Abstract
The tumor suppressor WW domain-containing oxidoreductase (WWOX) exhibits regulatory interactions with an array of transcription factors and signaling molecules that are positioned at the well-known crossroads between inflammation and cancer. WWOX is also subject to downregulation by genotoxic environmental exposures, making it of potential interest to the study of lung pathobiology. Knockdown of lung WWOX expression in mice was observed to cause neutrophil influx and was accompanied by a corresponding vascular leak and inflammatory cytokine production. In cultured human alveolar epithelial cells, loss of WWOX expression resulted in increased c-Jun- and IL-8-dependent neutrophil chemotaxis toward cell monolayers. WWOX was observed to directly interact with c-Jun in these cells, and its absence resulted in increased nuclear translocation of c-Jun. Finally, inhibition of the c-Jun-activating kinase JNK abrogated the lung neutrophil influx observed during WWOX knockdown in mice. Altogether, these observations represent a novel mechanism of pulmonary neutrophil influx that is highly relevant to the pathobiology and potential treatment of a number of different lung inflammatory conditions.
Keywords: inflammation, lung injury, neutrophils, WWOX
wwox is a tumor suppressor protein containing an enzymatic carboxy-terminal short-chain dehydrogenase domain (SDR) and two amino-terminal WW domains that are characterized by a pair of conserved tryptophan (W) residues (7, 55). The majority of WWOX functional studies describe modulation of cancer-related pathways via protein-protein interactions between the first WW domain and various transcription factors and signaling molecules containing proline-tyrosine-rich motifs (19). The WWOX gene resides at the second most common chromosomal fragile site (FRA16D), making it highly susceptible to homozygous deletions and translocation breakpoints in response to genotoxic environmental exposures (63). Decreased WWOX expression in several different cancers is associated with increased tumor metastases and worse prognosis (22, 50, 53), but the mechanistic underpinning for this association is largely unknown.
Based on what is already known about the genetics and functional attributes of WWOX, it appears to be of particular interest to the study of acute respiratory distress syndrome (ARDS) and other lung diseases. ARDS is a critical illness consisting of inflammatory alveolar damage and disruption of the pulmonary vascular barrier resulting in pulmonary edema and respiratory failure (66). Associated triggering conditions of ARDS include a variety of local and systemic inflammatory insults with the most common causes being pneumonia, sepsis, and trauma (66). At least eight of the known binding partners of WWOX (4, 19) have been observed to participate in signaling pathways that modulate multiple facets of ARDS pathobiology. These include NF-κB-activating protein (21), ErbB4 (57), c-Jun (5, 30, 35, 42), ezrin (1), Dvl2 (54), p53 (6, 41), and the R-SMADs (3, 38). Many of these molecules also play critical roles in the pathogenesis of other lung diseases such as asthma, chronic obstructive pulmonary disease (COPD), pulmonary hypertension, and pulmonary fibrosis (8, 10, 11, 17, 25, 27, 31, 37, 43, 47, 48). Furthermore, the expression of common chromosomal fragile site genes, including WWOX/FRA16D, is known to be downregulated by environmental carcinogens as well as benzopyrene diol epoxide (63). In modern industrial societies, the lung is exquisitely susceptible to these exposures and is therefore likely particularly amenable to loss of WWOX expression.
One of the well-studied models of ARDS is a murine model of neutrophilic alveolitis induced by intratracheal instillation of bacterial lipopolysaccharide (LPS) (46). The effects of LPS in this model are modulated by many of the WWOX-binding partners already mentioned. For example, Liu et al. (41) have shown that stabilization of p53 attenuates LPS-induced NF-κB activation in neutrophils and macrophages with p53−/− mice being more susceptible to ARDS in this model. Both NF-κB and p53 are subject to regulation by functional interactions with WWOX (4, 15). In another example, the membrane-cytoskeletal linker protein ezrin has been implicated in the regulation of endothelial cell permeability in response to various ARDS-relevant stimuli (1, 2), whereas WWOX has been shown to participate in a cortical membrane-localizing interaction with ezrin where it modulates critical cytoskeletal remodeling events (34). Finally, the transcription factor c-Jun and its upstream activator, JNK, have been described as key regulators of neutrophil influx during LPS-induced ARDS in mice (5). WWOX exerts an inhibitory interaction with c-Jun preventing its activation by upstream kinases and subsequent nuclear translocation to participate in activator protein-1 (AP-1) transcription factor activity (26).
Based on these observations, we hypothesized that WWOX modulates multiple facets of ARDS pathobiology via its documented interactions with a number of ARDS-relevant transcription factors and key signaling molecules. To test this hypothesis, we began the current study by examining the effects of global lung silencing of WWOX in a murine model of LPS-induced ARDS.
MATERIALS AND METHODS
Reagents.
Antibodies against WWOX and c-Jun; ELISA kits for measuring human IL-8, IL-6, and IL-1 levels; and the NF-κB p65 transcription factor activity assay were purchased from Abcam. TF-Detect AP-1/c-Jun transcription factor activity assay kit was from GeneCopoeia. Scrambled control siRNA and siRNA targeting mouse WWOX expression were from Dharmacon/GE. ELISA kits to measure mouse IL-6 and IL-1β were from Biolegend, whereas kits to measure mouse keratinocyte-derived chemokine (KC) and macrophage inflammatory protein-2 (MIP-2) were from Sigma-Aldrich. FITC-dextran, SP100625, A549 cells, LPS, red blood cell (RBC) lysis buffer, RIPA buffer, and anti-DDK M2 magnetic beads were also from Sigma-Aldrich. Human neutrophil isolation kit was from StemCell Technologies. The BCA protein assay kit, anti-HA magnetic beads, NE-PER nuclear and cytoplasmic extraction reagent kit, Shandon Kwik-Diff kit, BLOCK-iT Pol II miR RNAi expression vector kit with EmGFP, Lipofectamine 2000 transfection reagent, anti-HA epitope tag (16B12) Alexa Fluor 594 conjugate antibody, ProLong Gold antifade mountant with DAPI, and neutralizing anti-IL-8 antibody were from Thermo Fisher. Mini-PROTEAN TGX precast gels were from Bio-Rad. pCMV6 entry vector containing the ORF corresponding to (Myc-DDK-tagged)-human WW domain-containing oxidoreductase (WWOX), transcript variant 1 was purchased from Origene. pcDNA3.1 containing open reading frame-expressing HA-tagged Homo sapiens Jun proto-oncogene (JUN) was purchased from Genscript.
Animals.
All experiments and animal care procedures were approved by the University of Illinois at Chicago Animal Care and Use Committee. Six- to eight-week-old male C57BL/6 mice were purchased from Jackson Laboratories. They were housed in cages in a temperature-controlled room with a 12-h:12-h dark-light cycle and with free access to food and water. A murine model of LPS induced neutrophilic alveolitis and silencing of lung WWOX expression. Six- to eight-week-old male C57BL/6 mice were anesthetized with isoflurane and underwent tracheal cannulation. Ten milligrams per kilograms of scrambled control vs. WWOX-silencing RNA were instilled into the airway in saline. The WWOX siRNA sequence was 5′-CCACAGAUUUACAGAUAUU-3′, and the scrambled control sequence was 5′-AUGUAUUGGCCUGUAUUAG-3′. The mice were housed in cages as described above for 54 h after which they underwent reanesthetization and tracheal cannulation for instillation of 1 mg/kg LPS vs. an equivalent volume of sterile saline. Eighteen hours later, they underwent testing and lung harvesting as described below.
FITC-dextran permeability assay.
