Abstract
Mineralocorticoid and glucocorticoid receptors are closely related steroid hormone receptors that regulate gene expression through many of the same hormone response elements. However, their transcriptional activities and effects in skeletal muscles are largely unknown. We recently identified mineralocorticoid receptors (MR) in skeletal muscles after finding that combined treatment with the angiotensin-converting enzyme inhibitor lisinopril and MR antagonist spironolactone was therapeutic in Duchenne muscular dystrophy mouse models. The glucocorticoid receptor (GR) agonist prednisolone is the current standard-of-care treatment for Duchenne muscular dystrophy because it prolongs ambulation, likely due to its anti-inflammatory effects. However, data on whether glucocorticoids have a beneficial or detrimental direct effect on skeletal muscle are controversial. Here, we begin to define the gene expression profiles in normal differentiated human skeletal muscle myotubes treated with MR and GR agonists and antagonists. The MR agonist aldosterone and GR agonist prednisolone had highly overlapping gene expression profiles, supporting the notion that prednisolone acts as both a GR and MR agonist that may have detrimental effects on skeletal muscles. Co-incubations with aldosterone plus either nonspecific or selective MR antagonists, spironolactone or eplerenone, resulted in similar numbers of gene expression changes, suggesting that both drugs can block MR activation to a similar extent. Eplerenone treatment alone decreased a number of important muscle-specific genes. This information may be used to develop biomarkers to monitor clinical efficacy of MR antagonists or GR agonists in muscular dystrophy, develop a temporally coordinated treatment with both drugs, or identify novel therapeutics with more specific downstream targets.
Keywords: mineralocorticoid receptor, glucocorticoid receptor, Duchenne muscular dystrophy, aldosterone, eplerenone, spironolactone, prednisolone, mifepristone
drugs that target mineralocorticoid (MR) and glucocorticoid (GR) receptors are being used clinically in patients with Duchenne muscular dystrophy (DMD), a degenerative and fatal striated muscle disease. The GR agonist prednisolone is the current standard-of-care treatment for DMD because it prolongs ambulation, likely due to its anti-inflammatory and immunosuppressive effects (8, 13). We have recently shown treatment with the MR antagonist spironolactone and angiotensin converting enzyme inhibitor lisinopril improved both skeletal and cardiac muscle function and pathology in a mouse model of DMD (45). This preclinical study has been translated into a published clinical trial in DMD patients demonstrating significant efficacy of an MR antagonist on cardiac outcomes (46). We have recently demonstrated that MR are present in skeletal muscle myotubes and in a wide variety of skeletal muscle tissues. Although MR antagonists are widely used to treat heart failure (21–23, 26), the presence of MR in skeletal muscles supports the potential for a direct therapeutic effect of MR antagonists on skeletal muscle pathology in muscular dystrophy (9). MR and GR are structurally similar nuclear hormone receptors that regulate gene transcription as ligand-dependent transcription factors (4, 15, 21, 56), but the direct effects of these receptors on gene expression in skeletal muscle are largely unknown.
Upon ligand binding, MR and GR dissociate from chaperone proteins and translocate from the cytosol to the nucleus where they bind hormone response elements (HRE) on DNA to modulate gene transcription (58). MR and GR bind many of the same HRE and therefore interact to regulate transcription of many of the same genes (21). Steroid hormone receptors typically bind their HRE as homodimers. However, MR and GR are highly homologous, can form functional heterodimers, and exhibit a broad overlap of target genes in some cell types (26, 43).
MR and GR exhibit cross-reactivity with endogenous glucocorticoids, which have a similar affinity for MR as the endogenous mineralocorticoid aldosterone and have a 10-fold greater affinity for MR than for GR (16, 21, 33, 47, 52). Aldosterone can only bind GR at supraphysiological concentrations, but this binding may contribute to its action (49). In aldosterone selective tissues, MR are protected from glucocorticoids by the type 2 11β-hydroxysteroid dehydrogenase enzyme that converts glucocorticoids to inactive metabolites unable to bind MR (42, 54). We have recently demonstrated that skeletal muscle fibers contain type 2 11β-hydroxysteroid dehydrogenase, supporting the notion that skeletal muscle is an aldosterone selective tissue (10). Aldosterone levels within dystrophic muscles are higher than normal, and evidence suggests that aldosterone is being produced locally from infiltrating inflammatory cells present in dystrophic muscle tissue (10). These data suggest that MR are continually activated in injured skeletal muscles and further support the possibility that MR antagonists may be therapeutic for DMD.
Although glucocorticoids are used therapeutically in DMD, whether they have a beneficial or detrimental direct effect on skeletal muscle is controversial, and several laboratories have shown that prednisolone actually worsens both skeletal and cardiac muscle damage in DMD mouse models (18, 27, 29, 48). GR regulate glucose homeostasis in skeletal muscle by increasing protein degradation and decreasing protein synthesis. However, continued GR-mediated protein degradation results in skeletal muscle atrophy and weakness in populations without muscle disease (34). Since treatment with the nonspecific MR antagonist spironolactone can also bind GR and was therapeutic for dystrophic skeletal muscles, these data suggest that optimizing modulation of MR and GR activity may improve therapeutic interventions for skeletal muscle disorders (45, 59). We recently demonstrated that high levels of aldosterone changed gene expression in normal differentiated human skeletal muscle myotubes (9). However, more detailed comparisons between MR and GR agonists and antagonists are crucial to optimize therapies targeting these receptors for skeletal muscle diseases and to develop biomarkers of treatment.
In the current study we begin to define the molecular changes in differentiated skeletal muscle myotubes that may underlie the observed in vivo efficacy of MR antagonists and differentiate between MR- and GR-responsive genes and drugs that target MR or GR. We performed gene expression microarray on normal human myotubes treated with the endogenous MR agonist aldosterone, the nonspecific MR antagonist spironolactone, which can also bind GR at higher concentrations (59), the selective MR antagonist eplerenone, and the GR antagonist mifepristone. Since prednisone has been shown to worsen skeletal muscle damage in dystrophic mice, we also treated normal human myotubes with prednisolone to determine whether it leads to detrimental gene expression changes.
MATERIALS AND METHODS
Mammalian cell culture.
Human skeletal muscle myoblasts (lot #0000421209; Lonza) isolated from normal healthy males were grown in skeletal muscle cell growth medium (SkGM-2 bullet kit, Lonza) as previously described (9). Cells were serum restricted for 5 days in high-glucose Dulbecco’s modified medium (DMEM, Invitrogen) and supplemented with 2% horse serum and 100 U/ml penicillin-streptomycin, followed by 48 h treatments with: aldosterone (10 µM; MR EC50: 1.3 nM; GR EC50: 80 nM), eplerenone (10 µM; MR IC50: 81 nM; Pfizer Compound Transfer Program), mifepristone (1 µM; GR IC50: 2.6 nM; Cayman Chemical), prednisolone (1 µM; GR EC50: 4.4 nM), spironolactone (10 µM; MR IC50: 1.6 nM; GR IC50: 2.9 µM), or vehicle (drugs were purchased from Sigma and dissolved in 100% DMSO unless specified otherwise). We chose 48 h as a time-point to identify both primary and secondary gene expression changes in this high-dose treatment experiment. In a separate experiment, the same primary cells were differentiated as described above and treated with a 100-fold lower concentration of aldosterone (100 nM), or co-incubuated with 100 nM aldosterone plus an excess of eplerenone (10 µM), spironolactone (10 µM), or mifepristone (1 µM) for 24 h to block the MR, MR and GR, and GR, respectively. A shorter time-point was used to ensure that the lower dosage of the drugs remained stable throughout the experiment but was still long enough to ensure effects on both early- and late-phase genes. Three biological replicates for each treatment were conducted. Drugs were added directly to existing differentiation media, and cells were harvested in 200 µl of cellular extract buffer as previously described (9).
