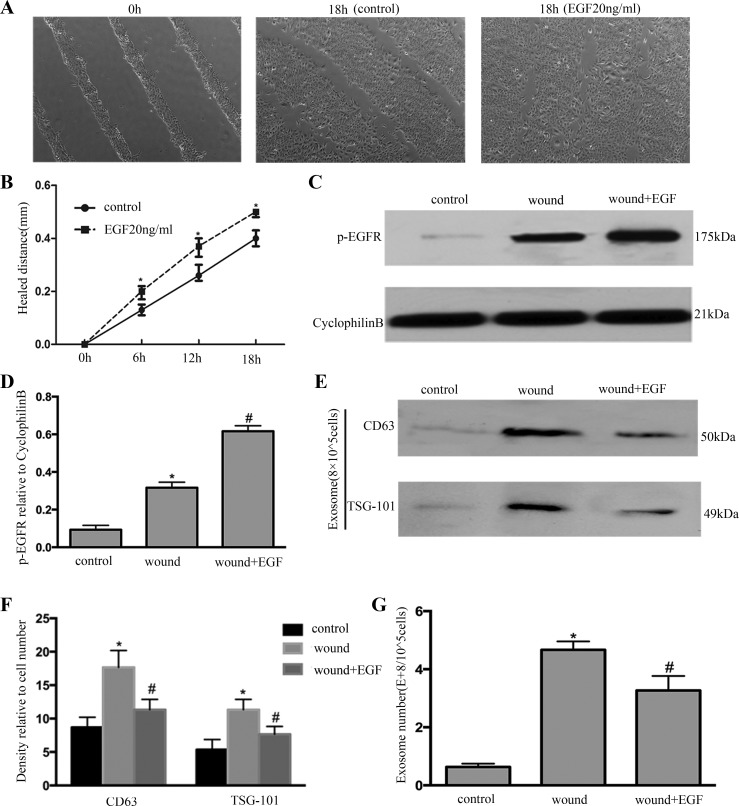
To verify the connection between exosome induction and EGFR activation during wound healing, we used EGF as an inducer of EGFR activation on wounding cells. As shown in Fig. 3, A and B, EGF treatment promoted wound healing. Furthermore, EGF treatment activated EGFR (Fig. 3C); the result was further confirmed by quantification via densitometry (Fig. 3D). Interestingly, EGF treatment decreased the exosome release according to CD63 and TSG101 of immunoblots (Fig. 3E); the result was further confirmed by quantification via densitometry (Fig. 3F). The exosome release was decreased after EGF treatment according to NTA (Fig. 3G).
Fig. 3.
EGFR activation by EGF decreases exosome production and promotes wound healing. A: BUMPT were scratch-wounded with the device and then incubated with or without EGF, and healing distance was recorded with a phase-contrast microscope. B: the wound width was measured at 6, 12, and 18 h after scratching to determine the healed distance. *P < 0.05 vs. control group. C: whole cell lysate was collected for immunoblot analysis of P-EGFR. D: densitometric analysis of P-EGFR expression. *P < 0.05 vs. control group; #P < 0.05 vs. wound-only group. E: exosomes were collected for immunoblot analysis; EGF treatment decreased the expression of CD63 and TSG101 in exosomes. F: densitometric analysis of CD63 and TSG101 expression. *P < 0.05 vs. control group; #P < 0.05 vs. wound-only group. G: exosome number was measured by nanoparticle tracking analysis. *P < 0.05 vs. control; #P < 0.05 vs. wound-only group.
Inhibition of EGFR increases exosome production accompanied by inhibition of wound healing.
