Coadministraton of haptoglobin but not hemopexin with cell-free hemoglobin prevents hemoglobin-induced systemic hypertension in mice with a normal endothelium. In contrast, treatment with the same amount of haptoglobin is unable to prevent hemoglobin-induced vasoconstriction in mice with hyperlipidemia or diabetes mellitus, disorders that are associated with endothelial dysfunction.
Keywords: cell-free hemoglobin, haptoglobin, hemopexin, nitric oxide, endothelial dysfunction
Abstract
Intravascular hemolysis produces injury in a variety of human diseases including hemoglobinopathies, malaria, and sepsis. The adverse effects of increased plasma hemoglobin are partly mediated by depletion of nitric oxide (NO) and result in vasoconstriction. Circulating plasma proteins haptoglobin and hemopexin scavenge extracellular hemoglobin and cell-free heme, respectively. The ability of human haptoglobin or hemopexin to inhibit the adverse effects of NO scavenging by circulating murine hemoglobin was tested in C57Bl/6 mice. In healthy awake mice, the systemic hemodynamic effects of intravenous coinfusion of cell-free hemoglobin and exogenous haptoglobin or of cell-free hemoglobin and hemopexin were compared with the hemodynamic effects of infusion of cell-free hemoglobin or control protein (albumin) alone. We also studied the hemodynamic effects of infusing hemoglobin and haptoglobin as well as injecting either hemoglobin or albumin alone in mice fed a high-fat diet (HFD) and in diabetic (db/db) mice. Coinfusion of a 1:1 weight ratio of haptoglobin but not hemopexin with cell-free hemoglobin prevented hemoglobin-induced systemic hypertension in healthy awake mice. In mice fed a HFD and in diabetic mice, coinfusion of haptoglobin mixed with an equal mass of cell-free hemoglobin did not reverse hemoglobin-induced hypertension. Haptoglobin retained cell-free hemoglobin in plasma, but neither haptoglobin nor hemopexin affected the ability of hemoglobin to scavenge NO ex vivo. In conclusion, in healthy C57Bl/6 mice with normal endothelium, coadministration of haptoglobin but not hemopexin with cell-free hemoglobin prevents acute hemoglobin-induced systemic hypertension by compartmentalizing cell-free hemoglobin in plasma. In murine diseases associated with endothelial dysfunction, haptoglobin therapy appears to be insufficient to prevent hemoglobin-induced vasoconstriction.
NEW & NOTEWORTHY Coadministraton of haptoglobin but not hemopexin with cell-free hemoglobin prevents hemoglobin-induced systemic hypertension in mice with a normal endothelium. In contrast, treatment with the same amount of haptoglobin is unable to prevent hemoglobin-induced vasoconstriction in mice with hyperlipidemia or diabetes mellitus, disorders that are associated with endothelial dysfunction.
intravascular hemolysis contributes to the pathophysiology of a variety of human diseases including hemoglobinopathies, malaria, and sepsis and triggers adverse effects after cardiopulmonary bypass and after transfusion of packed red blood cells that have been stored for prolonged intervals (19). Some of the adverse effects caused by cell-free hemoglobin are a result of depletion of nitric oxide (NO), which may lead to vasoconstriction, inflammation, and platelet activation (10, 24).
Haptoglobin and hemopexin are plasma proteins that bind to plasma hemoglobin and its breakdown product heme, respectively. Haptoglobin binds to hemoglobin dimers and forms a high-molecular-weight haptoglobin-hemoglobin complex, which is cleared from the circulation after binding to the CD163 receptor on hepatic and splenic macrophages (12). By binding cell-free hemoglobin, haptoglobin prevents glomerular filtration of cell-free hemoglobin and subsequent kidney injury (2, 7). Hemopexin binds to cell-free heme and facilitates uptake of heme into liver and spleen by the LDL receptor-related protein (14).
Because cell-free hemoglobin is a potent scavenger of endothelial NO, intravenous administration of tetrameric hemoglobin causes vasoconstriction in a variety of species including mice, rats, guinea pigs, and humans (5, 6, 11, 24). Coadministration of haptoglobin with cell-free hemoglobin attenuates hemoglobin-induced hypertension in guinea pigs and rats (2, 6, 11). Schaer and colleagues showed that administration of exogenous haptoglobin prevents extravasation of cell-free hemoglobin and vasoconstriction in rats (18). Similarly, we previously reported that high-molecular-weight hemoglobin-based oxygen carriers (HBOCs) do not cause systemic hypertension in healthy awake mice. The extensive cross-linkage of cell-free hemoglobin in a high-molecular-weight HBOC retains cell-free hemoglobin dimers in the intravascular compartment, thereby preventing extravasation of cell-free hemoglobin into the muscular layer of the arteries where local NO consumption can trigger vasoconstriction (25). Mice with diabetes mellitus or hyperlipidemia have evidence of endothelial dysfunction, which is associated with reduced NO synthase (NOS)3 activity (15, 16). Compared with wild-type (WT) mice, mice with endothelial dysfunction have an increased vasoconstrictor response to infusion of cell-free hemoglobin (25). Furthermore, mice with endothelial dysfunction are more sensitive to the NO scavenging effects of HBOCs. An infusion of high-molecular-weight HBOCs caused hypertension in diabetic mice or mice fed a high-fat diet (HFD) but not in mice fed a normal diet (25).
The current study had three objectives: first, to investigate whether the systemic hypertension associated with injection of free tetrameric hemoglobin into mice can be prevented by coinfusion of human haptoglobin; second, to investigate the hemodynamic effects of coinfusion of hemopexin during injection of murine tetrameric hemoglobin; and third, to determine whether infusion of human haptoglobin attenuates cell-free hemoglobin-induced systemic hypertension in murine models of endothelial dysfunction, including mice with hyperlipidemia or diabetes mellitus.