RNA isolation.
RNA was isolated from three biological replicates from each group of treated human myotubes or mouse gastrocnemius muscles with TRIzol reagent (Life Technologies), according to manufacturer’s instructions. Samples were DNase-treated using RQ1 DNase (Promega); samples for microarray analysis were further purified using the RNeasy mini kit (Qiagen) cleanup protocol as previously described (9).
Microarray.
The integrity of RNA samples was verified using an Agilent 2100 Bioanalyzer (Agilent Technologies). Samples with RNA integrity numbers > 7.9 were assayed as follows. A 100 ng aliquot of total RNA was linearly amplified and 5.5 µg of cDNA was labeled and fragmented using the GeneChip WT PLUS reagent kit (Affymetrix) following the manufacturer's instructions. Labeled cDNA targets were hybridized to an Affymetrix GeneChip Human Transcriptome Array 2.0 for 16 h at 45°C rotating at 60 rpm. Microarrays were washed and stained using the Affymetrix Fluidics Station 450 and scanned using the GeneChip Scanner 3000. Initial intensities were assessed visually and through sample quality metrics such as positive vs. negative area under the curve, all probe set mean, and all probe set relative log expression mean. Quality standards were met on all metrics. Resulting data were normalized using Affymetrix Expression Console software. A gene level algorithm involving signal space transformation and robust multiarray analysis (57) was applied using default settings that included the criteria of a consistent increase/decrease across all three replicate comparisons with a P value of ≤ 0.015 based upon the Wilcoxon’s signed rank test. Tukey’s biweight average algorithm was used to determine a robust average unaffected by outliers resulting in a biweight average shown in a log2 scale. Fold-change comparisons between samples were made in Transcriptome Analysis Console (Affymetrix) using ANOVA calculated using NMATH Package P ≤ 0.5. A cutoff of twofold was used for comparisons between high-dose individual treatments, and a cutoff of 1.5-fold was used for comparisons between low-dose aldosterone and low-dose aldosterone plus antagonists. Microarray data have been deposited in the National Center for Biotechnology Information's Gene Expression Omnibus under accession number GSE84992. Gene groups were assigned using ontology annotation clustering tools from ToppGene and the Database for Annotation, Visualization and Integrated Discovery (DAVID) (11, 28). The list of genes for each pairwise comparison was first run through ToppGene using the ToppFun function. Biological Processes were used to classify the genes. Genes that were duplicated in more than one category were assigned to the category that contained the largest gene groups. Classification groups for each gene were kept consistent between comparisons. Any gene that was not identified in ToppGene was separately searched using the Functional Annotation Clustering tool in DAVID.
Western blot analysis.
Protein concentration was determined by Dc Protein Assay (Bio-Rad) as previously described (9). We probed 35 µg per lane of total protein from cell extracts with a combination of MR-specific monoclonal antibodies, MRN 2B7 and rMR 1-18 6G1[monoclonal mouse (20)], or GAPDH (polyclonal rabbit, Proteintech # 10494-1-AP) followed by anti- mouse or rabbit horseradish peroxidase (Jackson Immunoresearch) secondary antibodies. Signals were detected with ECL 2 Western blotting substrate (Pierce) followed by film (blue ultra, GeneMate) exposure.
Mice.
All protocols were approved by the Institutional Laboratory Animal Care and Use Committee of The Ohio State University (OSU). Three mouse models of DMD were used for RNA isolation: dystrophin-deficient mdx mice (7, 51), dystrophin-deficient utrophin haplo-insufficent (utrn+/−; mdx) “het” mice (60) and dystrophin/utrophin-deficient double knockout “dko” mice (14), in addition to C57BL/10 (Harlan) wild-type control mice. Gastrocnemius muscles were removed from 8 wk old mice bred in-house and genotyped as described previously (14, 29, 60).
Real-time PCR.
We used 1 µg of DNased RNA to generate cDNA using the Reverse Transcriptase High Capacity cDNA Reverse Transcription kit (Applied Biosystems), as previously described (9). Relative quantitation RT-PCR was performed using Power SYBR Green PCR Master Mix (Applied Biosystems) inoculated with 1 µl of the cDNA reaction and 40 nM of each primer. Technical triplicates of three biological replicates for each treatment or mouse genotype were performed. Primers used to amplify PRG4 were: 5′-TTTGGCCGGGAGACTCAATC-3′ (forward) and 5′-ATTCTGCGTGGTGGAGATGG-3′ (reverse) for mouse gastrocnemius muscles, and 5′-GCAGCGCTTTCAACAGCTAA-3′ (forward) and 5′-GCGACGTCTCCTAACCTGTG-3′ (reverse) for human myotubes. Expression levels of PRG4 were normalized to a β-actin control in mice 5′-ACCAGTTCGCCATGGATGAC-3′ (forward) and 5′-TGCCGGAGCCGTTGTC-3′ (reverse) and GAPDH in human 5′-ATGTTCGTCATGGGTGTGAA-3′ (forward) and 5′-GGTGCTAAGCAGTTGGTGGT-3′ (reverse). The C57 wild-type mouse tissue or aldosterone treated human myotubes with the highest level of PRG4 expression was normalized to 1× and used to determine fold-changes in the other samples. Nonreverse-transcribed RNA was used as a negative control for each sample, as well as a nontemplate control for each reaction mixture. Data were analyzed by one-way ANOVA. If the overall ANOVA indicated statistical significance, either a nonparametric Dunnett post hoc test was used to test for significant differences between each dystrophic group compared with the wild-type control group or a Student's t-test was used to compare between aldosterone- treated and aldosterone plus spironolactone-treated human myotubes (2 groups). A P value ≤ 0.05 was considered statistically significant.
RESULTS
MR protein levels are not decreased with aldosterone treatment in normal human myotubes.
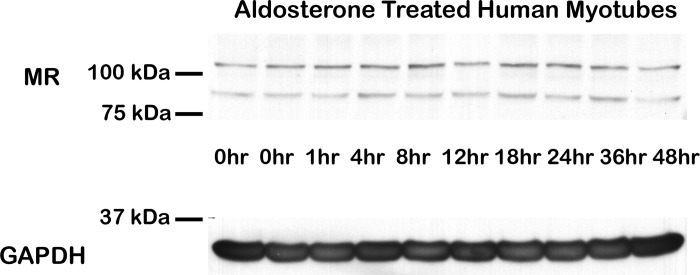
Ligand binding and transcriptional activation of nuclear hormone receptors are directly coupled to degradation of the receptor through the ubiquitin-proteasome pathway (2, 37, 50). To analyze the effect of ligands on MR degradation and protein levels in skeletal muscle, we treated normal human myotubes with aldosterone for time periods between 1 and 48 h. Western blot analysis revealed no observable change in MR protein levels or shift in molecular weight (Fig. 1) (21).
Fig. 1.
Mineralocorticoid receptor (MR) protein levels are maintained in aldosterone-treated human myotubes. Normal human myotubes were treated with 100 nM aldosterone from 0 to 48 h. Representative Western comparing MR protein levels from equivalent amounts (35 µg) of cell lysates shown. Western blots used a combination of MR-specific monoclonal antibodies MR1-18 1D5 and MRN 2B7 (20) (full-length MR predicted molecular weight ~107 kDa) or a GAPDH antibody (loading control; predicted molecular weight ~36 kDa).
Ninety-five percent of gene expression changes resulting from aldosterone treatment is also changed with prednisolone treatment.