FITC-dextran (70 kDa) was used to measure pulmonary vascular leak as described previously (29). Briefly, FITC-dextran was mixed to a concentration of 30 mg/ml in sterile PBS and sterile filtered. Thirty minutes before euthanasia, 100 µl of FITC-dextran solution were injected into the retro-orbital venous plexus. Bronchoalveolar lavage fluid (BALF) was collected as described below. Blood was collected during lung harvest and centrifuged to isolate serum. Fluorescence of equal volumes of BALF supernatant and serum was measured, and relative changes in permeability were expressed as a ratio of FluoBALF to FluoSerum normalized to the control condition.
BALF collection and analysis.
Mice were anesthetized with ketamine-xylazine. The trachea was exposed surgically, and a small incision was made on the anterior surface. An 18-gauge, blunt-end cannula was inserted into this opening and secured with a suture tied around the trachea. One milliliter of sterile saline was infused through this cannula and slowly withdrawn. This BALF was centrifuged to pellet cells and remove supernatant. RBCs were removed from the cell pellet using RBC lysis buffer according to the manufacturer’s instructions. The remaining cells (leukocytes) were pelleted and resuspended in a fixed volume of saline, and a small aliquot was used to measure cell concentration with an automated cell counter. The remaining cell suspension was used to make a cytospin preparation on a glass slide for staining with the Shandon Kwik-Diff kit. A manual differential cell count was performed on 10 high-power fields to determine relative percentages of neutrophils in the leukocyte population. Protein concentration was measured using the BCA protein assay kit, and the corresponding ELISA kits were used to measure concentrations of IL-1β, IL-6, KC, and MIP-2.
Lung harvesting for Western blot analysis and histology.
Following BALF collection, the right ventricle was infused with sterile saline until the lungs were free of intravascular blood. The right middle lobe was excised and snap frozen in liquid nitrogen for Western blot analysis. The remaining lobes were excised and placed in 10% formalin for 24 h followed by transfer to 70% ethanol for storage until paraffin embedding and sectioning for hematoxylin-eosin staining.
Snap-frozen lungs were thawed and homogenized in RIPA buffer. Homogenized samples were centrifuged to pellet debris and isolate protein lysates. The protein concentration of lysates was measured using the BCA protein assay kit, and samples were diluted in RIPA buffer until all had the same protein concentration. After dilution with SDS buffer, samples were loaded into 4–20% Mini-PROTEAN TGX precast gels and subjected to SDS-PAGE. Gels were transferred to nitrocellulose membranes that were then subjected to Western blot analysis using antibodies targeting proteins of interest in accordance with the manufacturer’s instructions. Densitometric analysis was performed using ImageJ software.
In vitro silencing of WWOX and c-Jun in A549 cells.
In vitro silencing of WWOX or c-Jun both individually and dually was achieved using the BLOCK-iT Pol II miR RNAi expression vector kit with EmGFP made by Invitrogen/Thermo Fisher. In this system, a plasmid is generated that encodes for a Pol II pre-miRNA transcript, which when processed by the cellular machinery generates an RNAi corresponding to the gene of interest. In addition, the Pol II expression of miRNA allows dual expression of WWOX and c-Jun-targeting silencers on the same transcript as well as cocistronic expression of a GFP reporter. The final RNAi sequences generated by Invitrogen’s BLOCK-IT RNAi designer were as follows: WWOX: 5′-TTCCCACTGAGTCTTCTCCTC-3′; and c-Jun: 5′-TTAACGTGGTTCATGACTTTC-3′. PcDNA6.2-GW/EmGFP-miR plasmids containing these sequences were generated as per manufacturer’s instructions. Additionally, a plasmid expressing both silencers on one transcript was generated as per manufacturer’s instructions. A negative control plasmid was provided by the manufacturer. These were transfected into A549 cells using Lipofectamine 2000 as per manufacturer’s instructions with silencing confirmed by Western blot analysis.
Coimmunoprecipitation and coimmunofluorescence studies.
A549 cells were grown under manufacturer-recommended conditions and transfected with a Myc-FLAG-tagged WWOX-expressing construct using Lipofectamine 2000 in accordance with manufacturer’s instructions. Cells used for a control mock immunoprecipitation were treated with transfection reagent without plasmid. Cells were washed and collected in NP-40 buffer for coimmunoprecipitation as described previously. Clarified lysates were subjected to coimmunoprecipitation using anti-DDK M2 magnetic beads as per manufacturer’s instructions.
A549 cells grown on coverslips were cotransfected with either a GFP-tagged negative control or GFP-tagged WWOX-silencing BLOCK-iT Pol II miR RNAi expression vector and an HA-tagged c-Jun-expressing construct. Cells were washed and fixed with ice-cold methanol, followed by staining with anti-HA epitope tag (16B12) Alexa Fluor 594 conjugate antibody according to manufacturer’s instructions. Coverslips were mounted to glass slides using ProLong Gold antifade mountant with DAPI and imaged using the Zeiss LSM710 confocal microscope.
In vitro neutrophil chemotaxis studies.
A neutrophil chemotaxis assay was developed based on small modifications to a previously published method (58). Human neutrophils were prepared from blood donated by healthy volunteers using the human neutrophil isolation kit made by StemCell Technologies and diluted to a concentration of 1 × 105 cells/ml. Control vs. WWOX-silenced A549 cells were grown to confluence in six-well plates and overlaid with a polycarbonate Transwell containing 3-μm pores. One millilter of the neutrophil suspension was added to the Transwell overlying the cell layer. The entire assembly was incubated at 37°C and 5% CO2 for 30 min, after which the Transwells were removed and the media overlying the cells were extracted and centrifuged to obtain a cell pellet. Pellets were resuspended in a fixed volume of saline, cytospun onto slides, and stained with the Shandon Kwik-Diff kit for manual examination and counting under a light microscope.
c-Jun/AP-1 and NF-κB activity assay.
Nuclear fractions from A549 cells were prepared using the NE-PER nuclear and cytoplasmic extraction reagent kit. Ten micrograms of nuclear protein were used to determine transcription factor activity.
For the c-Jun/AP-1 assay, the corresponding kit from GeneCopoeia was used according to manufacturer’s instructions. Briefly, the kit supplied a 96-well plate containing immobilized oligonucleotides that included the c-Jun/AP-1 consensus binding site. Activated c-Jun was captured by the immobilized nucleotides, and Ser-73 phosphorylated c-Jun was detected by a phospho-c-Jun antibody and a horseradish peroxidase (HRP)-conjugated secondary antibody. Quantification was performed using the HRP substrate 3,3′,5,5′-tetramethylbenzidine and spectrophotometry.
For the NF-κB assay, the corresponding kit from Abcam was used according to manufacturer’s instructions. The principle underlying the assay in this kit was similar to that of the one used for c-Jun/AP-1. A 96-well plate contained immobilized oligonucleotides that included the NF-κB consensus binding sequence. After capture of NF-κB from nuclear extract, p65 was detected using a primary antibody, and a colorimetric readout was obtained using and HRP-conjugated secondary antibody and 3,3′,5,5′-tetramethylbenzidine.
ELISA for human IL-8, IL-1, and IL-6.
The corresponding kits purchased from Abcam were used according to manufacturer’s instructions to determine cytokine concentration in media supernatant extracted from A549 cultures under various conditions.
Statistical analysis.
Either a Student’s t-test or an ANOVA were performed where applicable on all data using GraphPad Prism software. Significance was set at *P < 0.05.
RESULTS
Global loss of murine lung WWOX expression causes neutrophilic alveolitis.