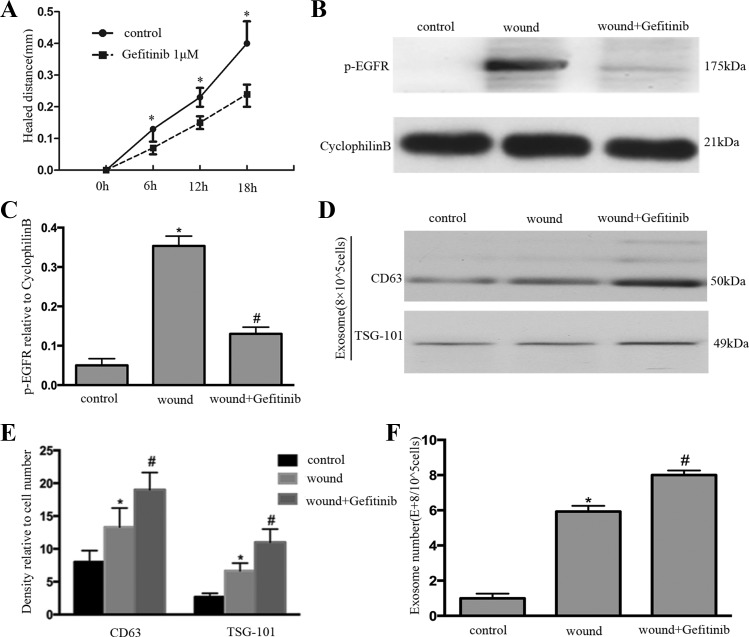
To verify further the connection between exosome induction and EGFR activation during wound healing, EGFR inhibitor gefitinib was applied in the wounding process. As shown in Fig. 4A, gefitinib treatment delayed wound healing. Furthermore, gefitinib treatment dramatically inhibited EGFR activation (Fig. 4B); the result was further confirmed by quantification via densitometry (Fig. 4C). Gefitinib treatment increased the exosome release according to immunoblots of CD63 and TSG101 (Fig. 4D); the result was further confirmed by quantification via densitometry (Fig. 4E). The exosome release was increased after gefitinib treatment according to NTA (Fig. 4F).
Fig. 4.
EGFR inhibitors increase exosome production and blocks wound healing. A: BUMPT were scratch-wounded with the device and then incubated with or without 1 μM gefitinib, and healing distance was recorded with a phase-contrast microscope. The wound width was measured at 6, 12, and 18 h after scratching to determine the healed distance. *P < 0.05 vs. gefitinib group. B: whole cell lysate was collected for immunoblot analysis of P-EGFR. C: densitometric analysis of P-EGFR expression. *P < 0.05 vs. control; #P < 0.05 vs. wound-only group. D: exosome was collected for immunoblot analysis; gefitinib treatment increased the expression of CD63 and TSG101 in exosomes after wounding. E: densitometric analysis of CD63 and TSG101 expression. *P < 0.05 vs. control group; #P < 0.05 vs. wound-only group. F: exosome number was measured by nanoparticle tracking analysis. *P < 0.05 vs. control; #P < 0.05 vs. wound-only group.
Inhibition of exosome release increases the expression of EGFR and promotes wound healing.
