Abstract
The heart oxidizes fatty acids, carbohydrates, and ketone bodies inside the tricarboxylic acid (TCA) cycle to generate the reducing equivalents needed for ATP production. Competition between these substrates makes it difficult to estimate the extent of pyruvate oxidation. Previously, hyperpolarized pyruvate detected propionate-mediated activation of carbohydrate oxidation, even in the presence of acetate. In this report, the optimal concentration of propionate for the activation of glucose oxidation was measured in mouse hearts perfused in Langendorff mode. This study was performed with a more physiologically relevant perfusate than the previous work. Increasing concentrations of propionate did not cause adverse effects on myocardial metabolism, as evidenced by unchanged O2 consumption, TCA cycle flux, and developed pressures. Propionate at 1 mM was sufficient to achieve significant increases in pyruvate dehydrogenase flux (3×), and anaplerosis (6×), as measured by isotopomer analysis. These results further demonstrate the potential of propionate as an aid for the correct estimation of total carbohydrate oxidative capacity in the heart. However, liquid chromotography/mass spectroscopy-based metabolomics detected large changes (~30-fold) in malate and fumarate pool sizes. This observation leads to a key observation regarding mass balance in the TCA cycle; flux through a portion of the cycle can be drastically elevated without changing the O2 consumption.
Keywords: substrate selection, carbohydrates, fatty acids, isotopomer analysis, metabolomics, metabonomics
oxidative phosphorylation is tightly coupled with the rate of ATP hydrolysis to accommodate the large need for energy for mechanical work done by the heart (43). The major source of reducing equivalents for oxidative phosphorylation in healthy hearts is fatty acid (FA) oxidation (FAO), while the contribution of glycolysis in the generation of reducing equivalents is comparatively minor (14). However, in the case of failing hearts, it has been observed that glucose oxidation is elevated compared with the utilization of fatty acids (2, 15, 24, 34), a phenomenon that has been attributed to fetal gene reprogramming (13). Therapeutic approaches based on upregulating either glucose or FAO have been proposed for the treatment of heart failure (19, 20, 27). Studies performed on mice with congenital overexpression of glucose transporter 1 (GLUT1) resulting in a significant increase in glucose oxidation have shown normal cardiac function even under pressure overload conditions. However, inhibition of FAO without a tandem increase in glucose oxidation has been shown to be detrimental, as observed in carnitine palmitoyl transferase-1 (CPT-1) knockout mice (1). In another study (23), it was shown that prevention of substrate switching due to fetal gene reprogramming by deletion of cardiac-specific acetyl-CoA carboxylase (ACC2) resulted in hypertrophied hearts exhibiting function similar to control hearts. These varied observations illustrate the need for approaches that address the issue of myocardial substrate preferences in detail.
Hyperpolarized (HP) carbon-13-based imaging is a new, metabolically sensitive technique that can be used for human imaging (10, 33). HP imaging can detect changes in substrate preference in real time (5). The selectivity of carbon-13 imaging paired with the short time needed for the protocol suggests that it can evolve into a clinically relevant means of assessing myocardial metabolism in humans (10). For robust identification of altered enzyme activity in the myocardium, the addition of exogenous agents that modulate metabolic flux can be useful (26). A similar approach was illustrated in the work by Purmal et al. (36), in which propionate was shown to increase pyruvate utilization >30 times even in the presence of 2 mM acetate, a condition that maximally restricts carbohydrate oxidation. Propionate also undergoes carboxylation to form methyl-malonyl CoA and eventually succinyl-CoA, thereby providing an anaplerotic flux into the tricarboxylic acid (TCA) cycle (7, 18, 32, 41a). Based on these results, it was hypothesized that a pretreatment of propionate given intravenously to a subject should activate pyruvate dehydrogenase (PDH), allowing a more accurate assessment of the maximal PDH flux in the heart, specifically, in the presence of competing physiological substrates like FAs. The optimal concentration of propionate required for such a modulation of PDH flux is, however, not clear from the literature.
In this study, the concentration of propionate was varied to identify the condition enabling maximal activation of both anaplerotic and PDH flux. This was done in mouse hearts perfused in Langendorff mode under conditions that mimic the native physiological state, as opposed to the previous work using acetate perfusion alone (36). We used a high temperature superconducting (HTS) probe (37) to overcome sensitivity challenges in measuring 13C NMR spectra for isotopomer analysis in mouse hearts. As part of the research design, liquid chromotography (LC)/mass spectrometry (MS)-based metabolomics of TCA cycle intermediates was also used to build a more complete picture of the effect of propionate on heart metabolism. The data indicated that anaplerosis and cataplerosis can be modulated significantly without affecting myocardial O2 consumption and, by extension, its capacity for mechanical work.
MATERIALS AND METHODS
Perfusions.
Mice were euthanized by cervical dislocation, and the heart was excised from the thoracic cavity. The heart was rapidly transferred to ice-cold saline to arrest beating. Fat deposits and connective tissues were cleaned off the heart surgically, and the aorta was cannulated. Cannulated hearts were perfused under constant-pressure (100 cmH2O) Langendorff mode (42) for 30 min and freeze clamped immediately after. The perfusate was composed of Krebs-Henseleit buffer (pH 7.4), 5.6 mM [1,6-13C]glucose (Sigma-Aldrich), 0.63 mM [U-13C]free FAs (FFA; Cambridge Isotope Laboratories) [FFA concentration chosen to mimic circulating concentrations (39)], and 1 µU/ml insulin. Control perfusions without propionate and perfusions under four different concentrations (0.25, 1, 3, and 6 mM) of [1-13C]sodium propionate (Cambridge Isotope Laboratories) were carried out. Before and during the perfusion, the perfusate was constantly aerated using a mixture of 95% O2 and 5% CO2 at 37°C. O2 consumption of the hearts and pH of the perfusate during the perfusion were monitored using a blood gas analyzer. A total of 41 mice were used for perfusions using the labeled substrates. Breakdown for each group is provided in the figure legends.
MS.
Pool sizes of TCA cycle intermediates were determined using MS as previously described in the literature (12). Eighteen mice (breakdown for each group is provided in the figure legends) were used for the MS experiments. These perfusions were carried out using the same perfusate but without the labeled substrates.
Perchloric acid extraction.
Metabolites in the heart were isolated using perchloric acid (PCA) extraction. Briefly, tissues were crushed in a liquid nitrogen-cooled pestle and mortar. The crushed powder was mixed with ice-cold 6% (vol/vol) PCA, agitated using a vortex, and centrifuged at 4°C. The supernatant was decanted, neutralized using potassium hydroxide (initially using 15 M KOH and subsequently using 2 M KOH), and centrifuged to remove the insoluble potassium perchlorate and lyophilized. To prepare salt-free NMR samples, the lyophilized powder was dissolved in ~200 µl deionized water and pH was readjusted using sodium hydroxide and hydrochloric acid, centrifuged to remove excess salt, and lyophilized again.