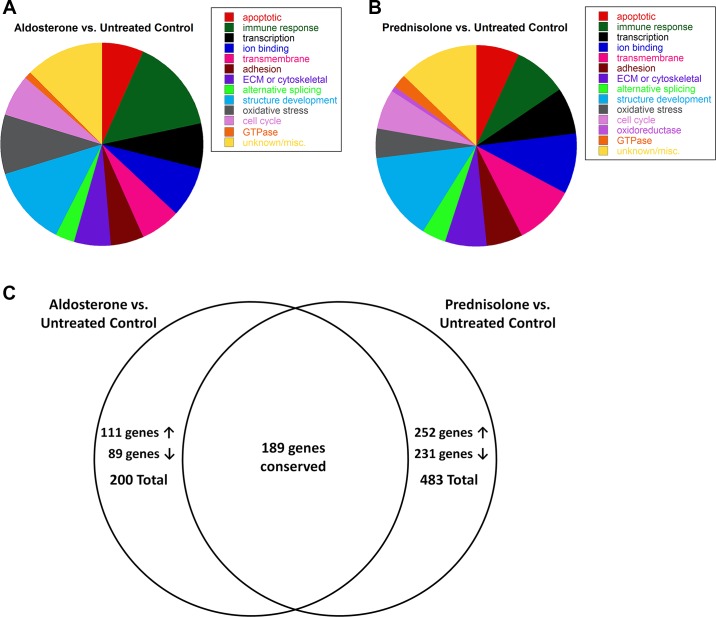
Normal human myotubes were first treated with supraphysiological concentrations of MR and GR agonists and antagonists for 48 h to examine maximal effects on gene expression and ensure the microarray data encompassed both early- and late-stage gene expression changes. Treatment of normal human myotubes with aldosterone resulted in 200 gene expression changes; 111 genes were increased (2- to 18-fold) with aldosterone treatment, and 89 genes were decreased (2- to 10-fold) (Supplementary Table S1). (The online version of this article contains supplemental material.) The majority of aldosterone-responsive genes were functionally categorized as: apoptotic (6.5%), immune response (15.0%), regulation of transcription (7.0%), ion binding (8.0%), transmembrane (6.5%), cell adhesion (5.0%), extracellular matrix or cytoskeletal binding (6.0%), alternative splicing (3.0%), vasculature or muscle structure development (12.5%), oxidative stress response (9.5%), regulation of cell differentiation (6.5%), or GTPases (1.0%) (Fig. 2A).
Fig. 2.
Gene expression changes resulting from high-dose aldosterone treatment were conserved with prednisolone treatment. A: treatment with the endogenous MR agonist aldosterone resulted in 200 gene expression changes. Functional clustering of these genes revealed: 13 apoptotic, 30 immune or defense response, 14 transcriptional regulators, 16 ion binding, 13 transmembrane, 10 cell adhesion, 12 extracellular matrix (ECM) or cytoskeletal binding, 6 alternative splicing, 25 vasculature or muscle structure development, 19 oxidative stress responsive, 15 regulators of cell differentiation, 2 GTPases, and 25 genes with unknown or specific functions. B: prednisolone treatment resulted in 483 gene expression changes and functional clustering of these genes revealed: 34 apoptotic, 41 immune or defense response, 36 transcriptional regulators, 47 ion binding, 47 transmembrane, 29 cell adhesion, 31 ECM or cytoskeletal binding, 19 alternative splicing, 69 vasculature or muscle structure development, 23 oxidative stress responsive, 30 cell cycle, 5 oxidoreductases, 10 GTPase, and 62 genes with unknown or specific functions. C: treatment with aldosterone increased expression of 111 genes and decreased expression of 89 genes compared with untreated normal human myotubes. Of these gene changes, 189 (95%) were conserved in prednisolone-treated vs. untreated human myotubes. Treatment with standard-of-care prednisolone increased expression of 252 genes and decreased 231 genes. About 40% of these changes (189 genes) were conserved with aldosterone-treated myotubes; the remaining 294 genes are specific to prednisolone treatment.
Prednisolone treatment resulted in 483 gene expression changes; prednisolone increased expression of 252 genes (2- to 50-fold) and decreased 231 genes (2- to 26-fold) (Supplementary Table S1). Interestingly, 189 of the 200 genes changed with aldosterone treatment were also changed in the same direction with prednisolone treatment. Prednisolone treatment compared with untreated controls resulted in a similar gene expression profile as that for aldosterone treatment compared with untreated controls: apoptotic (7.0%), immune response (8.5%), regulation of transcription (7.5%), ion binding (9.7%), transmembrane (9.7%), cell adhesion (6.0%), extracellular matrix or cytoskeletal binding (6.4%), alternative splicing (3.9%), vasculature or muscle structure development (14.3%), oxidative stress response (4.8%), cell cycle (6.2%), oxidoreductase (1.0%), or GTPase activity (2.1%) (Fig. 2, B and C). The remaining 11 genes changed with aldosterone treatment that were not changed with prednisolone treatment include: C3, TNFSF10, JUNB, ABCA6, ABCA8, FBLN1, FBLN2, CENPF, TOP2A, SMOC1, and TMEM45A.
Treatment of normal human myotubes with spironolactone results in very few gene expression changes compared with eplerenone and mifepristone.
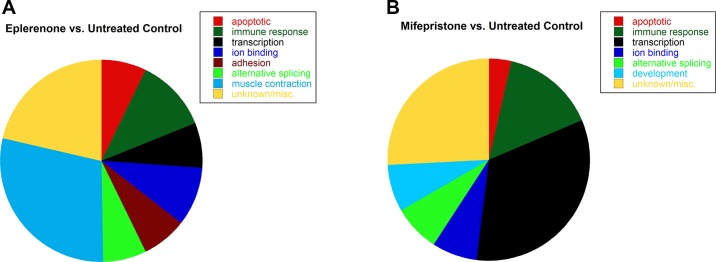
Only three genes were increased twofold with spironolactone treatment compared with untreated controls. However, treatment of myotubes with the selective MR antagonist eplerenone alone resulted in 42 gene expression changes, with seven genes increased (2- to 3-fold) by eplerenone treatment and 35 genes decreased (2- to 4-fold) compared with untreated controls (Supplementary Table S2). Over one-quarter of these genes were functionally categorized as genes involved in muscle contraction (28.6%); the remaining genes were categorized as: apoptotic (7.1%), immune response (11.9%), regulation of transcription (7.1%), ion binding (9.5%), cell adhesion (7.1%), or alternative splicing (7.1%) (Fig. 3A). Treatment with mifepristone compared with untreated normal human myotubes resulted in 27 gene expression changes (Supplementary Table S3). Mifepristone is a known GR antagonist that also antagonizes the progesterone receptor but was used for these studies since no GR-specific antagonists are available. Only one gene was decreased (2-fold) with mifepristone treatment, and 26 genes were increased (2- to 10-fold). A large percentage of genes changed by mifepristone treatment were functionally categorized as regulation of transcription (33.3%), and the remaining genes were categorized as: apoptotic (3.7%), immune response (14.8%), ion binding (7.4%), alternative splicing (7.4%), or developmental (7.4%) (Fig. 3B). Only PRG4 was changed (increased) by both mifepristone and eplerenone treatment compared with untreated controls.
Fig. 3.
Treatment with eplerenone or mifepristone alone caused gene expression changes in normal human myotubes. A: treatment with the selective MR antagonist eplerenone resulted in 42 gene expression changes and functional clustering of these genes revealed: 3 apoptotic, 5 immune or defense response, 3 transcriptional regulators, 4 ion binding, 3 cell adhesion, 3 alternative splicing, 12 muscle contraction, and 9 genes with unknown or specific functions. B: treatment with the GR antagonist mifepristone resulted in 27 gene expression changes and functional clustering of these genes revealed: 1 apoptotic, 4 immune or defense response, 9 regulators of transcription, 2 ion binding, 2 alternative splicing, 2 developmental, and 7 genes with unknown or specific functions.
