We used proteomics and metadata analysis software to identify contributors to metabolic changes in striated muscle during cancer cachexia. We found increased expression of hypoxia-inducible factor-1α in the heart and skeletal muscle, suggesting a potential target for the therapeutic treatment of cancer cachexia.
Keywords: hypoxia-inducible factor, cancer cachexia, heart, hypoxia, proteomics, skeletal muscle
Abstract
Cancer cachexia is a progressive wasting disease resulting in significant effects on the quality of life and high mortality. Most studies on cancer cachexia have focused on skeletal muscle; however, the heart is now recognized as a major site of cachexia-related effects. To elucidate possible mechanisms, a proteomic study was performed on the left ventricles of colon-26 (C26) adenocarcinoma tumor-bearing mice. The results revealed several changes in proteins involved in metabolism. An integrated pathway analysis of the results revealed a common mediator in hypoxia-inducible factor-1α (HIF-1α). Work by other laboratories has shown that extensive metabolic restructuring in the C26 mouse model causes changes in gene expression that may be affected directly by HIF-1α, such as glucose metabolic genes. M-mode echocardiography showed progressive decline in heart function by day 19, exhibited by significantly decreased ejection fraction and fractional shortening, along with posterior wall thickness. Using Western blot analysis, we confirmed that HIF-1α is significantly upregulated in the heart, whereas there were no changes in its regulatory proteins, prolyl hydroxylase domain-containing protein 2 (PHD2) and von Hippel-Lindau protein (VHL). PHD2 requires both oxygen and iron as cofactors for the hydroxylation of HIF-1α, marking it for ubiquination via VHL and subsequent destruction by the proteasome complex. We examined venous blood gas values in the tumor-bearing mice and found significantly lower oxygen concentration compared with control animals in the third week after tumor inoculation. We also examined select skeletal muscles to determine whether they are similarly affected. In the diaphragm, extensor digitorum longus, and soleus, we found significantly increased HIF-1α in tumor-bearing mice, indicating a hypoxic response, not only in the heart, but also in skeletal muscle. These results indicate that HIF-1α may contribute, in part, to the metabolic changes that occur during cancer cachexia.
NEW & NOTEWORTHY We used proteomics and metadata analysis software to identify contributors to metabolic changes in striated muscle during cancer cachexia. We found increased expression of hypoxia-inducible factor-1α in the heart and skeletal muscle, suggesting a potential target for the therapeutic treatment of cancer cachexia.
in the united states, cancer is the second leading cause of death behind cardiovascular disease, whereas in the United Kingdom, cancer is the leading cause of death (38, 47). As the quality of life index and longevity continue to increase, cancer, with a higher incidence after midlife, will likely become a more prevalent problem in developing and third world countries (19). In the United States, ~40% of men and women will be diagnosed with cancer at some point in their lifetime (14). Cancer, a heavy burden on its own, can also lead to the development of cachexia (43, 44), a wasting syndrome in which muscle and adipose tissue experience marked loss and breakdown, which is not nutritionally reversible. In addition to the fat and muscle wasting, there are significant appetite and metabolic complications in cachectic patients. Cachectic patients also suffer a reduced quality of life, experiencing greater fatigue, exercise intolerance, and depression (45, 46). Cachexia is inherently difficult to treat because of the lack of Federal Drug Administration-approved drugs, and cachexia causes patients to be refractory to conventional cancer treatments. For patients with cancer, there is a 30–80% chance that they will develop cachexia, with certain cancers such as gastric, prostate, colon, and lung cancer having higher incidences of cachexia (13). Among the patients who develop cachexia, it is the primary cause of death in 20–40% of them (44). The extensive burden and complications of cachexia warrant further investigation into the mechanisms of progression to allow development of new therapeutic strategies.
The heart was not typically considered at risk for wasting during the development of cancer-induced cachexia because of normalized heart weight showing no significant loss. However, recent research has shown that cardiac function is compromised in a tumor-burdened setting. Heart samples from patients who died as a direct result of cachexia showed significant fibrotic remodeling, indicative of heart failure (39). With the use of an experimental model of cancer cachexia, the colon-26 (C26) adenocarcinoma cell line, it has also been shown that tumor burden can significantly impact cardiac function (52). CD2F1 mice subcutaneously injected with this cell line show reduced ejection fraction and fractional shortening with a reduction in posterior wall thickness during systole (52). Studies have implicated the ubiquitin proteasome system, autophagy, ANG II, and matrix metalloproteinases in this cardiac dysfunction (5, 40, 42). However, other possible contributors to cardiac and skeletal muscle dysfunction remain unknown.
To uncover possible mechanisms of cardiac and skeletal muscle dysfunction, a proteomics approach was used. The proteomics approach, however, creates several problems that must be overcome, namely, sorting and analyzing the data. A data analyzer from Qiagen (Valencia, CA) was used [Ingenuity Pathway Analysis (IPA)] to sort the data and determine connections. Both metabolic and cytoskeletal differences were linked to hypoxia-inducible factor-1α (HIF-1α). Until this study, HIF-1α has not been extensively studied outside of the native tumor environment. A study by Tian et al. (42), using the C26 model of cachexia, demonstrated that cardiac tissue expresses several RNA markers consistent with increased reliance on glucose metabolism and decreased fatty acid oxidation. Increased reliance on glucose is a hallmark of anaerobic metabolism because of the inability to perform fatty acid oxidation. These effects have not been limited to animal models, as metabolism is also significantly altered in human patients suffering from cancer. A cause of cachexia is thought to be increased reliance on the Cori cycle. Tumors secrete inflammatory factors and, thereby, cause the breakdown of fat stores and increased gluconeogenesis by the liver in an anaerobic fashion (48). Because this process is highly inefficient, it creates wasted energy and futile cycling, possibly contributing to the cachectic state (9). HIF-1α is capable of causing these metabolic reprogramming events, but the degree to which it is involved in cancer-induced cachexia is unknown.
HIF-1α is a transcription factor that responds to the amount of oxygen available in the environment, increasing in expression during hypoxia (50). HIF-1α is a transcription factor for more than 60 genes (50). In normoxic conditions, HIF-1α is hydroxylated by prolyl hydroxylase-2 (PHD2); this posttranslational modification is then recognized by the ubiquitin ligase von Hippel-Lindau tumor suppressor (VHL). VHL ubiquinates the hydroxylated HIF-1α, marking it for destruction by the proteasome complex (18). PHD2 itself requires several cofactors to perform the hydroxylation reaction, namely, oxygen and iron (8). In hypoxic conditions, HIF-1α expression increases as PHD2 is no longer able to mark HIF-1α for destruction. HIF-1α is known to be upregulated in human tumors, contributing to the development of large tumors that have a hypoxic core (35, 53). Increased hypoxia in other tissues during cancer progression has been understudied, and it is possibly confounded with the normal restriction of peripheral blood supply during the end stages of cancer. Because of the ability of HIF-1α to cause substantial metabolic reprogramming, there may be a threshold in which even a minor amount of increased HIF-1α could cause significant effects. Further study will need to be performed to understand when HIF-1α becomes relevant in this biological system.
