Abstract
Direct intracerebroventricular injection of angiotensin II (ANG II) causes increases in blood pressure and salt and water intake, presumably mimicking an effect mediated by an endogenous mechanism. The subfornical organ (SFO) is a potential source of cerebrospinal fluid (CSF), ANG I, and ANG II, and thus we hypothesized that the SFO has a secretory function. Endogenous levels of angiotensinogen (AGT) and renin are very low in the brain. We therefore examined the immunohistochemical localization of angiotensin peptides and AGT in the SFO, and AGT in the CSF in two transgenic models that overexpress either human AGT (A+ mice), or both human AGT (hAGT) and human renin (SRA mice) in the brain. Measurements were made at baseline and following volumetric depletion of CSF. Ultrastructural analysis with immunoelectron microscopy revealed that superficially located ANG I/ANG II and AGT immunoreactive cells in the SFO were vacuolated and opened directly into the ventricle. Withdrawal of CSF produced an increase in AGT in the CSF that was accompanied by a large decline in AGT immunoreactivity within SFO cells. Our data provide support for the hypothesis that the SFO is a secretory organ that releases AGT and possibly ANG I/ANG II into the ventricle at least under conditions when genes that control the renin-angiotensin system are overexpressed in mice.
Keywords: angiotensinogen, cerebrospinal fluid, subfornical organ, secretion, transgenic mice
the circumventricular organs (CVOs) are neural structures located beneath the ependyma of the cerebral ventricles, mostly at the junctions between the different components of the ventricular system (7). They are all highly vascular with a modified or deficient blood-brain barrier (25). Three CVOs, the subfornical organ (SFO), organum vasculosum of the lamina terminalis (OVLT), and area postrema (AP), possess neuronal cell bodies with afferent and efferent neuronal connections with the hypothalamus and other brain regions (23, 25). Not only are neurons in the SFO, OVLT, and AP directly exposed to serum contents but they also are exposed to diffusible elements in the cerebrospinal fluid (CSF) via the ventricular ependyma and the Virchow-Robin spaces (13). In particular, the SFO possesses a sodium level sensor that can detect changes in CSF sodium ion concentration (14). Thus, the SFO, OVLT, and AP have been viewed essentially as sensory CVOs that can detect changes in osmolarity and ion concentrations (e.g., sodium) in the blood and CSF (10), as well as hormonal factors that cannot cross the blood-brain barrier, and they can effect changes in cardiovascular function and hypothalamic control of water intake through their axonal projections (17).
The SFO has access to circulating ANG II, and systemic administration of ANG II causes drinking that is mediated by the SFO (8, 35, 36), though the pressor effects of systemic ANG II appear to require the hypothalamic median preoptic area. On the other hand, intracerebroventricular (ICV) ANG II causes a 10–15% rise in mean arterial pressure and a bradycardia, effects that are blocked by losartan, suggesting that AT1 receptors either on the CVOs themselves or on intraventricular structures such as the choroid plexus are involved (11, 29). It has also been reported that small doses of ANG I or ANG II introduced into the third ventricle of sodium-depleted rats restores the intake of 1.0% NaCl after bilateral nephrectomy (4). Intracerebroventricular injection of angiotensin receptor antagonists (18) or antibodies directed against ANG II (9) greatly reduces drinking in normally hydrated rats. Furthermore, local overproduction of central ANG II by overexpression of human angiotensinogen (AGT) and renin transgenes in the SFO in mice causes elevated drinking and blood pressure, and increased resting metabolic rate (12, 26, 32, 37), effects that are blocked by intracerebroventricular losartan. Moreover, the dipsogenic response in these mice is blocked by genetic ablation of human AGT (hAGT) in the SFO (32). Expression of components of the renin-angiotensin system (RAS) occurs in the SFO (11, 19, 20, 46). These results suggest that the SFO may influence blood pressure and water homeostasis by intraventricular secretion of AGT, ANG I, or ANG II.
The subcellular mechanisms of ANG II synthesis in the SFO and possible secretion into the ventricles remain largely unknown. Increases in ANG II restricted to the SFO are dipsogenic, although whether this is mediated through neural connections or intraventricular release is unclear (5). Although there are neurons in the SFO, and ependymal cells cover its ventricular surface, putative neurosecretory cells are also present (6). The present study sought to determine whether the SFO secretes ANG I/ANG II and AGT into the ventricle and whether depletion of CSF acts as a stimulus for SFO secretion. Because of relatively low endogenous levels of brain RAS components, we utilized two transgenic mice strains with 1) overexpression of hAGT alone (termed A+), or 2) both human renin and hAGT targeted specifically to the central nervous system (termed SRA).
METHODS
Animal models.
Transgenic mice encoding the human renin gene under the control of neuron-specific promoter synapsin-I (SR mice), and human AGT under its endogenous promoter were generated as previously described (26, 32). All mice were maintained by successive generations of back-cross breeding to C57BL/6J mice (The Jackson Laboratory). SRA double-transgenic mice were generated by cross-breeding heterozygous SR with heterozygous hAGT transgenic mice. Double-transgenic mice were identified via PCR in tail DNA using human renin and hAGT-specific primers as previously described (26, 47). All mice (n = 58) were fed standard mouse chow (LM-485; Teklad Premier Laboratory Diets) and water ad libitum. Care of the mice used in the experiments met the standards set forth in Guide for the Care and Use of Laboratory Animals (8th ed., Washington, DC: National Academies Press). All procedures were approved by the University of Iowa Institutional Animal Care and Use Committee.
AGT and ANG I/II immunohistochemistry.
Nontransgenic (NT), SRA, and A+ mice were anesthetized with ketamine-xylazine solution (91 and 9.1 mg/kg) and perfused with PBS containing 4% paraformaldehyde and 1.0% glutaraldehyde. Brains were removed, postfixed in the same fixative for 24 h at 4°C, and cut with a vibratome into 35- to 50-μm-thick coronal sections. Preembedding immunohistochemistry was performed in pairs: brain sections from SRA or A+ mice were developed in the same bottle as brain sections from NT mice. Sections were incubated overnight in a 1:300 dilution of primary antibody designed to specifically detect ANG I/II (N-10 angiotensin-I/II antisera, sc-7419; Santa Cruz Biotechnology) or in a 1:300 dilution of primary anti-hAGT antibody provided by Dr. Duane Tewksbury (Marshfield Medical Research Foundation, Marshfield, WI). Anti-ANG I/II (Santa Cruz Biotechnology) is an affinity-purified goat polyclonal antibody developed against a peptide representing full-length ANG I of human origin and was designed to specifically detect ANG I/II of mouse, rat, and human origin, but it also detects full-length hAGT. The polyclonal hAGT antibody was generated by inoculating rabbits with purified hAGT followed by affinity purification (39). Sections were then washed with PBS and placed in 1:200 biotinylated secondary antibody, followed by biotin detection with solutions from an ABC Elite kit (Vector Laboratories). Visualization was made possible by treatment with 3,3′-diaminobenzidine (DAB) plus hydrogen peroxide.
