Abstract
Vascular endothelial protein tyrosine phosphatase (VE-PTP) stabilizes endothelial adherens junctions (AJs) through constitutive dephosphorylation of VE-cadherin. Here we investigated the role of stromal interaction molecule 1 (STIM1) activation of store-operated Ca2+ entry (SOCE) in regulating AJ assembly. We observed that SOCE induced by STIM1 activated Pyk2 in human lung microvascular endothelial cells (ECs) and induced tyrosine phosphorylation of VE-PTP at Y1981. Pyk2-induced tyrosine phosphorylation of VE-PTP promoted Src binding to VE-PTP, Src activation, and subsequent VE-cadherin phosphorylation and thereby increased the endothelial permeability response. The increase in permeability was secondary to disassembly of AJs. Pyk2-mediated responses were blocked in EC-restricted Stim1 knockout mice, indicating the requirement for STIM1 in initiating the signaling cascade. A peptide derived from the Pyk2 phosphorylation site on VE-PTP abolished the STIM1/SOCE-activated permeability response. Thus Pyk2 activation secondary to STIM1-induced SOCE causes tyrosine phosphorylation of VE-PTP, and VE-PTP, in turn, binds to and activates Src, thereby phosphorylating VE-cadherin to increase endothelial permeability through disassembly of AJs. Our results thus identify a novel signaling mechanism by which STIM1-induced Ca2+ signaling activates Pyk2 to inhibit the interaction of VE-PTP and VE-cadherin and hence increase endothelial permeability. Therefore, targeting the Pyk2 activation pathway may be a potentially important anti-inflammatory strategy.
Keywords: thrombin, stromal interaction molecule 1, store-operated Ca2+ entry, Pyk2, vascular endothelial protein tyrosine phosphatase, VE-cadherin, permeability increase
endothelial barrier dysfunction results in fluid extravasation and tissue edema (2, 17). VE-cadherin forms Ca2+-dependent homophilic cis and trans dimers at adherens junctions (AJs) to establish cell-to-cell adhesion, which is essential for endothelial barrier integrity (11, 22). The cytoplasmic domain of VE-cadherin interacts with intracellular proteins, β-catenin, plakoglobin, and p120-catenin, which transfer intracellular signals and modulate the interactions with the actin cytoskeleton (11, 22). Antibodies that specifically target the extracellular domain of VE-cadherin destabilized AJs in vivo, resulting in increased vascular permeability and leukocyte extravasation (5, 9, 20). Furthermore, a knockin mouse model, in which the VE-cadherin-β-catenin-α-catenin complex was replaced with a VE-cad-α-catenin fusion protein, showed stabilized endothelial AJs and inhibition of increased permeability and leukocyte extravasation in response to VEGF and LPS (36). These findings suggested a key role for AJs in regulating endothelial permeability to fluid and protein as well as circulating blood cells.
Phosphorylation of VE-cadherin and its associated proteins, β-catenin and p120-catenin, destabilized AJs (7, 30). Inflammatory mediators (e.g., VEGF, bradykinin, TNF-α, and LPS) functioned in part by inducing the phosphorylation of VE-cadherin at tyrosine residues (Y658, Y685, and Y731) and thereby increased vascular permeability and leukocyte extravasation (18, 19, 32, 47). Wessel et al. (50) showed that knockin mice expressing the Y685F mutant VE-cadherin exhibited impaired vascular permeability responses, whereas mice expressing the Y731F mutant showed decreased neutrophil extravasation, suggesting that phosphorylation of VE-cadherin at these distinct tyrosine residues could differentially regulate vascular permeability and leukocyte extravasation. Permeability-increasing mediators induced endothelial barrier dysfunction by disassembling AJs via c-Src-dependent phosphorylation of VE-cadherin and its binding partner p120-catenin in endothelial cells (ECs) (7, 30). Several protein tyrosine phosphatases (PTPs) appear to regulate endothelial barrier function by regulating the phosphorylation of AJ proteins (47). Of these, vascular endothelial PTP (VE-PTP), expressed in ECs, interacts with VE-cadherin at AJs to maintain endothelial barrier integrity in a phosphatase-dependent manner (14, 38, 47).
VE-PTP (also known as PTPRB or PTP-β) is a receptor-type PTP (RPTP) of the R3 subtype (28). The extracellular region of VE-PTP contains fibronectin type III-like domains, and the cytoplasmic region has a single catalytic domain (25). VE-PTP binds to VE-cadherin via the extracellular domain of VE-PTP (29). Binding of a phosphorylated VE-PTP substrate to the cytosolic domain of VE-PTP may induce detachment of the extracellular domain of VE-PTP from VE-cadherin (48). The R3 subtype RPTPs contain a conserved amino acid motif YxNФ (where Ф is a hydrophobic amino acid and x is any amino acid) in the COOH terminus (25). The YxNФ motif undergoes tyrosine phosphorylation and, in turn, binds the Src homology-2 (SH2) domain of Src family kinases (SFKs). This binding has the potential to activate SFK (25, 27). Both human (h) and mouse (m) deduced sequences of VE-PTP contain the amino acid motif “YENV” (h residues 1981–1984; m residues 1982–1985) in the COOH terminus. However, it is not known whether permeability-increasing mediators induce VE-PTP phosphorylation at Y1981 and, if so, whether phosphorylated VE-PTP can thereby bind and activate Src and whether Src, in turn, can phosphorylate VE-cadherin to disassemble AJs.
Thrombin, a permeability-increasing mediator that acts at AJs, is generated at the site of infection during sepsis (12, 16, 26) and promotes vascular leak by activating the G protein-coupled receptor protease-activated receptor 1 (PAR-1) expressed in ECs (13, 33, 43, 49). We showed that thrombin-induced endothelial permeability required store-operated Ca2+ entry (SOCE) through store-operated Ca2+ channels (SOCs) (42, 43). Furthermore, we and others showed that the endoplasmic reticulum (ER)-localized Ca2+ sensor protein, stromal interaction molecule 1 (STIM1), was essential for activating SOCE (1, 10, 39, 41). Importantly, ECs from EC-restricted STIM1 knockout (Stim1ΔEC) mice showed loss of SOCE (15). Consistent with this observation, LPS-induced lung vascular leak and leukocyte infiltration were abrogated in lungs of Stim1ΔEC mice (15). These findings support the fundamental role of STIM1-activated SOCE in ECs as a critical mechanism of endothelial barrier dysfunction. However, the signaling pathway downstream of SOCE inducing endothelial barrier dysfunction has not been identified. Because Pyk2 is activated secondary to SOCE (8, 37) and may interfere with the association between VE-PTP and VE-cadherin (48), we investigated whether Pyk2-induced tyrosine phosphorylation of VE-PTP per se was required for signaling disassembly of AJs and thereby increasing endothelial permeability. Thus we determined whether Pyk2 activation downstream of SOCE mediated tyrosine phosphorylation of VE-PTP and whether this, in turn, facilitated Src binding to and activation of Src tyrosine kinase to induce the phosphorylation of VE-cadherin disassembly of AJs.
MATERIALS AND METHODS
Reagents.
Hanks balanced salt solution (HBSS), L-glutamine, trypsin, and fura-2 AM were purchased from Invitrogen (Carlsbad, CA). FBS was from Hyclone (Logan, UT). Human α-thrombin was obtained from Enzyme Research Laboratories (South Bend, IN). All peptides used for experiments were synthesized by Genscript (Piscataway, NJ). Peptide purity and amino acid sequences were determined by high-performance liquid chromatography and MS, respectively. PAR-1-activating peptide (TFLLRNPNDK-NH2) was synthesized as the COOH-terminal amide with a purity of >95%. The COOH-terminal peptides of VE-PTP [h residues, 1977–1988; wild-type (WT)-LFPIYENVNPEY; mutant (Mut)- LFPIFENVNPEY] were synthesized as a fusion peptide to the COOH terminus of the antennapedia (RQIKIWFQNRRMKWKK) internalization sequence with >95% purity (23). Scrambled-siRNA (sc-siRNA), human (h)-specific siRNA against STIM1 (ON-TARGETplus SMARTpool siRNA containing sequences: GGUGGUGUCUAUCGUUAUU, UACAGUGGCUGAUCACAUA, CAAUUACCAUGACCCAACA, and UCUCUUGACUCGCCAUAAU) and mouse (m)-specific siRNA against Pyk2 (ON-TARGETplus SMARTpool siRNA containing sequences: GACACUACCUGGAACGAAA, UCAUGGAACUGUAUCCUUA, UAAGGGCUCUCUCAUCAUG, and GAAGUUGGCUCAGCAGAAC) were purchased from Dharmacon (Lafayette, CO). Goat polyclonal antibody (pAb) against VE-cadherin (cat. no. sc-6458) (19), rabbit pAb against VE-PTP (cat. no. sc-28905) (19), siRNA specific to human Pyk2, and transfection reagents were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit pAb against STIM1 (cat no. 11565-1-AP) (10) was from Proteintech Group (Chicago, IL). Rabbit pAb specific to phospho-Pyk2 Y402 (cat. no. 3291) (48), rabbit pAb against Pyk2 (cat. no. 3090) (32), rabbit mAb against phospho-Src Y416 (cat. no. 6943) (24), and rabbit mAb against Src (cat. no. 2123) (3) were purchased from Cell Signaling (Danvers, MA). Mouse mAb against β-actin (cat. no. A5441) (10) and Pyk2 inhibitor (PF-431396 hydrate, 1 µM working solution) were from Sigma (St. Louis, MO). Src tyrosine kinase-specific inhibitor 4-amino-5-(4-methylphenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (PP1) and anti-phospho-tyrosine mAb (pY20) (cat. no. 525295) (51) were from Calbiochem (La Jolla, CA). Rabbit pAb specific to phospho-VE-cadherin Y731 (cat. no. 44-1145G) (24) was from Invitrogen. Rabbit pAb against phospho-VE-cadherin Y685 (cat. no. ab119785) (24) was from Abcam (Cambridge, MA).
Animals.