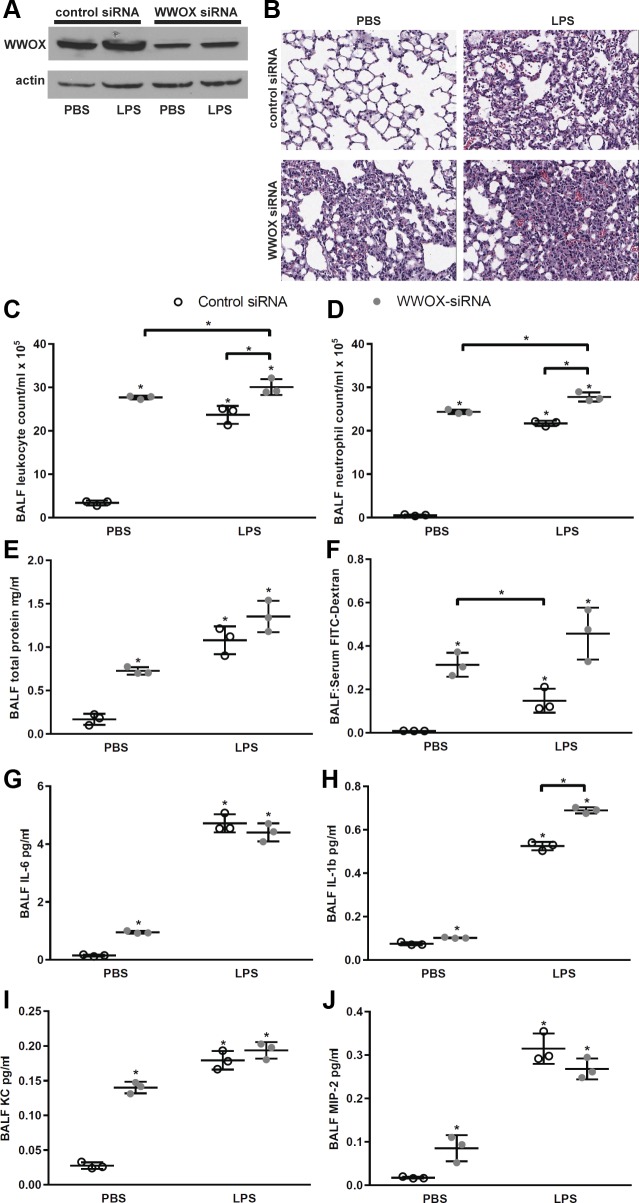
We intratracheally instilled control vs. WWOX-targeting siRNA in C57Bl/6 mice and induced ARDS using LPS as described previously (73). We achieved significant silencing of WWOX expression as measured in whole lung homogenates (Fig. 1A). Surprisingly, WWOX silencing alone caused neutrophil influx (Fig. 1, B–D) and a corresponding vascular leak (Fig. 1, E and F) to a similar degree that LPS did in wild-type mice pretreated with scrambled control siRNA. WWOX-silenced mice treated with LPS exhibited a greater degree of neutrophil influx (Fig. 1, B–D) and vascular leak (Fig. 1, E and F) than their wild-type counterparts treated with LPS.
Fig. 1.
Knockdown of WW domain-containing oxidoreductase (WWOX) expression in mouse lung causes neutrophil influx, vascular leak, and inflammatory cytokine production. Twelve C57Bl/6 mice were treated with intratracheal instillation of either control nontargeting (n = 6) or WWOX-targeting siRNA (n = 6). Seventy-two hours later 3 mice in each group received 40 µl of PBS via intratracheal instillation, and the remaining mice received 1 mg/kg LPS in a 40-µl volume. Eighteen hours later all mice underwent bronchoalveolar lavage (BAL) with 1 ml of PBS, followed by harvesting of the lungs for homogenization and Western blotting as well as histologic examination. A: representative Western blot for WWOX in mouse lung homogenates resolved by SDS-PAGE. B: representative hematoxylin-eosin-stained lung histologic sections from each of the 4 experimental conditions. BALF, bronchoalveolar lavage fluid. C–J: scatter plots depict BAL leukocyte counts, neutrophil counts, protein concentration, and FITC-dextran flux as a measure of permeability, and IL-1, IL-6, keratinocyte-derived chemokine (KC), and macrophage inhibitory-2 (MIP-2) concentrations, respectively. Results are shown as means ± SD in n = 3 independent experiments. A two-way ANOVA for PBS vs. LPS and control vs. WWOX siRNA was performed followed by a Student’s t-test for all significant comparisons. *P < 0.05, significantly different from control except for comparisons indicated by brackets.
We next considered the mechanism by which loss of WWOX expression led to neutrophil influx in the lung. As such, we examined levels of inflammatory cytokines in the BALF of these mice including the levels of the most potent chemoattractants for neutrophils, the mouse analogs of human IL-8, KC, and MIP-2. As shown in Fig. 1, G–J, the levels of both KC and MIP-2 were significantly elevated in the BALF of WWOX-silenced mice as were those of IL-1β and IL-6.
WWOX is a key basal binding inhibitor of c-Jun/AP-1 activity.
IL-8 is synthesized and secreted by lung macrophages, epithelial cells, and endothelial cells in response to various inflammatory stimuli (58–60). The primary mode of upregulation is increased gene transcription mediated by a number of different transcription factors binding to the IL-8 promoter (9). Among these are c-Jun/AP-1 and NF-κB, both of which may be subject to WWOX functional activity (4, 19). Of the two, c-Jun directly interacts with, and is inhibited by, WWOX. Gaudio et al. (26) report that the effect of the WWOX-c-Jun interaction is cytoplasmic sequestration of c-Jun whereby WWOX exerts an inhibition of c-Jun nuclear translocation and therefore its transcriptional activity as part of the AP-1 complex. We sought to examine whether this functional interaction takes place in at least one lung cell type known to secrete IL-8. We chose to use A549 alveolar epithelial cell lines as these are known potent producers of IL-8 and since their neutrophil chemotactic properties could be directly studied while they form adherent monolayers.
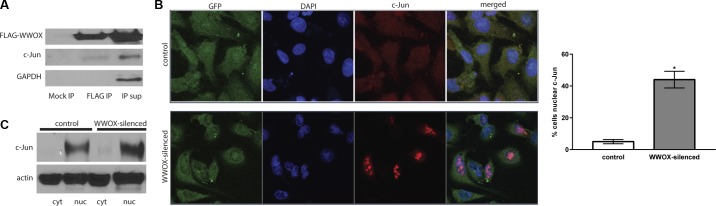
We transfected A549 cells with a FLAG-tagged WWOX-expressing plasmid and performed coimmunoprecipitation as in Gaudio et al. The eluent exhibited the presence of c-Jun, confirming the binding interaction already described previously (Fig. 2A). Next, we silenced WWOX in these cells using a plasmid coexpressing a miR silencer and GFP. We examined cellular localization of c-Jun in control GFP-only vs. WWOX-silenced GFP-expressing cells using fluorescent confocal microscopy. As illustrated in the images in Fig. 2B, silencing of WWOX was associated with a significant increase in activated c-Jun translocating to the nucleus. This was also confirmed by Western blotting of nuclear vs. cytoplasmic fractions of control vs. WWOX-silenced A549 cells (Fig. 2C).
Fig. 2.