For antisera control studies, adjacent sections were either processed using the same protocol but without primary antibody or processed with primary antisera that was preabsorbed with immunizing peptide. Preabsorption eliminated ANG or hAGT immunoreactivity in the SFO from NT, SRA, and A+ animals. Coverslips were placed over the sections and observed with a Nikon light microscope.
Sections taken for electron microscopic analysis were postfixed with 1% OsO4, rinsed, dehydrated in alcohol and acetone, and flat embedded in a mixture of Epon-812 and Araldite-502. The SFO was photographed with a Nikon microscope then cut out from flat sections using beveled cuts to preserve orientation. Ultrathin sections were cut using a Leica ultramicrotome, following which the sections were stained with uranyl acetate and lead citrate, and examined with a Jeol-1230 electron microscope. Under electron microscopic examination, we considered a structure as labeled (immunoreactive) if it showed a highly electron-dense DAB reaction product in the cytoplasm.
To confirm that release of AGT was through synaptic release, we undertook immunohistochemical detection of synaptobrevin-2 (VAMP2) using the same method as described above, though taking sections from SRA mice only. The polyclonal VAMP2 antibody (Osenses, Keswick, Australia) was generated by inoculating rabbits with a synthetic peptide corresponding to amino acids 10 through 60 of human VAMP2. The peptide is homologous to the same region of mouse VAMP2.
Cerebrospinal fluid collection.
Mice (n = 21), anesthetized as above, were placed in a stereotaxic frame (David Kopf Instruments), the skin on the neck was opened, and the neck muscles were retracted away from the midline, exposing the atlanto-occipital membrane. This membrane was carefully punctured, and a glass micropipette was inserted into the cisterna magna. CSF was allowed to flow into the micropipette by capillary action for 5 min initially. The micropipette was then replaced for a further 35 min of CSF collection for a second sample of CSF. Approximately 5 μl of CSF were removed in the 5-min period and ~15 μl were removed in the 35-min period. Animals were then perfused, and brain sections were processed for light and electron microscopic immunohistochemistry against ANG I/ANG II or hAGT.
Western blotting.
Western blotting of CSF of wild-type, SRA, and A+ mice was performed using the same antibody that was used to immunochemically detect hAGT.
Digital imaging.
All light microscopic images were taken with a digital camera (Sciscope Instrument) attached to a Nikon light microscope. Electron microscopic images were taken with a Gatan UltraScan 1000 2k × 2k CCD digital camera. Postprocessing of all images was performed with Adobe Photoshop CS3 software but was limited to minor contrast and color balancing and the removal of dirt and other blemishes.
Estimation of percentage of ANG I–II/AGT immunoreactivity in SFO cells.
A total of 72 digital electron micrographs (12 from each group) were randomly selected from wild-type, SRA, and A+ animals with or without CSF drainage (six groups) and stained with the sc-7419 antibody. Each digital micrograph was blind coded so the investigator performing the quantitative analysis was unaware of treatment or animal type. On one cell from each micrograph, the black value of 25 pieces of clearly identifiable intracellular AGT immunochemical reaction product was measured and the lowest (most white) value was used as a threshold value to digitally partition the original image into a black-and-white binary image in which the reaction product was represented by black and the remainder as white. An inverted digital “stencil” of the nucleus and mitochondria was created from the original image and digitally added to the binary image to eliminate any immunoreactivity associated with these organelles. The boundary of the cell was then carefully outlined and the percentage area of reaction product in each cell was calculated from the ratio of black pixels to white pixels within the cell outline. Percentage area data were compared by two-way ANOVA followed by a Bonferroni post hoc multiple comparisons procedure.
RESULTS
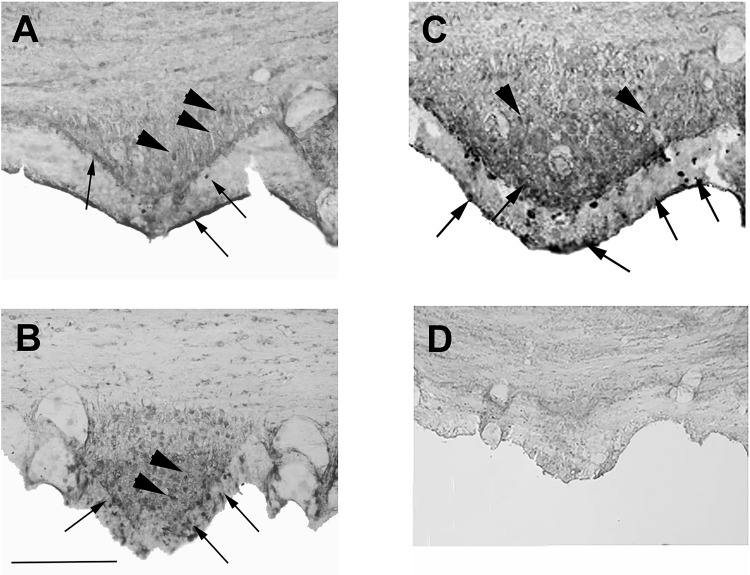
Our initial goal was to assay for ANG I and ANG II peptides in the SFO by immunohistochemistry. A weak immunohistochemical signal was detected in the SFO of NT mice using an antibody that is designed to detect both ANG I and ANG II (Fig. 1A). Using Western blots, we determined that this antiserum could detect full-length AGT, particularly when AGT is overexpressed (data not shown). A qualitatively stronger immunohistochemical signal was reproducibly observed in the SFO of A+ (Fig. 1B) and SRA (Fig. 1C) mice when we used an antiserum designed to detect full-length hAGT. In all cases, immunoreactive ANG I and ANG II peptide-positive cells (Fig. 1A) and immunoreactive hAGT-positive cells (Fig. 1, B and C) were located in the central portion of the SFO, in the subependymal zone, between that and the ependyma, and within the ependyma itself. Occasionally, immunoreactive cells were observed on the surface of the ependyma covering the SFO (see arrows in Fig. 1). Similar to our previous study (32), control immunohistochemical experiments using primary ANG I and ANG II antibody preabsorbed with the immunizing peptide (Fig. 1D) or in the absence of primary antibody (data not shown) did not produce any specific immunostaining in SRA mice. There was no evidence of any morphological differences in the SFO when comparing NT mice with A+ and SRA mice.