Co-incubation with aldosterone plus eplerenone or spironolactone results in a similar number of gene expression changes.
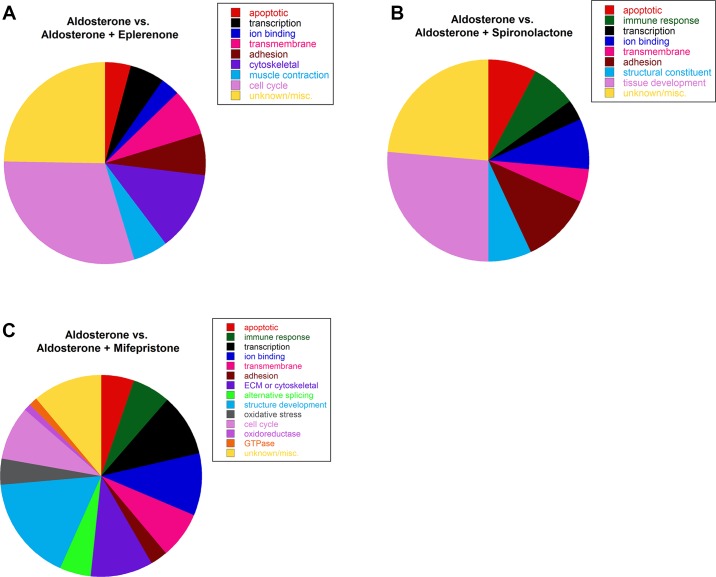
We next wanted to uncover whether aldosterone induces gene expression changes through MR or GR and determine if eplerenone and spironolactone blocked MR from binding aldosterone and becoming activated to the same extent. Therefore, we treated the same normal primary human myotubes with a 100-fold lower concentration of aldosterone more similar to physiological levels. Gene expression changes in normal human myotubes resulting from this aldosterone treatment alone were compared with changes resulting from myotubes incubated with aldosterone in combination with either eplerenone, spironolactone, or mifepristone to determine the effects of MR and GR antagonism in the presence of aldosterone activation of gene expression. There were 93 gene expression changes between aldosterone vs. aldosterone plus eplerenone; eight genes were increased (1.5- to 4-fold), and 85 genes were decreased (1.5- to 3-fold) with eplerenone treatment (Supplementary Table S4). Seventeen of the 42 genes changed by eplerenone treatment compared with untreated controls were also changed in this data set, suggesting that some gene expression differences between aldosterone vs. aldosterone plus eplerenone may be due to the effects of eplerenone alone rather than blocking MR from binding aldosterone. The observation that spironolactone alone did not lead to many gene expression differences suggests that they are not due to constitutive activation of MR in these cells. The majority of genes changed between aldosterone and aldosterone plus eplerenone were functionally categorized as: apoptotic (4.3%), regulation of transcription (5.4%), ion binding (3.2%), transmembrane (7.5%), cell adhesion (6.5%), cytoskeletal protein binding (12.9%), muscle contraction (5.4%), or cell cycle (30.1%) (Fig. 4A).
Fig. 4.
Gene expression changes with low-dose aldosterone compared with aldosterone plus antagonists in normal human myotubes. A: co-incubation with aldosterone plus eplerenone increased 8 genes and decreased 85 genes (93 total) compared with myotubes treated with aldosterone alone. Functional clustering revealed: 4 apoptotic, 7 transcriptional regulators, 5 ion binding, 7 transmembrane, 6 cell adhesion, 11 cytoskeletal protein binding, 4 muscle contraction, 27 cell cycle, and 22 genes with unknown or specific functions. B: co-incubation with aldosterone plus spironolactone increased 24 genes and decreased 90 genes (114 total) compared with myotubes treated with aldosterone alone. Functional clustering revealed: 9 apoptotic, 8 immune or defense response, 4 transcriptional regulators, 9 ion binding, 6 transmembrane, 13 cell adhesion, 8 structural constituents of muscle, 30 tissue development, and 27 genes with unknown or specific functions. C: co-incubation with aldosterone plus mifepristone increased 251 genes and decreased 370 genes (621 total) compared with myotubes treated with aldosterone alone. Functional clustering revealed: 33 apoptotic, 38 immune or defense response, 62 transcriptional regulators, 63 ion binding, 45 transmembrane, 18 cell adhesion, 63 ECM or cytoskeletal protein binding, 30 alternative splicing, 106 vasculature or muscle structure development, 25 oxidative stress responsive, 54 cell cycle, 7 oxidoreductase, 9 GTPase, and 68 genes with unknown or specific functions.
Expression of 114 genes were changed between aldosterone vs. aldosterone plus spironolactone; 24 genes were increased (1.5- to 3-fold) with spironolactone treatment and 90 genes were decreased (1.5- to 3-fold) (Supplementary Table S4). Thirty-six of these genes were also changed in the aldosterone vs. untreated control data set and are likely aldosterone responsive genes. This data, together with the observation that spironolactone treatment alone results in almost no gene expression changes, supports that gene expression changes between aldosterone and aldosterone plus spironolactone result from spironolactone inhibiting aldosterone induced genes. Genes were functionally categorized as: apoptotic (7.9%), immune response (7.0%), regulation of transcription (3.5%), ion binding (7.9%), transmembrane (5.3%), cell adhesion (11.4%), structural constituent of muscle (7.0%), or tissue development (26.3%) (Fig. 4B).
We found 621 genes were changed between aldosterone vs. aldosterone plus mifepristone; 251 genes were increased (1.5- to 8-fold) with mifepristone treatment, and 370 genes were decreased (1.5- to 16-fold), including dystrophin (Supplementary Table S5). In addition, 143 of the 200 genes changed in microarray data comparing aldosterone vs. untreated control were also changed in the aldosterone vs. aldosterone plus mifepristone data set; 14 of the 27 genes changed by mifepristone treatment alone were also changed in the larger aldosterone vs. aldosterone plus mifepristone data set. These data suggest that gene expression changes resulting from aldosterone vs. aldosterone plus mifepristone are due to both mifepristone blocking aldosterone-induced gene expression changes and the effects of mifepristone treatment alone. Genes changed between aldosterone vs. aldosterone plus mifepristone were functionally categorized as: apoptotic (5.3%), immune response (6.1%), regulation of transcription (10.0%), ion binding (10.1%), transmembrane (7.2%), cell adhesion (2.9%), extracellular matrix or cytoskeletal protein binding (10.1%), alternative splicing (4.8%), vasculature or muscle structure development (17.1%), response to oxidative stress (4.0%), cell cycle (8.7%), oxidoreductase (1.1%), or GTPase activity (1.4%) (Fig. 4C).
PRG4 expression is normalized by treatment with MR antagonists in vitro and in vivo.
