Abstract
Heat stress evokes significant increases in muscle sympathetic nerve activity (MSNA) in healthy individuals. The MSNA response to heat stress in chronic heart failure (CHF) is unknown. We hypothesized that the MSNA response to heat stress is attenuated in CHF. Passive whole body heating was applied with water-perfused suits in 13 patients (61 ± 2 yr) with stable class II-III CHF, 12 age-matched (62 ± 2 yr) healthy subjects, and 14 young (24 ± 1 yr) healthy subjects. Mild heating (i.e., increases in skin temperature ΔTsk ~2–4°C, internal temperature ΔTcore <0.3°C) significantly decreased MSNA in CHF patients; however, it did not significantly alter the MSNA in the age-matched and young healthy subjects. Heat stress (i.e., ΔTsk ~4°C and ΔTcore ~0.6°C) raised MSNA in the age-matched (32.9 ± 3.2 to 45.6 ± 4.2 bursts/min; P < 0.001) and young (14.3 ± 1.7 to 26.3 ± 2.4 bursts/min; P < 0.001) controls, but not in CHF (46.2 ± 5.3 to 50.5 ± 5.3 bursts/min; P = 0.06). The MSNA increase by the heat stress in CHF (Δ4.2 ± 2.0 bursts/min) was significantly less than those seen in the age-matched (Δ12.8 ± 1.7 bursts/min, P < 0.05) and young (Δ12.0 ± 2.7 bursts/min, P < 0.05) control groups. These data suggest that the MSNA response to heat stress is attenuated in CHF patients. We speculate that the attenuated MSNA response to heat stress may contribute to impaired cardiovascular adjustments in CHF in a hot environment.
Keywords: heart failure, hemodynamics, nervous system, sympathetic, stress
clinical reports suggest that thermal tolerance to extreme heat exposure is impaired in patients with cardiovascular diseases (14). For example, heat waves are associated with “excess” death and injury (40), especially in those with prior medical conditions, such as chronic heart failure (CHF) or hypertension (39, 40).
We (9, 11) and other authors (22) have demonstrated that the rise in skin blood flow (SkBF) and cutaneous vascular conductance (CVC) evoked by whole body heating is less in CHF patients than that in healthy subjects. However, our recent report (11) suggests that the skin sympathetic nerve activity response to heat stress is not attenuated in CHF patients. Although the attenuated rise in SkBF may be one of the key factors contributing to the heat-related injury seen in those with cardiovascular disease, other neural and cardiovascular adjustments to heat stress may also be altered in patients with CHF.
In healthy humans, arterial blood pressure (BP) maintenance in the presence of the thermally induced cutaneous vasodilation requires an increase in cardiac output and a reduction in blood flow to noncutaneous beds. This redistribution of blood flow to the skin is accomplished, in part, by the reduction in splanchnic and renal blood flow (36–38). Several reports have shown that passive whole body heating induces a significant elevation in muscle sympathetic nerve activity (MSNA) in young healthy (8, 13) and older healthy (20, 30) subjects. The mechanism(s) for the MSNA elevation during whole body component of the autonomic adjustments to heat stress is not clear (32). The ability to increase MSNA in response to varied stimuli [e.g., unloading baroreceptors (21) and stimulation of muscle metaboreceptors (43)] is attenuated in CHF patients. Additionally, it has been shown that renal sympathoexcitatory responses to heat stress are significantly reduced in heart failure rats, as compared with sham rats (26). However, it is unclear whether whole body heat stress evokes MSNA increases in humans with CHF. We hypothesized that the MSNA response during whole body passive heat stress would be attenuated in CHF patients. The attenuated sympathetic response can be one component of the altered autonomic adjustments to heat stress in this disease. To examine this question, one has to also be cognizant of prior work showing that aging alters autonomic control and thermal regulation (27). For example, a recent report demonstrated a smaller heart rate (HR) increase in older healthy subjects than that in young healthy subjects during heat stress (20). Thus, in our report, both young healthy and older age-matched subjects were compared with the CHF subjects.
METHODS
Subjects.
Thirteen male patients with CHF (age, 61 ± 2 yr; height, 177 ± 2 cm; weight, 96 ± 6 kg), 12 age- and race-matched male healthy control subjects (age, 62 ± 2 yr; height, 177 ± 1 cm; weight, 81 ± 3 kg), and 14 young male healthy control subjects (age, 24 ± 1 yr; height, 174 ± 3 cm; weight, 73 ± 3 kg) participated in this study. The experimental protocol was approved by the Institutional Review Board of the Milton S. Hershey Medical Center and conformed to the Declaration of Helsinki. The purposes and risks of the protocol were explained to each subject before written informed consent was obtained.
All control subjects were healthy, and none was taking any medication. As described in our previous reports (11), patients with CHF were eligible on the basis of the following inclusion criteria: 1) New York Heart Association heart failure class II–III after stabilization; 2) ejection fraction <40% determined by two-dimensional echocardiography; and 3) no underlying aortic outflow obstruction, as assessed by echocardiography. Patients with ischemic and nonischemic pathology were considered eligible for the study. Patients were excluded if they had a recent myocardial infarction, unstable angina, or any angina without dyspnea or exertional fatigue. Patients with other systemic diseases, such as liver disease, renal failure, lung diseases, including asthma and chronic obstructive pulmonary disease, were also excluded. The CHF subjects were class II and III. The mean ejection fraction of the group was 25 ± 3%. Ten CHF subjects had an ischemic etiology for their heart failure, whereas three had a nonischemic cause. Subjects had suffered from CHF for 8 ± 2 yr at the time they were studied. Ten of the patients were diagnosed with hypertension, three had atrial fibrillation, and 12 had hyperlipidemia.
Table 1 lists the patients’ medications by class. Because of safety concerns for the CHF patients, β-blockers were not withheld. All other medications, including angiotensin-converting enzyme (ACE) inhibitors and diuretics were held after the midnight before participating in the whole body heating or the handgrip exercise studies. All patients and the control subjects refrained from caffeine, alcohol, and exercise 24 h before the study.
Table 1.
Class of medications for the patients with chronic heart failure
| Medication Class | n | % |
|---|---|---|
| β-Blocker | 13 | 100 |
| ACEI | 10 | 77 |
| Loop diuretic | 12 | 92 |
| Aldosterone antagonist | 8 | 62 |
| Statin | 10 | 77 |
| Digitalis glycosides | 2 | 15 |
| Anti-platelet | 11 | 85 |
ACEI, angiotensin-converting enzyme inhibitor; n, subject number; %, percentage in all patients.
Measurements.
As described in our previous reports (11), each volunteer was dressed in a tube-lined suit that permitted the control of mean skin temperature (Tsk) by changing the temperature of the water perfusing the suit. Tsk was measured via the weighted average of six thermocouples attached to the skin on the chest (22%), abdomen (14%), upper back (19%), lower back (19%), thigh (14%), and calf (11%) (20). Internal temperature (Tcore) was measured with an ingestible pill telemetry system (HTI Technologies, Palmetto, FL) (n = 31) or from a thermocouple placed in the sublingual sulcus (n = 8, 6 patients and 2 controls). To indicate the increase in both skin and internal temperatures, mean body temperature (Tbody) was calculated as follows: 0.9 × Tcore + 0.1 × Tsk (11, 49). SkBF was indexed from dorsal forearm skin using the mean values of two integrating flow probes of laser-Doppler flowmetry (MoorLab, Moor Instruments, Devon, UK). CVC was calculated from the ratio of the SkBF to mean arterial blood pressure (MAP). The final CVC was expressed as a percentage of the normothermic baseline. Forearm sweat rate (mg·cm−2·min−1) was indexed from forearm skin via capacitance hygrometry (Vaisala, Woburn, MA) using the ventilated capsule method (surface area = 2.0 cm2). The areas from which SkBF and sweat rate were measured were not covered by the suit, and the local temperature of these areas were not controlled.
