Calcium-activated chloride channel anoctamin1 (Ano1) is necessary for normal slow waves in the gastrointestinal interstitial cells of Cajal. Exon “0” encodes the NH2 terminus of full-length human Ano1 [Ano1(0)], while exon 13 encodes residues EAVK on its first intracellular loop. Splice variants lack EAVK more often in the stomach than other tissues. Ano1(0) without EAVK [Ano1(0)ΔEAVK] has reduced sensitivity for intracellular calcium, attributable to slower kinetics. Differential expression of EAVK may function as a calcium-sensitive switch in the human stomach.
Keywords: TMEM16A, ion channels, electrophysiology
Abstract
Anoctamin1 (Ano1 and TMEM16A) is a calcium-activated chloride channel specifically expressed in the interstitial cells of Cajal (ICC) of the gastrointestinal tract muscularis propria. Ano1 is necessary for normal electrical slow waves and ICC proliferation. The full-length human Ano1 sequence includes an additional exon, exon “0,” at the NH2 terminus. Ano1 with exon 0 [Ano1(0)] had a lower EC50 for intracellular calcium ([Ca2+]i) and faster chloride current (ICl) kinetics. The Ano1 alternative splice variant with segment “c” encoding exon 13 expresses on the first intracellular loop four additional amino acid residues, EAVK, which alter ICl at low [Ca2+]i. Exon 13 is expressed in 75–100% of Ano1 transcripts in most human tissues but only 25% in the human stomach. Our aim was to determine the effect of EAVK deletion on Ano1(0) ICl parameters. By voltage-clamp electrophysiology, we examined ICl in HEK293 cells transiently expressing Ano1(0) with or without the EAVK sequence [Ano1(0)ΔEAVK]. The EC50 values of activating and deactivating ICl for [Ca2+]i were 438 ± 7 and 493 ± 9 nM for Ano1(0) but higher for Ano1(0)ΔEAVK at 746 ± 47 and 761 ± 26 nM, respectively. Meanwhile, the EC50 values for the ratio of instantaneous to steady-state ICl were not different between variants. Congruently, the time constant of activation was slower for Ano1(0)ΔEAVK than Ano1(0) currents at intermediate [Ca2+]i. These results suggest that EAVK decreases the calcium sensitivity of Ano1(0) current activation and deactivation by slowing activation kinetics. Differential expression of EAVK in the human stomach may function as a switch to increase sensitivity to [Ca2+]i via faster gating of Ano1.
NEW & NOTEWORTHY Calcium-activated chloride channel anoctamin1 (Ano1) is necessary for normal slow waves in the gastrointestinal interstitial cells of Cajal. Exon 0 encodes the NH2 terminus of full-length human Ano1 [Ano1(0)], while exon 13 encodes residues EAVK on its first intracellular loop. Splice variants lack EAVK more often in the stomach than other tissues. Ano1(0) without EAVK [Ano1(0)ΔEAVK] has reduced sensitivity for intracellular calcium, attributable to slower kinetics. Differential expression of EAVK may function as a calcium-sensitive switch in the human stomach.
the interstitial cells of Cajal (ICC) generate electrical slow waves that drive rhythmic contractions in the gastrointestinal tract (11, 34, 37). Several ion channels generate and pattern the electrical slow wave, and among these is a Ca2+-activated Cl− current (10, 12, 15, 21, 22, 42). Anoctamin1 (Ano1, TMEM16A, and DOG1) is an 8-transmembrane Ca2+-activated Cl− channel that, along with the receptor tyrosine kinase Kit, is selectively expressed in ICC in the gastrointestinal tract (2, 8, 9, 14, 27, 33, 41). Ano1 is localized at the plasma membrane (26, 35) and functions in ICC to regulate proliferation (18, 30) and contribute to slow wave generation (13, 25, 42). In tissues other than gastrointestinal smooth muscle, Ano1 contributes to cellular excitability in vascular smooth muscle (1) and peripheral nerves (3), functions that require rapid gating and can involve movement of small numbers of ions. In contrast to Ano1 functions in electrically excitable cells, Ano1 also contributes to Cl− secretion in epithelia of the intestine (20), lung (2), and salivary gland acinar cells (24). These functions require bulk movement of Cl− ions but do not necessarily depend on rapid gating.
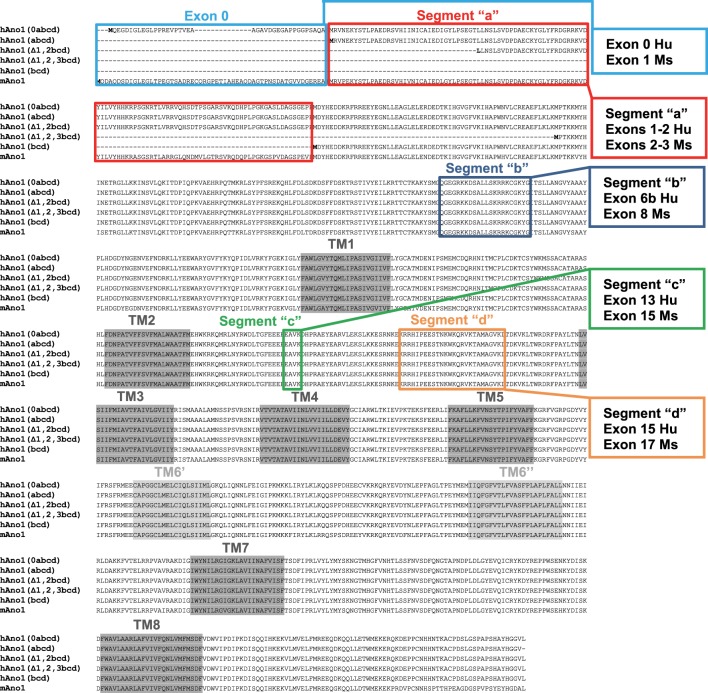
The underlying mechanisms for the differences in Ano1-gating and ionic conductances that are needed to fulfill the disparate Ano1 functions are not clear. However, it is evident that regulation by protein kinases (6), lipids (23), and intracellular Ca2+ (2, 27, 41) can alter the biophysical properties of Ano1. In addition to the impact of these molecules on Ano1 function, several transcriptional variants of Ano1 are generated from the mouse and human Ano1 gene (Fig. 1). The properties of these segments vary with respect to anion selectivity, inhibitor sensitivity, intracellular Ca2+ regulation, and activation and deactivation kinetics (31, 32, 40). The many possible transcriptional variants of Ano1 result from either alternative splicing of exons or the use of differing NH2-terminal sequences (7, 19, 29). The splice variants can be generated by in-frame deletion of three exons, referred to as segments “b,” “c,” and “d” of the channel protein and coding for 22, 4, and 26 amino acids (exons 6b, 13, and 15, respectively, in the human ANO1 gene) (7). At least three segments with differing NH2-terminal amino acid sequences have been reported (17–19, 29). Thus tissue-specific expression of any of the multiple isoforms of Ano1 proteins can confer differing biophysical properties on the expressed channels. Furthermore, altered expression of specific Ano1 segments can have pathological effects (17, 31).