NMR spectroscopy.
All spectra were measured using a 14.1-T NMR magnet equipped with either a CP-DUL cryoprobe (Bruker Biospin, Billerca, MA) or a home-built superconducting (HTS) probe (37). NMR samples were prepared by dissolving the lyophilized powder in 90% (vol/vol) of 50 mM sodium phosphate buffer in D2O (pH 7.0) containing 2 mM EDTA. To this solution, 10% (vol/vol) internal standard (5 mM DSS-D6 and 0.2% NaN3 in D2O, Chenomx) was added. Total sample volumes were either 200 µl (CP-DUL probe) or 50 µl (HTS probe). 1H spectra were measured with a spectral width of 12 ppm and acquisition time of 4 s. 13C spectra were measured with an acquisition time of 1.4 s, a spectral width of 240 ppm, and 1H decoupling field strength of 4800 Hz.
Data analysis.
13C NMR spectra were processed (zero filled to 131,072 points, 0.5-Hz exponential line broadening, and polynomial or spline baseline correction as needed) using ACD NMR processor (ACD Laboratories, Toronto, ON, Canada). Chemical shifts were calibrated by setting the singlet resonance of taurine to 48.4 ppm. Analysis was carried out by fitting glutamate and aspartate peak multiplets to a mixed Gaussian/Lorentzian shape to extract relative peak areas. Areas obtained from the results of the fitting procedure were used as input to a metabolic model and solved numerically using tcaCALC (8, 31, 41). Fluxes from different substrates were calculated from the model results and O2 consumption data using appropriate weighting factors reported in the literature (29). Fractional contributions to acetyl-CoA from endogenous, unlabeled sources were estimated assuming that Fc0 + Fc2 + Fc3 = 1, where Fc0 is the fraction of acetyl-CoA with no 13C labeling, Fc2 is the fraction of [2-13C]acetyl-CoA, and Fc3 is the fraction of [1,2-13C2]acetyl-CoA. With the chosen perfusion conditions, Fc2 and Fc3 correspond to contributions from [1,6-13C2]glucose and [U-13C]long-chain FAs (LCFAs), respectively.
Statistical analysis.
ANOVA was performed to understand the statistical significance of observed changes to various fluxes (17). This analysis took into account all measured variables simultaneously. The ANOVA model used here was as follows:
where yij is the value of the response (relative intensities of the carbon multiplets, aspartate 1S, aspartate 1D, etc., or the calculated fluxes) for group i (i = 1, 2, . . ., g) and sample j (j = 1, 2, . . ., n). μ is the overall mean with errors εij assumed to be independent and identically distributed N (0,σ2) (16). When the number of groups g = 2, the ANOVA and t-test produce equivalent results.
However, other important variables may affect the experiment. These variables can also be modeled in this framework. Mouse weight, heart weight, and O2 consumption after 30 min were considered as potentially important covariates in the model. Models with covariates were evaluated using a combination of Bayesian information criterion (40) and residual analysis. The ANOVA model with covariate [also called the ANCOVA model (44)] used for analysis had the following form:
where yij is the value of the response for group i (i = 1, 2, . . ., g) and sample j (j = 1, 2, . . ., n). μ is the overall mean, and xj is the value for the covariate recorded for sample j. If the covariate has no effect, parameter β is not different from zero. As with the single factor model, the null hypothesis that τ1 = τ2 = . . . = τg can be tested. If the covariate x and the treatment are independent, then the test of the effect of the treatment will be the same, regardless of whether the ANOVA or ANCOVA model is used.
Results from the overall (ANCOVA) model reflect a test of equivalence between means (for the effect of propionate dose) or association between quantity measured and the measured covariate (mouse weight, heart weight, O2). When the best model included a significant effect for amount of propionate, contrasts were performed to assess the pairwise differences between conditions: 0 vs. 0.25, 0 vs. 1, 0 vs. 3, or 0 vs. 6 for the changes in fluxes observed.
Ethics approval.
Studies involving animals were carried out using the protocol approved by the Institutional Animal Care and Use Committees of the University of Florida and University of Texas Southwestern Medical Center (Dallas, TX).
Availability of data and materials.
The data sets and/or analysis from the present study are available from the corresponding author on reasonable request.
RESULTS
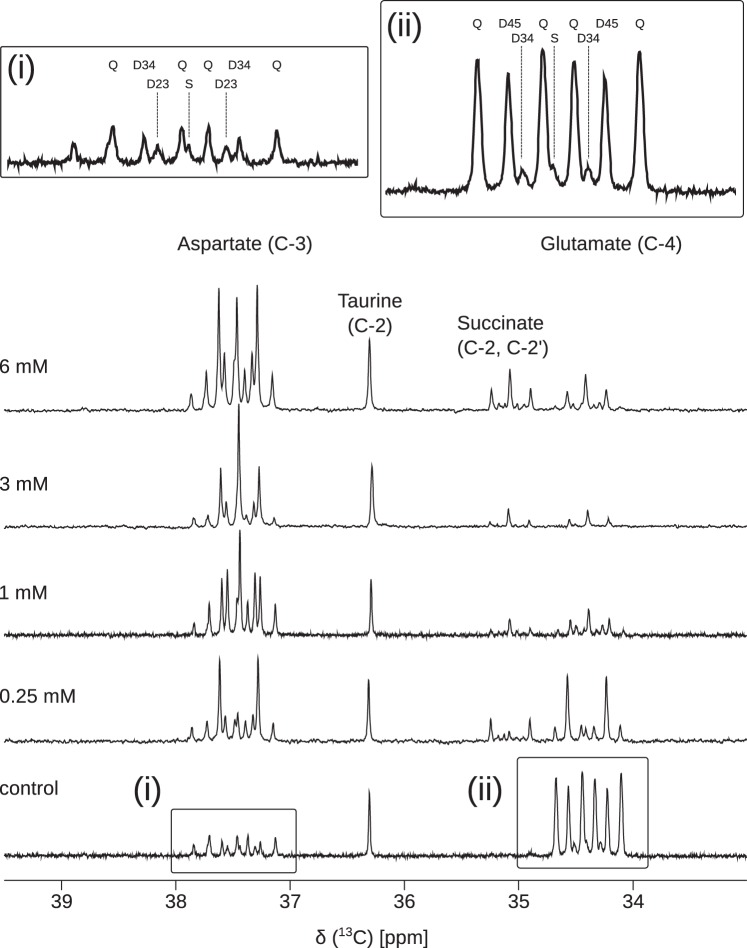
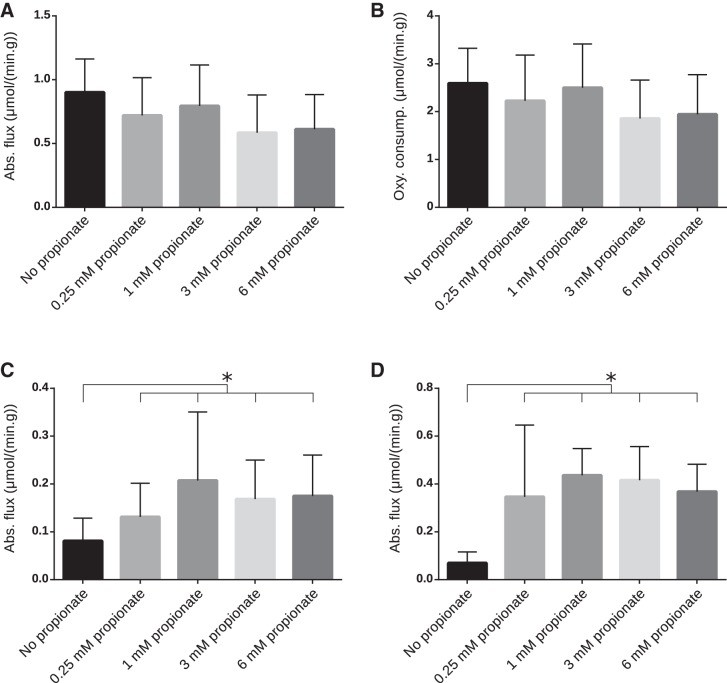