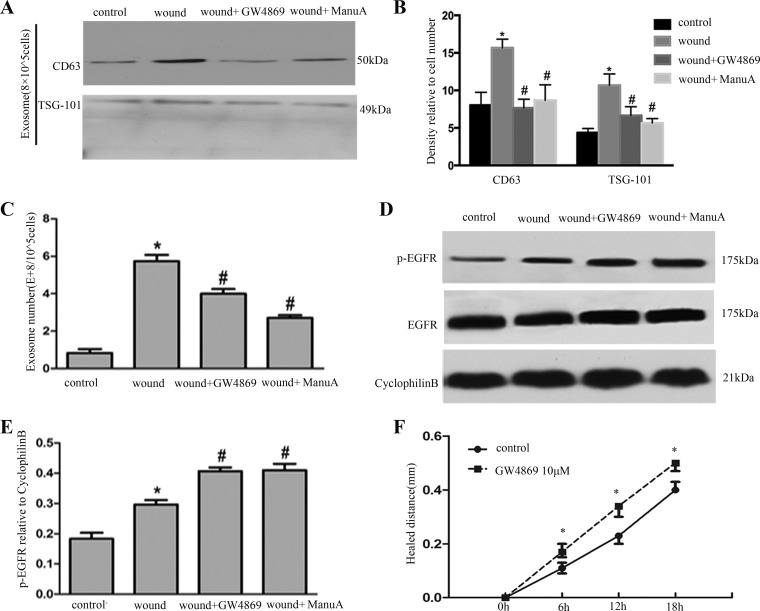
Although we confirmed the release of exosomes, the role of exosomes in wound healing was still poorly understood. To address this question, two pharmacological inhibitors of exosome secretion, GW4869 and manumycin A, were tested. As shown in Fig. 5A, the exosome release was significantly inhibited after GW4869 and manumycin A administration according to immunoblots tests; the result was further confirmed by quantification via densitometry (Fig. 5B). The exosome release was significantly inhibited after GW4869 and manumycin A administration according to NTA (Fig. 5C). Meanwhile, GW4869 and manumycin A treatment also increased EGFR activation during scratch-wound healing (Fig. 5D). The changes in EGFR activation were further confirmed by quantification via densitometry (Fig. 5E). Notably, inhibition of exosome release by GW4869 promoted wounding healing (Fig. 5F).
Fig. 5.
Inhibition of exosome release increases EGFR activation and promotes wound healing. A: exosomes were collected for immunoblot analysis, and exosome inhibitor GW4869 and manumycin A (ManuA) decreased exosome secretion. B: densitometric analysis of CD63 and TSG101 expression. *P < 0.05 vs. control group; #P < 0.05 vs. wound-only group. C: exosome number was determined by nanoparticle tracking analysis. *P < 0.05 vs. control; #P < 0.05 vs. wound-only group. D: whole cell lysate was collected for immunoblot analysis of EGFR. E: densitometric analysis of P-EGFR expression. *P < 0.05 vs. control; #P < 0.05 vs. wound-only group. F: BUMPT were scratch-wounded with the device and then incubated with or without GW4869, and healing distance was recorded with a phase-contrast microscope. The wound width was measured at 6, 12, and 18 h after scratching to determine the healed distance. *P < 0.05 vs. control.
Exosomes derived from scratch-wounding cell delayed wounding healing.
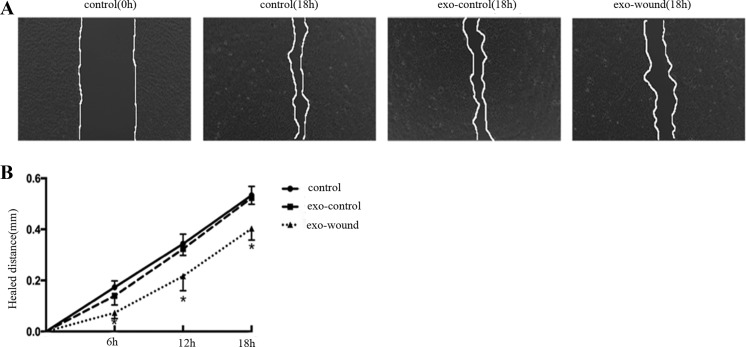
To demonstrate further the role of exosomes in the wound-healing process, exosomes derived from multiple-wounding cells or normal control cells were added in linearly scratched plates. As shown in Fig. 6, A and B, compared with exosomes from normal cell, exosomes from scratch wounding reduced the rate of healing.
Fig. 6.
Exosomes derived from scratch-wounded cells suppress wound healing. A: BUMPT were scratch-wounded with a sterile pipette tip and then incubated with 20-μg exosomes in 12-well plates. The exosomes were from culture medium of wounding cell (exo-wound) and normal cell (exo-control). The healing was recorded with a phase-contrast microscope. B: the wound width was measured at 6, 12, and 18 h after scratching to determine the healed distance. *P < 0.05 vs. control and exo-control.
