Cellular action potential measurements within tissue using glass micropipette electrodes usually require tissue immobilization, potentially influencing the physiological relevance of the measurement. Here, we addressed this limitation with novel 100-µg detachable glass microelectrodes that can be precisely positioned to provide long-term measurements of action potential duration during unconstrained tissue movement.
Keywords: transmembrane potential, electrophysiology, glass micropipette electrodes, action potential duration
Abstract
Here, we describe new detachable floating glass micropipette electrode devices that provide targeted action potential recordings in active moving organs without requiring constant mechanical constraint or pharmacological inhibition of tissue motion. The technology is based on the concept of a glass micropipette electrode that is held firmly during cell targeting and intracellular insertion, after which a 100-µg glass microelectrode, a “microdevice,” is gently released to remain within the moving organ. The microdevices provide long-term recordings of action potentials, even during millimeter-scale movement of tissue in which the device is embedded. We demonstrate two different glass micropipette electrode holding and detachment designs appropriate for the heart (sharp glass microdevices for cardiac myocytes in rats, guinea pigs, and humans) and the brain (patch glass microdevices for neurons in rats). We explain how microdevices enable measurements of multiple cells within a moving organ that are typically difficult with other technologies. Using sharp microdevices, action potential duration was monitored continuously for 15 min in unconstrained perfused hearts during global ischemia-reperfusion, providing beat-to-beat measurements of changes in action potential duration. Action potentials from neurons in the hippocampus of anesthetized rats were measured with patch microdevices, which provided stable base potentials during long-term recordings. Our results demonstrate that detachable microdevices are an elegant and robust tool to record electrical activity with high temporal resolution and cellular level localization without disturbing the physiological working conditions of the organ.
NEW & NOTEWORTHY Cellular action potential measurements within tissue using glass micropipette electrodes usually require tissue immobilization, potentially influencing the physiological relevance of the measurement. Here, we addressed this limitation with novel 100-µg detachable glass microelectrodes that can be precisely positioned to provide long-term measurements of action potential duration during unconstrained tissue movement.
measurements of cellular transmembrane voltage and current using glass microelectrodes are of fundamental importance for multiple scientific disciplines, including neuroscience and cardiology (33, 40). Micrometer-scale spatial resolution, submillisecond time resolution, picoamp current and millivolt voltage sensitivities, along with the relative ease of preparing microelectrodes using glass micropipettes have made the technique invaluable for studies of single cell electrophysiology. Two similar, but technically distinct, glass microelectrode technologies have emerged for electrophysiology experiments: the sharp glass microelectrode (23) and the patch glass microelectrode (12).
Sharp glass electrodes have tip diameters in the 10s of nanometers range, are filled with solutions having high salt concentration, and have relatively high impedance (10–100 MΩ). Their nanoscale tips provide simple and, for the most part, nondestructive transmembrane probing with minimal dialysis of the cell and without the need for pressure handling of the electrode filling solution. In contrast, patch glass electrodes have tip diameters in the range of 1–2 μm and therefore have low impedance (3–10 MΩ) (4, 7). In the measurement of action potentials (APs) using a whole cell patch, the electrode solution closely matches the ionic composition and osmolarity of the cytosol. Glass patch electrodes are popular because they provide high-quality seals to the cell membrane (gigaseals) and, for whole cell patches, provide a well-controlled membrane break to access the intercellular space. One caveat is that the micrometer scale tip of patch glass electrode makes the cell prone to a higher rate of dialysis, and sealing to and breaking through the cell membrane require an electrode holder that has an additional port for the appropriate pressure handling of the electrode filling solution (12).
Transmembrane measurements using glass microelectrodes are extremely sensitive to motion due to the nanometer-scale distance across which the measurement is performed, necessitating care in sample handling and probe stability during measurement. Motion compensation is the main challenge when performing measurements from single cells within active organs. To overcome this challenge, the organ is often mechanically inactivated through restraint or pharmacological inhibition of motion (14, 42, 45), which can alter normal organ function, thereby limiting the physiological insight provided by the measurement.
The challenge of motion compensation is exemplified by the sheer orders of magnitude difference between the interface of the electrode and cell membrane and the motion of an active organ. For example, the local epicardial deformation of a healthy beating rodent heart can be several millimeters, whereas local deformations within a rodent brain caused by blood flow, respiration, and chewing can be several micrometers. Such deformations are orders of magnitude larger than the nanometer thick cell membrane across which transmembrane recordings occur. Further compounding the challenge of local deformation is that living organs, such as the brain, can be extremely soft, with Young’s modulus values between 0.1 and 1 kPa (41) relative to the glass of micropipettes, which have Young’s modulus values between 10 and 100 GPa. Several technologies have been developed to accommodate the enormous differences (in both space and mechanical properties) between glass electrodes and the cell membrane, with the aim of maintaining the interface between the electrode tip and cell membrane while an organ is active and in motion. Such technologies include floating, flexible, and pneumatic mounting of glass electrodes (2, 32, 36–38, 44), electrode fixation to the tissue and complex fixtures to hold electrodes (19, 20, 22), and optical feedback-based electrode stabilization (9).
In contrast to previous floating glass microelectrode designs (17, 18, 29, 45), we have developed detachable free-standing floating glass microelectrodes that can be implanted within active tissue with precision. These microelectrodes are at least an order of magnitude lighter than previously reported floating glass microelectrodes (100 µg vs. 4.5–75.5 mg; Table 1). Furthermore, these microelectrodes are simple to build and handle, and we have found that they provide long-term AP recordings from cells within active unconstrained organs.
Table 1.
Physical parameters of floating microdevices and other commonly constructed floating glass micropipette electrodes
| Parameter | Our Floating Microdevices | Other Glass Microelectrodes |
|---|---|---|
| Electrode length, mm | 6 ± 0.1 | 9–56* |
| Electrode mass, mg | 0.1 ± 0.015 | 4.5–75.5* |
| Sharp electrode impedance, MΩ | 13 ± 0.3 | 10–20 |
| Patch electrode impedance, MΩ | 6 ± 1 | 1–10 |
These parameters were measured by building the different types of floating glass micropipette electrodes that have been described in the literature.
Our design is based on the concept of a glass micropipette electrode that is held firmly while, under macroscopic observation, a cell within active tissue is targeted and the membrane is impaled. The innovation is that, once a desired cellular connection is achieved, an extremely lightweight (~100 µg) and small (~6-mm length and 100-μm diameter) sharp or patch glass microelectrode is gently released to remain within the tissue. This “microdevice,” akin to a “bee’s stinger,” then remains floating within the moving organ while providing continuous and stable AP timing measurements. The microdevices operate in a manner similar to that of conventional glass micropipette electrodes and are used with conventional micromanipulators, glass micropipette electrode holders, and intracellular electrode preamplifiers while at the same time providing for control over the precise location of impalement within active-moving organs.
MATERIALS AND METHODS
Heart preparation.
Excised perfused hearts from Sprague-Dawley rats (304 ± 23.4 g) and Hartley guinea pigs (968 ± 31.4 g) were studied as well as human left ventricular (LV) wedge preparations from deidentified discarded donor hearts. Animal protocols were approved by the Institutional Animal Care and Use Committee of The George Washington University. All animal experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Human protocols were approved by the Institutional Review Board of George Washington University.
Rats were anesthetized with an intraperitoneal injection of Telazol (50 mg/kg) and then subjected to inhalation of 2–4% isoflurane. Guinea pigs were anesthetized with an intraperitoneal injection of ketamine (50 mg/kg) + xylazine (5 mg/kg) and then subjected to inhalation of 2–4% isoflurane (if needed). After cessation of the toe pinch reflex, the heart was quickly excised and submerged in ice-cold perfusate solution, and the aorta was cannulated for retrograde perfusion. Human LV wedge preparations were prepared as previously described (11). Briefly, a lateral marginal artery was isolated and cannulated from a transmural segment of the posterior-lateral LV free wall. Any major arterial leak was ligated with silk suture. After cannulation, hearts and LV wedges were perfused at constant pressure (60–70 mmHg) using a Langendorff perfusion system with oxygenated (95% O2-5% CO2) modified Krebs-Henseleit solution containing (in mM) 118 NaCl, 4.7 KCl, 1.25 CaCl2, 0.57 MgSO4, 1.17 KH2PO4, 25 NaHCO3, and 6 glucose with 500 mU/l insulin. Hearts and LV wedges were maintained at constant temperature (37°C) throughout each study. Baseline rhythm in animal hearts was maintained by the sinus node, whereas human LV wedges were continuously paced at 1 Hz (60 beats/min) using an electrode touching the endocardial surface.