Fig. 1.
Sequence alignment of human and mouse anoctamin1 (Ano1) isoforms. The human Ano1 isoforms Ano1(0abcd) (19, 31), Ano1(abcd) (19, 31), hAno1(Δ1,2bcd) (19, 29), hAno1(Δ1,2,3bcd) (19), and hAno1(bcd) (29) were previously characterized by electrophysiology and are aligned using sequences from the current human annotation (#108). The mouse Ano1 sequence (annotation #106) is aligned to the human sequences using the transcript XM_006508458.3 that contains the commonly used full-length sequence. This alignment does not include the recent mouse transcript XM_006508463.3 (that has an alternative 5′-end with an alternative exon 1 that joins to exon 2 by skipping the current exon 1) that has not been previously studied by electrophysiology. In colors are highlighted the segments “a,” “b,” “c,” and “d” with the exon correspondence between mouse and human genes. The putative transmembrane domains are highlighted in gray.
Our studies on Ano1 variants in human gastric muscularis propria identified differential expression of the three spliced exons as well as three alternative NH2-terminal sequences (17, 29). One of the NH2-terminal segments is generated using an exon 93-kb upstream of, and in frame with, the annotated first exon in the human ANO1 gene (19). This upstream exon “0” [hAno1(0abcd), Fig. 1] is homologous to the annotated first exon of the mouse Ano1 gene (mAno1, Fig. 1), and its expression is driven by a functional promoter sequence upstream of exon 0 (19). The functional effect of including the sequence from exon 0 in heterologously expressed full-length clones of Ano1 (including exons 6b, 13, and 15) was a significant increase in the current density, a greater sensitivity to [Ca2+]i, and changes to the Ca2+ dependence of activation and deactivation kinetics (31).
The effect of this extended NH2-terminal sequence of Ano1 in the context of transcriptional variants generated by exon splicing is not known. Several groups have determined that shorter constructs without exon 0 exhibit differences in [Ca2+]i sensitivity and gating kinetics when the exon usage is changed (4, 7, 36, 40). Studies showed that the variant segment c has significantly lower Cl− current densities at all voltages and altered channel kinetics at low or no intracellular Ca2+ (7, 40). This exon is found in Ano1 transcripts isolated from human gastric muscularis propria at a much lower abundance (25% of Ano1 transcripts; Ref. 17) than that typically found in other tissues (>75% of transcripts; Ref. 7). Interestingly, segment c abundance was higher in the gastric muscularis propria of patients with diabetic gastroparesis compared with healthy controls, indicating an association between Ano1 function and disease (17). Due to the contribution of Ano1 to normal electrical slow wave activity (13, 28), it is important to understand how these properties of Ca2+ sensitivity and altered channel density and kinetics are affected by differential splicing of exon 13 in the context of exon 0-containing transcripts of the gene. Therefore, the aim of this study was to determine the effect that segment c expression has on voltage, time, and [Ca2+]I dependence of full-length Ano1 variants containing exon 0.
METHODS
Cloning of Ano1(0) and Ano1(0)ΔEAVK.
An expression construct for full-length Ano1 with the alternative 5′-transcriptional start site encoding exon 0 (Ano1(0)) was described previously (19, 31). A second construct was made from Ano1(0) truncating exon 13, which encodes four amino acids, EAVK, to create a splice variant in the Ano1(0) genetic background: Ano1(0)ΔEAVK.
HEK293 cell transfection.
Constructs for Ano1(0) or Ano1(0)ΔEAVK were transiently cotransfected along with green fluorescent protein as a reporter (pEGFP-C1; Clontech, Palo Alto, CA) by the reagent Lipofectamine 2000 (Invitrogen, Carlsbad, CA) into HEK293 cells (American Type Culture Collection, Manassas, VA) as previously described (17, 18).
Solutions.
To record Ca2+-activated Cl− current, intra- and extracellular solutions were made as described previously (31) with equimolar Cl− in and outside of the cell to set the reversal potential of Cl− (ECl) to an estimated 0 mV. Principal cations in the solutions were N-methyl-d-glucamine (NMDG+) or Cs+ so that visible current could be readily attributed to Cl−. The extracellular solution for whole cell patch-clamp experiments was as follows (in mM): 155 Cl−, 150 NMDG+ or 150 Cs+, 10 HEPES, 5.5 glucose, and 2.5 Ca2+ at pH 7.35 and 300 mmol/kg. The intracellular solution was as follows (in mM): 150.1–151.5 Cl−, 150 Cs+, 2 EGTA and 10 HEPES at pH 7.0 and 300 mmol/kg. Stock CaCl2 was diluted in the EGTA-buffered intracellular solution at volumes calculated with Maxchelator software (http://www.stanford.edu/~cpatton/) to reach 30–3,000 nM free intracellular Ca2+. The predicted liquid junction potential was +1.0 mV.
Electrophysiology.
Patch-clamp electrodes were pulled from KG12 glass on a Sutter P97 puller (Sutter Instruments) to a resistance of 1–3 MΩ as described previously. Data were recorded with pClamp 10.4 software on a CyberAmp320, Digidata 1440A, and Axopatch 200B (Molecular Devices). Whole cell Cl− currents from Ano1(0)- or Ano1(0)ΔEAVK-transfected HEK cells were elicited at 20 mV intervals from a holding potential of −100 mV by twelve 1-s steps from −100 through +120 mV. Families of current traces were averaged up to 10 times with an intersweep time of 5–10 s. Samples were taken at a rate of 10 kHz and filtered at 1–2 kHz.
Analysis.
Ca2+-activated Cl− currents were normalized to the whole cell capacitance dialed in during recording and then analyzed with Clampfit 10.4, Excel 2010, and SigmaPlot 12.3 as described previously (31). Families of Cl− current densities were measured at three time points; instantaneous (IINST) or steady-state (IACT) Cl− current densities were measured, respectively, at 25 or 975 ms after activation by the voltage stimulus, while tail currents were measured 25 ms after cessation of each voltage stimulus. Activation (τACT) or deactivation (τDEACT) time constants were calculated by fitting currents to a weighted exponential function, f(t) = K0(f1e−t/τi) + C, as previously described (17, 31). The effect of [Ca2+]i was analyzed by a four-parameter logistic function, y = min + (max − min)/[1 + (x/EC50) − Hill slope], in which the min or max are the lower or upper asymptotes, the Hill slope is a reflection of the steepness of the curve, and the EC50 is the concentration of half response to [Ca2+]i. Data are expressed as means ± SE, and significance was assigned at P < 0.05 by an unpaired two-tailed Student’s t-test with Welch’s correction (see Figs. 2 and 3) or a two-way ANOVA with Bonferroni posttest (see Fig. 5).
