Abstract
The increasing prevalence of multifocal cerebral microhemorrhages (CMHs, also known as “cerebral microbleeds”) is a significant, newly recognized problem in the aging population of the Western world. CMHs are associated with rupture of small intracerebral vessels and are thought to progressively impair neuronal function, potentially contributing to cognitive decline, geriatric psychiatric syndromes, and gait disorders. Clinical studies show that aging and hypertension significantly increase prevalence of CMHs. CMHs are also now recognized by the National Institutes of Health as a major factor in Alzheimer’s disease pathology. Moreover, the presence of CMHs is an independent risk factor for subsequent larger intracerebral hemorrhages. In this article, we review the epidemiology, detection, risk factors, clinical significance, and pathogenesis of CMHs. The potential age-related cellular mechanisms underlying the development of CMHs are discussed, with a focus on the structural determinants of microvascular fragility, age-related alterations in cerebrovascular adaptation to hypertension, the role of oxidative stress and matrix metalloproteinase activation, and the deleterious effects of arterial stiffening, increased pulse pressure, and impaired myogenic autoregulatory protection on the brain microvasculature. Finally, we examine potential treatments for the prevention of CMHs based on the proposed model of aging- and hypertension-dependent activation of the reactive oxygen species-matrix metalloproteinases axis, and we discuss critical questions to be addressed by future studies.
Keywords: vascular contributors to cognitive impairment and dementia, vascular cognitive impairment, vascular aging, cerebrovascular, cerebromicrovascular, stroke, transient ischemic attack
in recent years, significant advances in imaging technologies enabled the detection of previously undetected small chronic intracerebral hemorrhages [<5 to 10 mm in diameter (78)] termed cerebral microhemorrhages (CMHs; also described as microbleeds, multifocal signal loss lesions, petechial hemorrhages). CMHs are due to the rupture of small arteries, arterioles, and/or capillaries [in human CMHs, the diameter of ruptured vessels is estimated to be less than 200 µm, with many bleeds occurring at the arteriole and capillary levels (67)]. CMHs appear as small, oval hypointense lesions corresponding to focal hemosiderin depositions, which can be best detected using T2*-weighted Gradient-Recall Echo (T2*-GRE) MRI sequences (Fig. 1). Because hemosiderin remains present for a long time at the location of a previous bleeding, these methods enable the assessment of chronic, cumulative CMH burden.
Fig. 1.
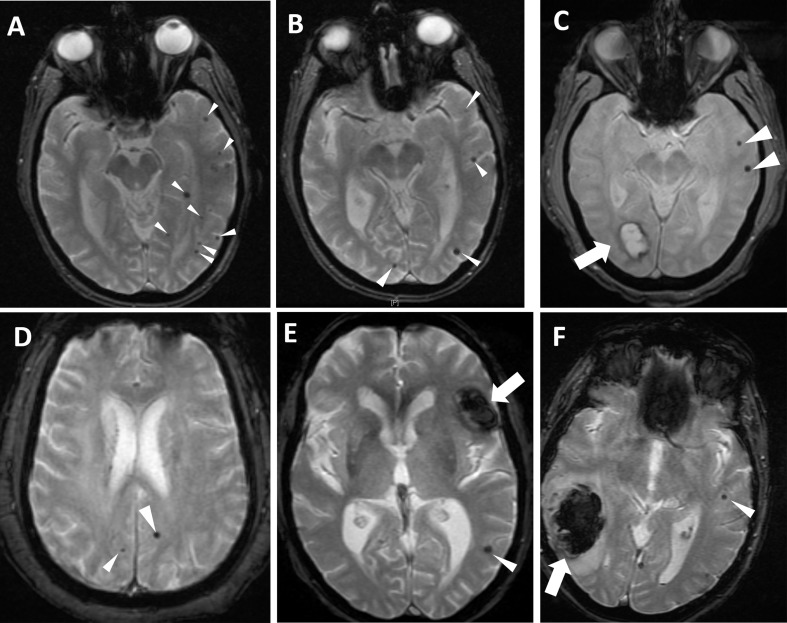
Cerebral microhemorrhages visible on T2*-GRE MRI sequences in the axial plane (please see white arrowheads). All images were obtained on a 1.5 T field strength scanner (GE Medical Systems). A: 76-yr-old man with chronic untreated hypertension and mild cognitive impairment presenting with transient ischemic attack manifested as right hemiparesis. Imaging was negative for acute cerebral infarction. Although at least one lesion involves deeper brain regions, many of the cerebral microhemorrhages (CMHs) involve the gray-white matter junction. B: 82-yr-old woman with a diagnosis of probable Alzheimer's disease in the moderate stage of diabetes, coronary artery disease, and hypertension. Imaging was obtained as part of the outpatient assessment for memory loss and risk prediction for future risk of intracranial hemorrhage, as it was preceded by a concurrent new diagnosis of deep venous thrombosis. Several lobar CMHs are noted, suggesting amyloid angiopathy. C: 73-yr-old man with amnestic mild cognitive impairment, hypertension, coronary artery disease, and current smoking, presenting with right visual field deficit of unclear duration. Note the large occipital bleed (thick arrow) and additional two left hemispheric CMHs (arrowheads). D: 57-yr-old man with long-standing smoking, hypercholesterolemia, and untreated hypertension. He presented with transient ischemic attack manifested as hemisensory loss and dysarthria. E: 61-yr-old man presenting with new-onset mild aphasia and headache. He had a prior history of chronic anticoagulation for cardiac valvular disease, with therapeutic levels of warfarin upon presentation. Note the large intracerebral hemorrhage (thick arrow) and additional CMH (arrowhead), illustrating the potential association between CMHs and larger intraparenchymal bleeds. F: 86-yr-old woman with prior stroke, atrial fibrillation (not on anticoagulation), and hypertension presenting with acute stroke, treated with intravenous thrombolytics with subsequent development of intraparenchymal hemorrhage (thick arrow). Note the presence of CMH (thin arrow) in the contralateral hemisphere.
In this review, the pathophysiology of CMHs is considered in terms of potential mechanisms involved, known risk factors and their clinical significance. The cellular and molecular mechanisms underlying structural weakening of cerebral vessels, the pathophysiological events promoting rupture of the vascular wall and the impact of risk factors (in particular, the synergistic effects of aging and hypertension) on the mechanical properties of the extracellular matrix and smooth muscle cells, which all contribute to the pathogenesis of CMHs, are critically discussed, and possible targets for intervention for prevention are identified.
Epidemiology and Risk Factors for CMHs
Age is the most significant independent risk factor for CMHs (21, 22, 95). Prevalence of CMHs is low in younger subjects and progressively increases with age (145, 148). The majority of studies report that the prevalence of CMHs is 24% to 56% in elderly patients (92, 104, 143, 145, 175, 235, 242). Because of the rapid development and increased availability of imaging methods, this figure is expected to significantly increase in the near future (232). Approximately half of the patients present with multiple CMHs (92). Despite their clinical importance, the pathogenesis of CMHs and the mechanisms responsible for the dramatic age-related increases in CMH incidence in human studies are not well understood, and there are no effective treatments available for prevention.
Hypertension is another major independent risk factor for CMHs (Fig. 2) (27, 95, 148, 150, 174, 222). The key role of the interaction of aging and hypertension in the development of CMHs is indicated by the findings that hypertension almost exclusively increases CMHs in elderly patients and aged laboratory animals (194).
Fig. 2.
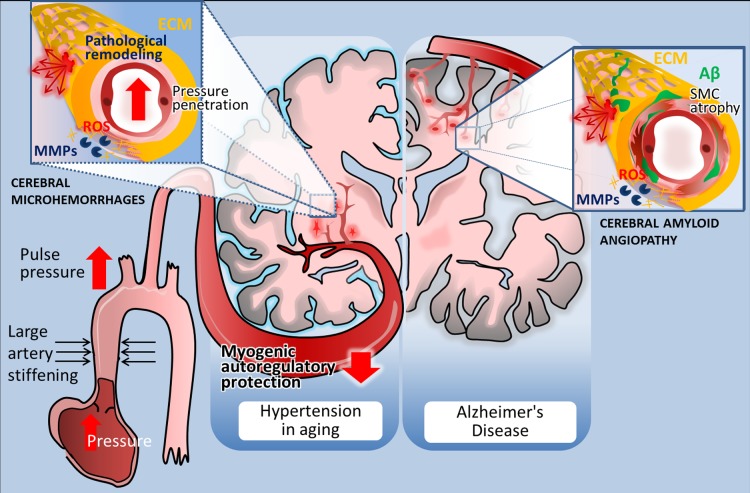
Distribution and pathogenesis of cerebral microhemorrhages (CMHs) associated with hypertension and Alzheimer’s disease in elderly patients. Left: in elderly patients CMHs associated with hypertensive vasculopathy typically affect the small perforating end-arteries located in the deep gray nuclei, brain stem, cerebellum, and deep white matter. The scheme highlights potential factors determining location and mechanisms involved in microvascular fragility. Accordingly, age-related large conduit artery stiffening increases pulsatile pressure, which can penetrate into the vulnerable portion of the microcirculation due to an impairment of myogenic autoregulatory protection in the proximal resistance arteries. The predilection of brain regions to hypertension-induced CMHs is determined by the branching pattern of penetrating arterioles. Increased pressure-induced vascular reactive oxygen species (ROS) production activates matrix metalloproteinases (MMPs), which degrade collagens and other components of the extracellular matrix (ECM), compromising the structural integrity of the cerebral microvasculature and promoting CMHs (inset). Right: in Alzheimer’s disease, CMHs develop in vessels affected by cerebral amyloid angiopathy (CAA). Location of the CMHs is determined by the predilection of brain regions for CAA pathology, which preferentially affects the small arteries and arterioles located in the cerebral cortex and at the junction of white and gray matter. The scheme depicts that deposition of Aβ in the vascular wall (green) promotes vascular smooth muscle cell atrophy and oxidative stress and exacerbates MMP-mediated degradation of the ECM, compromising the structural integrity of the vessels. The model predicts that because similar cellular mechanisms are involved in the pathogenesis of CMHs in both conditions, similar interventions should be effective for prevention as well.
Cerebral amyloid angiopathy and Alzheimer’s disease (AD) also constitute an important risk factor for CMHs (Fig. 2). The prevalence of CMHs is 26–48% in AD patients (16, 132, 243, 244). AD patients often exhibit multiple CMHs (74, 132). CMHs in AD patients are clinically important, as they may exacerbate cognitive decline, predict risk of future intracerebral hemorrhage, and indicate immunotherapy-related adverse events (74, 165). CMHs in AD patients typically show a cortical/subcortical localization (Fig. 2) (76, 120, 244). Cerebral amyloid angiopathy (CAA) in AD patients show a similar distribution (224), and it is believed that most CMHs in AD patients develop in vessels affected by CAA. Animal models of CAA also exhibit spontaneous CMHs (68).
The link between diabetes mellitus and CMH pathogenesis is not well understood (157). Initial analysis of the data obtained in the Northern Manhattan Study suggests that diabetes medication use is negatively associated with CMHs, but fasting blood glucose is not (21). In type 1 diabetic patients with proliferative diabetic retinopathy, an increased prevalence of CMHs was reported (237), suggesting that generalized microangiopathy may contribute to both the cerebral and retinal microvascular injury. Larger studies are warranted to elucidate the exact role of diabetes in the pathogenesis of CMHs and to determine whether those with medication‐controlled diabetes are, indeed, more protected from microvascular injury.
CMHs are also found in close to half of individuals affected by cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), which is an adult-onset hereditary stroke disorder caused by mutations of the Notch 3 gene (prevalence: 1–9 per 100,000) (219).
Clinical Significance of CMHs
Detection of CMHs using advanced imaging modalities.
Continued advances in MRI technology, including a continued increase in the imaging sequences available for clinical imaging, as well as access to MRI studies, have allowed for an increased detection of CMHs over the last two decades (for an excellent overview, see Ref. 223). These lesions, which typically are not seen on CT, were originally described (124) as flow void area on T2*-GRE MRI sequences and comprise small perivascular hemosiderin deposits surrounded by essentially normal brain tissue (78). After extravasation of erythrocytes into the perivascular space, within hours, polymorphic nuclear leukocytes and scavenger cells appear. Degradation of hemoglobin contained in erythrocytes by these cells leads to the formation of hemosiderin. Hemosiderin is paramagnetic, which allows for its detection by T2*-GRE MRI sequences.
Guidelines proposed by a panel of experts for detecting CMHs take into account the size, shape, and signal characteristics (78). More specifically, CMHs are defined as round or ovoid lesions, that appear black and with a blooming effect on T2*-GRE sequences, devoid of signal hyperintensity on T1-weighted or T2-weighted sequences and are distinct from potential CMH “mimics,” such as vessel flow voids, bone, or iron or calcium deposits. In addition, the clinical history should exclude traumatic diffuse axonal injury. Although there has been a lot of variability in defining cut-off points to classify CMHs, the guidelines recommend the range of 5–10 mm as an upper size limit. Of note, this upper range allows for the best distinction from larger bleeds (macrobleeds). A potential limitation in detecting CMHs is represented by disagreement among raters about the number and presence of lesion, a problem that could be addressed by the future use of validated standardized rating scales (79). The detection of CMHs is sensitive to many MRI imaging variables, such as field strength, echo times, and resolution. Recent studies using the susceptibility-weighted imaging (SWI) approach reported an increased sensitivity of detection of small lesions, resulting in a threefold increase in the number of CMHs detected (119). The development of high-resolution novel imaging methods and prospective studies using serial scans is expected to promote the better understanding of the pathogenesis of CMHs and shed new light on the clinical consequences of this currently underdiagnosed disease entity (81).
Consequences of CMHs.
There is substantial evidence that CMHs are clinically not silent. Here, we provide an overview of the scientific and clinical evidence for the role of CMHs in cognitive decline and gait abnormalities and the link between CMHs and small vessel disease, subsequent stroke, and increased mortality.
role of cmhs in cognitive decline.
Patients with CMHs have higher incidence of cognitive dysfunction (22, 92, 144, 220, 233, 234, 238, 240, 241). The behavioral consequences of CMHs likely depend on multiple factors, including CMH number and size, location, and/or cooccurrence of other diseases. There is strong evidence that a higher number of CMHs is associated with more severe cognitive dysfunction (238). Cross-sectional studies of the cognitive consequences of CMHs suggest that CMHs are associated with impaired executive function, decreased attention and processing speed, and impaired global cognition (245). CMHs have also been linked to significant behavioral problems, such as post-stroke emotional lability (181), depression (177, 179, 180, 184), suicidality (178), and fatigue (183), as well as impaired post-stroke cognitive recovery (182). Thus, further studies are needed to elucidate how CMHs affect the clinical presentation, treatment response, and outcome of geriatric psychological disorders. Future studies should also determine whether prevention of CMHs in high-risk patients would prevent or delay cognitive decline and/or alter the incidence of psychological disorders in the elderly.
The behavioral consequences of CMHs clearly depend on their locations (220). A recent meta-analysis shows that CMHs in deep brain regions, lobar regions, basal ganglia, and thalamus are associated with significant cognitive decline (238). Temporal lobe-located CMHs were reported to be associated with memory and attention impairment, whereas frontal lobe CMHs results in impairment of memory, concept shifting, psychomotor speed, and attention (220). CMHs in deep and lobar regions were shown to associate with attention/executive function and fluency domains (218). In other studies, lobar CMHs and CMHs located predominantly in the left hemisphere were shown to associate with late-onset depression (64). Despite these advances, it should be noted that there are several limitations to establishing direct cognitive associations, according to location of CMHs, including limitations inherent to imaging modalities used, study demographics, and presence of confounding variables (e.g., education) that affect patient performance on commonly used cognitive tests, and limited sensitivity of available cognitive measures. In many cross-sectional clinical studies, cognitive tests and sample size lack the sensitivity to measure subtle changes in performance due to a small number of CMHs. Cognitive consequences of CMHs are also influenced by other brain pathologies, such as the presence of periventricular leukoaraiosis (59).
The mechanisms by which CMHs impair cognitive function are likely to include focal brain damage and secondary disruption of neuronal and astrocytic communication in neighboring brain regions. CMHs likely cause a persisting local inflammatory response, focal blood-brain barrier disruption, activation of microglia, and perivascular macrophages (152). Recent studies propose that multiple CMHs lead to disruption of structural networks in the brain (90). It is less understood whether CMHs can resolve, although disappearance of imaging signs of CMHs has been documented in the literature (245).