The ECG.
Needle electrodes recorded the bath-conducted ECG in the excised animal heart experiments. One electrode (negative) was positioned close to the right atrium, a second electrode (positive) was positioned close to the apex, and the third electrode (ground) was positioned close to the left atrium (LA). In human LV wedge experiments, one needle electrode was placed near the endocardial surface and a second electrode was placed near the epicardial surface. In all experiments, the ECG was amplified (2122i Bioamplifier, UFI) and filtered before acquisition (PowerLab 8/35, AD Instruments) and was monitored in real time using LabChart software (AD Instruments). Activation sequence consistency was checked by the superposition of multiple QRST complexes to ensure adequate tissue perfusion and electrophysiological stability throughout the experiments.
Sharp microdevice preparation.
Standard sharp glass micropipettes were heat pulled using a conventional micropipette puller (model P-97, Sutter Instrument) and filamented glass capillary tubes (BF100-58-10, Sutter Instruments). The micropipettes were back filled with 3M KCl solution and mounted horizontally under an inspection microscope using an XYZ positioner. The sharp microdevice was constructed by holding each micropipette using tweezers jacketed with soft tubing (Small Parts Part no. IRI-U2016C-01), scoring the tip with a scribe (Ted Pella Model 13663), breaking off the tip, and fitting the tip with a 25-µm-diameter insulated silver wire (California Fine Wire). Before insertion, a 1-mm length of insulation was heat stripped (Master Appliance model MT-51) from the end of the wire and chlorided in bleach for several minutes to facilitate stable electrical connection with the electrode salt solution. The other end of the wire (typically 3–5 in. long) was heat stripped and attached to a connector pin using silver paint (volume resistivity: 0.0002 Ω·cm, Ted Pella), and the pin was connected to the headstage of an intracellular preamplifier (IX2-700 Dual Intracellular Preamp, Dagan).
The proximal end (back) of the microdevice was sealed with a bead of melted wax (Aquabond 55, Aquabond Technologies), which was applied using a handheld dental mini-waxer (Almore model Mini-Waxer). Using the mini-waxer, noncontact heat was applied to the wax bead so that it formed (due to surface tension) a smooth and hard wax sphere, typically 0.5 mm in diameter. A fire-polished 1-mm-outer diameter glass capillary holding tube (the “holding micropipette”) was then mounted on the XYZ positioner, and the proximal end was connected to the laboratory vacuum line (suction pressure: −2.3 ± 0.1 psi) using small-diameter laboratory tubing. The proximal end of the holding micropipette was then advanced toward the wax seal of the microdevice and pressed against it. The microdevice was then released from the tweezers and held by the vacuum at the proximal end of the holding micropipette. At this point, the device (holding micropipette + microdevice) could be used for measurements by positioning the microdevice within the tissue via the holding micropipette, impaling a cell, and turning off the vacuum to release the wax bead, thereby deploying the microdevice. With some practice and care, these devices were prepared within minutes. They could also be easily constructed at the bench top for immediate use during an experiment.
Cardiac myocyte AP recordings.
Holding micropipettes were mounted on a XYZ micromanipulator that allowed for precise positioning of the impalement site. The silver wire was then connected to the headstage of the intracellular preamplifier. Normally pipette holders are used with headstages, but since this is a detachable microdevice, with only a silver wire to carry the signal, a pin at the headstage was used instead of a pipette holder. A reference electrode (Ag/AgCl pellet) was submersed in the tissue superfusate and connected to the ground terminal of the headstage. The microdevice was then slowly advanced to contact the perfusate on the surface of the tissue, and the initial offset was calibrated at the preamplifier to zero any direct current potential. After impalement and deployment of the microdevice, APs were acquired from the 10× output channel of the intracellular preamplifier using a PowerLab data acquisition unit (PowerLab 8/35, AD Instruments). APs were observed in real time using LabChart software for the duration of each experiment.
Induction of hypoxia.
Changes in AP duration (APD) during gradual hypoxia in rat and guinea pig hearts were measured using sharp glass microdevices. Normoxic APDs were measured before hearts were subjected to hypoxia. The perfusate was then deoxygenated by bubbling with N2 gas, with full deoxygenation usually occurring within 5 min. In other experiments, sharp microdevices were used to measure changes in APD during global no-flow ischemia and reperfusion in rat hearts. This was done by continuously recording APs while stopping the flow of perfusate to the aorta for 4 min and then restoring aortic flow.
Activation of sarcolemmal ATP-sensitive K+ channels.
In human LV wedge experiments, APD was shortened by administering the ATP-sensitive K+ (KATP) channel agonist pinacidil to the perfusate at a circulating concentration of 5 µM. Changes in APD before and after the administration of pinacidil were continuously measured using sharp microdevices to assess temporal reductions in human ventricular APD caused by pinacidil.
Data analysis.
All AP signals were analyzed using custom Matlab algorithms, as we have previously described (15, 43). APD was measured for each AP, within sequences of APs, as the time from the maximum positive derivative [max(dV/dt)] during depolarization to the time corresponding to either 50%, 90%, or 100% repolarization (APD50, APD90, or APD, respectively). AP amplitudes were also measured as the voltage difference (in mV) from the resting potential (APmin) at the time of the maximum second derivative [max(d2V/dt2)], just before depolarization, to the peak of the AP during phase 0 (APmax). APs were normalized using the following equation:
| (1)) |
where AP(t) is the full AP (mV) acquired during a study. Only APs from continuous and stable recordings of at least 1 min were considered during steady-state conditions, such as baseline (control) perfusion and normoxic perfusion.
Membrane patch microdevice preparation.
The initial steps for constructing membrane patch microdevices were similar to those for constructing sharp microdevices: heat pulling a glass capillary tube, back filling with a conducting solution, and mounting the micropipette horizontally under an inspection microscope using an XYZ positioner. The patch microdevice was then constructed by scoring the micropipette tip, breaking the tip, and inserting a 25-µm-diameter chlorided silver wire into the proximal end of the tip. A 1-mm-outer diameter holding micropipette was fire polished, mounted on the XYZ positioner, and brought over the proximal end of the patch microdevice. Melted wax from the tip of a handheld dental wax heater was then applied to the proximal end of the holding micropipette and heated until it sealed the joint between the holding micropipette and the proximal end of the patch microdevice. The wax did not obstruct the inner channel connecting the holding micropipette and the microdevice, maintaining a contiguous inner channel between the patch microdevice and the holding micropipette. This was required to control the pressure of the solution within the microdevice, a necessary requirement for producing a cellular membrane patch. The last step was to thread the holding micropipette through a small heater coil (Omega, Nickel-Chromium Alloy, 80% Nickel-20% Chromium Resistance Heating Wire model NI80-010), position the coil over the wax junction between the microdevice and the holding micropipette, and attach the heater leads to the heater power source. After the patch microdevice was positioned within the brain and a successful cell membrane patch was confirmed, the heater coil was powered to melt the wax, thereby simultaneously releasing the microdevice from the holding micropipette and sealing the proximal of the end of the microdevice with wax. As with the sharp microdevice, the patch microdevices were prepared within minutes and could also be easily constructed at the bench top for immediate use during an experiment. The typical resistance of these devices was 5–7 MΩ.
Neuron AP recordings from the hippocampus of rats.