Fig. 2.
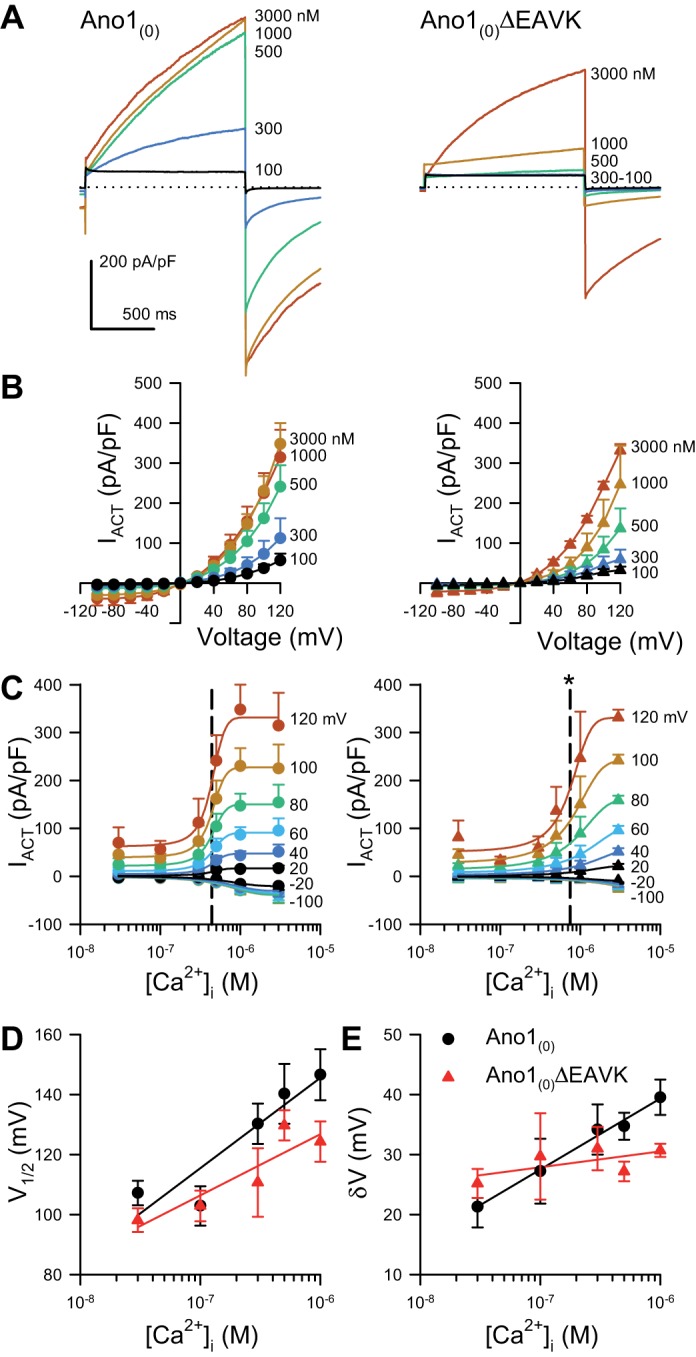
Activating currents from the Ano1(0)ΔEAVK splice variant have a higher EC50 of Ca2+ dependence than Ano1(0). A: representative Cl− current traces activated by a voltage step to +80 mV, recorded from HEK293 cells transfected with Ano1(0) (left) or Ano1(0)ΔEAVK (right), and dialyzed with intracellular Ca2+ concentrations ([Ca2+]i) ranging from 100 to 3,000 nM as indicated by the spectrum. Dotted line; 0 pA/pF. B: Cl− currents activated at the 1-s time point (IACT) from Ano1(0) (left) or Ano1(0)ΔEAVK (right), plotted vs. voltage stimulus with 100–3,000 nM [Ca2+]i (spectrum). C: IACT from Ano1(0) (left) or Ano1(0)ΔEAVK (right), plotted as a function of [Ca2+]i at the indicated voltage steps (spectrum). EC50 values (dashed lines): Ano1(0), 438 ± 7 nM; Ano1(0)ΔEAVK, 746 ± 47 nM; n = 11; *P < 0.05, compared with Ano1(0) control by an unpaired t-test with Welch’s correction. D–E: voltage of half-activation (V1/2; D) or slope (δV; E) plotted as a function of [Ca2+]i (P < 0.05, [Ca2+]i as a source of variation of V1/2 or δV values; P > 0.05, interaction between the EAVK sequence and [Ca2+]i for either V1/2 or δV by two-way ANOVA with Bonferroni posttest).
Fig. 3.
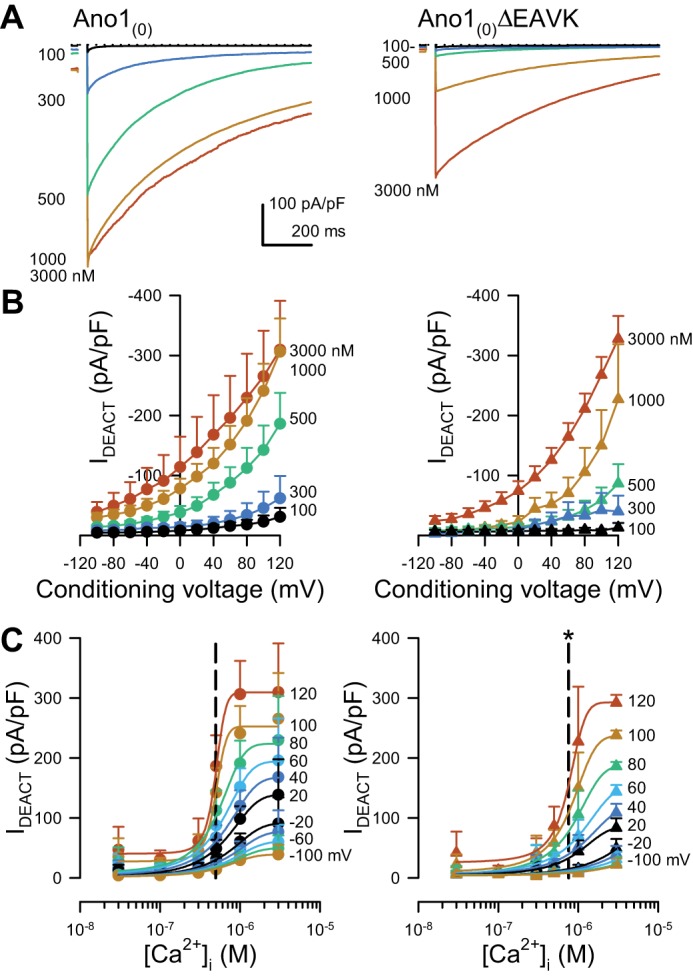
Deactivating currents from the Ano1(0)ΔEAVK splice variant have a higher EC50 of Ca2+ dependence than Ano1(0). A: representative Cl− current traces deactivating from a conditioning voltage step to +80 mV, recorded from HEK293 cells transfected with Ano1(0) (left) or Ano1(0)ΔEAVK (right) and dialyzed with intracellular Ca2+ concentrations ([Ca2+]i) ranging from 100 to 3,000 nM as indicated by the spectrum. Dotted line: 0 pA/pF. B: peak Cl− currents deactivating at the holding voltage (IDEACT) from Ano1(0) (left) or Ano1(0)ΔEAVK (right), plotted vs. the conditioning voltage with 100–3,000 nM [Ca2+]i (spectrum). C: IDEACT from Ano1(0) (left) or Ano1(0)ΔEAVK (right), plotted as a function of [Ca2+]i after the indicated voltage steps (spectrum). EC50 values (dashed lines): Ano1(0), 493 ± 9 nM; Ano1(0)ΔEAVK, 761 ± 26 nM; n = 11, *P < 0.05, compared with Ano1(0) control by an unpaired t-test with Welch’s correction.