In this study, we demonstrated, through a proteomics approach, coupled with data analysis, that HIF-1α is a possible contributor to heart and skeletal muscle pathology of tumor-bearing mice. We also demonstrated possible mechanisms contributing to HIF-1α increases and discuss possible consequences of HIF-1α upregulation.
MATERIALS AND METHODS
Animal husbandry and care.
Adult female mice (~10 wk old), weighing 20–22 g, were obtained from Charles River Laboratories (Charles River, Wilmington, MA). Mice were housed one to three per cage and were maintained on a 12:12-h light-dark cycle at 25°C. Mice were provided ad libitum access to water and standard rodent chow throughout the duration of the study. All animal care and procedures were approved by the Ohio State University Institutional Animal Care and Use Committee.
Animal study and exclusion criteria.
Animal studies were carefully monitored during the study to avoid complications arising from a moribund state, which may complicate hypoxia-related findings. Despite losing ~10–20% body mass, mice remained active and alert. Mice showing signs of tumor ulceration, impaired mobility, hunched posture, or guarding behavior were removed from the study and were not used for any further biochemical studies, and their final weight values were not reported for statistical analysis.
Tumor model.
C26 cells were maintained as previously described (52). Approximately 1 wk after arrival, mice were randomly assigned to receive an injection of tumor cells or saline vehicle. Cells (5 × 105) were injected subcutaneously between the scapulae so that tumor growth would not disrupt locomotion. Tumor growth was evident as early as day 7 post injection. Mice became moribund at approximately day 21. After blood collection, a cardiectomy was performed, and the left ventricle (LV) was dissected, snap frozen in liquid nitrogen, and stored at −80°C until further biochemical analyses.
Proteomics and bioinformatics.
Proteomics analysis was performed by the OSU Proteomics Core, as previously described (25). Briefly, samples were marked with dye and subjected to two-dimensional electrophoretic separation (Fig. 1). The gels were then scanned using a Typhoon 9400 variable mode scanner (GE Healthcare, Aurora, OH) to visualize the dye-labeled proteins. After identification of differences in samples between control and tumor-bearing mice, the proteins were extracted from the gel and prepared for mass spectrometry. Capillary liquid chromatography-tandem mass spectrometry was used to identify peptide sequences. These peptide sequences were compared with the National Center for Biotechnology Information NR database and then checked to ensure that only proteins with a Mascot score of 100 or higher were accepted.
Fig. 1.
2-D SDS-PAGE scan used for proteomics analysis, provided by the Ohio State University Proteomics Core.
Proteomics data analysis.
IPA software (Qiagen) was used to sort and analyze the proteomics data. An IPA compatible data set from the proteomics results was uploaded into the IPA Knowledge Database, and an interactive report was generated. To determine connections, Pathway Builder software, provided as part of the IPA analysis software, was used to grow connections from the identified proteins. These networks were continually expanded and combined until common targets were identified for further analysis.
Echocardiography.
Cardiac function was determined using echocardiography with a VEVO 2100 Ultrasound System (Visual Sonics, Toronto, ON, Canada). Mice were anesthetized using isoflurane: 3% in 100% oxygen for induction and 1% in 100% oxygen for maintenance. During echocardiography, animals were monitored for heart rate measurements to ensure that their rate was ~500 beats/min during maintenance, and the percentage of isoflurane was changed to maintain this rate without waking the animal. Animals were placed on a warm table, fur around the chest was removed using a depilatory agent, and temperature was measured via a rectal probe. Prewarmed ultrasound gel was applied to a 15-mHz transducer probe optimized for mouse echocardiography. The transducer was placed on the parasternal short axis to obtain a view of the LV at the midpapillary level for image capture and measurements. LV dimensions [LV end-diastolic dimension and LV end-systolic dimension (LVEDd and LVEDs)] as well as posterior wall thickness during systole and diastole (PWTs and PWTd) were acquired using the leading edge method, as recommended by the American Society for Echocardiography. Fractional shortening (in %) was calculated using the following formula: fractional shortening = (LVEDd − LVEDs/LVEDd) × 100. Ejection fraction was also measured and calculated using the following formula: ejectional fraction = (stroke volume/end-diastolic volume) × 100. For serial measurements, the same mice were used consecutively for each time point using the same conditions. Mice whose fur had grown back had their fur removed using a depilatory agent as previously stated, and animals were checked during each session in case of adverse reactions that could affect echocardiographic measurements. For echocardiography results, n = 6 was used for each experimental group.
SDS-PAGE and Western blot analysis.
Proteins were isolated from sample tissue and run as previously described (5, 49). Briefly, gels were run at a constant voltage until the tracking dye ran off the gel. Protein was then transferred onto a nitrocellulose membrane, which was blocked with BSA for an hour at room temperature. Blots were incubated with primary antibody [HIF-1α (MGC3) (ThermoScientific, Grand Island, NY), PHD2 (ab109088) (Abcam, Cambridge, MA), VHL (ab28434) (Abcam), and c-Kit (C-19) (Santa Cruz Biotechnology, Dallas, TX)] for an hour at room temperature or at 4°C overnight. Blots were washed multiple times with Tris-buffered saline-Tween 20 before the addition of secondary antibody for incubation for an hour at room temperature. After being washed with Tris-buffered saline-Tween 20, blots were visualized using the 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium color reaction. Digital images of the blots were quantitated using ImageJ [National Institutes of Health (NIH), Bethesda, MD] software.
For loading control, separate gels were run under the same conditions and were stained using Coomassie blue instead of being transferred. The region containing α/β-actin was used as the loading control. Digital images of the gels were quantitated using ImageJ, which was used to calculate the protein fold change.
Blood gas measurements.
To measure blood gas values, an iStat blood analyzer (Abbott, Princeton, NJ) was used along with the CG8+ Cartridge (Abbott). Blood was drawn each week from the submandibular vein, and ~100 µl were dispensed into the cartridge sample port. Blood draws were performed on conscious, unanesthetized mice to avoid complications that anesthesia could have on respiratory rate. Mice were gripped at the scruff and held so that the submandibular vein was easily accessible. A needle was then used to pierce the skin over the submandibular vein, and blood was collected in a dropwise fashion into an Eppendorf tube. Blood was immediately transferred to the iSTAT biochip in a process that took no more than a few seconds to keep blood gases as close to physiological values as possible. Values were recorded and then analyzed for the significance of differences between groups.