Measures of myocardial function including heart rate, coronary flow, and developed pressure showed no significant changes as a function of propionate concentration (see Supplemental Figures S1–S4 in the Supplemental Material; Supplemental Material for this article is available at the American Journal of Physiology-Heart and Circulatory Physiology website). Representative 13C NMR spectra of the PCA extracts displayed excellent signal-to-noise (SNR) ratios (Fig. 1). Figure 1 shows, in detail, changes in metabolite pool sizes as well as dilution of TCA cycle intermediates by increasing concentrations of propionate. Glutamate concentration decreased with the addition of propionate, whereas the aspartate concentration increased. Downregulation of LCFA oxidation and increased propionate anaplerosis manifests in distinct ways. The depletion of the quartet in glutamate, which must arise from doubly labeled acetyl-CoA, signals a switch to greater glucose oxidation. The development of intense singlets in aspartate C3 and glutamate C4 as a function of propionate concentration is indicative not only of increased glucose oxidation, which produces only methyl-labeled acetyl-CoA, but also a fractional decrease in carbon-13 enrichment in the interior carbons of TCA cycle intermediates. If glutamate C3 were more highly enriched in carbon-13, the fraction of C4D34 would increase and the singlet resonance would decrease in glutamate C4. Despite the large changes in the pool sizes and differences in substrate selection, the total TCA cycle flux and O2 consumption in the presence of propionate did not significantly change (Fig. 2, A and B) This observation suggests that the addition of propionate does not adversely affect the energy metabolism in the perfused tissue even at the highest concentrations used.
Fig. 1.
Representative 13C NMR spectra of perchloric acid extracts of hearts perfused with and without four different concentrations of [1-13C]sodium propionate (indicated in the figure). [1,6-13C]glucose (5.6 mM) and uniformly 13C-enriched free fatty acids (0.63 mM) were also present in the perfusate. Resonances corresponding to C4 of glutamate (~34.5 ppm) and C3 of aspartate (~37.5 ppm) are shown. Insets i and ii show the spectral components for each metabolite. S is singlet, D is the doublet assigned to the coupling indicated in each subscript, and Q is the quartet, actually a doublet of doublets associated with isotopomers that have three consecutive carbons enriched in 13C. Aspartate C3 shares the same atom mapping as glutamate C4 and hence reports on acetyl-CoA labeling in an analogous manner.
Fig. 2.
A and B: tricarboxylic acid (TCA) cycle flux with varying concentrations of propionate (A) and measured O2 consumption during perfusion (B). No change in TCA cycle flux or O2 consumption was significant (see also Table 1). C and D: absolute values of pyruvate dehydrogenase (PDH) flux (C) and anaplerotic flux (D) calculated by isotopomer analysis. *Statistical significance (P < 0.05). Statistical significance was measured using the analysis of covariance (ANCOVA) model detailed in methods. Numbers of mice for each group were as follows: 9 (control, 0.25 mM, and 1 mM), 8 (3 mM), and 7 (6 mM).
To understand the statistical significance of the results, the data were analyzed using ANOVA methods. Using ANCOVA models (see methods for details), it was established that the effect of O2 consumption was significant for TCA cycle turnover and fractional oxidation of glucose versus FAs but not for endogenous sources of acetyl-CoA or anaplerotic flux. The P values for the test of groups were lower in the ANCOVA model compared with the ANOVA model. This leads to the conclusion that the O2 consumption, TCA cycle flux, and fractional oxidation of fatty acids and glucose are interdependent. There was no evidence that mouse and heart weights were important covariates and so were excluded from further analysis. The summary of P values for pairwise comparison adjusted for O2 consumption using the ANCOVA model are shown in Table 1. The differences in endogenous sources of acetyl-CoA for increasing propionate concentrations were not significant and are not presented in the summary.
Table 1.
ANCOVA of various flux values calculated based on the NMR spectra and O2 consumption measurements
| Comparison | P Value |
|---|---|
| Glucose flux | |
| Control vs. 0.25 mM | 0.0129 |
| Control vs. 1 mM | 0.0005 |
| Control vs. 3 mM | 0.0004 |
| Control vs. 6 mM | 0.0007 |
| Fatty acid flux | |
| Control vs. 0.25 mM | 0.2216 |
| Control vs. 1 mM | 0.0038 |
| Control vs. 3 mM | 0.0025 |
| Control vs. 6 mM | 0.0059 |
| Pyruvate dehydrogenase flux | |
| Control vs. 0.25 mM | 0.0129 |
| Control vs. 1 mM | 0.0005 |
| Control vs. 3 mM | 0.0004 |
| Control vs. 6 mM | 0.0007 |
| Propionate anaplerosis | |
| Control vs. 0.25 mM | 0.0014 |
| Control vs. 1 mM | 0.0001 |
| Control vs. 3 mM | 0.0003 |
| Control vs. 6 mM | 0.0020 |
| Total tricarboxylic acid flux | |
| Control vs. 0.25 mM | 0.5501 |
| Control vs. 1 mM | 0.2580 |
| Control vs. 3 mM | 0.1957 |
| Control vs. 6 mM | 0.1556 |
Group (propionate treatment) and O2 consumption values were the variables used in the model. Numbers of mice for each group were as follows: 9 (control, 0.25 mM, and 1 mM), 8 (3 mM), and 7 (6 mM). ANCOVA, analysis of covariance.