Fig. 5.
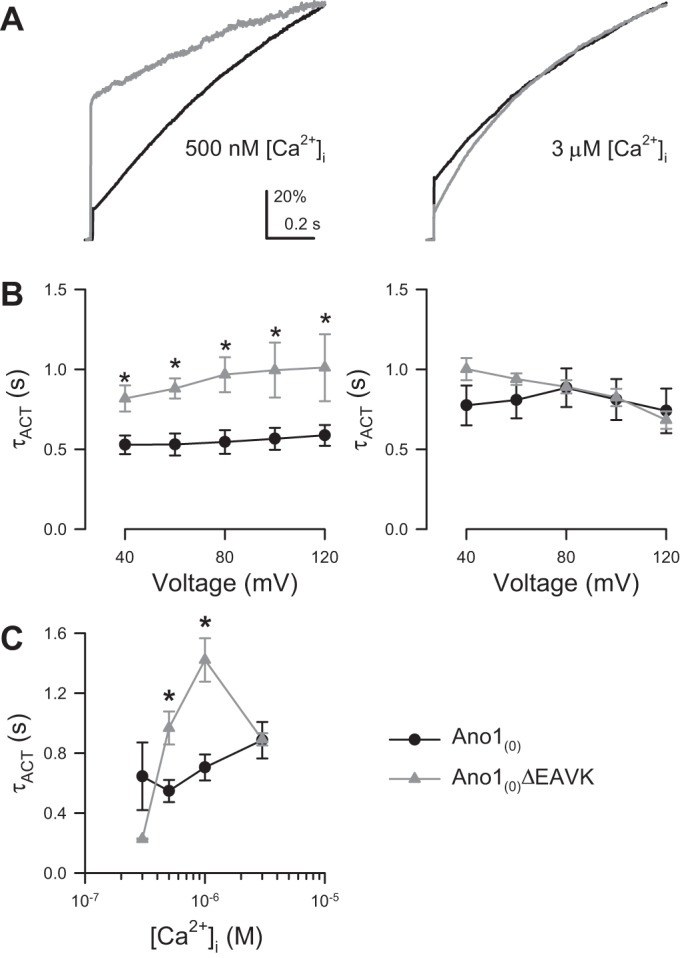
The Ano1(0)ΔEAVK splice variant has slower activation kinetics than Ano1(0) at intermediate [Ca2+]i. A: normalized Cl− current traces activated by a step pulse to +80 mV from Ano1(0) (black) or Ano1(0)ΔEAVK (gray) at 500 nM (left) or 3 µM (right) [Ca2+]i. B: time constant of activation (τACT) vs. voltage stimulus of Ano1(0) (black) or Ano1(0)ΔEAVK (gray) with 500 nM (left) or 3 µM (right) [Ca2+]i. P < 0.05, variation from EAVK. P > 0.05, variation from or interaction with voltage stimulus by a two-way ANOVA. *P < 0.05, by Bonferroni posttest. C: τACT of Ano1(0) (black) or Ano1(0)ΔEAVK (gray) vs. [Ca2+]i. P < 0.05, variation from and interaction with [Ca2+]i by a two-way ANOVA. *P < 0.05, by Bonferroni posttest; n = 5–16 cells per point.
RESULTS
We first aimed to determine whether Ano1(0) and Ano1(0)ΔEAVK constructs had differences in calcium sensitivities of Cl− currents. Ano1(0)ΔEAVK channels were voltage clamped, and Cl− current densities were compared with those of Ano1(0) across a range of intracellular Ca2+ concentrations ([Ca2+]i). Both constructs produced robust Cl− currents (Fig. 2A). The activation of both constructs was voltage and Ca2+ dependent (Fig. 2B). Ano1(0) activated at lower [Ca2+]i than Ano1(0)ΔEAVK (Fig. 2C). We compared plateau currents (the current at 1 s per step voltage) vs. [Ca2+]i. These data showed that Ano1(0)ΔEAVK currents had a significantly higher EC50 for Ca2+ dependence of Cl− currents than Ano1(0) controls (Ano1(0), 438 ± 7 nM; Ano1(0)ΔEAVK, 746 ± 47 nM; n = 11; P < 0.05, compared with control by an unpaired t-test with Welch’s correction) (Fig. 2C). Interestingly, the voltage dependence was calcium sensitive, but this calcium sensitivity of voltage dependence was not significantly different between of these two constructs (P < 0.05, [Ca2+]i as source of variation of V1/2 or δV values; P > 0.05, interaction between the EAVK sequence and [Ca2+]i for either V1/2 or δV; Fig. 2, D and E).
To look at the calcium sensitivity of Cl− current independent of the voltage dependence of each Ano1 variant, we tested whether Ano1(0)ΔEAVK Ca2+ sensitivity was also different using peak tail currents at the holding potential (−100 mV) immediately after steps to the conditioning pulse (Fig. 3). The Ano1(0) tail current, like steady state, was activated at lower [Ca2+]i than Ano1(0)ΔEAVK (Fig. 3B). The EC50 for [Ca2+]i of deactivating Cl− current was right-shifted for Ano1(0)ΔEAVK compared with full length [Ano1(0), 493 ± 9 nM vs. Ano1(0)ΔEAVK, 761 ± 26 nM; n = 11; P < 0.05, by an unpaired t-test with Welch’s correction; Fig. 3C]. Our data show that the calcium sensitivities of Ano1 tail currents sensitivity were similar to those obtained using voltage-dependent activation. These results suggest that calcium sensitivity and not voltage dependence is different between the Ano1(0)ΔEAVK and Ano1(0) constructs.
