The ScheBo Pancreatic Elastase 1 stool test is widely used to assess pancreatic exocrine function, yet its molecular targets have been poorly defined. We demonstrate that, among the human pancreatic proteinases, the test measures the elastase isoform chymotrypsin-like elastase (CELA) 3B (CELA3B) and, to a lesser extent, CELA3A. Genetic variants of the human CELA3 isoforms have no significant effect on test performance.
Keywords: pancreas, digestive proteinase, elastase, chronic pancreatitis, pancreatic insufficiency
Abstract
Determination of fecal pancreatic elastase content by ELISA is a reliable, noninvasive clinical test for assessing exocrine pancreatic function. Despite the widespread use of commercial tests, their exact molecular targets remain poorly characterized. This study was undertaken to clarify which human pancreatic elastase isoforms are detected by the ScheBo Pancreatic Elastase 1 Stool Test and whether naturally occurring genetic variants influence the performance of this test. Using recombinantly expressed and purified human pancreatic proteinases, we found that the test specifically measured chymotrypsin-like elastases (CELA) 3A and 3B (CELA3A and CELA3B), while CELA2A was not detected. Inactive proelastases, active elastases, and autolyzed forms were detected with identical efficiency. CELA3B elicited approximately four times higher ELISA signal than CELA3A, and we identified Glu154 in CELA3B as the critical determinant of detection. Common genetic variants of CELA3A and CELA3B had no effect on test performance, with the exception of the CELA3B variant W79R, which increased detection by 1.4-fold. Finally, none of the human trypsin and chymotrypsin isoforms were detected. We conclude that the ScheBo Pancreatic Elastase 1 Stool Test is specific for human CELA3A and CELA3B, with most of the ELISA signal attributable to CELA3B.
NEW & NOTEWORTHY The ScheBo Pancreatic Elastase 1 Stool Test is widely used to assess pancreatic exocrine function, yet its molecular targets have been poorly defined. We demonstrate that, among the human pancreatic proteinases, the test measures the elastase isoform CELA3B and, to a lesser extent, CELA3A. Genetic variants of the human CELA3 isoforms have no significant effect on test performance.
diseases of the pancreas that result in loss of functional acinar cells can compromise digestive enzyme production and eventually lead to maldigestion. Clinical laboratory tests that quantify decreased digestive enzyme output can aid in the diagnosis of pancreatic insufficiency. The most widely used tests measure levels of a pancreatic elastase enzyme in the stool (1, 7, 8, 12, 13, 15, 18-20, 23, 30, 32, 44). The chymotrypsin-like elastases (CELAs) are digestive serine proteinases secreted by the pancreas. CELA1 was first described in 1949 by the Hungarian scientists Baló and Banga as an enzyme in the pig pancreas capable of hydrolyzing insoluble elastin (3, 4). Because of its cationic character, CELA1 can absorb to the surface of the negatively charged elastin fibers and cleave multiple Ala-Ala and Ala-Gly peptide bonds (9, 10, 43). Despite its name, CELA1 is not a specific elastin-degrading enzyme, and it readily digests a variety of dietary protein substrates. The primary specificity pocket of CELA1 accommodates small (Ala and Ser) and aliphatic (Ile, Leu, Met, and Val) amino acid side chains at the so-called P1 position of its substrates [Schechter-Berger nomenclature (29) of proteinase-substrate interactions, where P1-P1′ corresponds to the scissile peptide bond] (Ref. 5 and references therein). Curiously, while the human CELA1 gene appears to be potentially functional, it is not expressed in the pancreas because of evolutionary mutations in its promoter and enhancer regions (26, 41). A second pancreatic elastase (CELA2) was identified on the basis of its ability to solubilize elastin (Ref. 17; for a complete list of references see Ref. 5). Unlike CELA1, this elastase exhibits chymotrypsin-like P1 specificity and prefers to cleave after aromatic (Tyr and Phe) and aliphatic (Leu and Met) P1 amino acids (6, 17, 34). In humans, evolutionary duplication of CELA2 gave rise to the CELA2A and CELA2B genes. Even though both genes are expressed at the mRNA level (14), only the CELA2A enzyme is functional, as CELA2B seems to have accumulated inactivating evolutionary mutations (35). The CELA2A content of pancreatic juice corresponds to ~10% of total protein (24).
Arguably, CELA3 has the most interesting history and characteristics among the human elastases. This elastase gene is also duplicated in humans, and the two closely related isoforms were designated CELA3A and CELA3B (31, 42). Both are expressed in the pancreas at comparable mRNA and protein levels (31, 42). Substrate specificity of human CELA3A and CELA3B appears to be similar to that of porcine CELA1, broadly directed toward aliphatic P1 side chains (Ref. 5 and references therein). CELA3B was first described in 1975 as protease E, an anionic pancreatic proteinase devoid of elastolytic activity (21). A subsequent study in 1976 isolated human CELA3B and CELA2A and designated these enzymes elastase 1 and elastase 2, respectively (17). For reasons that remain unclear, the authors found that CELA3B was capable of solubilizing elastin, an erroneous observation, which, at the time, justified the elastase 1 name. Finally, in a number of studies starting in 1982, Sziegoleit and co-workers characterized a so-called cholesterol-binding protein with proteolytic activity, which eventually was found to correspond to CELA3B (36, 37, 39). These authors also determined that the CELA3B content of human pancreatic juice accounts for 4–6% of total protein (36). Thus the combined levels of CELA3A and CELA3B are similar to that of CELA2A. Spurred by the observation that CELA3B suffers no proteolytic degradation during intestinal transit and appears in the stool in high concentrations (38), ELISA tests have been developed for the detection of stool elastase, and their clinical utility in the diagnosis of pancreatic insufficiency has been demonstrated (7, 8, 12, 15, 18-20, 23, 30, 32). One of the most widely used assays is the ScheBo Pancreatic Elastase 1 Stool Test (ScheBo Biotech, Giessen, Germany), which utilizes two monoclonal antibodies raised against CELA3B to measure enzyme levels. However, it remains unclear whether the test also detects other elastases, CELA3A in particular, and the extent to which the homologous pancreatic trypsins and chymotrypsins might interfere with the assay. More importantly, the potential confounding effect of natural CELA3 variants on test performance has not been evaluated. In the present study we set out to fill these knowledge gaps, and using well-defined recombinant pancreatic proteinases, we characterized the detection specificity of the ScheBo ELISA test.
MATERIALS AND METHODS
Materials.
The ScheBo Pancreatic Elastase 1 Stool Test was purchased from the manufacturer. For some of the experiments, we used the ScheBo Pancreatic Elastase 1 Serum Test, which contains essentially the same ELISA components.