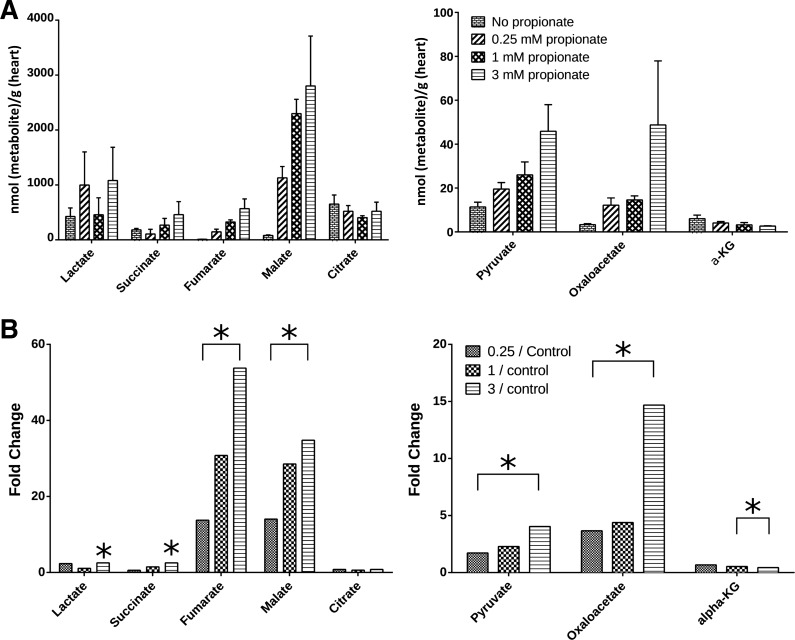
Pool sizes of TCA cycle intermediates were measured using MS. It was immediately apparent that the addition of propionate caused profound changes in the pool sizes of several intermediates (Fig. 3A). There was a large increase in the pool size for four-carbon TCA cycle intermediates upon the addition of just 0.25 mM propionate to the perfusate, whereas subsequent increases in the pool sizes appeared to be incremental with higher concentrations of propionate. This can be readily seen by the fourfold increase in fumarate between 0.25 and 3 mM propionate perfusions (Fig. 3B). Among the TCA cycle intermediates, malate and fumarate showed the largest increase in pool sizes with 50 and 35 times increases in the presence of 3 mM propionate compared with the control (no propionate) samples.
Fig. 3.
A: pool sizes of TCA cycle intermediates and related metabolites as determined by mass spectrometry. B: relative changes in the pool sizes with respect to changes in propionate concentration in the perfusate. Significant changes were identified using paired t-tests (*P < 0.05). Numbers of mice for each group were as follows: 6 (control and 1 mM) and 3 (0.25 and 3 mM).
DISCUSSION
The necessary role of pyruvate anaplerosis in the maintenance of TCA cycle turnover was first established in the perfused rat heart (38). Alternatively, propionate metabolism has also been studied extensively in the rat heart using multiple methods. Initial results using carbon-14 powerfully demonstrated propionate’s effect on PDH flux (25) and were the motivation for our initial study (36). However, radiotracer studies cannot easily report on isotopomer distributions due to the need for carbon-by-carbon degradation of the metabolic products, sacrificing much of the encoded information. The chemical selectivity of NMR and its ability to detect multiple isotopomers simultaneously through 13C-13C homonuclear J-couplings means it can powerfully address propionate metabolism, but early studies focused on properly developing the metabolic modeling as opposed to measuring results using a physiological mixture of substrates (30). The perfusion conditions used in this report are our best approximation of myocardial metabolism in the fed state. Similarly, the well-executed experiments of Kasumov et al. (22) using MS focused on quantitative measures of propionate anaplerosis as opposed to substrate selection for acetyl-CoA production, also in a perfusion condition not matched to our hypothesis. Other experiments from the same group (6) paired propionate and oleate in the perfusate but did not supply glucose as an alternative substrate. Our central hypothesis is that propionate can be used to more accurately assess the active fraction of PDH in the heart using hyperpolarized [1-13C]pyruvate. Confirmation of this hypothesis necessitates a quantitative understanding of substrate selection. While we do not report on results with hyperpolarization here, we consider the results presented to be essential for the correct interpretation of future experiments using hyperpolarized species. The data presented here are also distinguished by its quality; to our knowledge, results like these have not been achieved in the perfused mouse heart, as opposed to previous results in the rat heart. The inclusion of a HTS NMR probe in the research design enabled the acquisition of suitable carbon-13 NMR spectra from extracted mouse hearts in ~25% of the time using conventional cryoprobe technology (37). Generalization of carbon-13 NMR isotopomer methods to mouse hearts enables the future study of genetically modified animals with the consequent increase in knowledge of metabolic regulation.
It has been shown that propionate dramatically increases pyruvate flux through the PDH complex in the heart perfused in the presence of acetate (36). Since acetate is not β-oxidized and does not require active transport into the mitochondria, it should be the most avidly consumed of all possible substrates for acetyl-CoA production. In this work, we used physiologically relevant LCFAs in the perfusate for the hearts. It can be seen from the control perfusions (no propionate) that the heart prefers FAs as the primary substrate for oxidative flux (Fig. 4), as is well known (28). Addition of propionate shifts the substrate preference in the heart from FA to glucose oxidation, showing an additive behavior with increasing concentration. Fitting the data to a quantitative model of TCA cycle flux shows that higher concentrations of propionate appear to suppress FAO and upregulate glucose oxidation efficiently. On average, FA flux contributed to >60% of the total TCA cycle flux without propionate. This contribution dropped by 30% in the presence of 3 mM propionate. The suppression of FAO is likely due to weak inhibition of the CPT-1 complex by methylmalonyl-CoA (21). Due to the abundance of propionyl-CoA carboxylase in the heart (18), propionyl-CoA is readily converted to methylmalonyl-CoA, which, in turn, can inhibit CPT-I. Concurrently, glucose oxidation increased almost threefold to 24% of the total TCA cycle flux in the presence of 1 mM propionate (in comparison, control was ~8% of the total flux). In terms of absolute flux, glucose oxidation increased from 0.081 µmol·min−1·g−1 in the absence of propionate to 0.208 µmol·min−1·g−1 in the presence of 1 mM propionate. These changes, however, did not appreciably change the total TCA turnover across the conditions involving different propionate concentrations. In addition to increases in glucose oxidation, PDH flux almost tripled in the presence of 1 mM propionate (Fig. 2C). However, if modulation of CPT-I activity is responsible for increased glucose oxidation, it is not readily apparent why propionate should so effectively upregulate pyruvate oxidation in the presence of acetate (36). A more direct role of propionate in PDH activation is still possible.