Finally, the lack of agreement regarding cognitive assessment methods is likely responsible for the variability in the reported rate of occurrence and/or severity of cognitive impairment in patients presenting with CMHs. Studies using complex batteries of neuropsychological tests (including tests to assess intellectual function, verbal and visual memory, naming and perceptual skills, speed and attention, and executive function) demonstrate significant association between CMHs and cognitive decline (233). Although there are studies that fail to demonstrate a clear association between CMHs and cognitive impairment, this is usually due to the lack of sensitivity of the memory assessment used. For example, many studies rely on the mini-mental state examination (MMSE) (220), which is known to lack sensitivity to mild cognitive impairment. In addition, MMSE scores are affected by educational level, such that higher education may lead to false-negatives among patients with CMHs. Thus, we would like to emphasize the pressing need for the development and use of validated, sensitive, comprehensive cognitive neurological assessment methods in studies that attempt to evaluate the pathogenic role of CMHs in cognitive impairment.
role of cmhs in gait abnormalities.
There are clinical studies extant linking CMHs with gait dysfunction (25, 46, 134). Using an animal model of CMHs, we provided proof-of-concept showing that CMHs in aged mice cause gait disorder, which correlates with the severity and number of the CMHs (194). Gait and balance are governed by an interaction of the brain stem, sensory systems (vision, touch, vestibular input), somatosensory cortex, basal ganglia system, frontal subcortex, and the cerebellum. Because of these complex regulatory interactions, a large variety of motor and sensory problems associated with CMHs in multiple brain regions may lead to gait and balance disorders. High-level gait disorders may be caused by CMHs affecting cognitive and attentional processes governing balance and gait. The clinical importance of identifying potentially preventable microvascular mechanisms of gait abnormalities in the elderly cannot be overestimated. Gait abnormalities, which are present in more than one-third of older adults, increase the risk of falls with often tragic consequences. Falls are the fifth most common cause of death in the elderly, and only 25% of patients who suffer hip fractures regain their former level of function. Further studies are needed to adopt sensitive methods of gait analysis to detect even slight, subclinical gait abnormalities to be able to predict CMHs in high-risk patients. Incorporating gait as an end point in future studies designed to evaluate treatments for CMH prevention will also be highly informative.
cmhs, subsequent stroke, and mortality.
The presence of CMHs appears to predict subsequent intracerebral hemorrhage in elderly patients (198), or in patients with ischemic stroke (58, 121), AD, or those receiving anticoagulants (107, 110) (17). These associations suggest that similar cellular and molecular mechanisms may underlie vascular fragility, leading to both CMHs and ensuing larger intracerebral hemorrhages with significant clinical impact. There are several studies extant showing that the presence of CMHs is associated with an increased risk for cardiovascular mortality (2, 17, 77).
cmhs and small vessel disease.
White matter hyperintensities (WMHs; or leukoaraiosis) are nonspecific hyperintense changes localized to the cerebral white matter, which are frequently seen on T2/FLAIR sequences in elderly individuals. White matter damage in leukoaraiosis develops as a consequence of small vessel disease, and there is growing evidence from imaging studies, suggesting that CMHs contribute to the pathogenesis of leukoaraiosis and its functional consequences (16, 70, 93, 217). Presence and progression of small-vessel disease are strong predictors of new CMHs (73).
Pathophysiology of CMHs
The cellular and molecular mechanisms underlying microvascular fragility associated with the development of CMHs are not well understood. Here, we provide an overview of possible mechanisms inferred from studies on both animal models of CMHs (Fig. 2) and studies on pathological structural alterations in the peripheral vasculature induced by known risk factors of CMHs in the cerebral circulation (Fig. 3). Special emphasis is placed on age-related structural and functional maladaptation to hypertension and the underlying mechanisms.
Fig. 3.
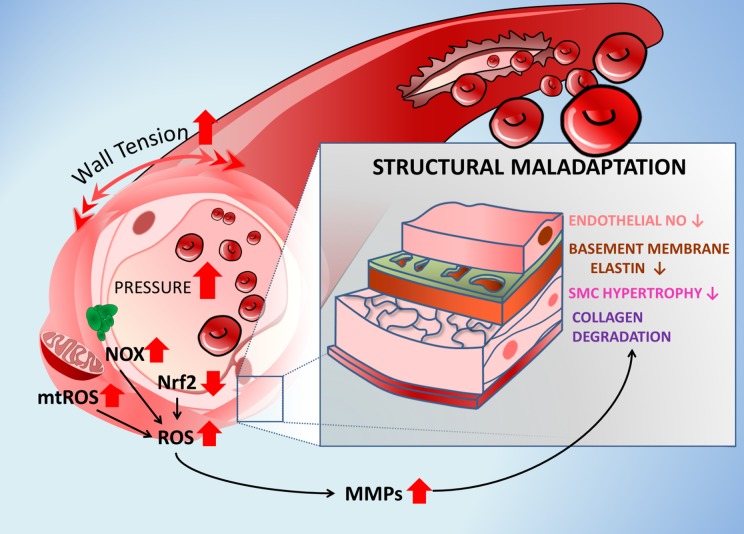
Cellular mechanisms by which aging exacerbates development of CMHs. The scheme highlights the role of age-related exacerbation of pressure-induced vascular oxidative stress, MMP activation, and structural maladaptation to hypertension in pathogenesis of CMHs. Accordingly, the increased intraluminal pressure and consequential increases in wall tension activate NADPH oxidases and promote mitochondria-derived production of ROS (mtROS) in the aged vascular smooth muscle cells. Pressure-induced vascular oxidative stress is exacerbated in aging due to a homeostatic failure due to dysregulation of NRF2-mediated antioxidant response. Vascular oxidative stress is responsible for endothelial dysfunction and the increase in MMP activity, which is implicated in collagen degradation, smooth muscle cell atrophy, and degradation of elastic components of the basement membrane. These structural changes weaken the microvascular wall and increase vulnerability to the formation of CMHs. Arrows indicate the effects of aging.
Structural determinants of microvascular fragility.
role of alterations in the extracellular matrix of cerebral vasculature in the pathogenesis of cmhs.
The extracellular matrix contributes significantly to the tensile strength of the vascular wall. The components of the extracellular matrix that account for the majority of the mechanical properties of cerebral vessels are collagen and elastin deposited by smooth muscle cells in the medial layer. The extracellular matrix is organized in a three-dimensional network connected to smooth muscle cells and distributes tensile stress in the vascular wall. Originally, detection of localized microaneurysms on brain vessels that were associated with spontaneous cerebral hemorrhages in early studies (23) led to the hypothesis that degenerative or inflammatory changes affecting small arterioles cause structural weakening and eventually rupture of the vascular wall. It is believed that disruption/weakening of the extracellular matrix network is also a critical factor in the development of CMHs. In experimental animals injection of extracellular matrix-degrading enzymes [e.g., collagenases (229)] result in the development of intracerebral hemorrhages. Further, human patients with genetic disorders affecting components of the extracellular matrix often present with intracerebral hemorrhages, including CMHs (221). These include both collagenopathies such as COL4A1 gene mutations (1, 101, 141, 215), COL3A1 gene mutation/Ehlers-Danlos syndrome (122, 131), mutations of COL1A1 or COL1A2 [osteogenesis imperfecta (60)], and elastinopathies, such as the Marfan syndrome [mutation in the FBN1 gene encoding the ECM protein fibrillin 1 (128)] and mutation in the ELN gene-encoding elastin [supravalvular aortic stenosis/Williams-Beuren syndrome (97)].
In the vascular wall, matrix metalloproteinases (MMPs) are known to degrade collagen and elastin and other components of the basal lamina and extracellular matrix, compromising the structural integrity of the cerebral vasculature. Cerebral arteries express MMP2 (collagens I, II, III, IV, VII, X), MMP3 (collagens II, IV, IX, X, X), MMP8 (collagens I, II, III, VII, VIII, X), MMP9 (collagens IV, V), MMP12 (elastin, fibronectin, collagen IV) and MMP13 (collagens I, II, III, IV, IX, X, XIV). Studies on mouse models of hypertension-induced CMHs demonstrate that the hypertension-related microvascular fragility and pathogenesis of CMHs involve weakening of the vessel wall by upregulation and/or oxidative stress-dependent activation of MMPs, such as MMP2 and MMP9 (226, 227). Importantly, aging was shown to promote the development of CMHs in mouse models by exacerbating hypertension-induced oxidative stress and redox-sensitive activation of MMPs in the cerebrovasculature (Fig. 3). The mechanisms by which hypertension promotes vascular oxidative stress in the cerebral vasculature are multifaceted (194). Multiple lines of evidence suggest that in addition to circulating factors (e.g., angiotensin II), changes in the hemodynamic environment (high pressure, increased circumferential wall tension) associated with hypertension activate ROS production in the vascular smooth muscle cells (166, 194, 205, 206). Recent studies demonstrate that high pressure elicits increased mitochondrial production of ROS, as well as activation of NADPH oxidases in smooth muscle cells located in the cerebral arteries and that these effects are significantly exacerbated in aging (166, 194). Aging per se is known to promote vascular oxidative stress (7–12, 30–33, 35–45, 99, 100, 111, 130, 166, 194, 196, 200, 201, 203, 204, 207, 209–212, 216) and to impair adaptive activation of Nrf2-driven antioxidant response pathways (7, 30, 37, 40, 203). We have recently reported that treatment of aged mice with the naturally occurring polyphenol resveratrol inhibits the development of hypertension-induced CMHs by attenuating oxidative stress-induced MMP activation (194). Importantly, resveratrol inhibits vascular oxidative stress by attenuating mitochondrial ROS production, inhibiting NADPH oxidases, and by upregulating Nrf2-dependent pathways (34, 37, 40, 130, 194, 195, 202, 208, 209, 247). Multiple lines of evidence suggest that activation/expression of MMPs are suppressed by NO and that decreased NO bioavailability promotes MMP activation in the vascular wall (47, 51, 80, 83, 214). Thus, it is important that hypertension in young mice can induce CMHs only after the inhibition of NO synthesis (226–228). Because aging is known to significantly decrease bioavailability of NO (9, 11, 30, 31, 42, 195, 199), we predict that age-related endothelial dysfunction may contribute to the pathogenesis of CMHs in the elderly.
MMP activation may provide a potential common mechanism of CMHs associated with multiple diverse pathophysiological conditions. In mouse models increased MMP9 activity was implicated in cerebral amyloid angiopathy-related hemorrhages (102, 103). Recent findings also suggest involvement of MMPs and lower levels of tissue inhibitors of matrix metalloproteinases (or TIMPs) in the pathogenesis of CMHs associated with AD (50, 248) in human patients. Interestingly, increased activity of the MMP system was also proposed to be a major contributing factor to increased CMH occurrence associated with anti-Aβ immunotherapy (236). High methionine diet and/or the resulting hyperhomocysteinemia were also reported to increase CMHs in mouse models, which are associated with increased expression and MMP2 and MMP9 (172). On the basis of the available evidence, future studies are warranted to test the efficacy of MMP inhibitors in animal models of various disorders that are characterized by increased incidence of CMHs.
In response to hypertension, there is a significant mechanical/structural adaptation process of the vascular wall (49, 135–140, 146). The vascular smooth muscle cells sense the increases in wall tensile stress. In healthy young organisms, by changing the synthesis and/or turnover of components of the extracellular matrix, stress distribution and stress concentration factor within the vascular wall can be attenuated, decreasing the susceptibility for rupture. However, in aging or under pathological conditions, these mechanical/structural adaptation processes are likely impaired (Tarantini S and Ungvari Z, unpublished observation), and intracerebral hemorrhages/CMHs thus ensue. Similar remodeling processes are thought to determine fragility of cerebral microvessels and vessels of the peripheral circulation. Thus, potential mechanisms that may be involved in the pathogenesis of CMHs are inferred here from the studies on the age-related changes in the cerebral circulation and the studies on hypertension-induced remodeling in diverse vascular beds. There is evidence that with aging, cerebral arterioles undergo atrophy, which is associated with a decline in elastin content (82). Age-related elastin degradation is also evident in the human cerebral vasculature (69). Further, cerebral amyloid angiopathy is also associated with significant elastin degradation, which is attributed to an upregulation of elastase expression in the smooth muscle cells (116). Collagen fibers in cerebral arterioles play a critical role in preventing damaging overextension of elastic components of the vascular wall. Despite their importance, it is not well understood how hypertension and aging affect the expression/synthesis/organization of various collagens in the wall of cerebral vessels (82, 89). As both injection of exogenous elastase and collagenase is known to induce intracerebral hemorrhages and activated MMPs degrade both elastin and collagens, the specific roles of altered elastin and collagen content/functionality in increased susceptibility of aged cerebral arterioles to rupture should be elucidated in future studies.
In angiotensin II-treated mice lysyl oxidase mediated ECM cross-linking was shown to contribute significantly to hypertension-induced remodeling and stiffening of arteries (52). Other studies demonstrate that the enhanced sensitivity to stroke in the stroke-prone spontaneously hypertensive rat is associated with an increased susceptibility of the arterial internal elastic lamina to rupture and dysregulation of lysyl oxidase (28). Importantly, β-aminopropionitrile, an inhibitor of lysyl oxidase-mediated cross-link formation in collagen and elastic fibers, exacerbates vascular rupture in spontaneously hypertensive rats (28). Thus, further studies are warranted to elucidate the role of lysyl oxidase also in the pathogenesis of CMHs, determining whether aging and other conditions that promote CMHs are associated with altered expression/activity of lysyl oxidases.
role of alterations in vascular smooth muscle cells in the pathogenesis of cmhs.
Unlike in elastic conduit arteries, in small cerebral arteries and arterioles, the smooth muscle cells significantly contribute to the passive mechanical properties of the vascular wall. Advanced age is associated with atrophy in cerebral arterioles, which results in a decreased cross-sectional area of smooth muscle cells (82), likely weakening the vessel wall. Smooth muscle cell loss is also prevalent in vessels affected by cerebral amyloid angiopathy (116). Possible mechanisms that may contribute to cerebral arteriolar atrophy involve circulating IGF-1 deficiency, which is the most prominent endocrine change with aging. Importantly, mouse models of circulating IGF-1 deficiency exhibit arteriolar atrophy, mimicking the aging phenotype (185). In healthy subjects in response to hypertension, cerebral vessels exhibit adaptive media hypertrophy (135), which likely increase the resistance of the vascular wall to rupture. There is evidence that in aging and IGF-1 deficiency, this adaptive response is impaired, which likely exacerbates CMHs (185). The concept that smooth muscle atrophy in cerebral arterioles contributes significantly to their increased susceptibility to rupture is supported by the histological findings from patients affected by CADASIL and animal models of CADASIL, showing that the underlying pathology for CADASIL-related CMHs is a progressive degeneration of the smooth muscle cells and the consequential structural weakening of cerebral vessels (4, 6, 29, 225). In addition to affecting the smooth muscle coverage of arterioles and activating ROS/MMP axis, age-related alterations in smooth muscle phenotype can exert multifaceted effects on the pathogenesis of CMHs. On the basis of the findings that radiation-induced vasculopathy is also associated with CMHs, further studies should elucidate the role of cellular senescence-related pathways age-related CMHs (19, 160). New research is also needed to address the role of proinflammatory changes and the interaction of vascular smooth muscle cells and MMP-expressing perivascular macrophages (3) as well.
Lipohyalinosis (also known as “fibrinoid necrosis”) is a complex form of small-vessel disease prevalent in hypertensive elderly patients affecting deep-perforating arteries, which are small arteries branching off directly from relatively large arteries in the basal ganglia, white matter, the brain stem, and the cerebellum (65). The “fibrinoid deposition” in the vessel wall characteristic to this disease is attributed to the effusion of plasma components due to inflammation and consequential blood-brain barrier disruption (the histological term “fibrinoid” refers to the positive fibrin staining of the amorphous eosinophilic material). Because of the phenotypic changes in smooth muscles and remodeling of the ECM associated with lipohyalinosis, the structural integrity of the vascular wall is compromised, promoting the formation of microaneurysms and intracerebral hemorrhages (151). Hyaline arteriolosclerosis (also known as arteriolar hyalinosis) is a simple form of small-vessel disease manifested in hypertensive elderly individuals, which is characterized by thickening of the arteriolar wall by massive collagen deposits around the basement membrane. Arteriosclerosis associates with CMHs (161), as arteriolar walls affected by hyalinosis are presumably weakened and are, therefore, more vulnerable to rupture.