Venous oxygen content () was calculated using the following equation: = [Hb × 1.34 × (So2/100)] + (Po2 × 0.031), where Hb is hemoglobin, So2 is the percentage of hemoglobin saturated with oxygen, and Po2 is the partial pressure of oxygen. Hb, So2, and Po2 were measured directly by the iSTAT, whereas the other values are constants representing the amount of oxygen carried by hemoglobin and the amount of oxygen dissolved in plasma, respectively.
Oil red O staining and quantitation.
Hearts from control and tumor-bearing mice were preserved in cryo-embedding media (OCT), frozen in liquid nitrogen, and stored at −80°C until sectioned. The OCT blocks were sectioned using a cryostat and fixed onto glass slides. Sections were stained in oil red O stain (Abcam) for 45 min. Sections were rinsed in distilled water three times. Slides were stained in Harris hematoxylin, washed in tap water, and washed in ammonia water to contrast the nuclei followed by another wash in tap water. Slides were then mounted with aqueous mounting media.
Slide images were captured using an Olympus IX73 microscope (Olympus, Pittsburgh, PA) at ×20 magnification. The stained slides were quantitated as previously described (26). Briefly, the images were opened in ImageJ (NIH) and converted into an eight-bit grayscale image. The threshold limit was set using a control slide, and this threshold limit was applied to every slide. The measure feature on the software was used to determine the area of red staining on the slide, and the area was used to perform statistical analyses as well as graphing.
RNA isolation and quantitative PCR.
RNA isolation and quantitative PCR were performed as previously described (52). Briefly, the free wall of the LV was homogenized in TRIzol (Life Technologies, Carlsbad, CA) using a TissueLyser (Qiagen) for 3 min. A TRIzol-chloroform extraction was performed, following the manufacturer guidelines using the RNEasy Mini-Kit (Qiagen). cDNA synthesis was performed as previously described (52) using the iScript Reverse Transcription Supermix (Bio-Rad, Hercules, CA). Quantitative PCR was done using SYBR Master Mix (Bio-Rad) with a three-step amplification protocol, as previously described (52). Results were calculated using the Livak method (23).
Statistical analyses.
Values are reported as the average ± SE for control and tumor-bearing mice. For the RNA, protein, and iron measurements, n = 6 for both groups. For blood measurements, n = 5 for each group. The histological analyses were n = 3 for each group. To determine significant differences, a Student’s t-test was used using Prism 6 (Graphpad Software, La Jolla, CA). Differences were considered statistically significant if P < 0.05.
RESULTS
Identification of downstream targets and upstream effectors of HIF-1α levels.
IPA analysis of the proteomics results indicated connections to HIF-1α. The HIF-1α-suppressive protein HSP68 was decreased in tumor-bearing mice, whereas several downstream HIF-1α proteins, such as transferrin, albumin, thrombin, and transglutaminase, were increased in tumor-bearing mice (Table 1).
Table 1.
Effects of HIF-1α on transferrin, albumin, thrombin, and transglutaminase in tumor-bearing mice
| Protein Name | Fold Change | Effect on HIF-1α | Reference |
|---|---|---|---|
| Albumin | +10.5 | Increases HIF-1α | 27 |
| Thrombin | +3.7 | Increases HIF-1α | 12 |
| Transglutaminase | +4.2 | Increases HIF-1α | 21 |
| Transferrin | +3.8 | HIF-1α increases expression | 33 |
HIF-1α, hypoxia-inducible factor-1α.
Body and tissue weights.
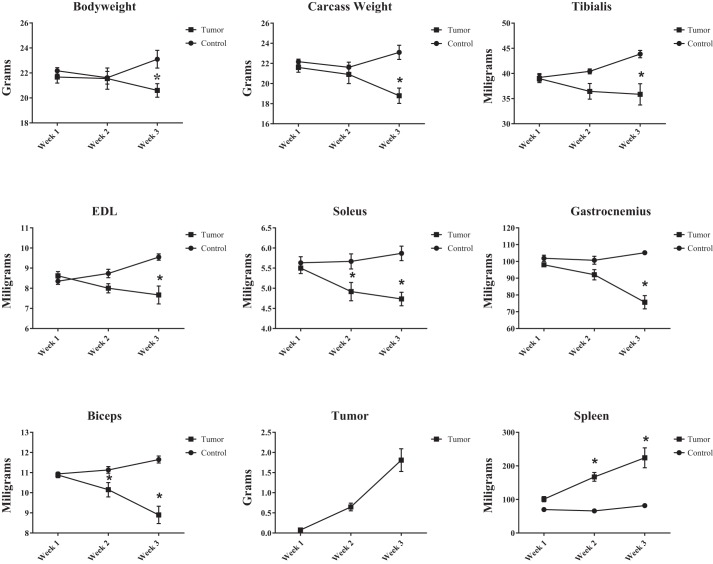
Time course measurements showing the weight of the mouse (body weight), weight of the mouse minus the tumor weight (i.e., carcass weight), soleus weight, extensor digitorum longus (EDL) weight, heart weight, tumor weight, and spleen weight are shown in Fig. 2.
Fig. 2.
Time course measurements showing the weight of the mouse (body weight), weight of the mouse minus the tumor weight (i.e., carcass weight), soleus weight, extensor digitorum longus (EDL) weight, heart weight, tumor weight, and spleen weight. *P < 0.05.
Depressed myocardial function in late tumor development.
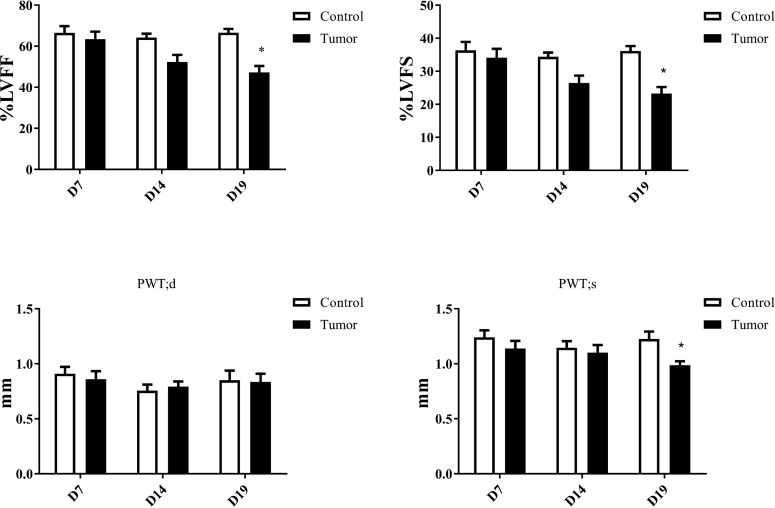
Short-axis M-mode echocardiography of the hearts of control and tumor-burdened mice revealed significant myocardial function depression at 19 days post tumor inoculation. In addition to depressed fractional shortening and ejection fraction, there was a significant decrease in PWTs at 19 days postinjection without a change in PWTd (Fig. 3).