Fig. 4.
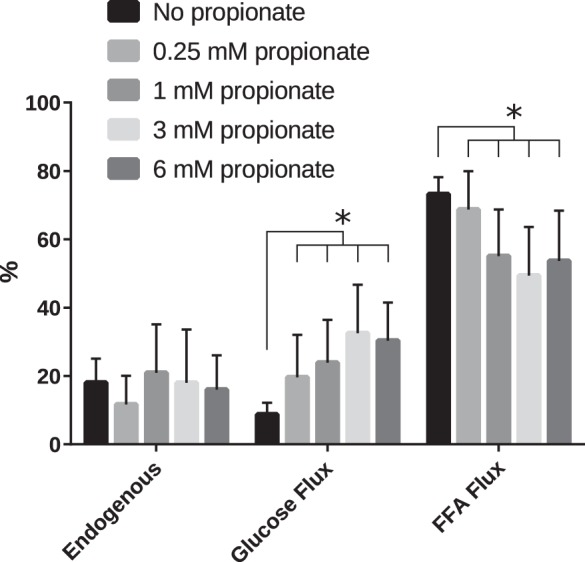
Contribution of fluxes through different pathways to the total TCA cycle flux expressed as percentages. *Significant changes (P < 0.05). Significance was established using the ANCOVA model including O2 consumption. Even the lowest concentration of propionate significantly upregulated glucose oxidation.
Exposure of the heart to propionate would be expected to result in an increase in anaplerosis due to the abundance of propionyl-CoA carboxylase in the heart (18). Indeed, there was a greater than sixfold increase in anaplerosis in the presence of propionate (Fig. 2D). Interestingly, at the lowest concentration of propionate used (0.25 mM), flux due to anaplerosis increased fivefold. This suggests that the enzymes needed for propionate anaplerosis are always active at some level, “waiting” for substrate to arrive. Indeed, this is the same enzyme complex that is responsible for producing the readily oxidized CoA forms of ketone bodies (4). Subsequent increases in propionate concentration provided only marginal increases in anaplerosis (<30% increase between 0.25 mM and any of the higher concentrations).
When examined in isolation, the effect of increasing propionate concentration on fluxes other than FAO was not apparent, due to the large amount of variance among individual observations within each group. However, once the variation due to O2 consumption was accounted for in the ANCOVA models, the effect of propionate was clearly significant for estimates of substrate selection (Figs. 2 and 4), emphasizing the importance of considering covariates in these analyses (40). The models that estimate substrate selection and flux depend on the relative areas of the glutamate and aspartate resonances. To evaluate which individual resonances measured were responding to propionate, ANCOVA of the aspartate peak ratios was carried out. Since [1-13C]propionate is the anaplerotic substrate, significant changes in peak ratios for carbons 1 and 4 of the four-carbon TCA cycle intermediates were expected. Additionally, the quartet peaks for the interior carbons of aspartate would also be expected to change significantly. Indeed, by comparing 0.25 mM propionate treatment with the higher concentrations, it was clear that these resonances showed the most significant changes (Table 2). These results were internally consistent with the changes in flux estimated using tcaCALC.
Fig. 5.
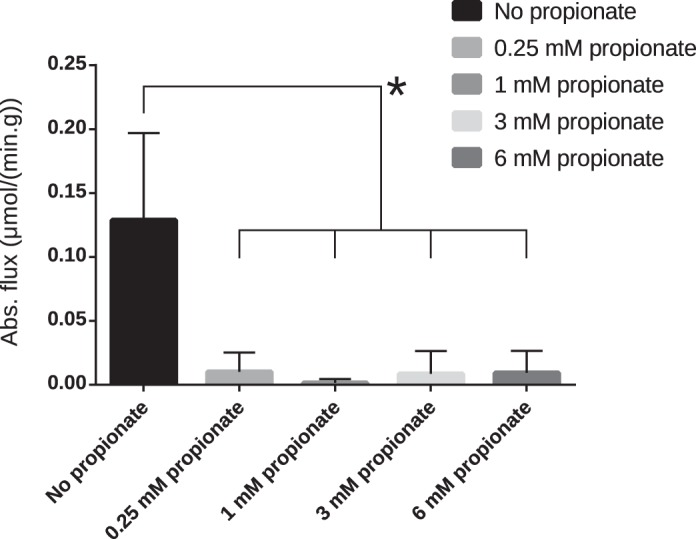
Absolute values of pyruvate carboxylase (PC) flux calculated by isotopomer analysis. *Statistical significance (P < 0.05). Numbers of mice for each group were as follows: 9 (control, 0.25 mM, and 1 mM), 8 (3 mM), and 7 (6 mM).
Table 2.
ANCOVA of aspartate peak ratios
| Peak Identity | P Value |
|---|---|
| 0.25 vs. 1 mM | |
| ASP1S | 0.0005 |
| ASP2S | 0.0349 |
| ASP2Q | 0.0042 |
| ASP3S | 0.0101 |
| ASP3Q | 0.0008 |
| ASP4S | 0.0005 |
| 0.25 vs. 3 mM | |
| ASP1S | 0.0001 |
| ASP2S | 0.0036 |
| ASP2Q | 0.0021 |
| ASP3S | 0.0017 |
| ASP3Q | 0.0004 |
| ASP4S | <0.0001 |
| 0.25 vs. 6 mM | |
| ASP1S | 0.0019 |
| ASP2S | 0.0449 |
| ASP2Q | 0.0169 |
| ASP3S | 0.0101 |
| ASP3Q | 0.0017 |
| ASP4S | 0.0003 |
Group (propionate treatment) and O2 consumption values were the variables used in the model. Only significant (P value < 0.05) peak ratios are shown. ASP, aspartate.