Systolic blood pressure (SBP), diastolic blood pressure (DBP), and MAP were measured with an automated sphygmomanometer from the brachial artery (SureSigns VS3, Philips, Philip Medical System, Highland Heights, OH). HR was monitored from the electrocardiogram (Cardicap/5, Datex-Ohmeda, GE Healthcare, Piscataway, NJ). As described in our previous report (10), multifiber recordings of MSNA were obtained with a tungsten microelectrode inserted in the peroneal nerve of the nontreated leg. A reference electrode was placed subcutaneously 2–3 cm from the recording electrode. The recording electrode was adjusted until a site was found in which muscle sympathetic bursts were clearly identified (using previously established criteria) (47). The nerve signal was amplified, band-pass filtered with a bandwidth of 500–5,000 Hz, and integrated with a time constant of 0.1 s (Iowa Bioengineering, Iowa City, IA). The nerve signal was also routed to a loudspeaker and a computer for monitoring. MSNA was successfully recorded throughout the heating procedure in 11 of the 13 CHF patients and in all of the control subjects.
Protocols.
The study was conducted in a temperature-controlled laboratory (~23°C) in the morning. After the instrumentation, the suit was perfused with 34°C water, as applied in previous studies in young (8, 13), older subjects (9, 20), and CHF patients (9, 11). After ~5 min of rest, 6 min of data were collected as a normothermic baseline. Thereafter, whole body heating was applied by perfusing warm (~46°C) water through the water-perfused suit. Whole body heating continued until Tcore increased a minimum of 0.5°C. Once Tcore was elevated ~0.5°C, the temperature of the water perfusing the suit was slightly reduced to attenuate the rate of increase in Tcore. Six minutes of data were then obtained as whole body heat stress data. During the whole body heating procedure, the subjects were questioned regarding their sensations as “cool, neutral, warm, or hot,” “comfortable or uncomfortable” and “tolerable or intolerable.”
In a separate visit, five CHF patients performed a fatiguing static handgrip exercise at 30% of the maximal voluntary contraction in the laboratory at ~23°C. The subjects were not heated during this visit. This protocol was used to examine whether the attenuated increase in MSNA seen with heat stress was due to a “ceiling effect.”
Data analysis.
Data were sampled at 200 Hz via a data acquisition system (MacLab, AD Instruments, Castle Hill, Australia). As described in our previous report (10), MSNA bursts were first identified in real time by visual inspection of the data, coupled with the burst sound from the audio amplifier. These bursts were further evaluated by a computer program that identified bursts based upon fixed criteria, including an appropriate latency following the R-wave of the electrocardiogram and having a signal-to-noise ratio of at least 2:1 (10). Integrated MSNA was normalized by assigning a value of 100 to the mean amplitude of the top 10% largest bursts during the 6-min normothermic baseline (10). Total MSNA activity for each cardiac cycle was calculated from burst area of the integrated neurogram. If no burst were detected for a cardiac cycle, a zero was assigned to that cycle. For each data segment, the beat-by-beat HR, R-R interval (RRI), SBP, DBP, MAP, and MSNA were calculated simultaneously using a computer program (10). Mean values of MSNA data were reported as burst rate (i.e., bursts/min), bursts incidence (i.e., bursts/100 heart beats), and total activity (i.e., total burst area/min).
The mean values of MSNA, thermal and hemodynamic variables during the 6-min normothermic baseline, and the 6-min heat stress data were calculated. In addition, the mean values of the variables during mild heating, in which Tsk was greater than 36°C, while the increase in Tcore was less than 0.3°C, also were calculated (see Fig. 1). These data were analyzed because MSNA in CHF patients was lower than the baseline over this period.
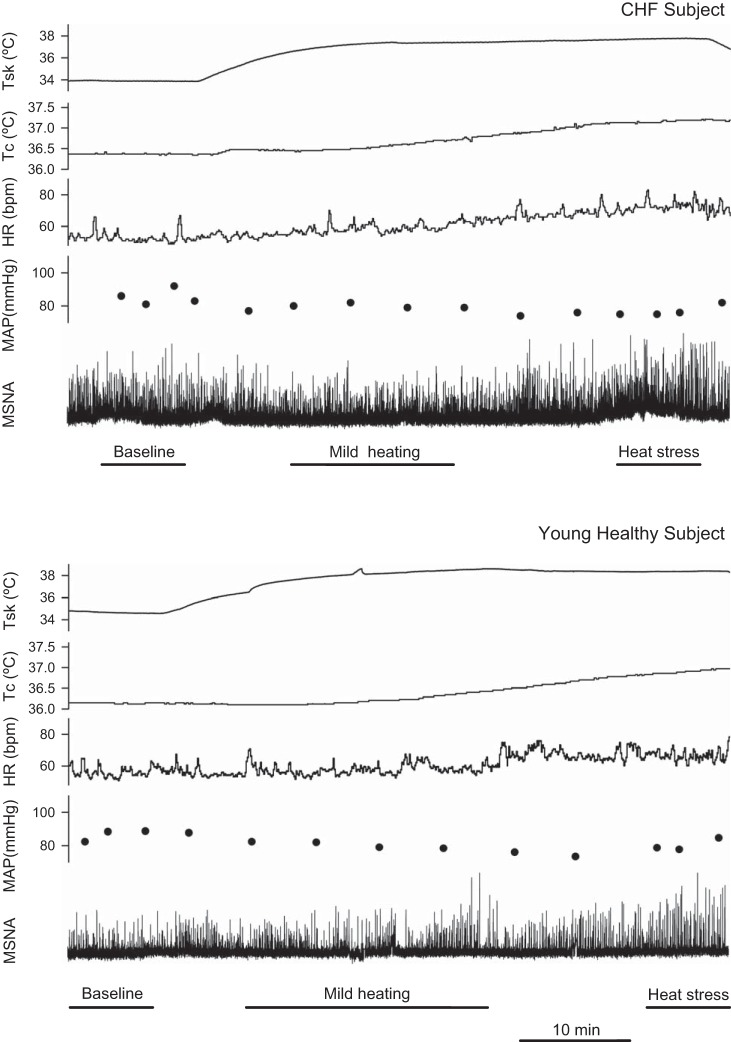
Fig. 1.
Representative tracings of mean skin temperature (Tsk), internal temperature (Tcore), heart rate (HR), mean arterial blood pressure (MAP) and muscle sympathetic nerve activity (MSNA) during whole body heating in a CHF patient (top) and a young healthy control subject (bottom). Mild heating: Tsk > ~36°C, while ΔTcore <0.3°C. Heat stress: ΔTcore >0.5°C when Tsk was ~38°C.
In this report, we evaluated baroreflex control of MSNA (burst incidence, burst area, and total activity) by analyzing the relationship between spontaneous beat-to-beat variations in DBP and MSNA during each of the thermal conditions. We employed previously described methods (25, 28). In brief, DBP for each cardiac cycle were grouped into 3-mmHg interval bins for each thermal condition. “Burst incidence” (i.e., number of bursts per 100 cardiac cycles) for a given 3-mmHg DBP bin was determined and was expressed as the percentage of cardiac cycles in which a burst occurred for a given DBP bin. For a given 3-mmHg DBP interval, the total burst area was calculated. Then, this total burst area was divided by the number of MSNA bursts occurring within the described DBP group. This value presented the mean “burst area” for a given DBP bin. Similarly, the total burst area within a DBP bin was divided by the number of cardiac cycles that occurred within that interval. This represented the “total MSNA” for a given DBP bin. Using all such binned data obtained for each condition, we identified the slopes for the relationship between the “burst incidence” and mean DBP, between the “burst area” and mean DBP, and between “total MSNA” and mean DBP, respectively, using linear regression analyses. For all linear regression analyses, the data were weighted for the number of cardiac cycles within each DBP bin. These slopes were used as indices for the sensitivity of baroreflex control of MSNA.
Cardiac baroreflex control was estimated with the sequence technique (24, 42) for each of the thermal conditions. The beat-to-beat time series of SBP and RRI were analyzed using Hemolab software (Harald Stauss Scientific, Iowa City, IA). Briefly, sequences of four or more consecutive beats where SBP and RRI changed in the same direction were identified as arterial baroreflex sequences (24, 42) A linear regression was applied to each individual sequence, and only those sequences in which R2 > 0.80 were accepted. The slopes of these sequences were calculated as a measure of spontaneous cardiac baroreflex sensitivity.