Effects of arterial stiffening and increased pulse pressure on the brain microvasculature.
In healthy young individuals, the Windkessel effect of the elastic conduit arteries (aorta and carotid arteries) helps in damping hemodynamic pulsatility over the cardiac cycle (18, 189). Both the vascular aging process and exposure to vascular risk factors (including hypertension, hyperlipidemia, and diabetes) in the elderly are associated with increases in aortic stiffness, which impairs the Windkessel function, resulting in consequential increases in the amplitude of systolic pressure and amplification of pressure pulsatility in the elderly (48, 133). Cerebromicrovascular injury has long been hypothesized to result from the transmission of increased pulsatile pressure into the thinned-walled distal portion of the aging cerebral microcirculation (reviewed in Refs. 123, 164). Excessive arterial pressure wave reflections returning from the peripheral resistance arteries may augment pressure pulsatility transmitted to the cerebral microcirculation in the elderly. There is now increasing experimental and clinical evidence that pressure pulsatility, indeed, penetrates the cerebral microcirculation in aging (155, 187), which likely represents an important contributing factor to the pathogenesis of CMHs, as well as microvascular injury leading to blood brain barrier disruption, neuroinflammation, and white matter disease. The existing data are consistent with the hypothesis that age-related autoregulatory dysfunction of proximal resistance arteries allow the pressure waves to travel without substantial attenuation within the cerebral circulation and reach the vulnerable portion of the microcirculatory network, exacerbating microvascular damage (see below).
Role of impaired myogenic protection in the pathogenesis of CMHs.
Cerebral autoregulatory mechanisms are vested with the responsibility of protecting the fragile cerebral microvessels from transmission of increased pulsatile pressure (Fig. 2) (for a review, see Ref. 193). Particularly, the myogenic response of proximal resistance arteries plays a crucial role in dampening of high-pressure waves and reducing the likelihood of pressure-induced microvascular injury (14, 61, 62, 84, 125). Myogenic responses in healthy individuals involve powerful pressure/wall tension-induced constriction of small cerebral arteries that significantly increases segmental hydrodynamic resistance. Because of a larger pressure drop along the proximal resistance arteries, transmission of transient or chronic surges in pulsatile pressure is prevented from reaching the distal brain microcirculation (Fig. 2). Recently, we have shown that in laboratory animals, advanced age is associated with impaired myogenic adaptation to pulsatile pressure in isolated middle cerebral arteries (167). If this newly found evidence holds true for proximal resistance arteries in aged patients, then the age-related decrease of cerebral myogenic protection from hypertensive events could potentially increase the pressure-induced stress imposed on the thin-walled cerebral microvessels of elder individuals. The observation that pressure pulsatility penetrates the cerebral microcirculation in aging (155, 187, 187) supports this concept.
Anecdotal evidence indicates that certain otherwise common activities may impose an undue burden upon the aged cerebral circulation with impaired autoregulatory mechanisms. For example the transient arterial pressure increase caused by the Valsalva maneuver (defined as a forced expiratory blow against a closed glottis) is fairly common in many activities that involve moderate exertion. Such activities include weight lifting, blowing air into inflatable devices, intense coughing, vomiting, nose blowing, and strain during defecation. Depending on the levels of expiratory strain, intrathoracic pressure in these conditions may increase well over 150 mmHg. For instance, strain associated with coughing may increase intrathoracic pressure to ~300 mmHg for several seconds (159). The intrathoracic pressure is transmitted to the circulatory system, and the resulting pressure wave readily reaches the brain. While normally harmless, such transient rises in cerebral arterial pressure along with anger, sexual intercourse, and startling may impose excessive pressure to the brain microvessels of elder individuals due to the impaired myogenic responses of aged proximal arterioles, which allows the pressure wave to penetrate to the distal microvasculature, contributing to the development of microhemorrhages. We posit that in elderly patients, when microvascular pressure exceeds the threshold for structural injury in weakened microvessels, CMHs ensue (Fig. 2). Genetic mouse models of microvascular fragility provide proof-of-concept showing that short-term exercise (which is known to increase blood pressure) can significantly exacerbate CMHs (94). Rapid development of severe hypertension in cats with reduced renal mass results in autoregulatory dysfunction (as suggested by the marked brain edema), promoting CMHs (20). It should be noted that during acute hypertensive episodes, venular pressure can also increase significantly (112, 114), which has been linked to disruption of the blood-brain-barrier. For example, while during phenylephrine-induced acute hypertension in rats, pial arteriolar pressure increases by ~47 mmHg and pial venous pressure increases by ~20 mmHg (113). Given the much thinner wall of small veins, it is logical to predict that this pressure increase may predispose to venular CMHs as well.
Importantly, during the Valsalva maneuver, the central venous pressure also increases considerably (239); thus, a retrograde venous pressure wave may also reach the vulnerable cerebral microcirculation, exacerbating damage. In the internal jugular vein, valves are present to prevent the penetration of backward venous pressure into the cerebral venous system during increases in intrathoracic pressure (and consequentially, central venous pressure) associated with the Valsalva maneuver (26, 66, 249, 250). However, in aged individuals, the vein valves are often insufficient, and without competent vein valves, retrograde transmission of venous pressure occurs. Indirect evidence for a potentially important link between venous pressure increase and CMHs comes from studies on victims of domestic violence who survived manual strangulation (5). In these patients, venous obstruction (and consequential increase in venous and microvascular pressure) leads to petechial hemorrhages in the skin, conjunctiva of the eyes, and deep internal organs, including the brain (142, 246).
There is ample evidence that in hypertension, young cerebral arterial vessels exhibit functional adaptation to the altered hemodynamic environment, which includes augmentation of pressure-induced myogenic constriction (87, 98, 125, 190, 196). The hemodynamic effect of this functional adaptation is synergistic with the structural adaptation of resistance vessels to hypertension [hypertrophic inward remodeling (15)]. Together, these adaptive structural changes and functional changes in autoregulatory mechanisms increase segmental resistance in proximal resistance vessels, protecting the injury-prone distal portion of the cerebral microcirculation from pressure overload (115, 118, 129, 153, 154, 168–170, 191, 196, 231). Because of these adaptive functional changes, pressure in the thin-walled microvessels is maintained relatively constant, protecting them from pressure-induced injury (Fig. 2). The cellular mechanisms that underlie development of pressure-induced myogenic constriction of cerebral resistance arteries include increased production of the vasoconstrictor eicosanoid 20-HETE, inhibition of BKCa channels, and activation of LCa and TRPC channels (53–55, 57, 71, 72, 85, 86, 117, 126, 127, 147). There is strong evidence that aging cerebral arteries do not exhibit a hypertension-induced adaptive increase in myogenic constriction due to the dysregulation of the 20-HETE/TRPC6-mediated pathway of mechanotransduction in the vascular smooth muscle cells (190, 191, 196). We posit that loss of myogenic autoregulatory protection in the brain of hypertensive elderly subjects likely allows high blood pressure to penetrate the distal, injury-prone portion of the cerebral microcirculation, contributing to the pathogenesis of CMHs (Fig. 2). Direct experimental evidence in support for this concept comes from the recent elegant series of studies by Drs. Fan Fan and Richard Roman, who demonstrated that rats with impaired myogenic constriction (due to genetic deficiencies in 20-HETE production) exhibit increased penetration of pressure to the distal portion of the microcirculation, resulting in increased propensity to microvascular injury (56). Specifically, because of deficient myogenic constriction in the proximal arteries of the Fawn Hooded Hypertensive rat model, the pressure in terminal cerebral arteries was shown to be substantially increased (from ~37 to ~63 mmHg) when mean arterial pressure was increased from 100 to 160 mmHg (56). In contrast, in animals with intact myogenic constriction, the same increases in mean arterial pressure were not associated with significant changes in the pressure in terminal cerebral arteries (56). In general, these data correspond well to those obtained in earlier studies, demonstrating that pressure in pial arteries is ~50% of systemic pressure, as proximal larger intracranial vessels contribute significantly to total cerebral vascular resistance (13, 14, 61–63, 87, 88, 158, 171, 176). It should be noted that some earlier studies demonstrated larger increases in pressure in small pial arteries during hypertension (176), which may be attributed to the differences in the experimental models used [e.g., anesthetized cat vs. rat (13), methods for induction of hypertension etc.].
Novel findings implicate endocrine changes that occur during aging in functional and structural alterations in the aged cerebral circulation (163, 204). Of particular interest is the age-related decline in circulating levels of insulin-like growth factor-1 (IGF-1), which have been causally linked to vascular aging phenotypes, including the pathophysiological alterations in the brain microvasculature occurring with age (7, 163, 192, 197, 204). Experimental evidence suggesting that IGF-1 deficiency promotes autoregulatory dysfunction was recently obtained using a novel mouse model of circulating IGF-1 deficiency (adeno-associated viral knockdown of IGF-1 specifically in the mouse liver using Cre-lox technology; Igf1f/f + TBG-Cre-AAV8) (7). Specifically, studies using this novel murine model have shown that low IGF-1 levels in the circulation impair myogenic autoregulatory adaptation of the cerebral resistance arteries to hypertension, which likely exacerbate hypertension-induced cerebromiscrovascular damage and injury (197). It appears that IGF-1-deficient mice fail to adapt to hypertension by upregulating vascular TRPC6 expression, augmenting the 20-HETE/TRPC-mediated component of pressure-induced myogenic constriction (197), which mimics the aging phenotype. These findings have important clinical relevance, as in population-based studies, lower circulating IGF-1 levels were also shown to associate with aorta stiffening and higher aortic pulse wave velocity (91, 156). Further, experimentally induced IGF-1 deficiency was demonstrated to mimic additional aspects of cerebromicrovascular aging as well (7, 8, 163, 186, 192).
CMHs in patients with and without hypertension have different distribution patterns (173). It appears that hypertension predominantly promotes deep or infratentorial CMHs (96, 105, 106, 157, 173, 222). Recent studies lead to the hypothesis that regional differences in vascular anatomy and myogenic autoregulatory protection determine the predilection site of CMHs associated with hypertensive vasculopathy. Specifically, it was proposed that in deep and infratentorial regions, penetrating branches arise directly from the posterior cerebral artery and the middle cerebral artery and because of their branching pattern, the segment of proximal vessels, whose resistance protects the microcirculation, is shorter than in other brain regions (109). These anatomical properties would render these vascular supply areas more vulnerable to sudden changes in blood pressure (109). In addition, there are known regional differences in the cellular and molecular mechanisms involved in regulation of myogenic constriction of cerebral arteries. Future studies should elucidate regional variation in the combined effects of age and hypertension in cerebral arteries as well.
Perspectives: From Diagnosis to Prevention
Despite the dramatic increase of publications on the detection and diagnostic and prognostic values of CMHs, several fundamental questions relayed to their pathogenesis and prevention remain to be clarified. Future studies should characterize what percentage of CMHs occurs in arterioles, in capillaries or in venules and determine how this pattern changes in hypertension, aging, and other risk factors for CMHs. Developing novel genetic models with smooth muscle cell-specific depletion of factors involved in microvascular fragility would greatly facilitate the understanding of the pathogenesis of CMHs in aging and in AD. Future work should focus on specific treatments to inhibit or reduce the development of CMHs. Many studies have pointed toward cellular ROS production as an attractive cellular target for intervention to increase microvascular resilience. New research using novel approaches to attenuate age-related microvascular oxidative stress and disrupt oxidative stress-driven pathways involved in the formation of CMHs is very much needed. A growing body of evidence indicates that targeting redox-sensitive MMP activation could be an important treatment option for the prevention of CMHs. The role of cellular senescence programs, contribution of pericytes and perivascular macrophages, as well as inflammatory factors in the pathogenesis of CMHs are still to be fully characterized (67, 94, 149, 162). Alterations in myogenic autoregulatory protection and changes in endothelial tight junctions are likely to exacerbate CMHs at the capillary level. Future studies should determine additional structural factors promoting capillary bleeds (including alterations in biomechanical properties of the basal membrane) and identify targets for intervention (94). In addition to capillaries, tight junctions are also present in arterioles and venules. It is known that different pathophysiological conditions (including diabetes mellitus) result in dysregulation of tight junction proteins, but it is yet unclear how this affects pressure-induced injury in those segments of the vasculature. It is also unclear how retinal microvascular alterations visible on ophthalmoscopy (e.g., hypertensive retinopathy) and CMHs relate. Mechanisms involved in resolution/repair of CMHs, such as a potential role of macrophages suggested by studies on zebrafish models of cerebrovascular injury (108), are not well understood and require further investigation. Randomized controlled trials have been designed to determine the long-term effects of established medications (230) on CMHs, and it is expected that, using similar approaches, application of a mechanism-based therapy targeting pathways identified in experimental studies [e.g., polyphenol compounds (194)] will be also tested in the future. Several compounds that have potentially relevant pharmacological action are approved for other disorders, offering the possibility of drug repurposing for novel prophylactic strategies for CMHs. In the clinical practice, the importance of blood pressure control (determining optimal intensity of treatment; elucidating differences between the effects of various antihypertensive agents with different mechanisms of actions) and lifestyle interventions controlling modifiable risk factors (e.g., to limit temporal blood pressure surges, dietary interventions) should also be emphasized. Since influencing multiple targets (e.g., disrupting the ROS-MMP axis, improving endothelial function, attenuating inflammation, preventing pressure overload) may be most effective, using a combination of vasoprotective drugs and other interventions may be preferable. Clinical studies should also elucidate the link between side-effects of commonly used medications [e.g., statins (148), anticoagulants, aspirin (75)] or even thrombolytics (24) and CMHs. Finally, longitudinal studies should improve our understanding of the prognostic value of CMHs in various pathological conditions associated with aging and the causal link among CMHs, cognitive performance, and gait abnormalities.
GRANTS
This work was supported by grants from the American Heart Association (to S. Tarantini, A. Csiszar, A. Kirkpatrick, and Z. Ungvari), National Institutes of Health (NIH) Grants R01-AT-006526 (to Z. Ungvari), R01-AG-047879, R01-AG-038747, and U54-GM-104938 (to A. Kirkpatrick), R01-NS-056218 (to A. Csiszar), the Oklahoma Nathan Shock Center (NIH Grant 3-P30-AG-050911-02S1), the Arkansas Claude Pepper Older Americans Independence Center at University of Arkansas Medical Center (NIH Grant P30-AG-028718, to Z. Ungvari), the Oklahoma Center for the Advancement of Science and Technology (to A. Csiszar and Z. Ungvari), the Oklahoma IDeA Network for Biomedical Research Excellence (to A. Csiszar), Department of Veterans Affairs Grant CX000340 (to C. Prodan), and the Reynolds Foundation (to Z. Ungvari and A. Csiszar).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Z.I.U., S.T., A.C., and C.I.P. prepared figures; Z.I.U., A.C., and C.I.P. drafted manuscript; Z.I.U., S.T., A.C.K., A.C., and C.I.P. edited and revised manuscript; Z.I.U., S.T., A.C.K., A.C., and C.I.P. approved final version of manuscript.
ACKNOWLEDGMENTS
Portions of this work were presented and published in thesis form in fulfillment of the PhD requirements for S. Tarantini from the University of Oklahoma Health Sciences Center.