WWOX binds c-Jun and prevents its nuclear translocation. A549 cells were transfected with a FLAG-tagged WWOX expression construct, and a coimmunoprecipitation was performed using anti-FLAG antibody conjugated to magnetic beads in n = 3 experiments. A: representative results of the coimmunoprecipitation experiment. A mock immunoprecipitation (IP) was performed in A549 cells transfected with a control plasmid to ensure that resulting immunoprecipitation bands were not due to nonspecific protein binding to the beads. The Western blot shown here illustrates pulldown of c-Jun along with FLAG-WWOX. Note that since WWOX and c-Jun are of similar molecular weight, the bands for each are derived from separate gels that were run at the same time and that contained the same fractions of the same eluents, supernatants, and lysates. In separate experiments, A549 cells were cotransfected with a HA-tagged c-Jun expression construct and either a control miR-GFP- or WWOX-silencing miR-GFP-expressing plasmid. Cells were then stained with anti-HA-Alexa Fluor 594-conjugated antibody (red) along with DAPI counterstain. B: confocal microscopic images illustrate increased nuclear translocation of c-Jun during WWOX silencing vs. control observed in n = 3 experiments. Cells in 10 high-power fields (hpf) were counted and the percentage showing strong nuclear staining are depicted in the accompanying bar graph. C: these results were confirmed by Western blotting of nuclear (nuc) vs. cytoplasmic (cyt) fractions derived from cells transfected with control vs. WWOX-silencing miR-expressing plasmids in n = 3 experiments. *P < 0.05, significantly different from control by Student’s t-test.
WWOX knockdown in A549 cells increases IL-8-dependent neutrophil chemotaxis.
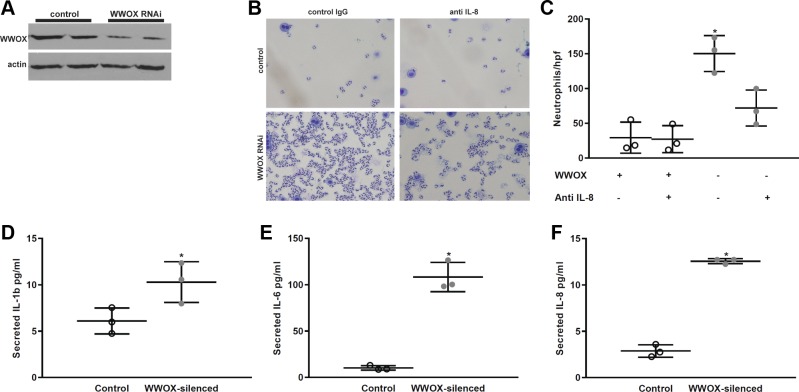
We next examined the effects of WWOX knockdown on epithelial cytokine secretion and neutrophil chemotaxis toward A549 cell monolayers. We silenced WWOX in A549 cells (Fig. 3A) and measured the levels of secreted cytokines in the media supernatant. Consistent with what was observed in the BALF of lung WWOX-silenced mice, the levels of IL-8, IL-1, and IL-6 were elevated in control vs. silenced cells (Fig. 3, B–D). We performed an assay for neutrophil chemotaxis in parallel to these experiments in which A549 cells were grown in a monolayer on the bottom chamber of a Transwell plate and transfected with control vs. WWOX-silencing plasmids. After 18–24 h when silencing was known to be optimal, 1 × 105 human neutrophils freshly isolated from healthy donors were placed in the top chamber of the Transwell, which contained 3-μm pores. Thirty minutes later the Transwell was removed and the media supernatant was examined for the presence of neutrophils that transmigrated through the pores and toward the monolayer. As shown in Fig. 3, E and F, WWOX silencing significantly increased neutrophil transmigration. To confirm that this effect was due to increased IL-8 secretion, we repeated the experiment by pretreating the media supernatant with control IgG vs. neutralizing anti-IL-8 antibody and observed an abrogation in the number of neutrophils transmigrating through the Transwell in the cells treated with anti-IL-8 (Fig. 3, B and C).
Fig. 3.
WWOX knockdown in human alveolar epithelial cells results in increased neutrophil chemotaxis. A549 cells grown in culture were transfected with control vs. WWOX-silencing miR-expressing plasmids. A: representative Western blot for WWOX in cell lysates. B and C: transfected A549 cells were grown in the bottom chamber of a Transwell plate containing 3-μm pores. Control IgG vs. neutralizing anti-IL-8 antibody was added to the media. Then, 1 × 105 human neutrophils were placed in the top chamber. Thirty minutes later, neutrophils were pelleted from the media in the bottom chamber and resuspended in a fixed volume, and a cytospin preparation was stained and examined under light microscopy. A representative image is shown here along with a scatter plot depicting the number of neutrophils counted per high-powered field. D–F: concentrations of IL-1β, IL-6, and IL-8 were measured by ELISA in the media supernatant of control vs. WWOX-silenced cells without anti-IL-8 neutralizing antibody or control IgG. Results are shown as means ± SD in n = 3 independent experiments. *P < 0.05, compared with control by Student’s t-test.
c-Jun activation during loss of WWOX expression drives an increase in NF-κB activity, cytokine secretion, and neutrophil chemotaxis.
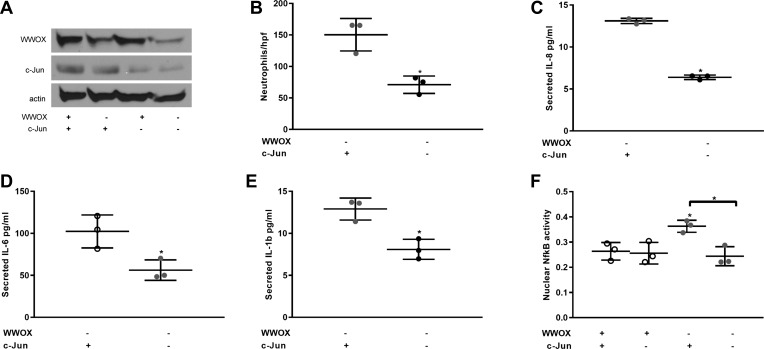
To determine the role of activated c-Jun in these phenomena, we used single and dual silencing of WWOX and c-Jun in a repeat examination of neutrophil chemotaxis and cytokine secretion by A549 cells. A dual silencer for WWOX and c-Jun was developed using the BLOCK-iT Pol II miR RNAi expression vector kit. Single and dual silencing of these gene products was confirmed by Western blotting as shown in Fig. 4A. The experiments of Fig. 3 were then repeated with the additional variable of c-Jun silencing with or without WWOX knockdown. The increased levels of neutrophil chemotaxis and cytokine secretion observed during WWOX silencing were all abrogated by concomitant silencing of c-Jun expression (Fig. 4, B–E).
Fig. 4.
Increased neutrophil chemotaxis in WWOX-silenced cells is dependent on IL-8 and c-Jun transcription factor activity. A549 cells grown in culture were transfected with either a single or dual WWOX- and c-Jun-silencing miR-expressing plasmid. A: representative Western blot for WWOX and c-Jun in cell lysates resolved by SDS-PAGE is shown. Note that since WWOX and c-Jun are of similar molecular weight, the bands for each are derived from separate gels that were run at the same time and that contained the same fractions of the same lysates. B: scatter plot illustrates the results of a neutrophil chemotaxis assay in n = 3 experiments. C–E: scatterplots depict measured concentrations of IL-8, IL-1β, and IL-6. F: results of an NF-kB assay on nuclear extracts. Results are shown as means ± SD in n = 3 independent experiments. *P < 0.05, compared with control except where brackets indicate another comparison by Student’s t-test.
The level of IL-8 secretion by alveolar epithelial cells in response to mechanical stimulation has been previously shown to be dependent on concomitant c-Jun and NF-κB activity with loss of either one associated with abrogation of the IL-8 response (39). WWOX is also known to interact with NF-κB activation in cancer cells (24). We therefore examined whether NF-κB activation was occurring simultaneously with c-Jun activation during WWOX knockdown. As shown in Fig. 4F, nuclear NF-κB activity was indeed increased during loss of WWOX expression. However, simultaneous knockdown of c-Jun abrogated this increase, suggesting that the primary driver of these proinflammatory effects during WWOX knockdown was c-Jun activation.