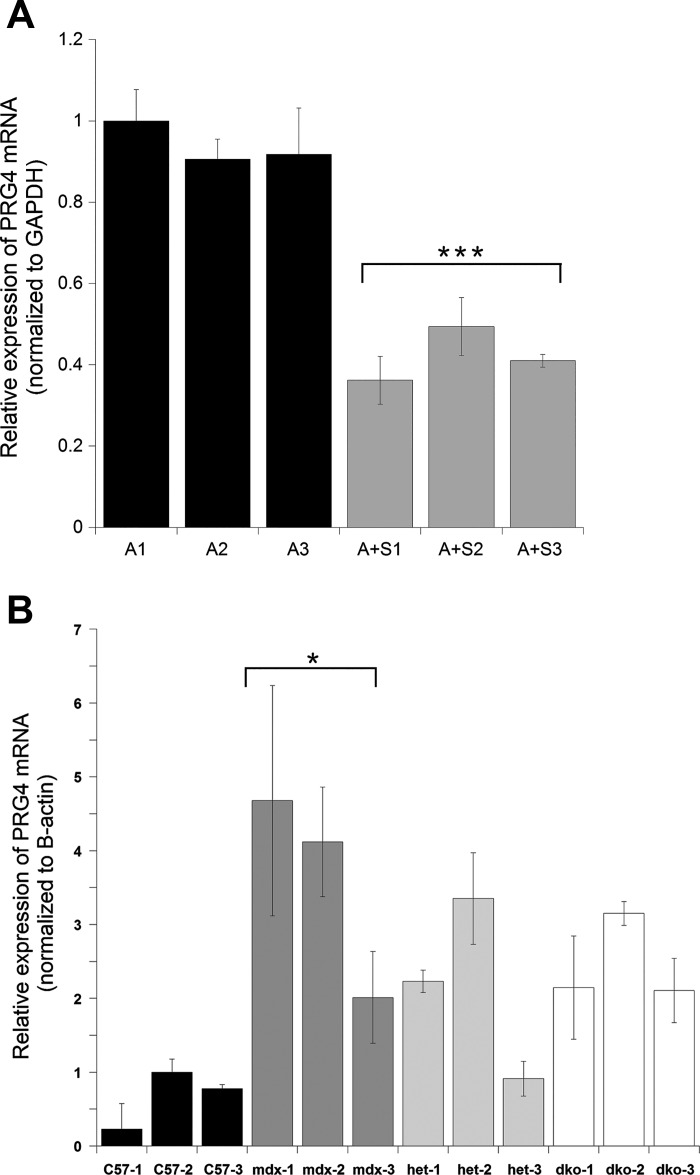
PRG4 was selected for additional analysis because it was robustly increased by aldosterone treatment in vitro across multiple microarray data sets and decreased in vivo in skeletal muscles of dystrophic mice treated with lisinopril plus spironolactone compared with untreated controls (9). Quantitative PCR (qPCR) was used to validate microarray results. Relative PRG4 transcript levels were significantly higher (P = 0.004) in aldosterone-treated human myotubes compared with myotubes treated with aldosterone plus spironolactone (Fig. 5A). Since Prg4 encodes a secreted extracellular matrix protein that could influence the fibrotic phenotype in muscular dystrophy, we next used qPCR to determine whether Prg4 transcript levels differ between muscles from normal and dystrophic mice. Prg4 transcript levels were higher in dystrophic mouse muscle relative to wild-type (Fig. 5B), supporting the idea that Prg4 expression is increased in DMD mouse models and normalized by MR antagonist treatment.
Fig. 5.
Real-time PCR revealed PRG4 transcripts are decreased with spironolactone treatment and upregulated in dystrophic muscle. A: PRG4 is significantly decreased (P = 0.0004) in human myotube samples co-incubated with 100 nM aldosterone plus 10 µM spironolactone compared with myotubes treated with 100 nM aldosterone only. B: Prg4 is increased in gastrocnemius muscles from mdx (dystrophin-deficient), het (utrn+/−, mdx) and dko (double knockout, utrn−/−; mdx) mice relative to C57 (wild type). Prg4 expression was significantly increased (P = 0.0175) in mdx gastrocnemius muscle compared with wild type. Each bar represents the mean of 3 technical triplicates ± SE. Three independent biological replicates are shown for each genotype or treatment group. Human myotube samples were normalized to the mean of A-1 and mouse gastrocnemius samples were normalized to the mean of C57-2. GAPDH was used as the normalization control for human myotube samples and β-actin was used as the normalization control for mouse gastrocnemius samples. A, aldosterone; S, spironolactone. ***P < 0.001 and *P < 0.05.
DISCUSSION
Similar to other nuclear transcription factors, ligand-activated MR alters expression of target genes in an early phase, at 1–3 h, and a late phase containing both early-phase genes and their downstream targets, in addition to nongenomic effects mediated by MR (6, 44, 55). Since MR and GR gene expression effects in skeletal muscle are largely unknown, we first treated normal human myotubes with supraphysiological concentrations of MR and GR agonists and antagonists for 48 h to identify maximal effects on gene expression and ensure microarray data encompassed both early- and late-stage gene expression changes. Although treatment of human kidney cells with aldosterone is known to result in ligand-induced phosphorylation of the receptor and decreased MR protein levels within 4 h (1), no decrease of MR was observed over the 48 h treatment of normal human skeletal muscle myotubes (Fig. 1). These data suggest either the absence of MR degradation in differentiated muscle or a balance between degradation and new MR expression.
In our previous microarray experiment, we treated normal human myotubes with 10 µM aldosterone for 48 h, which resulted in only 14 gene expression changes (9). When we repeated this microarray under the same conditions, 200 genes were changed with aldosterone, likely due to differences in the primary human myoblast cells used. For our first experiment we combined two lots of normal human myoblasts from healthy adult males (from Lonza) to limit individual bias. However, for the current microarray study we were only able to obtain normal human myoblasts from a single younger healthy adolescent male (from Lonza). Ten of the 14 previously observed gene changes were repeated in the current data set (9). This difference between donors could also explain the large proportion of genes functionally categorized as cell cycle or developmental. Since DMD is a pediatric disease affecting young boys, microarray data obtained from normal adolescent male muscle myotubes should provide better and more comprehensive data sets for future studies.
Treatment with the glucocorticoid prednisolone resulted in 483 gene expression changes, which included 189 of the 200 genes changed with aldosterone treatment. These data suggest that prednisolone is both a GR and MR agonist in normal human myotubes, which could be detrimental to muscle tissue since MR antagonists benefit skeletal muscles in dystrophic mice and prednisolone increases damage (9, 29, 39, 45). Even though prednisolone is classified as a GR agonist, previous studies have shown it can also activate MR at commonly used therapeutic doses (5, 24, 35). Transactivation studies in a CV-1 monkey kidney cell line demonstrated similar EC50 values for MR (EC50: 3.7 nM) and GR (EC50: 6.9 nM) activation by prednisolone (25). A caveat is that the transcript encoding the glucocorticoid-responsive GR α-isoform has several alternative translation initiation sites that produce several truncated isoforms, but functional studies have focused on the full-length GRα-A (41). Future studies will need to be conducted to define the contribution of these truncated isoforms as well as the nonglucocorticoid-binding dominant negative GR β-isoforms in muscle.
Treatment of normal human myotubes with spironolactone alone resulted in almost no gene expression changes, consistent with our previously published microarray data (GSE70822). These data support the notion that gene expression changes observed between separate antagonist (spironolactone)- and agonist (aldosterone)-treated myotubes were aldosterone dependent and not caused by the presence of the glucocorticoid cortisol in the media. These data also suggest that even high-dose spironolactone has minimal off-target effects in skeletal muscle. Surprisingly, treatment with eplerenone, the selective MR antagonist, resulted in many gene expression changes including reductions of muscle-specific genes. These data suggest the potential for off-target effects of eplerenone, although lower doses would need to be tested. Aldosterone co-incubations with spironolactone or eplerenone resulted in similar numbers of gene expression changes, suggesting both drugs can block MR binding to aldosterone to a similar extent. However, a proportion of gene expression changes between aldosterone and aldosterone plus eplerenone appears to be caused by eplerenone treatment alone.