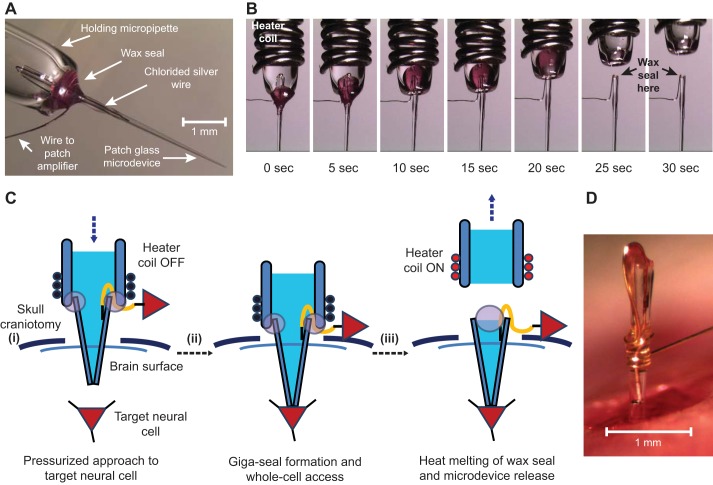
APs were measured from cortical neurons in anesthetized rats (Wistar male rats, postnatal day 25) using methods approved by the Janelia Institutional Animal Care and Use Committee (protocol no. 15-122). Animals were placed in a stereotactic rig (Narishige Model SR-6R), maintained at 1.5–2% isofluorane aneasthesia, and warmed to 37°C. A small craniotomy (~1-mm diameter) was drilled in the skull (position coordinates for the dorsal hippocampus: 3.5 mm posterior of the bregma and 2.5 mm lateral of midline). The dura was removed from a small region (~0.25-mm diameter) in the center of the craniotomy.
Before whole cell patching, the depth of the target pyramidal CA1 cell layer was mapped using an extracellular recording electrode. The signal from this electrode was monitored in current-clamp mode using an audio amplifier (A-M Systems model 3300). The electrode was advanced through the cortex, and the pyramidal CA1 cell layer was identified by characteristic spike bursts, typically 2.1–2.3 mm below the surface of the brain. Membrane patch microdevices were then constructed and filled with a solution containing (in mM) 135 K-gluconate, 10 HEPES, 4 KCl, 10 Na2-phosphocreatine, 4 MgATP, and 0.3 Na3GTP with ∼0.05% (wt/vol) biocytin in distilled water (with pH adjusted to 7.3 using KOH, 295 mosM).
A patch microdevice, attached to a holding micropipette, was then advanced through the cortex with 10–12 psi of pressure (monitored by WPI Pressure Monitor model PM015D) to prevent the microdevice tip from clogging. Microdevice impedance was also monitored by measuring the current while applying square wave pulses (5 mV, 100 Hz). Upon entry into the hippocampus (~100–150 μm above the pyramidal CA1 cell layer), the pressure was reduced to 0.5 psi. The microdevice was then advanced in 1-μm steps toward the pyramidal CA1 cell layer, and contact between the tip and a neuron was identified by reproducible increases in electrode tip resistance. At that point, the outward pressure was released, a bias voltage of −60 mV was applied to the tip-cell interface, and a gigaseal was achieved with or without mild suction. The intracellular access and whole cell patch was achieved by slow ramp suction up to −1 psi. The wax seal between the holding micropipette and the proximal end of the microdevice was then melted by powering the heater coil (1 amp) using a BK Precision model 1550 power supply. This gently released the microdevice to float within the hippocampus while maintaining an intracellular electrical connection with a neuron. Whole cell APs were measured using a computer controlled intracellular amplifier (Molecular Devices Multiclamp 700B) and digitized at a sample rate of 20 kHz (Molecular Devices Digidata 1440A) using pCLAMP 10 software (Molecular Devices).
Statistical analyses.
Statistical analyses were performed using Minitab 16 and Microsoft Excel 2010. Data are presented as means ± SE. Paired t-tests were used to compare differences between groups. Significance was determined as P < 0.05. The sample size for cardiac experiments was n = 6 guinea pigs, providing a power level of 80%, assuming a SD of 21 ms; n = 4 rats, providing a power level of 80%, assuming a SD of 3 ms; and n = 4 human LV wedges, providing a power level of 80%, assuming a SD of 50 ms.
RESULTS AND DISCUSSION
We developed two types of microdevices, constructed from conventional pulled glass micropipettes, each suitable for specific operational requirements of either a sharp glass microelectrode for the heart or a patch glass microelectrode for the brain. Typical microdevice characteristics and the characteristics of other floating glass microelectrodes are shown in Table 1. A unique aspect of the microdevices are that they are at least an order of magnitude lighter than previously reported floating glass microelectrodes (100 µg vs. 4.5–75.5 mg). AP parameters for LV myocytes measured with our microdevices as well as parameters reported in the literature for similar experimental conditions are shown in Table 2. The values indicate that our microdevice approach provided robust cardiac AP parameters that align with those of other approaches for measuring cardiac APs.
Table 2.
Electrophysiological parameters of ventricular action potentials measured from perfused guinea pig hearts
| Parameter | Our Floating Microdevices | Other Glass Microelectrodes | Optical Mapping |
|---|---|---|---|
| Action potential amplitude, mV | 90.3 ± 2.1 | 100 (39) | Not applicable |
| Upstroke velocity dV/dt, V/s | 75.4 ± 0.9 | 80–150 (39) | Not available |
| Rise time, ms | 3.1 ± 0.4 | 2–4 (10) | 8.2 ± 0.7 (5) |
| Suppression of motion | No | Often (13, 14, 45) | Yes |
| Action potential duration, ms | |||
| Sinus rate | 180 ± 8.2 | ~183 (16) | 162 ± 14 (10) |
| Paced | 171 ± 2.9 (at 3.5 Hz) | ~135–208 (at 3–4 Hz) (3, 35) | 174 ± 7 (at 3.3 Hz) (5) |
| ~168–173 (at 3.3–3.5 Hz) (30) |
Values are means ± SD, when available. Values were extracted from data reported within the referenced publications.
Cardiac myocyte AP recordings with sharp microdevices.
Sharp microdevices were tested within the contracting myocardium of three species (rat, guinea pig, and human LV). APs were continuously recorded while the myocardium was subjected to metabolic stress, including hypoxia, ischemia and reperfusion, and the activation of KATP channels using pinacidil. The overall scheme for using a sharp microdevice within contracting myocardium is shown in Fig. 1. The sequence of steps to fabricate the device is provided in the materials and methods above and in Supplemental Video S1 in the Supplemental Material (Supplemental Material for this article is available at the American Journal of Physiology-Heart and Circulatory Physiology website). Sharp microdevices consisted of a 6-mm-long 3 M-KCl-filled sharp glass electrode fitted with a chloridized silver wire (25 µm in diameter) and sealed with a hard, smooth wax ball (~0.7 mm in diameter). Microdevices were suction held by a fire-polished ~0.65-mm-inner diameter glass micropipette that was mounted in a standard electrophysiological micromanipulator with course XYZ axes and a fine Z-axis. The end of the silver wire that was fitted inside the device was connected to the headstage of a standard intracellular preamplifier.
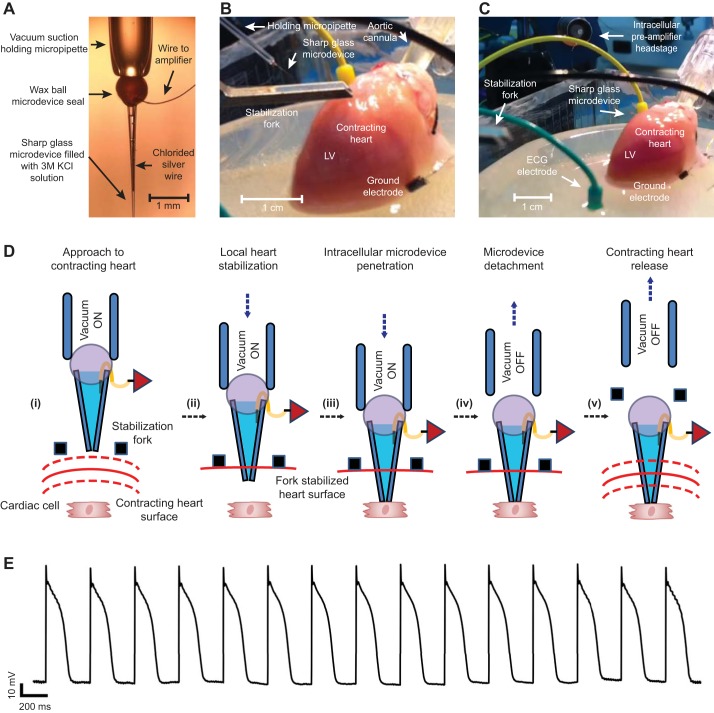
Fig. 1.