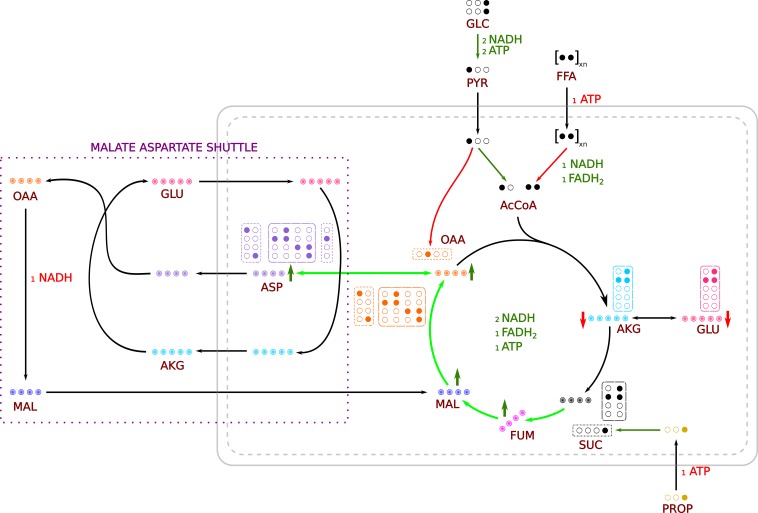
In addition to changes in PDH and anaplerotic flux, pool sizes of several TCA cycle intermediates were altered upon the addition of propionate (Fig. 3). MS measurements revealed a large increase in malate and fumarate and a comparatively smaller increase in the succinate pool size. It is apparent from the NMR spectra (Fig. 1) that the amount of glutamate in the perfused hearts was lower in the presence of propionate, whereas the aspartate levels were higher. It should be expected that propionate anaplerosis would result in an increase in concentration for the four-carbon intermediates of the TCA cycle, as reverse flux through α-ketoglutarate dehydrogenase is energetically unfavorable. These changes in pool sizes can be explained by the malate-aspartate shuttle (shown in Fig. 6). It is apparent that, in this case, glutamate and α-ketoglutarate are the rate-limiting factors. Indeed, at 3 mM propionate, the oxaloacetate pool size increases several times while the α-ketoglutarate pool size is at its nadir. One of the functions of malate-aspartate shuttle is to replenish mitochondrial NADH. As propionate is added, the shuttle presumably functions to deplete glutamate in the tissue in favor of maintaining the NADH pool size in the mitochondria. Further, since the heart lacks phosphoenolpyruvate carboxykinase, the shuttle is likely to be the most dominant pathway of oxaloacetate consumption. This is borne out in the increasing concentration of aspartate. In addition, with increasing propionate concentration, the pyruvate carboxylase pathway is almost completely shut down (Figs. 5 and 6). This effect is likely dependent on the increased oxidation of the available pyruvate.
Fig. 6.
Reaction scheme showing changes in pool sizes, pathways, and label propagation. Filled circles denote 13C labeling, and gradient filled circles represent various isotopomers that are possible for each metabolite. Green and red colored arrows represent an increase and a decrease, respectively, of fluxes or pool sizes as appropriate. Green and red colors for cofactors (NADH, ATP, and FADH2) represent their generation and consumption, respectively. Possible isotopomers of α-ketoglutarate, glutamate, succinate, and oxaloacetate from a forward turn of the TCA cycle, anaplerosis, and PC flux are shown in dashed, dotted-dashed, and dotted boxes, respectively. Oxaloacetate produced is converted to aspartate and exported out of the mitochondria along with α-ketoglutarate. In the cytoplasm, α-ketoglutarate and aspartate regenerate oxaloacetate, which is then converted to malate. Malate produced in the cytoplasm is exported into the mitochondria, and the shuttle proceeds. The highlighted span of the TCA cycle indicates increased anaplerotic/cataplerotic flux associated with propionate metabolism.
Since propionate anaplerosis should consume ATP (propionyl-CoA carboxylase is an ATP-dependent enzyme), it is expected that the TCA cycle must either turnover more quickly to compensate or the needed reducing equivalents must be produced more efficiently. Our O2 consumptions did not significantly change, implying that increased glucose oxidation is the source of the needed reducing equivalents (29). Compared with FAs, glucose produces ATP more efficiently per mole of O2 consumed. Increased ATP consumption on propionate administration at these levels is consistent with what has been shown in the literature in the rat liver (9). It has also been shown that in the presence of 2 mM propionate, the ratio of acetyl-CoA to coenzyme A (CoASH) is ~0.1 (25). This suggests that at 6 mM propionate, we should expect a severe lack of acetyl-CoA, leading to a drop in TCA cycle flux in the tissue. This was not observed in our experiments. Even though the propionate concentrations used here did not result in a decrease in TCA cycle flux, it is plausible that higher concentrations are likely to have an adverse effect on heart metabolism (3). However, given that maximal activation of PDH flux is seen at 1 mM propionate, there is no need to pursue higher doses of propionate when planning the hyperpolarized pyruvate experiments.
It is well understood that metabolomic analysis, like that performed here with LC/MS, produces a different view of metabolism compared with flux analysis. However, such significant differences in pool sizes (Fig. 3) lead to a reanalysis of the flux data. Without a change in TCA cycle turnover, the increased anaplerotic flux must be balanced by cataplerosis of oxaloacetate to aspartate. LC/MS metabolomics reports on this phenomenon and allows a different interpretation of the flux data than would otherwise be possible.
It is clear that propionate has a profound effect on myocardial metabolism, although its perturbation on oxidative flux is minimal. The previous work suggested that strong compartmentation between the cytosolic and mitochondrial pyruvate pools existed, as enrichments of the lactate and alanine pools were divergent. This work seems to corroborate this effect, as glucose utilization only doubled when 1 mM propionate was added. A partial explanation of the difference between the HP studies and the results shown here comes from the relative changes in PDH flux. The heart perfused with acetate showed greater suppression of carbohydrate oxidation. Therefore, when propionate was used to restore PDH flux, it had a greater possible dynamic range. Experiments using LCFA-perfused hearts and HP pyruvate are underway.
In conclusion, the upregulation of PDH flux as a result of the presence of propionate should enable a more straightforward estimate of PDH activity even under challenging conditions (35). Use of propionate has all the advantages of glucose-insulin-potassium treatment (45) while avoiding any detrimental side effects, such as possible hyperglycermia. Further experiments in vivo will establish the efficacy of propionate for activating PDH flux as detected using HP [1-13C]pyruvate.
GRANTS
This work was funded by National Institutes of Health (NIH) grant R21-EB-016197 (to M. E. Merritt). Salary support for M. E. Merritt was supplied by NIH Grants R01-DK-105346 and U24-DK-097209. Personnel support was provided in part by NIH Grant P41-EB-015908. A portion of this work was performed in the McKnight Brain Institute at the National High Magnetic Field Laboratory’s AMRIS Facility, which is supported by National Science Foundation Cooperative Agreement DMR-1157490 and the State of Florida, and was carried out using a 1.5-mm High Temperature Superconducting Cryogenic Probe developed with support from NIH Grant R01-EB-009772. A. Kirpich was supported by a University of Florida Informatics Institute Fellowship. L. M. McIntyre was supported by NIH Grant U24-DK-097209.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.R. and X.F. performed experiments; M.R., A.K., and L.M.M. analyzed data; M.R., S.C.B., and M.E.M. interpreted results of experiments; M.R. prepared figures; M.R. drafted manuscript; M.R., A.K., S.C.B., L.M.M., and M.E.M. edited and revised manuscript; M.R., A.K., S.C.B., L.M.M., and M.E.M. approved final version of manuscript; M.E.M. conceived and designed research.