DISCUSSION
In the present study, we have demonstrated that scratch wounding can activate EGFR and promote exosome production in renal tubular cells. Furthermore, EGFR activation can suppress exosome release during wound healing. Importantly, exosomes from scratch-wounding tubular cells reduces the rate of healing. EGFR activation has been suggested to promote the repair of injured renal epithelium by increasing cell proliferation and migration (10). Also, EGF and its receptor, EGFR, have been shown to mediate the recovery of renal function after kidney injury in vivo (33). Consistently, our present work confirms that EGFR activation can promote wound healing in mechanically injured BUMPT cells.
Exosomes are membrane-bound vesicles of 30–100 nm in diameter that can be produced by almost all renal cell types and may be critical for cell-to-cell communication (19). Release of exosomes was found to be regulated in several cellular model systems, and stress conditions or injury can stimulate the cellular release of exosomes (1). Membrane stretch and cardiac pressure overload from the heart also increased exosome secretion (25). In breast cancer cells, hypoxia enhanced exosome release that may involve HIF (14). In kidney, unilateral ureteral obstruction (UUO) and hypoxia induced the production of exosomes (2). In mesothelial cells, scratch wounding induced budding of exosomes (15). Our present study shows that scratch wounding increases exosome secretion in renal tubular cells. Exosome production occurs via an endosomal sorting complex required for transport (ESCRT)-dependent or -independent mechanism in different cell type (31). The release of exosome is also associated with the activation of specific cell surface receptors. Glebov et al. (9) demonstrated that serotonin receptors may modulate exosome release in microglial cells. Heijnen et al. (11) demonstrated that the release of exosomes was a response to thrombin receptor activation in platelets. In the present study, we show evidence that exosome secretion is negatively regulated by EGFR activation. EGFR activation decreased exosome production, whereas inhibition of EGFR increased exosome production (Figs. 3–5). These results are consistent with several studies in cancer research. In epidermoid carcinoma cells, after EGFR stimulation by EGF, the cellular uptake of exosomes was enhanced ~27-fold (23). Inhibition of oncogenic EGFR kinase triggered the release of exosome-like extracellular vesicles (21). However, Koistinen et al. (15) suggested that epithelial-mesenchymal transition (EMT) induced by EGF and wounding activates exosome shedding in rat primary mesothelial cells.
Exosomes may have biological function to the cells from which they are derived, and direct administration of these vesicles may affect renal physiology and the pathogenesis of kidney diseases (16, 35). Whether it is a protective or pathogenic remains elusive. In a protective role, exosomes may help the cells to get rid of pathogenic or oncogenic proteins/RNA to minimize the damage within. Alternatively, the exosomes could be used as amplifiers to spread pathogenic molecules between cells (8). To understand the effect of exosomes on wound healing, Zhang et al. (34) reported human umbilical cord mesenchymal stem cell-derived exosomes significantly enhanced reepithelialization of the wound in a rat burn injury model. However, we found exosomes from scratch-wounding cells reduced healing (Fig. 6). The exact role of exosomes on wound healing might depend on the precise conditions and functional state, especially the source of exosomes.
In conclusion, this study has demonstrated EGFR activation and the release of exosomes in scratch-wounded renal tubular cells. Although EGFR activation promotes wound healing, released exosomes may suppress wound healing. Interestingly, EGFR activation and exosomes appear to antagonize each other under this condition.
GRANTS
This study was supported in part by grants from the National Natural Science Foundation of China (81528004 and 81430017), the National Institute of Diabetes and Digestive and Kidney Diseases (DK058831 and DK087843), and U.S. Department of Veterans Affairs (BX000319).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
X.Z., W.Z., and Z.D. conceived and designed research; X.Z. performed experiments; X.Z., W.Z., H.Z., G.D., Y.L., J.-K.C., and Z.D. analyzed data; X.Z., W.Z., Q.Y., H.Z., G.D., M.Z., Y.L., J.-K.C., and Z.D. interpreted results of experiments; X.Z. prepared figures; X.Z. drafted manuscript; W.Z., Q.Y., H.Z., G.D., M.Z., Y.L., J.-K.C., and Z.D. edited and revised manuscript; X.Z., W.Z., Q.Y., H.Z., G.D., M.Z., Y.L., J.-K.C., and Z.D. approved final version of manuscript.