Stim1-floxed (Stim1fl/fl) mice (31) were a gift from Dr. Masatsugu Oh-Hora (Tokyo Medical and Dental University, Tokyo, Japan). C57BL/6 (WT) and B6.Cg-Tg(Cdh5-Cre)7Mlia/J (VE-cadh Cre+) mice were obtained from Jackson Laboratories (Bar Harbor, ME). Stim1fl/fl mice were crossed with VE-cadh Cre+ (VE-cadherin promoter driving the expression of Cre) mice to generate endothelial-restricted Stim1 knockout (Stim1ΔEC) mice as described previously (15). All mice were housed in the University of Illinois Animal Care Facility in accordance with institutional guidelines of the US National Institutes of Health. All animal experiments were performed under the protocol approved by the Institutional Animal Care and Use Committee of the University of Illinois at Chicago.
Cell culture.
Human lung microvascular endothelial cells (HLMVECs) and endothelial growth media-2 (EGM-2) were purchased from Lonza (Walkersville, MD). Mouse (C57BL/6) lung ECs (mLECs) were isolated and cultured as described previously (43). HLMVECs were cultured in EGM-2MV supplemented with 15% FBS, and mLECs were grown in EGM-2 supplemented with 5% FBS. Both cell types were used between passages 3 and 6.
Immunoprecipitation.
ECs grown to confluence treated with or without agonists were washed three times with PBS at 4°C and lysed in lysis buffer (50.00 mM Tris·HCl, pH 7.5, 150.00 mM NaCl, 1.00 mM EGTA, 1.00% Triton X-100, 0.25% sodium deoxycholate, 0.10% SDS, 10.00 μM orthovanadate, and protease-inhibitor mixture) as previously described (40). Mouse lungs were homogenized in lysis buffer (45). EC lysates or mouse lung homogenates were centrifuged (13,000 g for 10 min) to remove insoluble materials. Clear supernatant (300 μg protein) was subjected to immunoprecipitation. Each sample was incubated overnight with 1 μg/ml of the indicated antibody at 4°C. The next day, protein A/G beads were added to the sample and incubated for 1 h at 4°C. Immunoprecipitates were then washed three times with wash buffer (Tris-buffered saline containing 0.05% Triton X-100, 1.00 mM Na3VO4, 1.00 mM NaF, 2.00 μg/ml leupeptin, 2.00 μg/ml pepstatin A, 2.00 μg/ml aprotinin, and 44.00 μg/ml phenylmethylsulfonyl fluoride). Immunoprecipitated proteins were used for immunoblotting.
Immunoblotting.
EC lysates, lung tissue homogenates, or immunoprecipitated proteins were resolved by SDS-PAGE on a 4–15% gradient separating gel under reducing conditions and transferred to a Duralose membrane. Membranes were blocked with 5% dry milk in TBST (10.00 mM Tris·HCl pH 7.5, 150.00 mM NaCl, and 0.05% Tween-20) for 1 h at room temperature and then incubated with the indicated primary antibody diluted in blocking buffer overnight at 4°C. For phospho-specific blots, the membranes were incubated overnight at 4°C with the primary antibody diluted in TBST containing 5% bovine serum albumin. Membranes were next washed three times and then incubated with appropriate horseradish peroxidase P-conjugated secondary antibody. Protein bands were detected by enhanced chemiluminescence.
Cytosolic Ca2+ measurements.
The cytoplasmic Ca2+ ([Ca2+]i) in ECs was measured using the Ca2+-sensitive fluorescent dye fura-2 AM as previously described (10, 43).
Transendothelial electrical resistance measurements.
Real-time changes in transendothelial monolayer electrical resistance (TER) were measured to assess endothelial barrier function (44). Confluent endothelial monolayers were serum starved for 2 h with 1% FBS-containing medium. Thrombin-induced real-time changes in TER were next measured.
Immunostaining.
Confluent ECs grown on glass coverslips were subjected either to thrombin treatment or left untreated. After treatment, cells were washed quickly with cold PBS and fixed with 2% paraformaldehyde for 15 min at 4°C. Following fixation, cells were permeabilized with 0.05% Triton X-100 for 1 min at 4°C. Cells were next washed three times with PBS and then incubated with blocking buffer (PBS containing 5% horse serum and 1% BSA) for 1 h at room temperature. Cells were then incubated overnight with the indicated primary antibody (in PBS containing 1% BSA) at 4°C. The next day, cells were washed three times and incubated with specific Alexa-Fluor-conjugated secondary antibody and DAPI for 1 h at 4°C. Finally, cells were washed three times and mounted on glass slides for viewing. Images were acquired with the Zeiss LSM 510 confocal microscope. VE-cadherin immunoreactivity at cell-cell junctions was quantified using ImageJ software (version 1.50i; National Institutes of Health, Bethesda, MD).
siRNA transfection.
ECs grown to 70–80% confluence on gelatin-coated culture dishes were transfected with target siRNAs or sc-siRNA as described (10). At 72 h after transfection, cells were used for experiments.
In vivo siRNA delivery in mouse lungs.
Cationic liposomes were prepared with dimethyldioctadecylammonium bromide and cholesterol (1:1) as previously described (45, 53). The liposomes and siRNA (control-siRNA or mouse Pyk2-siRNA) were then mixed (8 mol lipid:1 μg siRNA), and the mixture (1 μg siRNA per gram body weight) was injected retroorbitally into mice under anesthesia (2.5% isoflurane in room air). At 48 h after delivery, the mice were used for experiments.
Mouse lung capillary filtration coefficient measurements.
Pulmonary capillary filtration coefficient (Kf,c) was measured to determine microvascular permeability to liquid as described previously (43, 49). To measure PAR-1 induced changes in endothelial barrier function, PAR-1 agonist peptide (30 μM) containing perfusion buffer was infused via a side port at a rate of 0.2 ml/min. Kf,c measurements were made at baseline and after 20 min of exposure to PAR-1 agonist peptide. The values are expressed as the ratio of experimental:basal Kf,c values in the same lung preparation.
We also determined mouse lung microvessel permeability in vivo. Here mice were anesthetized with 2.5% isoflurane in room air for retroorbital injections. The mice were then injected with saline (100 μl) or PAR-1 agonist peptide 100 μl (1 mg/mouse). After 15 min, mice received 100 μl of Evans blue dye conjugated with albumin (EBA) (20 mg/kg) (10). Thirty minutes after EBA injection, mice were euthanized, and lungs were harvested. The EBA concentration in lung tissue was measured as described previously (10). For VE-PTP peptide pretreatment experiments, vehicle (100 μl), WT-VE-PTP peptide (100 μl of 0.1 mM per mouse), or Mut-VE-PTP peptide (100 μl of 0.1 mM per mouse) was administered 60 min before PAR-1 agonist peptide injection.
Statistical analysis.
Western blot bands were quantified using NIH ImageJ software. ANOVA and Student’s t-test (2-tailed) were used to determine statistical significance with a P value threshold set at <0.05.
RESULTS
SOCE is required for tyrosine phosphorylation of VE-PTP and disassembly of VE-cadherin junctions.
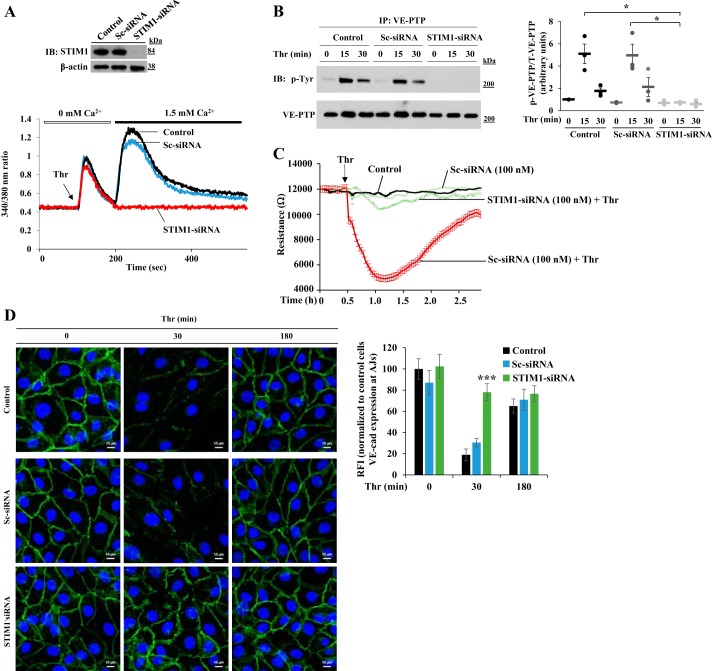
We investigated first whether SOCE in ECs induces tyrosine phosphorylation of VE-PTP, which, in turn, promotes destabilization of VE-cadherin at endothelial AJs. Because ER-localized STIM1 is critical for activating SOCE, we suppressed STIM1 expression using siRNA in HLMVECs. STIM1 expression as well as thrombin-induced Ca2+ entry were substantially reduced in STIM1-siRNA-transfected cells compared with that in cells transfected with control siRNA (sc-siRNA) or untreated cells (Fig. 1A). We observed that thrombin-induced tyrosine phosphorylation of VE-PTP was also blocked in STIM1 knockdown HLMVECs (Fig. 1B) but not in control or sc-siRNA-treated HLMVECs (Fig. 1B), suggesting that PAR-1-induced SOCE is essential for tyrosine phosphorylation of VE-PTP in ECs. To address the functional relevance of tyrosine phosphorylation of VE-PTP, we determined endothelial permeability responses by measuring TER in control and STIM1-siRNA-transfected HLMVECs. In STIM1-siRNA-treated HLMVECs, thrombin-induced decrease in TER was markedly reduced compared with control cells (Fig. 1C). We also measured VE-cadherin expression at endothelial AJs in control and STIM1 knockdown cells by immunostaining. In sc-siRNA-transfected cells, we observed VE-cadherin depletion from endothelial AJs 30 min after thrombin challenge (Fig. 1D) and restoration of VE-cadherin expression at endothelial AJs to near control levels 3 h after thrombin challenge (Fig. 1D). Interestingly, in STIM1-siRNA-transfected cells, thrombin challenge failed to deplete EC AJs of VE-cadherin (Fig. 1D), suggesting that SOCE-induced VE-PTP tyrosine phosphorylation was essential for PAR-1-induced depletion of VE-cadherin from endothelial AJs.
Fig. 1.