Action potential (AP) signals were recorded from excised perfused unconstrained beating hearts using detachable sharp microdevices. A: the fire-polished end of a glass capillary tube (the “holding micropipette”) holds the sharp microdevice via gentle suction. The microdevice is akin to a “bee stinger” and has a thin silver wire that attaches to the headstage of the preamplifier. B: the holding micropipette, sharp microdevice, and stabilization fork are advanced toward the epicardial surface of a beating guinea pig heart. C: The microdevice was deployed to float within the epicardial tissue. The stabilization fork and holding micropipette have been retracted. D: five diagrams (i−v) showing the steps for implanting and releasing a sharp microdevice into myocardial tissue to record APs from a cardiac myocyte. E: a representative signal from a sharp microdevice floating within the left ventricular (LV) epicardium of a beating guinea pig heart. This signal exhibited stable and consistent APs.
When we implanted a sharp microdevice, we found that temporary (1–2 min) and localized compression of the myocardium was necessary for stable AP recordings. This was done using a custom machined fork that was mounted to the coarse Z-axis of the micromanipulator. The holding micropipette, with an attached microdevice, was mounted on the fine Z-axis and moved along with the stabilization fork. The entire assembly was then advanced via the coarse Z-axis toward the heart while observing the approach using a macroscope, providing accurate and reproducible control of the target location of the recording. The advancement of a microdevice and the stabilization fork toward a contracting guinea pig heart is shown in Fig. 1B.
Myocardial stabilization, epicardial penetration, and the release of a sharp microdevice are schematically detailed in Fig. 1D,i−v. After approaching the heart (Fig. 1D,i), the stabilization fork constrained the epicardial surface (Fig. 1D,ii). The fine Z-axis of the micromanipulator advanced the holding micropipette, which passed the microdevice through the open groove of the fork and into epicardial tissue. The electrical potential at the tip of the microdevice was continuously monitored during this process. A stable myocyte AP signal was usually detected within 1–2 min of initiating compression (Figs. 1D,iii, and E). The microdevice was then deployed by slowly releasing the suction of the holding glass micropipette to detach the wax ball. The holding micropipette was retracted (Fig. 1D,iv) followed by retraction of the stabilization fork. The microdevice remained floating within contracting epicardial tissue (Fig. 1D,v), providing a continuous AP signal (Fig. 1E). Microdevices floating within contracting rat and guinea pig hearts are shown in Supplemental Videos S2 and S3.
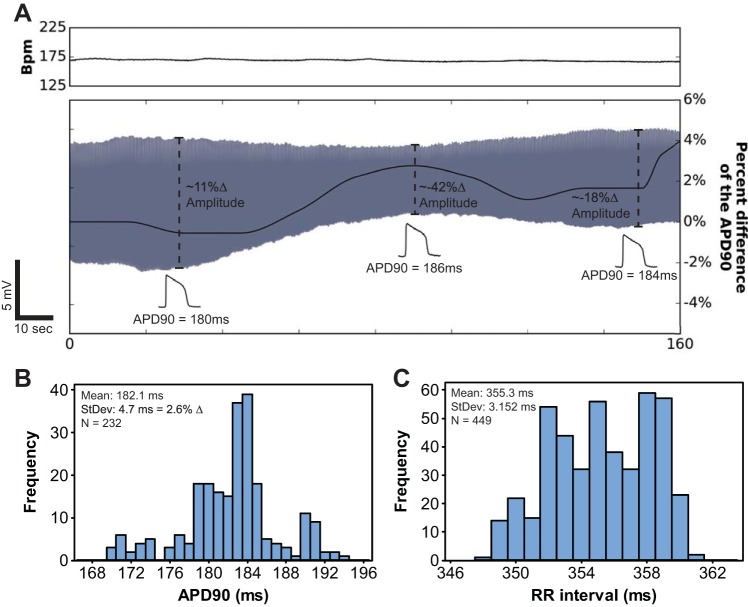
In many experiments, sharp microdevices in the LV provided stable AP amplitudes that averaged 90.3 ± 2.1 mV and had average rise times of 3.1 ± 0.4 ms, as shown in Fig. 1E and Table 2. However, stable AP amplitudes could not be obtained in every signal and exhibited slow amplitude drift. Even if AP amplitudes slowly drifted, as long as there were no physiological perturbations, measurements of APD were impressively consistent and stable, as shown in Fig. 2. For example, APD90 fluctuated no more than 4%, with a SD of 2.6%, in the 160-s signal shown in Fig. 2. Normal variation in sinus rate was likely a large contribution to the SD of APD90 in that signal (Fig. 2C). APD measured from microdevice signals was analogous to previously published values (Table 2) (16). Overall, these results indicate that, even if AP amplitudes drift, APD measured from microdevice signals can be stable and reliable.
Fig. 2.
AP duration (APD) was stable even if the AP amplitude drifted. A: instantaneous heart rate [in beats/min (bpm)] during normal sinus rhythm was measured from the ECG and is plotted above the simultaneously acquired AP signal (gray) generated by a sharp microdevice embedded in a guinea pig LV. APD measured for each beat is plotted in black on top of the AP signal. This image illustrates an extreme example of AP amplitude drift. These data address a potential limitation of microdevice recordings, which is that consistent AP amplitudes within the setting of significant contractile motion were sometimes difficult to achieve. However, for this 160-s signal, APD at 90% repolarization (APD90) fluctuated no more than 4%, with a SD of 2.6%. B: even though amplitudes drifted by as much as 42% (A), the level of APD fluctuation was small compared with APD changes observed during metabolic perturbations, such as those shown in Fig. 3. C: histogram of RR intervals for the heart rate signal shown in A. This indicates that APD fluctuations could be attributed to subtle changes in heart rate.
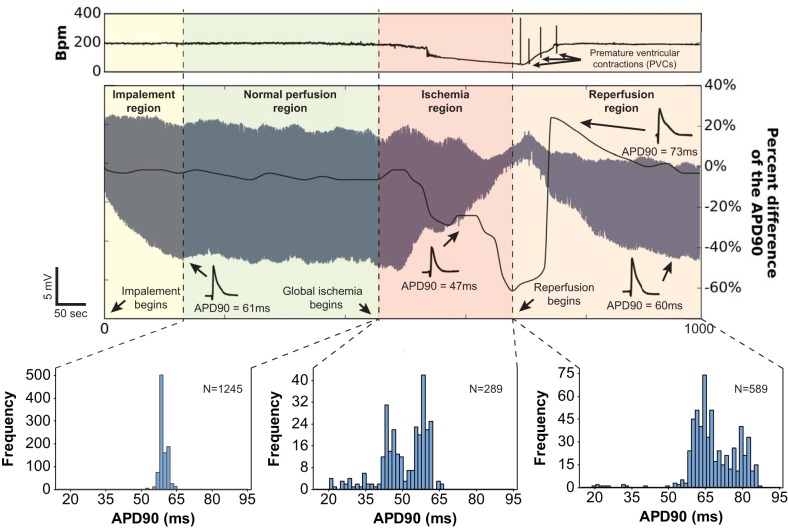
In unconstrained contracting rat hearts, we found that a microdevice implanted in the LV robustly measured the expected changes in APD that occurs during global ischemia and reperfusion, even during the substantial myocardial shrinkage and expansion that occurs during global ischemia and reperfusion. In those experiments, perfusate flow to the aorta was temporarily interrupted (ischemia) and restored (reperfusion) while APs were continuously recorded over intervals lasting as long as 15 min (Fig. 3). APD measured from microdevice signals shortened dramatically during ischemia, reflecting the increased efflux of cytosolic K+ resulting from sarcolemmal KATP activation (27). Indeed, KATP channel activation, and the resulting repolarizing K+ current, is known to shorten APD, which reduces Ca2+ influx via L-type Ca2+ channels, thus preventing cytosolic Ca2+ overload, shortening the Ca2+ transient and limiting ATP consumption (31, 34). Sarcolemmal KATP channels close and APD lengthens upon reoxygenation of the ischemic myocardium that is still viable. This sequence of events was observed in long-duration microdevice signals and is clearly indicated in the beat-to-beat measurement of APD shown in Fig. 3. APD shortened up to 60% after the cessation of aortic flow. Upon resumption of aortic flow (at minute 9), APD gradually (after ~3 min) increased to preischemic levels. The significance of this result is that APD was measured without interruption during ischemia and reperfusion in an unconstrained contracting heart, a process during which the myocardium dramatically changes shape: shrinking when the vasculature is depressurized when coronary flow is stopped and expanding when coronary flow and vascular pressure are restored.