Statistical analyses.
Statistical analyses were performed with the use of SigmaPlot software (version 13, Systat Software, San Jose, CA). MSNA and thermoregulatory and hemodynamic variables were used to examine two main effects: 1) the effect of the heating (i.e., baseline, mild heating, and heat stress), 2) the effects of group (CHF, age-matched control and young control), and the interaction between the two factors via two-way ANOVA. When appropriate, a Tukey post hoc test was used to adjust for multiple comparisons. The differences in the changes (delta) from the baseline to the mild heating or the heat stress between the groups were evaluated via one-way ANOVA. When appropriate, a Tukey post hoc test was used to adjust for multiple comparisons. When the normality test failed, one-way ANOVA was run on ranks, and Dunn’s post hoc test was used for multiple comparisons. MSNA during the last minute of handgrip was compared with baseline via paired t-test. The level of significance was set at P < 0.05. Values are reported as means ± SE.
RESULTS
Differences in baseline between the groups.
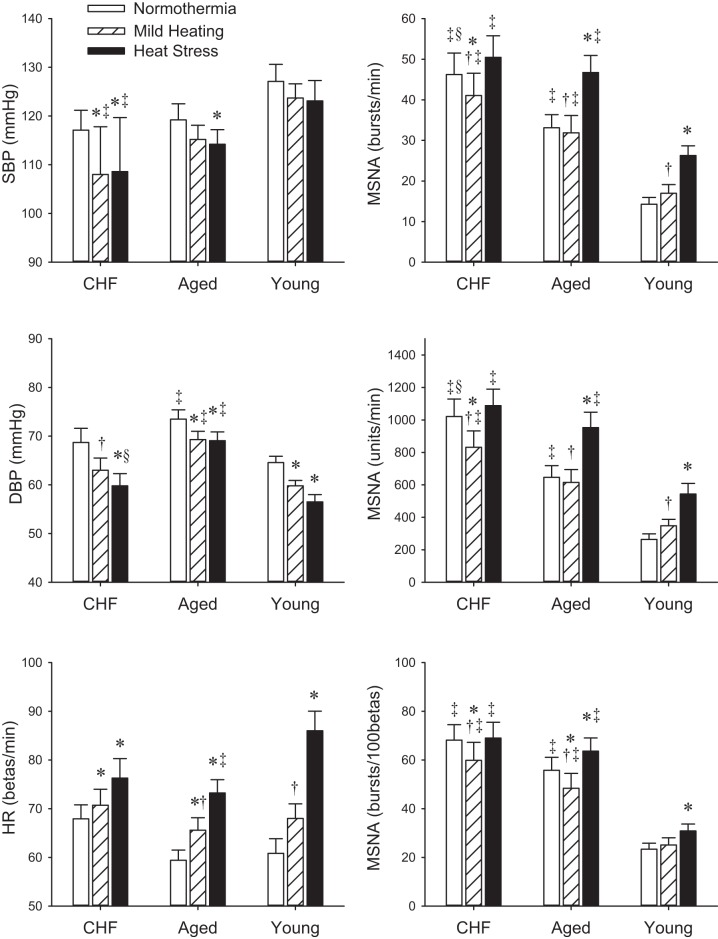
Figure 1 shows examples of Tsk, Tcore, HR, MAP, and MSNA traces during whole body heating in a CHF patient and a young healthy control subject. Absolute hemodynamic and thermal variables are reported in Fig. 2 and Table 2. Baseline Tsk was not different between the groups. Baseline Tcore in CHF was not different from that in age-matched controls. There were no significant differences in baseline SBP, MAP, and HR between the groups. DBP in CHF was not different from the age-matched controls. Baseline MSNA burst rate in CHF (46.2 ± 5.3 bursts/min) was significantly greater than those in the age-matched control groups (33.1 ± 3.2 bursts/min, P < 0.05) and young (14.3 ± 1.7 bursts/min, P < 0.001), and the MSNA burst rate in the age-matched controls was greater than that in the young controls (P = 0.001). Baseline MSNA burst incidence was significantly lower in the young controls than in the age-matched controls (P < 0.001) and in the CHF subjects (P < 0.001).
Fig. 2.
Systolic blood pressure (SBP), diastolic blood pressure (DBP), HR, and MSNA during baseline, mild heating, and heat stress. *P < 0.05 vs. baseline. †P < 0.05 vs. heat stress within the subject group. ‡P < 0.05 vs. young control subjects. §P < 0.05 vs. age-matched control subjects under the respective thermal condition.
Table 2.
Absolute hemodynamic and thermal variables during whole body heating
| Normothermia | Mild Heating | Heat Stress | P | |
|---|---|---|---|---|
| CHF patients | ||||
| MAP, mmHg | 84.8 ± 3.1 | 78.0 ± 2.4* | 76.1 ± 2.6*§ | <0.001 |
| SkBFarm, % | 100 | 123 ± 7† | 227 ± 39*‡§ | <0.001 |
| CVCarm, % | 100 | 134 ± 8† | 251 ± 41*‡§ | <0.001 |
| Tsk, °C | 33.9 ± 0.2 | 37.2 ± 0.2*†‡§ | 38.1 ± 0.1* | <0.001 |
| Tcore, °C | 36.7 ± 0.1‡ | 36.9 ± 0.1*† | 37.2 ± 0.1*‡ | <0.001 |
| Tbody, °C | 36.4 ± 0.1‡ | 36.9 ± 0.1*† | 37.3 ± 0.1*‡ | <0.001 |
| SR, mg·cm−2·min−1 | 0.187 ± 0.034‡ | 0.318 ± 0.051*† | 0.552 ± 0.076*‡ | <0.001 |
| Age-matched healthy subjects | ||||
| MAP, mmHg | 88.7 ± 2.2 | 84.6 ± 1.7* | 84.2 ± 1.9* | 0.007 |
| SkBFarm, % | 100 | 193 ± 32† | 545 ± 45*‡ | <0.001 |
| CVCarm, % | 100 | 204 ± 35† | 580 ± 55*‡ | <0.001 |
| Tsk, °C | 34.0 ± 0.1 | 37.6 ± 0.1* | 38.1 ± 0.1* | <0.001 |
| Tcore, °C | 36.7 ± 0.1 | 36.9 ± 0.1† | 37.3 ± 0.1* | <0.001 |
| Tbody, °C | 36.5 ± 0.1 | 36.9 ± 0.1*† | 37.4 ± 0.1* | <0.001 |
| SR, mg·cm−2·min−1 | 0.118 ± 0.009 | 0.175 ± 0.015† | 0.571 ± 0.089*‡ | <0.001 |
| Young healthy subjects | ||||
| MAP, mmHg | 85.3 ± 1.7 | 81.1 ± 1.3* | 78.5 ± 2.1* | <0.001 |
| SkBFarm, % | 100 | 227 ± 21*† | 681 ± 102* | <0.001 |
| CVCarm, % | 100 | 239 ± 22*† | 753 ± 122* | <0.001 |
| Tsk, °C | 34.2 ± 0.1 | 38.1 ± 0.2* | 38.3 ± 0.1* | <0.001 |
| Tcore, °C | 37.0 ± 0.1 | 37.1 ± 0.1† | 37.5 ± 0.1* | <0.001 |
| Tbody, °C | 36.7 ± 0.1 | 37.1 ± 0.1*† | 37.6 ± 0.1* | <0.001 |
| SR, mg·cm−2·min | 0.101 ± 0.005 | 0.212 ± 0.049† | 0.948 ± 0.102* | <0.001 |
Values are means ± SE. CHF, chronic heart failure; MAP, mean arterial pressure; SkBF, skin blood flow; CVC, cutaneous vascular conductance; Tsk, skin temperature; Tcore, core temperature; SR, sweat rate.
P < 0.05 vs. normothermia.
P < 0.05 vs. heat stress within the subject group.
P < 0.05 vs. young control subjects.
P < 0.05 vs. age-matched control subjects under the respective thermal condition.