The [Ca2+]i is known to alter the kinetics of Ano1 activation (steady state) currents, such that the current shape at low [Ca2+]i is “box-like,” as reflected quantitatively by the ratio between total current (IACT) and instantaneous current (IINST), IINST/IACT of ~1. At high [Ca2+]i, this ratio is ~0.3; rather, the current shape is no longer box like. Importantly, Ano1 splice variants missing segment c have been reported to have greater instantaneous current relative to steady-state current at low [Ca2+]i (7). Indeed, our data show that despite a higher EC50 for [Ca2+]i (Figs. 2C and 3C), instantaneous currents of Ano1(0)ΔEAVK were larger than Ano1(0) at low [Ca2+]i (Fig. 4B). Thus we looked for whether Ca2+ dependence of the instantaneous currents was different between splice variants. The ratio of instantaneous-to-steady-state currents (IINST/IACT) was voltage and [Ca2+]I dependent with currents more box-like (approaching IINST/IACT = 1) at lesser [Ca2+]i and/or lower voltages (Fig. 4, C and D). Interestingly, although the steady-state Ano1(0) current was more Ca2+ sensitive (438 ± 7 nM EC50) than Ano1(0)ΔEAVK (746 ± 47 nM EC50; Fig. 2C), instantaneous Cl− currents of the isoforms had similar calcium sensitivities [EC50: Ano1(0), 413 ± 15 nM; Ano1(0)ΔEAVK, 395 ± 22 nM; n = 11, P > 0.05, compared with by an unpaired t-test with Welch’s correction; Fig. 4D].
Fig. 4.
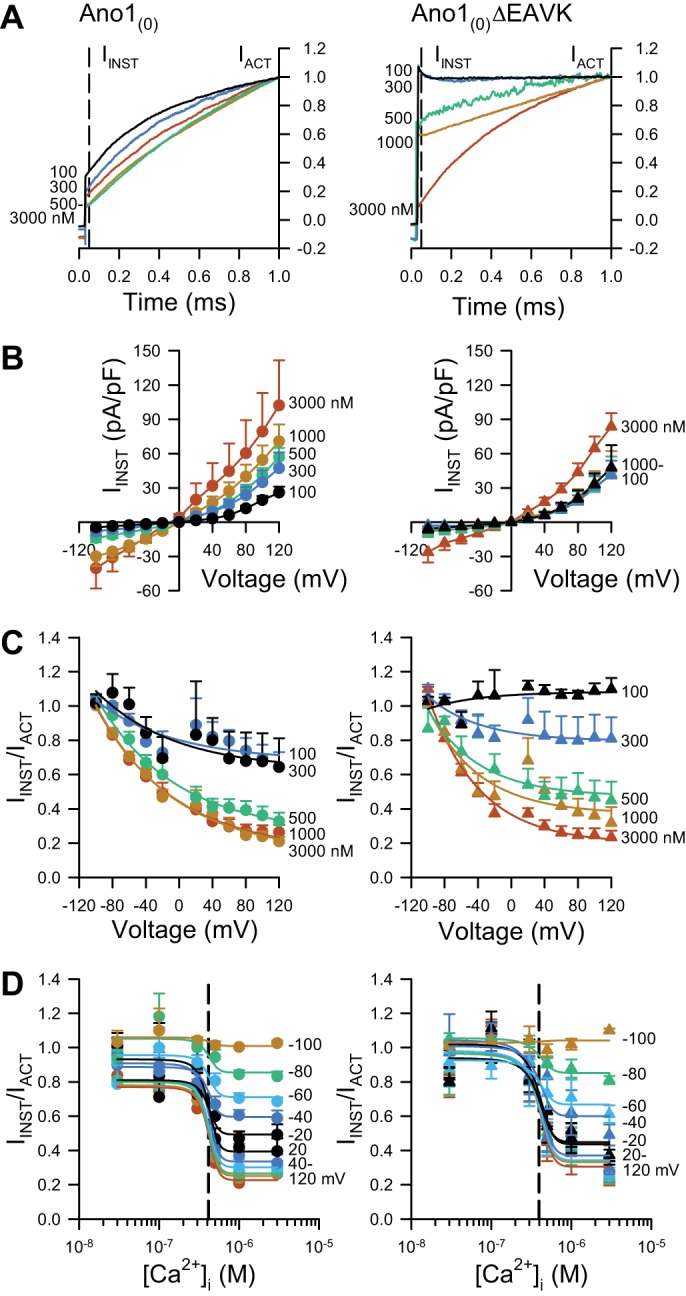
The Ano1(0)ΔEAVK splice variant has a higher ratio of instantaneous to steady-state Cl− current at low [Ca2+]i than Ano1(0). A: normalized Cl− current traces activated by a voltage step to +80 mV, recorded from HEK293 cells transfected with Ano1(0) (left) or Ano1(0)ΔEAVK (right), and dialyzed with [Ca2+]i ranging from 100 to 3,000 nM as indicated by the spectrum. IACT, steady-state Cl− current at 1 s is normalized to 1; IINST, instantaneous Cl− current at 25 ms is indicated by the dashed line. B: instantaneous Cl− current densities from Ano1(0) (left) or Ano1(0)ΔEAVK (right), plotted as a function of the voltage step and dialyzed with 100–3,000 nM [Ca2+]i (spectrum). C: ratio of IINST to IACT (IINST/IACT), plotted vs. the voltage stimulus from Ano1(0) (left) or Ano1(0)ΔEAVK (right) with 100–3,000 nM [Ca2+]i (spectrum), where IINST/IACT ~1 indicates a “box-like” Cl− current. D, IINST/IACT of Ano1(0) (left) or Ano1(0)ΔEAVK (right), plotted as a function of [Ca2+]i at the indicated voltage steps (spectrum). EC50 values (dashed lines): Ano1(0), 413 ± 15 nM; Ano1(0)ΔEAVK, 395 ± 22 nM; n = 11; P > 0.05, compared with Ano1(0) control by an unpaired t-test with Welch’s correction.
Our data show that compared with Ano1(0), Ano1(0)ΔEAVK has a significantly different [Ca2+]i EC50 of IACT but not [Ca2+]i EC50 of IINST/IACT. Therefore, we predicted that the differences in IACT are due to faster time constants of activation (τACT) for Ano1(0) than Ano1(0)ΔEAVK. This is consistent with previous studies using an Ano1 construct without exon 0, where Ano1ΔEAVK activated slower than wild-type Ano1 (40). Here, we find that at 500–1,000 nM [Ca2+]i, for which Ano1(0) current was larger than Ano1(0)ΔEAVK, the latter channels opened significantly slower than full length {Fig. 5, A and B, left, and C; 500 nM [Ca2+]i: Ano1(0), 547 ± 74 ms, n = 15; Ano1(0)ΔEAVK, 968 ± 110 ms, n = 6; 1,000 nM [Ca2+]i: Ano1(0), 705 ± 87 ms, n = 13; Ano1(0)ΔEAVK, 1,422 ± 144 ms, n = 4; P < 0.05, variation from the EAVK sequence by a two-way ANOVA with Bonferroni posttest}. At the highest [Ca2+]i tested (3 µM), The Ano1(0)ΔEAVK current was sloped (τACT, 892 ± 41 ms) and not different from Ano1(0) (τACT, 886 ± 121 ms; Fig. 5, A and B, right, and C). These data suggest that the changes in activation kinetics of Ano1(0)ΔEAVK compared with Ano1(0) lead to the shift in the Ca2+ dependence of steady-state IACT of Ano1(0)ΔEAVK.