Stromal interaction molecule 1 (STIM1) knockdown in human lung microvascular endothelial cells (HLMVECs) prevents thrombin-induced store-operated Ca2+ entry (SOCE), vascular endothelial protein tyrosine phosphatase (VE-PTP) phosphorylation, permeability increase, and disassembly of VE-cadherin at endothelial adherens junctions (AJs). A: HLMVECs transfected with 100 nM sc-siRNA or STIM1-siRNA. 48 h after transfection, cells were used to determine STIM1 protein expression by immunoblot (IB) (top) or used to measure thrombin-induced endoplasmic reticulum (ER)-store Ca2+ release and Ca2+ release-activated Ca2+ entry (bottom). B: nontransfected HLMVECs or HLMVECs transfected with sc-siRNA or STIM1-siRNA were challenged with thrombin (25 nM). Cells were used for immunoprecipitation with VE-PTP pAb and blotted with phosphotyrosine mAb. Quantified data from 3 experiments are presented as the means ± SE ratio of phosphorylated:total protein. *P < 0.05; transfected compared with nontransfected or sc-siRNA-treated cells. C: HLMVECs were transfected with sc-siRNA or STIM1-siRNA. At 24 h after transfection, cells were plated on gold electrodes (see materials and methods). After 24 h, cells were washed and incubated in 1% FBS containing medium for 2 h, and then cells were challenged with medium or thrombin (25 nM). Values from 3 experiments are presented as means ± SE. D: HLMVECs transfected with 100 nM sc-siRNA or STIM1-siRNA and then challenged with thrombin (25 nM) were stained with anti-VE-cadherin pAb. Experiment was repeated 3 times. The bar graph represents quantitative analysis of VE-cadherin staining at cell-cell junctions. Data shown are means ± SE of relative fluorescent intensity (RFI) compared with untreated cells. n = 8–10 cells per group; ***P < 0.001, transfected compared with nontransfected or sc-siRNA-treated cells.
EC-specific STIM1 deletion inhibits phosphorylation of VE-PTP and VE-cadherin and increases in lung vascular permeability.
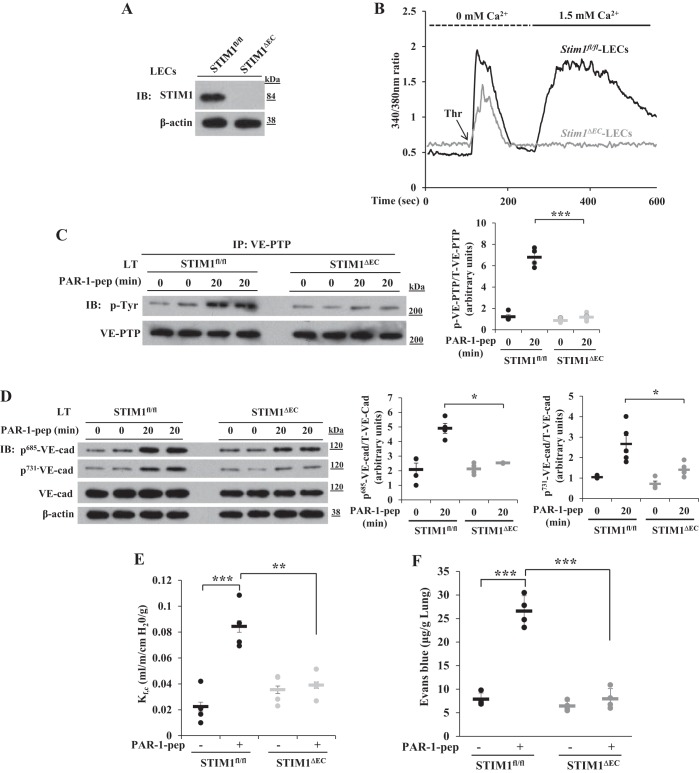
To further address the role of SOCE in inducing tyrosine phosphorylation of VE-PTP to disassemble AJs, we generated endothelial cell-restricted STIM1 knockout (Stim1ΔEC) mice (see materials and methods). We observed a loss of STIM1 expression in LECs obtained from Stim1ΔEC mice (Fig. 2A). Thrombin-induced Ca2+ entry (i.e., SOCE) was absent in LECs of Stim1ΔEC mice (Fig. 2B). We next measured PAR-1-induced tyrosine phosphorylation of VE-PTP in vivo in lungs of WT and Stim1ΔEC mice following intravenous injection of PAR-1 agonist peptide TFLLRNPNDK-NH2 into mice. At 20 min after PAR-1 peptide injection, the lungs harvested from WT and Stim1ΔEC mice were used to determine tyrosine phosphorylation of VE-PTP. PAR-1 induced tyrosine phosphorylation of VE-PTP in lungs of WT mice (Fig. 2C), whereas PAR-1-induced tyrosine phosphorylation of VE-PTP was significantly reduced in lungs of Stim1ΔEC mice (Fig. 2C). We also observed that PAR-1-induced phosphorylation of VE-cadherin at Y685 and Y731 was suppressed in lungs of Stim1ΔEC mice (Fig. 2D), indicating that SOCE-dependent tyrosine phosphorylation of VE-PTP was responsible for VE-cadherin phosphorylation.
Fig. 2.
Protease-activated receptor 1 (PAR-1)-induced Ca2+ entry, phosphorylation of VE-PTP, phosphorylation of VE-cadherin, and increase in lung vascular permeability are impaired in Stim1ΔEC mice. A: lung endothelial cells (LECs) from Stim1fl/fl and Stim1ΔEC mice were used for immunoblot to determine STIM1 expression. B: LECs from Stim1fl/fl and Stim1ΔEC mice were used to measure thrombin-induced ER-store Ca2+ release and Ca2+ release-activated Ca2+ entry. Data in A and B are representative of 3 independent experiments. C: Stim1fl/fl and Stim1ΔEC mice were intravenously injected with PAR-1 agonist peptide (see materials and methods). 0 and 20 min after administration of PAR-1 agonist peptide, lungs were harvested, and lung tissue extracts were prepared for IP with VE-PTP pAb and blotted with phosphotyrosine mAb. D: lung tissue extracts from Stim1fl/fl and Stim1ΔEC mice were immunoblotted with the indicated phospho-tyrosine-specific VE-cadherin antibodies. Quantified results are presented as the ratio of phosphorylated:total protein in C and D. n = 4 mice for each group. *P < 0.05; ***P < 0.001; Stim1fl/fl vs. Stim1ΔEC. E: lungs from Stim1fl/fl and Stim1ΔEC mice were isolated to determine lung vascular permeability (see materials and methods). PAR-1 agonist peptide (25 μM) was included in the perfusion buffer to determine PAR-1-induced microvessel filtration coefficient (Kf,c). n = 4 mice per group; **P < 0.01; ***P < 0.001; control Stim1fl/fl vs. PAR-1 peptide Stim1fl/fl or Stim1fl/fl PAR-1 peptide vs. Stim1ΔEC PAR-1 peptide. F: Stim1fl/fl and Stim1ΔEC mice received PAR-1 agonist peptide intravenously and were subsequently used to measure Evans blue dye conjugated with albumin (EBA) uptake in the lungs. Results are means ± SE n = 4 mice per group; ***P < 0.001; control Stim1fl/fl vs. PAR-1 peptide Stim1fl/fl or Stim1fl/fl PAR-1 peptide vs. Stim1ΔEC PAR-1 peptide.
To address the in vivo role of STIM1-activated SOCE in mediating lung vascular leak through disassembly of VE-cadherin at AJs, we measured PAR-1-induced lung microvascular liquid permeability (Kf,c) in intact lungs. We observed that the PAR-1 agonist peptide induced a threefold increase in Kf,c over basal level in Stim1fl/fl (WT) mice (Fig. 2E). PAR-1 agonist peptide, however, failed to significantly increase Kf,c in Stim1ΔEC mice (Fig. 2E). We next determined PAR-1-induced lung vascular leak in vivo by measuring uptake of EBA into lungs of WT and Stim1ΔEC mice. The PAR-1 agonist peptide induced a threefold increase in lung EBA uptake over basal level in WT mice (Fig. 2F), whereas PAR-1 agonist peptide failed to induce EBA uptake in lungs of Stim1ΔEC mice (Fig. 2F). These results together demonstrate that STIM1-dependent VE-PTP tyrosine phosphorylation signaled VE-cadherin phosphorylation, which, in turn, destabilized VE-cadherin junctions.
SOCE-induced Pyk2 activation phosphorylates VE-PTP to induce dissociation of VE-PTP from VE-cadherin.
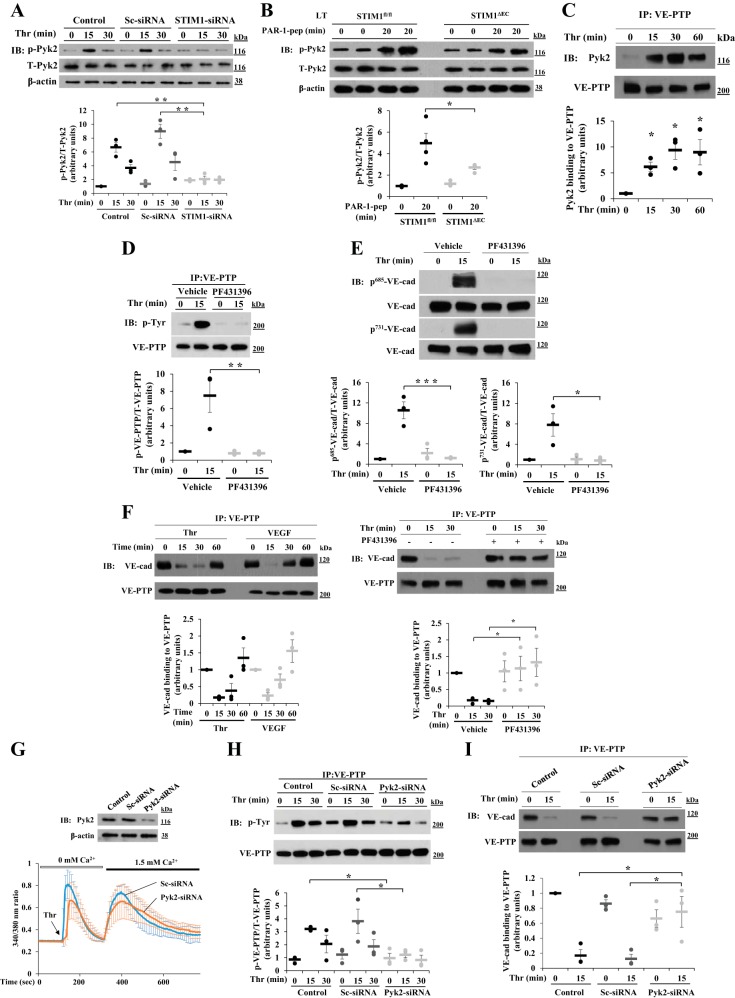
Because Pyk2 is activated by Ca2+ signaling, we studied the possibility that thrombin-induced SOCE activated Pyk2 in ECs. We measured thrombin-induced phosphorylation of Pyk2 at Y402 as a measure of Pyk2 activation (8, 37). Thrombin induced phosphorylation of Pyk2 at Y402 in control and sc-siRNA-transfected HLMVECs (Fig. 3A). However, thrombin-induced Pyk2 phosphorylation at Y402 was blocked in STIM1-siRNA-transfected HLMVECs (Fig. 3A). In other experiments, we measured PAR-1-induced Pyk2 activation in vivo in lungs of WT and Stim1ΔEC mice as described above (Fig. 2). PAR-1 activation induced a fourfold increase in phosphorylation of Pyk2 at Y402 over basal level in lungs of WT mice (Fig. 3B), whereas PAR-1-induced Pyk2 phosphorylation at Y402 was markedly reduced in Stim1ΔEC mice (Fig. 3B). We also observed that thrombin caused Pyk2 binding to VE-PTP in HLMVECs (Fig. 3C). Thus SOCE signaling promoted Pyk2 activation and the association of activated Pyk2 to VE-PTP.