Fig. 3.
APD measured from sharp microdevice signals during sinus rhythm reproduced the expected electrophysiological effects of ischemia and reperfusion. Instantaneous heart rate (in bpm) measured from the ECG of a perfused contracting rat heart is plotted above a simultaneously acquired 1,000-s AP signal generated by a microdevice embedded in the LV epicardium. APD measured for each beat is plotted in black on top of the AP signal. Specific time periods (impalement, normal perfusion, ischemia, and reperfusion) are indicated as different colors. APD90 histograms for the normal perfusion, ischemia, and reperfusion periods are shown to reveal the correlation between coronary flow and temporal changes in APD.
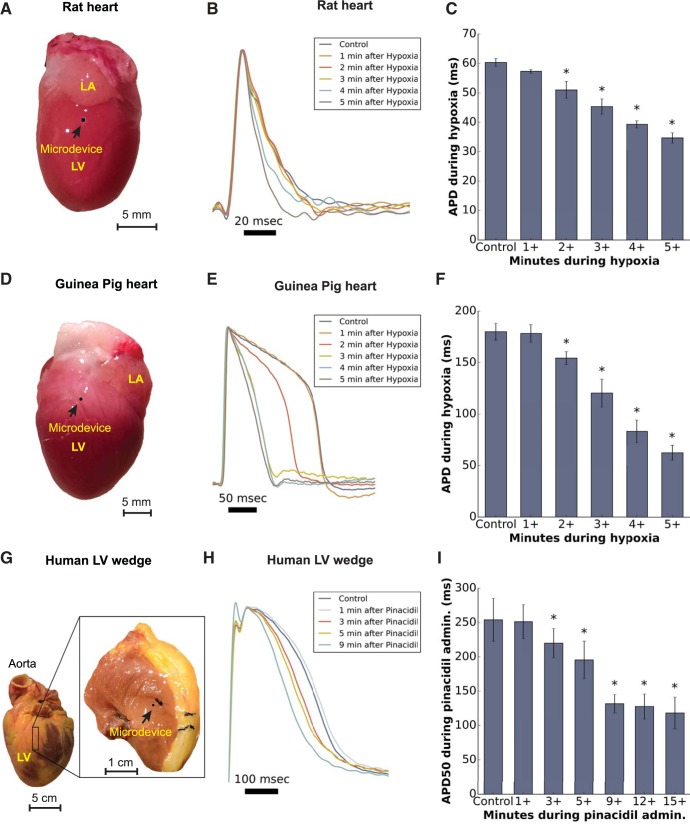
In another set of experiments, APD was measured from rat, guinea pig, and human LV myocardium before and after either hypoxic or pharmacological activation of KATP channels (Fig. 4). In rats and guinea pigs, APD90 changes were continuously monitored during gradual hypoxia induced by bubbling the perfusate with N2 gas. During hypoxia, as during ischemia, myocyte sarcolemmal KATP channels activated to shorten APD (28). We found that APD90 did not shorten significantly in the first minute after the initiation of perfusate deoxygenation [rats: 60.3 ± 1.3 ms during normoxia and 57.3 ± 0.7 ms 1 min after the initiation of deoxygenation (n = 4, P = 0.124); guinea pigs: 180 ± 8.2 ms during normoxia and 178.3 ± 8.7 ms 1 min after the initiation of deoxygenation (n = 6, P = 0.565)]. This result is consistent with previous studies of ischemia and hypoxia in perfused hearts and cells (8, 25, 34). Significant APD90 reductions (P < 0.05) were observed as deoxygenation progressed, reaching steady state after 5 min (Fig. 4, B, C, E, and F).
Fig. 4.
AP signals recorded using sharp microdevices embedded within the contracting LV of three species. APD measured from these signals reproduced the effect of ATP-sensitive K+ (KATP) channel activation, either due to hypoxia or a KATP channel agonist (pinacidil). Bar plots denote averages ± SE. A paired t-test compared the APD of APs with those of control perfusion. *Significant differences (P < 0.05). A, D, and G: images showing representative hearts from a rat and guinea pig as well as a human LV wedge preparation. The black circle denotes the location of a microdevice. B and E: representative APs from a rat and guinea pig heart during 5 min of hypoxia. C and F: APDs progressively decreased (n = 4 and n = 6 for rats and guinea pigs, respectively, P < 0.05) after 2 min of hypoxia. H: representative APs from a human LV wedge preparation during a 9-min recording after pharmacological activation of KATP channels using pinacidil. I: APDs progressively decreased (n = 4, P < 0.05) after 3 min of administration of pinacidil.
A similar trend was observed when KATP channels were activated in perfused human LV wedge preparations using pinacidil (n = 4). As shown in Fig. 4, H and I, APD50 did not significantly shorten until 3 min after the administration of pinacidil (254.0 ± 31.1 ms during normoxia and 251.3 ± 24.7 ms 1 min after the administratration of pinacidil, P = 0.741), which may, in part, reflect the time required for pinacidil to reach the heart. On average, APD50 reductions stabilized at approximately half the APD50 measured before pinacidil, and this occurred within 9 min after the administration of pinacidil. We did not observe additional statistically significant APD50 reductions after 9 min (P > 0.05). These results are consistent with previous studies in canine hearts that administered a similar dose of pinacidil (1, 6).
A useful aspect of the microdevice approach is that multiple microdevices can be sequentially deployed to provide AP signals from multiple locations without requiring a dedicated micromanipulator and micropipette holder for each electrode. In this way, AP signals measured from multiple sites provide fundamental electrophysiological parameters, such as atrioventricular (AV) conduction delay and myocardial conduction time. For example, Fig. 5 shows results from the implantation of two microdevices within a perfused contracting rat heart. One microdevice recorded AP signals from the LA and the other recorded AP signals from the LV. Signals from these microdevices were simultaneously recorded with the bath-conducted ECG (Fig. 5B). Depolarization of the LA APs was precisely aligned with the P wave, and depolarization of the LV APs was precisely aligned with the R wave, representing the typical cardiac activation sequence. Full repolarization of LV APs corresponded to the middle of the T wave (Fig. 5B). LA APDs were shorter than LV APDs (43.9 ± 0.85 vs. 64.1 ± 0.95 ms, respectively), as expected, and the AV conduction time was 56.7 ± 1.1 ms.
Fig. 5.