Fig. 1.
Light microscopy of angiotensin (ANG) peptides in the subfornical organ (SFO). Immunostained coronal sections through the SFO of a nontransgenic (NT) mouse (A), a mouse with overexpression of the human angiotensinogen (hAGT) gene (B), and a mouse with overexpression of hAGT gene and a human renin gene under a synapsin promoter (C and D). The section in A was stained with an anti-ANG I/II antibody (sc7419) that detects AGT as well, whereas the sections in B and C were stained with an anti-hAGT antibody. Note the general increase in intensity of immunochemical reaction product in the A+ and SRA animals overexpressing the human genes. Immunoreactive neurons (arrowheads) can be seen in the central part of the SFO as can neuron-like cells in the periphery and within and beneath the ependyma layer (arrows). The section in D was processed using antibody sc7419 preabsorbed with angiotensin II. Scale bar in B = 100 μm (it also applies to A and C).
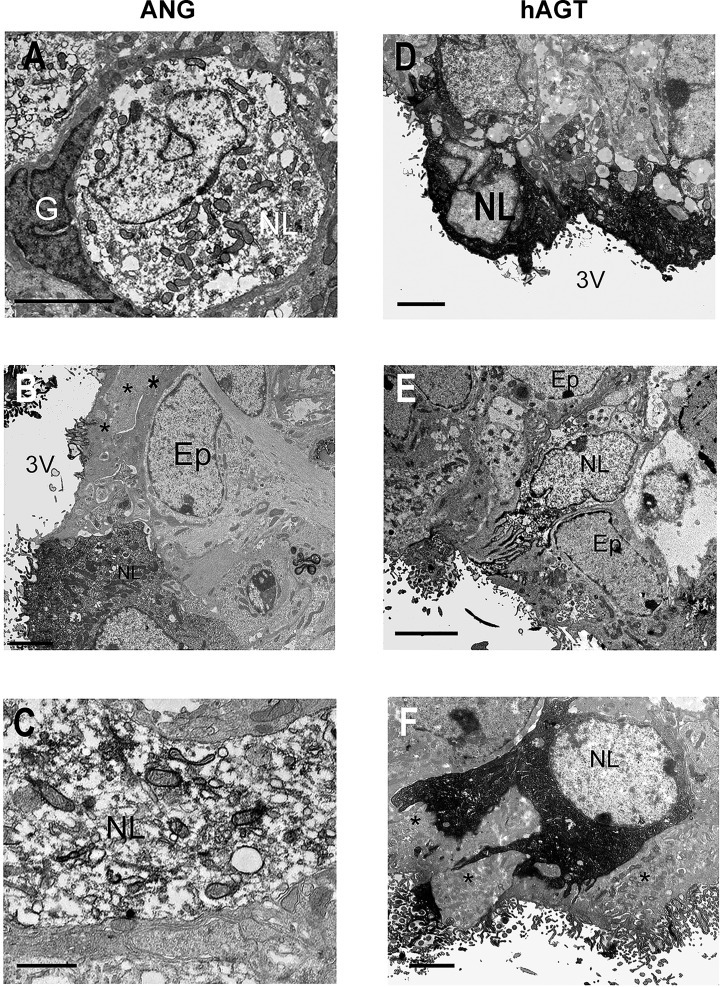
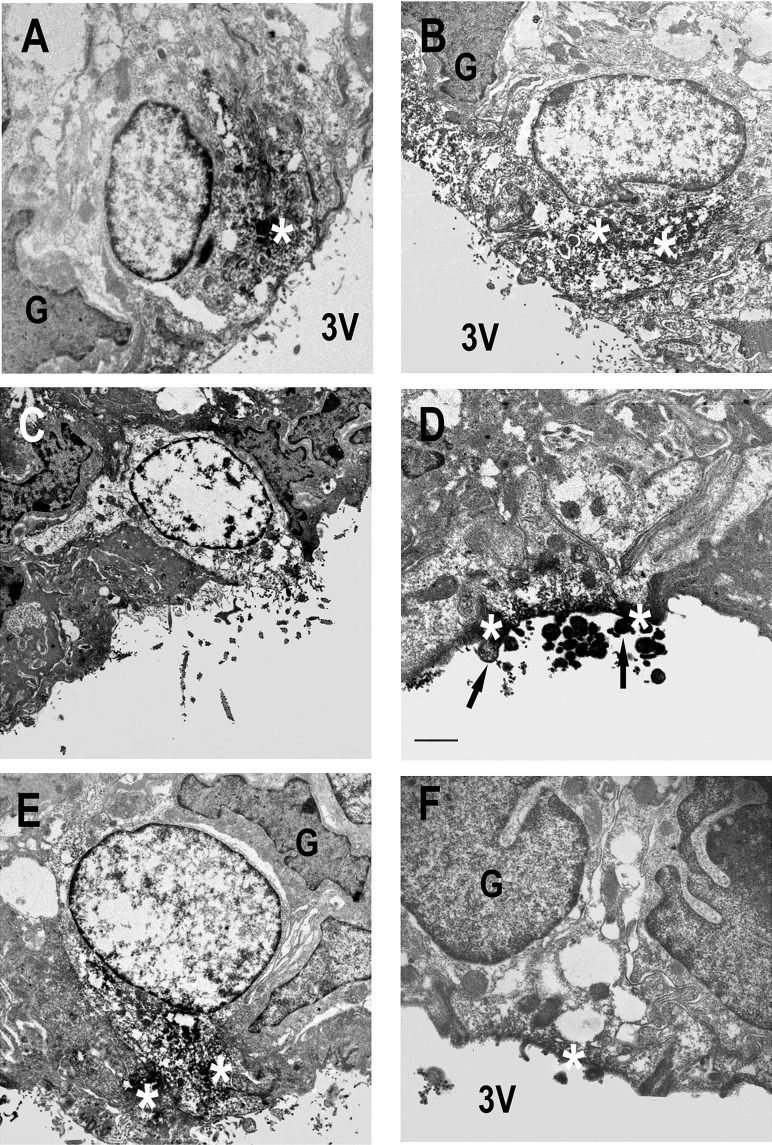
Electron microscopy confirmed immunoreactivity for total ANG I and ANG II peptides in the centrally located neuron population in NT and SRA mice, but a sparser population of strongly ANG-immunoreactive neuron-like cells was evident in the ependyma and subependymal region (Fig. 2, A–C). Most of the subependymal ANG I and ANG II peptide immunoreactive cells were located immediately beneath the ependyma, and in many cases, had processes extending to the surface (Fig. 2B). A similar pattern of immunoreactivity was observed when the antibody for hAGT was used, except we also observed microvilli projecting into the third ventricle (Fig. 2, D–F). All these cells possessed a round, electron-lucent nucleus that contrasted well with adjacent ependymal and glial cell nuclei, and immunoreactivity was located around the nucleus in the perikaryon and in apical processes (Fig. 2F). When we specifically examined hAGT staining in SRA mice, we observed that there was a qualitative increase in the density of the staining particularly in cells located nearer to the ventricular surface, either within the ependyma layer itself or on the surface that opens directly into the ventricle (Fig. 2D). Importantly, we did not observe immunohistochemical staining in cells in the SFO with morphological characteristics of astrocytes, glia, or ependymal cells. Human AGT immunoreactivity showed a similar cellular and subcellular distribution in A+ and SRA mice (Fig. 2, D–F).