Fig. 3.
STIM1-dependent Pyk2 activation induces tyrosine phosphorylation of VE-PTP in ECs. A: HLMVECs transfected with sc-siRNA or STIM1-siRNA as in Fig. 1 were used to measure thrombin-induced phosphorylation of Pyk2 at Y402 as a measure of Pyk2 activation. Quantified results are means ± SE of 3 experiments and are presented as the ratio of phosphorylated:total protein. **P < 0.01; compared with control or sc-siRNA-treated cells. B: Stim1fl/fl and Stim1ΔEC mice received PAR-1 agonist peptide as described in Fig. 2B. 0 and 20 min after administration of PAR-1 agonist peptide, the lungs were used for IB to determine tyrosine phosphorylation of Pyk2 at Y402. Quantified results are means ± SE and are shown as the ratio of phosphorylated:total protein. n = 4 mice for each group. *P < 0.05; Stim1fl/fl vs. Stim1ΔEC. C: HLMVECs exposed to thrombin were immunoprecipitated with VE-PTP pAb and blotted with anti-Pyk2 pAb. Quantified results are means ± SE of 3 experiments. *P < 0.05; control vs. thrombin-treated. D: HLMVECs were pretreated with PF431396 (1 μM) or vehicle (DMSO) for 15 min and then challenged with thrombin (25 nM). After thrombin treatment, cell lysates were immunoprecipitated with anti-VE-PTP pAb and immunoblotted with a phospho-tyrosine-specific mAb. The blot was reprobed with anti-VE-PTP pAb. E: HLMVECs challenged with thrombin as in D were immunoblotted with the indicated phospho-tyrosine-specific VE-cadherin antibodies. Quantified results are means ± SE of 3 experiments presented as the ratio of phosphorylated:total protein in D and E. *P < 0.05; **P < 0.01; ***P < 0.001; control (vehicle) vs. PF431396. F: HLMVECs challenged with thrombin (25 nM) or VEGF A (50 ng/ml) were immunoprecipitated with anti-VE-PTP pAb and immunoblotted with anti-VE-cadherin pAb (left). Quantified results are means ± SE of 3 experiments. HLMVECs were pretreated with PF431396 (1 μM) or vehicle (DMSO) for 30 min and then exposed to thrombin; cells were immunoprecipitated with anti-VE-PTP pAb and immunoblotted with anti-VE-cad pAb (right). Quantified results from 3 experiments are shown. *P < 0.05; control vs. PF431396-treated. G: LECs from wild-type (C57BL/6) mice transfected with 100 nM sc-siRNA or Pyk2-siRNA were immunoblotted with anti-Pyk2 pAb to determine Pyk2 expression (top), or cells were used to measure thrombin-induced ER-store Ca2+ release and Ca2+ release-activated Ca2+ entry (bottom). Values are means ± SD, n = 15 cells per group. H: control LECs, sc-siRNA-transfected LECs, and Pyk2-siRNA-transfected LECs were exposed to thrombin for 0, 15, and 30 min. After thrombin treatment, cell lysates were immunoprecipitated with anti-VE-PTP pAb and immunoblotted with anti-phospho-tyrosine mAb. The blot was reprobed with anti-VE-PTP pAb. Quantified data from 3 experiments are presented as the ratio of phosphorylated:total protein. *P < 0.05; significantly different from control or sc-siRNA-transfected. I: control LECs, sc-siRNA-transfected LECs, and Pyk2-siRNA-transfected LECs were exposed to thrombin for 0 and 15 min. After thrombin treatment, cell lysates were immunoprecipitated with anti-VE-PTP pAb and immunoblotted with anti-VE-cad pAb. The blot was reprobed with anti-VE-PTP pAb. Quantified results from 3 experiments are shown. *P < 0.05; significantly different from control or sc-siRNA-transfected.
We next used the Pyk2 selective inhibitor PF431396 (48) to study effects of Pyk2 inhibition on thrombin-induced VE-PTP tyrosine phosphorylation in HLMVECs. PF431396 treatment of ECs prevented thrombin-induced tyrosine phosphorylation of VE-PTP compared with control cells (Fig. 3D). PF431396 treatment also prevented thrombin-induced phosphorylation of VE-cadherin at Y685 and Y731 (Fig. 3E). To address the relationship between tyrosine phosphorylation of VE-PTP and VE-PTP binding to VE-cadherin, we next performed immunoprecipitation. We observed that thrombin as well as VEGF [known to induce dissociation of VE-PTP from VE-cadherin (48)] caused dissociation of VE-PTP from VE-cadherin in control cells (Fig. 3F). This response was blocked in PF431396-treated cells, (Fig. 3F), indicating that VE-PTP tyrosine phosphorylation is essential for the dissociation of VE-PTP from VE-cadherin at the level of AJs.
To address the role of Pyk2 in regulating AJ assembly, we suppressed Pyk2 expression with siRNA in LECs obtained from WT mice and studied PAR-1 responses. In Pyk2-siRNA-transfected LECs, Pyk2 protein expression was markedly reduced (Fig. 3G, top). We measured PAR-1-induced SOCE in control and Pyk2-siRNA-transfected cells. Pyk2 knockdown had no significant effect on SOCE activated by thrombin (Fig. 3G, bottom). We next determined VE-PTP phosphorylation and VE-PTP binding to VE-cadherin in control and Pyk2-siRNA-transfected cells. Thrombin-induced tyrosine phosphorylation of VE-PTP and VE-PTP dissociation from VE-cadherin were prevented in Pyk2 knockdown LECs (Fig. 3, H and I), indicating that Pyk2 signaling downstream of SOCE phosphorylated VE-PTP, which, in turn, promoted the dissociation of VE-PTP from VE-cadherin.
In vivo silencing of Pyk2 in mouse lung microvascular ECs prevents VE-PTP tyrosine phosphorylation, VE-cadherin phosphorylation, and increased endothelial permeability.
To address the in vivo role of Pyk2 in regulating endothelial barrier integrity secondary to VE-PTP tyrosine phosphorylation, we silenced Pyk2 expression in mouse lung microvascular ECs through the use of siRNA by liposome-mediated delivery, which targets lung ECs (45, 53). At 48 h after siRNA delivery, Pyk2 protein expression was markedly reduced in murine lungs (Fig. 4A). Because both VE-PTP and VE-cadherin are exclusively expressed in ECs, we studied the effects of Pyk2 silencing on PAR-1-induced tyrosine phosphorylation of VE-PTP and VE-cadherin. PAR-1 agonist peptide administration resulted in tyrosine phosphorylation of VE-PTP and phosphorylation of VE-cadherin at Y685 and Y731 in lungs of mice receiving control si-RNA (Fig. 4, B and C). In contrast, PAR-1 agonist peptide-induced tyrosine phosphorylation of VE-PTP and VE-cadherin phosphorylation at Y685 and Y731 was suppressed in lungs of mice receiving Pyk2-siRNA (Fig. 4, B and C). PAR-1-induced lung vascular leak as assessed by EBA uptake was also prevented in lungs of Pyk2-silenced mice (Fig. 4D). Thus Pyk2 induced vascular leak through phosphorylation of VE-PTP and VE-cadherin in ECs.
Fig. 4.
In vivo silencing of Pyk2 in mouse lung microvascular ECs abrogates PAR-1-induced phosphorylation of VE-PTP and VE-cadherin and lung vascular leak. C57BL/6 mice injected with sc-siRNA or Pyk2-siRNA were used for experiments (see materials and methods). A: immunoblot analysis of the lungs from mice 48 h after injection of sc-siRNA or Pyk2-siRNA to determine Pyk2 protein expression. Quantified data are presented in arbitrary units. n = 8–10 mice per group. ***P < 0.001, sc-siRNA vs. Pyk2-siRNA. B: mice injected with sc-siRNA or Pyk2-siRNA were injected with PAR-1 agonist peptide (see materials and methods) for 0 or 20 min. The lungs harvested after PAR-1 agonist peptide injection were used for lung extract preparation and then immunoprecipitated with anti-VE-PTP pAb. The immunoprecipitated samples were immunoblotted with anti-phosphotyrosine mAb. Blots were reprobed with anti-VE-PTP pAb. Quantified results are presented as the ratio of phosphorylated:total protein. n = 4–6 mice in each group. ***P < 0.001; sc-siRNA vs. Pyk2-siRNA. C: lung extracts from the above experiment were used for immunoblot analysis with anti-phospho-Y685-VE-cadherin or anti-phospho-Y731-VE-cadherin pAb. Quantified results are presented as the ratio of phosphorylated:total protein. n = 4–6 mice in each group. **P < 0.01; ***P < 0.001; sc-siRNA vs. Pyk2-siRNA. D: mice injected with sc-siRNA or Pyk2-siRNA were intravenously injected with PAR-1 agonist peptide (see materials and methods) to measure EBA uptake in the lungs. Results are means ± SE of changes in lung EBA uptake. n = 4 mice per group; ***P < 0.001; sc-siRNA control vs. sc-siRNA PAR-1 pep or Sc-siRNA PAR-1 pep vs. Pyk2-siRNA PAR-1 pep.