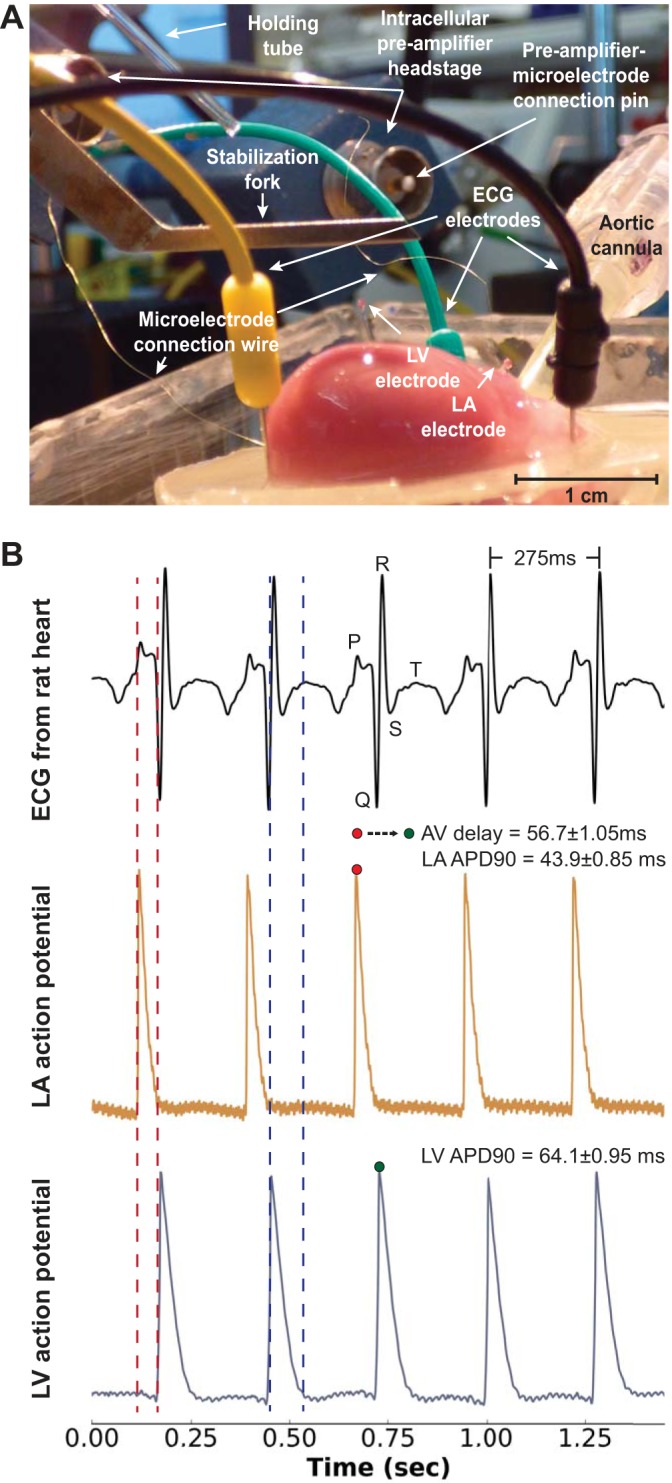
Simultaneous measurements from two sharp microdevices and the bath-conducted ECG. A: image showing an excised perfused contracting rat heart and the three ECG electrodes. A microdevice embedded in the LV and another microdevice embedded in the left atrium (LA) are also shown. B: the ECG signal (top), APs recorded from the LA (middle), and APs recorded from the LV (bottom). The activation sequence of AP signals follows the morphology of the ECG. The R-R interval was 275 ms, the average LA APD90 was 43.9 ± 0.85 ms, and the average LV APD90 was 64.1 ± 0.95 ms.
Neuron AP recordings with patch microdevices.
In neuroscience, patch glass micropipette electrodes are the dominant electrode technology for intracellular recordings from neurons. Therefore, we developed an insertion and deployment approach specifically for whole cell patch recordings and used it to record signals from neurons within the pyramidal CA1 cell layer of the brain of anesthetized animals. Figure 6 shows the overall design and use of the patch microdevices that we built. The fabrication steps are shown in Supplemental Video S1. A patch microdevice attached to a holding micropipette is shown in Fig. 6A. The proximal end of the holding micropipette was attached to a standard patch micropipette holder equipped with a standard pressure port. The silver wire was connected to a conventional patch-clamp headstage that was connected to a patch-clamp amplifier to record transmembrane potential. Our patch clamp transmembrane potential measurement using microdevices closely followed previously reported whole cell recording protocols (26), as explained in materials and methods.
Fig. 6.
AP signals were recorded from the hippocampus of anesthetized rats using patch microdevices whereby the sealing wax was melted to deploy the microdevice. A: each patch microdevice was held by inserting it into the end of a fire-polished glass capillary tube (the “holding micropipette”) and sealing the junction with wax. This maintained a contiguous pathway between the microdevice and the holding micropipette so that pressure or suction could be applied to the inside of the microdevice. B: Sequential images showing the release of a patch microdevice using a small heater coil to melt the wax seal. C: three diagrams (i−iii) showing the steps for implanting and releasing a patch microdevice into the brain to record APs from a CA1 hippocampal neuron. D: patch microdevice floating within the brain craniotomy of an anesthetized rat.
A critical design requirement for the patch microdevice was that the inner volume of the microdevice must remain contiguous with the inner volume of the holding micropipette (Fig. 6A), as explained in materials and methods. This was achieved by sealing the outside of the joint between the holding micropipette and the proximal end of the microdevice with wax that had a low melting point (Fig. 6A). We found this wax seal to be durable, never rupturing as a neuron was targeted and patched, and did not soften within the range of experimental temperatures (34–42°C). In general, and as is common in conventional practice, patch microdevices are not reusable and had to be prepared for each measurement attempt. Once a neuron patch was achieved, the microdevice was deployed to float within brain tissue by gently heating the wax seal, as shown in Fig. 6, B and C. Melting the wax gently released the microdevice while maintaining an intracellular electrical connection (Fig. 6C, iii). A video of the deployment sequence is provided in Supplemental Video S4. A patch microdevice floating in the center of a skull craniotomy of an anesthetized rat is shown in Fig. 6D.
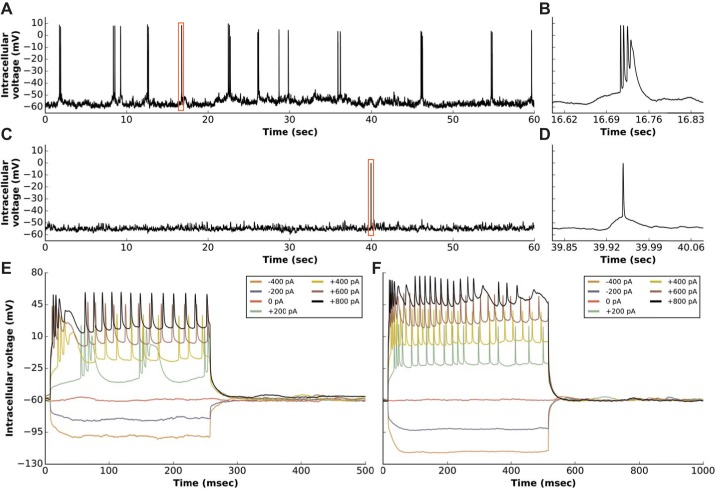
Typical whole cell AP signals recorded from two different CA1 layer neurons using a patch microdevice are shown in Fig. 7. The signals were acquired in current-clamp mode after the establishment of whole cell recording mode. One neuron had a base potential of Vm = −60 mV and exhibited active millivolt-scale subthreshold activity (Fig. 7A). This neuron bursted spontaneously, revealing prototypical triple or quadruple AP bursts (24). Another neuron was patched and represented a well-known “silent” cell (21) that fired an AP only once during the recording (Fig. 7C). That neuron had a base potential of Vm = −58 mV and also exhibited active millivolt-scale subthreshold activity that would otherwise not have been observed with an extracellular electrode or optical imaging. Both neurons could be hyperpolarized or depolarized in the standard current-clamp recording mode, and representative current injection curves for both neurons are shown in Fig. 7, E and F.
Fig. 7.