METHODS
Preparation of murine tetrameric hemoglobin solution and hemoglobin scavenger proteins.
The Institutional Animal Care and Use Committee of Massachusetts General Hospital (Boston, MA) approved these murine studies. Whole blood was obtained from WT mice as previously described and collected into a syringe containing heparin (24). After dilution with PBS in a 1:2 ratio, blood was frozen at −80°C and thawed at room temperature three times followed by centrifugation at 21,600 g for 1.5 h at 4°C. The supernatant was collected and passed through a 0.22-μm filter (Nalgene, Rochester, NY). The solution was dialyzed against 0.9% saline overnight at 4°C. After dialysis, the solution was concentrated using an Amicon Ultra-0.5 Centrifugal filter (Fisher Scientific, Suwanee, GA). Hemopexin and haptoglobin were obtained from CSL Behring (Bern, Switzerland). Both proteins were purified from a Cohn Fraction IV precipitate. Details of the preparation are described in United States Patent No. 9,534,029 B2. The haptoglobin preparation consisted of a mixture of Hp 2-1 and 2-2 with an approximate mean molecular mass of 360 kDa. Albumin was purchased from Kedrion Biopharma (Fort Lee, NJ).
Biochemical assays.
Plasma and urine samples were obtained from mice 1 h after infusion of proteins by cardiac puncture and bladder puncture, respectively. Plasma and urine hemoglobin concentrations were measured using a Hb assay kit (QuantiChrom, BioAssaySystems, Hayward, CA).
NO consumption assay.
Plasma NO consumption was measured as previously reported (23). In an air-tight oxygen-free reaction chamber, NO was produced by DETA-NONOate dissolved in PBS (pH 7.4). NO was continuously measured with a Sievers chemiluminescence analyzer (Sievers 280i, Boulder, CO). NO depletion was measured after injection of murine tetrameric hemoglobin, hemoglobin mixed with haptoglobin, and hemoglobin mixed with hemopexin in a 1:1 and 1:10 weight ratio.
Measurement of systolic blood pressure in awake mice.
Systolic blood pressure (SBP) was measured with a CODA noninvasive blood pressure system (Kent Scientific, Torrington, CT) in awake male C57Bl/6J WT mice, mice fed a HFD, and B6.Cg-m+/+Leprdb/J (on the C57Bl/6J background) diabetic (db/db) mice. To acclimate to the restrainer and blood pressure measurements, mice were trained as previously described (24). Murine tetrameric hemoglobin was administered over 1 min via tail vein injection. Intravenous infusion of murine tetrameric hemoglobin at 0.24 g/kg body wt consistently and reproducibly caused hypertension (24, 25).
Four groups of WT mice (n = 8 each) were studied. The first group received 0.24 g/kg albumin as control for nonspecific effects of the protein infusion. A second group received an infusion of 0.24 g/kg murine tetrameric hemoglobin. A third group received a coinfusion of 0.24 g/kg murine tetrameric hemoglobin and 0.24 g/kg human haptoglobin (1:1 weight ratio). A fourth group received 0.24 g/kg murine tetrameric hemoglobin mixed with 0.24 g/kg human hemopexin (1:1 weight ratio).
Mice in the HFD-fed group were fed a diet that consisted of 60% of calories from fat (Research Diets, New Brunswick, NJ) for 4–6 wk. Three groups of HFD-fed mice (n = 8 each) and db/db mice (n = 6 each) were studied. The first groups of HFD-fed and db/db mice received an infusion of albumin. The second cohorts of HFD-fed and db/db mice received an infusion of murine tetrameric hemoglobin. The third groups received murine tetrameric hemoglobin mixed with human haptoglobin in a 1:1 weight ratio.
Statistical analysis.
Data are presented as means ± SE. The Shapiro-Wilk test was used to assess normal distribution of measured variables. Continuous variables of two independent groups were compared using Student’s t-test with Welch’s correction or Mann-Whitney U-test. Statistical comparison between more than two groups was assessed by one-way ANOVA or a Kruskal-Wallis test followed by post hoc pairwise comparisons adjusted with a Bonferroni correction for multiple testing. Hemodynamic data were analyzed using two-way ANOVA with repeated measures. Correlations between plasma hemoglobin levels and plasma NO consumption were tested using Pearson’s correlation coefficient. Statistical analyses were performed using STATA-12 (StataCorp LP, College Station, TX) and GraphPad Prism software (GraphPad Software, La Jolla, CA).
RESULTS
Effects of haptoglobin or hemopexin on hemoglobin-induced hypertension in vivo.