Fig. 3.
Echocardiography measurements showing differences between control and tumor-bearing mice over time. Measurements were taken at the midpapillary level of the short axis to clearly identify the location of the papillary muscles for accurate M-mode measurements. *P < 0.05. %LVEF, percentage of left ventricular ejection fraction; %LVFS, percentage of left ventricular fractional shortening; PWTs, posterior wall thickness during systole; PWTd, posterior wall thickness during diastole.
Increase in HIF-1α and c-Kit protein levels in the myocardium.
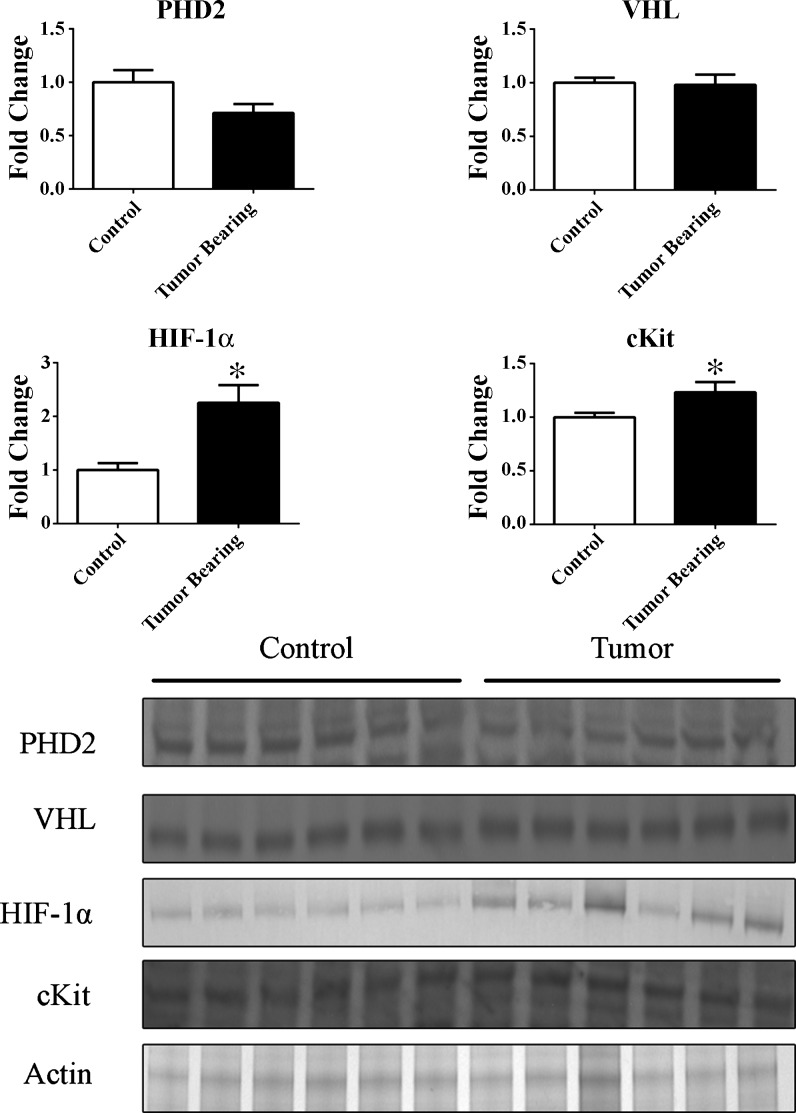
Densitometry scans of the immunoblots showed that HIF-1α was significantly increased in the myocardium of tumor-bearing mice. Regulators of HIF-1α, PHD2, and VHL were unchanged in the myocardium between control and tumor-bearing mice. Additionally, c-Kit, a marker of differentiation and downstream of HIF-1α, was slightly but significantly increased in the myocardium (Fig. 4).
Fig. 4.
Representative immunoblots of LVs of control and tumor-bearing mice. Protein fold changes in the LVs of control and tumor-bearing mice were determined by densitometry using actin as a loading control. *P < 0.05. PHD2, prolyl hydroxylase domain-containing protein 2; VHL, von Hippel-Lindau protein; HIF-1α, hypoxia-inducible factor-1α.
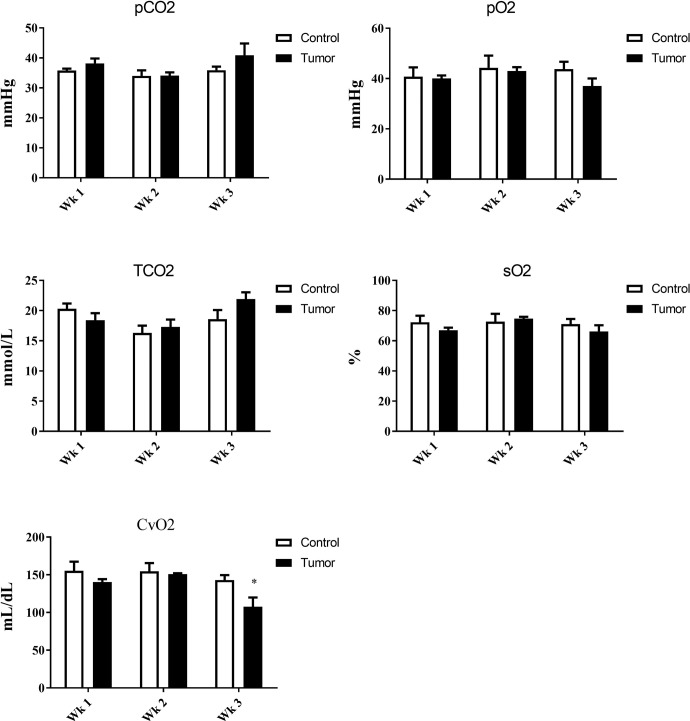
Decreased oxygen levels in venous blood.
Submandibular venous blood from control and tumor-burdened mice showed a reduction of oxygen concentration at the third week after tumor inoculation. No changes were observed in Pco2 or total CO2 in either control or tumor-burdened mice (Fig. 5).
Fig. 5.
Venous blood gas measurements from control and tumor-bearing mice. *P < 0.05.