Baroreflex sensitivity indices are reported in Table 3. The slope of the relationship between the “burst incidence” and mean DBP in CHF patients (n = 12) was significantly lower than that in young (n = 14) and age-matched control (n = 10) groups. Representative linear regression analysis data in a CHF patient, an aged-matched control subject, and a young control subject during normothermic condition are presented in Fig. 3. The baseline cardiac baroreflex sensitivity in CHF was significantly lower than those in young and aged-matched control subjects.
Table 3.
Baroreflex sensitivity during whole body heating
| Normothermia | Mild Heating | Heat Stress | P | |
|---|---|---|---|---|
| CHF patients | ||||
| BRS BI | −1.26 ± 0.31‡§ | −1.27 ± 0.28‡ | −1.38 ± 0.39‡ | 0.758 |
| BRS Area | −0.150 ± 0.070 | −0.421 ± 0.099 | −0.308 ± 0.090 | 0.139 |
| BRS total | −0.352 ± 0.068 | −0.418 ± 0.060 | −0.504 ± 0.099 | 0.377 |
| BRS RRI | 7.2 ± 1.8‡§ | 8.7 ± 2.8 | 4.3 ± 1.2‡ | 0.076 |
| Age-matched healthy subjects | ||||
| BRS BI | −2.74 ± 0.57 | −2.05 ± 0.39 | −2.27 ± 0.27 | 0.045 |
| BRS Area | −0.327 ± 0.075 | −0.241 ± 0.041 | −0.182 ± 0.105 | 0.257 |
| BRS total | −0.678 ± 0.106 | −0.461 ± 0.064 | −0.502 ± 0.046 | 0.081 |
| BRS RRI | 13.5 ± 1.6 | 8.9 ± 1.5* | 7.7 ± 1.3* | 0.001 |
| Young healthy subjects | ||||
| BRS BI | −2.43 ± 0.32 | −2.85 ± 0.54 | −3.07 ± 0.24* | 0.045 |
| BRS Area | −0.657 ± 0.173 | −0.660 ± 0.134 | −0.457 ± 0.273 | 0.257 |
| BRS total | −0.455 ± 0.063 | −0.671 ± 0.156 | −0.560 ± 0.136 | 0.395 |
| BRS RRI | 23.6 ± 2.8 | 15.5 ± 2.0* | 13.1 ± 1.9* | 0.001 |
Values are means ± SE. Units for the baroreflex sensitivity (BRS) indices: muscle sympathetic nerve activity (MSNA) burst incidence (BRS BI), bursts·100 beats−1·mmHg−1; MSNA burst area (BRS area), U·burst−1·mmHg−1; MSNA total activity (BRS total), U·beats−1·mmHg−1; cardiac interval (BRS RRI), ms/mmHg.
P < 0.05 vs. normothermia.
P < 0.05 vs. young control subjects under the respective thermal condition.
P < 0.05 vs. age-matched control subjects under the respective thermal condition.
Fig. 3.
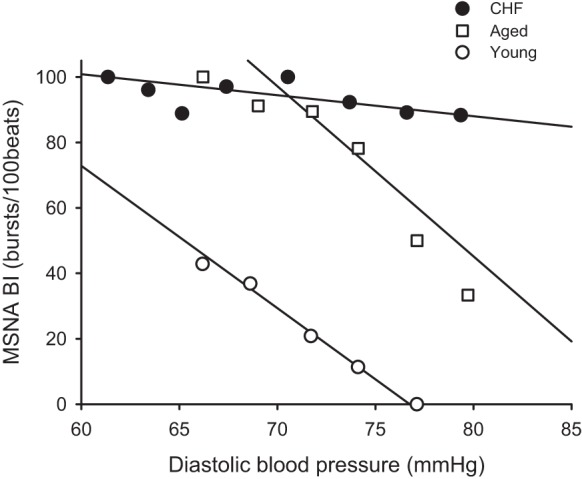
Slopes of the relationship between MSNA burst incidence (BI) and DBP during normothermia in a chronic heart failure (CHF) patient, an aged-matched control subject, and a young control subject.
Effects of mild heating.
We observed that in CHF patients MSNA during mild heating actually decreased from the baseline (Fig. 1). This mild heating period occurred when Tsk was more than ~36°C and when ΔTcore was <0.3°C. During this period, the Tcore was not altered at first, and then it gradually increased in some subjects. In other subjects, Tcore decreased slightly at first and then increased. During this period, all subjects reported their sensation as “warm,” “comfortable” (or “not uncomfortable”), and “tolerable.”
During this mild heating period, the absolute MSNA values (bursts rate, incidence, and total activity) in CHF were significantly lower than normothermic baseline, while the MAP was also lower than the baseline. In contrast, MSNA values in young controls were not significantly greater than the baseline (Fig. 2). In age-matched controls, the responses for MSNA burst rate and total activity varied among the subjects, while the MSNA burst incidence was lower than baseline. Mild heating did not alter the sensitivity of baroreflex control of MSNA. The cardiac baroreflex sensitivity in young and older healthy subjects during mild heating was lower than that in normothermic baseline. The MSNA changes from baseline in CHF were significantly different from those in young controls (Table 4). HR increased during mild heating in all groups compared with the normothermic baseline. There was no difference in the changes in SBP, DBP, MAP, and HR (Table 4) between the groups. The SkBF and CVC increased significantly from the baseline in control groups, but not in CHF patients (Table 2). The elevation (the delta from the baseline) in SkBF and CVC in CHF was significantly lower than those in young controls (Table 4).
Table 4.
Changes from the baseline during mild heating
| CHF | Aged | Young | P | |
|---|---|---|---|---|
| ΔSBP mmHg | −9.1 ± 2.3 | −4.1 ± 1.9 | −3.4 ± 2.0 | 0.121 |
| ΔDBP mmHg | −5.7 ± 1.3 | −4.2 ± 1.7 | −4.9 ± 1.0 | 0.725 |
| ΔMAP, mmHg | −6.8 ± 1.5 | −4.1 ± 1.5 | −4.3 ± 1.0 | 0.278 |
| ΔHR, beats/min | 2.8 ± 2.3 | 6.2 ± 1.0 | 7.2 ± 1.3 | 0.153 |
| ΔMSNA, BR | −5.2 ± 1.6* | −2.1 ± 1.7 | 2.7 ± 1.8 | 0.008 |
| ΔMSNA, BI | −8.3 ± 2.4* | −8.7 ± 2.3* | 1.7 ± 2.7 | 0.003 |
| ΔMSNA, Total | −190 ± 44* | −52 ± 33 | 84 ± 45 | <0.001 |
| ΔSkBFarm, % | 23 ± 7*† | 93 ± 32 | 127 ± 21 | <0.001 |
| ΔCVCarm, % | 34 ± 8*† | 104 ± 35 | 139 ± 22 | 0.011 |
| ΔTsk, °C | 3.3 ± 0.3 | 3.7 ± 0.2 | 3.9 ± 0.2 | 0.176 |
| ΔTcore, °C | 0.20 ± 0.03* | 0.10 ± 0.05 | 0.04 ± 0.05 | 0.031 |
| ΔTbody, °C | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.4 ± 0.1 | 0.401 |
| ΔSR, mg/cm2/min | 0.132 ± 0.042 | 0.056 ± 0.014 | 0.112 ± 0.051 | 0.426 |
| Period, min | 11.6 ± 1.6*† | 16.1 ± 1.2 | 14.1 ± 1.3 | <0.001 |
Values are means ± SE. Units for muscle sympathetic nerve activity: BR, bursts/min; BI, bursts/100 heartbeats; total, U/min. SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate.
P < 0.05 vs. young control subjects.
P < 0.05 vs. age-matched control subjects.
Effects of heat stress.
As a result of controlling the Tsk and the increase in Tcore, the elevation in Tcore or the Tbody during the heat stress was not different between the groups (Table 2). Thus, the heat stress challenge was similar in the three groups. Heat stress induced significant increases in SkBF and CVC in all groups (Table 2), but the elevation in SkBF and CVC in CHF patients was significantly attenuated when compared with age-matched and young controls (Table 5). Heat stress increased sweating index in all groups, but the elevation in sweat rate in CHF was lower than that in young controls (Table 5). All subjects reported their sensation as “hot” and “uncomfortable” but “tolerable” for the heat stress condition.