Since the levels of MR relative to GR has been shown to be different between human and rodents, these experiments conducted on human myotubes are likely to have relevance for monitoring direct skeletal muscle effects of MR and GR drugs in DMD patients (30). However, since treatment of patients will also include targeting MR from immune cells and fibroblasts contained within dystrophic muscles, the comparison with gene expression data generated from dystrophic mice may highlight some of the most biologically relevant changes from MR antagonism. Several of the gene expression changes between high-dose aldosterone alone compared with spironolactone treatment in the current study were conserved in our previously published in vitro microarray (GSE70822) and in vivo gene expression data from quadriceps muscles of dystrophic mice treated with lisinopril plus an MR antagonist vs. untreated controls (GSE70984 and GSE84876). ADAMTS1, ANKRD1, CFD, CIDEC, FOS, NPR3, PRG4, and TJP2 are decreased by MR antagonists, and CHAC1, PER3, and TNFAIP6 are decreased by aldosterone (9, 45).
Several of these upregulated genes are likely to play a role in exacerbating muscle damage and fibrosis in dystrophic skeletal muscles, and reducing their expression may underlie the efficacy of MR antagonist treatment on dystrophic pathogenesis. ANKRD1 is a transcriptional cofactor and sarcomeric component involved in mechanosignaling transduction in response to injury (32). ADAMST1 is regulated by MR and GR and plays roles in extracellular matrix remodeling and inflammation (53). FOS is involved in aldosterone-dependent cardiac remodeling, hypertrophy, and fibrosis (17). Prg4 is an extracellular matrix protein involved in organizing articular cartilage and has previously been shown to be decreased in cardiomyocyte-specific MR knockout mice following myocardial infarction (19, 31). Two additional extracellular matrix genes, COL8A1 and MGP, were consistently increased by aldosterone compared with spironolactone treatment in vitro in our studies and have been shown to be decreased in MR knockout mice following myocardial infarction (19). These secreted extracellular matrix proteins may modify fibroblast signaling to promote a fibrotic phenotype, which is an important pathological feature of muscular dystrophy.
Other conserved MR-regulated genes may be involved in cell metabolism, but their role in muscle and muscular dystrophy is less clear and will require further investigation. PER3 is a molecular clock gene that may contribute to muscle metabolism, and Per3 knockout mice have reduced muscle and increased adipose mass (12). NPR3 function has not been investigated in skeletal muscle but is known to help maintain blood pressure and extracellular fluid volume (3), which are both MR-regulated pathways (38). TJP2 is an important molecular target in vascular remodeling but has not been studied in muscle (40).
ANKRD1 has been recognized as a potential biomarker for several muscle diseases including: muscular dystrophies, muscle wasting, motor neuron diseases, and congenital myopathies (36). Proteins expressed from the other genes changed by MR antagonism may also be useful to develop as biomarkers for monitoring clinical efficacy of these drugs.
This study identifies several potential gene targets that may underlie the demonstrated preclinical efficacy of mineralocorticoid receptor antagonists in dystrophic skeletal muscles. We have also shown that treatment with the glucocorticoid prednisolone results in a large number of gene expression changes, including both MR- and GR-responsive genes, supporting the notion that prednisolone acts as an MR and GR agonist and may have detrimental effects on skeletal muscle. Mifepristone is not a viable treatment option for DMD because it leads to a large number of gene expression changes and decreases dystrophin expression, the causative gene for DMD. Treatment with the nonspecific MR antagonist spironolactone alone resulted in very few gene expression changes compared with the selective MR antagonist eplerenone. Furthermore, spironolactone was able to inhibit a larger proportion of aldosterone-induced genes and had higher conservation across in vitro and in vivo microarray data sets compared with eplerenone. Together, these data suggest spironolactone may demonstrate more long-term efficacy than eplerenone in dystrophic skeletal muscles.
GRANTS
This work was supported by National Institutes of Health Grants R01 NS-082868 and R01 HL-116533 and Department of Defense Grant MD120063 to J. A. Rafael-Fortney.
DISCLOSURES
The authors have no conflict of interest relevant to this study.
AUTHOR CONTRIBUTIONS
J.A.C. and J.A.R.-F. conceived and designed research; J.A.C., J.S.H., and C.E.G.-S. performed experiments; J.A.C., J.S.H., and J.A.R.-F. analyzed data; J.A.C., E.P.G.-S., and J.A.R.-F. interpreted results of experiments; J.A.C. and J.S.H. prepared figures; J.A.C. drafted manuscript; J.A.C., J.S.H., C.E.G.-S., E.P.G.-S., and J.A.R.-F. approved final version of manuscript; J.S.H., C.E.G.-S., E.P.G.-S., and J.A.R.-F. edited and revised manuscript.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank OSU's Genomics Shared Resource, and in particular S. Warner for microarray processing; Pfizer for supplying eplerenone under their compound transfer program; J. Lowe (OSU) for technical assistance in maintaining mouse colonies, dissections, and genotyping.
REFERENCES
- 1.Amazit L, Le Billan F, Kolkhof P, Lamribet K, Viengchareun S, Fay MR, Khan JA, Hillisch A, Lombès M, Rafestin-Oblin ME, Fagart J. Finerenone impedes aldosterone-dependent nuclear import of the mineralocorticoid receptor and prevents genomic recruitment of steroid receptor coactivator-1. J Biol Chem 290: 21876–21889, 2015. doi: 10.1074/jbc.M115.657957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amazit L, Roseau A, Khan JA, Chauchereau A, Tyagi RK, Loosfelt H, Leclerc P, Lombès M, Guiochon-Mantel A. Ligand-dependent degradation of SRC-1 is pivotal for progesterone receptor transcriptional activity. Mol Endocrinol 25: 394–408, 2011. doi: 10.1210/me.2010-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anand-Srivastava MB. Natriuretic peptide receptor-C signaling and regulation. Peptides 26: 1044–1059, 2005. doi: 10.1016/j.peptides.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 4.Arriza JL, Weinberger C, Cerelli G, Glaser TM, Handelin BL, Housman DE, Evans RM. Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor. Science 237: 268–275, 1987. doi: 10.1126/science.3037703. [DOI] [PubMed] [Google Scholar]
- 5.Bauer R, Blain A, Greally E, Lochmüller H, Bushby K, MacGowan GA, Straub V. Attenuation of adverse cardiac effects in prednisolone-treated delta-sarcoglycan-deficient mice by mineralocorticoid-receptor-antagonism. Neuromuscul Disord 20: 21–28, 2010. doi: 10.1016/j.nmd.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Bhalla V, Soundararajan R, Pao AC, Li H, Pearce D. Disinhibitory pathways for control of sodium transport: regulation of ENaC by SGK1 and GILZ. Am J Physiol Renal Physiol 291: F714–F721, 2006. doi: 10.1152/ajprenal.00061.2006. [DOI] [PubMed] [Google Scholar]