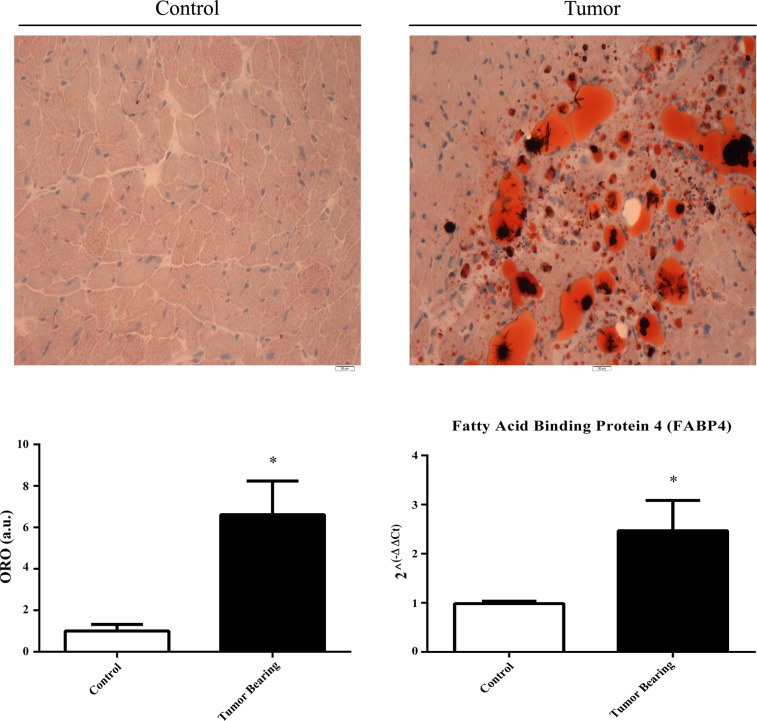
Increased lipid deposition in the myocardium and fatty acid-binding protein-4 RNA expression.
Stained sections revealed significant lipid deposition in the myocardium of tumor-bearing mice compared with control mice (Fig. 6). To determine whether the lipid deposition represented immature adipocytes, the RNA expression of fatty acid-binding protein-4 (FABP4) was measured and was significantly increased in tumor-bearing mice compared with control mice (Fig. 6).
Fig. 6.
Representative images of lipid deposition in the myocardium of control and tumor-bearing mice with quantitation of lipid density using the red stain of oil red O (ORO); also included are the RNA levels of fatty acid-binding protein-4 (FABP4) determined by quantitative RT-PCR. *P < 0.05.
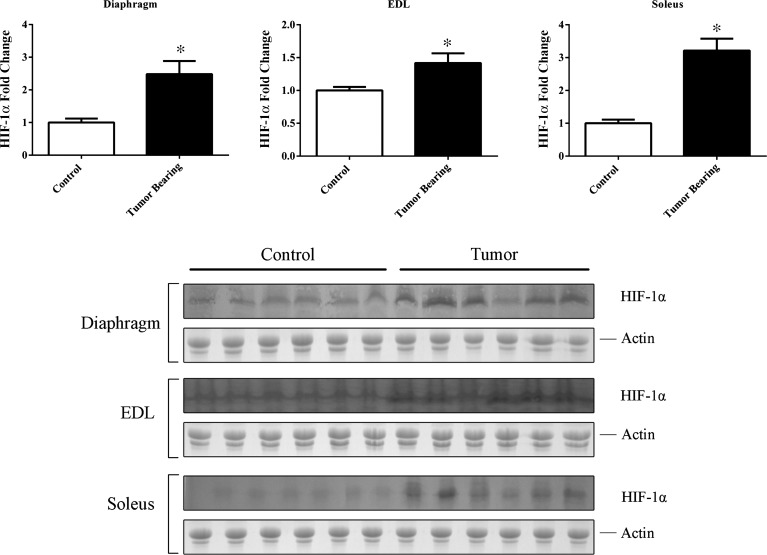
Increase in HIF-1α protein levels in skeletal muscle.
HIF-1α was significantly increased in the soleus, EDL, and diaphragm of tumor-bearing mice compared with control mice, as determined by densitometry of immunoblots (Fig. 7).
Fig. 7.
Protein fold changes in the diaphragm, EDL, and soleus of control and tumor-bearing mice as determined by densitometry using the actin-stained gel as a loading control. *P < 0.05.
DISCUSSION
Our findings demonstrate increased HIF-1α in the heart, EDL, soleus, and diaphragm of tumor-bearing mice. Although proteomics was originally performed only on the heart, leading to the focus on HIF-1α, it became evident that other tissues might be affected as well. Finding increased amounts of HIF-1α, without a compensatory increase in PHD2 or VHL, necessitated looking for a plausible mechanism to explain the increased HIF-1α. Because PHD2 requires both oxygen and iron as a cofactor to hydroxylate HIF-1α so that it can be recognized by VHL for ubiquination, we determined the amount of oxygen through blood gas measurements as well as the amount of iron present in the myocardium. There was a significantly lower oxygen concentration in the venous blood of tumor-bearing mice, indicating a hypoxic environment. We also examined the EDL, soleus, and diaphragm, and all were found to have significantly increased expression of HIF-1α. The effect of hypoxia and subsequent increase in HIF-1α in the heart has both beneficial and detrimental effects. Transient hypoxia and induction of HIF-1α have been shown to be cardioprotective in high-altitude training and in myocardial infarction (20, 31, 51). A plausible mechanism for this is that HIF-1α regulates the oxygen consumption of mitochondria, providing protection during hypoxic events and decreased oxygen availability (36). In the heart, c-Kit has been implicated as a progenitor marker leading to cardiomyocyte differentiation (24, 34). Previous studies have shown that hypoxia and the action of HIF-1α can induce c-Kit expression, possibly as a protective mechanism to replenish apoptotic cells as a response to hypoxia (22). Our findings show an increased amount of c-Kit in the myocardium of tumor-bearing mice, indicating that HIF-1α may be involved in progenitor cell activation. Sustained hypoxia and a subsequent increase in HIF-1α are associated with detrimental effects on the heart (11). The lack of available oxygen impacts the β-oxidation of fatty acids, preventing lipids from being used as an energy source; this, in turn, leads to lipid accumulation in the myocardium (4, 30, 32).