Fig. 2.
Electron microscopic localization of ANG peptides and hAGT in SFO. Ultrastructural appearance of ANG peptide (A–C) and hAGT (D–F) cell immunoreactivity in the SFO in NT (A and B), A+ (E), and SRA (C, D, and F) mice. ANG peptide immunoreactivity was fairly sparsely distributed in the perikarya (A) and dendrites (C) of neuron-like (NL) cells in both NT (A) and transgenic (C) animals. In contrast, more superficially located neuron-like cells exhibited stronger immunoreactivity (D and F). Immunoreactive cell processes could be observed passing between ependymal cell (Ep) processes (* in F) to the surface of the third ventricle (3V). Note the absence of ANG peptide and hAGT immunoreactivity in glia (G) and Ep cells adjacent to immunoreactive NL cells (A, B, and F). Scale bars A and C–F = 2 μm; scale bar B = 0.5 μm.
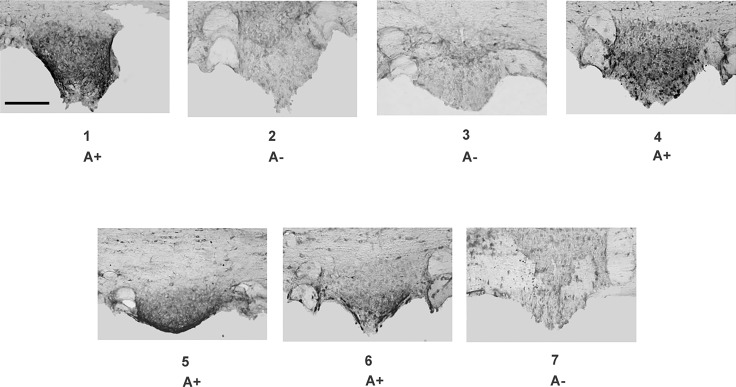
We wondered whether CSF withdrawal promoted release of hAGT from the SFO. As a pilot study to determine the feasibility of doing so, we first correlated the presence of hAGT in the CSF with hAGT immunostaining in the SFO of A+ mice. To accomplish this, CSF was obtained from two groups of transgenic and NT littermates, but researchers were blinded as to genotype. Mice were then perfused and the SFO was removed and sectioned. Human AGT protein in the CSF was scored by Western blot (data not shown) and then correlated with genotype. The positive signal on the Western blot correlated perfectly with genotype when the identity of the mice was decoded. Importantly, human AGT immunostaining was easily detected in the SFO of A+ mice, which exhibited hAGT in the CSF (Fig. 3).
Fig. 3.
AGT in SFO. Immunohistochemistry of hAGT expression in SFO of seven littermates of an A+ × C57 breeding (A+ and A− mice). The littermate animals were not identified before immunohistochemistry, and sections from different animals were developed in the same vial with the same concentration of antibody. Scale bar = 100 μm for all panels.
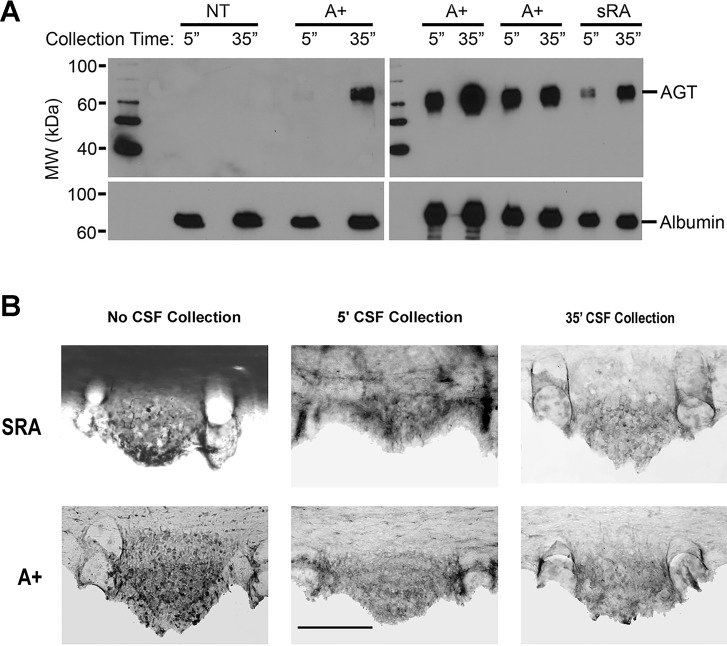
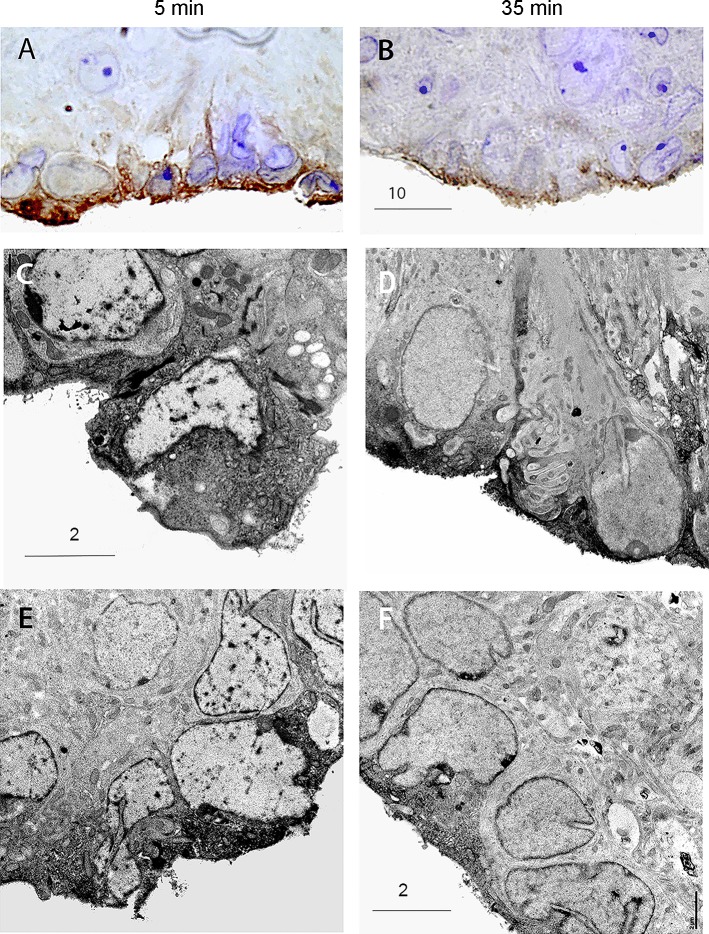
Next, CSF was withdrawn from the cisterna magna of NT, SRA, and A+ mice for 5 min, and then again from the same animals for an additional 35 min. Western blots indicated a robust increase in hAGT protein (approximately 4.3-fold, n = 4) in the CSF after the 35-min collection period compared with the initial 5-min collection period in the A+ and SRA mice (Fig. 4A). As expected, there was no detectable expression of hAGT in CSF from control NT mice. With light microscopy, the SFO of both SRA and A+ mice exhibited, even after 5 min of CSF collection, a distinct reduction in hAGT immunoreactivity compared with “nondrained” mice, particularly in the superficial aspects of the SFO (Fig. 4B). A further decrease in hAGT immunoreactivity was evident after 35 min of CSF collection. Electron microscopy indicated that in all NT, SRA, and A+ animals, immunoreactivity in superficially located SFO cells was located primarily in the half of the cell closest to the ventricular surface (Fig. 5). These parts of the cell were packed with reaction product, and the apical membranes of AGT-immunoreactive cells always showed numerous exocytotic vesicles (Fig. 5, D and F).