VE-PTP COOH-terminal phospho-Y1981 binds to and activates Src to increase vascular permeability.
Our results showed that Pyk2 signaling was essential for PAR-1-induced tyrosine phosphorylation of the cytosolic region of VE-PTP and VE-cadherin phosphorylation. However, the link between Pyk2-mediated tyrosine phosphorylation of VE-PTP and VE-cadherin phosphorylation was not established. VE-PTP contains the amino acid motif “1981YENV1984” in the COOH terminus, suggesting that COOH-terminal Y1981 of VE-PTP could be the target of Pyk2. Thus we investigated whether the COOH-terminal phospho-Y1981 of VE-PTP binds the SH2-domain of Src, which, in turn, activates Src to phosphorylate its substrate VE-cadherin. To address this question, we first determined whether Src activation is required for VE-cadherin phosphorylation in response to thrombin. We observed that inhibition of Src with PP1 blocked thrombin-induced phosphorylation of VE-cadherin at Y685 and Y731 (Fig. 5A). We next determined whether PAR-1-induced Pyk2 activation promotes Src activation. Thrombin-induced Src phosphorylation at Y416 was indicative of Src activation in ECs (Fig. 5B). In Pyk2 knockdown ECs, thrombin-induced Src phosphorylation was prevented (Fig. 5B). We next determined whether STIM1-dependent Pyk2 activation induced Src binding to the COOH-terminal phospho-tyrosine of VE-PTP in vivo (see above, Fig. 4). In control mouse lungs, PAR-1 agonist peptide induced Src binding to VE-PTP (Fig. 5C), whereas Src binding to VE-PTP was abolished in Pyk2-siRNA-treated lungs (Fig. 5C). We also observed that Pyk2 inhibition prevented thrombin-induced binding of Src to VE-PTP (Fig. 5D).
Fig. 5.
COOH-terminal tyrosine phosphorylation of VE-PTP triggers Src activation in ECs. A: confluent HLMVECs pretreated with Src inhibitor 4-amino-5-(4-methylphenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (PP1) (10 μM) for 30 min were exposed to thrombin for different time intervals. Then cells were used to determine VE-cadherin phosphorylation at Y685 or Y731. Quantified results shown are from 3 experiments as the ratio of phosphorylated:total protein. **P < 0.01; ***P < 0.001; control vs. PP1-treated. B: HLMVECs transfected with sc-siRNA or Pyk2-siRNA were treated with thrombin and immunoblotted with an antibody specific to phospho-Y416-Src. Quantified results shown are from 3 experiments as the ratio of phosphorylated:total protein. **P < 0.01; sc-siRNA vs. Pyk2-siRNA. C: C57BL/6 mice injected with sc-siRNA or Pyk2-siRNA (see above in Fig. 4) were intravenously injected with PAR-1 agonist peptide for 0, 20, and 60 min. Lung extracts prepared after PAR-1 agonist peptide treatment were immunoprecipitated with anti-VE-PTP pAb. The immunoprecipitated samples were immunoblotted with anti-Src mAb. Quantified results shown are from 3 experiments. **P < 0.01; sc-siRNA vs. Pyk2-siRNA. D: HLMVECs treated with Pyk2 inhibitor followed by thrombin exposure were immunoprecipitated with anti-VE-PTP pAb. The immunoprecipitated samples were immunoblotted with anti-Src mAb. Quantified results shown are from 3 separate experiments. *P < 0.05; vehicle vs. PF431396. E: confluent HLMVECs were incubated for 60 min with the indicated concentrations of either cell-permeable WT-VE-PTP or Mut-VE-PTP peptide and then exposed to thrombin for 30 min. Cell lysates were then immunoblotted with an antibody specific to phos-Y416-Src. Results are means ± SE of 3 experiments quantified as the ratio of phosphorylated:total protein. *P < 0.05; **P < 0.01; control (vehicle-treated) vs. WT-VE-PTP peptide-treated. F: HLMVECs, pretreated with WT-VE-PTP or Mut-VE-PTP peptide and then exposed to thrombin, were immunoprecipitated with anti-VE-PTP pAb. The immunoprecipitated samples were immunoblotted with anti-phosphotyrosine mAb. Results are means ± SE of 3 experiments quantified as the ratio of phosphorylated to total protein. *P < 0.05; **P < 0.01; control (vehicle-treated) vs. WT-VE-PTP peptide-treated.
To determine the relationship between Pyk2-mediated tyrosine phosphorylation of VE-PTP and VE-PTP binding to and activation of Src, we used a synthetic cell-permeable peptide derived from the COOH terminus (1977-LFPIYENVNPEY−1988) of VE-PTP, which contains the conserved amino acid motif “YENV”, to block Src activation. It is noteworthy that the phospho-Y1981 of VE-PTP can bind to signaling molecules containing the SH2 domain, including Src itself (25, 27, 28). We synthesized this sequence 1977LFPIYENVNPEY1988 (WT) as a fusion peptide to the COOH terminus of the antennapedia (RQIKIWFQNRRMKWKK) cell-permeable sequence (23). We also designed a cell-permeable mutant 1981Y/F peptide (1977LFPIFENVNPEY1988), which served as a negative control. The WT-VE-PTP peptide but not the Mut-VE-PTP peptide blocked PAR-1-induced Src activation in ECs (Fig. 5E). Consistent with this observation, WT-VE-PTP peptide prevented PAR-1-induced tyrosine phosphorylation of VE-PTP (Fig. 5F). These results collectively demonstrate that Pyk2 induced phosphorylation of Y1981 in the COOH-terminal region of VE-PTP and that phospho-Y1981, in turn, bound to and activated Src to phosphorylate VE-cadherin.
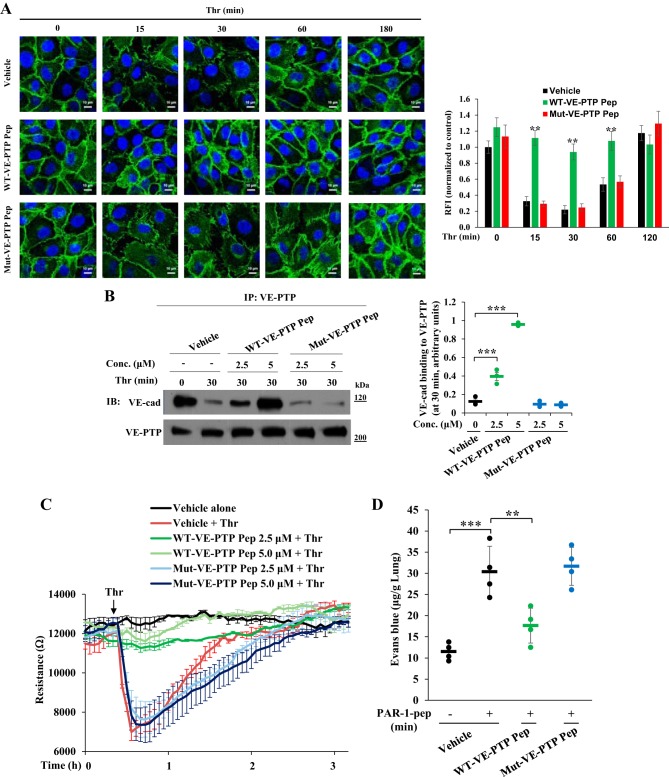
We next investigated whether treatment with the cell-permeable WT-VE-PTP peptide could inhibit the PAR-1-induced permeability response. We pretreated confluent EC monolayers with either WT-VE-PTP or Mut-VE-PTP peptide and then exposed them to thrombin for different time intervals. After thrombin exposure, cells were used to measure the expression of VE-cadherin at cell-cell junctions by staining with an anti-VE-cadherin pAb (Fig. 6A). Thrombin addition caused profound disassembly of VE-cadherin at cell-cell junctions in control and Mut-VE-PTP peptide-treated cells at 15 min and 30 min (Fig. 6A), which were restored to basal level 3 h after thrombin stimulation (Fig. 6A). In WT-VE-PTP peptide-treated ECs, thrombin failed to disassemble VE-cadherin at cell-cell junctions (Fig. 6A). We observed by immunoprecipitation that WT-VE-PTP peptide pretreatment prevented thrombin-induced dissociation of VE-PTP from VE-cadherin (Fig. 6B). In line with these results, pretreatment of the monolayer with WT-VE-PTP but not Mut-VE-PTP peptide inhibited the thrombin-induced decrease in TER (Fig. 6C), indicating that WT-VE-PTP peptide prevented thrombin-induced permeability increase. We also measured the effects of these peptides on PAR-1-induced lung vascular leak in mice by measuring EBA uptake. WT-VE-PTP peptide administration blocked PAR-1-induced lung vascular leak in mice (Fig. 6D). On the basis of these key findings, we propose a model (Fig. 7) in which phosphorylation of VE-PTP at Y1981 in the cytosolic COOH-terminal region via the STIM1/SOCE-Pyk2 axis regulates binding and activation of Src. Subsequently, Src induces VE-cadherin phosphorylation to disassemble AJs and increase endothelial permeability.
Fig. 6.
Peptide derived from the Pyk2 phosphorylation site on VE-PTP prevents PAR-1-induced disassembly of VE-cadherin at endothelial AJs and permeability increase. A: confluent HLMVECs pretreated with vehicle (DMSO), WT-VE-PTP peptide (5 μM), or mut-VE-PTP (5 μM) peptide for 60 min and then challenged with thrombin for different time intervals were immunostained with anti-VE-cadherin pAb. The bar graph represents quantitative analysis of VE-cadherin staining at cell-cell junctions. Data shown are means ± SE RFI compared with untreated control cells. n = 6–8 cells per group. **P < 0.01; compared with vehicle-treated or Mut-VE-PTP peptide-treated cells. B: confluent HLMVECs pretreated with either 5 μM WT-VE-PTP or mut-VE-PTP peptide for 60 min were exposed to thrombin for 30 min. After thrombin treatment, cell lysates were immunoprecipitated with anti-VE-PTP pAb and immunoblotted with anti-VE-cadherin pAb. Results are means ± SE of 3 experiments quantified. ***P < 0.001; control (vehicle-treated) vs. WT-VE-PTP peptide-treated. C: HLMVECs on gold electrodes (see materials and methods) were washed and incubated in medium containing 1% FBS for 2 h, and the cells were incubated with the indicated concentrations of WT-VE-PTP or mut-VE-PTP peptide for 60 min. Then the cells were challenged with thrombin (25 nM). Values represent means ± SD of 3 experiments. D: C57BL/6 mice weighing 25 g were intravenously injected with vehicle (100 μl), WT-VE-PTP peptide (100 μl of 0.1 mM per mouse), or mut-VE-PTP peptide (100 μl of 0.1 mM per mouse). 60 min after peptide injection, PAR-1 agonist peptide was injected intravenously to determine EBA uptake (see materials and methods). Results are means ± SE changes in lung EBA uptake. n = 4 mice per group; **P < 0.01; ***P < 0.001; untreated vs. PAR-1 peptide-treated, untreated vs. Mut-VE-PTP pep + PAR-1 peptide-treated, or PAR-1 peptide alone vs. WT-VE-PTP peptide + PAR-1 peptide-treated.