Table 5.
Changes from the baseline during heat stress
| CHF | Aged | Young | P | |
|---|---|---|---|---|
| ΔSBP, mmHg | −8.4 ± 2.7 | −5.0 ± 2.5 | −4.0 ± 2.0 | 0.381 |
| ΔDBP, mmHg | −8.9 ± 1.5 | −4.3 ± 1.8 | −8.1 ± 1.5 | 0.127 |
| ΔMAP mmHg | −8.8 ± 1.8 | −4.6 ± 1.8 | −6.7 ± 1.3 | 0.214 |
| ΔHR, beats/min | 8.3 ± 2.7* | 13.8 ± 1.4* | 25.2 ± 4.3 | 0.002 |
| ΔMSNA, BR | 4.2 ± 2.0*† | 12.8 ± 1.7 | 12.0 ± 2.7 | 0.025 |
| ΔMSNA, BI | 0.9 ± 2.4* | 7.1 ± 2.0 | 7.5 ± 3.3 | 0.029 |
| ΔMSNA, total | 67 ± 72*† | 291 ± 35 | 280 ± 74 | 0.031 |
| ΔSkBFarm, % | 127 ± 39*† | 445 ± 45 | 581 ± 102 | <0.001 |
| ΔCVCarm, % | 151 ± 41*† | 480 ± 55 | 653 ± 122 | <0.001 |
| ΔTsk, °C | 4.2 ± 0.2 | 4.1 ± 0.2 | 4.1 ± 0.2 | 0.952 |
| ΔTcore, °C | 0.56 ± 0.04 | 0.60 ± 0.05 | 0.53 ± 0.05 | 0.569 |
| ΔTbody, °C | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.614 |
| ΔSR, mg·cm−2·min | 0.365 ± 0.080* | 0.453 ± 0.088 | 0.847 ± 0.104 | 0.005 |
Values are means ± SE. Units for MSNA: BR, bursts/min; BI, bursts/100 beats. Total, U/min.
P < 0.05 vs. young control subjects.
P < 0.05 vs. age-matched control subjects.
Heat stress decreased MAP and raised HR from the baseline in all groups. The MAP drop was not different between the groups (Table 5). However, the increase in HR in CHF patients and age-matched controls was lower than that in young controls. Heat stress evoked significant MSNA increases in young and age-matched controls, but not in CHF patients (Fig. 2). The changes in MSNA by the heat stress in CHF patients (4.2 ± 2.0 bursts/min, 0.9 ± 2.4 bursts/100 beats, 67 ± 72 U/min) were significantly lower than that in age-matched (12.0 ± 1.7 bursts/min, 7.1 ± 2.0 bursts/100 beats, 291 ± 35 U/min, all P < 0.05) and young (12.0 ± 2.7 bursts/min, 7.5 ± 3.3 bursts/100 beats, 280 ± 74 U/min, all P < 0.05) control subjects. Heat stress significantly increased the slope of the relationship between the “burst incidence” and DBP in young healthy control subjects, but it did not significantly alter this slope in CHF patients and age-matched control subjects (Table 3). The slope of the relationship between the “burst area” and DBP or between the “total activity” and DBP was not significantly different than the baseline values in the three groups. The cardiac baroreflex sensitivity was not different from the normothermic baseline in CHF, but it was lower than the baseline in the young and aged-matched control subjects.
MSNA response to handgrip exercise.
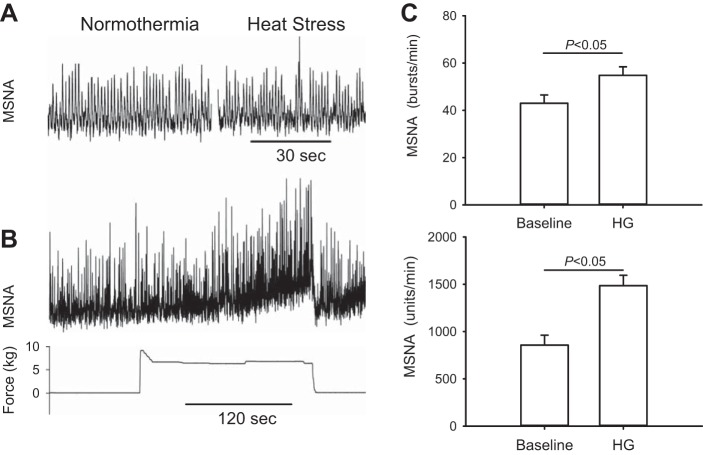
The MSNA recording during handgrip exercise in a representative subject is shown in Fig. 4. Fatiguing handgrip exercise evoked significant MSNA increase the CHF patients (Fig. 4).
Fig. 4.
Representative tracings of MSNA under normothermia and heat stress conditions (A), the MSNA response to a fatiguing handgrip (B) during a separate visit in one CHF patient. The patients were not heated during the visit for the handgrip exercise. Fatiguing handgrip exercise evoked significant MSNA increase in the CHF patients (C; n = 5). HG denotes last minute of the handgrip exercise.
DISCUSSION
The novel findings of the present study are that 1) mild whole body heating decreases MSNA in CHF patients, but does not significantly alter MSNA in the age-matched or young healthy subjects; and 2) the MSNA response to whole body heat stress is smaller in CHF when compared with those in the healthy control subjects. The data support our hypothesis that MSNA response to heat stress is attenuated in CHF patients.
Effects of mild heating.
It is well known that whole body heat stress is a potent activator of the sympathetic nervous system (35). In young healthy humans, heat stress (i.e., ΔTcore >0.5°C) is associated with pronounced increases in HR and MSNA (8, 13, 20). However, it was unknown whether the mild heating evokes MSNA activation. It should be noted that whole body heating at a mild level was unlikely to induce “stress,” because only small increase in Tcore was induced, and subjects described their sensation as “comfortable.” The presented data show that the mild whole body heating with ΔTcore <0.3°C did not significantly raise MSNA in young healthy subjects, although the mean value was greater than the baseline value. In contrast to young healthy subjects, the mild heating condition led to a fall in MSNA in the CHF subjects. A decrease in MSNA was also observed in some (7/12) age-matched control subjects. This is interesting and is not a predicted finding.
The mechanisms responsible for the decrease in MSNA in CHF “during mild heat stress” are not known. We suggest that the mild heating might alter some factor(s) that contributes to the high resting MSNA in CHF (21, 31, 33). Although it is believed that the heightened sympathetic nervous system engagement represents a systemic adaptation to impaired cardiac function, the mechanisms for the high resting MSNA in CHF are complicated and are not fully understood (18). It has been reported that the resting muscle and peripheral venous blood temperatures in CHF patients are lower than healthy individuals (41). Fagius and Kay (15) have shown that whole body cooling increases MSNA. Thus, if the lower peripheral temperatures (e.g., muscle temperature) contribute, in part, to the high resting MSNA in CHF, the MSNA would be decreased to some extent when a mild heating raises the peripheral temperatures. On the other hand, the MSNA decrease during mild heating might pose the question of whether the Tsk at ~34°C is the perfect “normothermic” temperature for CHF patients. Further studies are necessary to determine the normothermic condition for varied populations. We emphasize that the original purpose of this study was not to investigate the effects of mild heating. The possible mechanisms (e.g., muscle temperature) for the MSNA decrease should be examined in future studies.
Regardless of the mechanism(s), we speculate that decreasing MSNA in CHF may have physiological and clinical implications, since sympathetic overactivity in CHF eventually worsens cardiac performance, and it is associated with a reduced survival (3, 7, 34). Although extreme heat exposure [e.g., heat wave (14, 39, 40)] raises mortality, the mortality and the incidence of heart failure are in general lower during warm seasons than during cold seasons (5, 44). Recent data from our laboratory demonstrate that resting MSNA in healthy individuals is lower in summer than in winter (12). Further studies are necessary to systemically examine the effects of the mild heat exposure in CHF patients.
Effects of heat stress.
Because the Tsk and the increase in Tcore were controlled during the last period of the whole body heating protocol, the heat stress challenge was similar in the three groups. Consistent with previous reports (9, 11, 22), the present data show that the cutaneous vasodilator responses to heat stress were significantly attenuated in CHF, as compared with the age-matched and young controls. The sweat rate was also significantly lower in CHF than that in young healthy controls.