Fig. 4.
Human AGT in cerebrospinal fluid (CSF) 5 and 35 min after collection. A: Western blot of hAGT in CSF collected from A+, NT, and SRA animals 5 and 35 min after cannulation of the cisterna magna. The increased release of hAGT into the CSF of A+ and SRA mice after CSF withdrawal is clearly shown. B: hAGT immunoreactivity in SFO of SRA and A+ mice with and without CSF collection. hAGT immunoreactivity in SFO of SRA and A+ mice after 5 min CSF collection shows a decline from animals with no CSF withdrawal. After 35 min of withdrawal, an almost complete depletion of hAGT immunoreactivity in the superficial part of the SFO of SRA and A+ is evident.
Fig. 5.
Localization of ANG and AGT immunoreactivity near the ventricular surface. Superficial cells of SFO from NT (A and B), A+ (C), and SRA (D–F) mice showing accumulation of ANG peptide (A and B) and hAGT (C–F) immunoreactivity (white *) at the ventricular surface after 35 min of CSF withdrawal. The presence of reaction product in microvilli (arrows in D) is clearly shown. Scale bars in A–C and E = 2 μm; scale bar in D = 0.5 μm; scale bar in F = 1 μm.
Light microscopy showed strong VAMP2 immunostaining in cells of the SFO (Fig. 6A) after 5 min of CSF collection. In contrast, VAMP2 immunostaining was clearly much diminished in SFO cells after 35 min of CSF withdrawal (Fig. 6B). Similar findings were observed with electron microscopy (Fig. 6, C–F). In the SFO from animals after 35 min of collection, VAMP2 immunostaining was confined to the ventricular surface of cells (Fig. 6, D and F). The cell types appeared to be similar to those observed with ANG I/ANG II/hAGT immunostaining. We next measured the percent area of combined ANG I/II/AGT immunoreactivity in the SFO of A+ and SRA mice (Fig. 7). Following CSF withdrawal, all animals exhibited a significant loss of immunocytochemical reaction product.
Fig. 6.
Immunocytochemical detection of synaptobrevin-2 (VAMP2) in SFO. Immunocytochemical detection of VAMP2 immunoreactivity in SFO after 5 min (A, C, and E) and after 35 min (B, D, and F) of CSF withdrawal. Note the shift in VAMP2 immunoreactivity to the surface following 35 min of CSF withdrawal and the similar morphology of immunoreactive cells to those in Figs. 2 and 5. Scale bars are as indicated in the figure (in μm).
Fig. 7.
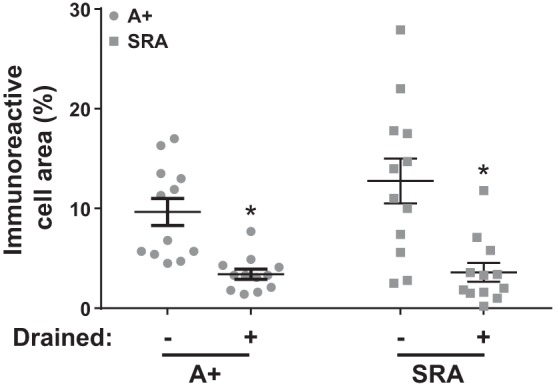
Quantification of ANG I/II/AGT immunoreactivity. Changes in ANG I/II/AGT immunoreactivity as a percentage of cell fragment area before and after CSF withdrawal. *P < 0.05 vs. undrained genotype P = 0.129, CSF withdrawal P < 0.001, interaction P = 0.179. Sample size was n = 12 per group. Two-way ANOVA with Tukey’s multiple comparison test.
DISCUSSION
It was postulated nearly 40 years ago that ANG II from the periphery or synthesized in brain tissue could be released into the ventricles (16, 33). However, it was unclear where the brain-derived peptide was synthesized and how it found its way into the CSF. The present data suggest that the SFO may be one of the sources of AGT/ANG II secretion into the CSF, at least under conditions of human RAS gene overexpression. We have identified a population of superficially located cells in the SFO that extend their processes to the ventricular surface, and that when CSF is withdrawn, appear to release most of their ANG I/II or AGT immunoreactivity into the ventricle.
Secretory cells in the SFO.
The cells producing and releasing ANG I/II or AGT into the CSF, even though they are located just within or deep in the ependyma, appeared to be neuron-like cells, not glia or typical ependymal cells. These cells appear to be similar in shape and location as SFO ANG II immunoreactive neurons in rats (30). However, the presence of numerous intracellular vacuoles suggests that these cells may be the relatively scarce SFO neuronal cell type previously described in the rodent SFO (6). These are thought to resemble hypothalamic neurosecretory cells, although further characterization is necessary.