REFERENCES
- 1.Alamowitch S, Plaisier E, Favrole P, Prost C, Chen Z, Van Agtmael T, Marro B, Ronco P. Cerebrovascular disease related to COL4A1 mutations in HANAC syndrome. Neurology 73: 1873–1882, 2009. doi: 10.1212/WNL.0b013e3181c3fd12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altmann-Schneider I, Trompet S, de Craen AJ, van Es AC, Jukema JW, Stott DJ, Sattar N, Westendorp RG, van Buchem MA, van der Grond J. Cerebral microbleeds are predictive of mortality in the elderly. Stroke 42: 638–644, 2011. doi: 10.1161/STROKEAHA.110.595611. [DOI] [PubMed] [Google Scholar]
- 3.Aoki T, Kataoka H, Morimoto M, Nozaki K, Hashimoto N. Macrophage-derived matrix metalloproteinase-2 and -9 promote the progression of cerebral aneurysms in rats. Stroke 38: 162–169, 2007. doi: 10.1161/01.STR.0000252129.18605.c8. [DOI] [PubMed] [Google Scholar]
- 4.Arboleda-Velasquez JF, Manent J, Lee JH, Tikka S, Ospina C, Vanderburg CR, Frosch MP, Rodríguez-Falcón M, Villen J, Gygi S, Lopera F, Kalimo H, Moskowitz MA, Ayata C, Louvi A, Artavanis-Tsakonas S. Hypomorphic Notch 3 alleles link Notch signaling to ischemic cerebral small-vessel disease. Proc Natl Acad Sci USA 108: E128–E135, 2011. doi: 10.1073/pnas.1101964108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armstrong M Jr, Strack GB. Recognition and documentation of strangulation crimes: a review. JAMA Otolaryngol Head Neck Surg 142: 891–897, 2016. doi: 10.1001/jamaoto.2016.0293. [DOI] [PubMed] [Google Scholar]
- 6.Ayata C. CADASIL: experimental insights from animal models. Stroke 41, Suppl: S129–S134, 2010. doi: 10.1161/STROKEAHA.110.595207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bailey-Downs LC, Mitschelen M, Sosnowska D, Toth P, Pinto JT, Ballabh P, Valcarcel-Ares MN, Farley J, Koller A, Henthorn JC, Bass C, Sonntag WE, Ungvari Z, Csiszar A. Liver-specific knockdown of IGF-1 decreases vascular oxidative stress resistance by impairing the Nrf2-dependent antioxidant response: a novel model of vascular aging. J Gerontol A Biol Sci Med Sci 67: 313–329, 2012. doi: 10.1093/gerona/glr164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bailey-Downs LC, Sosnowska D, Toth P, Mitschelen M, Gautam T, Henthorn JC, Ballabh P, Koller A, Farley JA, Sonntag WE, Csiszar A, Ungvari Z. Growth hormone and IGF-1 deficiency exacerbate high-fat diet-induced endothelial impairment in obese Lewis dwarf rats: implications for vascular aging. J Gerontol A Biol Sci Med Sci 67: 553–564, 2012. doi: 10.1093/gerona/glr197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bailey-Downs LC, Tucsek Z, Toth P, Sosnowska D, Gautam T, Sonntag WE, Csiszar A, Ungvari Z. Aging exacerbates obesity-induced oxidative stress and inflammation in perivascular adipose tissue in mice: a paracrine mechanism contributing to vascular redox dysregulation and inflammation. J Gerontol A Biol Sci Med Sci 68: 780–792, 2013. doi: 10.1093/gerona/gls238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banki E, Sosnowska D, Tucsek Z, Gautam T, Toth P, Tarantini S, Tamas A, Helyes Z, Reglodi D, Sonntag WE, Csiszar A, Ungvari Z. Age-related decline of autocrine pituitary adenylate cyclase-activating polypeptide impairs angiogenic capacity of rat cerebromicrovascular endothelial cells. J Gerontol A Biol Sci Med Sci 70: 665–674, 2015. doi: 10.1093/gerona/glu116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bátkai S, Rajesh M, Mukhopadhyay P, Haskó G, Liaudet L, Cravatt BF, Csiszár A, Ungvári Z, Pacher P. Decreased age-related cardiac dysfunction, myocardial nitrative stress, inflammatory gene expression, and apoptosis in mice lacking fatty acid amide hydrolase. Am J Physiol Heart Circ Physiol 293: H909–H918, 2007. doi: 10.1152/ajpheart.00373.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baumbach GL, Heistad DD. Cerebral circulation in chronic arterial hypertension. Hypertension 12: 89–95, 1988. doi: 10.1161/01.HYP.12.2.89. [DOI] [PubMed] [Google Scholar]
- 14.Baumbach GL, Heistad DD. Regional, segmental, and temporal heterogeneity of cerebral vascular autoregulation. Ann Biomed Eng 13: 303–310, 1985. doi: 10.1007/BF02584248. [DOI] [PubMed] [Google Scholar]
- 15.Baumbach GL, Heistad DD. Remodeling of cerebral arterioles in chronic hypertension. Hypertension 13: 968–972, 1989. doi: 10.1161/01.HYP.13.6.968. [DOI] [PubMed] [Google Scholar]
- 16.Benedictus MR, Goos JD, Binnewijzend MA, Muller M, Barkhof F, Scheltens P, Prins ND, van der Flier WM. Specific risk factors for microbleeds and white matter hyperintensities in Alzheimer’s disease. Neurobiol Aging 34: 2488–2494, 2013. doi: 10.1016/j.neurobiolaging.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 17.Benedictus MR, Prins ND, Goos JD, Scheltens P, Barkhof F, van der Flier WM. Microbleeds, mortality, and stroke in Alzheimer disease: the MISTRAL study. JAMA Neurol 72: 539–545, 2015. doi: 10.1001/jamaneurol.2015.14. [DOI] [PubMed] [Google Scholar]
- 18.Bergsneider M, Alwan AA, Falkson L, Rubinstein EH. The relationship of pulsatile cerebrospinal fluid flow to cerebral blood flow and intracranial pressure: a new theoretical model. Acta Neurochir Suppl 71: 266–268, 1998. [DOI] [PubMed] [Google Scholar]
- 19.Bian W, Banerjee S, Kelly DA, Hess CP, Larson PE, Chang SM, Nelson SJ, Lupo JM. Simultaneous imaging of radiation-induced cerebral microbleeds, arteries and veins, using a multiple gradient echo sequence at 7 Tesla. J Magn Reson Imaging 42: 269–279, 2015. doi: 10.1002/jmri.24802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown CA, Munday JS, Mathur S, Brown SA. Hypertensive encephalopathy in cats with reduced renal function. Vet Pathol 42: 642–649, 2005. doi: 10.1354/vp.42-5-642. [DOI] [PubMed] [Google Scholar]
- 21.Caunca MR, Del Brutto V, Gardener H, Shah N, Dequatre-Ponchelle N, Cheung YK, Elkind MS, Brown TR, Cordonnier C, Sacco RL, Wright CB. Cerebral microbleeds, vascular risk factors, and magnetic resonance imaging markers: the Northern Manhattan Study. J Am Heart Assoc 5: e003477, 2016. doi: 10.1161/JAHA.116.003477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chai C, Wang Z, Fan L, Zhang M, Chu Z, Zuo C, Liu L, Mark Haacke E, Guo W, Shen W, Xia S. Increased number and distribution of cerebral microbleeds is a risk factor for cognitive dysfunction in hemodialysis patients: a longitudinal study. Medicine (Baltimore) 95: e2974, 2016. doi: 10.1097/MD.0000000000002974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Charcot JM, Bouchard C. Nouvelles recherches sur la pathogénie de l’hemorrhagie cerebrale. Arch Physiol Norm Pathol 642: 110–127, 1868. [Google Scholar]
- 24.Charidimou A, Shoamanesh A; International META-MICROBLEEDS Initiative . Clinical relevance of microbleeds in acute stroke thrombolysis: comprehensive meta-analysis. Neurology 87: 1534–1541, 2016. doi: 10.1212/WNL.0000000000003207. [DOI] [PubMed] [Google Scholar]
- 25.Choi P, Ren M, Phan TG, Callisaya M, Ly JV, Beare R, Chong W, Srikanth V. Silent infarcts and cerebral microbleeds modify the associations of white matter lesions with gait and postural stability: population-based study. Stroke 43: 1505–1510, 2012. doi: 10.1161/STROKEAHA.111.647271. [DOI] [PubMed] [Google Scholar]
- 26.Chung CP, Beggs C, Wang PN, Bergsland N, Shepherd S, Cheng CY, Ramasamy DP, Dwyer MG, Hu HH, Zivadinov R. Jugular venous reflux and white matter abnormalities in Alzheimer’s disease: a pilot study. J Alzheimers Dis 39: 601–609, 2014. doi: 10.3233/JAD-131112. [DOI] [PubMed] [Google Scholar]
- 27.Cordonnier C, Al-Shahi Salman R, Wardlaw J. Spontaneous brain microbleeds: systematic review, subgroup analyses and standards for study design and reporting. Brain 130: 1988–2003, 2007. doi: 10.1093/brain/awl387. [DOI] [PubMed] [Google Scholar]
- 28.Coutard M, Osborne-Pellegrin M. Rupture of the internal elastic lamina and vascular fragility in stroke-prone spontaneously hypertensive rats. Stroke 22: 510–515, 1991. doi: 10.1161/01.STR.22.4.510. [DOI] [PubMed] [Google Scholar]
- 29.Craggs LJ, Yamamoto Y, Deramecourt V, Kalaria RN. Microvascular pathology and morphometrics of sporadic and hereditary small vessel diseases of the brain. Brain Pathol 24: 495–509, 2014. doi: 10.1111/bpa.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Csiszar A, Gautam T, Sosnowska D, Tarantini S, Banki E, Tucsek Z, Toth P, Losonczy G, Koller A, Reglodi D, Giles CB, Wren JD, Sonntag WE, Ungvari Z. Caloric restriction confers persistent anti-oxidative, pro-angiogenic, and anti-inflammatory effects and promotes anti-aging miRNA expression profile in cerebromicrovascular endothelial cells of aged rats. Am J Physiol Heart Circ Physiol 307: H292–H306, 2014. doi: 10.1152/ajpheart.00307.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Csiszar A, Labinskyy N, Jimenez R, Pinto JT, Ballabh P, Losonczy G, Pearson KJ, de Cabo R, Ungvari Z. Anti-oxidative and anti-inflammatory vasoprotective effects of caloric restriction in aging: role of circulating factors and SIRT1. Mech Ageing Dev 130: 518–527, 2009. doi: 10.1016/j.mad.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Csiszar A, Labinskyy N, Orosz Z, Xiangmin Z, Buffenstein R, Ungvari Z. Vascular aging in the longest-living rodent, the naked mole rat. Am J Physiol Heart Circ Physiol 293: H919–H927, 2007. doi: 10.1152/ajpheart.01287.2006. [DOI] [PubMed] [Google Scholar]
- 33.Csiszar A, Labinskyy N, Perez V, Recchia FA, Podlutsky A, Mukhopadhyay P, Losonczy G, Pacher P, Austad SN, Bartke A, Ungvari Z. Endothelial function and vascular oxidative stress in long-lived GH/IGF-deficient Ames dwarf mice. Am J Physiol Heart Circ Physiol 295: H1882–H1894, 2008. doi: 10.1152/ajpheart.412.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Csiszar A, Labinskyy N, Podlutsky A, Kaminski PM, Wolin MS, Zhang C, Mukhopadhyay P, Pacher P, Hu F, de Cabo R, Ballabh P, Ungvari Z. Vasoprotective effects of resveratrol and SIRT1: attenuation of cigarette smoke-induced oxidative stress and proinflammatory phenotypic alterations. Am J Physiol Heart Circ Physiol 294: H2721–H2735, 2008. doi: 10.1152/ajpheart.00235.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Csiszar A, Labinskyy N, Smith K, Rivera A, Orosz Z, Ungvari Z. Vasculoprotective effects of anti-TNF-α treatment in aging. Am J Pathol 170: 388–698, 2007. doi: 10.2353/ajpath.2007.060708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Csiszar A, Labinskyy N, Zhao X, Hu F, Serpillon S, Huang Z, Ballabh P, Levy RJ, Hintze TH, Wolin MS, Austad SN, Podlutsky A, Ungvari Z. Vascular superoxide and hydrogen peroxide production and oxidative stress resistance in two closely related rodent species with disparate longevity. Aging Cell 6: 783–797, 2007. doi: 10.1111/j.1474-9726.2007.00339.x. [DOI] [PubMed] [Google Scholar]
- 37.Csiszar A, Pinto JT, Gautam T, Kleusch C, Hoffmann B, Tucsek Z, Toth P, Sonntag WE, Ungvari Z. Resveratrol encapsulated in novel fusogenic liposomes activates Nrf2 and attenuates oxidative stress in cerebromicrovascular endothelial cells from aged rats. J Gerontol A Biol Sci Med Sci 70: 303−313, 2015. doi: 10.1093/gerona/glu029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Csiszar A, Podlutsky A, Wolin MS, Losonczy G, Pacher P, Ungvari Z. Oxidative stress and accelerated vascular aging: implications for cigarette smoking. Front Biosci 14: 3128–3144, 2009. doi: 10.2741/3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Csiszar A, Sosnowska D, Tucsek Z, Gautam T, Toth P, Losonczy G, Colman RJ, Weindruch R, Anderson RM, Sonntag WE, Ungvari Z. Circulating factors induced by caloric restriction in the nonhuman primate Macaca mulatta activate angiogenic processes in endothelial cells. J Gerontol A Biol Sci Med Sci 68: 235–249, 2013. doi: 10.1093/gerona/gls158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Csiszar A, Sosnowska D, Wang M, Lakatta EG, Sonntag WE, Ungvari Z. Age-associated proinflammatory secretory phenotype in vascular smooth muscle cells from the non-human primate Macaca mulatta: reversal by resveratrol treatment. J Gerontol A Biol Sci Med Sci 67: 811–820, 2012. doi: 10.1093/gerona/glr228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Csiszar A, Tucsek Z, Toth P, Sosnowska D, Gautam T, Koller A, Deak F, Sonntag WE, Ungvari Z. Synergistic effects of hypertension and aging on cognitive function and hippocampal expression of genes involved in β-amyloid generation and Alzheimer’s disease. Am J Physiol Heart Circ Physiol 305: H1120–H1130, 2013. doi: 10.1152/ajpheart.00288.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Csiszar A, Ungvari Z, Edwards JG, Kaminski P, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res 90: 1159–1166, 2002. doi: 10.1161/01.RES.0000020401.61826.EA. [DOI] [PubMed] [Google Scholar]
- 43.Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Aging-induced proinflammatory shift in cytokine expression profile in coronary arteries. FASEB J 17: 1183–1185, 2003. doi: 10.1096/fj.02-1049fje. [DOI] [PubMed] [Google Scholar]
- 44.Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Proinflammatory phenotype of coronary arteries promotes endothelial apoptosis in aging. Physiol Genomics 17: 21–30, 2004. doi: 10.1152/physiolgenomics.00136.2003. [DOI] [PubMed] [Google Scholar]
- 45.Dai DF, Rabinovitch PS, Ungvari Z. Mitochondria and cardiovascular aging. Circ Res 110: 1109–1124, 2012. doi: 10.1161/CIRCRESAHA.111.246140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Laat KF, van den Berg HA, van Norden AG, Gons RA, Olde Rikkert MG, de Leeuw FE. Microbleeds are independently related to gait disturbances in elderly individuals with cerebral small vessel disease. Stroke 42: 494–497, 2011. doi: 10.1161/STROKEAHA.110.596122. [DOI] [PubMed] [Google Scholar]
- 47.Dey NB, Lincoln TM. Possible involvement of cyclic-GMP-dependent protein kinase on matrix metalloproteinase-2 expression in rat aortic smooth muscle cells. Mol Cell Biochem 368: 27–35, 2012. doi: 10.1007/s11010-012-1339-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Diaz-Otero JM, Garver H, Fink GD, Jackson WF, Dorrance AM. Aging is associated with changes to the biomechanical properties of the posterior cerebral artery and parenchymal arterioles. Am J Physiol Heart Circ Physiol 310: H365–H375, 2016. doi: 10.1152/ajpheart.00562.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dorrance AM, Rupp NC, Nogueira EF. Mineralocorticoid receptor activation causes cerebral vessel remodeling and exacerbates the damage caused by cerebral ischemia. Hypertension 47: 590–595, 2006. doi: 10.1161/01.HYP.0000196945.73586.0d. [DOI] [PubMed] [Google Scholar]