DISCUSSION
We report presently that for the full-length Ano1, including the recently discovered exon 0 [Ano1(0)], omission of segment c (exon 13 or EAVK) leads to a decrease in the calcium sensitivity of Ano1 Cl− currents at all examined voltages. Compared with Ano1(0), Ano1(0)ΔEAVK had a slower rate of activation and a higher ratio of instantaneous to steady-state Cl− current. The changes in kinetics were not uniform across all [Ca2+]i; there was relatively more Cl− current at low [Ca2+]i early upon depolarization of Ano1(0)ΔEAVK, while Ano1(0)ΔEAVK had less Ca2+-activated Cl− current late after depolarization. The codependence of the parameters of voltage, [Ca2+]i, and time leads to a shift in the Ca2+ dependence of Cl− current density of Ano1(0)ΔEAVK compared with Ano1(0).
Previous studies on the splicing of segment c focused on both the EAVK sequence encoded by exon 13 and the adjacent amino acids, especially a repeat sequence of glutamate residues that together with exon 13 create the sequence motif, EEEEEAVK. This concentration of negatively charged residues has similarities to calcium-binding sites in other proteins, such as the large conductance Ca2+-activated K+ channels (BK) and bestrophin (5, 39), and might be expected to underlie calcium sensitivity of Ano1. However, Ferrera et al. (7) reported that ΔEAVK or ΔVK but not ΔEA in the human Ano1(a,b,c) construct (lacking segment d and exon 0) increased the relative instantaneous Ano1 current and ΔEAVK changed the voltage but not calcium dependence of Ano1. Xiao et al. (40) found that ΔEAVK from the mouse Ano1(a,c) clone (lacking exon 0 and segments b and d) resulted in higher current densities at 0 M Ca2+ and, therefore, concluded EAVK affects Ca2+-independent gating of Ano1. Yet, they found that EAVK was important for Ano1 activation in the presence of intracellular Ca2+. The affinity of mouse Ano1(a,c) for Ca2+ was 15-fold lower when segment c was deleted, which is a larger effect than the twofold change that we observed here in our studies on the full-length human Ano1(0,a,b,c,d) construct (40). Furthermore, compared with Ano1(a,c), Ano1(a)ΔEAVK had an increased τSLOW without change to τFAST, suggesting that EAVK sped up the slow-gating mode of Ano1 activation (38). These studies suggest that EAVK contributes to but is not necessary for voltage and calcium sensitivity of Ano1, but the effects are variable depending on the specific Ano1 construct used and intracellular Ca2+ concentration employed. The dependence of mouse Ano1(a,c) on EAVK for the dual-gating modes (fast in milliseconds and slow in seconds) exhibited by this channel is not evident when extracellular Cl− is lowered to 30 mM (4), so we did these studies in physiological Cl− concentrations. In our study, compared with Ano1(0), Ano1(0)ΔEAVK increased τACT, suggesting that EAVK speeds up activation time even in the exon 0 background in human Ano1(a,b,c,d). Our data are also consistent with other published studies, in that ΔEAVK in the human Ano1(0,a,b,c,d) background resulted in currents with a large, fast instantaneous component that is voltage sensitive and less dependent on Ca2+ than the less prominent slow outward component, due to the increased time constant for the slow component (τSLOW) as reported by Xiao et al. (40). The main difference observed by including exon 0 and segment d in the construct was a reduced shift in the EC50 value for [Ca2+]i when segment c is removed. One needs to also take into account that human Ano1(0,a,b,c,d) is already more Ca2+ sensitive than Ano1(a,b,c,d) (31).
Our studies are also consistent with a requirement for negatively charged amino acids on the COOH-terminal end of the Ano1 protein for effective activation of mouse Ano1(a,c) (lacking mouse exon 1 and segments b and d) by intracellular Ca2+ (36). For mouse Ano1(a,c), mutations in any one of six glutamate and aspartate residues to alanine residues between the fifth and seventh α-helical structures on the COOH-terminal end of the protein result in effectively blocking intracellular Ca2+ regulation (up to 1,000-fold increase in EC50 value) of the Ano1 channels, indicating that these residues form a Ca2+-binding site on the intracellular side of the protein (36). Our data support the concept that exon 0 and segment c are intracellular domains that contribute to the effective coupling of the Ca2+-binding site to channel gating and that transcriptional variants with or without these sites alter the Ca2+ sensitivity within the physiological range of 100 to 1,000+ nM [Ca2+]i.
The EAVK sequence expressed by exon 13 is a segment that is present in only 25% of all Ano1 transcripts from smooth muscle strips of the human stomach (17), where Ano1 is expressed in ICC and has a pacemaking function (8). The expression of EAVK in human stomach at 25% is low compared with >75% of Ano1 transcripts that include this exon in other tissues including intestinal mucosa, brain, and lung (7), where Ano1 is expressed in the epithelia and plays a role in secretion. In the present work, we used HEK293 cells for transient Ano1 transfection. This is an established and well-validated heterologous expression system that has minimal endogenous Cl− currents and robustly expresses heterologous Ano1. Our findings will need to be verified in primary ICC that express Ano1 transcripts with and without the EAVK sequence, such as those from nondiabetic human gastric muscularis propria.
In the context of regulation of pacemaking by ICC in the gastrointestinal tract, the low expression of EAVK means that the predominant Ano1 channels in gastric smooth muscle that lack EAVK will exhibit a reduced sensitivity to Ca2+ but faster instantaneous Ca2+-activated Cl− currents that deactivate more slowly. We propose that these properties contribute to the rapid activation of the pacemaker potential but only when intracellular Ca2+ reaches the necessary level for activation. In addition, there is evidence that Ca2+-activated Cl− currents also contribute to the width of the plateau of the electrical slow wave (16); therefore, slower deactivation of Ano1 channels means that the channels will contribute to a sustained Cl− current, broadening of the electrical slow wave, and more effective electrical coupling between adjacent ICC. Our studies on Ano1 knockout mice show a requirement for Ano1 in the synchronization of Ca2+ transients within pacemaker ICC of the mouse small intestine (28). We propose that this synchronization will be facilitated in Ano1 channels that express human exon 0 or mouse exon 1 and the EAVK sequence.