In agreement with previous findings, we found depressed myocardial function in the hearts of tumor-bearing mice that reached significance at 19 days postinjection (7, 41). This is most likely due to the LV wall being compromised, as evidenced by the reduction in PWTs. There were no changes to PWTd, however, indicating that the compromised function of the heart is not solely due to atrophy as a dilated cardiomyopathy phenotype, in which both walls would have reduced thickness. Although previous findings have looked at only day 21 posttumor cell injection, we examined the mice at three time points. By doing this, we detected that there is a progressive decline in the tumor-burdened heart, beginning at approximately day 14. Speckle tracking is a relatively new echocardiographic method that examines wall movement in a short-axis view and can reveal deficiencies before overt functional depression is detected. Speckle tracking could be used in future studies to reveal structural changes in the heart that occur before significantly decreased fractional shortening and ejection fraction. This may also resolve some questions concerning the reduction in PWTs without the corresponding decrease in PWTd, as speckle tracking may show changes in the structure of the LV wall that would otherwise be missed by conventional M-mode and B-mode measurements.
Our results confirm that there is lipid accumulation in the hearts of tumor-bearing mice, which could contribute to cardiac dysfunction through lipid toxicity. Lipid accumulation typically results in an increase in peroxisome proliferator-activated receptor (PPAR)-α; however, hypoxia has been shown to decrease PPAR-α expression with a consequent shift from lipid to glucose utilization (28). PPAR-α has been shown to be decreased in the C26 model of cancer cachexia (41). Studies have shown that HIF-1α acts as an adipocyte mobilization factor. Increases in HIF-1α cause the release of preadipocyte factors into the bloodstream (3, 15). The extensive lipid deposition in the myocardium, along with the increase in HIF-1α, raised the question of whether adipocyte cells had infiltrated the myocardium. FABP4 is a marker of adipocyte progenitor cells, and RNA expression is associated with terminal differentiation into an adipocyte. We found significantly increased RNA expression of FABP4 in the LV of tumor-bearing mice, indicating the presence of adipocyte progenitor cells. PPAR-α has also been shown to suppress the expression of adipocyte differentiation factors; its decrease in the LV of tumor-bearing mice may be contributing to the increased FAPB4 expression. An increase in FABP4 may impact the expression and activity of PPAR-γ, which would affect the ATP processing ability in the hearts of tumor-bearing mice. Further work is required to understand how adipocyte deposition and changes to the PPAR family of receptors are affecting the hearts of tumor-bearing mice, but it appears that HIF-1α may play a prominent role in these changes.
Because of the decreased oxygen concentrations in the arterial blood of tumor-bearing mice, we determined whether HIF-1α was also increased in skeletal muscle. Our findings show that HIF-1α is significantly increased in the EDL, soleus, and diaphragm. This indicates that there might be a broad-based hypoxic response in skeletal muscle. The role of HIF-1α in skeletal muscle is not as well understood compared with its role in the myocardium. Most of the available knowledge is based on endurance and exercise training studies, in which transient increases in HIF-1α promote increased oxidative capacity, angiogenesis, and muscle repair (1, 17). However, HIF-1α expression quickly returns to normal after the induction of repair and regeneration. Chronic cardiac hypoxia is associated with decreased mitochondrial capacity and content (16). In the C26 model, skeletal muscle has distorted mitochondria, although no studies have been reported on the oxidative capacity of these mitochondria (37). The diaphragm in mice is primarily composed of fast fibers and also shows increased expression of HIF-1α, similar to the EDL and soleus. This represents a paradox in which the diaphragm would be needed to increase respiration to restore oxygen levels to normal. It was thought that the diaphragm might have protective mechanisms to maintain function during hypoxia. However, a study involving exposure of mice to a hypoxic environment showed that the diaphragm has reduced mitochondrial capacity and integrity (10). The most common cause of death of cachectic patients is respiratory failure. Muscle weakness is most likely the primary cause, and HIF-1α may contribute to this through the suppression and loss of mitochondria (44).
A limitation of our study is that we presently have no mechanism to explain the decrease in oxygen pressure and concentration associated with tumor burden. Hypoxia and HIF-1α have been extensively studied in relation to the tumor and its hypoxic core, but there is little information on hypoxia experienced by patients with cancer. One possible explanation is that there was no change in Pco2 in tumor-bearing mice compared with control mice. Despite the reliance on oxygen, the body regulates its respiration rate through the sensing of CO2 (29). Without a change in Pco2, there may be no change in respiration, advancing the hypoxic phenotype. We also found significantly increased calcium, , and base excess in the arterial blood of tumor-bearing mice. The increase in calcium has been associated with increased hypoxia as a result of calcium moving from the intracellular to the extracellular space (2). The increase in and base excess could indicate metabolic alkalosis, which is normally compensated by hypoventilation. The respiratory ability in patients with cancer may be compromised. Renal compensation, while less efficient, is another method to restore normal bicarbonate levels. With increased calcium, as well as and base excess, it seems evident that the kidneys are no longer adequately filtering the blood.
Our findings demonstrate a novel pathway in the setting of cancer-induced cachexia and reveal the possibility of new mechanisms as well as therapeutic targets. We show that HIF-1α is increased in several striated muscles and is likely the result of a systemic hypoxic phenotype in the C26 model. These findings also implicate other, possibly physiologically relevant systems, such as the respiratory and renal systems. The heart was thought to be spared from the wasting experienced during cancer cachexia, a thought that has recently been refuted by multiple models as well as by human studies (39, 41, 42, 52). Cancer cachexia could be causing unknown effects in tissue types, which, if explored, could provide new mechanisms and therapeutic treatments for a wide range of cachexic patients. Our findings show that there could be systemic effects attributable to hypoxia and provide new mechanistic/therapeutic insight.
GRANTS
This work was supported by National Institute of Nursing Research Grant R01-NR-012618 (to L. Wold and P. Reiser).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.D., S.B., P.J.R., and L.E.W. performed experiments; R.D., S.B., P.J.R., and L.E.W. analyzed data; R.D., S.B., P.J.R., and L.E.W. interpreted results of experiments; R.D., S.B., P.J.R., and L.E.W. prepared figures; R.D., P.J.R., and L.E.W. drafted manuscript; R.D., S.B., P.J.R., and L.E.W. edited and revised manuscript; R.D., S.B., P.J.R., and L.E.W. approved final version of manuscript.