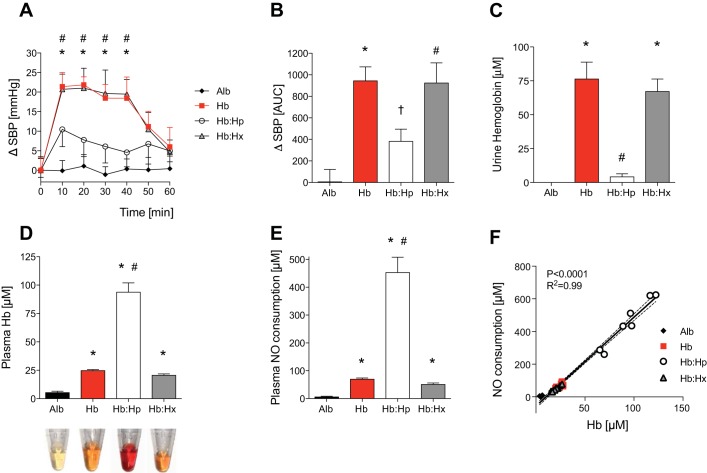
Intravenous infusion of cell-free hemoglobin induces hypertension in mice (24). The following studies were performed to consider the possibility that coinfusion of human haptoglobin or hemopexin with cell-free hemoglobin might prevent hemoglobin-induced hypertension. To establish the baseline effects of free hemoglobin on systemic blood pressure in mice, blood pressure was measured in awake mice during the 40 min after the infusion of murine tetrameric hemoglobin (0.24 mg/kg). Compared with baseline blood pressure, infusion of free hemoglobin was associated with an average increase in SBP of 20 ± 2 mmHg. Intravenous injection of albumin (0.24 mg/kg) did not affect the SBP of awake mice (Fig. 1A). Coadministration of haptoglobin attenuated the hemoglobin-induced increase in SBP during the 40 min after coinjection by an average of 13 ± 3 mmHg (Fig. 1A). The area under the curve (AUC) for the increase in SBP (ΔSBP) over time was significantly higher in mice injected with hemoglobin than in mice injected with albumin (AUCHb = 934 ± 130 vs. AUCAlb = 5 ± 115, P < 0.001; Fig. 1B). The AUC for ΔSBP was significantly lower in mice infused with hemoglobin together with haptoglobin than in mice infused with hemoglobin alone (AUCHb:Hp = 381 ± 114 vs. AUCHb = 943 ± 130, P < 0.01; Fig. 1B). In contrast to the effect of haptoglobin, coadministration of hemopexin did not attenuate the average hemoglobin-induced increase in SBP during the 40 min after coinjection (Fig. 1A). SBP increased on average 20 ± 2 mmHg in mice injected with hemoglobin mixed with hemopexin and therefore was similar to the increase after hemoglobin injection alone. In addition, the AUC for ΔSBP over time in mice infused with hemoglobin mixed with hemopexin was the same as that for hemoglobin alone [AUCHb:Hx = 923 ± 187 vs. AUCHb = 943 ± 130, P = not significant (NS)] and significantly higher compared with the AUC for ΔSBP in mice injected with albumin (AUCHb:Hx = 923 ± 187 vs. AUCAlb = 5 ± 115, P < 0.01; Fig. 1B). These results suggest that coinjection of haptoglobin but not hemopexin with cell-free hemoglobin can prevent hemoglobin-induced hypertension in awake, healthy mice.
Fig. 1.
Effects of haptoglobin or hemopexin on the free hemoglobin-induced increase of systemic blood pressure in awake mice. Infusion of cell-free hemoglobin (Hb) but not albumin (Alb) increased systolic blood pressure (SBP) in awake mice (n = 8 mice/group). Haptoglobin (Hp) but not hemopexin (Hx) attenuated the Hb-induced increase in SBP. The increase (Δ) in SBP in mmHg (A) and results presented as the area under the curve (AUC) (B) are shown. *P < 0.05, Hb differed vs. Alb; #P < 0.05, Hb:Hx differed vs. Alb; †P < 0.05, Hb:Hp differed vs. Hb. Urine Hb concentration was increased in urine of wild-type mice collected at 1 h after infusion of free Hb or free Hb and Hx but not after infusion of free Hb and Hp (C). Infusion of Hb was associated with an increase in plasma Hb measured at 1 h after infusion. Plasma Hb was markedly increased in animals that received coinfusion of Hp (D). Representative plasma samples obtained at 1 h after injection shown at the bottom. There was a correlation between plasma nitric oxide (NO) consumption and plasma Hb levels at 1 h after injection (E and F). Numbers of mice (n) in each treatment group were as follows: Alb (n = 4), Hb (n = 11), Hb:Hp (n = 7), and Hb:Hx (n = 9). *P < 0.05 vs. Alb, #P < 0.05 vs. Hb. Data represent means ± SE.
Effect of haptoglobin and hemopexin on renal hemoglobin clearance, plasma hemoglobin levels, and ex vivo NO consumption.
Plasma hemoglobin is primarily removed from the circulation by renal filtration. Haptoglobin binds to cell-free hemoglobin and thereby prevents glomerular filtration (6). To confirm that human haptoglobin can bind to infused murine tetrameric hemoglobin, urine hemoglobin concentration was measured in mice coinjected with hemoglobin and haptoglobin and in mice infused with murine tetrameric hemoglobin alone (0.24 mg/kg). At 1 h after injection, hemoglobinuria was detected in mice infused with cell-free hemoglobin but not in mice infused with cell-free hemoglobin mixed with haptoglobin (Fig. 1C). Hemoglobinuria was also detected in mice infused with cell-free hemoglobin mixed with hemopexin. At 1 h after injection, plasma hemoglobin concentrations were 3.8 ± 0.4-fold higher in mice infused with cell-free hemoglobin and haptoglobin compared with mice infused with cell-free hemoglobin alone (Fig. 1D). These results show that, in contrast to haptoglobin, hemopexin was unable to prevent free hemoglobin-induced hemoglobinuria. In addition, compared with mice treated with cell-free hemoglobin alone, infusion of haptoglobin together with cell-free hemoglobin increased the plasma hemoglobin level at 1 h after injection.
To test whether infusion of human haptoglobin or hemopexin affects the ability of murine cell-free hemoglobin to convert endothelium-derived NO into nonvasodilatory nitrate, plasma NO consumption was measured ex vivo as a surrogate for the NO scavenging capacity of the plasma (23). Plasma NO consumption increased in all mice infused with cell-free hemoglobin at 60 min after injection compared with mice infused with albumin alone (Fig. 1E). Mice that received cell-free hemoglobin together with haptoglobin had significantly higher plasma NO consumption than mice that received cell-free hemoglobin alone at 60 min after injection. Plasma NO consumption at 60 min after injection correlated with plasma hemoglobin concentrations in all mice infused with albumin, hemoglobin, hemoglobin mixed with haptoglobin, and hemoglobin mixed with hemopexin (Fig. 1F).