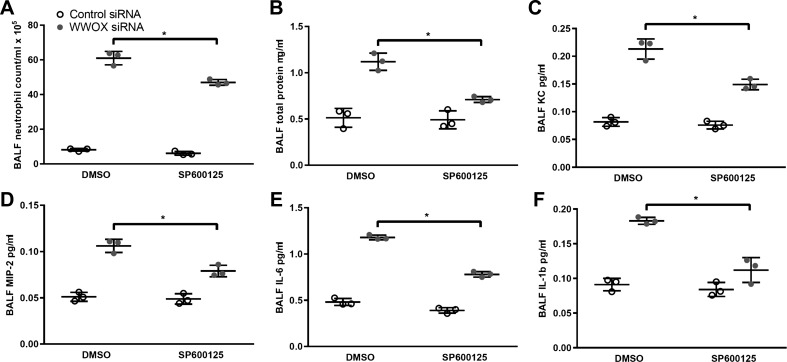
Pretreatment of mice with the JNK inhibitor SP600125 attenuates neutrophilic inflammation associated with loss of WWOX expression.
We instilled control vs. WWOX-silencing siRNA intratracheally in C57Bl/6 mice that were administered either DMSO vehicle or the JNK inhibitor SP600125 (30 mg/kg) via subcutaneous injection. A moderate increase in BALF neutrophils was associated with DMSO alone. However, the level of neutrophilic inflammation associated with WWOX knockdown was significantly attenuated by pretreatment with the JNK inhibitor as evidenced by BAL neutrophil counts, total protein concentration, and cytokine concentrations (Fig. 5).
Fig. 5.
Pretreatment of mice with the JNK-inhibitor, SP600125, attenuates neutrophilic inflammation associated with loss of WWOX expression. Twelve C57Bl/6 mice were treated with intratracheal instillation of either control nontargeting (n = 6) or WWOX-targeting siRNA (n = 6). Three mice in each group were then administered the JNK inhibitor SP500125 (30 mg/kg) or an equivalent volume of DMSO subcutaneously. Bronchoalveolar lavage with 1 ml of PBS was performed. A–F: BAL neutrophil counts, protein concentration, and KC, MIP-2, IL-6, and IL-1β concentrations were measured. Results are shown as means ± SD in n = 3 independent experiments. *P < 0.05, comparison as indicated by brackets by Student’s t-test.
DISCUSSION
Knockdown of WWOX expression in murine lungs causes neutrophil influx. This finding alone is of great interest to the pathobiology of pulmonary neutrophilic inflammation, which occurs in a number of lung diseases including those exacerbated by environmental exposures such as asthma, chronic bronchitis, and COPD (40). Also interesting to note is that both tumor-associated neutrophils and loss of tumor WWOX expression have been independently associated with greater metastatic potential (4, 16, 18, 28). Furthermore, lung neutrophil accumulation both in response to distant tumor secreted factors and in the context of preexisting inflammatory lung disease is thought to create a “premetastatic niche,” priming areas for the colonization of incoming distant cancer cells by a number of different mechanisms (16, 67). Evidence of preexisting inflammatory lung disease playing a role in the establishment of the premetastatic niche exists in the observed association between cigarette smoking and increased risk of lung metastases (56) as well as in observations of preclinical animal models of pneumonia (70), ARDS (20), and asthma (62), all exhibiting heightened frequency of lung metastases when combined with numerous models of cancer.
Genotoxic environmental exposures known to be associated with WWOX downregulation may exacerbate many of the neutrophil-associated pulmonary diseases listed above. For example, cigarette smoke exposure downregulates WWOX expression (71) and has been independently observed to increase the risk for development of ARDS (12, 13, 32), asthma (64), COPD (72), and extrapulmonary neoplastic metastases to the lung (49).
Our in vitro findings suggest the presence of a basal c-Jun-activating capability that is held in check by WWOX-mediated sequestration of c-Jun in A549 cells. The upstream c-Jun-activating kinase JNK is normally activated via a MAP kinase cascade by a variety of inflammatory and stress stimuli (65). However, basal levels of JNK activity have been documented in other cell lines (14, 33, 36, 51), including epithelial cells where it is relevant for cell survival, proliferation, and tonic maintenance of epithelial ionic permeability (14). In the current study, it appears to be responsible for c-Jun activation during acute loss of WWOX expression.
There are a few minor limitations to this study. First, only one WWOX-targeting siRNA was used in the mouse studies, raising the possibility that off-target effects might be responsible for the observed increase in lung neutrophil influx. However, this limitation is partially addressed by the fact that in separate in vitro studies, an siRNA targeting a different species-specific WWOX gene yielded the congruent phenomenon of increased neutrophil chemotaxis toward human epithelial cell monolayers. While it is possible that lung WWOX silencing produced by intratracheal instillation of siRNA targets other significant cell types that may contribute to the observed effect on neutrophil influx, it is known through studies using GFP-targeting siRNA in GFP-overexpressing mice that the predominant cell type affected by intratracheal siRNA delivery is the alveolar epithelial cell (52). A second potential limitation of our study is the use of the A549 cell line as our model for alveolar epithelium. While conflicting evidence exists as to whether A549 cells are phenotypically more closely related to primary type I or type II alveolar cells (23, 61), both primary cell types as well as A549 cells are known to secrete IL-8 in response to infectious or inflammatory stimuli (44, 45, 58, 68, 69). Therefore, for the focus of this study on neutrophil chemotaxis, A549 cells appear to have been a sufficient model of the effects of WWOX knockdown on epithelial IL-8 secretion. This is further supported by the finding that our observations of the role of c-Jun in these phenomena, which took place in the A549 cell line, were confirmed by our later in vivo study using a JNK inhibitor in our mouse model.
In addition to the observations regarding neutrophilic inflammation during WWOX knockdown, another finding described in this study but not yet explored is a potential role of WWOX in mediating vascular barrier function. As shown in Fig. 1, D and F, the degree of LPS-induced pulmonary vascular leak observed during WWOX knockdown was significantly greater than that observed in wild-type mice and well out of proportion to the corresponding degree of neutrophilic inflammation seen in these two groups of mice. This suggests an influence of WWOX deficiency on mechanisms of endothelial barrier dysfunction during LPS-TLR4 signaling events.
In summary, we have discovered a novel mechanism of pulmonary neutrophil influx that is highly relevant to the pathobiology and potential treatment of a number of different lung inflammatory conditions. The clinical translation of our findings may be reduced by the fact that, in our disease model, we studied acute, global knockdown of lung WWOX expression. In human lungs, the timing and extent of exposure-induced WWOX downregulation are not yet defined but are likely to accrue heterogeneously over chronic periods of recurrent toxic respiratory exposure. Therefore, further study of the role of WWOX in the interaction between environmental exposures and lung disease-specific models is warranted and may lead to novel anti-inflammatory WWOX-targeted therapies desperately needed in pulmonary medicine.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants 1R01-HL-133951-01, 1R01-HL-127342-01A1, and 4R01-HL-111656-04 (to R. F. Machado).
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the author(s).
AUTHOR CONTRIBUTIONS
S. Singla and R.F.M. conceived and designed research; S. Singla, S. Sethuraman, A.G., and S.Z. performed experiments; S. Singla, J.R.S., and R.F.M. analyzed data; S. Singla, J.C., J.R.S., and R.F.M. interpreted results of experiments; S. Singla prepared figures; S. Singla drafted manuscript; S. Singla, J.C., J.R.S., S.Z., and R.F.M. edited and revised manuscript; S. Singla, J.C., S. Sethuraman, J.R.S., A.G., S.Z., and R.F.M. approved final version of manuscript.