As seen in the previous studies (8, 13, 20), heat stress evoked significant MSNA increase in young healthy controls. It is well known that aging increases basal MSNA (16). In the present study, although the baseline MSNA in age-matched control subjects was greater than the young healthy controls, heat stress evoked similar MSNA increases in the age-matched and young control groups. This observation is consistent with a report by Gagnon et al. (20). Their data show that increases in MSNA and plasma catecholamine concentrations were similar in young and aged healthy individuals as passive heating increased Tcore by 0.6°C and 1.2°C. These data, as well as that report (20), suggest that aging itself does not attenuate the MSNA response to heat stress.
In the present study, the drops in MAP were similar in age-matched controls and young controls, while the HR increase in age-matched controls was lower than that in young controls (see Table 5). These observations are consistent with the report by Gagnon et al. (20). These authors speculated that altered cardiac vagal responses might have contributed to the differing HR responses (20). We speculate that the altered cardiac vagal responses evoked by heating may contribute to the decrease in cardiac baroreflex sensitivity in the healthy controls. The cardiac baroreflex sensitivity did not change significantly during heating in CHF. We suggest that this was due to very low baseline sensitivity of this reflex process seen in CHF.
In contrast to the young and age-matched controls, the MSNA response (i.e., the change from baseline) to heat stress in CHF was significantly smaller than that in the age-matched controls and that in the young controls. These observations demonstrate that the MSNA response to whole body heat stress is attenuated in CHF. The results of the current study are consistent with data from studies in rats (26), which demonstrated attenuated renal sympathetic discharge during heating in heart failure rats, as compared with sham rats.
In the present study, the BP drop evoked by heat stress in CHF was not significantly different from that seen in age-matched controls (see Table 5). Thus, the attenuated MSNA response to heat stress in CHF was not due to a smaller BP drop in CHF. Moreover, the HR increase in CHF was not greater than the age-matched controls. Thus, it was unlikely that heat stress induced a greater increase in cardiac output in CHF and, in turn, might have suppressed the MSNA response (48). The baseline MSNA in CHF was greater than that in age-matched controls. Thus, one might argue that attenuated MSNA response to heat stress in CHF could be due to a “ceiling effect.” Handgrip exercise evoked significant increases in MSNA in the CHF patients (see Fig. 4), whereas heat stress did not. Thus, it is unlikely that the attenuated MSNA response to heat stress was due to a ceiling effect. One may postulate that the decrease in MSNA during the mild heating in CHF itself may contribute to the attenuated MSNA response to heat stress. However, although the MSNA decrease during the mild heating was also observed in some age-matched control subjects, the heat stress did statistically increase MSNA above normothermic baseline in the age-matched healthy subjects.
A number of studies have already observed the MSNA increase to heat stress in healthy subjects (8, 13, 20). In healthy individuals, the increases in MSNA during heat stress are thought to reflect general sympathetic activation that may play an important role in the flow redistribution during heat stress (32). However, the mechanisms for this increase are not well understood. A previous study (8) suggests that this MSNA increase is not simply due to the decrease in central blood volume associated with the heat stress, and central mechanisms have been suggested. Thus, it is possible that central (and/or whole body) mechanisms, which play a role in the MSNA increases with heat stress in healthy individuals, are impaired in CHF. Interestingly, the aforementioned study (26) showed that the attenuated renal sympathetic nerve response to heating in CHF rats was partially restored by bilateral lesion of the hypothalamic paraventricular nucleus. These data suggest that central mechanisms may be responsible for altered sympathetic responses to heating seen in heart failure.
Additionally, the cutaneous vasodilation by heat stress was significantly less in CHF than in the aged-matched control subjects. This attenuation in CHF may have been due to impaired vascular function (22). When the relative flow redistribution to skin is lower, we cannot exclude a possibility that a higher vasoconstrictor activity in muscle is not necessary for the system. Moreover, the increase in sweat rate by heating in CHF patients was lower than that in young controls. The role of a low fluid loss cannot be excluded.
It is known that many autonomic control systems [e.g., baroreflex (1, 17, 21)] are impaired in CHF. Our data show that baroreflex control of MSNA burst incidence and the cardiac baroreflex sensitivity are impaired in CHF. Of note, heat stress increased baroreflex control of MSNA burst incidence in the young control group. This is consistent with a prior report (25). However, in the CHF group, the baroreflex control of MSNA burst incidence was not enhanced by heat stress. Whatever the cause of impaired baroreflex control in CHF, heat stress does not restore reflex control in this disease. This attenuated sympathetic response to heat stress has not been reported previously.
Of note, heat stress did not significantly alter the baroreflex control of MSNA burst area or total activity in any of the three groups. This is consistent with prior reports in young healthy subjects that showed that heat stress did not significantly alter the baroreflex control of MSNA burst area (25).
Study limitations.
β-Blockers were taken by all CHF patients on the day of the study. β-Blockers may alter baseline MSNA in healthy individuals (6, 45), but they do not in CHF patients (2). It is unknown whether β-blockers would alter the MSNA response to heat stress. However, it is known that β-blockers may have less of an effect on MSNA response to stresses such as a cold pressor test (45) and exercise (4). In healthy subjects, acute β-blockade decreases the HR (and raises the stroke volume) response to exercise performed in a hot environment (19). Thus, in our studies, the attenuated rise in HR evoked by heat stress in CHF could have been related to the therapy the CHF subjects were receiving. In turn, this could have reduced the bursts rate (i.e., bursts/min). However, we observed similar effects of heat stress on bursts incidence (i.e., bursts/100 beats) in CHF. This result suggests that the β-blocker therapy-induced restraint of HR was not the primary determinant of attenuated sympathetic outflow with heat stress in CHF. On the other hand, the effect of chronic β-blockade on stroke volume during heat stress in CHF cannot be excluded. Other medicines, including angiotensin-converting enzyme inhibitors and diuretics, were held after midnight before the study. However, when one considers the half-lives of these agents, it is possible that these agents could still have influenced our results.
We note that the body weight of the CHF patients tended to be greater than that of the age-matched controls (96 ± 6 vs. 81 ± 3 kg, P > 0.05). There is no report regarding how obesity alters the MSNA response to passive whole body heat stress. We cannot exclude the possible influence of this factor on our results. On the other hand, we would like to note that the body weight of the age-matched controls also tended to be greater than that of the young controls (81 ± 3 vs. 73 ± 3 kg, P > 0.05), while there was no difference in the MSNA response to passive whole body heat stress between the two control groups.
The maximal increase in Tcore was ~0.6°C in this study due to safety concerns for the CHF patients. Thus, we cannot exclude the possibility that a greater heat challenge (e.g., ΔTcore >1°C) may evoke significant MSNA increases from the normothermic baseline in CHF. Moreover, the subject numbers in the present study were limited, which could also affect the statistical significance of the MSNA increases from the normothermic baseline in CHF. Nevertheless, the presented data suggest that the MSNA response to whole body heating (i.e., changes) in CHF is significantly less than that seen in healthy individuals.
In conclusion, our data demonstrate that in CHF, MSNA response to whole body heating is different from those seen in age-matched and young control subjects. In CHF, MSNA during mild heat exposure is decreased, and during heat stress, the MSNA response is attenuated. The attenuated MSNA response to heat stress can be one component of the altered autonomic adjustments to stress in CHF.
Perspectives and Significance
It is well known that heart disease is associated with chronic high levels of resting sympathetic activity (21, 23, 31). In CHF, increased sympathetic nerve activity and the associated rise in vascular resistance eventually lead to worsening cardiac performance (50) and is associated with a reduced survival (7, 34). It is also known that the autonomic control mechanisms such as baroreflex function (1, 17, 21) and the muscle metaboreflex (43) are impaired in CHF. The presented data suggest for the first time that the MSNA response to passive whole body heating in CHF is different from the MSNA response noted in healthy controls. The clinical implications of the presented findings are twofold. First, mild heating may induce a decrease in resting MSNA in CHF. If this preliminary finding can be further verified and examined, it may have important clinical implications. Thermal interventions have been previously proposed as therapy for some cardiovascular diseases (29, 46). Further studies examining this intervention are warranted. Second, the MSNA response to heat stress is attenuated. We speculate that this attenuated response may contribute to the altered cardiovascular adjustments (e.g., flow redistribution) seen in CHF in response to extreme heat exposure. Altered autonomic regulation of sympathetic outflow during heat stress may make heart failure patients more vulnerable to extreme changes in weather patterns.