Nomenclature.
Coding DNA numbering starts with the first nucleotide of the translation initiator codon, which was designated c.1. Amino acid residues were numbered starting with the initiator methionine of the primary translation product. Note that the CELA3A and CELA3B genomic reference sequences (chromosome 1 primary assembly, NC_000001.11) contain the minor allelic variants G241A and W79R, respectively. In the present study we used the common alleles at these positions as the reference and designated G241A and W79R the minor variants.
Plasmid construction and mutagenesis.
Expression plasmids for human elastases CELA2A, CELA3A, and CELA3B and chymotrypsins CTRB1, CTRB2, CTRC, and CTRL1 constructed in the pcDNA3.1(−) vector (Ref. 33 and references therein) and for human trypsins PRSS1, PRSS2, and PRSS3 in the pTrapT7 vector are described elsewhere (16, 27, 28, 40). The plasmids contain the coding DNA for the proenzyme (zymogen) form of the indicated pancreatic proteinases. Mutations in CELA3A and CELA3B were introduced by overlap extension PCR mutagenesis. The coding DNA for autolyzed forms of CELA3B carrying nine His residues at their COOH terminus was created by gene synthesis and cloned into the pcDNA3.1(−) plasmid using EcoRI and BamHI restriction sites. In the CELA3B-9del construct, the NH2-terminal nine amino acids from Tyr18 to Ser26 were deleted. In the CELA3B-13del construct, the NH2-terminal 13 amino acids from Tyr18 to Val30 were removed.
Cell culture and transfection.
For small-scale expression studies, HEK 293T cells were grown in six-well tissue culture plates (1.5 × 106 cells per well) in Dulbecco's modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum, 4 mM glutamine, and 1% penicillin-streptomycin at 37°C. Transfections were performed with 4 µg of expression plasmid with 10 µl of Lipofectamine 2000 in 2 ml of DMEM. After overnight incubation, cells were rinsed and covered with 2 ml of Opti-MEM reduced-serum medium. The conditioned media were harvested after 48 h.
Purification of pancreatic proteinases.
Human proelastases and chymotrypsinogens were expressed in transiently transfected HEK 293T cells and purified from the conditioned medium through their COOH-terminal His tags by nickel-affinity chromatography, as reported elsewhere (33). Human trypsinogens were expressed in Escherichia coli as insoluble inclusion bodies. Refolding and purification on immobilized ecotin were performed according to our published protocol (16, 27, 28, 40). Concentrations of proenzyme solutions were determined on the basis of their UV absorbance at 280 nm with use of the following extinction coefficients, calculated with the web-based ProtParam tool (in M−1·cm−1): 73,505 for CELA2A, 76,025 for CELA3A, 74,535 for CELA3B, 69,035 for CELA3B mutant W79R, 47,605 for CTRB1 and CTRB2, 64,565 for CTRC, 37,525 for PRSS1, 38,890 for PRSS2, and 41,535 for PRSS3. Proteinase solutions were diluted to 1 nM working stocks in assay buffer [0.1 M Tris·HCl (pH 8.0), 1 mM CaCl2, and 0.05% Tween 20].
Expression and purification of autolyzed CELA3B forms.
The CELA3B-9del and CELA3B-13del constructs were produced in HEK 293T cells and purified by nickel-affinity chromatography as described for the wild-type proenzyme (33). NH2-terminal sequencing of the purified proteins revealed that ~90% of the CELA3B-9del preparation contained the expected NH2 terminus of Ser27 and minor contaminants with NH2-terminal amino acids of Asn31 (5%) and Glu33 (5%). The purified CELA3B-13del contained a ~50:50 mixture of two forms, one with the expected NH2 terminus of Asn31 and another with an NH2-terminal Glu33, indicating that the signal peptidase processed this construct at two sites. This preparation was suitable for the purpose of our experiments, and further purification was not attempted.
Enzyme activity measurements.
Enzyme activity of human CELA3A and CELA3B in the conditioned medium of transfected cells was determined using the Suc-Ala-Ala-Pro-Ala-p-nitroanilide substrate (5). To activate proelastases, aliquots of conditioned media (100 µl) were supplemented with 10 µl of 1 M Tris·HCl (pH 8.0) and 1 µl of 0.1 M CaCl2 and incubated with 100 nM human cationic trypsin at 37°C for 30 min (final concentrations). Activated elastases (20 μl) were then mixed with 175 μl of assay buffer [0.1 M Tris·HCl (pH 8.0), 1 mM CaCl2, and 0.05% Tween 20], and elastase activity was measured by addition of 5 μl of 6 mM substrate. The increase in absorbance at 405 nm was followed for 5 min in a microplate reader at 22°C. Rates of substrate cleavage were calculated from the linear portion of the curves and expressed in milli-optical density units per minute.
ELISAs.
Detection of human pancreatic proteinases by the ScheBo test was performed as follows according to the manufacturer’s instructions with the ready-to-use reagents supplied. The 5× wash buffer [phosphate-buffered saline (pH 7.2) with unspecified detergent] stock was diluted with water before use. Aliquots (50 µl) of purified proteinases or conditioned media diluted in assay buffer [0.1 M Tris·HCl (pH 8.0), 1 mM CaCl2, and 0.05% Tween 20] were added to the ELISA strips containing an immobilized anti-elastase antibody (Fig. 1). As blank, 50 µl of assay buffer were used. After 30 min of incubation at 22°C, the enzyme solutions were removed from the wells. The wells were rinsed three times for 2 min each with 250 µl of wash buffer. An aliquot (50 µl) of a biotinylated anti-elastase antibody complexed with peroxidase-conjugated streptavidin was added to the wells, and the sample was incubated for 30 min in darkness at 22°C. The antibody solution was discarded, and the wells were rinsed three times for 2 min each with 250 µl of wash buffer. For color development, 100 µl of 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)-peroxidase substrate solution were added, and the sample was incubated in darkness for 20 min at 22°C. The reaction was terminated by addition of 100 µl of stop solution and incubation for 10 min. The dark-green ELISA signal (absorbance) was measured in a plate reader at 405 nm. All assays were performed in duplicates. Data points represent absorbance readings corrected for the average of two blank values.
Fig. 1.