- 50.Duits FH, Hernandez-Guillamon M, Montaner J, Goos JD, Montañola A, Wattjes MP, Barkhof F, Scheltens P, Teunissen CE, van der Flier WM. Matrix metalloproteinases in Alzheimer’s disease and concurrent cerebral microbleeds. J Alzheimers Dis 48: 711–720, 2015. doi: 10.3233/JAD-143186. [DOI] [PubMed] [Google Scholar]
- 51.Eagleton MJ, Peterson DA, Sullivan VV, Roelofs KJ, Ford JA, Stanley JC, Upchurch GR Jr. Nitric oxide inhibition increases aortic wall matrix metalloproteinase-9 expression. J Surg Res 104: 15–21, 2002. doi: 10.1006/jsre.2002.6396. [DOI] [PubMed] [Google Scholar]
- 52.Eberson LS, Sanchez PA, Majeed BA, Tawinwung S, Secomb TW, Larson DF. Effect of lysyl oxidase inhibition on angiotensin II-induced arterial hypertension, remodeling, and stiffness. PLoS One 10: e0124013, 2015. doi: 10.1371/journal.pone.0124013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fan F, Ge Y, Lv W, Elliott MR, Muroya Y, Hirata T, Booz GW, Roman RJ. Molecular mechanisms and cell signaling of 20-hydroxyeicosatetraenoic acid in vascular pathophysiology. Front Biosci 21: 1427–1463, 2016. doi: 10.2741/4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fan F, Geurts AM, Murphy SR, Pabbidi MR, Jacob HJ, Roman RJ. Impaired myogenic response and autoregulation of cerebral blood flow is rescued in CYP4A1 transgenic Dahl salt-sensitive rat. Am J Physiol Regul Integr Comp Physiol 308: R379–R390, 2015. doi: 10.1152/ajpregu.00256.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fan F, Geurts AM, Pabbidi MR, Smith SV, Harder DR, Jacob H, Roman RJ. Zinc-finger nuclease knockout of dual-specificity protein phosphatase-5 enhances the myogenic response and autoregulation of cerebral blood flow in FHH.1BN rats. PLoS One 9: e112878, 2014. doi: 10.1371/journal.pone.0112878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fan F, Pabbidi M, Lin RC, Ge Y, Gomez-Sanchez EP, Rajkowska GK, Moulana M, Gonzalez-Fernandez E, Sims J, Elliott MR, Paul IA, Alexander AP, Mosley TH, Harder DR, Roman RJ. Impaired myogenic response of MCA elevates transmission of pressure to penetrating arterioles and contributes to cerebral vascular disease in aging hypertensive FHH rats. FASEB J 30: 953.957, 2016. [Google Scholar]
- 57.Fan F, Pabbidi MR, Ge Y, Li L, Wang S, Mims PN, Roman RJ. Knockdown of Add3 impairs the myogenic response of renal afferent arterioles and middle cerebral arteries. Am J Physiol Renal Physiol. In press. doi: 10.1152/ajprenal.00529.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fan YH, Zhang L, Lam WW, Mok VC, Wong KS. Cerebral microbleeds as a risk factor for subsequent intracerebral hemorrhages among patients with acute ischemic stroke. Stroke 34: 2459–2462, 2003. doi: 10.1161/01.STR.0000090841.90286.81. [DOI] [PubMed] [Google Scholar]
- 59.Fang M, Feng C, Xu Y, Hua T, Jin AP, Liu XY. Microbleeds and silent brain infarctions are differently associated with cognitive dysfunction in patients with advanced periventricular leukoaraiosis. Int J Med Sci 10: 1307–1313, 2013. doi: 10.7150/ijms.6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Faqeih E, Roughley P, Glorieux FH, Rauch F. Osteogenesis imperfecta type III with intracranial hemorrhage and brachydactyly associated with mutations in exon 49 of COL1A2. Am J Med Genet A 149A: 461–465, 2009. doi: 10.1002/ajmg.a.32653. [DOI] [PubMed] [Google Scholar]
- 61.Faraci FM, Baumbach GL, Heistad DD. Myogenic mechanisms in the cerebral circulation. J Hypertens Suppl 7: S61–S65, 1989. [PubMed] [Google Scholar]
- 62.Faraci FM, Heistad DD. Regulation of large cerebral arteries and cerebral microvascular pressure. Circ Res 66: 8–17, 1990. doi: 10.1161/01.RES.66.1.8. [DOI] [PubMed] [Google Scholar]
- 63.Faraci FM, Mayhan WG, Schmid PG, Heistad DD. Effects of arginine vasopressin on cerebral microvascular pressure. Am J Physiol Heart Circ Physiol 255: H70–H76, 1988. [DOI] [PubMed] [Google Scholar]
- 64.Feng C, Fang M, Xu Y, Hua T, Liu XY. Microbleeds in late-life depression: comparison of early- and late-onset depression. BioMed Res Int 2014: 682092, 2014. doi: 10.1155/2014/682092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fisher CM. Cerebral miliary aneurysms in hypertension. Am J Pathol 66: 313–330, 1972. [PMC free article] [PubMed] [Google Scholar]
- 66.Fisher J, Vaghaiwalla F, Tsitlik J, Levin H, Brinker J, Weisfeldt M, Yin F. Determinants and clinical significance of jugular venous valve competence. Circulation 65: 188–196, 1982. doi: 10.1161/01.CIR.65.1.188. [DOI] [PubMed] [Google Scholar]
- 67.Fisher M, French S, Ji P, Kim RC. Cerebral microbleeds in the elderly: a pathological analysis. Stroke 41: 2782–2785, 2010. doi: 10.1161/STROKEAHA.110.593657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fisher M, Vasilevko V, Passos GF, Ventura C, Quiring D, Cribbs DH. Therapeutic modulation of cerebral microhemorrhage in a mouse model of cerebral amyloid angiopathy. Stroke 42: 3300–3303, 2011. doi: 10.1161/STROKEAHA.111.626655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fonck E, Feigl GG, Fasel J, Sage D, Unser M, Rüfenacht DA, Stergiopulos N. Effect of aging on elastin functionality in human cerebral arteries. Stroke 40: 2552–2556, 2009. doi: 10.1161/STROKEAHA.108.528091. [DOI] [PubMed] [Google Scholar]
- 70.Gao Z, Wang W, Wang Z, Zhao X, Shang Y, Guo Y, Gong M, Yang L, Shi X, Xu X, An N, Wu W. Cerebral microbleeds are associated with deep white matter hyperintensities, but only in hypertensive patients. PLoS One 9: e91637, 2014. doi: 10.1371/journal.pone.0091637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ge Y, Murphy SR, Fan F, Williams JM, Falck JR, Liu R, Roman RJ. Role of 20-HETE in the impaired myogenic and TGF responses of the Af-Art of Dahl salt-sensitive rats. Am J Physiol Renal Physiol 307: F509–F515, 2014. doi: 10.1152/ajprenal.00273.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gebremedhin D, Lange AR, Lowry TF, Taheri MR, Birks EK, Hudetz AG, Narayanan J, Falck JR, Okamoto H, Roman RJ, Nithipatikom K, Campbell WB, Harder DR. Production of 20-HETE and its role in autoregulation of cerebral blood flow. Circ Res 87: 60–65, 2000. doi: 10.1161/01.RES.87.1.60. [DOI] [PubMed] [Google Scholar]
- 73.Goos JD, Henneman WJ, Sluimer JD, Vrenken H, Sluimer IC, Barkhof F, Blankenstein MA, Scheltens PH, van der Flier WM. Incidence of cerebral microbleeds: a longitudinal study in a memory clinic population. Neurology 74: 1954–1960, 2010. doi: 10.1212/WNL.0b013e3181e396ea. [DOI] [PubMed] [Google Scholar]
- 74.Goos JD, Kester MI, Barkhof F, Klein M, Blankenstein MA, Scheltens P, van der Flier WM. Patients with Alzheimer disease with multiple microbleeds: relation with cerebrospinal fluid biomarkers and cognition. Stroke 40: 3455–3460, 2009. doi: 10.1161/STROKEAHA.109.558197. [DOI] [PubMed] [Google Scholar]
- 75.Gorelick PB. Cerebral microbleeds: evidence of heightened risk associated with aspirin use. Arch Neurol 66: 691–693, 2009. doi: 10.1001/archneurol.2009.85. [DOI] [PubMed] [Google Scholar]
- 76.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S; American Heart Association Stroke Council, Council on Epidemiology and Prevention, Council on Cardiovascular Nursing, Council on Cardiovascular Radiology and Intervention, Council on Cardiovascular Surgery and Anesthesia . Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 42: 2672–2713, 2011. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Greenberg SM, Eng JA, Ning M, Smith EE, Rosand J. Hemorrhage burden predicts recurrent intracerebral hemorrhage after lobar hemorrhage. Stroke 35: 1415–1420, 2004. doi: 10.1161/01.STR.0000126807.69758.0e. [DOI] [PubMed] [Google Scholar]
- 78.Greenberg SM, Vernooij MW, Cordonnier C, Viswanathan A, Al-Shahi Salman R, Warach S, Launer LJ, Van Buchem MA, Breteler MM; Microbleed Study Group . Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol 8: 165–174, 2009. doi: 10.1016/S1474-4422(09)70013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gregoire SM, Chaudhary UJ, Brown MM, Yousry TA, Kallis C, Jäger HR, Werring DJ. The microbleed anatomical rating scale (MARS): reliability of a tool to map brain microbleeds. Neurology 73: 1759–1766, 2009. doi: 10.1212/WNL.0b013e3181c34a7d. [DOI] [PubMed] [Google Scholar]
- 80.Gurjar MV, Sharma RV, Bhalla RC. eNOS gene transfer inhibits smooth muscle cell migration and MMP-2 and MMP-9 activity. Arterioscler Thromb Vasc Biol 19: 2871–2877, 1999. doi: 10.1161/01.ATV.19.12.2871. [DOI] [PubMed] [Google Scholar]
- 81.Haacke EM, Boikov AS, Barnes S, Neelavalli J, Khan MA. Susceptibility-weighted imaging. In: Cerebral Microbleeds, edited by Werring DJ. New York: Cambridge University Press, 2011, p. 22–33. [Google Scholar]
- 82.Hajdu MA, Heistad DD, Siems JE, Baumbach GL. Effects of aging on mechanics and composition of cerebral arterioles in rats. Circ Res 66: 1747–1754, 1990. doi: 10.1161/01.RES.66.6.1747. [DOI] [PubMed] [Google Scholar]
- 83.Hale SA, Weger L, Mandala M, Osol G. Reduced NO signaling during pregnancy attenuates outward uterine artery remodeling by altering MMP expression and collagen and elastin deposition. Am J Physiol Heart Circ Physiol 301: H1266–H1275, 2011. doi: 10.1152/ajpheart.00519.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Halpern W, Osol G. Resistance vessels in hypertension. Prog Clin Biol Res 219: 211–223, 1986. [PubMed] [Google Scholar]
- 85.Harder DR, Kauser K, Roman RJ, Lombard JH. Mechanisms of pressure-induced myogenic activation of cerebral and renal arteries: role of the endothelium. J Hypertens Suppl 7: S11–S16, 1989. [PubMed] [Google Scholar]
- 86.Harder DR, Narayanan J, Gebremedhin D. Pressure-induced myogenic tone and role of 20-HETE in mediating autoregulation of cerebral blood flow. Am J Physiol Heart Circ Physiol 300: H1557–H1565, 2011. doi: 10.1152/ajpheart.01097.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Harper SL, Bohlen HG. Microvascular adaptation in the cerebral cortex of adult spontaneously hypertensive rats. Hypertension 6: 408–419, 1984. doi: 10.1161/01.HYP.6.3.408. [DOI] [PubMed] [Google Scholar]
- 88.Harper SL, Bohlen HG, Rubin MJ. Arterial and microvascular contributions to cerebral cortical autoregulation in rats. Am J Physiol Heart Circ Physiol 246: H17–H24, 1984. [DOI] [PubMed] [Google Scholar]
- 89.Hayenga HN, Hu JJ, Meyer CA, Wilson E, Hein TW, Kuo L, Humphrey JD. Differential progressive remodeling of coronary and cerebral arteries and arterioles in an aortic coarctation model of hypertension. Front Physiol 3: 420, 2012. doi: 10.3389/fphys.2012.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Heringa SM, Reijmer YD, Leemans A, Koek HL, Kappelle LJ, Biessels GJ; Utrecht Vascular Cognitive Impairment (VCI) Study Group . Multiple microbleeds are related to cerebral network disruptions in patients with early Alzheimer’s disease. J Alzheimers Dis 38: 211–221, 2014. doi: 10.3233/JAD-130542. [DOI] [PubMed] [Google Scholar]
- 91.Higashi Y, Sukhanov S, Anwar A, Shai SY, Delafontaine P. Aging, atherosclerosis, and IGF-1. J Gerontol A Biol Sci Med Sci 67: 626–639, 2012. doi: 10.1093/gerona/gls102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hilal S, Saini M, Tan CS, Catindig JA, Koay WI, Niessen WJ, Vrooman HA, Wong TY, Chen C, Ikram MK, Venketasubramanian N. Cerebral microbleeds and cognition: the epidemiology of dementia in Singapore study. Alzheimer Dis Assoc Disord 28: 106–112, 2014. doi: 10.1097/WAD.0000000000000015. [DOI] [PubMed] [Google Scholar]
- 93.Hommet C, Mondon K, Constans T, Beaufils E, Desmidt T, Camus V, Cottier JP. Review of cerebral microangiopathy and Alzheimer’s disease: relation between white matter hyperintensities and microbleeds. Dement Geriatr Cogn Disord 32: 367–378, 2011. doi: 10.1159/000335568. [DOI] [PubMed] [Google Scholar]
- 94.Jeanne M, Jorgensen J, Gould DB. Molecular and genetic analyses of collagen type IV mutant mouse models of spontaneous intracerebral hemorrhage identify mechanisms for stroke prevention. Circulation 131: 1555–1565, 2015. doi: 10.1161/CIRCULATIONAHA.114.013395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jeerakathil T, Wolf PA, Beiser A, Hald JK, Au R, Kase CS, Massaro JM, DeCarli C. Cerebral microbleeds: prevalence and associations with cardiovascular risk factors in the Framingham Study. Stroke 35: 1831–1835, 2004. doi: 10.1161/01.STR.0000131809.35202.1b. [DOI] [PubMed] [Google Scholar]
- 96.Jia Z, Mohammed W, Qiu Y, Hong X, Shi H. Hypertension increases the risk of cerebral microbleed in the territory of posterior cerebral artery: a study of the association of microbleeds categorized on a basis of vascular territories and cardiovascular risk factors. J Stroke Cerebrovasc Dis 23: e5–e11, 2014. doi: 10.1016/j.jstrokecerebrovasdis.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 97.Kitchiner D, Jackson M, Walsh K, Peart I, Arnold R. Prognosis of supravalve aortic stenosis in 81 patients in Liverpool (1960–1993). Heart 75: 396–402, 1996. doi: 10.1136/hrt.75.4.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kontos HA, Wei EP, Navari RM, Levasseur JE, Rosenblum WI, Patterson JL Jr. Responses of cerebral arteries and arterioles to acute hypotension and hypertension. Am J Physiol Heart Circ Physiol 234: H371–H383, 1978. [DOI] [PubMed] [Google Scholar]
- 99.Labinskyy N, Csiszar A, Orosz Z, Smith K, Rivera A, Buffenstein R, Ungvari Z. Comparison of endothelial function, O2•− and H2O2 production, and vascular oxidative stress resistance between the longest-living rodent, the naked mole rat, and mice. Am J Physiol Heart Circ Physiol 291: H2698–H2704, 2006. doi: 10.1152/ajpheart.00534.2006. [DOI] [PubMed] [Google Scholar]
- 100.Labinskyy N, Mukhopadhyay P, Toth J, Szalai G, Veres M, Losonczy G, Pinto JT, Pacher P, Ballabh P, Podlutsky A, Austad SN, Csiszar A, Ungvari Z. Longevity is associated with increased vascular resistance to high glucose-induced oxidative stress and inflammatory gene expression in Peromyscus leucopus. Am J Physiol Heart Circ Physiol 296: H946–H956, 2009. doi: 10.1152/ajpheart.00693.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lanfranconi S, Markus HS. COL4A1 mutations as a monogenic cause of cerebral small vessel disease: a systematic review. Stroke 41: e513–e518, 2010. doi: 10.1161/STROKEAHA.110.581918. [DOI] [PubMed] [Google Scholar]