REFERENCES
- 1.Ameln H, Gustafsson T, Sundberg CJ, Okamoto K, Jansson E, Poellinger L, Makino Y. Physiological activation of hypoxia inducible factor-1 in human skeletal muscle. FASEB J 19: 1009–1011, 2005. doi: 10.1096/fj.04-2304fje. [DOI] [PubMed] [Google Scholar]
- 2.Arnould T, Michiels C, Alexandre I, Remacle J. Effect of hypoxia upon intracellular calcium concentration of human endothelial cells. J Cell Physiol 152: 215–221, 1992. doi: 10.1002/jcp.1041520127. [DOI] [PubMed] [Google Scholar]
- 3.Cao Y. Angiogenesis modulates adipogenesis and obesity. J Clin Invest 117: 2362–2368, 2007. doi: 10.1172/JCI32239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chabowski A, Górski J, Calles-Escandon J, Tandon NN, Bonen A. Hypoxia-induced fatty acid transporter translocation increases fatty acid transport and contributes to lipid accumulation in the heart. FEBS Lett 580: 3617–3623, 2006. doi: 10.1016/j.febslet.2006.05.045. [DOI] [PubMed] [Google Scholar]
- 5.Devine RD, Bicer S, Reiser PJ, Velten M, Wold LE. Metalloproteinase expression is altered in cardiac and skeletal muscle in cancer cachexia. Am J Physiol Heart Circ Physiol 309: H685–H691, 2015. doi: 10.1152/ajpheart.00106.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Devine RD, Eichenseer CM, Wold LE. Minocycline attenuates cardiac dysfunction in tumor-burdened mice. J Mol Cell Cardiol 100: 35–42, 2016. doi: 10.1016/j.yjmcc.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fong GH, Takeda K. Role and regulation of prolyl hydroxylase domain proteins. Cell Death Differ 15: 635–641, 2008. doi: 10.1038/cdd.2008.10. [DOI] [PubMed] [Google Scholar]
- 9.Fukawa T, Yan-Jiang BC, Min-Wen JC, Jun-Hao ET, Huang D, Qian CN, Ong P, Li Z, Chen S, Mak SY, Lim WJ, Kanayama HO, Mohan RE, Wang RR, Lai JH, Chua C, Ong HS, Tan KK, Ho YS, Tan IB, Teh BT, Shyh-Chang N. Excessive fatty acid oxidation induces muscle atrophy in cancer cachexia. Nat Med 22: 666–671, 2016. doi: 10.1038/nm.4093. [DOI] [PubMed] [Google Scholar]
- 10.Gamboa JL, Andrade FH. Mitochondrial content and distribution changes specific to mouse diaphragm after chronic normobaric hypoxia. Am J Physiol Regul Integr Comp Physiol 298: R575–R583, 2010. doi: 10.1152/ajpregu.00320.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest 115: 500–508, 2005. doi: 10.1172/JCI200524408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Görlach A, Diebold I, Schini-Kerth VB, Berchner-Pfannschmidt U, Roth U, Brandes RP, Kietzmann T, Busse R. Thrombin activates the hypoxia-inducible factor-1 signaling pathway in vascular smooth muscle cells: role of the p22phox-containing NADPH oxidase. Circ Res 89: 47–54, 2001. doi: 10.1161/hh1301.092678. [DOI] [PubMed] [Google Scholar]
- 13.Granda-Cameron C, DeMille D, Lynch MP, Huntzinger C, Alcorn T, Levicoff J, Roop C, Mintzer D. An interdisciplinary approach to manage cancer cachexia. Clin J Oncol Nurs 14: 72–80, 2010. doi: 10.1188/10.CJON.72-80. [DOI] [PubMed] [Google Scholar]
- 14.Hayat MJ, Howlader N, Reichman ME, Edwards BK. Cancer statistics, trends, and multiple primary cancer analyses from the Surveillance, Epidemiology, and End Results (SEER) Program. Oncologist 12: 20–37, 2007. doi: 10.1634/theoncologist.12-1-20. [DOI] [PubMed] [Google Scholar]
- 15.He Q, Gao Z, Yin J, Zhang J, Yun Z, Ye J. Regulation of HIF-1α activity in adipose tissue by obesity-associated factors: adipogenesis, insulin, and hypoxia. Am J Physiol Endocrinol Metab 300: E877–E885, 2011. doi: 10.1152/ajpendo.00626.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoppeler H, Kleinert E, Schlegel C, Claassen H, Howald H, Kayar SR, Cerretelli P. Morphological adaptations of human skeletal muscle to chronic hypoxia. Int J Sports Med 11, Suppl 1: S3–S9, 1990. doi: 10.1055/s-2007-1024846. [DOI] [PubMed] [Google Scholar]
- 17.Hoppeler H, Vogt M, Weibel ER, Flück M. Response of skeletal muscle mitochondria to hypoxia. Exp Physiol 88: 109–119, 2003. doi: 10.1113/eph8802513. [DOI] [PubMed] [Google Scholar]
- 18.Huang LE, Gu J, Schau M, Bunn HF. Regulation of hypoxia-inducible factor 1alpha is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc Natl Acad Sci USA 95: 7987–7992, 1998. doi: 10.1073/pnas.95.14.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev 19: 1893–1907, 2010. doi: 10.1158/1055-9965.EPI-10-0437. [DOI] [PubMed] [Google Scholar]
- 20.Kido M, Du L, Sullivan CC, Li X, Deutsch R, Jamieson SW, Thistlethwaite PA. Hypoxia-inducible factor 1-alpha reduces infarction and attenuates progression of cardiac dysfunction after myocardial infarction in the mouse. J Am Coll Cardiol 46: 2116–2124, 2005. doi: 10.1016/j.jacc.2005.08.045. [DOI] [PubMed] [Google Scholar]
- 21.Kumar S, Mehta K. Tissue transglutaminase constitutively activates HIF-1α promoter and nuclear factor-κB via a non-canonical pathway. PLoS One 7: e49321, 2012. doi: 10.1371/journal.pone.0049321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Litz J, Krystal GW. Imatinib inhibits c-Kit-induced hypoxia-inducible factor-1alpha activity and vascular endothelial growth factor expression in small cell lung cancer cells. Mol Cancer Ther 5: 1415–1422, 2006. doi: 10.1158/1535-7163.MCT-05-0503. [DOI] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔCT) method. Methods 25: 402–408, 2001. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Magenta A, Avitabile D, Pompilio G, Capogrossi MC. c-kit-Positive cardiac progenitor cells: the heart of stemness. Circ Res 112: 1202–1204, 2013. doi: 10.1161/CIRCRESAHA.113.301317. [DOI] [PubMed] [Google Scholar]
- 25.Martin-Vaquero P, da Costa RC, Allen MJ, Moore SA, Keirsey JK, Green KB. Proteomic analysis of cerebrospinal fluid in canine cervical spondylomyelopathy. Spine 40: 601–612, 2015. doi: 10.1097/BRS.0000000000000831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mehlem A, Hagberg CE, Muhl L, Eriksson U, Falkevall A. Imaging of neutral lipids by oil red O for analyzing the metabolic status in health and disease. Nat Protoc 8: 1149–1154, 2013. doi: 10.1038/nprot.2013.055. [DOI] [PubMed] [Google Scholar]