REFERENCES
- 1.Adyshev DM, Dudek SM, Moldobaeva N, Kim KM, Ma SF, Kasa A, Garcia JG, Verin AD. Ezrin/radixin/moesin proteins differentially regulate endothelial hyperpermeability after thrombin. Am J Physiol Lung Cell Mol Physiol 305: L240–L255, 2013. doi: 10.1152/ajplung.00355.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adyshev DM, Moldobaeva NK, Elangovan VR, Garcia JG, Dudek SM. Differential involvement of ezrin/radixin/moesin proteins in sphingosine 1-phosphate-induced human pulmonary endothelial cell barrier enhancement. Cell Signal 23: 2086–2096, 2011. doi: 10.1016/j.cellsig.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akbarshahi H, Sam A, Chen C, Rosendahl AH, Andersson R. Early activation of pulmonary TGF-β1/Smad2 signaling in mice with acute pancreatitis-associated acute lung injury. Mediators Inflamm 2014: 148029, 2014. doi: 10.1155/2014/148029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aldaz CM, Ferguson BW, Abba MC. WWOX at the crossroads of cancer, metabolic syndrome related traits and CNS pathologies. Biochim Biophys Acta 1846: 188–200, 2014 10.1016/j.bbcan.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arndt PG, Young SK, Lieber JG, Fessler MB, Nick JA, Worthen GS. Inhibition of c-Jun N-terminal kinase limits lipopolysaccharide-induced pulmonary neutrophil influx. Am J Respir Crit Care Med 171: 978–986, 2005. doi: 10.1164/rccm.200406-712OC. [DOI] [PubMed] [Google Scholar]
- 6.Barabutis N, Dimitropoulou C, Birmpas C, Joshi A, Thangjam G, Catravas JD. p53 protects against LPS-induced lung endothelial barrier dysfunction. Am J Physiol Lung Cell Mol Physiol 308: L776–L787, 2015. doi: 10.1152/ajplung.00334.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bednarek AK, Laflin KJ, Daniel RL, Liao Q, Hawkins KA, Aldaz CM. WWOX, a novel WW domain-containing protein mapping to human chromosome 16q23.3-24.1, a region frequently affected in breast cancer. Cancer Res 60: 2140–2145, 2000. [PubMed] [Google Scholar]
- 8.Bennett BL. c-Jun N-terminal kinase-dependent mechanisms in respiratory disease. Eur Respir J 28: 651–661, 2006. doi: 10.1183/09031936.06.00012106. [DOI] [PubMed] [Google Scholar]
- 9.Bezzerri V, Borgatti M, Finotti A, Tamanini A, Gambari R, Cabrini G. Mapping the transcriptional machinery of the IL-8 gene in human bronchial epithelial cells. J Immunol 187: 6069–6081, 2011. doi: 10.4049/jimmunol.1100821. [DOI] [PubMed] [Google Scholar]
- 10.Bhandary YP, Shetty SK, Marudamuthu AS, Ji HL, Neuenschwander PF, Boggaram V, Morris GF, Fu J, Idell S, Shetty S. Regulation of lung injury and fibrosis by p53-mediated changes in urokinase and plasminogen activator inhibitor-1. Am J Pathol 183: 131–143, 2013. doi: 10.1016/j.ajpath.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonner JC. Mesenchymal cell survival in airway and interstitial pulmonary fibrosis. Fibrogenesis Tissue Repair 3: 15, 2010. doi: 10.1186/1755-1536-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calfee CS, Matthay MA, Eisner MD, Benowitz N, Call M, Pittet JF, Cohen MJ. Active and passive cigarette smoking and acute lung injury after severe blunt trauma. Am J Respir Crit Care Med 183: 1660–1665, 2011. doi: 10.1164/rccm.201011-1802OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calfee CS, Matthay MA, Kangelaris KN, Siew ED, Janz DR, Bernard GR, May AK, Jacob P, Havel C, Benowitz NL, Ware LB. Cigarette smoke exposure and the acute respiratory distress syndrome. Crit Care Med 43: 1790–1797, 2015. doi: 10.1097/CCM.0000000000001089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carrozzino F, Pugnale P, Féraille E, Montesano R. Inhibition of basal p38 or JNK activity enhances epithelial barrier function through differential modulation of claudin expression. Am J Physiol Cell Physiol 297: C775–C787, 2009. doi: 10.1152/ajpcell.00084.2009. [DOI] [PubMed] [Google Scholar]
- 15.Chen SJ, Huang SS, Chang NS. Role of WWOX and NF-κB in lung cancer progression. Transl Respir Med 1: 15, 2013. doi: 10.1186/2213-0802-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer 16: 431–446, 2016. doi: 10.1038/nrc.2016.52. [DOI] [PubMed] [Google Scholar]
- 17.de Jesus Perez V, Yuan K, Alastalo TP, Spiekerkoetter E, Rabinovitch M. Targeting the Wnt signaling pathways in pulmonary arterial hypertension. Drug Discov Today 19: 1270–1276, 2014. doi: 10.1016/j.drudis.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Del Mare S, Aqeilan RI. Tumor suppressor WWOX inhibits osteosarcoma metastasis by modulating RUNX2 function. Sci Rep 5: 12959, 2015. doi: 10.1038/srep12959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Del Mare S, Salah Z, Aqeilan RI. WWOX: its genomics, partners, and functions. J Cell Biochem 108: 737–745, 2009. doi: 10.1002/jcb.22298. [DOI] [PubMed] [Google Scholar]
- 20.El Rayes T, Catena R, Lee S, Stawowczyk M, Joshi N, Fischbach C, Powell CA, Dannenberg AJ, Altorki NK, Gao D, Mittal V. Lung inflammation promotes metastasis through neutrophil protease-mediated degradation of Tsp-1. Proc Natl Acad Sci USA 112: 16000–16005, 2015. doi: 10.1073/pnas.1507294112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Everhart MB, Han W, Sherrill TP, Arutiunov M, Polosukhin VV, Burke JR, Sadikot RT, Christman JW, Yull FE, Blackwell TS. Duration and intensity of NF-kappaB activity determine the severity of endotoxin-induced acute lung injury. J Immunol 176: 4995–5005, 2006. doi: 10.4049/jimmunol.176.8.4995. [DOI] [PubMed] [Google Scholar]
- 22.Fabbri M, Iliopoulos D, Trapasso F, Aqeilan RI, Cimmino A, Zanesi N, Yendamuri S, Han SY, Amadori D, Huebner K, Croce CM. WWOX gene restoration prevents lung cancer growth in vitro and in vivo. Proc Natl Acad Sci USA 102: 15611–15616, 2005. doi: 10.1073/pnas.0505485102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foster KA, Oster CG, Mayer MM, Avery ML, Audus KL. Characterization of the A549 cell line as a type II pulmonary epithelial cell model for drug metabolism. Exp Cell Res 243: 359–366, 1998. doi: 10.1006/excr.1998.4172. [DOI] [PubMed] [Google Scholar]
- 24.Fu J, Qu Z, Yan P, Ishikawa C, Aqeilan RI, Rabson AB, Xiao G. The tumor suppressor gene WWOX links the canonical and noncanonical NF-κB pathways in HTLV-I Tax-mediated tumorigenesis. Blood 117: 1652–1661, 2011. doi: 10.1182/blood-2010-08-303073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fujimoto H, D’Alessandro-Gabazza CN, Palanki MS, Erdman PE, Takagi T, Gabazza EC, Bruno NE, Yano Y, Hayashi T, Tamaki S, Sumida Y, Adachi Y, Suzuki K, Taguchi O. Inhibition of nuclear factor-kappaB in T cells suppresses lung fibrosis. Am J Respir Crit Care Med 176: 1251–1260, 2007. doi: 10.1164/rccm.200609-1288OC. [DOI] [PubMed] [Google Scholar]