- 102.Lee JM, Yin K, Hsin I, Chen S, Fryer JD, Holtzman DM, Hsu CY, Xu J. Matrix metalloproteinase-9 in cerebral-amyloid-angiopathy-related hemorrhage. J Neurol Sci 229-230: 249–254, 2005. doi: 10.1016/j.jns.2004.11.041. [DOI] [PubMed] [Google Scholar]
- 103.Lee JM, Yin KJ, Hsin I, Chen S, Fryer JD, Holtzman DM, Hsu CY, Xu J. Matrix metalloproteinase-9 and spontaneous hemorrhage in an animal model of cerebral amyloid angiopathy. Ann Neurol 54: 379–382, 2003. doi: 10.1002/ana.10671. [DOI] [PubMed] [Google Scholar]
- 104.Lee SH, Bae HJ, Ko SB, Kim H, Yoon BW, Roh JK. Comparative analysis of the spatial distribution and severity of cerebral microbleeds and old lacunes. J Neurol Neurosurg Psychiatry 75: 423–427, 2004. doi: 10.1136/jnnp.2003.015990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lee SH, Kwon SJ, Kim KS, Yoon BW, Roh JK. Cerebral microbleeds in patients with hypertensive stroke. topographical distribution in the supratentorial area. J Neurol 251: 1183–1189, 2004. doi: 10.1007/s00415-004-0500-6. [DOI] [PubMed] [Google Scholar]
- 106.Lee SH, Park JM, Kwon SJ, Kim H, Kim YH, Roh JK, Yoon BW. Left ventricular hypertrophy is associated with cerebral microbleeds in hypertensive patients. Neurology 63: 16–21, 2004. doi: 10.1212/01.WNL.0000132525.36804.A1. [DOI] [PubMed] [Google Scholar]
- 107.Leys D, Cordonnier C. Brain microbleeds as a potential risk factor for antiplatelet-related intracerebral haemorrhage. J Neurol Neurosurg Psychiatry 81: 589–590, 2010. doi: 10.1136/jnnp.2010.206417. [DOI] [PubMed] [Google Scholar]
- 108.Liu C, Wu C, Yang Q, Gao J, Li L, Yang D, Luo L. Macrophages mediate the repair of brain vascular rupture through direct physical adhesion and mechanical traction. Immunity 44: 1162–1176, 2016. doi: 10.1016/j.immuni.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 109.Liu W, Liu R, Sun W, Peng Q, Zhang W, Xu E, Cheng Y, Ding M, Li Y, Hong Z, Wu J, Zeng J, Yao C, Huang Y; CASISP Study Group . Different impacts of blood pressure variability on the progression of cerebral microbleeds and white matter lesions. Stroke 43: 2916–2922, 2012. doi: 10.1161/STROKEAHA.112.658369. [DOI] [PubMed] [Google Scholar]
- 110.Lovelock CE, Cordonnier C, Naka H, Al-Shahi Salman R, Sudlow CL, Sorimachi T, Werring DJ, Gregoire SM, Imaizumi T, Lee SH, Briley D, Rothwell PM. Antithrombotic drug use, cerebral microbleeds, and intracerebral hemorrhage: a systematic review of published and unpublished studies. Stroke 41: 1222–1228, 2010. doi: 10.1161/STROKEAHA.109.572594. [DOI] [PubMed] [Google Scholar]
- 111.Mattison JA, Wang M, Bernier M, Zhang J, Park SS, Maudsley S, An SS, Santhanam L, Martin B, Faulkner S, Morrell C, Baur JA, Peshkin L, Sosnowska D, Csiszar A, Herbert RL, Tilmont EM, Ungvari Z, Pearson KJ, Lakatta EG, de Cabo R. Resveratrol prevents high fat/sucrose diet-induced central arterial wall inflammation and stiffening in nonhuman primates. Cell Metab 20: 183–190, 2014. doi: 10.1016/j.cmet.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mayhan WG, Faraci FM, Heistad DD. Disruption of the blood-brain barrier in cerebrum and brain stem during acute hypertension. Am J Physiol Heart Circ Physiol 251: H1171–H1175, 1986. [DOI] [PubMed] [Google Scholar]
- 113.Mayhan WG, Faraci FM, Heistad DD. Mechanisms of protection of the blood-brain barrier during acute hypertension in chronically hypertensive rats. Hypertension 9: III101–III105, 1987. doi: 10.1161/01.HYP.9.6_Pt_2.III101. [DOI] [PubMed] [Google Scholar]
- 114.Mayhan WG, Heistad DD. Role of veins and cerebral venous pressure in disruption of the blood-brain barrier. Circ Res 59: 216–220, 1986. doi: 10.1161/01.RES.59.2.216. [DOI] [PubMed] [Google Scholar]
- 115.Mayhan WG, Werber AH, Heistad DD. Protection of cerebral vessels by sympathetic nerves and vascular hypertrophy. Circulation 75: I107–I112, 1987. [PubMed] [Google Scholar]
- 116.Merlini M, Wanner D, Nitsch RM. Tau pathology-dependent remodelling of cerebral arteries precedes Alzheimer’s disease-related microvascular cerebral amyloid angiopathy. Acta Neuropathol 131: 737–752, 2016. doi: 10.1007/s00401-016-1560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Miyata N, Roman RJ. Role of 20-hydroxyeicosatetraenoic acid (20-HETE) in vascular system. J Smooth Muscle Res 41: 175–193, 2005. doi: 10.1540/jsmr.41.175. [DOI] [PubMed] [Google Scholar]
- 118.Mueller SM, Heistad DD. Effect of chronic hypertension on the blood-brain barrier. Hypertension 2: 809–812, 1980. doi: 10.1161/01.HYP.2.6.809. [DOI] [PubMed] [Google Scholar]
- 119.Nandigam RN, Viswanathan A, Delgado P, Skehan ME, Smith EE, Rosand J, Greenberg SM, Dickerson BC. MR imaging detection of cerebral microbleeds: effect of susceptibility-weighted imaging, section thickness, and field strength. Am J Neuroradiol 30: 338–343, 2009. doi: 10.3174/ajnr.A1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ni J, Auriel E, Martinez-Ramirez S, Keil B, Reed AK, Fotiadis P, Gurol EM, Greenberg SM, Viswanathan A. Cortical localization of microbleeds in cerebral amyloid angiopathy: an ultra high-field 7T MRI study. J Alzheimers Dis 43: 1325–1330, 2015. doi: 10.3233/JAD-140864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nighoghossian N, Hermier M, Adeleine P, Blanc-Lasserre K, Derex L, Honnorat J, Philippeau F, Dugor JF, Froment JC, Trouillas P. Old microbleeds are a potential risk factor for cerebral bleeding after ischemic stroke: a gradient-echo T2*-weighted brain MRI study. Stroke 33: 735–742, 2002. doi: 10.1161/hs0302.104615. [DOI] [PubMed] [Google Scholar]
- 122.North KN, Whiteman DA, Pepin MG, Byers PH. Cerebrovascular complications in Ehlers-Danlos syndrome type IV. Ann Neurol 38: 960–964, 1995. doi: 10.1002/ana.410380620. [DOI] [PubMed] [Google Scholar]
- 123.O’Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension 46: 200–204, 2005. doi: 10.1161/01.HYP.0000168052.00426.65. [DOI] [PubMed] [Google Scholar]
- 124.Offenbacher H, Fazekas F, Schmidt R, Koch M, Fazekas G, Kapeller P. MR of cerebral abnormalities concomitant with primary intracerebral hematomas. AJNR Am J Neuroradiol 17: 573–578, 1996. [PMC free article] [PubMed] [Google Scholar]
- 125.Osol G, Halpern W. Myogenic properties of cerebral blood vessels from normotensive and hypertensive rats. Am J Physiol Heart Circ Phsiol 249: H914–H921, 1985. [DOI] [PubMed] [Google Scholar]
- 126.Pabbidi MR, Juncos J, Juncos L, Renic M, Tullos HJ, Lazar J, Jacob HJ, Harder DR, Roman RJ. Identification of a region of rat chromosome 1 that impairs the myogenic response and autoregulation of cerebral blood flow in fawn-hooded hypertensive rats. Am J Physiol Heart Circ Physiol 304: H311–H317, 2013. doi: 10.1152/ajpheart.00622.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pabbidi MR, Mazur O, Fan F, Farley JM, Gebremedhin D, Harder DR, Roman RJ. Enhanced large conductance K+ channel activity contributes to the impaired myogenic response in the cerebral vasculature of Fawn Hooded Hypertensive rats. Am J Physiol Heart Circ Physiol 306: H989–H1000, 2014. doi: 10.1152/ajpheart.00636.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Passalacqua M, Grasso G, Alafaci C, Collufio D, Morabito A, Salpietro FM, Tomasello F. Cerebellar hematoma in a patient with Marfan syndrome. Med Sci Monit 9: CS77–CS79, 2003. [PubMed] [Google Scholar]
- 129.Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovasc Brain Metab Rev 2: 161–192, 1990. [PubMed] [Google Scholar]
- 130.Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab 8: 157–168, 2008. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pepin MG, Schwarze U, Rice KM, Liu M, Leistritz D, Byers PH. Survival is affected by mutation type and molecular mechanism in vascular Ehlers-Danlos syndrome (EDS type IV). Genet Med 16: 881–888, 2014. doi: 10.1038/gim.2014.72. [DOI] [PubMed] [Google Scholar]
- 132.Pettersen JA, Sathiyamoorthy G, Gao FQ, Szilagyi G, Nadkarni NK, St George-Hyslop P, Rogaeva E, Black SE. Microbleed topography, leukoaraiosis, and cognition in probable Alzheimer disease from the Sunnybrook dementia study. Arch Neurol 65: 790–795, 2008. doi: 10.1001/archneur.65.6.790. [DOI] [PubMed] [Google Scholar]
- 133.Phan TS, Li JK, Segers P, Chirinos JA. Misinterpretation of the determinants of elevated forward wave amplitude inflates the role of the proximal aorta. J Am Heart Assoc 5: e003069, 2016. doi: 10.1161/JAHA.115.003069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pinter D, Ritchie SJ, Doubal F, Gattringer T, Morris Z, Bastin ME, Del C Valdés Hernández M, Royle NA, Corley J, Muñoz Maniega S, Pattie A, Dickie DA, Staals J, Gow AJ, Starr JM, Deary IJ, Enzinger C, Fazekas F, Wardlaw J. Impact of small vessel disease in the brain on gait and balance. Sci Rep 7: 41637, 2017. doi: 10.1038/srep41637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pires PW, Dams Ramos CM, Matin N, Dorrance AM. The effects of hypertension on the cerebral circulation. Am J Physiol Heart Circ Physiol 304: H1598–H1614, 2013. doi: 10.1152/ajpheart.00490.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pires PW, Deutsch C, McClain JL, Rogers CT, Dorrance AM. Tempol, a superoxide dismutase mimetic, prevents cerebral vessel remodeling in hypertensive rats. Microvasc Res 80: 445–452, 2010. doi: 10.1016/j.mvr.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pires PW, Girgla SS, McClain JL, Kaminski NE, van Rooijen N, Dorrance AM. Improvement in middle cerebral artery structure and endothelial function in stroke-prone spontaneously hypertensive rats after macrophage depletion. Microcirculation 20: 650–661, 2013. doi: 10.1111/micc.12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pires PW, Girgla SS, Moreno G, McClain JL, Dorrance AM. Tumor necrosis factor-α inhibition attenuates middle cerebral artery remodeling but increases cerebral ischemic damage in hypertensive rats. Am J Physiol Heart Circ Physiol 307: H658–H669, 2014. doi: 10.1152/ajpheart.00018.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pires PW, Jackson WF, Dorrance AM. Regulation of myogenic tone and structure of parenchymal arterioles by hypertension and the mineralocorticoid receptor. Am J Physiol Heart Circ Physiol 309: H127–H136, 2015. doi: 10.1152/ajpheart.00168.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pires PW, Rogers CT, McClain JL, Garver HS, Fink GD, Dorrance AM. Doxycycline, a matrix metalloprotease inhibitor, reduces vascular remodeling and damage after cerebral ischemia in stroke-prone spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 301: H87–H97, 2011. doi: 10.1152/ajpheart.01206.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Plaisier E, Gribouval O, Alamowitch S, Mougenot B, Prost C, Verpont MC, Marro B, Desmettre T, Cohen SY, Roullet E, Dracon M, Fardeau M, Van Agtmael T, Kerjaschki D, Antignac C, Ronco P. COL4A1 mutations and hereditary angiopathy, nephropathy, aneurysms, and muscle cramps. N Engl J Med 357: 2687–2695, 2007. doi: 10.1056/NEJMoa071906. [DOI] [PubMed] [Google Scholar]
- 142.Plattner T, Bolliger S, Zollinger U. Forensic assessment of survived strangulation. Forensic Sci Int 153: 202–207, 2005. doi: 10.1016/j.forsciint.2004.09.106. [DOI] [PubMed] [Google Scholar]
- 143.Poels MM, Ikram MA, van der Lugt A, Hofman A, Krestin GP, Breteler MM, Vernooij MW. Incidence of cerebral microbleeds in the general population: the Rotterdam Scan Study. Stroke 42: 656–661, 2011. doi: 10.1161/STROKEAHA.110.607184. [DOI] [PubMed] [Google Scholar]
- 144.Poels MM, Ikram MA, van der Lugt A, Hofman A, Niessen WJ, Krestin GP, Breteler MM, Vernooij MW. Cerebral microbleeds are associated with worse cognitive function: the Rotterdam Scan Study. Neurology 78: 326–333, 2012. doi: 10.1212/WNL.0b013e3182452928. [DOI] [PubMed] [Google Scholar]
- 145.Poels MM, Vernooij MW, Ikram MA, Hofman A, Krestin GP, van der Lugt A, Breteler MM. Prevalence and risk factors of cerebral microbleeds: an update of the Rotterdam scan study. Stroke 41, Suppl: S103–S106, 2010. doi: 10.1161/STROKEAHA.110.595181. [DOI] [PubMed] [Google Scholar]
- 146.Rigsby CS, Ergul A, Portik Dobos V, Pollock DM, Dorrance AM. Effects of spironolactone on cerebral vessel structure in rats with sustained hypertension. Am J Hypertens 24: 708–715, 2011. doi: 10.1038/ajh.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev 82: 131–185, 2002. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- 148.Romero JR, Preis SR, Beiser A, DeCarli C, Viswanathan A, Martinez-Ramirez S, Kase CS, Wolf PA, Seshadri S. Risk factors, stroke prevention treatments, and prevalence of cerebral microbleeds in the Framingham Heart Study. Stroke 45: 1492–1494, 2014. doi: 10.1161/STROKEAHA.114.004130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Romero JR, Preis SR, Beiser AS, DeCarli C, Lee DY, Viswanathan A, Benjamin EJ, Fontes J, Au R, Pikula A, Wang J, Kase CS, Wolf PA, Irrizary MC, Seshadri S. Lipoprotein phospholipase A2 and cerebral microbleeds in the Framingham Heart Study. Stroke 43: 3091–3094, 2012. doi: 10.1161/STROKEAHA.112.656744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Roob G, Schmidt R, Kapeller P, Lechner A, Hartung HP, Fazekas F. MRI evidence of past cerebral microbleeds in a healthy elderly population. Neurology 52: 991–994, 1999. doi: 10.1212/WNL.52.5.991. [DOI] [PubMed] [Google Scholar]
- 151.Rosenblum WI. Fibrinoid necrosis of small brain arteries and arterioles and miliary aneurysms as causes of hypertensive hemorrhage: a critical reappraisal. Acta Neuropathol 116: 361–369, 2008. doi: 10.1007/s00401-008-0416-9. [DOI] [PubMed] [Google Scholar]
- 152.Rosidi NL, Zhou J, Pattanaik S, Wang P, Jin W, Brophy M, Olbricht WL, Nishimura N, Schaffer CB. Cortical microhemorrhages cause local inflammation but do not trigger widespread dendrite degeneration. PLoS One 6: e26612, 2011. doi: 10.1371/journal.pone.0026612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sadoshima S, Fujishima M, Yoshida F, Ibayashi S, Shiokawa O, Omae T. Cerebral autoregulation in young spontaneously hypertensive rats. Effect of sympathetic denervation. Hypertension 7: 392–397, 1985. [PubMed] [Google Scholar]