Taken together, these results suggest that infusion of cell-free hemoglobin mixed with an equal mass of haptoglobin is sufficient to bind cell-free hemoglobin and prevent renal clearance of extracellular hemoglobin. Furthermore, haptoglobin retained cell-free hemoglobin in plasma and led to an increased capacity of the plasma to scavenge NO at 60 min after injection.
Effect of haptoglobin and hemopexin on NO dioxygenation in vitro.
In vitro studies and the NO consumption assay were used to further examine whether human haptoglobin or hemopexin affects the ability of murine cell-free hemoglobin to scavenge NO (23). Cell-free hemoglobin was mixed with human haptoglobin or hemopexin in a 1:1 and 1:10 weight ratio. Neither haptoglobin nor hemopexin reduced NO consumption of cell-free hemoglobin in vitro (Fig. 2). These data suggest that coadministration of human haptoglobin or hemopexin does not attenuate the conversion of NO to nitrate that is mediated by cell-free murine hemoglobin.
Fig. 2.
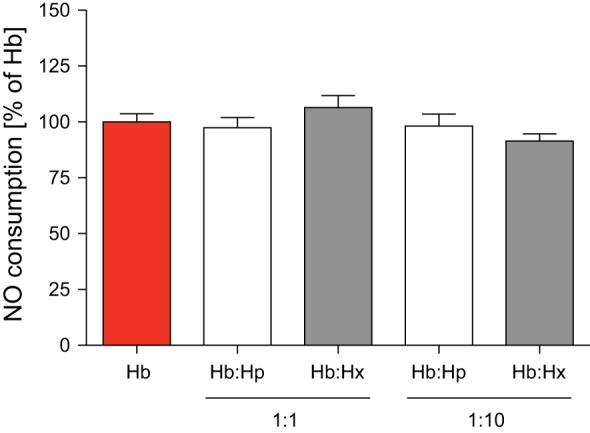
The amount of NO consumption by plasma after the addition of free Hb was not affected by coinfusion of either Hp or Hx. A 10-fold excess (weight ratio) of Hp or Hx also did not affect NO consumption by free Hb. NO consumption by Hb was set at 100%. Data represent means ± SE.
Effect of haptoglobin on hemoglobin-induced hypertension in mice with endothelial dysfunction.
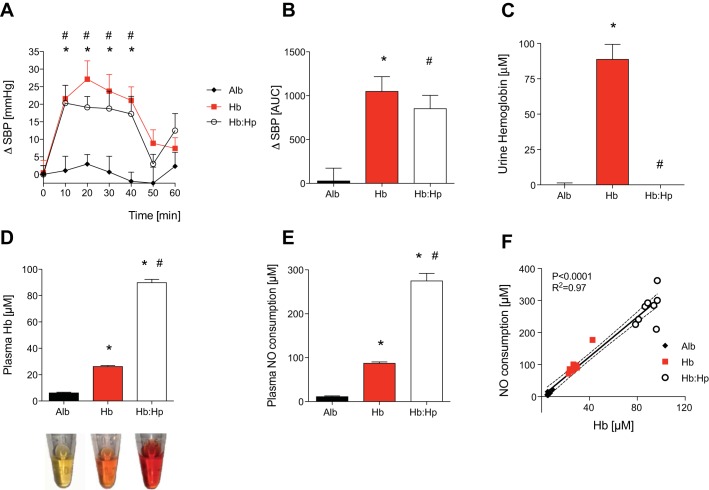
Endothelial dysfunction, a condition associated with reduced bioavailability of vascular NO, occurs in humans and mice with hyperlipidemia (16). To test whether infusion of haptoglobin can prevent hemoglobin-induced hypertension in mice with endothelial dysfunction, mice fed a HFD for 4–6 wk received an infusion of murine tetrameric hemoglobin alone, a coinfusion of hemoglobin and haptoglobin, or an infusion of albumin alone. During the 40 min after the infusion of murine tetrameric hemoglobin alone, SBP in mice fed a HFD increased on average by 23 ± 3 mmHg compared with baseline SBP measurements (Fig. 3A). In the same period, the increase in SBP after infusion of murine tetrameric hemoglobin mixed with haptoglobin was similar to the increase after infusion of murine tetrameric hemoglobin alone (ΔSBPHb = 23 ± 3 mmHg vs. ΔSBPHb:Hp = 19 ± 3 mmHg, P = NS). In mice with endothelial dysfunction that were infused with albumin, SBP did not increase compared with baseline (Fig. 3A). The AUC for ΔSBP over time was similar between HFD mice infused with cell-free hemoglobin and HFD mice infused with cell-free hemoglobin mixed with haptoglobin (AUCHb = 1048 ± 168 vs. AUCHb:Hp = 852 ± 150, P = NS; Fig. 3B).
Fig. 3.
Coinfusion of Hp mixed with an equal mass of cell-free Hb did not attenuate the Hb-induced increase in SBP in mice with endothelial dysfunction caused by a high-fat diet (HFD). ΔSBP in mmHg (A) and as the AUC (B) was measured in awake wild-type mice maintained on a HFD (n = 8 mice/group) after the infusion of Alb, murine tetrameric Hb, and Hb mixed with Hp (Hb:Hp). Each protein was infused at a dose of 0.24 mg/kg. *P < 0.05, Hb differed vs. Alb; #P < 0.05, Hb:Hp differed vs. Alb. Infusion of Hb but not Hb and Hp increased urine Hb concentration in the urine of mice maintained on a HFD collected at 1 h after infusion (C). One hour after infusion, plasma Hb was increased in mice infused with free Hb and was markedly increased in mice infused with both Hb and Hp (D). Representative plasma samples obtained at 1 h after injection are shown at the bottom. Plasma NO consumption at 1 h after injection correlated with the level of plasma Hb (E and F). Numbers of mice in each treatment group were as follows: Alb (n = 8), Hb (n = 9), and Hb:Hp (n = 8). *P < 0.05 vs. Alb; #P < 0.05 vs. Hb. Data represent means ± SE.