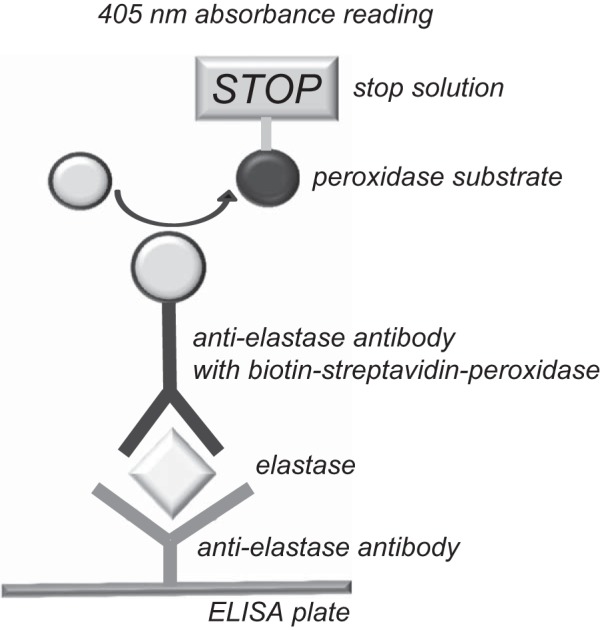
Schematic diagram of elastase detection by the sandwich ELISA method of the ScheBo Pancreatic Elastase 1 Stool Test.
RESULTS
The ScheBo Pancreatic Elastase 1 Stool Test detects elastase 3 isoforms CELA3A and CELA3B.
To determine the specificity of the ScheBo test, we recombinantly expressed and purified human pancreatic serine proteinases and performed ELISAs according to the manufacturer’s instructions. CELA3A and CELA3B were tested at 100 pM final concentration; all other proteinases were tested at the 10-fold-higher 1 nM concentration (Fig. 2). We obtained strong signals for CELA3A and CELA3B, whereas none of the other proteinases was detected to a significant extent.
Fig. 2.

Detection of human pancreatic serine proteinases by the ScheBo Pancreatic Elastase 1 Stool Test. Proteinases were expressed recombinantly and purified as described in materials and methods. Chymotrypsin-like elastases 3A and 3B (CELA3A and CELA3B) were tested at 0.1 nM final concentration; all other proteinases were tested at 1 nM. For chymotrypsin-like enzyme 1 (CTRL1), conditioned medium diluted 1,000-fold was used. Values are means ± SD from 3 measurements. CTRB1, CTRB2, and CTRC, chymotrypsin B1, B2 and C, respectively; PRSS1, PRSS2, and PRSS3, human trypsin isoforms.
Proelastases, active elastases, and autolyzed elastase forms are measured with equal efficiency.
To characterize the detection of CELA3A and CELA3B by the ScheBo test in a more quantitative manner, we performed the ELISA using elastases over a concentration range of 20–200 pM. As shown in Fig. 3A, the ELISA signal strength for CELA3B was, on average, 4.3-fold (range 3.4- to 5.1-fold) greater than that for CELA3A over the concentration range tested. Identical signals were obtained when proelastases were compared with active elastases, indicating that the test measures the zymogen and active forms with equal efficiency (Fig. 3A). Autolyzed forms of CELA3B missing either 9 amino acids (9del) or 13 amino acids (13del) from the NH2 terminus also produced ELISA signals identical to that of the intact CELA3B proelastase (Fig. 3B). Since these results indicate that the ScheBo test does not discriminate between proelastase, active elastase, and autolyzed elastase, in all subsequent experiments we used the proelastase forms of CELA3A and CELA3B. In a control experiment we also ruled out the unlikely confounding effect of the His tag on elastase expression and detection by the ScheBo test. Although data are not shown, tagged and untagged forms of CELA3A and CELA3B were secreted at comparable levels into the conditioned medium of transfected cells and gave identical ELISA signals.
Fig. 3.
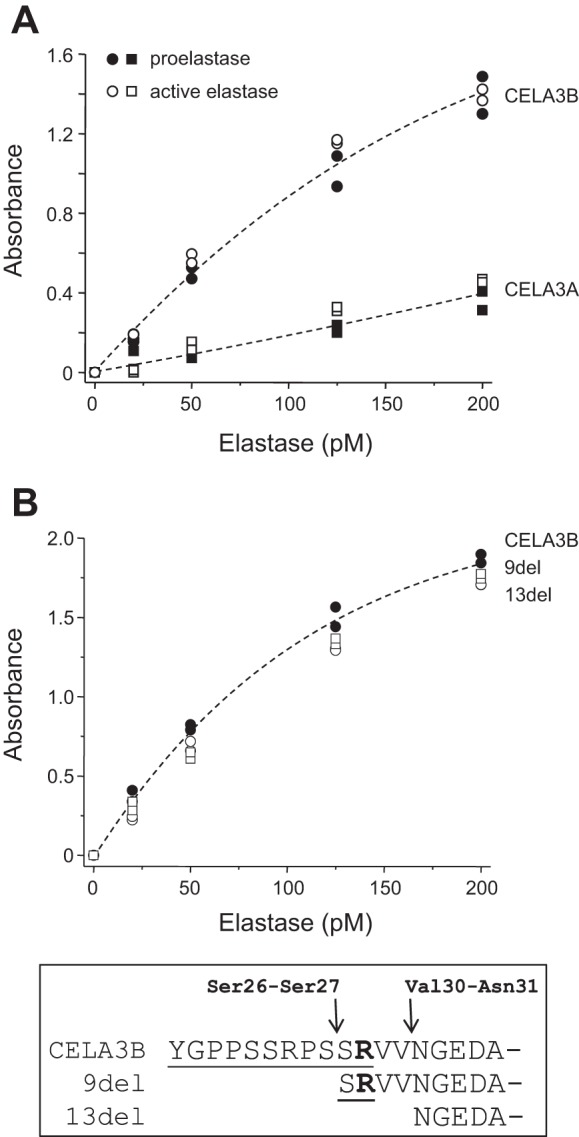
Detection of CELA3 isoforms by the ScheBo Pancreatic Elastase 1 Stool Test. A: test performance on proelastases vs. active elastases at 20–200 pM. B: detection of autolyzed forms of CELA3B. Inset: autolytic cleavage sites in the NH2-terminal part of CELA3B. The activation peptide is underlined. Assays were performed in duplicate, and both data points were plotted.
Effect of CELA3A and CELA3B genetic variants on performance of the ScheBo test.