- 154.Sadoshima S, Yoshida F, Ibayashi S, Shiokawa O, Fujishima M. Upper limit of cerebral autoregulation during development of hypertension in spontaneously hypertensive rats—effect of sympathetic denervation. Stroke 16: 477–481, 1985. doi: 10.1161/01.STR.16.3.477. [DOI] [PubMed] [Google Scholar]
- 155.Santisakultarm TP, Cornelius NR, Nishimura N, Schafer AI, Silver RT, Doerschuk PC, Olbricht WL, Schaffer CB. In vivo two-photon excited fluorescence microscopy reveals cardiac- and respiration-dependent pulsatile blood flow in cortical blood vessels in mice. Am J Physiol Heart Circ Physiol 302: H1367–H1377, 2012. doi: 10.1152/ajpheart.00417.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Schut AF, Janssen JA, Deinum J, Vergeer JM, Hofman A, Lamberts SW, Oostra BA, Pols HA, Witteman JC, van Duijn CM. Polymorphism in the promoter region of the insulin-like growth factor I gene is related to carotid intima-media thickness and aortic pulse wave velocity in subjects with hypertension. Stroke 34: 1623–1627, 2003. doi: 10.1161/01.STR.0000076013.00240.B0. [DOI] [PubMed] [Google Scholar]
- 157.Shams S, Martola J, Granberg T, Li X, Shams M, Fereshtehnejad SM, Cavallin L, Aspelin P, Kristoffersen-Wiberg M, Wahlund LO. Cerebral microbleeds: different prevalence, topography, and risk factors depending on dementia diagnosis—the Karolinska Imaging Dementia Study. AJNR Am J Neuroradiol 36: 661–666, 2015. doi: 10.3174/ajnr.A4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Shapiro HM, Stromberg DD, Lee DR, Wiederhielm CA. Dynamic pressures in the pial arterial microcirculation. Am J Physiol 221: 279–283, 1971. [DOI] [PubMed] [Google Scholar]
- 159.Sharpey-Schafer EP. The mechanism of syncope after coughing. BMJ 2: 860–863, 1953. doi: 10.1136/bmj.2.4841.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Shen Q, Lin F, Rong X, Yang W, Li Y, Cai Z, Xu P, Xu Y, Tang Y. Temporal cerebral microbleeds are associated with radiation necrosis and cognitive dysfunction in patients treated for nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 94: 1113–1120, 2016. doi: 10.1016/j.ijrobp.2015.11.037. [DOI] [PubMed] [Google Scholar]
- 161.Shimoyama T, Iguchi Y, Kimura K, Mitsumura H, Sengoku R, Kono Y, Morita M, Mochio S. Stroke patients with cerebral microbleeds on MRI scans have arteriolosclerosis as well as systemic atherosclerosis. Hypertens Res 35: 975–979, 2012. doi: 10.1038/hr.2012.84. [DOI] [PubMed] [Google Scholar]
- 162.Shoamanesh A, Preis SR, Beiser AS, Vasan RS, Benjamin EJ, Kase CS, Wolf PA, DeCarli C, Romero JR, Seshadri S. Inflammatory biomarkers, cerebral microbleeds, and small vessel disease: Framingham Heart Study. Neurology 84: 825–832, 2015. doi: 10.1212/WNL.0000000000001279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sonntag WE, Deak F, Ashpole N, Toth P, Csiszar A, Freeman W, Ungvari Z. Insulin-like growth factor-1 in CNS and cerebrovascular aging. Front Aging Neurosci 5: 27, 2013. doi: 10.3389/fnagi.2013.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sörös P, Whitehead S, Spence JD, Hachinski V. Antihypertensive treatment can prevent stroke and cognitive decline. Nat Rev Neurol 9: 174–178, 2013. doi: 10.1038/nrneurol.2012.255. [DOI] [PubMed] [Google Scholar]
- 165.Sperling RA, Jack CR Jr, Black SE, Frosch MP, Greenberg SM, Hyman BT, Scheltens P, Carrillo MC, Thies W, Bednar MM, Black RS, Brashear HR, Grundman M, Siemers ER, Feldman HH, Schindler RJ. Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: recommendations from the Alzheimer’s Association Research Roundtable Workgroup. Alzheimers Dement 7: 367–385, 2011. doi: 10.1016/j.jalz.2011.05.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Springo Z, Tarantini S, Toth P, Tucsek Z, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Aging exacerbates pressure-induced mitochondrial oxidative stress in mouse cerebral arteries. J Gerontol A Biol Sci Med Sci 70: 1355–1359, 2015. doi: 10.1093/gerona/glu244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Springo Z, Toth P, Tarantini S, Ashpole NM, Tucsek Z, Sonntag WE, Csiszar A, Koller A, Ungvari ZI. Aging impairs myogenic adaptation to pulsatile pressure in mouse cerebral arteries. J Cereb Blood Flow Metab 35: 527–530, 2015. doi: 10.1038/jcbfm.2014.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Strandgaard S. Autoregulation of cerebral blood flow in hypertensive patients. The modifying influence of prolonged antihypertensive treatment on the tolerance to acute, drug-induced hypotension. Circulation 53: 720–727, 1976. doi: 10.1161/01.CIR.53.4.720. [DOI] [PubMed] [Google Scholar]
- 169.Strandgaard S, Jones JV, MacKenzie ET, Harper AM. Upper limit of cerebral blood flow autoregulation in experimental renovascular hypertension in the baboon. Circ Res 37: 164–167, 1975. doi: 10.1161/01.RES.37.2.164. [DOI] [PubMed] [Google Scholar]
- 170.Strandgaard S, Olesen J, Skinhoj E, Lassen NA. Autoregulation of brain circulation in severe arterial hypertension. BMJ 1: 507–510, 1973. doi: 10.1136/bmj.1.5852.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Stromberg DD, Fox JR. Pressures in the pial arterial microcirculation of the cat during changes in systemic arterial blood pressure. Circ Res 31: 229–239, 1972. doi: 10.1161/01.RES.31.2.229. [DOI] [PubMed] [Google Scholar]
- 172.Sudduth TL, Powell DK, Smith CD, Greenstein A, Wilcock DM. Induction of hyperhomocysteinemia models vascular dementia by induction of cerebral microhemorrhages and neuroinflammation. J Cereb Blood Flow Metab 33: 708–715, 2013. doi: 10.1038/jcbfm.2013.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sun J, Soo YO, Lam WW, Wong KS, Zeng JS, Fan YH. Different distribution patterns of cerebral microbleeds in acute ischemic stroke patients with and without hypertension. Eur Neurol 62: 298–303, 2009. doi: 10.1159/000235850. [DOI] [PubMed] [Google Scholar]
- 174.Sveinbjornsdottir S, Sigurdsson S, Aspelund T, Kjartansson O, Eiriksdottir G, Valtysdottir B, Lopez OL, van Buchem MA, Jonsson PV, Gudnason V, Launer LJ. Cerebral microbleeds in the population based AGES-Reykjavik study: prevalence and location. J Neurol Neurosurg Psychiatry 79: 1002–1006, 2008. doi: 10.1136/jnnp.2007.121913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Takashima Y, Mori T, Hashimoto M, Kinukawa N, Uchino A, Yuzuriha T, Yao H. Clinical correlating factors and cognitive function in community-dwelling healthy subjects with cerebral microbleeds. J Stroke Cerebrovasc Dis 20: 105–110, 2011. doi: 10.1016/j.jstrokecerebrovasdis.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 176.Tamaki K, Heistad DD. Response of cerebral arteries to sympathetic stimulation during acute hypertension. Hypertension 8: 911–917, 1986. doi: 10.1161/01.HYP.8.10.911. [DOI] [PubMed] [Google Scholar]
- 177.Tang WK, Chen Y, Liang H, Chu WC, Mok VC, Ungvari GS, Wong KS. Cerebral microbleeds as a predictor of 1-year outcome of poststroke depression. Stroke 45: 77–81, 2014. doi: 10.1161/STROKEAHA.113.002686. [DOI] [PubMed] [Google Scholar]
- 178.Tang WK, Chen YK, Liang HJ, Chu WC, Mok VC, Ungvari GS, Wong KS. Cerebral microbleeds and suicidality in stroke. Psychosomatics 53: 439–445, 2012. doi: 10.1016/j.psym.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 179.Tang WK, Chen YK, Lu JY, Chu WC, Mok VC, Ungvari GS, Wong KS. Cerebral microbleeds and depression in lacunar stroke. Stroke 42: 2443–2446, 2011. doi: 10.1161/STROKEAHA.111.614586. [DOI] [PubMed] [Google Scholar]
- 180.Tang WK, Chen YK, Lu JY, Chu WC, Mok VC, Ungvari GS, Wong KS. Cerebral microbleeds and symptom severity of post-stroke depression: a magnetic resonance imaging study. J Affect Disord 129: 354–358, 2011. doi: 10.1016/j.jad.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 181.Tang WK, Chen YK, Lu JY, Mok VC, Xiang YT, Ungvari GS, Ahuja AT, Wong KS. Microbleeds and post-stroke emotional lability. J Neurol Neurosurg Psychiatry 80: 1082–1086, 2009. doi: 10.1136/jnnp.2009.175372. [DOI] [PubMed] [Google Scholar]
- 182.Tang WK, Chen YK, Lu JY, Wong A, Mok V, Chu WC, Ungvari GS, Wong KS. Absence of cerebral microbleeds predicts reversion of vascular ‘cognitive impairment no dementia’ in stroke. Int J Stroke 6: 498–505, 2011. doi: 10.1111/j.1747-4949.2011.00682.x. [DOI] [PubMed] [Google Scholar]
- 183.Tang WK, Liu XX, Chen YK, Abrigo J, Chu WC, Mok VC, Ungvari GS, Wong KS. Cerebral microbleeds and fatigue in stroke. Eur Neurol 71: 213–216, 2014. doi: 10.1159/000354845. [DOI] [PubMed] [Google Scholar]
- 184.Tang WK, Liu XX, Chen YK, Abrigo J, Chu WC, Mok VC, Ungvari GS, Wong KS. Pontine microbleeds and depression in stroke. J Geriatr Psychiatry Neurol 27: 159–164, 2014. doi: 10.1177/0891988714522699. [DOI] [PubMed] [Google Scholar]
- 185.Tarantini S, Valcarcel-Ares NM, Yabluchanskiy A, Springo Z, Fulop GA, Ashpole N, Gautam T, Giles CB, Wren JD, Sonntag WE, Csiszar A, Ungvari Z. IGF-1 deficiency exacerbates hypertension-induced cerebral microhemorrhages in mice, mimicking the aging phenotype. Aging Cell In press. doi: 10.1111/acel.12583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Tarantini S, Tucsek Z, Valcarcel-Ares MN, Toth P, Gautam T, Giles CB, Ballabh P, Wei JY, Wren JD, Ashpole NM, Sonntag WE, Ungvari Z, Csiszar A. Circulating IGF-1 deficiency exacerbates hypertension-induced microvascular rarefaction in the mouse hippocampus and retrosplenial cortex: implications for cerebromicrovascular and brain aging. Age (Dordr) 38: 273–289, 2016. doi: 10.1007/s11357-016-9931-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Tarumi T, Ayaz Khan M, Liu J, Tseng BY, Parker R, Riley J, Tinajero C, Zhang R. Cerebral hemodynamics in normal aging: central artery stiffness, wave reflection, and pressure pulsatility. J Cereb Blood Flow Metab 34: 971–978, 2014. doi: 10.1038/jcbfm.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Tomoto T, Sugawara J, Nogami Y, Aonuma K, Maeda S. The influence of central arterial compliance on cerebrovascular hemodynamics: insights from endurance training intervention. J Appl Physiol 119: 445–451, 2015. doi: 10.1152/japplphysiol.00129.2015. [DOI] [PubMed] [Google Scholar]
- 190.Toth P, Csiszar A, Tucsek Z, Sosnowska D, Gautam T, Koller A, Schwartzman ML, Sonntag WE, Ungvari ZI. Role of 20-HETE,TRP channels, and BKCa in dysregulation of pressure-induced Ca2+ signaling and myogenic constriction of cerebral arteries in aged hypertensive mice. Am J Physiol Heart Circ Physiol 305: H1698–H1708, 2013. doi: 10.1152/ajpheart.00377.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Toth P, Csiszar A, Tucsek Z, Sosnowska D, Gautam T, Koller A, Schwartzman ML, Sonntag WE, Ungvari Z. Role of 20-HETE, TRPC channels, and BKCa in dysregulation of pressure-induced Ca2+ signaling and myogenic constriction of cerebral arteries in aged hypertensive mice. Am J Physiol Heart Circ Physiol 305: H1698–H1708, 2013. doi: 10.1152/ajpheart.00377.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Toth P, Tarantini S, Ashpole NM, Tucsek Z, Milne GL, Valcarcel-Ares NM, Menyhart A, Farkas E, Sonntag WE, Csiszar A, Ungvari Z. IGF-1 deficiency impairs neurovascular coupling in mice: implications for cerebromicrovascular aging. Aging Cell 14: 1034–1044, 2015. doi: 10.1111/acel.12372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Toth P, Tarantini S, Csiszar A, Ungvari ZI. Functional vascular contributions to cognitive impairment and dementia: mechanisms and consequences of cerebral microvascular dysfunction in aging. Am J Physiol Heart Circ Physiol 312: H1−H20, 2017. doi: 10.1152/ajpheart.00581.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Toth P, Tarantini S, Springo Z, Tucsek Z, Gautam T, Giles CB, Wren JD, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Aging exacerbates hypertension-induced cerebral microhemorrhages in mice: role of resveratrol treatment in vasoprotection. Aging Cell 14: 400–408, 2015. doi: 10.1111/acel.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Toth P, Tarantini S, Tucsek Z, Ashpole NM, Sosnowska D, Gautam T, Ballabh P, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Resveratrol treatment rescues neurovascular coupling in aged mice: role of improved cerebromicrovascular endothelial function and downregulation of NADPH oxidase. Am J Physiol Heart Circ Physiol 306: H299–H308, 2014. doi: 10.1152/ajpheart.00744.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Toth P, Tucsek Z, Sosnowska D, Gautam T, Mitschelen M, Tarantini S, Deak F, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Age-related autoregulatory dysfunction and cerebromicrovascular injury in mice with angiotensin II-induced hypertension. J Cereb Blood Flow Metab 33: 1732–1742, 2013. doi: 10.1038/jcbfm.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Toth P, Tucsek Z, Tarantini S, Sosnowska D, Gautam T, Mitschelen M, Koller A, Sonntag WE, Csiszar A, Ungvari Z. IGF-1 deficiency impairs cerebral myogenic autoregulation in hypertensive mice. J Cereb Blood Flow Metab 34: 1887–1897, 2014. doi: 10.1038/jcbfm.2014.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Tsushima Y, Aoki J, Endo K. Brain microhemorrhages detected on T2*-weighted gradient-echo MR images. Am J Neuroradiol 24: 88–96, 2003. [PMC free article] [PubMed] [Google Scholar]