Similar to our observations in mice that received a normal diet, haptoglobin prevented hemoglobinuria in HFD-fed mice and retained extracellular hemoglobin in plasma at 60 min after injection (Fig. 3, C and D). In mice fed a HFD, plasma NO consumption after coinfusion of cell-free hemoglobin and haptoglobin was 2.8-fold higher than after infusion of cell-free hemoglobin alone at 1 h after infusion (Fig. 3E). Plasma NO consumption at 60 min after injection correlated with plasma hemoglobin concentrations in all mice infused with albumin, hemoglobin, or hemoglobin mixed with haptoglobin (Fig. 3F).
These results suggest that contrary to the findings in mice that received a normal diet, in mice fed a HFD, haptoglobin mixed with an equal mass of cell-free hemoglobin is unable to prevent cell-free hemoglobin-induced hypertension.
Effect of haptoglobin on hemoglobin-induced hypertension in diabetic mice.
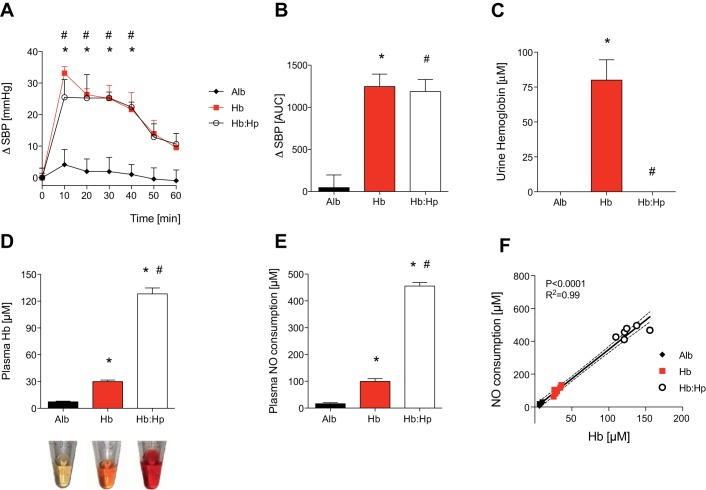
As with mice fed a HFD, diabetic db/db mice also have endothelial dysfunction (15). Previous studies have shown that db/db mice have enhanced susceptibility to hemoglobin-induced vasoconstriction (25). To investigate the effect of free hemoglobin on blood pressure in this second murine model of endothelial dysfunction, db/db mice were infused with murine tetrameric hemoglobin. SBP increased 26 ± 2 mmHg during the 40 min after the infusion of hemoglobin in db/db mice (Fig. 4A). A similar increase in SBP was observed in db/db mice that received a coinfusion of cell-free hemoglobin and haptoglobin. The AUC for ΔSBP over time did not differ between db/db mice infused with cell-free hemoglobin and db/db mice coinfused with cell-free hemoglobin and haptoglobin (AUCHb = 1247 ± 147 vs. AUCHb:Hp = 1186 ± 144, P = NS; Fig. 4B).
Fig. 4.
Coinfusion of Hp mixed with an equal mass of cell-free Hb did not attenuate the Hb-induced increase in SBP in mice with endothelial dysfunction caused by diabetes. ΔSBP in mmHg (A) and as the AUC (B) were measured in awake diabetic (db/db) mice (n = 6 mice/group) after the infusion of Alb, murine tetrameric Hb, and Hb mixed with Hp (Hb:Hp). Each protein was infused with a dose of 0.24 mg/kg. *P < 0.05, Hb differed vs. Alb; #P < 0.05, Hb:Hp differed vs. Alb. Infusion of Hb but not Hb and Hp increased the urine Hb concentration in db/db mice collected at 1 h after infusion (C). One hour after infusion, plasma Hb was increased in mice infused with free Hb and was markedly increased in mice infused with both Hb and Hp (D). Representative plasma samples obtained at 1 h after injection are shown at the bottom. Plasma NO consumption at 1 h after injection correlated with the level of plasma Hb (E and F). Numbers of mice in each treatment group were as follows: Alb (n = 5), Hb (n = 6), and Hb:Hp (n = 6). *P < 0.05 vs. Alb; #P < 0.05 vs. Hb. Data represent means ± SE.
Similar to results obtained in healthy WT mice, haptoglobin mixed in a 1:1 weight ratio with murine tetrameric hemoglobin prevented hemoglobinuria, retained extracellular hemoglobin in plasma at 60 min after injection, and did not interfere with the ability of plasma hemoglobin to scavenge NO in db/db mice (Fig. 4, C–F). Taken together, these results confirm that haptoglobin mixed with an equal mass of cell-free hemoglobin is unable to prevent cell-free hemoglobin-induced hypertension in mice with endothelial dysfunction.
DISCUSSION
In the current study, we tested whether intravenous administration of exogenous haptoglobin and hemopexin can attenuate acute systemic hypertension caused by infusion of cell-free hemoglobin in mice. Although administration of haptoglobin or hemopexin did not affect the NO scavenging ability of murine tetrameric hemoglobin, treatment with haptoglobin but not hemopexin prevented hemoglobin-induced hypertension in healthy awake mice. Furthermore, haptoglobin retained cell-free hemoglobin in plasma and prevented hemoglobinuria. In mice fed a HFD or in diabetic mice fed a normal diet, haptoglobin mixed with murine tetrameric hemoglobin in a 1:1 weight ratio also retained cell-free hemoglobin in plasma and prevented hemoglobinuria but did not reduce hemoglobin-induced hypertension. Therefore, in disease conditions that are associated with endothelial dysfunction, prevention of extra-vasation of cell-free hemoglobin by haptoglobin might be insufficient to prevent hemoglobin-induced vasoconstriction.