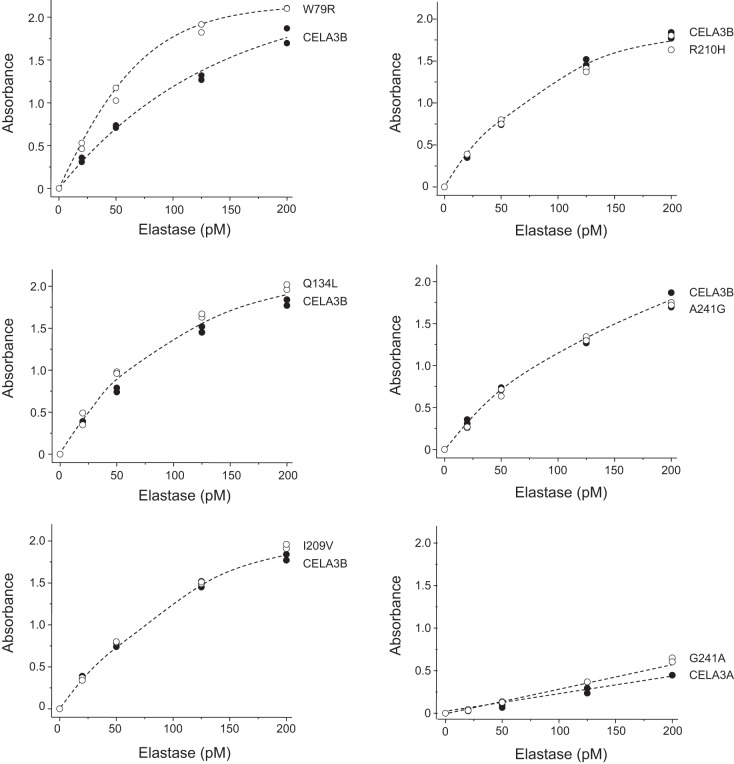
To identify genetic variants of CELA3A and CELA3B in the population, we interrogated the Exome Variant Server database of the National Heart, Lung, and Blood Institute Exome Sequencing Project. When considering missense variants with an allele frequency >1%, we found one CELA3A variant (G241A) and five CELA3B variants (W79R, Q134L, I209V, R210H, and A241G) (see Table 1 in Ref. 33 for further details). The occurrence of CELA3A variant G241A and CELA3B variant A241G was also confirmed by our recent genetic study in a Hungarian population (25). To evaluate whether common genetic variants of CELA3A and CELA3B might alter performance of the ScheBo test, we purified these six variants and tested their detection in the ELISA. With the exception of the CELA3B W79R variant, none of the variants had an appreciable effect on signal development (Fig. 4). The strength of the signal for variant W79R was, on average, 1.4-fold (range 1.2- to 1.5-fold) greater than that for wild-type CELA3B over the concentration range tested. However, if we consider that in most carriers the variant is heterozygous, this difference should have no meaningful impact on the clinical interpretation of the ScheBo test results.
Fig. 4.
Detection of naturally occurring CELA3A and CELA3B variants by the ScheBo Pancreatic Elastase 1 Stool Test. Wild-type CELA3A, CELA3B, and the indicated variants were purified and assayed at 20–200 pM. Assays were performed in duplicate, and both data points were plotted.
Glu154 in CELA3B is a critical determinant of recognition by the ScheBo test.
CELA3A and CELA3B share 92% identity at the amino acid level, yet the strength of the ELISA signal is nearly fourfold greater for CELA3B than CELA3A. To identify the reason for this difference, we aligned the two isoforms (Fig. 5A) and then individually mutated all divergent amino acid positions in CELA3B to the corresponding CELA3A amino acid. Positions where differences occurred in neighboring amino acids were mutated en bloc. Overall, 11 new CELA3B mutants were constructed and tested. For these qualitative screening experiments we used conditioned media of HEK 293T cells transfected with the mutant constructs. Remarkably, mutant E154K gave no ELISA signal, while all other mutants were robustly detected with some variations in signal strength (Fig. 5B). Since the mutations may alter secretion and enzyme activity of CELA3B, we also verified the expression of all mutants by SDS-PAGE and Coomassie staining (not shown) and by direct activity measurements after activation with trypsin. As shown in Fig. 5C, all mutants, including E154K, exhibited measurable elastase activity, and for the majority of mutants, activity was comparable to or even higher than that of the wild-type CELA3B. The higher activity of mutants S77R,S78D,W79L and D89N,R90L was due to greater amounts of elastase secreted into the conditioned medium (not shown), and this was also consistent with the stronger ELISA signal (Fig. 5B). Similarly, mutant A241G was secreted at higher levels (not shown) and produced a stronger ELISA signal, but this change was not obvious in the activity measurement, as this mutation decreases catalytic activity of CELA3B (25). This initial screen conclusively identified Glu154 in CELA3B as a major determinant of recognition by the ScheBo test.
Fig. 5.

Effect of amino acid differences between CELA3A and CELA3B on detection by the ScheBo Pancreatic Elastase 1 Stool Test. A: alignment of the amino acid sequences of the 2 human CELA3 isoforms. Active enzymes are shown; numbering starts with the initiator methionine of the pre-proelastases. Differences are underlined and boldface. B: ELISA of CELA3B mutants carrying substitutions with the corresponding CELA3A amino acids. HEK 293T cells were transfected with the indicated constructs, and conditioned medium was collected after 48 h. Elastase was assayed using 1,000-fold-diluted conditioned medium by the ScheBo test. C: elastase activity of CELA3B mutants in the conditioned medium after activation with trypsin. Values are means ± SD from 3 experiments. mOD, milli-optical density units.
To confirm the importance of Glu154, we purified the E154K mutant and compared detection with that of wild-type CELA3B over a concentration range of 20–200 pM. No signal was obtained with the mutant (Fig. 6A). The ScheBo test uses a sandwich assay format with separate capturing and detection antibodies directed at different regions of the elastase molecules (Fig. 1). To ascertain whether the defect in the E154K mutant is at the level of capturing or detection, we eliminated the capturing step by immobilizing wild-type and mutant CELA3B to nickel plates (Ni-NTA HisSorb plate, Qiagen, Valencia, CA) via their His tag. Under these assay conditions, both elastase forms were detected comparably, indicating that mutation E154K interferes with the capturing step in the ELISA protocol (Fig. 6B). Finally, structural modeling indicated that Glu154 is located on the surface of CELA3B far from the active site (Fig. 6C).
Fig. 6.