Supplementary Material
ACKNOWLEDGMENTS
Authors thank James Rocca, Nicholas Carpenter and Xiaodong Wen for excellent technical assistance.
REFERENCES
- 1.Abdurrachim D, Luiken JJ, Nicolay K, Glatz JF, Prompers JJ, Nabben M. Good and bad consequences of altered fatty acid metabolism in heart failure: evidence from mouse models. Cardiovasc Res 106: 194–205, 2015. doi: 10.1093/cvr/cvv105. [DOI] [PubMed] [Google Scholar]
- 2.Akki A, Smith K, Seymour A-M. Compensated cardiac hypertrophy is characterised by a decline in palmitate oxidation. Mol Cell Biochem 311: 215–224, 2008. doi: 10.1007/s11010-008-9711-y. [DOI] [PubMed] [Google Scholar]
- 3.Ando T, Rasmussen K, Nyhan WL, Hull D. 3-Hydroxypropionate: significance of β-oxidation of propionate in patients with propionic acidemia and methylmalonic acidemia. Proc Natl Acad Sci USA 69: 2807–2811, 1972. doi: 10.1073/pnas.69.10.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aubert G, Martin OJ, Horton JL, Lai L, Vega RB, Leone TC, Koves T, Gardell SJ, Krüger M, Hoppel CL, Lewandowski ED, Crawford PA, Muoio DM, Kelly DP. The failing heart relies on ketone bodies as a fuel. Circulation 133: 698–705, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bastiaansen JA, Merritt ME, Comment A. Measuring changes in substrate utilization in the myocardium in response to fasting using hyperpolarized [1-13C]butyrate and [1-13C]pyruvate. Sci Rep 6: 25573, 2016. doi: 10.1038/srep25573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bian F, Kasumov T, Jobbins KA, Minkler PE, Anderson VE, Kerner J, Hoppel CL, Brunengraber H. Competition between acetate and oleate for the formation of malonyl-CoA and mitochondrial acetyl-CoA in the perfused rat heart. J Mol Cell Cardiol 41: 868–875, 2006. doi: 10.1016/j.yjmcc.2006.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunengraber H, Roe CR. Anaplerotic molecules: current and future. J Inherit Metab Dis 29: 327–331, 2006. doi: 10.1007/s10545-006-0320-1. [DOI] [PubMed] [Google Scholar]
- 8.Carvalho RA, Babcock EE, Jeffrey FM, Sherry AD, Malloy CR. Multiple bond 13C-13C spin-spin coupling provides complementary information in a 13C NMR isotopomer analysis of glutamate. Magn Reson Med 42: 197–200, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- 9.Ciman M, Rossi CR, Siliprandi N. On the mechanism of the antiketogenic action of propionate and succinate in isolated rat liver mitochondria. FEBS Lett 22: 8–10, 1972. doi: 10.1016/0014-5793(72)80205-9. [DOI] [PubMed] [Google Scholar]
- 10.Cunningham CH, Lau JYC, Chen AP, Geraghty BJ, Perks WJ, Roifman I, Wright GA, Connelly KA. Hyperpolarized 13C metabolic MRI of the human heart: initial experience. Circ Res 119: 1177–1182, 2016. doi: 10.1161/CIRCRESAHA.116.309769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Des Rosiers C, Fernandez CA, David F, Brunengraber H. Reversibility of the mitochondrial isocitrate dehydrogenase reaction in the perfused rat liver. Evidence from isotopomer analysis of citric acid cycle intermediates. J Biol Chem 269: 27179–27182, 1994. [PubMed] [Google Scholar]
- 13.Dirkx E, da Costa Martins PA, De Windt LJ. Regulation of fetal gene expression in heart failure. Biochim Biophys Acta 1832: 2414–2424, 2013. doi: 10.1016/j.bbadis.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 14.Doenst T, Nguyen TD, Abel ED. Cardiac metabolism in heart failure: implications beyond ATP production. Circ Res 113: 709–724, 2013. doi: 10.1161/CIRCRESAHA.113.300376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fillmore N, Mori J, Lopaschuk GD. Mitochondrial fatty acid oxidation alterations in heart failure, ischaemic heart disease and diabetic cardiomyopathy. Br J Pharmacol 171: 2080–2090, 2014. doi: 10.1111/bph.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fisher RA. The Correlation Between Relatives on the Supposition of Mendelian Inheritance [Online]. https://digital.library.adelaide.edu.au/dspace/handle/2440/15097 [26 August 2016].
- 17.Fisher RA. Theory of statistical estimation. Math Proc Camb Philos Soc 22: 700–725, 1925. doi: 10.1017/S0305004100009580. [DOI] [Google Scholar]
- 18.Gibala MJ, Young ME, Taegtmeyer H. Anaplerosis of the citric acid cycle: role in energy metabolism of heart and skeletal muscle. Acta Physiol Scand 168: 657–665, 2000. doi: 10.1046/j.1365-201x.2000.00717.x. [DOI] [PubMed] [Google Scholar]
- 19.Horowitz JD, Chirkov YY, Kennedy JA, Sverdlov AL. Modulation of myocardial metabolism: an emerging therapeutic principle. Curr Opin Cardiol 25: 329–334, 2010. doi: 10.1097/HCO.0b013e328339f191. [DOI] [PubMed] [Google Scholar]
- 20.Jaswal JS, Keung W, Wang W, Ussher JR, Lopaschuk GD. Targeting fatty acid and carbohydrate oxidation−a novel therapeutic intervention in the ischemic and failing heart. Biochim Biophys Acta 1813: 1333–1350, 2011. doi: 10.1016/j.bbamcr.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 21.Kashfi K, Mynatt RL, Cook GA. Hepatic carnitine palmitoyltransferase-I has two independent inhibitory binding sites for regulation of fatty acid oxidation. Biochim Biophys Acta 1212: 245–252, 1994. doi: 10.1016/0005-2760(94)90259-3. [DOI] [PubMed] [Google Scholar]
- 22.Kasumov T, Cendrowski AV, David F, Jobbins KA, Anderson VE, Brunengraber H. Mass isotopomer study of anaplerosis from propionate in the perfused rat heart. Arch Biochem Biophys 463: 110–117, 2007. doi: 10.1016/j.abb.2007.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kolwicz SC Jr, Olson DP, Marney LC, Garcia-Menendez L, Synovec RE, Tian R. Cardiac-specific deletion of acetyl CoA carboxylase 2 prevents metabolic remodeling during pressure-overload hypertrophy. Circ Res 111: 728–738, 2012. doi: 10.1161/CIRCRESAHA.112.268128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kolwicz SC Jr, Tian R. Glucose metabolism and cardiac hypertrophy. Cardiovasc Res 90: 194–201, 2011. doi: 10.1093/cvr/cvr071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Latipää PM, Peuhkurinen KJ, Hiltunen JK, Hassinen IE. Regulation of pyruvate dehydrogenase during infusion of fatty acids of varying chain lengths in the perfused rat heart. J Mol Cell Cardiol 17: 1161–1171, 1985. doi: 10.1016/S0022-2828(85)80112-7. [DOI] [PubMed] [Google Scholar]