- 199.Tucsek Z, Gautam T, Sonntag WE, Toth P, Saito H, Salomao R, Szabo C, Csiszar A, Ungvari Z. Aging exacerbates microvascular endothelial damage induced by circulating factors present in the serum of septic patients. J Gerontol A Biol Sci Med Sci 68: 652–660, 2013. doi: 10.1093/gerona/gls232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Tucsek Z, Toth P, Sosnowska D, Gautam T, Mitschelen M, Koller A, Szalai G, Sonntag WE, Ungvari Z, Csiszar A. Obesity in aging exacerbates blood-brain barrier disruption, neuroinflammation, and oxidative stress in the mouse hippocampus: effects on expression of genes involved in β-amyloid generation and Alzheimer’s disease. J Gerontol A Biol Sci Med Sci 69: 1212–1226, 2014. doi: 10.1093/gerona/glt177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Tucsek Z, Toth P, Tarantini S, Sosnowska D, Gautam T, Warrington JP, Giles CB, Wren JD, Koller A, Ballabh P, Sonntag WE, Ungvari Z, Csiszar A. Aging exacerbates obesity-induced cerebromicrovascular rarefaction, neurovascular uncoupling, and cognitive decline in mice. J Gerontol A Biol Sci Med Sci 69: 1339–1352, 2014. doi: 10.1093/gerona/glu080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ungvari Z, Bagi Z, Feher A, Recchia FA, Sonntag WE, Pearson K, de Cabo R, Csiszar A. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am J Physiol Heart Circ Physiol 299: H18–H24, 2010. doi: 10.1152/ajpheart.00260.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ungvari Z, Bailey-Downs L, Sosnowska D, Gautam T, Koncz P, Losonczy G, Ballabh P, de Cabo R, Sonntag WE, Csiszar A. Vascular oxidative stress in aging: a homeostatic failure due to dysregulation of NRF2-mediated antioxidant response. Am J Physiol Heart Circ Physiol 301: H363–H372, 2011. doi: 10.1152/ajpheart.01134.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ungvari Z, Csiszar A. The emerging role of IGF-1 deficiency in cardiovascular aging: recent advances. J Gerontol A Biol Sci Med Sci 67: 599–610, 2012. doi: 10.1093/gerona/gls072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ungvari Z, Csiszar A, Huang A, Kaminski PM, Wolin MS, Koller A. High pressure induces superoxide production in isolated arteries via protein kinase C-dependent activation of NAD(P)H oxidase. Circulation 108: 1253–1258, 2003. doi: 10.1161/01.CIR.0000079165.84309.4D. [DOI] [PubMed] [Google Scholar]
- 206.Ungvari Z, Csiszar A, Kaminski PM, Wolin MS, Koller A. Chronic high pressure-induced arterial oxidative stress: involvement of protein kinase C-dependent NAD(P)H oxidase and local renin-angiotensin system. Am J Pathol 165: 219–226, 2004. doi: 10.1016/S0002-9440(10)63290-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ungvari Z, Kaley G, de Cabo R, Sonntag WE, Csiszar A. Mechanisms of vascular aging: new perspectives. J Gerontol A Biol Sci Med Sci 65: 1028–1041, 2010. doi: 10.1093/gerona/glq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ungvari Z, Labinskyy N, Mukhopadhyay P, Pinto JT, Bagi Z, Ballabh P, Zhang C, Pacher P, Csiszar A. Resveratrol attenuates mitochondrial oxidative stress in coronary arterial endothelial cells. Am J Physiol Heart Circ Physiol 297: H1876–H1881, 2009. doi: 10.1152/ajpheart.00375.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ungvari Z, Orosz Z, Labinskyy N, Rivera A, Xiangmin Z, Smith K, Csiszar A. Increased mitochondrial H2O2 production promotes endothelial NF-κB activation in aged rat arteries. Am J Physiol Heart Circ Physiol 293: H37–H47, 2007. doi: 10.1152/ajpheart.01346.2006. [DOI] [PubMed] [Google Scholar]
- 210.Ungvari Z, Parrado-Fernandez C, Csiszar A, de Cabo R. Mechanisms underlying caloric restriction and lifespan regulation: implications for vascular aging. Circ Res 102: 519–528, 2008. doi: 10.1161/CIRCRESAHA.107.168369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Ungvari Z, Sonntag WE. Brain and cerebrovascular aging—new mechanisms and insights. J Gerontol A Biol Sci Med Sci 69: 1307–1310, 2014. doi: 10.1093/gerona/glu187. [DOI] [PubMed] [Google Scholar]
- 212.Ungvari Z, Tucsek Z, Sosnowska D, Toth P, Gautam T, Podlutsky A, Csiszar A, Losonczy G, Valcarcel-Ares MN, Sonntag WE, Csiszar A. Aging-induced dysregulation of dicer1-dependent microRNA expression impairs angiogenic capacity of rat cerebromicrovascular endothelial cells. J Gerontol A Biol Sci Med Sci 68: 877–891, 2013. doi: 10.1093/gerona/gls242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Upchurch GR Jr, Ford JW, Weiss SJ, Knipp BS, Peterson DA, Thompson RW, Eagleton MJ, Broady AJ, Proctor MC, Stanley JC. Nitric oxide inhibition increases matrix metalloproteinase-9 expression by rat aortic smooth muscle cells in vitro. J Vasc Surg 34: 76–83, 2001. doi: 10.1067/mva.2001.115598. [DOI] [PubMed] [Google Scholar]
- 215.Vahedi K, Kubis N, Boukobza M, Arnoult M, Massin P, Tournier-Lasserve E, Bousser MG. COL4A1 mutation in a patient with sporadic, recurrent intracerebral hemorrhage. Stroke 38: 1461–1464, 2007. doi: 10.1161/STROKEAHA.106.475194. [DOI] [PubMed] [Google Scholar]
- 216.Valcarcel-Ares MN, Gautam T, Warrington JP, Bailey-Downs L, Sosnowska D, de Cabo R, Losonczy G, Sonntag WE, Ungvari Z, Csiszar A. Disruption of Nrf2 signaling impairs angiogenic capacity of endothelial cells: implications for microvascular aging. J Gerontol A Biol Sci Med Sci 67: 821–829, 2012. doi: 10.1093/gerona/glr229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Valdés Hernández M, Allerhand M, Glatz A, Clayson L, Muñoz Maniega S, Gow A, Royle N, Bastin M, Starr J, Deary I, Wardlaw J. Do white matter hyperintensities mediate the association between brain iron deposition and cognitive abilities in older people? Eur J Neurol 23: 1202–1209, 2016. doi: 10.1111/ene.13006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Valenti R, Del Bene A, Poggesi A, Ginestroni A, Salvadori E, Pracucci G, Ciolli L, Marini S, Nannucci S, Pasi M, Pescini F, Diciotti S, Orlandi G, Cosottini M, Chiti A, Mascalchi M, Bonuccelli U, Inzitari D, Pantoni L; VMCI-Tuscany Study Group . Cerebral microbleeds in patients with mild cognitive impairment and small vessel disease: The Vascular Mild Cognitive Impairment (VMCI)-Tuscany study. J Neurol Sci 368: 195–202, 2016. doi: 10.1016/j.jns.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 219.van den Boom R, Lesnik Oberstein SA, Ferrari MD, Haan J, van Buchem MA. Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy: MR imaging findings at different ages—3rd–6th decades. Radiology 229: 683–690, 2003. doi: 10.1148/radiol.2293021354. [DOI] [PubMed] [Google Scholar]
- 220.van Norden AG, van den Berg HA, de Laat KF, Gons RA, van Dijk EJ, de Leeuw FE. Frontal and temporal microbleeds are related to cognitive function: the Radboud University Nijmegen Diffusion Tensor and Magnetic Resonance Cohort (RUN DMC) Study. Stroke 42: 3382–3386, 2011. doi: 10.1161/STROKEAHA.111.629634. [DOI] [PubMed] [Google Scholar]
- 221.Vanakker OM, Hemelsoet D, De Paepe A. Hereditary connective tissue diseases in young adult stroke: a comprehensive synthesis. Stroke Res Treat 2011: 712903, 2011. doi: 10.4061/2011/712903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Vernooij MW, van der Lugt A, Ikram MA, Wielopolski PA, Niessen WJ, Hofman A, Krestin GP, Breteler MM. Prevalence and risk factors of cerebral microbleeds: the Rotterdam Scan Study. Neurology 70: 1208–1214, 2008. doi: 10.1212/01.wnl.0000307750.41970.d9. [DOI] [PubMed] [Google Scholar]
- 223.Versluis MJ, Webb AG, Van Buchem MA. Detection of cerebral microbleeds: physical principles, technical aspects and new developments. In: Cerebral Microbleeds, edited by Werring DJ. New York: Cambridge University Press, 2011, p. 13–21. doi: 10.1017/CBO9780511974892.005 [DOI] [Google Scholar]
- 224.Vinters HV, Gilbert JJ. Cerebral amyloid angiopathy: incidence and complications in the aging brain. II. The distribution of amyloid vascular changes. Stroke 14: 924–928, 1983. doi: 10.1161/01.STR.14.6.924. [DOI] [PubMed] [Google Scholar]
- 225.Vitali P, Boghen D, Daneault N, Guillon-Létourneau L, Poppe AY. Cerebral microbleed causing an acute stroke-like episode in a CADASIL patient. Can J Neurol Sci 41: 661–663, 2014. doi: 10.1017/cjn.2014.29. [DOI] [PubMed] [Google Scholar]
- 226.Wakisaka Y, Chu Y, Miller JD, Rosenberg GA, Heistad DD. Critical role for copper/zinc-superoxide dismutase in preventing spontaneous intracerebral hemorrhage during acute and chronic hypertension in mice. Stroke 41: 790–797, 2010. doi: 10.1161/STROKEAHA.109.569616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Wakisaka Y, Chu Y, Miller JD, Rosenberg GA, Heistad DD. Spontaneous intracerebral hemorrhage during acute and chronic hypertension in mice. J Cereb Blood Flow Metab 30: 56–69, 2010. doi: 10.1038/jcbfm.2009.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Wakisaka Y, Miller JD, Chu Y, Baumbach GL, Wilson S, Faraci FM, Sigmund CD, Heistad DD. Oxidative stress through activation of NAD(P)H oxidase in hypertensive mice with spontaneous intracranial hemorrhage. J Cereb Blood Flow Metab 28: 1175–1185, 2008. doi: 10.1038/jcbfm.2008.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Wang J, Fields J, Zhao C, Langer J, Thimmulappa RK, Kensler TW, Yamamoto M, Biswal S, Doré S. Role of Nrf2 in protection against intracerebral hemorrhage injury in mice. Free Radic Biol Med 43: 408–414, 2007. doi: 10.1016/j.freeradbiomed.2007.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Ward SA, Raniga P, Ferris NJ, Woods RL, Storey E, Bailey MJ, Brodtmann A, Yates PA, Donnan GA, Trevaks RE, Wolfe R, Egan GF, McNeil JJ. ASPREE-NEURO study protocol: A randomized controlled trial to determine the effect of low-dose aspirin on cerebral microbleeds, white matter hyperintensities, cognition, and stroke in the healthy elderly. Int J Stroke 12: 108−113, 2016. doi: 10.1177/1747493016669848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Werber AH, Heistad DD. Effects of chronic hypertension and sympathetic nerves on the cerebral microvasculature of stroke-prone spontaneously hypertensive rats. Circ Res 55: 286–294, 1984. doi: 10.1161/01.RES.55.3.286. [DOI] [PubMed] [Google Scholar]
- 232.Werring DJ. (editor). Mechanisms underlying microbleeds. In: Cerebral Microbleeds. New York: Cambridge University Press, 2011. doi: 10.1017/CBO9780511974892 [DOI] [Google Scholar]
- 233.Werring DJ, Frazer DW, Coward LJ, Losseff NA, Watt H, Cipolotti L, Brown MM, Jäger HR. Cognitive dysfunction in patients with cerebral microbleeds on T2*-weighted gradient-echo MRI. Brain 127: 2265–2275, 2004. doi: 10.1093/brain/awh253. [DOI] [PubMed] [Google Scholar]
- 234.Werring DJ, Gregoire SM, Cipolotti L. Cerebral microbleeds and vascular cognitive impairment. J Neurol Sci 299: 131–135, 2010. doi: 10.1016/j.jns.2010.08.034. [DOI] [PubMed] [Google Scholar]
- 235.Wiegman AF, Meier IB, Schupf N, Manly JJ, Guzman VA, Narkhede A, Stern Y, Martinez-Ramirez S, Viswanathan A, Luchsinger JA, Greenberg SM, Mayeux R, Brickman AM. Cerebral microbleeds in a multiethnic elderly community: demographic and clinical correlates. J Neurol Sci 345: 125–130, 2014. doi: 10.1016/j.jns.2014.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Wilcock DM, Morgan D, Gordon MN, Taylor TL, Ridnour LA, Wink DA, Colton CA. Activation of matrix metalloproteinases following anti-Aβ immunotherapy: implications for microhemorrhage occurrence. J Neuroinflammation 8: 115, 2011. doi: 10.1186/1742-2094-8-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Woerdeman J, van Duinkerken E, Wattjes MP, Barkhof F, Snoek FJ, Moll AC, Klein M, de Boer MP, Ijzerman RG, Serné EH, Diamant M. Proliferative retinopathy in type 1 diabetes is associated with cerebral microbleeds, which is part of generalized microangiopathy. Diabetes Care 37: 1165–1168, 2014. doi: 10.2337/dc13-1586. [DOI] [PubMed] [Google Scholar]
- 238.Wu R, Feng C, Zhao Y, Jin AP, Fang M, Liu X. A meta-analysis of association between cerebral microbleeds and cognitive impairment. Med Sci Monit 20: 2189–2198, 2014. doi: 10.12659/MSM.891004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Wysoki MG, Covey A, Pollak J, Rosenblatt M, Aruny J, Denbow N. Evaluation of various maneuvers for prevention of air embolism during central venous catheter placement. J Vasc Interv Radiol 12: 764–766, 2001. doi: 10.1016/S1051-0443(07)61451-1. [DOI] [PubMed] [Google Scholar]
- 240.Yakushiji Y, Noguchi T, Charidimou A, Eriguchi M, Nishihara M, Hara M, Nanri Y, Horikawa E, Nishiyama M, Werring DJ, Hara H. Basal ganglia cerebral microbleeds and global cognitive function: the Kashima Scan Study. J Stroke Cerebrovasc Dis 24: 431–439, 2015. doi: 10.1016/j.jstrokecerebrovasdis.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 241.Yakushiji Y, Werring DJ. Cerebrovascular disease: Lobar cerebral microbleeds signal early cognitive impairment. Nat Rev Neurol 12: 680–682, 2016. doi: 10.1038/nrneurol.2016.179. [DOI] [PubMed] [Google Scholar]
- 242.Yang Q, Yang Y, Li C, Li J, Liu X, Wang A, Zhao J, Wang M, Zeng X, Fan D. Quantitative assessment and correlation analysis of cerebral microbleed distribution and leukoaraiosis in stroke outpatients. Neurol Res 37: 403–409, 2015. doi: 10.1179/1743132815Y.0000000027. [DOI] [PubMed] [Google Scholar]
- 243.Yates PA, Desmond PM, Phal PM, Steward C, Szoeke C, Salvado O, Ellis KA, Martins RN, Masters CL, Ames D, Villemagne VL, Rowe CC; AIBL Research Group . Incidence of cerebral microbleeds in preclinical Alzheimer disease. Neurology 82: 1266–1273, 2014. doi: 10.1212/WNL.0000000000000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Yates PA, Sirisriro R, Villemagne VL, Farquharson S, Masters CL, Rowe CC; AIBL Research Group . Cerebral microhemorrhage and brain β-amyloid in aging and Alzheimer disease. Neurology 77: 48–54, 2011. doi: 10.1212/WNL.0b013e318221ad36. [DOI] [PubMed] [Google Scholar]
- 245.Yates PA, Villemagne VL, Ellis KA, Desmond PM, Masters CL, Rowe CC. Cerebral microbleeds: a review of clinical, genetic, and neuroimaging associations. Front Neurol 4: 205, 2014. doi: 10.3389/fneur.2013.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Yen K, Vock P, Christe A, Scheurer E, Plattner T, Schön C, Aghayev E, Jackowski C, Beutler V, Thali MJ, Dirnhofer R. Clinical forensic radiology in strangulation victims: forensic expertise based on magnetic resonance imaging (MRI) findings. Int J Legal Med 121: 115–123, 2007. doi: 10.1007/s00414-006-0121-y. [DOI] [PubMed] [Google Scholar]
- 247.Zhang H, Zhang J, Ungvari Z, Zhang C. Resveratrol improves endothelial function: role of TNFα and vascular oxidative stress. Arterioscler Thromb Vasc Biol 29: 1164–1171, 2009. doi: 10.1161/ATVBAHA.109.187146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Zhao L, Arbel-Ornath M, Wang X, Betensky RA, Greenberg SM, Frosch MP, Bacskai BJ. Matrix metalloproteinase 9-mediated intracerebral hemorrhage induced by cerebral amyloid angiopathy. Neurobiol Aging 36: 2963–2971, 2015. doi: 10.1016/j.neurobiolaging.2015.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Zivadinov R. Is there a link between the extracranial venous system and central nervous system pathology? BMC Med 11: 259, 2013. doi: 10.1186/1741-7015-11-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Zivadinov R, Chung CP. Potential involvement of the extracranial venous system in central nervous system disorders and aging. BMC Med 11: 260, 2013. doi: 10.1186/1741-7015-11-260. [DOI] [PMC free article] [PubMed] [Google Scholar]