The scavenging of endothelium-derived NO causes the systemic hypertension associated with administration of cell-free ferrous oxyhemoglobin (8, 24). When mice are infused with extracellular hemoglobin that has been extensively cross-linked and only contains a small fraction of monomeric hemoglobin (as in the HBOC PolyHeme), scavenging of NO is markedly reduced (25). An inverse correlation between the molecular size of HBOCs and the vasoconstrictive response of arteries has been demonstrated (17). Inhibition of extravasation of cross-linked extracellular hemoglobin has been postulated as a possible mechanism of decreased NO scavenging in the muscular layer of arteries by high-molecular-weight HBOCs. Recent data suggest that haptoglobin prevents hemoglobin-induced hypertension by a similar mechanism (18). By binding hemoglobin in a high-molecular-weight hemoglobin-haptoglobin complex, haptoglobin blocks extravasation of cell-free hemoglobin into the vascular wall in rats, guinea pigs, and dogs (2, 6, 18). In the current study, we demonstrated that administration of human haptoglobin retained cell-free hemoglobin in plasma, prevented hemoglobinuria, and reduced the hemoglobin-induced hypertension in healthy awake mice (see Fig. 1A). These findings support the concept that blocking extravasation of hemoglobin, and its endothelial translocation near the muscular layer of arteries, might be a key mechanism for the ability of haptoglobin to prevent hemoglobin-induced systemic hypertension in mice with normal endothelium.
In mice with endothelial dysfunction, the vasoconstrictor response to administration of cell-free hemoglobin is enhanced (25). In our previous study, we demonstrated that infusion of PolyHeme, which did not induce systemic hypertension in healthy mice, caused systemic hypertension in mice with endothelial dysfunction (25). In this study, using mice fed a HFD and diabetic mice, we found that infusion of haptoglobin mixed with murine tetrameric hemoglobin in a 1:1 weight ratio (an amount of haptoglobin that was sufficient to block hemoglobin-induced hypertension in healthy mice) was unable to attenuate hemoglobin-induced hypertension in mice with endothelial dysfunction. Schaer and colleagues observed similar results in a study using rats that were injected with the endothelial NOS inhibitor N-nitro-l-arginine methyl ester. Coinjection of cell-free hemoglobin and haptoglobin in N-nitro-l-arginine methyl ester-treated rats abolished the ability of haptoglobin to prevent hemoglobin-induced vasoconstriction (18).
The laminar flow of blood in vessels results in an erythrocyte-free plasma zone along the endothelium. When extracellular hemoglobin is present in plasma, NO scavenging in the erythrocyte-free plasma zone leads to an increased diffusion of NO from endothelium into blood (20). In conditions with endothelial dysfunction, in which the ability of the vascular endothelium to produce NO has been compromised, scavenging of residual endothelial NO by small amounts of cell-free hemoglobin might be sufficient to induce vasoconstriction (25). Consistent with a previous report, binding to haptoglobin does not affect the ability of murine cell-free hemoglobin to scavenge NO (1). It is possible that hypertension in mice with endothelial dysfunction is induced by small amounts of unbound cell-free hemoglobin. Alternatively, hemoglobin-haptoglobin complexes may deplete NO in the luminal, endothelial, or subendothelial portions of the vasculature of mice with endothelial dysfunction.
Increased plasma concentrations of cell-free heme, the breakdown product of hemoglobin, promote activation and inflammation of endothelial cells and enhance oxidative stress and vascular permeability (22). In mouse models associated with chronic hemolysis and heme overload, such as in mice with a sickle hemoglobin (HbS) gene knockin or mouse models of β-thalassemia, repeated treatment with hemopexin was shown to reduce chronically elevated blood pressure (21). Likewise, infusion of haptoglobin or hemopexin in HbS mice inhibited hemoglobin-dependent vasoocclusion (3). Haptoglobin acts by preventing release of heme from cell-free hemoglobin and hemoglobin S. The mechanism postulated for the beneficial effects of hemopexin in mouse models of chronic hemolysis involves inhibition of heme-dependent activation of Toll-like receptor 4 signaling and a combination of reduced endothelial formation of ROS and restored endothelial NOS activity (3). Toll-like receptor 4 activation by free heme would induce mobilization of P-selectin and von Willebrand factor to the endothelial surface that triggers VCAM-1, ICAM-1, and E-selectin-dependent hemostasis and vasoocclusion (3, 4). These proposed modes of hemopexin and haptoglobin action are relevant in states of chronic hemolysis. In the current study, only the rapid physiological effects of acute exposure to intravascular cell-free hemoglobin on the vasoconstrictor response of systemic blood vessels were analyzed. In this setting, coinfusion of hemopexin was unable to attenuate the hypertension associated with administration of murine tetrameric hemoglobin.
A potential limitation of this study is that we coinfused purified human haptoglobin or hemopexin with murine tetrameric hemoglobin into mice. Although the binding affinities of human haptoglobin for extracellular murine hemoglobin are high, translation of our findings into humans might be limited by differing plasma clearances of human haptoglobin bound to murine hemoglobin. Furthermore, plasma clearance of cell-free hemoglobin and hemoglobin bound to haptoglobin differs between humans and mice: mice clear hemoglobin bound to haptoglobin at a slower rate than humans (9). In addition, the effects of the haptoglobin-hemoglobin complex on vascular NO depletion in vivo should be the subject of further research. A second potential limitation of this study is that we administered human haptoglobin and hemopexin in an amount that was equivalent to that of hemoglobin, as determined by weight. Using a 1:1 equivalent weight dose in guinea pigs, Lipiski and colleagues demonstrated that coinfusion of haptoglobin with hemoglobin was able to prevent hemoglobin-induced hypertension irrespective of the haptoglobin phenotype (Hp1-1 or Hp2-2) (13). A 10-fold higher concentration of either haptoglobin or hemopexin did not change hemoglobin-induced NO consumption in vitro. It remains possible that higher doses of hemopexin in WT mice (i.e., greater than 1:1 weight equivalent) and higher doses of haptoglobin and hemopexin in mice fed a HFD or in diabetic mice could have a beneficial effect in vivo. Furthermore, it remains unclear if mixing haptoglobin with murine tetrameric hemoglobin at a 1:1 weight ratio leads to complete binding of cell-free hemoglobin. However, higher doses of haptoglobin and hemopexin would require injection of large volumes of fluid which is not feasible in a mouse model.