Our quantitative examination of AGT-immunoreactive SFO cells showed a greater than 50% decrease in immunoreactivity and an apparent change in the distribution of reaction product following CSF withdrawal. The greater concentration of AGT immunoreactivity closer to the apical (ventricular) part of the SFO in mice where the CSF was drained contrasted with the more uniform distribution of reaction product in the nondrained animals. This parallels our observation that the CSF from the same animal after 35 min of CSF collection showed an increase in CSF AGT vs. that found after 5 min of collection. CSF withdrawal thus appears to be a stimulus for AGT and possible ANG I/II release by the SFO. Secretion of AGT has been reported from rat brain neuronal cell culture (40) and brain tissue (38, 40, 41), and is consistent with our findings.
VAMP2 is a protein that is essential to the fusion of synaptic vesicles with the cell membrane and exocytosis. Real-time imaging of VAMP2-labeled vesicles has shown that it shifts from the cytosol to the plasma membrane during exocytosis (1). Thus the change in location of VAMP2 immunoreactivity from largely intracellular vesicles to the cell membrane following CSF withdrawal is consistent with an exocytotic event. Though we were unable to colocalize AGT peptides with VAMP2 immunoreactivity (due to technical problems), the close similarities between the AGT-positive and VAMP2-positive cells suggests that AGT-positive cells undergo exocytosis upon CSF withdrawal.
Functional significance of ventricular angiotensin secretion.
Intracerebroventricular injection of antibodies against ANG II in normally hydrated rats greatly reduces water intake, suggesting that endogenous CSF ANG II may be primarily responsible for dehydration-induced drinking (9). It has been established that lesions of the SFO decrease sodium depletion-induced salt intake, which is dependent on ANG II (27, 31, 42, 45). Local production of ANG II in the SFO of transgenic mice elevates drinking, and chronic intracerebroventricular administration of losartan dramatically decreases water intake, urinary volume, and salt appetite in SRA and NT mice (32). Most of these studies implicate the well-established neuronal pathway from the SFO to the median preoptic area and paraventricular hypothalamic nucleus, a pathway that is angiotensinergic (22). However, ANG II may act within the ventricular system itself, possibly through action on the SFO, other CVOs such as the area postrema or the choroid plexus, all of which possess AT1 receptors (28, 43), though more likely through the antero-ventral third ventricle (AV3V) region (15). Perhaps secretion of AGT or ANG II directly into the ventricle provides a more direct route for the SFO to alter salt and water intake via periventricular structures.
Intraventricular secretion of ANG II could account for why chronic intracerebroventricular infusion of losartan decreases water and salt intake in SRA and NT mice (32). Blockade by losartan of AT1 receptors localized on intraventricular/periventricular structures such as the AV3V region could abolish the natural interaction of secreted ANG II with these receptors. Physiological studies have shown that in mice with a “floxed” human AGT gene, SFO-directed AdCre injection diminishes ANG II immunoreactivity in the SFO and reduces water intake (32, 37). Furthermore, water and salt intake by rodents increases after intracerebroventricular ANG II injection (2, 3). If a decrease in CSF volume is a natural stimulus for promoting ANG II secretion by the SFO, such secretion may lead to increased CSF production by the choroid plexus and concomitant increases in salt and water intake and changes in cardiovascular function. Alternatively, volume decreases in CSF may alter its sodium concentration, activating the SFO sodium sensors (14) and causing intracerebroventricular release of ANG II. Intracerebroventricular infusion of sodium causes a pressor response that is blocked by losartan (44) as well as increased drinking (14). ANG II secreted by the SFO may restore CSF sodium levels by direct action on the choroid plexus, the main site of CSF sodium regulation (21) as well as by enhancing water intake.
Strengths and limitations of the study.
The main conclusion of this study was derived from studies performed using transgenic mice that overexpress components of the human RAS. Expression of human renin was directed by the synapsin promoter to limit transgene expression to neurons (12, 26). Although synapsin 1 has been found in neurosecretory cells (34), it is likely that the number of neurons expressing human renin in these mice is larger than those that endogenously express renin. Indeed, a long-standing question on the precise cellular localization of where renin is expressed in the brain remains unanswered. Importantly, however, the human AGT transgene was controlled by its an endogenous promoter, and many studies by us have provided strong evidence that the cellular localization of hAGT in A+ and SRA mice mirrors expression of endogenous AGT (24, 32, 47). Thus the cellular localization of AGT and consequently ANG I and ANG II derived from its precursor AGT, should emulate sites of endogenous ANG I and ANG II generation. The preservation of endogenous sites of AGT and ANG II production is a strength of the study. On the contrary, there can be no doubt that levels of AGT and ANG II generated in A+ and SRA mice, respectively, is much greater than under normal circumstances. A strength of this approach is that we can now easily detect AGT and ANG II, which would be difficult to detect under normal circumstances. Indeed, the immunohistochemical signal we detect in NT controls is weak and diffuse. Thus we recognize that the overexpression of AGT and ANG II may also represent a limitation because there is no way to ensure that our results are fully reproducible in normal physiology. Indeed, we cannot rule out the contribution of other regions of the brain, particularly other CVOs that also participate in ANG I/II and AGT release in our model. Future studies should employ more sensitive tools to detect endogenous ANG I and ANG II in normal mice or under conditions in which the brain RAS is induced, such as after infusion of deoxycorticosterone acetate (DOCA)-salt.
Perspectives and Significance
The SFO plays a key role in mediating ANG II-induced drinking. Currently, the general view is that the SFO is a sensory organ that can detect changes in osmolarity and ion concentration in the blood and CSF. Electron microscopic immunohistochemistry and Western blot analysis of CSF following withdrawal of CSF from wild-type and transgenic mice that overexpress hAGT and human renin has revealed release of ANG I/II or AGT into the CSF. The SFO may thus monitor physiological changes in the CSF and respond, in part, by secreting angiotensin-related peptides into the CSF itself.
GRANTS
Support for this work was provided by National Heart, Lung, and Blood Institute Grants HL-084207 to C. D. Sigmund and HL-098276 to J. L. Grobe, by American Heart Association Grant 15SFRN23480000 to C. D. Sigmund, and by a University of Iowa Fraternal Order of Eagles Diabetes Research Center grant to J. L. Grobe. We gratefully acknowledge the generous research support of the Roy J. Carver Trust.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.A., J.L.G., C.D.S., and M.D.C. conceived and designed research; K.A., X.L., M.A., A.P.T., and M.D.C. performed experiments; K.A., X.L., M.A., A.P.T., and M.D.C. analyzed data; K.A., M.A., C.D.S., and M.D.C. interpreted results of experiments; K.A., X.L., M.A., A.P.T., C.D.S., and M.D.C. prepared figures; K.A., J.L.G., C.D.S., and M.D.C. drafted manuscript; K.A., J.L.G., X.L., M.A., A.P.T., C.D.S., and M.D.C. edited and revised manuscript; K.A., J.L.G., X.L., M.A., A.P.T., C.D.S., and M.D.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank A. Kim Johnson for very useful comments regarding the manuscript.