In conclusion, the human full-length Ano1 that includes the recently discovered exon 0 [Ano1(0)], when lacking exon 13 (segment c or EAVK), has a decreased steady-state calcium sensitivity due to slower channel activation. These changes would alter Cl− flux in tissues where there is physiological decreased expression of the EAVK segment such as gastric smooth muscle.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grant DK-57061 and Mayo Clinic Center for Cell Signaling in Gastroenterology NIDDK Grant P30-DK-084567.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
P.R.S., S.J.G., A.M., and G.F. conceived and designed research; P.R.S., A.M., and C.E.B. performed experiments; P.R.S. and A.M. analyzed data; P.R.S., S.J.G., A.M., and A.B. interpreted results of experiments; P.R.S. and A.M. prepared figures; P.R.S. drafted manuscript; P.R.S., S.J.G., A.B., and G.F. edited and revised manuscript; P.R.S., S.J.G., A.M., C.E.B., A.B., and G.F. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Kristy Zodrow for secretarial assistance and Dr. David Linden for helpful advice.
REFERENCES
- 1.Bulley S, Neeb ZP, Burris SK, Bannister JP, Thomas-Gatewood CM, Jangsangthong W, Jaggar JH. TMEM16A/ANO1 channels contribute to the myogenic response in cerebral arteries. Circ Res 111: 1027–1036, 2012. doi: 10.1161/CIRCRESAHA.112.277145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, Pfeffer U, Ravazzolo R, Zegarra-Moran O, Galietta LJ. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science 322: 590–594, 2008. doi: 10.1126/science.1163518. [DOI] [PubMed] [Google Scholar]
- 3.Cho H, Yang YD, Lee J, Lee B, Kim T, Jang Y, Back SK, Na HS, Harfe BD, Wang F, Raouf R, Wood JN, Oh U. The calcium-activated chloride channel anoctamin 1 acts as a heat sensor in nociceptive neurons. Nat Neurosci 15: 1015–1021, 2012. doi: 10.1038/nn.3111. [DOI] [PubMed] [Google Scholar]
- 4.Cruz-Rangel S, De Jesús-Pérez JJ, Contreras-Vite JA, Pérez-Cornejo P, Hartzell HC, Arreola J. Gating modes of calcium-activated chloride channels TMEM16A and TMEM16B. J Physiol 593: 5283–5298, 2015. doi: 10.1113/JP271256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cui J, Yang H, Lee US. Molecular mechanisms of BK channel activation. Cell Mol Life Sci 66: 852–875, 2009. doi: 10.1007/s00018-008-8609-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dutta AK, Khimji AK, Liu S, Karamysheva Z, Fujita A, Kresge C, Rockey DC, Feranchak AP. PKCα regulates TMEM16A-mediated Cl− secretion in human biliary cells. Am J Physiol Gastrointest Liver Physiol 310: G34–G42, 2016. doi: 10.1152/ajpgi.00146.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrera L, Caputo A, Ubby I, Bussani E, Zegarra-Moran O, Ravazzolo R, Pagani F, Galietta LJ. Regulation of TMEM16A chloride channel properties by alternative splicing. J Biol Chem 284: 33360–33368, 2009. doi: 10.1074/jbc.M109.046607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gomez-Pinilla PJ, Gibbons SJ, Bardsley MR, Lorincz A, Pozo MJ, Pasricha PJ, Van de Rijn M, West RB, Sarr MG, Kendrick ML, Cima RR, Dozois EJ, Larson DW, Ordog T, Farrugia G. Ano1 is a selective marker of interstitial cells of Cajal in the human and mouse gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol 296: G1370–G1381, 2009. doi: 10.1152/ajpgi.00074.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hartzell HC, Yu K, Xiao Q, Chien LT, Qu Z. Anoctamin/TMEM16 family members are Ca2+-activated Cl− channels. J Physiol 587: 2127–2139, 2009. doi: 10.1113/jphysiol.2008.163709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirst GD, Dickens EJ, Edwards FR. Pacemaker shift in the gastric antrum of guinea-pigs produced by excitatory vagal stimulation involves intramuscular interstitial cells. J Physiol 541: 917–928, 2002. doi: 10.1113/jphysiol.2002.018614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huizinga JD, Thuneberg L, Klüppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature 373: 347–349, 1995. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- 12.Huizinga JD, Zhu Y, Ye J, Molleman A. High-conductance chloride channels generate pacemaker currents in interstitial cells of Cajal. Gastroenterology 123: 1627–1636, 2002. doi: 10.1053/gast.2002.36549. [DOI] [PubMed] [Google Scholar]
- 13.Hwang SJ, Blair PJ, Britton FC, O’Driscoll KE, Hennig G, Bayguinov YR, Rock JR, Harfe BD, Sanders KM, Ward SM. Expression of anoctamin 1/TMEM16A by interstitial cells of Cajal is fundamental for slow wave activity in gastrointestinal muscles. J Physiol 587: 4887–4904, 2009. doi: 10.1113/jphysiol.2009.176198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jung J, Nam JH, Park HW, Oh U, Yoon JH, Lee MG. Dynamic modulation of ANO1/TMEM16A HCO3(-) permeability by Ca2+/calmodulin. Proc Natl Acad Sci USA 110: 360–365, 2013. doi: 10.1073/pnas.1211594110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kito Y, Suzuki H. Properties of pacemaker potentials recorded from myenteric interstitial cells of Cajal distributed in the mouse small intestine. J Physiol 553: 803–818, 2003. doi: 10.1113/jphysiol.2003.051334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lees-Green R, Gibbons SJ, Farrugia G, Sneyd J, Cheng LK. Computational modeling of anoctamin 1 calcium-activated chloride channels as pacemaker channels in interstitial cells of Cajal. Am J Physiol Gastrointest Liver Physiol 306: G711–G727, 2014. doi: 10.1152/ajpgi.00449.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mazzone A, Bernard CE, Strege PR, Beyder A, Galietta LJ, Pasricha PJ, Rae JL, Parkman HP, Linden DR, Szurszewski JH, Ördög T, Gibbons SJ, Farrugia G. Altered expression of Ano1 variants in human diabetic gastroparesis. J Biol Chem 286: 13393–13403, 2011. doi: 10.1074/jbc.M110.196089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mazzone A, Eisenman ST, Strege PR, Yao Z, Ordog T, Gibbons SJ, Farrugia G. Inhibition of cell proliferation by a selective inhibitor of the Ca(2+)-activated Cl(-) channel, Ano1. Biochem Biophys Res Commun 427: 248–253, 2012. doi: 10.1016/j.bbrc.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mazzone A, Gibbons SJ, Bernard CE, Nowsheen S, Middha S, Almada LL, Ordog T, Kendrick ML, Reid Lombardo KM, Shen KR, Galietta LJ, Fernandez-Zapico ME, Farrugia G. Identification and characterization of a novel promoter for the human ANO1 gene regulated by the transcription factor signal transducer and activator of transcription 6 (STAT6). FASEB J 29: 152–163, 2015. doi: 10.1096/fj.14-258541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mroz MS, Keely SJ. Epidermal growth factor chronically upregulates Ca(2+)-dependent Cl(-) conductance and TMEM16A expression in intestinal epithelial cells. J Physiol 590: 1907–1920, 2012. doi: 10.1113/jphysiol.2011.226126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park SJ, Mckay CM, Zhu Y, Huizinga JD. Volume-activated chloride currents in interstitial cells of Cajal. Am J Physiol Gastrointest Liver Physiol 289: G791–G797, 2005. doi: 10.1152/ajpgi.00050.2005. [DOI] [PubMed] [Google Scholar]