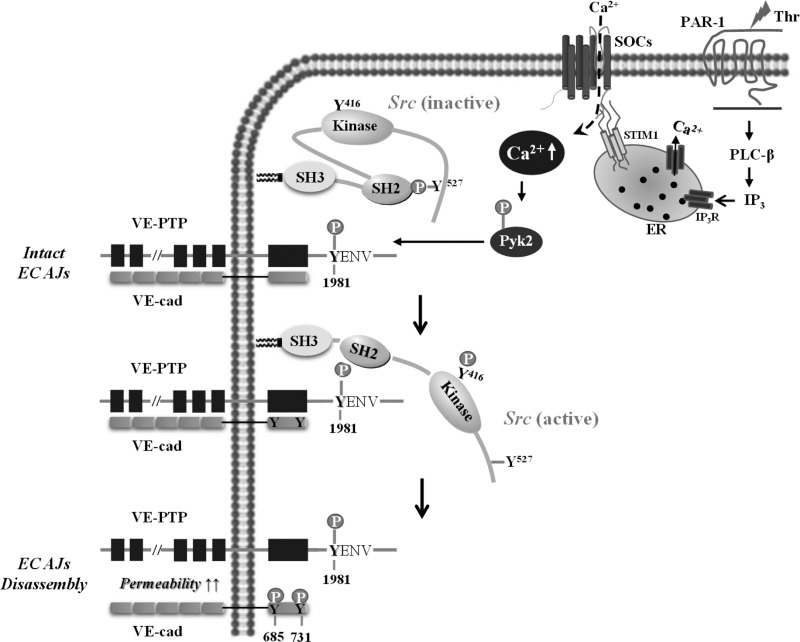
Fig. 7.
Model for induction of VE-PTP-dependent Src activation in ECs via PAR-1-SOCE-Pyk2 pathway. PAR-1-induced ER store Ca2+ depletion via phospholipase C (PLC)-inositol 1,4,5-triphosphate (IP3) activates SOCE, inducing autophosphorylation of Pyk2 at Y402 to activate Pyk2. The activated Pyk2 binds to and phosphorylates VE-PTP at Y1981 in the COOH-terminal region. The phospho-Y1981 of VE-PTP binds to the Src homology-2 (SH2) domain of Src (anchored to the membrane), which, in turn, promotes the autophosphorylation of Y416 in the kinase domain of Src to trigger Src activation. The activated Src can phosphorylate tyrosine residues in the cytosolic COOH-terminal region of VE-cadherin to promote endothelial barrier dysfunction.
DISCUSSION
We previously showed that PAR-1-induced SOCE in ECs signaled increased vascular permeability by inducing EC retraction secondary to actin-stress fiber formation and disassembly of AJs (34, 35, 43). An emerging concept is that VE-PTP plays a crucial role in mediating VE-cadherin homotypic interaction at AJs and thus has an essential role in regulating endothelial barrier function. VE-PTP interacts with VE-cadherin at AJs and maintains endothelial cell-cell contact in a phosphatase-dependent manner (19, 29, 38, 47, 48). Here we addressed the mechanisms by which PAR-1-induced SOCE regulated the activity of VE-PTP and hence mediated disassembly of AJs and increased endothelial permeability. We demonstrated that loss of STIM1-mediated Ca2+ entry prevented PAR-1-induced increased endothelial permeability, confirming our earlier findings (34, 35, 43). The novel point in this study is that STIM1-activated Ca2+ entry was required for PAR-1-induced Pyk2 activation, and Pyk2 activation, in turn, mediated tyrosine phosphorylation of the COOH-terminal region of VE-PTP. Importantly, tyrosine phosphorylation of the COOH terminus of VE-PTP induced by Pyk2 facilitated the binding and activation of Src tyrosine kinase and, in turn, phosphorylation of VE-cadherin at AJs to disassemble AJs and increase endothelial permeability. A cell-permeable peptide derived from the Pyk2 phosphorylation site on VE-PTP abrogated the SOCE-induced increase in endothelial permeability. These findings collectively support the model that, although VE-PTP constitutively maintains endothelial barrier integrity through dephosphorylation of VE-cadherin at AJs, Pyk2-induced phosphorylation of VE-PTP enables VE-PTP to function as a scaffold for Src and facilitate Src activation, thereby dissembling AJs through the phosphorylation of VE-cadherin.
STIM1 is known to be required for PAR-1-induced Ca2+ entry, which occurs primarily via activation of SOCs in ECs (1, 10, 39, 41). We showed that EC-restricted deletion of STIM1 (Stim1ΔEC) in mice prevented PAR-1-induced increase in lung vascular permeability. This was the result of inhibition of VE-cadherin internalization from the AJs. A similar role of STIM1 has been shown for LPS-induced vascular permeability (15). The present paradigm is that Src-mediated tyrosine phosphorylation of VE-cadherin and subsequent internalization of phosphorylated VE-cadherin increase endothelial permeability (18, 19, 32, 47). Because VE-PTP maintains the integrity of AJs by dephosphorylating VE-cadherin at Y658, Y685, and Y731, we investigated whether the signaling cascade activated by SOCE disrupted VE-PTP/VE-cadherin interaction and was thus required for increasing endothelial permeability. We observed that STIM1 depletion in HLMVECs prevented PAR-1-induced tyrosine phosphorylation of VE-PTP as well as of VE-cadherin at Y-685 and Y-731. Because VE-PTP and VE-cadherin are exclusively expressed in ECs, we determined PAR-1-induced phosphorylation of VE-PTP and VE-cadherin in vivo in lungs of WT and Stim1ΔEC mice. As with results in HLMVECs, the PAR-1-induced tyrosine phosphorylation of VE-PTP and VE-cadherin was seen in WT lungs but not in Stim1ΔEC mouse lungs. These results thus show that STIM1-activated SOCE is essential for tyrosine phosphorylation of both VE-PTP and VE-cadherin and thereby for the mechanism of increased endothelial permeability.
Pyk2 is a non-receptor tyrosine kinase, which does not contain SH2 or SH3 domains, but has a central catalytic domain flanked by an NH2-terminal FERM domain (37). Ca2+ and calmodulin binding to the NH2-terminal FERM domain of Pyk2 induce Pyk2 dimerization, which triggers autophosphorylation of Pyk2 at Y402 required for its catalytic activity (8, 21). Ca2+-dependent Pyk2 activation in monocytes (52) and ECs (4) induced inflammation through NF-κB activation. Reactive oxygen species (ROS) are also known to activate Pyk2 (37, 48). van Buul et al. (46) showed that anti-VE-cadherin antibody induced disassembly of AJs via the ROS-Pyk2 axis mediating β-catenin tyrosine phosphorylation. Another study showed that TNF-α-induced VE-cadherin phosphorylation in human umbilical vascular ECs was activated by Pyk2 via the p110α isoform of phosphoinositide 3-kinase (6). Vockel and Vestweber (48) reported that lymphocyte binding to VCAM-1 on the murine brain EC surface or stimulation of murine ECs with VEGF induced the dissociation of VE-PTP from VE-cadherin, which involved ROS-dependent Pyk2 activation. This study proposed a model in which Pyk2 induced the binding of phosphorylated VE-PTP substrate to the cytosolic domain of VE-PTP, thus inducing conformational changes across the membrane, resulting in detachment of the extracellular domain of VE-PTP from VE-cadherin.
Because Pyk2 can be activated by SOCE (8), we investigated whether PAR-1-induced SOCE signaling was capable of phosphorylating Pyk2 at Y402, thereby activating Pyk2. First, on depleting STIM1 in HLMVECs using siRNA, we observed that the PAR-1-induced Pyk2 activation was impaired in STIM1-depleted HLMVECs. Using the Stim1ΔEC mouse model, we next showed that STIM-mediated SOCE was essential for PAR-1-induced Pyk2 activation. Thus it appears that PAR-1-induced SOCE signaling mediated Pyk2 activation in ECs. To address whether SOCE-dependent Pyk2 activation was in fact responsible for VE-PTP tyrosine phosphorylation, we inhibited Pyk2 by treating ECs with PF431396. Pyk2 inhibition blocked thrombin-induced tyrosine phosphorylation of VE-PTP. Additionally, thrombin-induced tyrosine phosphorylation of VE-PTP was blocked in Pyk2-depleted ECs. To address the in vivo role of Pyk2, we silenced Pyk2 expression in mouse lung microvascular ECs through liposome-mediated delivery of siRNAs (45). Depletion of Pyk2 in mouse lung ECs in this manner abolished PAR-1-induced tyrosine phosphorylation of VE-PTP and VE-cadherin and increased lung vascular permeability. These results thus indicate the central role of the SOCE-Pyk2 axis in mediating VE-PTP and VE-cadherin phosphorylation, which, in turn, was responsible for increasing lung vascular permeability.