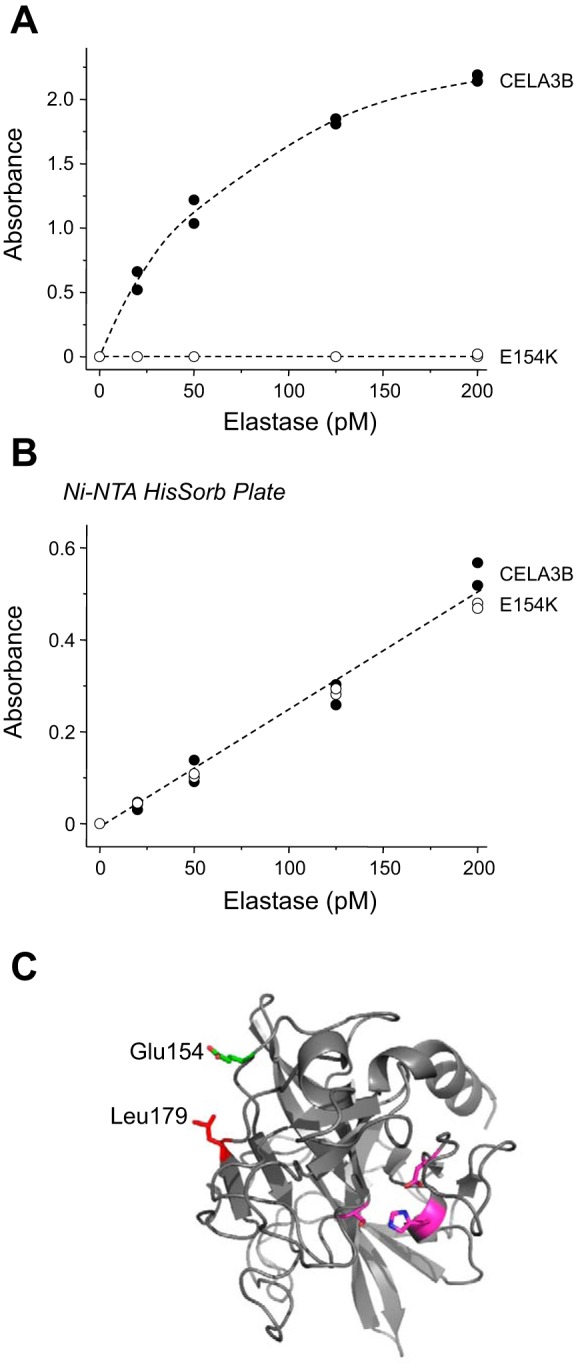
Detection of CELA3B mutant E154K by the ScheBo Pancreatic Elastase 1 Stool Test. A: purified wild-type and E154K mutant CELA3B were assayed at 20–200 pM. B: purified wild-type and E154K mutant CELA3B were immobilized through their COOH-terminal His tags to Ni-NTA HisSorb plates (Qiagen) and detected with the biotinylated antibody-streptavidin-peroxidase complex from the ScheBo test. Assays were performed in duplicate, and both data points were plotted. C: structural model of human CELA3B indicating the positions of Glu154 (green) and nearby Leu179 (red). Also shown for reference are residues of the catalytic triad (magenta). Structural model for active CELA3B was generated by the SWISS-MODEL protein structure homology-modeling server (2) with porcine elastase used as template (PDB file 3UOU). Image was rendered with PyMOL (Schrödinger, Cambridge, MA).
Mutations of Lys154 and nearby Arg179 improve detection of CELA3A.
The experiments presented above strongly indicate that detection of CELA3A should be improved by changing the Lys154 residue to Glu. However, we were surprised to find no improvement in detection over wild-type CELA3A when the CELA3A mutant K154E was purified and tested (Fig. 7A). To explain these puzzling observations, we speculated that the close proximity of another divergent amino acid interferes with the recognition of Glu154 in the CELA3A K154E mutant. Inspection of the CELA3B structural model indicated that amino acid 179, which is Arg in CELA3A and Leu in CELA3B, might be important in this regard (Fig. 6C). Indeed, when mutations K154E and R179L were introduced simultaneously in CELA3A, the strength of the ELISA signal was increased by ~2.5-fold and approximated that of CELA3B (Fig. 7B).
Fig. 7.
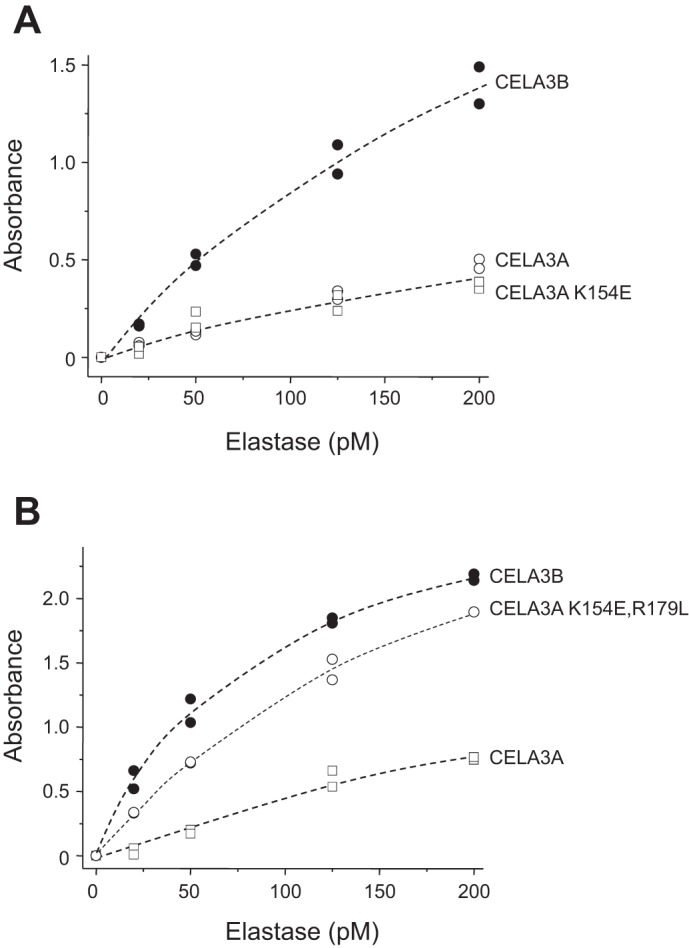
Detection of CELA3A mutant K154E (A) and double mutant K154E,R179L (B) by the ScheBo Pancreatic Elastase 1 Stool Test. Wild-type CELA3A, CELA3B, and the indicated CELA3A variants were purified and assayed at 20–200 pM. Assays were performed in duplicate, and both data points were plotted.