In summary, in mice with a normal endothelium, administration of haptoglobin but not hemopexin with cell-free hemoglobin prevents hemoglobin-induced systemic hypertension. Because haptoglobin retained hemoglobin in the vasculature, but did not alter the ability of hemoglobin to scavenge NO in the intravascular space, the effect of haptoglobin may have resulted from preventing hemoglobin from scavenging NO by decreasing the amount of cell-free hemoglobin that reaches subendothelial smooth muscle cells. In disease conditions that are associated with endothelial dysfunction, in which the ability of the endothelium to produce NO is compromised, the same dose of haptoglobin was unable to prevent hemoglobin-induced vasoconstriction. Therefore, although haptoglobin might be an effective treatment for hypertension associated with free hemoglobin in individuals with a normal endothelium, the therapy may not be effective in patients that have metabolic or vascular diseases associated with endothelial dysfunction.
GRANTS
This research was supported by Deutsche Forschungsgemeinschaft Grant 4446/1-1 (to J. A. Graw); National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-082971 and the Leducq Foundation (to D. B. Bloch); Shriners Hospital for Crippled Children Grant 87200 (to H. S. Warren); and funds from the Department of Anesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital (to W. M. Zapol).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.A.G., B.Y., H.S.W., D.B.B., and W.M.Z. conceived and designed research; J.A.G. and B.Y. performed experiments; J.A.G., B.Y., E.R., H.S.W., E.S.B., D.B.B., and W.M.Z. analyzed data; J.A.G., E.R., H.S.W., E.S.B., D.B.B., and W.M.Z. interpreted results of experiments; J.A.G. and E.R. prepared figures; J.A.G., E.S.B., and D.B.B. drafted manuscript; J.A.G., B.Y., E.R., H.S.W., E.S.B., D.B.B., and W.M.Z. edited and revised manuscript; J.A.G., B.Y., E.R., H.S.W., E.S.B., D.B.B., and W.M.Z. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank F. E. Riley (Infectious Disease Unit) and V. Yao (Anesthesia Center for Critical Care Research, Dept. of Anesthesia, Critical Care, and Pain Medicine, both at Massachusetts General Hospital, Harvard Medical School, Boston, MA) for helpful technical assistance. Purified human hemopexin and haptoglobin were a generous gift of CSL Behring, USA.
REFERENCES
- 1.Azarov I, He X, Jeffers A, Basu S, Ucer B, Hantgan RR, Levy A, Kim-Shapiro DB. Rate of nitric oxide scavenging by hemoglobin bound to haptoglobin. Nitric Oxide 18: 296–302, 2008. doi: 10.1016/j.niox.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baek JH, D’Agnillo F, Vallelian F, Pereira CP, Williams MC, Jia Y, Schaer DJ, Buehler PW. Hemoglobin-driven pathophysiology is an in vivo consequence of the red blood cell storage lesion that can be attenuated in guinea pigs by haptoglobin therapy. J Clin Invest 122: 1444–1458, 2012. doi: 10.1172/JCI59770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belcher JD, Chen C, Nguyen J, Milbauer L, Abdulla F, Alayash AI, Smith A, Nath KA, Hebbel RP, Vercellotti GM. Heme triggers TLR4 signaling leading to endothelial cell activation and vaso-occlusion in murine sickle cell disease. Blood 123: 377–390, 2014. doi: 10.1182/blood-2013-04-495887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belcher JD, Mahaseth H, Welch TE, Vilback AE, Sonbol KM, Kalambur VS, Bowlin PR, Bischof JC, Hebbel RP, Vercellotti GM. Critical role of endothelial cell activation in hypoxia-induced vasoocclusion in transgenic sickle mice. Am J Physiol Heart Circ Physiol 288: H2715–H2725, 2005. doi: 10.1152/ajpheart.00986.2004. [DOI] [PubMed] [Google Scholar]
- 5.Berra L, Pinciroli R, Stowell CP, Wang L, Yu B, Fernandez BO, Feelisch M, Mietto C, Hod EA, Chipman D, Scherrer-Crosbie M, Bloch KD, Zapol WM. Autologous transfusion of stored red blood cells increases pulmonary artery pressure. Am J Respir Crit Care Med 190: 800–807, 2014. doi: 10.1164/rccm.201405-0850OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boretti FS, Buehler PW, D’Agnillo F, Kluge K, Glaus T, Butt OI, Jia Y, Goede J, Pereira CP, Maggiorini M, Schoedon G, Alayash AI, Schaer DJ. Sequestration of extracellular hemoglobin within a haptoglobin complex decreases its hypertensive and oxidative effects in dogs and guinea pigs. J Clin Invest 119: 2271–2280, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deuel JW, Schaer CA, Boretti FS, Opitz L, Garcia-Rubio I, Baek JH, Spahn DR, Buehler PW, Schaer DJ. Hemoglobinuria-related acute kidney injury is driven by intrarenal oxidative reactions triggering a heme toxicity response. Cell Death Dis 7: e2064, 2016. doi: 10.1038/cddis.2015.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doherty DH, Doyle MP, Curry SR, Vali RJ, Fattor TJ, Olson JS, Lemon DD. Rate of reaction with nitric oxide determines the hypertensive effect of cell-free hemoglobin. Nat Biotechnol 16: 672–676, 1998. doi: 10.1038/nbt0798-672. [DOI] [PubMed] [Google Scholar]