It is now known that Src family tyrosine kinases induce VE-cadherin tyrosine phosphorylation to promote disassembly of endothelial AJs (18, 19, 32, 47). We observed that either pharmacological inhibition of Pyk2 or Pyk2 depletion in ECs prevented thrombin-induced Src activation, suggesting that Pyk2 is upstream of Src in the pathway mediating VE-cadherin tyrosine phosphorylation. In addition, we observed that thrombin-induced Src binding to VE-PTP and Src activation were dependent on Pyk2 activation. However, whether Pyk2-mediated tyrosine phosphorylation of VE-PTP is essential for thrombin-induced Src activation remains unknown. The cytosolic COOH terminus of hVE-PTP contains the putative tyrosine residue (Y1981), which can be phosphorylated by tyrosine kinases and binds and activates Src (25, 27, 28). Furthermore, it was recently shown that, in HEK293 cells expressing WT mVE-PTP, treatment with the tyrosine phosphatase inhibitor pervanadate (PV) induced VE-PTP tyrosine phosphorylation, which induced the binding of the SFK Fyn to VE-PTP (28). Interestingly, in HEK293 cells expressing the mutant Y1982F–mVE-PTP, tyrosine phosphorylation of VE-PTP and Fyn binding to VE-PTP were abolished by PV pretreatment, indicating that phosphorylation of Y-1982 in the COOH terminus of mVE-PTP plays an essential role in Src activation (28). To address whether Pyk2-mediated tyrosine phosphorylation of VE-PTP was essential for Src activation downstream of PAR-1-induced SOCE, we used a synthetic cell-permeable peptide derived from the COOH terminus of hVE-PTP (1977-LFPIYENVNPEY−1988) to block thrombin-induced Src activation. Here, we observed that the WT peptide, but not the Y1981F mutant-VE-PTP (control) peptide, prevented thrombin-induced Src activation, VE-PTP tyrosine phosphorylation, VE-PTP dissociation from VE-cadherin, and increased vascular permeability. These results together show that Pyk2-mediated Y1981 phosphorylation of VE-PTP induced VE-cadherin phosphorylation via Src activation to disassemble the AJs and increase endothelial permeability.
GRANTS
This work was supported by National Institutes of Health Grants R01 HL-128359, R01 GM-117028, R-01 HL-122157, R01HL-045638, and P01HL-060678.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.S., Y.-Y.Z., and C.T. conceived and designed research; D.S., S.C.R., D.-M.W., A.D., and S.M.V. performed experiments; D.S., S.M.V., A.B.M., and C.T. analyzed data; D.S., A.B.M., and C.T. interpreted results of experiments; D.S. and S.C.R. prepared figures; D.S., S.M.V., A.B.M., and C.T. drafted manuscript; D.S., S.M.V., and C.T. edited and revised manuscript; D.S. and C.T. approved final version of manuscript.
REFERENCES
- 1.Abdullaev IF, Bisaillon JM, Potier M, Gonzalez JC, Motiani RK, Trebak M. Stim1 and Orai1 mediate CRAC currents and store-operated calcium entry important for endothelial cell proliferation. Circ Res 103: 1289–1299, 2008. doi: 10.1161/01.RES.0000338496.95579.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baghai-Ravary R, Bellingan GJ. Pulmonary dysfunction in sepsis. Adv Sepsis 5: 42–51, 2006. [Google Scholar]
- 3.Begley MJ, Yun CH, Gewinner CA, Asara JM, Johnson JL, Coyle AJ, Eck MJ, Apostolou I, Cantley LC. EGF-receptor specificity for phosphotyrosine-primed substrates provides signal integration with Src. Nat Struct Mol Biol 22: 983–990, 2015. doi: 10.1038/nsmb.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bijli KM, Fazal F, Rahman A. Regulation of Rela/p65 and endothelial cell inflammation by proline-rich tyrosine kinase 2. Am J Respir Cell Mol Biol 47: 660–668, 2012. doi: 10.1165/rcmb.2012-0047OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breviario F, Caveda L, Corada M, Martin-Padura I, Navarro P, Golay J, Introna M, Gulino D, Lampugnani MG, Dejana E. Functional properties of human vascular endothelial cadherin (7B4/cadherin-5), an endothelium-specific cadherin. Arterioscler Thromb Vasc Biol 15: 1229–1239, 1995. doi: 10.1161/01.ATV.15.8.1229. [DOI] [PubMed] [Google Scholar]
- 6.Cain RJ, Vanhaesebroeck B, Ridley AJ. The PI3K p110alpha isoform regulates endothelial adherens junctions via Pyk2 and Rac1. J Cell Biol 188: 863–876, 2010. doi: 10.1083/jcb.200907135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen XL, Nam JO, Jean C, Lawson C, Walsh CT, Goka E, Lim ST, Tomar A, Tancioni I, Uryu S, Guan JL, Acevedo LM, Weis SM, Cheresh DA, Schlaepfer DD. VEGF-induced vascular permeability is mediated by FAK. Dev Cell 22: 146–157, 2012. doi: 10.1016/j.devcel.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen YF, Chiu WT, Chen YT, Lin PY, Huang HJ, Chou CY, Chang HC, Tang MJ, Shen MR. Calcium store sensor stromal-interaction molecule 1-dependent signaling plays an important role in cervical cancer growth, migration, and angiogenesis. Proc Natl Acad Sci USA 108: 15225–15230, 2011. doi: 10.1073/pnas.1103315108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corada M, Mariotti M, Thurston G, Smith K, Kunkel R, Brockhaus M, Lampugnani MG, Martin-Padura I, Stoppacciaro A, Ruco L, McDonald DM, Ward PA, Dejana E. Vascular endothelial-cadherin is an important determinant of microvascular integrity in vivo. Proc Natl Acad Sci USA 96: 9815–9820, 1999. doi: 10.1073/pnas.96.17.9815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DebRoy A, Vogel SM, Soni D, Sundivakkam PC, Malik AB, Tiruppathi C. Cooperative signaling via transcription factors NF-kappaB and AP1/c-Fos mediates endothelial cell STIM1 expression and hyperpermeability in response to endotoxin. J Biol Chem 289: 24188–24201, 2014. doi: 10.1074/jbc.M114.570051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dejana E, Orsenigo F, Lampugnani MG. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J Cell Sci 121: 2115–2122, 2008. doi: 10.1242/jcs.017897. [DOI] [PubMed] [Google Scholar]
- 12.Esmon CT. The interactions between inflammation and coagulation. Br J Haematol 131: 417–430, 2005. doi: 10.1111/j.1365-2141.2005.05753.x. [DOI] [PubMed] [Google Scholar]
- 13.Fazal F, Bijli KM, Murrill M, Leonard A, Minhajuddin M, Anwar KN, Finkelstein JN, Watterson DM, Rahman A. Critical role of non-muscle myosin light chain kinase in thrombin-induced endothelial cell inflammation and lung PMN infiltration. PLoS One 8: e59965, 2013. doi: 10.1371/journal.pone.0059965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frye M, Dierkes M, Küppers V, Vockel M, Tomm J, Zeuschner D, Rossaint J, Zarbock A, Koh GY, Peters K, Nottebaum AF, Vestweber D. Interfering with VE-PTP stabilizes endothelial junctions in vivo via Tie-2 in the absence of VE-cadherin. J Exp Med 212: 2267–2287, 2015. doi: 10.1084/jem.20150718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gandhirajan RK, Meng S, Chandramoorthy HC, Mallilankaraman K, Mancarella S, Gao H, Razmpour R, Yang XF, Houser SR, Chen J, Koch WJ, Wang H, Soboloff J, Gill DL, Madesh M. Blockade of NOX2 and STIM1 signaling limits lipopolysaccharide-induced vascular inflammation. J Clin Invest 123: 887–902, 2013. doi: 10.1172/JCI65647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gando S, Nanzaki S, Morimoto Y, Kobayashi S, Kemmotsu O. Systemic activation of tissue-factor dependent coagulation pathway in evolving acute respiratory distress syndrome in patients with trauma and sepsis. J Trauma 47: 719–723, 1999. doi: 10.1097/00005373-199910000-00017. [DOI] [PubMed] [Google Scholar]
- 17.Goldenberg NM, Steinberg BE, Slutsky AS, Lee WL. Broken barriers: a new take on sepsis pathogenesis. Sci Transl Med 3: 88ps25, 2011. doi: 10.1126/scitranslmed.3002011. [DOI] [PubMed] [Google Scholar]
- 18.Gong H, Gao X, Feng S, Siddiqui MR, Garcia A, Bonini MG, Komarova Y, Vogel SM, Mehta D, Malik AB. Evidence of a common mechanism of disassembly of adherens junctions through Gα13 targeting of VE-cadherin. J Exp Med 211: 579–591, 2014. doi: 10.1084/jem.20131190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gong H, Rehman J, Tang H, Wary K, Mittal M, Chaturvedi P, Zhao YY, Komarova YA, Vogel SM, Malik AB. HIF2α signaling inhibits adherens junctional disruption in acute lung injury. J Clin Invest 125: 652–664, 2015. doi: 10.1172/JCI77701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gotsch U, Borges E, Bosse R, Böggemeyer E, Simon M, Mossmann H, Vestweber D. VE-cadherin antibody accelerates neutrophil recruitment in vivo. J Cell Sci 110: 583–588, 1997. [DOI] [PubMed] [Google Scholar]
- 21.Kohno T, Matsuda E, Sasaki H, Sasaki T. Protein-tyrosine kinase CAKbeta/PYK2 is activated by binding Ca2+/calmodulin to FERM F2 alpha2 helix and thus forming its dimer. Biochem J 410: 513–523, 2008. doi: 10.1042/BJ20070665. [DOI] [PubMed] [Google Scholar]
- 22.Komarova Y, Malik AB. Regulation of endothelial permeability via paracellular and transcellular transport pathways. Annu Rev Physiol 72: 463–493, 2010. doi: 10.1146/annurev-physiol-021909-135833. [DOI] [PubMed] [Google Scholar]
- 23.Kwiatek AM, Minshall RD, Cool DR, Skidgel RA, Malik AB, Tiruppathi C. Caveolin-1 regulates store-operated Ca2+ influx by binding of its scaffolding domain to transient receptor potential channel-1 in endothelial cells. Mol Pharmacol 70: 1174–1183, 2006. doi: 10.1124/mol.105.021741. [DOI] [PubMed] [Google Scholar]