REFERENCES
- 1.Bang C, Batkai S, Dangwal S, Gupta SK, Foinquinos A, Holzmann A, Just A, Remke J, Zimmer K, Zeug A, Ponimaskin E, Schmiedl A, Yin X, Mayr M, Halder R, Fischer A, Engelhardt S, Wei Y, Schober A, Fiedler J, Thum T. Cardiac fibroblast-derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J Clin Invest 124: 2136–2146, 2014. doi: 10.1172/JCI70577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borges FT, Melo SA, Özdemir BC, Kato N, Revuelta I, Miller CA, Gattone VH 2nd, LeBleu VS, Kalluri R. TGF-β1-containing exosomes from injured epithelial cells activate fibroblasts to initiate tissue regenerative responses and fibrosis. J Am Soc Nephrol 24: 385–392, 2013. doi: 10.1681/ASN.2012101031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruno S, Porta S, Bussolati B. Extracellular vesicles in renal tissue damage and regeneration. Eur J Pharmacol 790: 83–91, 2016. doi: 10.1016/j.ejphar.2016.06.058. [DOI] [PubMed] [Google Scholar]
- 4.Burger D, Viñas JL, Akbari S, Dehak H, Knoll W, Gutsol A, Carter A, Touyz RM, Allan DS, Burns KD. Human endothelial colony-forming cells protect against acute kidney injury: role of exosomes. Am J Pathol 185: 2309–2323, 2015. doi: 10.1016/j.ajpath.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 5.Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 30: 255–289, 2014. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 6.Daßler-Plenker J, Reiners KS, van den Boorn JG, Hansen HP, Putschli B, Barnert S, Schuberth-Wagner C, Schubert R, Tüting T, Hallek M, Schlee M, Hartmann G, Pogge von Strandmann E, Coch C. RIG-I activation induces the release of extracellular vesicles with antitumor activity. OncoImmunology 5: e1219827, 2016. doi: 10.1080/2162402X.2016.1219827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dinkins MB, Enasko J, Hernandez C, Wang G, Kong J, Helwa I, Liu Y, Terry AV Jr, Bieberich E. Neutral sphingomyelinase-2 deficiency ameliorates Alzheimer’s disease pathology and improves cognition in the 5XFAD mouse. J Neurosci 36: 8653–8667, 2016. doi: 10.1523/JNEUROSCI.1429-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gangoda L, Boukouris S, Liem M, Kalra H, Mathivanan S. Extracellular vesicles including exosomes are mediators of signal transduction: are they protective or pathogenic? Proteomics 15: 260–271, 2015. doi: 10.1002/pmic.201400234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glebov K, Löchner M, Jabs R, Lau T, Merkel O, Schloss P, Steinhäuser C, Walter J. Serotonin stimulates secretion of exosomes from microglia cells. Glia 63: 626–634, 2015. doi: 10.1002/glia.22772. [DOI] [PubMed] [Google Scholar]
- 10.Hallman MA, Zhuang S, Schnellmann RG. Regulation of dedifferentiation and redifferentiation in renal proximal tubular cells by the epidermal growth factor receptor. J Pharmacol Exp Ther 325: 520–528, 2008. doi: 10.1124/jpet.107.134031. [DOI] [PubMed] [Google Scholar]
- 11.Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood 94: 3791–3799, 1999. [PubMed] [Google Scholar]