DISCUSSION
In the present study we evaluated the isoform specificity of the ScheBo Pancreatic Elastase 1 Stool Test. The clinical utility of the fecal elastase test in the evaluation of pancreatic insufficiency has been well established, and it has become widely used for routine indirect testing of pancreatic function (7, 8, 12, 15, 18-20, 23, 30, 32). Although limitations were also indicated by some studies (1, 20), the test gained popularity because it is noninvasive, relatively rapid, unaffected by pancreatic enzyme replacement therapy, and clearly superior to the previously used indirect test that measured fecal chymotrypsin activity. It is somewhat surprising that the exact molecular targets of this ELISA have not been characterized; it has been unclear which elastase isoform(s) is detected by the test and whether other homologous pancreatic proteinases interfere with the assay. The misnomer used in the test’s commercial name adds to the uncertainty as to what exactly it measures. As discussed in the introduction, CELA1 is not expressed in the human pancreas, and, in all likelihood, the test’s name refers to one of the several historic names used for CELA3B in the article published by Largman et al. in 1976 (17). Thus we expected that the test would detect CELA3B; yet two vexing questions remained. 1) Was the 92%-identical CELA3A isoform equally well detected? 2) Did natural genetic variants of CELA3A and CELA3B affect test performance? Taking advantage of developments in the genomic sequencing and annotation of human digestive enzymes, we used well-defined recombinant preparations of human elastases, chymotrypsins, and trypsins to characterize the specificity of the ScheBo test.
Our findings confirmed that the primary target of the test is CELA3B; however, CELA3A, with a signal strength nearly fourfold weaker than that of CELA3B, was also detected. Importantly, CELA2A, chymotrypsins CTRB1, CTRB2, CTRC, and CTRL1, and trypsins PRSS1, PRSS2, and PRSS3 produced minimal or no signal in this ELISA. In 1984, Sziegoleit measured CELA3B levels as 4–6% of total pancreatic juice protein (36), and other studies indicated that mRNA and protein levels for the two CELA3 isoforms are grossly comparable (31, 42). However, how the expression of CELA3A and CELA3B in the pancreas might vary under different physiological and pathological conditions is unknown. It is intriguing to speculate that test performance might be affected by changes in the CELA3 isoform ratio. For this reason, development of a test that specifically detects CELA3B without cross-reactivity with CELA3A could potentially offer a better diagnostic tool.
Elastases are secreted as inactive precursors (zymogens) by the pancreas, and these proelastases become activated in the duodenum by trypsin. Proelastases can also suffer autolysis due to their intrinsic zymogen activity or by active elastases (33). The most prominent autolytic cleavage takes place at the Val30-Asn31 peptide bond in CELA3B (and, to a lesser extent, at the Val30-His31 peptide bond in CELA3A), resulting in a catalytically inactive elastase species (11, 33). We compared test performance for all molecular forms of CELA3B and found no difference in detection efficiency, indicating that test results are unaffected by the activation state or the degree of autolysis of secreted elastases.
Another potentially confounding factor is the occurrence of natural genetic variants of the CELA3 isoforms that might demonstrate an ELISA reaction different from that of the wild-type targets. The test is based on the binding of elastase to two monoclonal antibodies (Fig. 1), and genetic variants can alter the surface epitopes where these antibodies bind, resulting in altered detection efficiency. We characterized the effect on test performance of all genetic variants of CELA3A and CELA3B that occur at or above 1% frequency in the population and found no clinically meaningful changes. Only one variant, CELA3B W79R, exhibited increased detection, by ~1.4-fold, which should be inconsequential in heterozygous carriers. Our studies do not rule out the possibility that rare or private genetic variants in certain patients may interfere with the test; however, for the large majority of the population, the ScheBo test should not be affected by common genetic variants in the CELA3 isoforms.
We performed limited epitope mapping to identify amino acids that are responsible for the preferential detection of CELA3B over CELA3A by the ScheBo test. We identified Glu154 in CELA3B, which is Lys in CELA3A, as a key determinant of recognition by the capturing monoclonal antibody. Mutation E154K in CELA3B abolished detection by ELISA. It was surprising, however, that the opposite mutation K154E in CELA3A did not improve detection by the test, and simultaneous mutation of the nearby Arg179 to Leu (R179L) was required to achieve signal levels that were comparable to those of CELA3B.
Position 154 is located within a potential N-glycosylation site, and we considered the possibility that the side chain of amino acid 154 may alter glycosylation and, thereby, affect antibody recognition. N-linked glycosylation occurs on Asn residues in Asn-Xaa-Ser/Thr sequons, where Xaa can be any amino acid except Pro. Importantly, however, if the amino acid following the sequon is Pro, N-glycosylation is inhibited (22). In CELA3A the sequon Asn153-Lys154-Thr155 and in CELA3B the sequon Asn153-Glu154-Thr155 are followed by Pro156, indicating that Asn153 is unlikely to undergo glycosylation in either isoform. This notion is consistent with earlier studies that characterized the glycosylation of CELA3B and found a single N-glycosylation site at Asn114 (45). Finally, although data are not shown, we observed no change in detection of CELA3A and CELA3B by the ScheBo test after treatment with peptide-N-glycosidase F.
In addition to the ScheBo test, which utilizes monoclonal antibodies, another clinical test developed and commercialized by Bioserv Diagnostics (Rostock, Germany) is based on detection by polyclonal antibodies against elastase 1 (12, 13, 23, 30). A recent study characterized the isoform specificity of the Bioserv test and found that CELA3A is a target, while CELA2A is not detected; however, other pancreatic proteinases were not evaluated in a comprehensive manner (44).
In summary, we characterized the molecular targets of the ScheBo Pancreatic Elastase 1 Stool Test and demonstrated that it predominantly measures CELA3B but also detects CELA3A with lower efficiency. Other pancreatic proteinases or genetic variants of the CELA3 isoforms have no appreciable impact on test performance.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R01 DK095753, R01 DK082412, and R01 DK058088 (to M. Sahin-Tóth) and Hungarian Academy of Sciences Momentum Grant LP2014-10/2014 (to P. Hegyi).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.Z.T., P.H., and M.S.-T. conceived and designed research; A.Z.T., A.S., and E.H. performed experiments; A.Z.T., E.H., and M.S.-T. analyzed data; A.Z.T., E.H., P.H., and M.S.-T. interpreted results of experiments; A.Z.T. and M.S.-T. prepared figures; A.Z.T. drafted manuscript; A.Z.T., A.S., E.H., P.H., and M.S.-T. edited and revised manuscript; A.Z.T., A.S., E.H., P.H., and M.S.-T. approved final version of manuscript.
ACKNOWLEDGMENTS
A. Z. Tóth gratefully acknowledges the support of Csaba Bereczki (Dept. of Pediatrics, University of Szeged, Szeged, Hungary). M. Sahin-Tóth thanks Evette Radisky (Mayo Clinic) for help with modeling.