- 9.Etzerodt A, Kjolby M, Nielsen MJ, Maniecki M, Svendsen P, Moestrup SK. Plasma clearance of hemoglobin and haptoglobin in mice and effect of CD163 gene targeting disruption. Antioxid Redox Signal 18: 2254–2263, 2013. doi: 10.1089/ars.2012.4605. [DOI] [PubMed] [Google Scholar]
- 10.Gladwin MT, Crawford JH, Patel RP. The biochemistry of nitric oxide, nitrite, and hemoglobin: role in blood flow regulation. Free Radic Biol Med 36: 707–717, 2004. doi: 10.1016/j.freeradbiomed.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 11.Irwin DC, Baek JH, Hassell K, Nuss R, Eigenberger P, Lisk C, Loomis Z, Maltzahn J, Stenmark KR, Nozik-Grayck E, Buehler PW. Hemoglobin-induced lung vascular oxidation, inflammation, and remodeling contribute to the progression of hypoxic pulmonary hypertension and is attenuated in rats with repeated-dose haptoglobin administration. Free Radic Biol Med 82: 50–62, 2015. doi: 10.1016/j.freeradbiomed.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kristiansen M, Graversen JH, Jacobsen C, Sonne O, Hoffman HJ, Law SK, Moestrup SK. Identification of the haemoglobin scavenger receptor. Nature 409: 198–201, 2001. doi: 10.1038/35051594. [DOI] [PubMed] [Google Scholar]
- 13.Lipiski M, Deuel JW, Baek JH, Engelsberger WR, Buehler PW, Schaer DJ. Human Hp1-1 and Hp2-2 phenotype-specific haptoglobin therapeutics are both effective in vitro and in guinea pigs to attenuate hemoglobin toxicity. Antioxid Redox Signal 19: 1619–1633, 2013. doi: 10.1089/ars.2012.5089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muller-Eberhard U, Javid J, Liem HH, Hanstein A, Hanna M. Plasma concentrations of hemopexin, haptoglobin and heme in patients with various hemolytic diseases. Blood 32: 811–815, 1968. [PubMed] [Google Scholar]
- 15.Pannirselvam M, Wiehler WB, Anderson T, Triggle CR. Enhanced vascular reactivity of small mesenteric arteries from diabetic mice is associated with enhanced oxidative stress and cyclooxygenase products. Br J Pharmacol 144: 953–960, 2005. doi: 10.1038/sj.bjp.0706121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roberts CK, Barnard RJ, Sindhu RK, Jurczak M, Ehdaie A, Vaziri ND. A high-fat, refined-carbohydrate diet induces endothelial dysfunction and oxidant/antioxidant imbalance and depresses NOS protein expression. J Appl Physiol 98: 203–210, 2005. doi: 10.1152/japplphysiol.00463.2004. [DOI] [PubMed] [Google Scholar]
- 17.Sakai H, Hara H, Yuasa M, Tsai AG, Takeoka S, Tsuchida E, Intaglietta M. Molecular dimensions of Hb-based O2 carriers determine constriction of resistance arteries and hypertension. Am J Physiol Heart Circ Physiol 279: H908–H915, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Schaer CA, Deuel JW, Schildknecht D, Mahmoudi L, Garcia-Rubio I, Owczarek C, Schauer S, Kissner R, Banerjee U, Palmer AF, Spahn DR, Irwin DC, Vallelian F, Buehler PW, Schaer DJ. Haptoglobin preserves vascular nitric oxide signaling during hemolysis. Am J Respir Crit Care Med 193: 1111–1122, 2016. doi: 10.1164/rccm.201510-2058OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schaer DJ, Buehler PW, Alayash AI, Belcher JD, Vercellotti GM. Hemolysis and free hemoglobin revisited: exploring hemoglobin and hemin scavengers as a novel class of therapeutic proteins. Blood 121: 1276–1284, 2013. doi: 10.1182/blood-2012-11-451229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Secomb TW. Red blood cell mechanics and capillary blood rheology. Cell Biophys 18: 231–251, 1991. doi: 10.1007/BF02989816. [DOI] [PubMed] [Google Scholar]
- 21.Vinchi F, De Franceschi L, Ghigo A, Townes T, Cimino J, Silengo L, Hirsch E, Altruda F, Tolosano E. Hemopexin therapy improves cardiovascular function by preventing heme-induced endothelial toxicity in mouse models of hemolytic diseases. Circulation 127: 1317–1329, 2013. doi: 10.1161/CIRCULATIONAHA.112.130179. [DOI] [PubMed] [Google Scholar]
- 22.Vinchi F, Gastaldi S, Silengo L, Altruda F, Tolosano E. Hemopexin prevents endothelial damage and liver congestion in a mouse model of heme overload. Am J Pathol 173: 289–299, 2008. doi: 10.2353/ajpath.2008.071130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X, Tanus-Santos JE, Reiter CD, Dejam A, Shiva S, Smith RD, Hogg N, Gladwin MT. Biological activity of nitric oxide in the plasmatic compartment. Proc Natl Acad Sci USA 101: 11477–11482, 2004. doi: 10.1073/pnas.0402201101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu B, Raher MJ, Volpato GP, Bloch KD, Ichinose F, Zapol WM. Inhaled nitric oxide enables artificial blood transfusion without hypertension. Circulation 117: 1982–1990, 2008. doi: 10.1161/CIRCULATIONAHA.107.729137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu B, Shahid M, Egorina EM, Sovershaev MA, Raher MJ, Lei C, Wu MX, Bloch KD, Zapol WM. Endothelial dysfunction enhances vasoconstriction due to scavenging of nitric oxide by a hemoglobin-based oxygen carrier. Anesthesiology 112: 586–594, 2010. doi: 10.1097/ALN.0b013e3181cd7838. [DOI] [PMC free article] [PubMed] [Google Scholar]