- 24.Lee CS, Kim YG, Cho HJ, Park J, Jeong H, Lee SE, Lee SP, Kang HJ, Kim HS. Dipeptidyl Peptidase-4 Inhibitor Increases Vascular Leakage in Retina through VE-cadherin Phosphorylation. Sci Rep 6: 29393, 2016. doi: 10.1038/srep29393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matozaki T, Murata Y, Mori M, Kotani T, Okazawa H, Ohnishi H. Expression, localization, and biological function of the R3 subtype of receptor-type protein tyrosine phosphatases in mammals. Cell Signal 22: 1811–1817, 2010. doi: 10.1016/j.cellsig.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Minami T, Sugiyama A, Wu SQ, Abid R, Kodama T, Aird WC. Thrombin and phenotypic modulation of the endothelium. Arterioscler Thromb Vasc Biol 24: 41–53, 2004. doi: 10.1161/01.ATV.0000099880.09014.7D. [DOI] [PubMed] [Google Scholar]
- 27.Mori M, Murata Y, Kotani T, Kusakari S, Ohnishi H, Saito Y, Okazawa H, Ishizuka T, Mori M, Matozaki T. Promotion of cell spreading and migration by vascular endothelial-protein tyrosine phosphatase (VE-PTP) in cooperation with integrins. J Cell Physiol 224: 195–204, 2010. doi: 10.1002/jcp.22122. [DOI] [PubMed] [Google Scholar]
- 28.Murata Y, Mori M, Kotani T, Supriatna Y, Okazawa H, Kusakari S, Saito Y, Ohnishi H, Matozaki T. Tyrosine phosphorylation of R3 subtype receptor-type protein tyrosine phosphatases and their complex formations with Grb2 or Fyn. Genes Cells 15: 513–524, 2010. doi: 10.1111/j.1365-2443.2010.01398.x. [DOI] [PubMed] [Google Scholar]
- 29.Nawroth R, Poell G, Ranft A, Kloep S, Samulowitz U, Fachinger G, Golding M, Shima DT, Deutsch U, Vestweber D. VE-PTP and VE-cadherin ectodomains interact to facilitate regulation of phosphorylation and cell contacts. EMBO J 21: 4885–4895, 2002. doi: 10.1093/emboj/cdf497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nottebaum AF, Cagna G, Winderlich M, Gamp AC, Linnepe R, Polaschegg C, Filippova K, Lyck R, Engelhardt B, Kamenyeva O, Bixel MG, Butz S, Vestweber D. VE-PTP maintains the endothelial barrier via plakoglobin and becomes dissociated from VE-cadherin by leukocytes and by VEGF. J Exp Med 205: 2929–2945, 2008. doi: 10.1084/jem.20080406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oh-Hora M, Yamashita M, Hogan PG, Sharma S, Lamperti E, Chung W, Prakriya M, Feske S, Rao A. Dual functions for the endoplasmic reticulum calcium sensors STIM1 and STIM2 in T cell activation and tolerance. Nat Immunol 9: 432–443, 2008. doi: 10.1038/ni1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orsenigo F, Giampietro C, Ferrari A, Corada M, Galaup A, Sigismund S, Ristagno G, Maddaluno L, Koh GY, Franco D, Kurtcuoglu V, Poulikakos D, Baluk P, McDonald D, Grazia Lampugnani M, Dejana E. Phosphorylation of VE-cadherin is modulated by haemodynamic forces and contributes to the regulation of vascular permeability in vivo. Nat Commun 3: 1208, 2012. doi: 10.1038/ncomms2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paria BC, Bair AM, Xue J, Yu Y, Malik AB, Tiruppathi C. Ca2+ influx induced by protease-activated receptor-1 activates a feed-forward mechanism of TRPC1 expression via nuclear factor-kappaB activation in endothelial cells. J Biol Chem 281: 20715–20727, 2006. doi: 10.1074/jbc.M600722200. [DOI] [PubMed] [Google Scholar]
- 34.Sandoval R, Malik AB, Minshall RD, Kouklis P, Ellis CA, Tiruppathi C. Ca(2+) signalling and PKCalpha activate increased endothelial permeability by disassembly of VE-cadherin junctions. J Physiol 533: 433–445, 2001. doi: 10.1111/j.1469-7793.2001.0433a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sandoval R, Malik AB, Naqvi T, Mehta D, Tiruppathi C. Requirement for Ca2+ signaling in the mechanism of thrombin-induced increase in endothelial permeability. Am J Physiol Lung Cell Mol Physiol 280: L239–L247, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Schulte D, Küppers V, Dartsch N, Broermann A, Li H, Zarbock A, Kamenyeva O, Kiefer F, Khandoga A, Massberg S, Vestweber D. Stabilizing the VE-cadherin-catenin complex blocks leukocyte extravasation and vascular permeability. EMBO J 30: 4157–4170, 2011. doi: 10.1038/emboj.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharma D, Kinsey WH. PYK2: a calcium-sensitive protein tyrosine kinase activated in response to fertilization of the zebrafish oocyte. Dev Biol 373: 130–140, 2013. doi: 10.1016/j.ydbio.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shen J, Frye M, Lee BL, Reinardy JL, McClung JM, Ding K, Kojima M, Xia H, Seidel C, Lima e Silva R, Dong A, Hackett SF, Wang J, Howard BW, Vestweber D, Kontos CD, Peters KG, Campochiaro PA. Targeting VE-PTP activates TIE2 and stabilizes the ocular vasculature. J Clin Invest 124: 4564–4576, 2014. doi: 10.1172/JCI74527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sundivakkam PC, Freichel M, Singh V, Yuan JP, Vogel SM, Flockerzi V, Malik AB, Tiruppathi C. The Ca(2+) sensor stromal interaction molecule 1 (STIM1) is necessary and sufficient for the store-operated Ca(2+) entry function of transient receptor potential canonical (TRPC) 1 and 4 channels in endothelial cells. Mol Pharmacol 81: 510–526, 2012. doi: 10.1124/mol.111.074658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sundivakkam PC, Kwiatek AM, Sharma TT, Minshall RD, Malik AB, Tiruppathi C. Caveolin-1 scaffold domain interacts with TRPC1 and IP3R3 to regulate Ca2+ store release-induced Ca2+ entry in endothelial cells. Am J Physiol Cell Physiol 296: C403–C413, 2009. doi: 10.1152/ajpcell.00470.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sundivakkam PC, Natarajan V, Malik AB, Tiruppathi C. Store-operated Ca2+ entry (SOCE) induced by protease-activated receptor-1 mediates STIM1 protein phosphorylation to inhibit SOCE in endothelial cells through AMP-activated protein kinase and p38β mitogen-activated protein kinase. J Biol Chem 288: 17030–17041, 2013. doi: 10.1074/jbc.M112.411272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tiruppathi C, Ahmmed GU, Vogel SM, Malik AB. Ca2+ signaling, TRP channels, and endothelial permeability. Microcirculation 13: 693–708, 2006. doi: 10.1080/10739680600930347. [DOI] [PubMed] [Google Scholar]
- 43.Tiruppathi C, Freichel M, Vogel SM, Paria BC, Mehta D, Flockerzi V, Malik AB. Impairment of store-operated Ca2+ entry in TRPC4(-/-) mice interferes with increase in lung microvascular permeability. Circ Res 91: 70–76, 2002. doi: 10.1161/01.RES.0000023391.40106.A8. [DOI] [PubMed] [Google Scholar]
- 44.Tiruppathi C, Malik AB, Del Vecchio PJ, Keese CR, Giaever I. Electrical method for detection of endothelial cell shape change in real time: assessment of endothelial barrier function. Proc Natl Acad Sci USA 89: 7919–7923, 1992. doi: 10.1073/pnas.89.17.7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tiruppathi C, Soni D, Wang DM, Xue J, Singh V, Thippegowda PB, Cheppudira BP, Mishra RK, Debroy A, Qian Z, Bachmaier K, Zhao YY, Christman JW, Vogel SM, Ma A, Malik AB. The transcription factor DREAM represses the deubiquitinase A20 and mediates inflammation. Nat Immunol 15: 239–247, 2014. doi: 10.1038/ni.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Buul JD, Anthony EC, Fernandez-Borja M, Burridge K, Hordijk PL. Proline-rich tyrosine kinase 2 (Pyk2) mediates vascular endothelial-cadherin-based cell-cell adhesion by regulating beta-catenin tyrosine phosphorylation. J Biol Chem 280: 21129–21136, 2005. doi: 10.1074/jbc.M500898200. [DOI] [PubMed] [Google Scholar]
- 47.Vestweber D, Wessel F, Nottebaum AF. Similarities and differences in the regulation of leukocyte extravasation and vascular permeability. Semin Immunopathol 36: 177–192, 2014. doi: 10.1007/s00281-014-0419-7. [DOI] [PubMed] [Google Scholar]
- 48.Vockel M, Vestweber D. How T cells trigger the dissociation of the endothelial receptor phosphatase VE-PTP from VE-cadherin. Blood 122: 2512–2522, 2013. doi: 10.1182/blood-2013-04-499228. [DOI] [PubMed] [Google Scholar]
- 49.Vogel SM, Gao X, Mehta D, Ye RD, John TA, Andrade-Gordon P, Tiruppathi C, Malik AB. Abrogation of thrombin-induced increase in pulmonary microvascular permeability in PAR-1 knockout mice. Physiol Genomics 4: 137–145, 2000. [DOI] [PubMed] [Google Scholar]
- 50.Wessel F, Winderlich M, Holm M, Frye M, Rivera-Galdos R, Vockel M, Linnepe R, Ipe U, Stadtmann A, Zarbock A, Nottebaum AF, Vestweber D. Leukocyte extravasation and vascular permeability are each controlled in vivo by different tyrosine residues of VE-cadherin. Nat Immunol 15: 223–230, 2014. doi: 10.1038/ni.2824. [DOI] [PubMed] [Google Scholar]
- 51.Wortmann A, He Y, Christensen ME, Linn M, Lumley JW, Pollock PM, Waterhouse NJ, Hooper JD. Cellular settings mediating Src Substrate switching between focal adhesion kinase tyrosine 861 and CUB-domain-containing protein 1 (CDCP1) tyrosine 734. J Biol Chem 286: 42303–42315, 2011. doi: 10.1074/jbc.M111.227462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamamoto S, Shimizu S, Kiyonaka S, Takahashi N, Wajima T, Hara Y, Negoro T, Hiroi T, Kiuchi Y, Okada T, Kaneko S, Lange I, Fleig A, Penner R, Nishi M, Takeshima H, Mori Y. TRPM2-mediated Ca2+influx induces chemokine production in monocytes that aggravates inflammatory neutrophil infiltration. Nat Med 14: 738–747, 2008. doi: 10.1038/nm1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou MY, Lo SK, Bergenfeldt M, Tiruppathi C, Jaffe A, Xu N, Malik AB. In vivo expression of neutrophil inhibitory factor via gene transfer prevents lipopolysaccharide-induced lung neutrophil infiltration and injury by a beta2 integrin-dependent mechanism. J Clin Invest 101: 2427–2437, 1998. doi: 10.1172/JCI407. [DOI] [PMC free article] [PubMed] [Google Scholar]