- 7.Bulfield G, Siller WG, Wight PA, Moore KJ. X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc Natl Acad Sci USA 81: 1189–1192, 1984. doi: 10.1073/pnas.81.4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bushby K, Finkel R, Birnkrant DJ, Case LE, Clemens PR, Cripe L, Kaul A, Kinnett K, McDonald C, Pandya S, Poysky J, Shapiro F, Tomezsko J, Constantin C; DMD Care Considerations Working Group . Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurol 9: 77–93, 2010. doi: 10.1016/S1474-4422(09)70271-6. [DOI] [PubMed] [Google Scholar]
- 9.Chadwick JA, Hauck JS, Lowe J, Shaw JJ, Guttridge DC, Gomez-Sanchez CE, Gomez-Sanchez EP, Rafael-Fortney JA. Mineralocorticoid receptors are present in skeletal muscle and represent a potential therapeutic target. FASEB J 29: 4544–4554, 2015. doi: 10.1096/fj.15-276782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chadwick JA, Swager SA, Lowe J, Welc SS, Tidball JG, Gomez-Sanchez CE, Gomez-Sanchez EP, Rafael-Fortney JA. Myeloid cells are capable of synthesizing aldosterone to exacerbate damage in muscular dystrophy. Hum Mol Genet 25: 5167–5177, 2016. doi: 10.1093/hmg/ddw331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen J, Bardes EE, Aronow BJ, Jegga AG. ToppGene Suite for gene list enrichment analysis and candidate gene prioritization. Nucleic Acids Res 37, Web Server: W305–W311, 2009. doi: 10.1093/nar/gkp427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costa MJ, So AY, Kaasik K, Krueger KC, Pillsbury ML, Fu YH, Ptacek LJ, Yamamoto KR, Feldman BJ. Circadian rhythm gene period 3 is an inhibitor of the adipocyte cell fate. J Biol Chem 286: 9063–9070, 2011. doi: 10.1074/jbc.M110.164558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol 335: 2–13, 2011. doi: 10.1016/j.mce.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deconinck AE, Rafael JA, Skinner JA, Brown SC, Potter AC, Metzinger L, Watt DJ, Dickson JG, Tinsley JM, Davies KE. Utrophin-dystrophin-deficient mice as a model for Duchenne muscular dystrophy. Cell 90: 717–727, 1997. doi: 10.1016/S0092-8674(00)80532-2. [DOI] [PubMed] [Google Scholar]
- 15.Evans RM. The steroid and thyroid hormone receptor superfamily. Science 240: 889–895, 1988. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farman N, Rafestin-Oblin ME. Multiple aspects of mineralocorticoid selectivity. Am J Physiol Renal Physiol 280: F181–F192, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Fiebeler A, Schmidt F, Müller DN, Park JK, Dechend R, Bieringer M, Shagdarsuren E, Breu V, Haller H, Luft FC. Mineralocorticoid receptor affects AP-1 and nuclear factor-kappab activation in angiotensin II-induced cardiac injury. Hypertension 37: 787–793, 2001. doi: 10.1161/01.HYP.37.2.787. [DOI] [PubMed] [Google Scholar]
- 18.Fisher I, Abraham D, Bouri K, Hoffman EP, Muntoni F, Morgan J. Prednisolone-induced changes in dystrophic skeletal muscle. FASEB J 19: 834–836, 2005.. [DOI] [PubMed] [Google Scholar]
- 19.Fraccarollo D, Berger S, Galuppo P, Kneitz S, Hein L, Schütz G, Frantz S, Ertl G, Bauersachs J. Deletion of cardiomyocyte mineralocorticoid receptor ameliorates adverse remodeling after myocardial infarction. Circulation 123: 400–408, 2011. doi: 10.1161/CIRCULATIONAHA.110.983023. [DOI] [PubMed] [Google Scholar]
- 20.Gomez-Sanchez CE, de Rodriguez AF, Romero DG, Estess J, Warden MP, Gomez-Sanchez MT, Gomez-Sanchez EP. Development of a panel of monoclonal antibodies against the mineralocorticoid receptor. Endocrinology 147: 1343–1348, 2006. doi: 10.1210/en.2005-0860. [DOI] [PubMed] [Google Scholar]
- 21.Gomez-Sanchez E, Gomez-Sanchez CE. The multifaceted mineralocorticoid receptor. Compr Physiol 4: 965–994, 2014. doi: 10.1002/cphy.c130044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gomez-Sanchez EP. Brain mineralocorticoid receptors: orchestrators of hypertension and end-organ disease. Curr Opin Nephrol Hypertens 13: 191–196, 2004. doi: 10.1097/00041552-200403000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Gomez-Sanchez EP, Gomez-Sanchez MT, de Rodriguez AF, Romero DG, Warden MP, Plonczynski MW, Gomez-Sanchez CE. Immunohistochemical demonstration of the mineralocorticoid receptor, 11beta-hydroxysteroid dehydrogenase-1 and -2, and hexose-6-phosphate dehydrogenase in rat ovary. J Histochem Cytochem 57: 633–641, 2009. doi: 10.1369/jhc.2009.953059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gross ND, Kempton JB, Trune DR. Spironolactone blocks glucocorticoid-mediated hearing preservation in autoimmune mice. Laryngoscope 112: 298–303, 2002. doi: 10.1097/00005537-200202000-00018. [DOI] [PubMed] [Google Scholar]
- 25.Grossmann C, Scholz T, Rochel M, Bumke-Vogt C, Oelkers W, Pfeiffer AF, Diederich S, Bahr V. Transactivation via the human glucocorticoid and mineralocorticoid receptor by therapeutically used steroids in CV-1 cells: a comparison of their glucocorticoid and mineralocorticoid properties. Eur J Endocrinol 151: 397–406, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Hawkins UA, Gomez-Sanchez EP, Gomez-Sanchez CM, Gomez-Sanchez CE. The ubiquitous mineralocorticoid receptor: clinical implications. Curr Hypertens Rep 14: 573–580, 2012. doi: 10.1007/s11906-012-0297-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heier CR, Damsker JM, Yu Q, Dillingham BC, Huynh T, Van der Meulen JH, Sali A, Miller BK, Phadke A, Scheffer L, Quinn J, Tatem K, Jordan S, Dadgar S, Rodriguez OC, Albanese C, Calhoun M, Gordish-Dressman H, Jaiswal JK, Connor EM, McCall JM, Hoffman EP, Reeves EK, Nagaraju K. VBP15, a novel anti-inflammatory and membrane-stabilizer, improves muscular dystrophy without side effects. EMBO Mol Med 5: 1569–1585, 2013. doi: 10.1002/emmm.201302621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4: 44–57, 2009. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 29.Janssen PM, Murray JD, Schill KE, Rastogi N, Schultz EJ, Tran T, Raman SV, Rafael-Fortney JA. Prednisolone attenuates improvement of cardiac and skeletal contractile function and histopathology by lisinopril and spironolactone in the mdx mouse model of Duchenne muscular dystrophy. PLoS One 9: e88360, 2014. doi: 10.1371/journal.pone.0088360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.John K, Marino JS, Sanchez ER, Hinds TD Jr. The glucocorticoid receptor: cause of or cure for obesity? Am J Physiol Endocrinol Metab 310: E249–E257, 2016. doi: 10.1152/ajpendo.00478.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klein TJ, Malda J, Sah RL, Hutmacher DW. Tissue engineering of articular cartilage with biomimetic zones. Tissue Eng Part B Rev 15: 143–157, 2009. doi: 10.1089/ten.teb.2008.0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kojic S, Radojkovic D, Faulkner G. Muscle ankyrin repeat proteins: their role in striated muscle function in health and disease. Crit Rev Clin Lab Sci 48: 269–294, 2011. doi: 10.3109/10408363.2011.643857. [DOI] [PubMed] [Google Scholar]