- 26.Gaudio E, Palamarchuk A, Palumbo T, Trapasso F, Pekarsky Y, Croce CM, Aqeilan RI. Physical association with WWOX suppresses c-Jun transcriptional activity. Cancer Res 66: 11585–11589, 2006. doi: 10.1158/0008-5472.CAN-06-3376. [DOI] [PubMed] [Google Scholar]
- 27.Gauldie J, Kolb M, Ask K, Martin G, Bonniaud P, Warburton D. Smad3 signaling involved in pulmonary fibrosis and emphysema. Proc Am Thorac Soc 3: 696–702, 2006. doi: 10.1513/pats.200605-125SF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gourley C, Paige AJ, Taylor KJ, Ward C, Kuske B, Zhang J, Sun M, Janczar S, Harrison DJ, Muir M, Smyth JF, Gabra H. WWOX gene expression abolishes ovarian cancer tumorigenicity in vivo and decreases attachment to fibronectin via integrin alpha3. Cancer Res 69: 4835–4842, 2009. doi: 10.1158/0008-5472.CAN-08-2974. [DOI] [PubMed] [Google Scholar]
- 29.Grommes J, Vijayan S, Drechsler M, Hartwig H, Mörgelin M, Dembinski R, Jacobs M, Koeppel TA, Binnebösel M, Weber C, Soehnlein O. Simvastatin reduces endotoxin-induced acute lung injury by decreasing neutrophil recruitment and radical formation. PLoS One 7: e38917, 2012. doi: 10.1371/journal.pone.0038917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo RF, Lentsch AB, Sarma JV, Sun L, Riedemann NC, McClintock SD, McGuire SR, Van Rooijen N, Ward PA. Activator protein-1 activation in acute lung injury. Am J Pathol 161: 275–282, 2002. doi: 10.1016/S0002-9440(10)64179-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hosokawa S, Haraguchi G, Sasaki A, Arai H, Muto S, Itai A, Doi S, Mizutani S, Isobe M. Pathophysiological roles of nuclear factor kappaB (NF-kB) in pulmonary arterial hypertension: effects of synthetic selective NF-kB inhibitor IMD-0354. Cardiovasc Res 99: 35–43, 2013. doi: 10.1093/cvr/cvt105. [DOI] [PubMed] [Google Scholar]
- 32.Hsieh SJ, Zhuo H, Benowitz NL, Thompson BT, Liu KD, Matthay MA, Calfee CS; National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome Network; National Heart Lung and Blood Institute Acute Respiratory Distress Syndrome Network . Prevalence and impact of active and passive cigarette smoking in acute respiratory distress syndrome. Crit Care Med 42: 2058–2068, 2014. doi: 10.1097/CCM.0000000000000418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Javelaud D, Laboureau J, Gabison E, Verrecchia F, Mauviel A. Disruption of basal JNK activity differentially affects key fibroblast functions important for wound healing. J Biol Chem 278: 24624–24628, 2003. doi: 10.1074/jbc.M301942200. [DOI] [PubMed] [Google Scholar]
- 34.Jin C, Ge L, Ding X, Chen Y, Zhu H, Ward T, Wu F, Cao X, Wang Q, Yao X. PKA-mediated protein phosphorylation regulates ezrin-WWOX interaction. Biochem Biophys Res Commun 341: 784–791, 2006. doi: 10.1016/j.bbrc.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 35.Lee HS, Kim HJ, Moon CS, Chong YH, Kang JL. Inhibition of c-Jun NH2-terminal kinase or extracellular signal-regulated kinase improves lung injury. Respir Res 5: 23, 2004. doi: 10.1186/1465-9921-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee S, Das HK. Inhibition of basal activity of c-jun-NH2-terminal kinase (JNK) represses the expression of presenilin-1 by a p53-dependent mechanism. Brain Res 1207: 19–31, 2008. doi: 10.1016/j.brainres.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 37.Li L, Wei C, Kim IK, Janssen-Heininger Y, Gupta S. Inhibition of nuclear factor-κB in the lungs prevents monocrotaline-induced pulmonary hypertension in mice. Hypertension 63: 1260–1269, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03220. [DOI] [PubMed] [Google Scholar]
- 38.Li LF, Lee CS, Liu YY, Chang CH, Lin CW, Chiu LC, Kao KC, Chen NH, Yang CT. Activation of Src-dependent Smad3 signaling mediates the neutrophilic inflammation and oxidative stress in hyperoxia-augmented ventilator-induced lung injury. Respir Res 16: 112, 2015. doi: 10.1186/s12931-015-0275-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li LF, Ouyang B, Choukroun G, Matyal R, Mascarenhas M, Jafari B, Bonventre JV, Force T, Quinn DA. Stretch-induced IL-8 depends on c-Jun NH2-terminal and nuclear factor-κB-inducing kinases. Am J Physiol Lung Cell Mol Physiol 285: L464–L475, 2003. doi: 10.1152/ajplung.00031.2003. [DOI] [PubMed] [Google Scholar]
- 40.Lindén A, Laan M, Anderson GP. Neutrophils, interleukin-17A and lung disease. Eur Respir J 25: 159–172, 2005. doi: 10.1183/09031936.04.00032904. [DOI] [PubMed] [Google Scholar]
- 41.Liu G, Park YJ, Tsuruta Y, Lorne E, Abraham E. p53 Attenuates lipopolysaccharide-induced NF-kappaB activation and acute lung injury. J Immunol 182: 5063–5071, 2009. doi: 10.4049/jimmunol.0803526. [DOI] [PubMed] [Google Scholar]
- 42.Liu M, Shi L, Chen M, Chen S, Zou X. Effects of c-Jun N-terminal kinase signaling pathway on severe acute pancreatitis-associated lung injury. Pancreas 41: 358–366, 2012. doi: 10.1097/MPA.0b013e3182297f09. [DOI] [PubMed] [Google Scholar]
- 43.Malenfant S, Neyron AS, Paulin R, Potus F, Meloche J, Provencher S, Bonnet S. Signal transduction in the development of pulmonary arterial hypertension. Pulm Circ 3: 278–293, 2013. doi: 10.4103/2045-8932.114752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manzer R, Wang J, Nishina K, McConville G, Mason RJ. Alveolar epithelial cells secrete chemokines in response to IL-1beta and lipopolysaccharide but not to ozone. Am J Respir Cell Mol Biol 34: 158–166, 2006. doi: 10.1165/rcmb.2005-0205OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mao P, Wu S, Li J, Fu W, He W, Liu X, Slutsky AS, Zhang H, Li Y. Human alveolar epithelial type II cells in primary culture. Physiol Rep 3: e12288, 2015. doi: 10.14814/phy2.12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol 295: L379–L399, 2008. doi: 10.1152/ajplung.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morrell NW, Adnot S, Archer SL, Dupuis J, Jones PL, MacLean MR, McMurtry IF, Stenmark KR, Thistlethwaite PA, Weissmann N, Yuan JX, Weir EK. Cellular and molecular basis of pulmonary arterial hypertension. J Am Coll Cardiol 54, Suppl: S20–S31, 2009. doi: 10.1016/j.jacc.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morrisey EE. Wnt signaling and pulmonary fibrosis. Am J Pathol 162: 1393–1397, 2003. doi: 10.1016/S0002-9440(10)64271-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murin S, Inciardi J. Cigarette smoking and the risk of pulmonary metastasis from breast cancer. Chest 119: 1635–1640, 2001. doi: 10.1378/chest.119.6.1635. [DOI] [PubMed] [Google Scholar]