REFERENCES
- 1.Amann ST, Bishop M, Curington C, Toskes PP. Fecal pancreatic elastase 1 is inaccurate in the diagnosis of chronic pancreatitis. Pancreas 13: 226–230, 1996. doi: 10.1097/00006676-199610000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Arnold K, Bordoli L, Kopp J, Schwede T. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics 22: 195–201, 2006. doi: 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]
- 3.Baló J, Banga I. Elastase and elastase-inhibitor. Nature 164: 491, 1949. doi: 10.1038/164491a0. [DOI] [PubMed] [Google Scholar]
- 4.Baló J, Banga I. The elastolytic activity of pancreatic extracts. Biochem J 46: 384–387, 1950. doi: 10.1042/bj0460384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boros E, Szabó A, Zboray K, Héja D, Pál G, Sahin-Tóth M. Overlapping specificity of duplicated human pancreatic elastase 3 isoforms and archetypal porcine elastase 1 provides clues to evolution of digestive enzymes. J Biol Chem 292: 2690–2702, 2017. doi: 10.1074/jbc.M116.770560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Del Mar EG, Largman C, Brodrick JW, Fassett M, Geokas MC. Substrate specificity of human pancreatic elastase 2. Biochemistry 19: 468–472, 1980. doi: 10.1021/bi00544a011. [DOI] [PubMed] [Google Scholar]
- 7.Domínguez-Muñoz JE, D Hardt P, Lerch MM, Löhr MJ. Potential for screening for pancreatic exocrine insufficiency using the fecal elastase-1 test. Dig Dis Sci 62: 1119–1130, 2017. doi: 10.1007/s10620-017-4524-z. [DOI] [PubMed] [Google Scholar]
- 8.Dominici R, Franzini C. Fecal elastase-1 as a test for pancreatic function: a review. Clin Chem Lab Med 40: 325–332, 2002. doi: 10.1515/CCLM.2002.051. [DOI] [PubMed] [Google Scholar]
- 9.Gertler A. The non-specific electrostatic nature of the adsorption of elastase and other basic proteins on elastin. Eur J Biochem 20: 541–546, 1971. doi: 10.1111/j.1432-1033.1971.tb01425.x. [DOI] [PubMed] [Google Scholar]
- 10.Gertler A, Weiss Y, Burstein Y. Purification and characterization of porcine elastase II and investigation of its elastolytic specificity. Biochemistry 16: 2709–2715, 1977. doi: 10.1021/bi00631a019. [DOI] [PubMed] [Google Scholar]
- 11.Gomis-Rüth FX, Gómez-Ortiz M, Vendrell J, Ventura S, Bode W, Huber R, Avilés FX. Cutting at the right place–the importance of selective limited proteolysis in the activation of proproteinase E. Eur J Biochem 251: 839–844, 1998. doi: 10.1046/j.1432-1327.1998.2510839.x. [DOI] [PubMed] [Google Scholar]
- 12.Hahn J-U, Bochnig S, Kerner W, Koenig H, Sporleder B, Lankisch PG, Maisonneuve P, Lowenfels AB. A new fecal elastase 1 test using polyclonal antibodies for the detection of exocrine pancreatic insufficiency. Pancreas 30: 189–191, 2005. doi: 10.1097/01.mpa.0000153617.40513.34. [DOI] [PubMed] [Google Scholar]
- 13.Hardt PD, Hauenschild A, Nalop J, Marzeion AM, Porsch-Ozcürümez M, Luley C, Sziegoleit A, Kloer HU; Binding Studies and Clinical Use in Patients with Exocrine Pancreatic Insufficiency . The commercially available ELISA for pancreatic elastase 1 based on polyclonal antibodies does measure an as yet unknown antigen different from purified elastase 1. Binding studies and clinical use in patients with exocrine pancreatic insufficiency. Z Gastroenterol 41: 903–906, 2003. doi: 10.1055/s-2003-41832. [DOI] [PubMed] [Google Scholar]
- 14.Kawashima I, Tani T, Shimoda K, Takiguchi Y. Characterization of pancreatic elastase II cDNAs: two elastase II mRNAs are expressed in human pancreas. DNA 6: 163–172, 1987. doi: 10.1089/dna.1987.6.163. [DOI] [PubMed] [Google Scholar]
- 15.Keim V, Teich N, Moessner J. Clinical value of a new fecal elastase test for detection of chronic pancreatitis. Clin Lab 49: 209–215, 2003. [PubMed] [Google Scholar]
- 16.Kukor Z, Tóth M, Sahin-Tóth M. Human anionic trypsinogen: properties of autocatalytic activation and degradation and implications in pancreatic diseases. Eur J Biochem 270: 2047–2058, 2003. doi: 10.1046/j.1432-1033.2003.03581.x. [DOI] [PubMed] [Google Scholar]
- 17.Largman C, Brodrick JW, Geokas MC. Purification and characterization of two human pancreatic elastases. Biochemistry 15: 2491–2500, 1976. doi: 10.1021/bi00656a036. [DOI] [PubMed] [Google Scholar]
- 18.Leeds JS, Oppong K, Sanders DS. The role of fecal elastase-1 in detecting exocrine pancreatic disease. Nat Rev Gastroenterol Hepatol 8: 405–415, 2011. doi: 10.1038/nrgastro.2011.91. [DOI] [PubMed] [Google Scholar]
- 19.Löser C, Möllgaard A, Fölsch UR. Faecal elastase 1: a novel, highly sensitive, and specific tubeless pancreatic function test. Gut 39: 580–586, 1996. doi: 10.1136/gut.39.4.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lüth S, Teyssen S, Forssmann K, Kölbel C, Krummenauer F, Singer MV. Fecal elastase-1 determination: “gold standard” of indirect pancreatic function tests? Scand J Gastroenterol 36: 1092–1099, 2001. doi: 10.1080/003655201750422729. [DOI] [PubMed] [Google Scholar]
- 21.Mallory PA, Travis J. Human pancreatic enzymes: purification and characterization of a nonelastolytic enzyme, protease E, resembling elastase. Biochemistry 14: 722–730, 1975. doi: 10.1021/bi00675a012. [DOI] [PubMed] [Google Scholar]
- 22.Mellquist JL, Kasturi L, Spitalnik SL, Shakin-Eshleman SH. The amino acid following an Asn-X-Ser/Thr sequon is an important determinant of N-linked core glycosylation efficiency. Biochemistry 37: 6833–6837, 1998. doi: 10.1021/bi972217k. [DOI] [PubMed] [Google Scholar]