GRANTS
This work was supported by the American Heart Association Grants 15GRNT24480051 and 0565399U (to J. Cui), National Institutes of Health Grants P01 HL-096570 (to L. I. Sinoway) and UL1 TR000127 (to L. I. Sinoway).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.C. and C.A.B. conceived and designed the research; J.C. and C.A.B. performed experiments; J.C. analyzed data; J.C., J.B., and L.I.S. interpreted results of experiments; J.C. prepared figures; J.C. and J.B. drafted manuscript; J.C., J.B., and L.I.S. edited and revised manuscript; J.C., J.B., C.A.B., and L.I.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We express appreciation to the subjects for their willingness to participate in this protocol. We thank Allan Probert for technical assistance. We are grateful to Jennifer L. Stoner for secretarial help in preparing this manuscript.
REFERENCES
- 1.Ando S, Dajani HR, Senn BL, Newton GE, Floras JS. Sympathetic alternans. Evidence for arterial baroreflex control of muscle sympathetic nerve activity in congestive heart failure. Circulation 95: 316–319, 1997. doi: 10.1161/01.CIR.95.2.316. [DOI] [PubMed] [Google Scholar]
- 2.Azevedo ER, Kubo T, Mak S, Al-Hesayen A, Schofield A, Allan R, Kelly S, Newton GE, Floras JS, Parker JD. Nonselective versus selective beta-adrenergic receptor blockade in congestive heart failure: differential effects on sympathetic activity. Circulation 104: 2194–2199, 2001. doi: 10.1161/hc4301.098282. [DOI] [PubMed] [Google Scholar]
- 3.Barretto AC, Santos AC, Munhoz R, Rondon MU, Franco FG, Trombetta IC, Roveda F, de Matos LN, Braga AM, Middlekauff HR, Negrão CE. Increased muscle sympathetic nerve activity predicts mortality in heart failure patients. Int J Cardiol 135: 302–307, 2009. doi: 10.1016/j.ijcard.2008.03.056. [DOI] [PubMed] [Google Scholar]
- 4.Beloka S, Gujic M, Deboeck G, Niset G, Ciarka A, Argacha JF, Adamopoulos D, Van de Borne P, Naeije R. Beta-adrenergic blockade and metabo-chemoreflex contributions to exercise capacity. Med Sci Sports Exerc 40: 1932–1938, 2008. doi: 10.1249/MSS.0b013e31817fbe11. [DOI] [PubMed] [Google Scholar]
- 5.Boulay F, Berthier F, Sisteron O, Gendreike Y, Gibelin P. Seasonal variation in chronic heart failure hospitalizations and mortality in France. Circulation 100: 280–286, 1999. doi: 10.1161/01.CIR.100.3.280. [DOI] [PubMed] [Google Scholar]
- 6.Cogliati C, Colombo S, Ruscone TG, Gruosso D, Porta A, Montano N, Malliani A, Furlan R. Acute beta-blockade increases muscle sympathetic activity and modifies its frequency distribution. Circulation 110: 2786–2791, 2004. doi: 10.1161/01.CIR.0000146335.69413.F9. [DOI] [PubMed] [Google Scholar]
- 7.Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS, Simon AB, Rector T. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med 311: 819–823, 1984. doi: 10.1056/NEJM198409273111303. [DOI] [PubMed] [Google Scholar]
- 8.Crandall CG, Etzel RA, Farr DB. Cardiopulmonary baroreceptor control of muscle sympathetic nerve activity in heat-stressed humans. Am J Physiol Heart Circ Physiol 277: H2348–H2352, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Cui J, Arbab-Zadeh A, Prasad A, Durand S, Levine BD, Crandall CG. Effects of heat stress on thermoregulatory responses in congestive heart failure patients. Circulation 112: 2286–2292, 2005. doi: 10.1161/CIRCULATIONAHA.105.540773. [DOI] [PubMed] [Google Scholar]
- 10.Cui J, Blaha C, Moradkhan R, Gray KS, Sinoway LI. Muscle sympathetic nerve activity responses to dynamic passive muscle stretch in humans. J Physiol 576: 625–634, 2006. doi: 10.1113/jphysiol.2006.116640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cui J, Boehmer JP, Blaha C, Lucking R, Kunselman AR, Sinoway LI. Chronic heart failure does not attenuate the total activity of sympathetic outflow to skin during whole-body heating. Circ Heart Fail 6: 271–278, 2013. doi: 10.1161/CIRCHEARTFAILURE.112.000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cui J, Muller MD, Blaha C, Kunselman AR, Sinoway LI. Seasonal variation in muscle sympathetic nerve activity. Physiol Rep 3: e12492, 2015. doi: 10.14814/phy2.12492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cui J, Wilson TE, Crandall CG. Phenylephrine-induced elevations in arterial blood pressure are attenuated in heat-stressed humans. Am J Physiol Regul Integr Comp Physiol 283: R1221–R1226, 2002. doi: 10.1152/ajpregu.00195.2002. [DOI] [PubMed] [Google Scholar]
- 14.Depasquale NP, Burch GE. The seasonal incidence of myocardial infarction in New Orleans. Am J Med Sci 242: 468–474, 1961. doi: 10.1097/00000441-196110000-00012. [DOI] [PubMed] [Google Scholar]
- 15.Fagius J, Kay R. Low ambient temperature increases baroreflex-governed sympathetic outflow to muscle vessels in humans. Acta Physiol Scand 142: 201–209, 1991. doi: 10.1111/j.1748-1716.1991.tb09148.x. [DOI] [PubMed] [Google Scholar]
- 16.Fagius J, Wallin BG. Long-term variability and reproducibility of resting human muscle nerve sympathetic activity at rest, as reassessed after a decade. Clin Auton Res 3: 201–205, 1993. doi: 10.1007/BF01826234. [DOI] [PubMed] [Google Scholar]
- 17.Ferguson DW, Berg WJ, Roach PJ, Oren RM, Mark AL. Effects of heart failure on baroreflex control of sympathetic neural activity. Am J Cardiol 69: 523–531, 1992. doi: 10.1016/0002-9149(92)90998-E. [DOI] [PubMed] [Google Scholar]
- 18.Floras JS. Sympathetic activation in human heart failure: diverse mechanisms, therapeutic opportunities. Acta Physiol Scand 177: 391–398, 2003. doi: 10.1046/j.1365-201X.2003.01087.x. [DOI] [PubMed] [Google Scholar]
- 19.Freund BJ, Joyner MJ, Jilka SM, Kalis J, Nittolo JM, Taylor JA, Peters H, Feese G, Wilmore JH. Thermoregulation during prolonged exercise in heat: alterations with β-adrenergic blockade. J Appl Physiol (1985) 63: 930–936, 1987. [DOI] [PubMed] [Google Scholar]
- 20.Gagnon D, Schlader ZJ, Crandall CG. Sympathetic activity during passive heat stress in healthy aged humans. J Physiol 593: 2225–2235, 2015. doi: 10.1113/JP270162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grassi G, Seravalle G, Cattaneo BM, Lanfranchi A, Vailati S, Giannattasio C, Del Bo A, Sala C, Bolla GB, Pozzi M, Mancia G. Sympathetic activation and loss of reflex sympathetic control in mild congestive heart failure. Circulation 92: 3206–3211, 1995. doi: 10.1161/01.CIR.92.11.3206. [DOI] [PubMed] [Google Scholar]
- 22.Green DJ, Maiorana AJ, Siong JH, Burke V, Erickson M, Minson CT, Bilsborough W, O’Driscoll G. Impaired skin blood flow response to environmental heating in chronic heart failure. Eur Heart J 27: 338–343, 2006. doi: 10.1093/eurheartj/ehi655. [DOI] [PubMed] [Google Scholar]