- 50.Nunez MI, Rosen DG, Ludes-Meyers JH, Abba MC, Kil H, Page R, Klein-Szanto AJ, Godwin AK, Liu J, Mills GB, Aldaz CM. WWOX protein expression varies among ovarian carcinoma histotypes and correlates with less favorable outcome. BMC Cancer 5: 64, 2005. doi: 10.1186/1471-2407-5-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parra E, Gutiérrez L, Ferreira J. Inhibition of basal JNK activity by small interfering RNAs enhances cisplatin sensitivity and decreases DNA repair in T98G glioblastoma cells. Oncol Rep 33: 413–418, 2015. doi: 10.3892/or.2014.3570. [DOI] [PubMed] [Google Scholar]
- 52.Perl M, Chung CS, Lomas-Neira J, Rachel TM, Biffl WL, Cioffi WG, Ayala A. Silencing of Fas, but not caspase-8, in lung epithelial cells ameliorates pulmonary apoptosis, inflammation, and neutrophil influx after hemorrhagic shock and sepsis. Am J Pathol 167: 1545–1559, 2005. doi: 10.1016/S0002-9440(10)61240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Płuciennik E, Kusińska R, Potemski P, Kubiak R, Kordek R, Bednarek AK. WWOX--the FRA16D cancer gene: expression correlation with breast cancer progression and prognosis. Eur J Surg Oncol 32: 153–157, 2006. doi: 10.1016/j.ejso.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 54.Pongracz JE, Stockley RA. Wnt signalling in lung development and diseases. Respir Res 7: 15, 2006. doi: 10.1186/1465-9921-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ried K, Finnis M, Hobson L, Mangelsdorf M, Dayan S, Nancarrow JK, Woollatt E, Kremmidiotis G, Gardner A, Venter D, Baker E, Richards RI. Common chromosomal fragile site FRA16D sequence: identification of the FOR gene spanning FRA16D and homozygous deletions and translocation breakpoints in cancer cells. Hum Mol Genet 9: 1651–1663, 2000. doi: 10.1093/hmg/9.11.1651. [DOI] [PubMed] [Google Scholar]
- 56.Scanlon EF, Suh O, Murthy SM, Mettlin C, Reid SE, Cummings KM. Influence of smoking on the development of lung metastases from breast cancer. Cancer 75: 2693–2699, 1995. doi:. [DOI] [PubMed] [Google Scholar]
- 57.Schmiedl A, Behrens J, Zscheppang K, Purevdorj E, von Mayersbach D, Liese A, Dammann CE. Lipopolysaccharide-induced injury is more pronounced in fetal transgenic ErbB4-deleted lungs. Am J Physiol Lung Cell Mol Physiol 301: L490–L499, 2011. doi: 10.1152/ajplung.00131.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Standiford TJ, Kunkel SL, Basha MA, Chensue SW, Lynch JP III, Toews GB, Westwick J, Strieter RM. Interleukin-8 gene expression by a pulmonary epithelial cell line. A model for cytokine networks in the lung. J Clin Invest 86: 1945–1953, 1990. doi: 10.1172/JCI114928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Standiford TJ, Strieter RM, Kasahara K, Kunkel SL. Disparate regulation of interleukin 8 gene expression from blood monocytes, endothelial cells, and fibroblasts by interleukin 4. Biochem Biophys Res Commun 171: 531–536, 1990. doi: 10.1016/0006-291X(90)91178-U. [DOI] [PubMed] [Google Scholar]
- 60.Strieter RM, Chensue SW, Basha MA, Standiford TJ, Lynch JP III, Baggiolini M, Kunkel SL. Human alveolar macrophage gene expression of interleukin-8 by tumor necrosis factor-alpha, lipopolysaccharide, and interleukin-1 beta. Am J Respir Cell Mol Biol 2: 321–326, 1990. doi: 10.1165/ajrcmb/2.4.321. [DOI] [PubMed] [Google Scholar]
- 61.Swain RJ, Kemp SJ, Goldstraw P, Tetley TD, Stevens MM. Assessment of cell line models of primary human cells by Raman spectral phenotyping. Biophys J 98: 1703–1711, 2010. doi: 10.1016/j.bpj.2009.12.4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Taranova AG, Maldonado D III, Vachon CM, Jacobsen EA, Abdala-Valencia H, McGarry MP, Ochkur SI, Protheroe CA, Doyle A, Grant CS, Cook-Mills J, Birnbaumer L, Lee NA, Lee JJ. Allergic pulmonary inflammation promotes the recruitment of circulating tumor cells to the lung. Cancer Res 68: 8582–8589, 2008. doi: 10.1158/0008-5472.CAN-08-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thavathiru E, Ludes-Meyers JH, MacLeod MC, Aldaz CM. Expression of common chromosomal fragile site genes, WWOX/FRA16D and FHIT/FRA3B is downregulated by exposure to environmental carcinogens, UV, and BPDE but not by IR. Mol Carcinog 44: 174–182, 2005. doi: 10.1002/mc.20122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thomson NC, Chaudhuri R, Livingston E. Asthma and cigarette smoking. Eur Respir J 24: 822–833, 2004. doi: 10.1183/09031936.04.00039004. [DOI] [PubMed] [Google Scholar]
- 65.Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer 9: 537–549, 2009. doi: 10.1038/nrc2694. [DOI] [PubMed] [Google Scholar]
- 66.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 342: 1334–1349, 2000. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 67.Wculek SK, Malanchi I. Neutrophils support lung colonization of metastasis-initiating breast cancer cells. Nature 528: 413–417, 2015. doi: 10.1038/nature16140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wickremasinghe MI, Thomas LH, Friedland JS. Pulmonary epithelial cells are a source of IL-8 in the response to Mycobacterium tuberculosis: essential role of IL-1 from infected monocytes in a NF-kappa B-dependent network. J Immunol 163: 3936–3947, 1999. [PubMed] [Google Scholar]
- 69.Wong MH, Johnson MD. Differential response of primary alveolar type I and type II cells to LPS stimulation. PLoS One 8: e55545, 2013. doi: 10.1371/journal.pone.0055545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yan L, Cai Q, Xu Y. The ubiquitin-CXCR4 axis plays an important role in acute lung infection-enhanced lung tumor metastasis. Clin Cancer Res 19: 4706–4716, 2013. doi: 10.1158/1078-0432.CCR-13-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang W, Cui S, Ma J, Lu Q, Kong C, Liu T, Sun Z. Cigarette smoking extract causes hypermethylation and inactivation of WWOX gene in T-24 human bladder cancer cells. Neoplasma 59: 216–223, 2012. doi: 10.4149/neo_2012_028. [DOI] [PubMed] [Google Scholar]
- 72.Yoshida T, Tuder RM. Pathobiology of cigarette smoke-induced chronic obstructive pulmonary disease. Physiol Rev 87: 1047–1082, 2007. doi: 10.1152/physrev.00048.2006. [DOI] [PubMed] [Google Scholar]
- 73.Zhao Y, Gorshkova IA, Berdyshev E, He D, Fu P, Ma W, Su Y, Usatyuk PV, Pendyala S, Oskouian B, Saba JD, Garcia JG, Natarajan V. Protection of LPS-induced murine acute lung injury by sphingosine-1-phosphate lyase suppression. Am J Respir Cell Mol Biol 45: 426–435, 2011. doi: 10.1165/rcmb.2010-0422OC. [DOI] [PMC free article] [PubMed] [Google Scholar]