Representative AP signals recorded from the pyramidal CA1 layer of the hippocampus of a rat using a patch microdevice. Eight different neurons were successfully patched with a microdevice. A and C: whole cell patch recordings from two different CA1 layer neural cells. B and D: zoomed in views of APs denoted by the red boxes shown within the signal on the left. E and F: representative current injection recordings measured using the same microdevice and neurons that were patched to acquire the signals shown in A and C.
Limitations.
Sharp and patch microdevices have some of the same limitations as conventional glass micropipette electrodes. For example, microdevices can be used only once and the best results require that they be built for each experiment, optimally the same day as an experiment. Doing so is usually not a problem because, with some practice, microdevices can be built within ~5 min. Also similar to glass micropipette electrodes, the tips of microdevices are at risk of breaking, especially when measuring signals from myocardium that has substantial contractile motion. To address this, we found it helpful to temporary immobilize the impailment site with the stabilization fork. Although this may be seen as a drawback, it reduced the risk of breaking the tip and also provided improved control in targetting a microdevice to impale a specific site. Finally, while one significant feature of microdevices is that they provide robust measurements of APD (Figs. 2−5), it can be difficult to achieve AP amplitudes that do not drift. We believe that this is due to the vigourous motion of contracting myocardium around a microdevice. This motion likely impedes the formation of a robust seal between a myocyte membrane and the microdevice tip, thereby causing amplitude drift.
Conclusions.
We have developed detachable floating glass microelectrodes that have a typical mass of 100 µg, which is at least an order of magnitude lighter than previously reported microelectrodes. These microdevices can also be targeted with precision to specific locations within active moving organs. Two forms of the microdevice (sharp and patch) provide AP signals without requiring constant mechanical constraint or pharmacological inhibition of the organ. With sharp microdevices, beat-to-beat changes in APD were continuously measured for at least 15 min in fully contracting hearts of multiple species. This was achieved during conditions of no-flow ischemia, reperfusion, hypoxia, and pharmacological activation of KATP channels. Patch microdevices provided continuous measurements of transmembrane potential from neurons within the CA1 layer of the intact hippocampus of rats. The ease of deploying microdevices enables simultaneous measurements from multiple locations, providing multicell analysis of electrical activity within intact organs during complex physiological scenarios. In conclusion, our results demonstrate that detachable microdevices are an elegant and robust tool to record electrical activity with high temporal resolution and cellular level localization without disturbing the physiological working conditions of the organ.
GRANTS
This work was supported by the Howard Hughes Medical Institute (to M. Barbic and T. D. Harris), National Heart, Lung, and Blood Institute Grants R01-HL-095828 and R21-HL-132618 (to M. W. Kay), and a Don J. Levy and Elma Levy Fellowship (to A. Moreno).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.B., A.M., T.D.H., and M.W.K. conceived and designed research; M.B. and A.M. performed experiments; M.B., A.M., and M.W.K. interpreted results of experiments; M.B. and A.M. drafted manuscript; M.B., A.M., and M.W.K. edited and revised manuscript; M.B., A.M., T.D.H., and M.W.K. approved final version of manuscript; A.M. analyzed data; A.M. prepared figures.
Supplementary Material
ACKNOWLEDGMENTS
We thank Albert Lee, Doyun Lee, and Bertalan Andrasfalvy for useful technical insights and training in patch-clamp electrophysiology and Bruce Bowers and Bill Biddle for high-quality machining work. We also thank Chaoyi Kang and Igor Efimov for their support in the cannulation of the human LV wedges.
REFERENCES
- 1.Abi-Gerges N, Valentin JP, Pollard CE. Dog left ventricular midmyocardial myocytes for assessment of drug-induced delayed repolarization: short-term variability and proarrhythmic potential. Br J Pharmacol 159: 77–92, 2010. doi: 10.1111/j.1476-5381.2009.00338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker FL, York DH. A micro-electrode suspension for intracellular recording from moving tissue. Electroencephalogr Clin Neurophysiol 32: 329–331, 1972. doi: 10.1016/0013-4694(72)90184-8. [DOI] [PubMed] [Google Scholar]
- 3.Bosch RF, Wang Z, Li GR, Nattel S. Electrophysiological mechanisms by which hypothyroidism delays repolarization in guinea pig hearts. Am J Physiol 277: H211–H220, 1999. [DOI] [PubMed] [Google Scholar]
- 4.Cauley E, Wang X, Dyavanapalli J, Sun K, Garrott K, Kuzmiak-Glancy S, Kay MW, Mendelowitz D. Neurotransmission to parasympathetic cardiac vagal neurons in the brain stem is altered with left ventricular hypertrophy-induced heart failure. Am J Physiol Heart Circ Physiol 309: H1281–H1287, 2015. doi: 10.1152/ajpheart.00445.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi BR, Salama G. Simultaneous maps of optical action potentials and calcium transients in guinea-pig hearts: mechanisms underlying concordant alternans. J Physiol 529: 171–188, 2000. doi: 10.1111/j.1469-7793.2000.00171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Diego JM, Cordeiro JM, Goodrow RJ, Fish JM, Zygmunt AC, Pérez GJ, Scornik FS, Antzelevitch C. Ionic and cellular basis for the predominance of the Brugada syndrome phenotype in males. Circulation 106: 2004–2011, 2002. doi: 10.1161/01.CIR.0000032002.22105.7A. [DOI] [PubMed] [Google Scholar]
- 7.Dyavanapalli J, Jameson H, Dergacheva O, Jain V, Alhusayyen M, Mendelowitz D. Chronic intermittent hypoxia-hypercapnia blunts heart rate responses and alters neurotransmission to cardiac vagal neurons. J Physiol 592: 2799–2811, 2014. doi: 10.1113/jphysiol.2014.273482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Efimov IR, Huang DT, Rendt JM, Salama G. Optical mapping of repolarization and refractoriness from intact hearts. Circulation 90: 1469–1480, 1994. doi: 10.1161/01.CIR.90.3.1469. [DOI] [PubMed] [Google Scholar]
- 9.Fee MS. Active stabilization of electrodes for intracellular recording in awake behaving animals. Neuron 27: 461–468, 2000. doi: 10.1016/S0896-6273(00)00057-X. [DOI] [PubMed] [Google Scholar]
- 10.Girouard SD, Laurita KR, Rosenbaum DS. Unique properties of cardiac action potentials recorded with voltage-sensitive dyes. J Cardiovasc Electrophysiol 7: 1024–1038, 1996. doi: 10.1111/j.1540-8167.1996.tb00478.x. [DOI] [PubMed] [Google Scholar]
- 11.Glukhov AV, Fedorov VV, Lou Q, Ravikumar VK, Kalish PW, Schuessler RB, Moazami N, Efimov IR. Transmural dispersion of repolarization in failing and nonfailing human ventricle. Circ Res 106: 981–991, 2010. doi: 10.1161/CIRCRESAHA.109.204891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch 391: 85–100, 1981. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 13.Huang J, Cheng K-A, Dosdall DJ, Smith WM, Ideker RE. Role of maximum rate of depolarization in predicting action potential duration during ventricular fibrillation. Am J Physiol Heart Circ Physiol 293: H2530–H2536, 2007. doi: 10.1152/ajpheart.00793.2007. [DOI] [PubMed] [Google Scholar]