- 26.Lauritzen MH, Laustsen C, Butt SA, Magnusson P, Søgaard LV, Ardenkjær-Larsen JH, Åkeson P. Enhancing the [13C]bicarbonate signal in cardiac hyperpolarized [1-13C]pyruvate MRS studies by infusion of glucose, insulin and potassium. NMR Biomed 26: 1496–1500, 2013. doi: 10.1002/nbm.2982. [DOI] [PubMed] [Google Scholar]
- 27.Lionetti V, Stanley WC, Recchia FA. Modulating fatty acid oxidation in heart failure. Cardiovasc Res 90: 202–209, 2011. doi: 10.1093/cvr/cvr038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopaschuk GD, Ussher JR, Folmes CDL, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Rev 90: 207–258, 2010. doi: 10.1152/physrev.00015.2009. [DOI] [PubMed] [Google Scholar]
- 29.Malloy CR, Jones JG, Jeffrey FM, Jessen ME, Sherry AD. Contribution of various substrates to total citric acid cycle flux and anaplerosis as determined by 13C isotopomer analysis and O2 consumption in the heart. MAGMA 4: 35–46, 1996. doi: 10.1007/BF01759778. [DOI] [PubMed] [Google Scholar]
- 30.Malloy CR, Sherry AD, Jeffrey FM. Evaluation of carbon flux and substrate selection through alternate pathways involving the citric acid cycle of the heart by 13C NMR spectroscopy. J Biol Chem 263: 6964–6971, 1988. [PubMed] [Google Scholar]
- 31.Malloy CR, Thompson JR, Jeffrey FMH, Sherry AD. Contribution of exogenous substrates to acetyl coenzyme A: measurement by 13C NMR under non-steady-state conditions. Biochemistry 29: 6756–6761, 1990. doi: 10.1021/bi00481a002. [DOI] [PubMed] [Google Scholar]
- 32.Martini WZ, Stanley WC, Huang H, Rosiers CD, Hoppel CL, Brunengraber H. Quantitative assessment of anaplerosis from propionate in pig heart in vivo. Am J Physiol Endocrinol Metab 284: E351–E356, 2003. doi: 10.1152/ajpendo.00354.2002. [DOI] [PubMed] [Google Scholar]
- 33.Nelson SJ, Kurhanewicz J, Vigneron DB, Larson PE, Harzstark AL, Ferrone M, van Criekinge M, Chang JW, Bok R, Park I, Reed G, Carvajal L, Small EJ, Munster P, Weinberg VK, Ardenkjaer-Larsen JH, Chen AP, Hurd RE, Odegardstuen L-I, Robb FJ, Tropp J, Murray JA. Metabolic imaging of patients with prostate cancer using hyperpolarized [1-13C]pyruvate. Sci Transl Med 5: 198ra108, 2013. doi: 10.1126/scitranslmed.3006070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neubauer S. The failing heart−an engine out of fuel. N Engl J Med 356: 1140–1151, 2007. doi: 10.1056/NEJMra063052. [DOI] [PubMed] [Google Scholar]
- 35.Le Page LM, Rider OJ, Lewis AJ, Ball V, Clarke K, Johansson E, Carr CA, Heather LC, Tyler DJ. Increasing pyruvate dehydrogenase flux as a treatment for diabetic cardiomyopathy: a combined 13C hyperpolarized magnetic resonance and echocardiography study. Diabetes 64: 2735–2743, 2015. doi: 10.2337/db14-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Purmal C, Kucejova B, Sherry AD, Burgess SC, Malloy CR, Merritt ME. Propionate stimulates pyruvate oxidation in the presence of acetate. Am J Physiol Heart Circ Physiol 307: H1134–H1141, 2014. doi: 10.1152/ajpheart.00407.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramaswamy V, Hooker JW, Withers RS, Nast RE, Brey WW, Edison AS. Development of a 13C-optimized 1.5-mm high temperature superconducting NMR probe. J Magn Reson 235: 58–65, 2013. doi: 10.1016/j.jmr.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Russell RR III, Taegtmeyer H. Pyruvate carboxylation prevents the decline in contractile function of rat hearts oxidizing acetoacetate. Am J Physiol Heart Circ Physiol 261: H1756–H1762, 1991. [DOI] [PubMed] [Google Scholar]
- 39.Salerno A, Fragasso G, Esposito A, Canu T, Lattuada G, Manzoni G, Del Maschio A, Margonato A, De Cobelli F, Perseghin G. Effects of short-term manipulation of serum FFA concentrations on left ventricular energy metabolism and function in patients with heart failure: no association with circulating bio-markers of inflammation. Acta Diabetol 52: 753–761, 2015. doi: 10.1007/s00592-014-0695-7. [DOI] [PubMed] [Google Scholar]
- 40.Schwarz G. Estimating the dimension of a model. Ann Stat 6: 461–464, 1978. doi: 10.1214/aos/1176344136. [DOI] [Google Scholar]
- 41.Sherry AD, Jeffrey FM, Malloy CR. Analytical solutions for 13C isotopomer analysis of complex metabolic conditions: substrate oxidation, multiple pyruvate cycles, and gluconeogenesis. Metab Eng 6: 12–24, 2004. doi: 10.1016/j.ymben.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 41a.Sherry AD, Malloy CR, Roby RE, Rajagopal A, Jeffrey FM. Propionate metabolism in the rat heart by 13C n.m.r. spectroscopy. Biochem J 254: 593−598, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Skrzypiec-Spring M, Grotthus B, Szeląg A, Schulz R. Isolated heart perfusion according to Langendorff−still viable in the new millennium. J Pharmacol Toxicol Methods 55: 113–126, 2007. doi: 10.1016/j.vascn.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 43.Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev 85: 1093–1129, 2005. doi: 10.1152/physrev.00006.2004. [DOI] [PubMed] [Google Scholar]
- 44.Steel GD, Torrie JH. Principles and procedures of statistics. New York: McGraw, 1960. [Google Scholar]
- 45.Taegtmeyer H. Improving energy metabolism in the postischemic heart−the story of GIK. Semin Cardiothorac Vasc Anesth 7: 67–76, 2003. doi: 10.1177/108925320300700113. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets and/or analysis from the present study are available from the corresponding author on reasonable request.