- 23.Hasking GJ, Esler MD, Jennings GL, Burton D, Johns JA, Korner PI. Norepinephrine spillover to plasma in patients with congestive heart failure: evidence of increased overall and cardiorenal sympathetic nervous activity. Circulation 73: 615–621, 1986. doi: 10.1161/01.CIR.73.4.615. [DOI] [PubMed] [Google Scholar]
- 24.Iellamo F, Hughson RL, Castrucci F, Legramante JM, Raimondi G, Peruzzi G, Tallarida G. Evaluation of spontaneous baroreflex modulation of sinus node during isometric exercise in healthy humans. Am J Physiol Heart Circ Physiol 267: H994–H1001, 1994. [DOI] [PubMed] [Google Scholar]
- 25.Keller DM, Cui J, Davis SL, Low DA, Crandall CG. Heat stress enhances arterial baroreflex control of muscle sympathetic nerve activity via increased sensitivity of burst gating, not burst area, in humans. J Physiol 573: 445–451, 2006. doi: 10.1113/jphysiol.2006.108662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kenney MJ, Musch TI, Weiss ML. Renal sympathetic nerve regulation to heating is altered in rats with heart failure. Am J Physiol Heart Circ Physiol 280: H2868–H2875, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Kenney WL, Munce TA. Aging and human temperature regulation. J Appl Physiol (1985) 95: 2598–2603, 2003. doi: 10.1152/japplphysiol.00202.2003. [DOI] [PubMed] [Google Scholar]
- 28.Kienbaum P, Karlsson T, Sverrisdottir YB, Elam M, Wallin BG. Two sites for modulation of human sympathetic activity by arterial baroreceptors? J Physiol 531: 861–869, 2001. doi: 10.1111/j.1469-7793.2001.0861h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kihara T, Biro S, Imamura M, Yoshifuku S, Takasaki K, Ikeda Y, Otuji Y, Minagoe S, Toyama Y, Tei C. Repeated sauna treatment improves vascular endothelial and cardiac function in patients with chronic heart failure. J Am Coll Cardiol 39: 754–759, 2002. doi: 10.1016/S0735-1097(01)01824-1. [DOI] [PubMed] [Google Scholar]
- 30.Klein JC, Crandall CG, Matthew Brothers RM, Carter JR. Combined heat and mental stress alters neurovascular control in humans. J Appl Physiol (1985) 109: 1880–1886, 2010. doi: 10.1152/japplphysiol.00779.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leimbach WN Jr, Wallin BG, Victor RG, Aylward PE, Sundlöf G, Mark AL. Direct evidence from intraneural recordings for increased central sympathetic outflow in patients with heart failure. Circulation 73: 913–919, 1986. doi: 10.1161/01.CIR.73.5.913. [DOI] [PubMed] [Google Scholar]
- 32.Low DA, Keller DM, Wingo JE, Brothers RM, Crandall CG. Sympathetic nerve activity and whole body heat stress in humans. J Appl Physiol (1985) 111: 1329–1334, 2011. doi: 10.1152/japplphysiol.00498.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mark AL. Sympathetic dysregulation in heart failure: mechanisms and therapy. Clin Cardiol 18, Suppl I: I3–I8, 1995. doi: 10.1002/clc.4960181303. [DOI] [PubMed] [Google Scholar]
- 34.Packer M. Neurohormonal interactions and adaptations in congestive heart failure. Circulation 77: 721–730, 1988. doi: 10.1161/01.CIR.77.4.721. [DOI] [PubMed] [Google Scholar]
- 35.Rowell LB. Hyperthermia: a hyperadrenergic state. Hypertension 15: 505–507, 1990. doi: 10.1161/01.HYP.15.5.505. [DOI] [PubMed] [Google Scholar]
- 36.Rowell LB. Thermal stress. In: Human Circulation Regulation During Physical Stress, edited by Rowell LB. London: Oxford University Press, 1986, p. 174–212. [Google Scholar]
- 37.Rowell LB, Brengelmann GL, Blackmon JR, Murray JA. Redistribution of blood flow during sustained high skin temperature in resting man. J Appl Physiol 28: 415–420, 1970. [DOI] [PubMed] [Google Scholar]
- 38.Rowell LB, Detry JR, Profant GR, Wyss C. Splanchnic vasoconstriction in hyperthermic man--role of falling blood pressure. J Appl Physiol 31: 864–869, 1971. [DOI] [PubMed] [Google Scholar]
- 39.Semenza JC, McCullough JE, Flanders WD, McGeehin MA, Lumpkin JR. Excess hospital admissions during the July 1995 heat wave in Chicago. Am J Prev Med 16: 269–277, 1999. doi: 10.1016/S0749-3797(99)00025-2. [DOI] [PubMed] [Google Scholar]
- 40.Semenza JC, Rubin CH, Falter KH, Selanikio JD, Flanders WD, Howe HL, Wilhelm JL. Heat-related deaths during the July 1995 heat wave in Chicago. N Engl J Med 335: 84–90, 1996. doi: 10.1056/NEJM199607113350203. [DOI] [PubMed] [Google Scholar]
- 41.Shellock FG, Swan HJC, Rubin SA. Muscle and femoral vein temperatures during short-term maximal exercise in heart failure. J Appl Physiol (1985) 58: 400–408, 1985. [DOI] [PubMed] [Google Scholar]
- 42.Stauss HM, Moffitt JA, Chapleau MW, Abboud FM, Johnson AK. Baroreceptor reflex sensitivity estimated by the sequence technique is reliable in rats. Am J Physiol Heart Circ Physiol 291: H482–H483, 2006. doi: 10.1152/ajpheart.00228.2006. [DOI] [PubMed] [Google Scholar]
- 43.Sterns DA, Ettinger SM, Gray KS, Whisler SK, Mosher TJ, Smith MB, Sinoway LI. Skeletal muscle metaboreceptor exercise responses are attenuated in heart failure. Circulation 84: 2034–2039, 1991. doi: 10.1161/01.CIR.84.5.2034. [DOI] [PubMed] [Google Scholar]
- 44.Stewart S, McIntyre K, Capewell S, McMurray JJ. Heart failure in a cold climate. Seasonal variation in heart failure-related morbidity and mortality. J Am Coll Cardiol 39: 760–766, 2002. doi: 10.1016/S0735-1097(02)01685-6. [DOI] [PubMed] [Google Scholar]
- 45.Tank J, Diedrich A, Schroeder C, Stoffels M, Franke G, Sharma AM, Luft FC, Jordan J. Limited effect of systemic beta-blockade on sympathetic outflow. Hypertension 38: 1377–1381, 2001. doi: 10.1161/hy1201.096120. [DOI] [PubMed] [Google Scholar]
- 46.Tei C, Horikiri Y, Park JC, Jeong JW, Chang KS, Toyama Y, Tanaka N. Acute hemodynamic improvement by thermal vasodilation in congestive heart failure. Circulation 91: 2582–2590, 1995. doi: 10.1161/01.CIR.91.10.2582. [DOI] [PubMed] [Google Scholar]
- 47.Vallbo AB, Hagbarth K-E, Torebjörk HE, Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev 59: 919–957, 1979. [DOI] [PubMed] [Google Scholar]
- 48.Gunnar Wallin BG. Interindividual differences in muscle sympathetic nerve activity: a key to new insight into cardiovascular regulation? Acta Physiol (Oxf) 190: 265–275, 2007. doi: 10.1111/j.1748-1716.2007.01730.x. [DOI] [PubMed] [Google Scholar]
- 49.Wenger CB, Roberts MF, Stolwijk JAJ, Nadel ER. Forearm blood flow during body temperature transients produced by leg exercise. J Appl Physiol 38: 58–63, 1975. [DOI] [PubMed] [Google Scholar]
- 50.Zelis R, Parilak L, Huber D, Hogeman C, Leuenberger U. The sympathetic nervous system in heart failure. In: Perspectives in Heart Failure Proceedings of the Congress Italian Society of Cardiology, edited by Dei Cas L, and Leier CV. Milan, Italy: 1995, p. 25–34. [Google Scholar]