- 12.Helwa I, Cai J, Drewry MD, Zimmerman A, Dinkins MB, Khaled ML, Seremwe M, Dismuke WM, Bieberich E, Stamer WD, Hamrick MW, Liu Y. A comparative study of serum exosome isolation using differential ultracentrifugation and three commercial reagents. PLoS One 12: e0170628, 2017. doi: 10.1371/journal.pone.0170628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, Singh S, Williams C, Soplop N, Uryu K, Pharmer L, King T, Bojmar L, Davies AE, Ararso Y, Zhang T, Zhang H, Hernandez J, Weiss JM, Dumont-Cole VD, Kramer K, Wexler LH, Narendran A, Schwartz GK, Healey JH, Sandstrom P, Labori KJ, Kure EH, Grandgenett PM, Hollingsworth MA, de Sousa M, Kaur S, Jain M, Mallya K, Batra SK, Jarnagin WR, Brady MS, Fodstad O, Muller V, Pantel K, Minn AJ, Bissell MJ, Garcia BA, Kang Y, Rajasekhar VK, Ghajar CM, Matei I, Peinado H, Bromberg J, Lyden D. Tumour exosome integrins determine organotropic metastasis. Nature 527: 329–335, 2015. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King HW, Michael MZ, Gleadle JM. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer 12: 421, 2012. doi: 10.1186/1471-2407-12-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koistinen V, Härkönen K, Kärnä R, Arasu UT, Oikari S, Rilla K. EMT induced by EGF and wounding activates hyaluronan synthesis machinery and EV shedding in rat primary mesothelial cells. Matrix Biol. First published December 30, 2016; doi: 10.1016/j.matbio.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 16.Krause M, Samoylenko A, Vainio SJ. Exosomes as renal inductive signals in health and disease, and their application as diagnostic markers and therapeutic agents. Front Cell Dev Biol 3: 65, 2015. doi: 10.3389/fcell.2015.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lan R, Geng H, Hwang Y, Mishra P, Skloss WL, Sprague EA, Saikumar P, Venkatachalam M. A novel wounding device suitable for quantitative biochemical analysis of wound healing and regeneration of cultured epithelium. Wound Repair Regen 18: 159–167, 2010. doi: 10.1111/j.1524-475X.2010.00576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu W, Hsu DK, Chen HY, Yang RY, Carraway KL 3rd, Isseroff RR, Liu FT. Galectin-3 regulates intracellular trafficking of EGFR through Alix and promotes keratinocyte migration. J Invest Dermatol 132: 2828–2837, 2012. doi: 10.1038/jid.2012.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maas SL, Breakefield XO, Weaver AM. Extracellular vesicles: unique intercellular delivery vehicles. Trends Cell Biol 27: 172–188, 2017. doi: 10.1016/j.tcb.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, LeBleu VS, Mittendorf EA, Weitz J, Rahbari N, Reissfelder C, Pilarsky C, Fraga MF, Piwnica-Worms D, Kalluri R. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 523: 177–182, 2015. doi: 10.1038/nature14581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montermini L, Meehan B, Garnier D, Lee WJ, Lee TH, Guha A, Al-Nedawi K, Rak J. Inhibition of oncogenic epidermal growth factor receptor kinase triggers release of exosome-like extracellular vesicles and impacts their phosphoprotein and DNA content. J Biol Chem 290: 24534–24546, 2015. doi: 10.1074/jbc.M115.679217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morrison EE, Bailey MA, Dear JW. Renal extracellular vesicles: from physiology to clinical application. J Physiol 594: 5735–5748, 2016. doi: 10.1113/JP272182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakase I, Kobayashi NB, Takatani-Nakase T, Yoshida T. Active macropinocytosis induction by stimulation of epidermal growth factor receptor and oncogenic Ras expression potentiates cellular uptake efficacy of exosomes. Sci Rep 5: 10300, 2015. doi: 10.1038/srep10300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pastar I, Stojadinovic O, Yin NC, Ramirez H, Nusbaum AG, Sawaya A, Patel SB, Khalid L, Isseroff RR, Tomic-Canic M. Epithelialization in wound