- 23.Miendje Y, Maisin D, Sipewa MJ, Deprez P, Buts JP, De Nayer P, Philippe M. Polyclonal versus monoclonal ELISA for the determination of fecal elastase 1: diagnostic value in cystic fibrosis and chronic pancreatic insufficiency. Clin Lab 50: 419–424, 2004. [PubMed] [Google Scholar]
- 24.Ohlsson K, Olsson AS. Purification and partial characterization of human pancreatic elastase. Hoppe Seylers Z Physiol Chem 357: 1153–1161, 1976. doi: 10.1515/bchm2.1976.357.2.1153. [DOI] [PubMed] [Google Scholar]
- 25.Párniczky A, Hegyi E, Tóth AZ, Szücs Á, Szentesi A, Vincze Á, Izbéki F, Németh BC, Hegyi P, Sahin-Tóth M. Genetic analysis of human chymotrypsin-like elastases 3A and 3B (CELA3A and CELA3B) to assess the role of complex formation between proelastases and procarboxypeptidases in chronic pancreatitis. Int J Mol Sci 17: 2148, 2016. doi: 10.3390/ijms17122148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rose SD, MacDonald RJ. Evolutionary silencing of the human elastase I gene (ELA1). Hum Mol Genet 6: 897–903, 1997. doi: 10.1093/hmg/6.6.897. [DOI] [PubMed] [Google Scholar]
- 27.Sahin-Tóth M. Human cationic trypsinogen. Role of Asn-21 in zymogen activation and implications in hereditary pancreatitis. J Biol Chem 275: 22750–22755, 2000. doi: 10.1074/jbc.M002943200. [DOI] [PubMed] [Google Scholar]
- 28.Sahin-Tóth M, Tóth M. Gain-of-function mutations associated with hereditary pancreatitis enhance autoactivation of human cationic trypsinogen. Biochem Biophys Res Commun 278: 286–289, 2000. doi: 10.1006/bbrc.2000.3797. [DOI] [PubMed] [Google Scholar]
- 29.Schechter I, Berger A. On the size of the active site in proteases. I. Papain. Biochem Biophys Res Commun 27: 157–162, 1967. doi: 10.1016/S0006-291X(67)80055-X. [DOI] [PubMed] [Google Scholar]
- 30.Schneider A, Funk B, Caspary W, Stein J. Monoclonal versus polyclonal ELISA for assessment of fecal elastase concentration: pitfalls of a new assay. Clin Chem 51: 1052–1054, 2005. doi: 10.1373/clinchem.2004.046888. [DOI] [PubMed] [Google Scholar]
- 31.Shirasu Y, Takemura K, Yoshida H, Sato Y, Iijima H, Shimada Y, Mikayama T, Ozawa T, Ikeda N, Ishida A, Tamai Y, Matsuki S, Tanaka J, Ikenaga H, Ogawa M. Molecular cloning of complementary DNA encoding one of the human pancreatic protease E isozymes. J Biochem 104: 259–264, 1988. doi: 10.1093/oxfordjournals.jbchem.a122454. [DOI] [PubMed] [Google Scholar]
- 32.Stein J, Jung M, Sziegoleit A, Zeuzem S, Caspary WF, Lembcke B. Immunoreactive elastase I: clinical evaluation of a new noninvasive test of pancreatic function. Clin Chem 42: 222–226, 1996. [PubMed] [Google Scholar]
- 33.Szabó A, Pilsak C, Bence M, Witt H, Sahin-Tóth M. Complex formation of human proelastases with procarboxypeptidases A1 and A2. J Biol Chem 291: 17706–17716, 2016. doi: 10.1074/jbc.M116.743237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Szabó A, Sahin-Tóth M. Determinants of chymotrypsin C cleavage specificity in the calcium-binding loop of human cationic trypsinogen. FEBS J 279: 4283–4292, 2012. doi: 10.1111/febs.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szepessy E, Sahin-Tóth M. Inactivity of recombinant ELA2B provides a new example of evolutionary elastase silencing in humans. Pancreatology 6: 117–122, 2006. doi: 10.1159/000090031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sziegoleit A. A novel proteinase from human pancreas. Biochem J 219: 735–742, 1984. doi: 10.1042/bj2190735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sziegoleit A. Purification and characterization of a cholesterol-binding protein from human pancreas. Biochem J 207: 573–582, 1982. doi: 10.1042/bj2070573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sziegoleit A, Krause E, Klör HU, Kanacher L, Linder D. Elastase 1 and chymotrypsin B in pancreatic juice and feces. Clin Biochem 22: 85–89, 1989. doi: 10.1016/S0009-9120(89)80003-7. [DOI] [PubMed] [Google Scholar]
- 39.Sziegoleit A, Linder D, Schlüter M, Ogawa M, Nishibe S, Fujimoto K. Studies on the specificity of the cholesterol-binding pancreatic proteinase and identification as human pancreatic elastase 1. Eur J Biochem 151: 595–599, 1985. doi: 10.1111/j.1432-1033.1985.tb09145.x. [DOI] [PubMed] [Google Scholar]
- 40.Szmola R, Kukor Z, Sahin-Tóth M. Human mesotrypsin is a unique digestive protease specialized for the degradation of trypsin inhibitors. J Biol Chem 278: 48580–48589, 2003. doi: 10.1074/jbc.M310301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tani T, Kawashima I, Furukawa H, Ohmine T, Takiguchi Y. Characterization of a silent gene for human pancreatic elastase I: structure of the 5′-flanking region. J Biochem 101: 591–599, 1987. doi: 10.1093/jb/101.3.591. [DOI] [PubMed] [Google Scholar]
- 42.Tani T, Ohsumi J, Mita K, Takiguchi Y. Identification of a novel class of elastase isozyme, human pancreatic elastase III, by cDNA and genomic gene cloning. J Biol Chem 263: 1231–1239, 1988. [PubMed] [Google Scholar]
- 43.Vered M, Burstein Y, Gertler A. Digestion of elastin by porcine pancreatic elastase I and elastase II. Int J Pept Protein Res 25: 76–84, 1985. doi: 10.1111/j.1399-3011.1985.tb02148.x. [DOI] [PubMed] [Google Scholar]
- 44.Weiss FU, Budde C, Lerch MM. Specificity of a polyclonal fecal elastase ELISA for CELA3. PLoS One 11: e0159363, 2016. doi: 10.1371/journal.pone.0159363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wendorf P, Geyer R, Sziegoleit A, Linder D. Localization and characterization of the glycosylation site of human pancreatic elastase 1. FEBS Lett 249: 275–278, 1989. doi: 10.1016/0014-5793(89)80640-4. [DOI] [PubMed] [Google Scholar]