- 22.Parsons SP, Sanders KM. An outwardly rectifying and deactivating chloride channel expressed by interstitial cells of cajal from the murine small intestine. J Membr Biol 221: 123–132, 2008. doi: 10.1007/s00232-007-9084-2. [DOI] [PubMed] [Google Scholar]
- 23.Pritchard HA, Leblanc N, Albert AP, Greenwood IA. Inhibitory role of phosphatidylinositol 4,5-bisphosphate on TMEM16A-encoded calcium-activated chloride channels in rat pulmonary artery. Br J Pharmacol 171: 4311–4321, 2014. doi: 10.1111/bph.12778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romanenko VG, Catalán MA, Brown DA, Putzier I, Hartzell HC, Marmorstein AD, Gonzalez-Begne M, Rock JR, Harfe BD, Melvin JE. Tmem16A encodes the Ca2+-activated Cl− channel in mouse submandibular salivary gland acinar cells. J Biol Chem 285: 12990–13001, 2010. doi: 10.1074/jbc.M109.068544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanders KM, Zhu MH, Britton F, Koh SD, Ward SM. Anoctamins and gastrointestinal smooth muscle excitability. Exp Physiol 97: 200–206, 2012. doi: 10.1113/expphysiol.2011.058248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schreiber R, Uliyakina I, Kongsuphol P, Warth R, Mirza M, Martins JR, Kunzelmann K. Expression and function of epithelial anoctamins. J Biol Chem 285: 7838–7845, 2010. doi: 10.1074/jbc.M109.065367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schroeder BC, Cheng T, Jan YN, Jan LY. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell 134: 1019–1029, 2008. doi: 10.1016/j.cell.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh RD, Gibbons SJ, Saravanaperumal SA, Du P, Hennig GW, Eisenman ST, Mazzone A, Hayashi Y, Cao C, Stoltz GJ, Ordog T, Rock JR, Harfe BD, Szurszewski JH, Farrugia G. Ano1, a Ca2+-activated Cl− channel, coordinates contractility in mouse intestine by Ca2+ transient coordination between interstitial cells of Cajal. J Physiol 592: 4051–4068, 2014. doi: 10.1113/jphysiol.2014.277152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sondo E, Scudieri P, Tomati V, Caci E, Mazzone A, Farrugia G, Ravazzolo R, Galietta LJ. Non-canonical translation start sites in the TMEM16A chloride channel. Biochim Biophys Acta 1838: 89–97, 2014. doi: 10.1016/j.bbamem.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stanich JE, Gibbons SJ, Eisenman ST, Bardsley MR, Rock JR, Harfe BD, Ordog T, Farrugia G. Ano1 as a regulator of proliferation. Am J Physiol Gastrointest Liver Physiol 301: G1044–G1051, 2011. doi: 10.1152/ajpgi.00196.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strege PR, Bernard CE, Mazzone A, Linden DR, Beyder A, Gibbons SJ, Farrugia G. A novel exon in the human Ca2+-activated Cl− channel Ano1 imparts greater sensitivity to intracellular Ca2. Am J Physiol Gastrointest Liver Physiol 309: G743–G749, 2015. doi: 10.1152/ajpgi.00074.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sung TS, O’Driscoll K, Zheng H, Yapp NJ, Leblanc N, Koh SD, Sanders KM. Influence of intracellular Ca2+ and alternative splicing on the pharmacological profile of ANO1 channels. Am J Physiol Cell Physiol 311: C437–C451, 2016. doi: 10.1152/ajpcell.00070.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Terashima H, Picollo A, Accardi A. Purified TMEM16A is sufficient to form Ca2+-activated Cl− channels. Proc Natl Acad Sci USA 110: 19354–19359, 2013. doi: 10.1073/pnas.1312014110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thuneberg L. Interstitial cells of Cajal: intestinal pacemaker cells? Adv Anat Embryol Cell Biol 71: 1–130, 1982. doi: 10.1007/978-3-642-68417-3_1. [DOI] [PubMed] [Google Scholar]
- 35.Tian Y, Schreiber R, Kunzelmann K. Anoctamins are a family of Ca2+-activated Cl− channels. J Cell Sci 125: 4991–4998, 2012. doi: 10.1242/jcs.109553. [DOI] [PubMed] [Google Scholar]
- 36.Tien J, Peters CJ, Wong XM, Cheng T, Jan YN, Jan LY, Yang H. A comprehensive search for calcium binding sites critical for TMEM16A calcium-activated chloride channel activity. eLife 3: 027723, 2014. doi: 10.7554/eLife.02772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ward SM, Burns AJ, Torihashi S, Sanders KM. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol 480: 91–97, 1994. doi: 10.1113/jphysiol.1994.sp020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiao Q, Cui Y. Acidic amino acids in the first intracellular loop contribute to voltage- and calcium-dependent gating of anoctamin1/TMEM16A. PLoS One 9: e99376, 2014. doi: 10.1371/journal.pone.0099376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiao Q, Prussia A, Yu K, Cui YY, Hartzell HC. Regulation of bestrophin Cl channels by calcium: role of the C terminus. J Gen Physiol 132: 681–692, 2008. doi: 10.1085/jgp.200810056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xiao Q, Yu K, Perez-Cornejo P, Cui Y, Arreola J, Hartzell HC. Voltage- and calcium-dependent gating of TMEM16A/Ano1 chloride channels are physically coupled by the first intracellular loop. Proc Natl Acad Sci USA 108: 8891–8896, 2011. doi: 10.1073/pnas.1102147108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, Park SP, Lee J, Lee B, Kim BM, Raouf R, Shin YK, Oh U. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature 455: 1210–1215, 2008. doi: 10.1038/nature07313. [DOI] [PubMed] [Google Scholar]
- 42.Zhu MH, Kim TW, Ro S, Yan W, Ward SM, Koh SD, Sanders KM. A Ca(2+)-activated Cl(-) conductance in interstitial cells of Cajal linked to slow wave currents and pacemaker activity. J Physiol 587: 4905–4918, 2009. doi: 10.1113/jphysiol.2009.176206. [DOI] [PMC free article] [PubMed] [Google Scholar]