REFERENCES
- 1.Allersma MW, Wang L, Axelrod D, Holz RW. Visualization of regulated exocytosis with a granule-membrane probe using total internal reflection microscopy. Mol Biol Cell 15: 4658–4668, 2004. doi: 10.1091/mbc.E04-02-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avrith DB, Fitzsimons JT. Increased sodium appetite in the rat induced by intracranial administration of components of the renin-angiotensin system. J Physiol 301: 349–364, 1980. doi: 10.1113/jphysiol.1980.sp013210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bryant RW, Epstein AN, Fitzsimons JT, Fluharty SJ. Arousal of a specific and persistent sodium appetite in the rat with continuous intracerebroventricular infusion of angiotensin II. J Physiol 301: 365–382, 1980. doi: 10.1113/jphysiol.1980.sp013211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiaraviglio E. Effect of renin-angiotensin system on sodium intake. J Physiol 255: 57–66, 1976. doi: 10.1113/jphysiol.1976.sp011269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coble JP, Cassell MD, Davis DR, Grobe JL, Sigmund CD. Activation of the renin-angiotensin system, specifically in the subfornical organ is sufficient to induce fluid intake. Am J Physiol Regul Integr Comp Physiol 307: R376–R386, 2014. doi: 10.1152/ajpregu.00216.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dellmann HD. Structure of the subfornical organ: a review. Microsc Res Tech 41: 85–97, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 7.Duvernoy HM, Risold PY. The circumventricular organs: an atlas of comparative anatomy and vascularization. Brain Res Brain Res Rev 56: 119–147, 2007. doi: 10.1016/j.brainresrev.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Fitzsimons JT. Angiotensin, thirst, and sodium appetite. Physiol Rev 78: 583–686, 1998. [DOI] [PubMed] [Google Scholar]
- 9.Franci CR, Kozlowski GP, McCann SM. Water intake in rats subjected to hypothalamic immunoneutralization of angiotensin II, atrial natriuretic peptide, vasopressin, or oxytocin. Proc Natl Acad Sci USA 86: 2952–2956, 1989. doi: 10.1073/pnas.86.8.2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fry M, Ferguson AV. The sensory circumventricular organs: brain targets for circulating signals controlling ingestive behavior. Physiol Behav 91: 413–423, 2007. doi: 10.1016/j.physbeh.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez AD, Wang G, Waters EM, Gonzales KL, Speth RC, Van Kempen TA, Marques-Lopes J, Young CN, Butler SD, Davisson RL, Iadecola C, Pickel VM, Pierce JP, Milner TA. Distribution of angiotensin type 1a receptor-containing cells in the brains of bacterial artificial chromosome transgenic mice. Neuroscience 226: 489–509, 2012. doi: 10.1016/j.neuroscience.2012.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grobe JL, Grobe CL, Beltz TG, Westphal SG, Morgan DA, Xu D, de Lange WJ, Li H, Sakai K, Thedens DR, Cassis LA, Rahmouni K, Mark AL, Johnson AK, Sigmund CD. The brain renin-angiotensin system controls divergent efferent mechanisms to regulate fluid and energy balance. Cell Metab 12: 431–442, 2010. doi: 10.1016/j.cmet.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gross PM. Circumventricular organ capillaries. Prog Brain Res 91: 219–233, 1992. doi: 10.1016/S0079-6123(08)62338-9. [DOI] [PubMed] [Google Scholar]
- 14.Hiyama TY, Watanabe E, Okado H, Noda M. The subfornical organ is the primary locus of sodium-level sensing by Nax sodium channels for the control of salt-intake behavior. J Neurosci 24: 9276–9281, 2004. doi: 10.1523/JNEUROSCI.2795-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffman WE, Phillips MI. The effect of subfornical organ lesions and ventricular blockade on drinking induced by angiotensin II. Brain Res 108: 59–73, 1976. doi: 10.1016/0006-8993(76)90164-5. [DOI] [PubMed] [Google Scholar]
- 16.Johnson AK, Epstein AN. The cerebral ventricles as the avenue for the dipsogenic action of intracranial angiotensin. Brain Res 86: 399–418, 1975. doi: 10.1016/0006-8993(75)90891-4. [DOI] [PubMed] [Google Scholar]
- 17.Johnson AK, Gross PM. Sensory circumventricular organs and brain homeostatic pathways. FASEB J 7: 678–686, 1993. [DOI] [PubMed] [Google Scholar]
- 18.Johnson AK, Schwob JE. Cephalic angiotensin receptors mediating drinking to systemic angiotensin II. Pharmacol Biochem Behav 3: 1077–1084, 1975. doi: 10.1016/0091-3057(75)90020-9. [DOI] [PubMed] [Google Scholar]
- 19.Lavoie JL, Cassell MD, Gross KW, Sigmund CD. Adjacent expression of renin and angiotensinogen in the rostral ventrolateral medulla using a dual-reporter transgenic model. Hypertension 43: 1116–1119, 2004. doi: 10.1161/01.HYP.0000125143.73301.94. [DOI] [PubMed] [Google Scholar]
- 20.Lavoie JL, Cassell MD, Gross KW, Sigmund CD. Localization of renin expressing cells in the brain, by use of a REN-eGFP transgenic model. Physiol Genomics 16: 240–246, 2004. doi: 10.1152/physiolgenomics.00131.2003. [DOI] [PubMed] [Google Scholar]
- 21.Leenen FH. The central role of the brain aldosterone-“ouabain” pathway in salt-sensitive hypertension. Biochim Biophys Acta 1802: 1132–1139, 2010. doi: 10.1016/j.bbadis.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Lind RW, Johnson AK. Subfornical organ-median preoptic connections and drinking and pressor responses to angiotensin II. J Neurosci 2: 1043–1051, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lind RW, Van Hoesen GW, Johnson AK. An HRP study of the connections of the subfornical organ of the rat. J Comp Neurol 210: 265–277, 1982. doi: 10.1002/cne.902100306. [DOI] [PubMed] [Google Scholar]