healing: a comprehensive review. Adv Wound Care (New Rochelle) 3: 445–464, 2014. doi: 10.1089/wound.2013.0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pironti G, Strachan RT, Abraham D, Mon-Wei Yu S, Chen M, Chen W, Hanada K, Mao L, Watson LJ, Rockman HA. Circulating exosomes induced by cardiac pressure overload contain functional angiotensin II type 1 receptors. Circulation 131: 2120–2130, 2015. doi: 10.1161/CIRCULATIONAHA.115.015687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci USA 101: 13368–13373, 2004. doi: 10.1073/pnas.0403453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rani S, O’Brien K, Kelleher FC, Corcoran C, Germano S, Radomski MW, Crown J, O’Driscoll L. Isolation of exosomes for subsequent mRNA, microRNA, and protein profiling. Methods Mol Biol 784: 181–195, 2011. doi: 10.1007/978-1-61779-289-2_13. [DOI] [PubMed] [Google Scholar]
- 28.Rossol-Allison J, Ward CJ. Exosomes to the rescue. J Am Soc Nephrol 26: 2303–2304, 2015. doi: 10.1681/ASN.2015030254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tamai K, Tanaka N, Nakano T, Kakazu E, Kondo Y, Inoue J, Shiina M, Fukushima K, Hoshino T, Sano K, Ueno Y, Shimosegawa T, Sugamura K. Exosome secretion of dendritic cells is regulated by Hrs, an ESCRT-0 protein. Biochem Biophys Res Commun 399: 384–390, 2010. doi: 10.1016/j.bbrc.2010.07.083. [DOI] [PubMed] [Google Scholar]
- 30.Tetta C, Ghigo E, Silengo L, Deregibus MC, Camussi G. Extracellular vesicles as an emerging mechanism of cell-to-cell communication. Endocrine 44: 11–19, 2013. doi: 10.1007/s12020-012-9839-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brügger B, Simons M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319: 1244–1247, 2008. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y, Zhang L, Li Y, Chen L, Wang X, Guo W, Zhang X, Qin G, He SH, Zimmerman A, Liu Y, Kim IM, Weintraub NL, Tang Y. Exosomes/microvesicles from induced pluripotent stem cells deliver cardioprotective miRNAs and prevent cardiomyocyte apoptosis in the ischemic myocardium. Int J Cardiol 192: 61–69, 2015. doi: 10.1016/j.ijcard.2015.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Z, Chen JK, Wang SW, Moeckel G, Harris RC. Importance of functional EGF receptors in recovery from acute nephrotoxic injury. J Am Soc Nephrol 14: 3147–3154, 2003. doi: 10.1097/01.ASN.0000098681.56240.1A. [DOI] [PubMed] [Google Scholar]
- 34.Zhang B, Wu X, Zhang X, Sun Y, Yan Y, Shi H, Zhu Y, Wu L, Pan Z, Zhu W, Qian H, Xu W. Human umbilical cord mesenchymal stem cell exosomes enhance angiogenesis through the Wnt4/β-catenin pathway. Stem Cells Transl Med 4: 513–522, 2015. doi: 10.5966/sctm.2014-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang W, Zhou X, Zhang H, Yao Q, Liu Y, Dong Z. Extracellular vesicles in diagnosis and therapy of kidney diseases. Am J Physiol Renal Physiol 311: F844–F851, 2016. doi: 10.1152/ajprenal.00429.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhuang S, Dang Y, Schnellmann RG. Requirement of the epidermal growth factor receptor in renal epithelial cell proliferation and migration. Am J Physiol Renal Physiol 287: F365–F372, 2004. doi: 10.1152/ajprenal.00035.2004. [DOI] [PubMed] [Google Scholar]
- 37.Zhuang S, Duan M, Yan Y. Src family kinases regulate renal epithelial dedifferentiation through activation of EGFR/PI3K signaling. J Cell Physiol 227: 2138–2144, 2012. doi: 10.1002/jcp.22946. [DOI] [PMC free article] [PubMed] [Google Scholar]