- 27.Nagai J, Yamamoto A, Yumoto R, Takano M. Albumin overload induces expression of hypoxia-inducible factor 1α and its target genes in HK-2 human renal proximal tubular cell line. Biochem Biophys Res Commun 434: 670–675, 2013. doi: 10.1016/j.bbrc.2013.03.140. [DOI] [PubMed] [Google Scholar]
- 28.Narravula S, Colgan SP. Hypoxia-inducible factor 1-mediated inhibition of peroxisome proliferator-activated receptor alpha expression during hypoxia. J Immunol 166: 7543–7548, 2001. doi: 10.4049/jimmunol.166.12.7543. [DOI] [PubMed] [Google Scholar]
- 29.Nattie E. CO2, brainstem chemoreceptors and breathing. Prog Neurobiol 59: 299–331, 1999. doi: 10.1016/S0301-0082(99)00008-8. [DOI] [PubMed] [Google Scholar]
- 30.Nielsen LB, Perko M, Arendrup H, Andersen CB. Microsomal triglyceride transfer protein gene expression and triglyceride accumulation in hypoxic human hearts. Arterioscler Thromb Vasc Biol 22: 1489–1494, 2002. doi: 10.1161/01.ATV.0000030199.06252.26. [DOI] [PubMed] [Google Scholar]
- 31.Ostadal B, Kolar F. Cardiac adaptation to chronic high-altitude hypoxia: beneficial and adverse effects. Respir Physiol Neurobiol 158: 224–236, 2007. doi: 10.1016/j.resp.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 32.Pantanowitz L. Fat infiltration in the heart. Heart 85: 253, 2001. doi: 10.1136/heart.85.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peyssonnaux C, Nizet V, Johnson RS. Role of the hypoxia inducible factors HIF in iron metabolism. Cell Cycle 7: 28–32, 2008. doi: 10.4161/cc.7.1.5145. [DOI] [PubMed] [Google Scholar]
- 34.Sanada F, Kim J, Czarna A, Chan NY, Signore S, Ogórek B, Isobe K, Wybieralska E, Borghetti G, Pesapane A, Sorrentino A, Mangano E, Cappetta D, Mangiaracina C, Ricciardi M, Cimini M, Ifedigbo E, Perrella MA, Goichberg P, Choi AM, Kajstura J, Hosoda T, Rota M, Anversa P, Leri A. c-Kit-positive cardiac stem cells nested in hypoxic niches are activated by stem cell factor reversing the aging myopathy. Circ Res 114: 41–55, 2014. doi: 10.1161/CIRCRESAHA.114.302500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene 29: 625–634, 2010. doi: 10.1038/onc.2009.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Semenza GL. Hypoxia-inducible factor 1: regulator of mitochondrial metabolism and mediator of ischemic preconditioning. Biochim Biophys Acta 1813: 1263–1268, 2011. doi: 10.1016/j.bbamcr.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shum AM, Mahendradatta T, Taylor RJ, Painter AB, Moore MM, Tsoli M, Tan TC, Clarke SJ, Robertson GR, Polly P. Disruption of MEF2C signaling and loss of sarcomeric and mitochondrial integrity in cancer-induced skeletal muscle wasting. Aging (Albany NY) 4: 133–143, 2012. doi: 10.18632/aging.100436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 65: 5–29, 2015. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 39.Springer J, Tschirner A, Haghikia A, von Haehling S, Lal H, Grzesiak A, Kaschina E, Palus S, Pötsch M, von Websky K, Hocher B, Latouche C, Jaisser F, Morawietz L, Coats AJ, Beadle J, Argiles JM, Thum T, Földes G, Doehner W, Hilfiker-Kleiner D, Force T, Anker SD. Prevention of liver cancer cachexia-induced cardiac wasting and heart failure. Eur Heart J 35: 932–941, 2014. doi: 10.1093/eurheartj/eht302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stevens SC, Velten M, Youtz DJ, Clark Y, Jing R, Reiser PJ, Bicer S, Devine RD, McCarthy DO, Wold LE. Losartan treatment attenuates tumor-induced myocardial dysfunction. J Mol Cell Cardiol 85: 37–47, 2015. doi: 10.1016/j.yjmcc.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tian M, Asp ML, Nishijima Y, Belury MA. Evidence for cardiac atrophic remodeling in cancer-induced cachexia in mice. Int J Oncol 39: 1321–1326, 2011. doi: 10.3892/ijo.2011.1150. [DOI] [PubMed] [Google Scholar]
- 42.Tian M, Nishijima Y, Asp ML, Stout MB, Reiser PJ, Belury MA. Cardiac alterations in cancer-induced cachexia in mice. Int J Oncol 37: 347–353, 2010. [DOI] [PubMed] [Google Scholar]
- 43.Tisdale MJ. Cachexia in cancer patients. Nat Rev Cancer 2: 862–871, 2002. doi: 10.1038/nrc927. [DOI] [PubMed] [Google Scholar]
- 44.Tisdale MJ. Cancer cachexia. Curr Opin Gastroenterol 26: 146–151, 2010. doi: 10.1097/MOG.0b013e3283347e77. [DOI] [PubMed] [Google Scholar]
- 45.Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev 89: 381–410, 2009. doi: 10.1152/physrev.00016.2008. [DOI] [PubMed] [Google Scholar]
- 46.Tisdale MJ. Molecular pathways leading to cancer cachexia. Physiology (Bethesda) 20: 340–348, 2005. doi: 10.1152/physiol.00019.2005. [DOI] [PubMed] [Google Scholar]
- 47.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 65: 87–108, 2015. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 48.Vaughan VC, Martin P, Lewandowski PA. Cancer cachexia: impact, mechanisms and emerging treatments. J Cachexia Sarcopenia Muscle 4: 95–109, 2013. doi: 10.1007/s13539-012-0087-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Velten M, Gorr MW, Youtz DJ, Velten C, Rogers LK, Wold LE. Adverse perinatal environment contributes to altered cardiac development and function. Am J Physiol Heart Circ Physiol 306: H1334–H1340, 2014. doi: 10.1152/ajpheart.00056.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang GL, Semenza GL. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc Natl Acad Sci USA 90: 4304–4308, 1993. doi: 10.1073/pnas.90.9.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xie Y, Zhu WZ, Zhu Y, Chen L, Zhou ZN, Yang HT. Intermittent high altitude hypoxia protects the heart against lethal Ca2+ overload injury. Life Sci 76: 559–572, 2004. doi: 10.1016/j.lfs.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 52.Xu H, Crawford D, Hutchinson KR, Youtz DJ, Lucchesi PA, Velten M, McCarthy DO, Wold LE. Myocardial dysfunction in an animal model of cancer cachexia. Life Sci 88: 406–410, 2011. doi: 10.1016/j.lfs.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL, Simons JW. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res 59: 5830–5835, 1999. [PubMed] [Google Scholar]