- 24.Littlejohn NK, Siel RB Jr, Ketsawatsomkron P, Pelham CJ, Pearson NA, Hilzendeger AM, Buehrer BA, Weidemann BJ, Li H, Davis DR, Thompson AP, Liu X, Cassell MD, Sigmund CD, Grobe JL. Hypertension in mice with transgenic activation of the brain renin-angiotensin system is vasopressin dependent. Am J Physiol Regul Integr Comp Physiol 304: R818–R828, 2013. doi: 10.1152/ajpregu.00082.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKinley MJ, McAllen RM, Davern P, Giles ME, Penschow J, Sunn N, Uschakov A, Oldfield BJ. The sensory circumventricular organs of the mammalian brain. Adv Anat Embryol Cell Biol 172: III–XII, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Morimoto S, Cassell MD, Sigmund CD. Neuron-specific expression of human angiotensinogen in brain causes increased salt appetite. Physiol Genomics 9: 113–120, 2002. doi: 10.1152/physiolgenomics.00007.2002. [DOI] [PubMed] [Google Scholar]
- 27.Morris MJ, Wilson WL, Starbuck EM, Fitts DA. Forebrain circumventricular organs mediate salt appetite induced by intravenous angiotensin II in rats. Brain Res 949: 42–50, 2002. doi: 10.1016/S0006-8993(02)02963-3. [DOI] [PubMed] [Google Scholar]
- 28.Phillips MI, Shen L, Richards EM, Raizada MK. Immunohistochemical mapping of angiotensin AT1 receptors in the brain. Regul Pept 44: 95–107, 1993. doi: 10.1016/0167-0115(93)90233-X. [DOI] [PubMed] [Google Scholar]
- 29.Phillips MI, Sumners C. Angiotensin II in central nervous system physiology. Regul Pept 78: 1–11, 1998. doi: 10.1016/S0167-0115(98)00122-0. [DOI] [PubMed] [Google Scholar]
- 30.Pickel VM, Chan J, Ganten D. Dual peroxidase and colloidal gold-labeling study of angiotensin converting enzyme and angiotensin-like immunoreactivity in the rat subfornical organ. J Neurosci 6: 2457–2469, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruhf AA, Starbuck EM, Fitts DA. Effects of SFO lesions on salt appetite during multiple sodium depletions. Physiol Behav 74: 629–636, 2001. doi: 10.1016/S0031-9384(01)00625-4. [DOI] [PubMed] [Google Scholar]
- 32.Sakai K, Agassandian K, Morimoto S, Sinnayah P, Cassell MD, Davisson RL, Sigmund CD. Local production of angiotensin II in the subfornical organ causes elevated drinking. J Clin Invest 117: 1088–1095, 2007. doi: 10.1172/JCI31242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schelling P, Ganten U, Sponer G, Unger T, Ganten D. Components of the renin-angiotensin system in the cerebrospinal fluid of rats and dogs with special consideration of the origin and the fate of angiotensin II. Neuroendocrinology 31: 297–308, 1980. doi: 10.1159/000123092. [DOI] [PubMed] [Google Scholar]
- 34.Senda T, Nishii Y, Fujita H. Immunocytochemical localization of synapsin I in the adrenal medulla of rats. Histochemistry 96: 25–30, 1991. doi: 10.1007/BF00266757. [DOI] [PubMed] [Google Scholar]
- 35.Simpson JB, Routtenberg A. Subfornical organ: a dipsogenic site of action of angiotensin II. Science 201: 379–381, 1978. doi: 10.1126/science.663664. [DOI] [PubMed] [Google Scholar]
- 36.Simpson JB, Routtenberg A. Subfornical organ: site of drinking elicitation by angiotensin II. Science 181: 1172–1175, 1973. doi: 10.1126/science.181.4105.1172. [DOI] [PubMed] [Google Scholar]
- 37.Sinnayah P, Lazartigues E, Sakai K, Sharma RV, Sigmund CD, Davisson RL. Genetic ablation of angiotensinogen in the subfornical organ of the brain prevents the central angiotensinergic pressor response. Circ Res 99: 1125–1131, 2006. doi: 10.1161/01.RES.0000250259.66683.f5. [DOI] [PubMed] [Google Scholar]
- 38.Sood PP, Richoux JP, Panigel M, Bouhnik J, Wegmann R. The existence of renin-angiotensinogen system in the rat fetal brain. II. Immunocytochemical localization of angiotensinogen in the telencephalon and the diencephalon. Cell Mol Biol 33: 681–689, 1987. [PubMed] [Google Scholar]
- 39.Sramek SJ, Wallow IH, Tewksbury DA, Brandt CR, Poulsen GL. An ocular renin-angiotensin system. Immunohistochemistry of angiotensinogen. Invest Ophthalmol Vis Sci 33: 1627–1632, 1992. [PubMed] [Google Scholar]
- 40.Thomas WG, Greenland KJ, Shinkel TA, Sernia C. Angiotensinogen is secreted by pure rat neuronal cell cultures. Brain Res 588: 191–200, 1992. doi: 10.1016/0006-8993(92)91575-Y. [DOI] [PubMed] [Google Scholar]
- 41.Thomas WG, Sernia C. Immunocytochemical localization of angiotensinogen in the rat brain. Neuroscience 25: 319–341, 1988. doi: 10.1016/0306-4522(88)90029-2. [DOI] [PubMed] [Google Scholar]
- 42.Thunhorst RL, Beltz TG, Johnson AK. Effects of subfornical organ lesions on acutely induced thirst and salt appetite. Am J Physiol Regul Integr Comp Physiol 277: R56–R65, 1999. [DOI] [PubMed] [Google Scholar]
- 43.Tsutsumi K, Saavedra JM. Quantitative autoradiography reveals different angiotensin II receptor subtypes in selected rat brain nuclei. J Neurochem 56: 348–351, 1991. doi: 10.1111/j.1471-4159.1991.tb02602.x. [DOI] [PubMed] [Google Scholar]
- 44.Van Huysse JW, Hou X. Pressor response to CSF sodium in mice: mediation by a ouabain-like substance and renin-angiotensin system in the brain. Brain Res 1021: 219–231, 2004. doi: 10.1016/j.brainres.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 45.Weisinger RS, Denton DA, Di Nicolantonio R, Hards DK, McKinley MJ, Oldfield B, Osborne PG. Subfornical organ lesion decreases sodium appetite in the sodium-depleted rat. Brain Res 526: 23–30, 1990. doi: 10.1016/0006-8993(90)90245-7. [DOI] [PubMed] [Google Scholar]
- 46.Yang G, Gray TS, Sigmund CD, Cassell MD. The angiotensinogen gene is expressed in both astrocytes and neurons in murine central nervous system. Brain Res 817: 123–131, 1999. doi: 10.1016/S0006-8993(98)01236-0. [DOI] [PubMed] [Google Scholar]
- 47.Yang G, Merrill DC, Thompson MW, Robillard JE, Sigmund CD. Functional expression of the human angiotensinogen gene in transgenic mice. J Biol Chem 269: 32497–32502, 1994. [PubMed] [Google Scholar]