- 33.Krozowski ZS, Funder JW. Renal mineralocorticoid receptors and hippocampal corticosterone-binding species have identical intrinsic steroid specificity. Proc Natl Acad Sci USA 80: 6056–6060, 1983. doi: 10.1073/pnas.80.19.6056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuo T, Harris CA, Wang JC. Metabolic functions of glucocorticoid receptor in skeletal muscle. Mol Cell Endocrinol 380: 79–88, 2013. doi: 10.1016/j.mce.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lan NC, Graham B, Bartter FC, Baxter JD. Binding of steroids to mineralocorticoid receptors: implications for in vivo occupancy by glucocorticoids. J Clin Endocrinol Metab 54: 332–342, 1982. doi: 10.1210/jcem-54-2-332. [DOI] [PubMed] [Google Scholar]
- 36.Laure L, Suel L, Roudaut C, Bourg N, Ouali A, Bartoli M, Richard I, Danièle N. Cardiac ankyrin repeat protein is a marker of skeletal muscle pathological remodelling. FEBS J 276: 669–684, 2009. doi: 10.1111/j.1742-4658.2008.06814.x. [DOI] [PubMed] [Google Scholar]
- 37.Lonard DM, Nawaz Z, Smith CL, O’Malley BW. The 26S proteasome is required for estrogen receptor-alpha and coactivator turnover and for efficient estrogen receptor-alpha transactivation. Mol Cell 5: 939–948, 2000. doi: 10.1016/S1097-2765(00)80259-2. [DOI] [PubMed] [Google Scholar]
- 38.Lother A, Moser M, Bode C, Feldman RD, Hein L. Mineralocorticoids in the heart and vasculature: new insights for old hormones. Annu Rev Pharmacol Toxicol 55: 289–312, 2015. doi: 10.1146/annurev-pharmtox-010814-124302. [DOI] [PubMed] [Google Scholar]
- 39.Lowe J, Wodarcyk AJ, Floyd KT, Rastogi N, Schultz EJ, Swager SA, Chadwick JA, Tran T, Raman SV, Janssen PM, Rafael-Fortney JA. The Angiotensin Converting Enzyme Inhibitor Lisinopril Improves Muscle Histopathology but not Contractile Function in a Mouse Model of Duchenne Muscular Dystrophy. J Neuromuscul Dis 2: 257–268, 2015. doi: 10.3233/JND-150099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Misior AM, Deshpande DA, Loza MJ, Pascual RM, Hipp JD, Penn RB. Glucocorticoid- and protein kinase A-dependent transcriptome regulation in airway smooth muscle. Am J Respir Cell Mol Biol 41: 24–39, 2009. doi: 10.1165/rcmb.2008-0266OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oakley RH, Cidlowski JA. The biology of the glucocorticoid receptor: new signaling mechanisms in health and disease. J Allergy Clin Immunol 132: 1033–1044, 2013. doi: 10.1016/j.jaci.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Odermatt A, Arnold P, Frey FJ. The intracellular localization of the mineralocorticoid receptor is regulated by 11beta-hydroxysteroid dehydrogenase type 2. J Biol Chem 276: 28484–28492, 2001. doi: 10.1074/jbc.M100374200. [DOI] [PubMed] [Google Scholar]
- 43.Odermatt A, Kratschmar DV. Tissue-specific modulation of mineralocorticoid receptor function by 11β-hydroxysteroid dehydrogenases: an overview. Mol Cell Endocrinol 350: 168–186, 2012. doi: 10.1016/j.mce.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 44.Pearce D, Bhargava A, Cole TJ. Aldosterone: its receptor, target genes, and actions. Vitam Horm 66: 29–76, 2003. doi: 10.1016/S0083-6729(03)01002-1. [DOI] [PubMed] [Google Scholar]
- 45.Rafael-Fortney JA, Chimanji NS, Schill KE, Martin CD, Murray JD, Ganguly R, Stangland JE, Tran T, Xu Y, Canan BD, Mays TA, Delfín DA, Janssen PM, Raman SV. Early treatment with lisinopril and spironolactone preserves cardiac and skeletal muscle in Duchenne muscular dystrophy mice. Circulation 124: 582–588, 2011. doi: 10.1161/CIRCULATIONAHA.111.031716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raman SV, Hor KN, Mazur W, Halnon NJ, Kissel JT, He X, Tran T, Smart S, McCarthy B, Taylor MD, Jefferies JL, Rafael-Fortney JA, Lowe J, Roble SL, Cripe LH. Eplerenone for early cardiomyopathy in Duchenne muscular dystrophy: a randomised, double-blind, placebo-controlled trial. Lancet Neurol 14: 153–161, 2015. doi: 10.1016/S1474-4422(14)70318-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rupprecht R, Reul JM, van Steensel B, Spengler D, Söder M, Berning B, Holsboer F, Damm K. Pharmacological and functional characterization of human mineralocorticoid and glucocorticoid receptor ligands. Eur J Pharmacol 247: 145–154, 1993. doi: 10.1016/0922-4106(93)90072-H. [DOI] [PubMed] [Google Scholar]
- 48.Sali A, Guerron AD, Gordish-Dressman H, Spurney CF, Iantorno M, Hoffman EP, Nagaraju K. Glucocorticoid-treated mice are an inappropriate positive control for long-term preclinical studies in the mdx mouse. PLoS One 7: e34204, 2012. doi: 10.1371/journal.pone.0034204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmidt TJ, Husted RF, Stokes JB. Steroid hormone stimulation of Na+ transport in A6 cells is mediated via glucocorticoid receptors. Am J Physiol Cell Physiol 264: C875–C884, 1993. [DOI] [PubMed] [Google Scholar]
- 50.Shen T, Horwitz KB, Lange CA. Transcriptional hyperactivity of human progesterone receptors is coupled to their ligand-dependent down-regulation by mitogen-activated protein kinase-dependent phosphorylation of serine 294. Mol Cell Biol 21: 6122–6131, 2001. doi: 10.1128/MCB.21.18.6122-6131.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sicinski P, Geng Y, Ryder-Cook AS, Barnard EA, Darlison MG, Barnard PJ. The molecular basis of muscular dystrophy in the mdx mouse: a point mutation. Science 244: 1578–1580, 1989. doi: 10.1126/science.2662404. [DOI] [PubMed] [Google Scholar]
- 52.Stockand JD. New ideas about aldosterone signaling in epithelia. Am J Physiol Renal Physiol 282: F559–F576, 2002. doi: 10.1152/ajprenal.00320.2001. [DOI] [PubMed] [Google Scholar]
- 53.Torres-Collado AX, Kisiel W, Iruela-Arispe ML, Rodríguez-Manzaneque JC. ADAMTS1 interacts with, cleaves, and modifies the extracellular location of the matrix inhibitor tissue factor pathway inhibitor-2. J Biol Chem 281: 17827–17837, 2006. doi: 10.1074/jbc.M513465200. [DOI] [PubMed] [Google Scholar]
- 54.van Uum SH, Hermus AR, Smits P, Thien T, Lenders JW. The role of 11 beta-hydroxysteroid dehydrogenase in the pathogenesis of hypertension. Cardiovasc Res 38: 16–24, 1998. doi: 10.1016/S0008-6363(97)00299-X. [DOI] [PubMed] [Google Scholar]
- 55.Viengchareun S, Le Menuet D, Martinerie L, Munier M, Pascual-Le Tallec L, Lombès M. The mineralocorticoid receptor: insights into its molecular and (patho)physiological biology. Nucl Recept Signal 5: e012, 2007. doi: 10.1621/nrs.05012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weinberger C, Giguère V, Hollenberg SM, Thompson C, Arriza J, Evans RM. Human steroid receptors and erb-A gene products form a superfamily of enhancer-binding proteins. Clin Physiol Biochem 5: 179–189, 1987. [PubMed] [Google Scholar]
- 57.Wu Z. A review of statistical methods for preprocessing oligonucleotide microarrays. Stat Methods Med Res 18: 533–541, 2009. doi: 10.1177/0962280209351924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang J, Young MJ. The mineralocorticoid receptor and its coregulators. J Mol Endocrinol 43: 53–64, 2009. doi: 10.1677/JME-09-0031. [DOI] [PubMed] [Google Scholar]
- 59.Yang J, Young MJ. Mineralocorticoid receptor antagonists-pharmacodynamics and pharmacokinetic differences. Curr Opin Pharmacol 27: 78–85, 2016. doi: 10.1016/j.coph.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 60.Zhou L, Rafael-Fortney JA, Huang P, Zhao XS, Cheng G, Zhou X, Kaminski HJ, Liu L, Ransohoff RM. Haploinsufficiency of utrophin gene worsens skeletal muscle inflammation and fibrosis in mdx mice. J Neurol Sci 264: 106–111, 2008. doi: 10.1016/j.jns.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.