- 14.Huang J, Zhou X, Smith WM, Ideker RE. Restitution properties during ventricular fibrillation in the in situ swine heart. Circulation 110: 3161–3167, 2004. doi: 10.1161/01.CIR.0000147618.93579.56. [DOI] [PubMed] [Google Scholar]
- 15.Jaimes R III, Walton RD, Pasdois P, Bernus O, Efimov IR, Kay MW. A technical review of optical mapping of intracellular calcium within myocardial tissue. Am J Physiol Heart Circ Physiol 310: H1388–H1401, 2016. doi: 10.1152/ajpheart.00665.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kléber AG. Resting membrane potential, extracellular potassium activity, and intracellular sodium activity during acute global ischemia in isolated perfused guinea pig hearts. Circ Res 52: 442–450, 1983. doi: 10.1161/01.RES.52.4.442. [DOI] [PubMed] [Google Scholar]
- 17.Konopacki J, Bland BH, Dyck R. Intracellular recording and labeling of neurons in midline structures of the rat brain in vivo using sharp electrodes. J Neurosci Methods 127: 85–93, 2003. doi: 10.1016/S0165-0270(03)00126-2. [DOI] [PubMed] [Google Scholar]
- 18.Kunze WA. A mobile intracellular microelectrode designed to record from neurons in contracting tissue. Brain Res Brain Res Protoc 3: 94–99, 1998. doi: 10.1016/S1385-299X(98)00029-4. [DOI] [PubMed] [Google Scholar]
- 19.Lee AK, Epsztein J, Brecht M. Head-anchored whole-cell recordings in freely moving rats. Nat Protoc 4: 385–392, 2009. doi: 10.1038/nprot.2009.5. [DOI] [PubMed] [Google Scholar]
- 20.Lee AK, Manns ID, Sakmann B, Brecht M. Whole-cell recordings in freely moving rats. Neuron 51: 399–407, 2006. doi: 10.1016/j.neuron.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Lee D, Lin BJ, Lee AK. Hippocampal place fields emerge upon single-cell manipulation of excitability during behavior. Science 337: 849–853, 2012. doi: 10.1126/science.1221489. [DOI] [PubMed] [Google Scholar]
- 22.Lee D, Shtengel G, Osborne JE, Lee AK. Anesthetized- and awake-patched whole-cell recordings in freely moving rats using UV-cured collar-based electrode stabilization. Nat Protoc 9: 2784–2795, 2014. doi: 10.1038/nprot.2014.190. [DOI] [PubMed] [Google Scholar]
- 23.Ling G, Gerard RW. The normal membrane potential of frog sartorius fibers. J Cell Comp Physiol 34: 383–396, 1949. doi: 10.1002/jcp.1030340304. [DOI] [PubMed] [Google Scholar]
- 24.Lisman JE. Bursts as a unit of neural information: making unreliable synapses reliable. Trends Neurosci 20: 38–43, 1997. doi: 10.1016/S0166-2236(96)10070-9. [DOI] [PubMed] [Google Scholar]
- 25.MacKenzie I, Saville VL, Waterfall JF. Differential class III and glibenclamide effects on action potential duration in guinea-pig papillary muscle during normoxia and hypoxia/ischaemia. Br J Pharmacol 110: 531–538, 1993. doi: 10.1111/j.1476-5381.1993.tb13843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Margrie TW, Brecht M, Sakmann B. In vivo, low-resistance, whole-cell recordings from neurons in the anaesthetized and awake mammalian brain. Pflugers Arch 444: 491–498, 2002. doi: 10.1007/s00424-002-0831-z. [DOI] [PubMed] [Google Scholar]
- 27.Nichols CG, Ripoll C, Lederer WJ. ATP-sensitive potassium channel modulation of the guinea pig ventricular action potential and contraction. Circ Res 68: 280–287, 1991. doi: 10.1161/01.RES.68.1.280. [DOI] [PubMed] [Google Scholar]
- 28.Nichols CG, Singh GK, Grange DK. KATP channels and cardiovascular disease: suddenly a syndrome. Circ Res 112: 1059–1072, 2013. doi: 10.1161/CIRCRESAHA.112.300514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Omichi C, Lee MH, Ohara T, Naik AM, Wang NC, Karagueuzian HS, Chen PS. Comparing cardiac action potentials recorded with metal and glass microelectrodes. Am J Physiol Heart Circ Physiol 279: H3113–H3117, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Osadchii OE. Effects of ventricular pacing protocol on electrical restitution assessments in guinea-pig heart. Exp Physiol 97: 807–821, 2012. doi: 10.1113/expphysiol.2012.065219. [DOI] [PubMed] [Google Scholar]
- 31.Powers SK, Murlasits Z, Wu M, Kavazis AN. Ischemia-reperfusion-induced cardiac injury: a brief review. Med Sci Sports Exerc 39: 1529–1536, 2007. doi: 10.1249/mss.0b013e3180d099c1. [DOI] [PubMed] [Google Scholar]
- 32.Pruett JK, Woods EF. Intracellular potentials recorded from the myocardium in situ: an improved technique. J Appl Physiol 21: 1071–1072, 1966. [DOI] [PubMed] [Google Scholar]
- 33.Sakmann B, Neher E. Single-Channel Recording (2nd ed.). New York: Springer, 1995. [Google Scholar]
- 34.Salama G, Kanai AJ, Huang D, Efimov IR, Girouard SD, Rosenbaum DS. Hypoxia and hypothermia enhance spatial heterogeneities of repolarization in guinea pig hearts: analysis of spatial autocorrelation of optically recorded action potential durations. J Cardiovasc Electrophysiol 9: 164–183, 1998. doi: 10.1111/j.1540-8167.1998.tb00897.x. [DOI] [PubMed] [Google Scholar]
- 35.Salata JJ, Jurkiewicz NK, Wallace AA, Stupienski RF III, Guinosso PJ Jr, Lynch JJ Jr. Cardiac electrophysiological actions of the histamine H1-receptor antagonists astemizole and terfenadine compared with chlorpheniramine and pyrilamine. Circ Res 76: 110–119, 1995. doi: 10.1161/01.RES.76.1.110. [DOI] [PubMed] [Google Scholar]
- 36.Sato K. Modifications of glass microelectrodes: a self-filling and a semifloating glass microelectrode. Am J Physiol 232: C207–C210, 1977. [DOI] [PubMed] [Google Scholar]
- 37.Schainbaum J. Muscle action potentials: a technique for recording in situ. Science 138: 905–906, 1962. [DOI] [PubMed] [Google Scholar]
- 38.Schmidt EM, Mutsuga N, Mcintosh JS, Kanda K, Goldstein SR. Intracellular recording from pulsating cerebral cortex. Electroencephalogr Clin Neurophysiol 42: 581–583, 1977. doi: 10.1016/0013-4694(77)90222-X. [DOI] [PubMed] [Google Scholar]
- 39.Schneider JA, Sperelakis N. Slow Ca2+ and Na+ responses induced by isoproterenol and methylxanthines in isolated perfused guinea pig hearts exposed to elevated K+. J Mol Cell Cardiol 7: 249–273, 1975. doi: 10.1016/0022-2828(75)90084-X. [DOI] [PubMed] [Google Scholar]
- 40.Standen NB, Gray PTA, Whitaker MJ. Microelectrode Techniques: the Plymouth Workshop Handbook. Cambridge: Company of Biologists, 1987. [Google Scholar]
- 41.Tyler WJ. The mechanobiology of brain function. Nat Rev Neurosci 13: 867–878, 2012. doi: 10.1038/nrn3383. [DOI] [PubMed] [Google Scholar]
- 42.Watanabe T, Delbridge LM, Bustamante JO, McDonald TF. Heterogeneity of the action potential in isolated rat ventricular myocytes and tissue. Circ Res 52: 280–290, 1983. doi: 10.1161/01.RES.52.3.280. [DOI] [PubMed] [Google Scholar]
- 43.Wengrowski AM, Wang X, Tapa S, Posnack NG, Mendelowitz D, Kay MW. Optogenetic release of norepinephrine from cardiac sympathetic neurons alters mechanical and electrical function. Cardiovasc Res 105: 143–150, 2015. doi: 10.1093/cvr/cvu258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Woodbury JW, Brady AJ. Intracellular recording from moving tissues with a flexibly mounted ultramicroelectrode. Science 123: 100−101, 1956. [DOI] [PubMed] [Google Scholar]
- 45.Zhou XH, Knisley SB, Wolf PD, Rollins DL, Smith WM, Ideker RE. Prolongation of repolarization time by electric field stimulation with monophasic and biphasic shocks in open-chest dogs. Circ Res 68: 1761–1767, 1991. doi: 10.1161/01.RES.68.6.1761. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.