Abstract
Pompe disease is an autosomal recessive disorder caused by a deficiency of acid α-glucosidase (GAA), an enzyme responsible for hydrolyzing lysosomal glycogen. Deficiency of GAA leads to systemic glycogen accumulation in the lysosomes of skeletal muscle, motor neurons, and smooth muscle. Skeletal muscle and motor neuron pathology are known to contribute to respiratory insufficiency in Pompe disease, but the role of airway pathology has not been evaluated. Here we propose that GAA enzyme deficiency disrupts the function of the trachea and bronchi and this lower airway pathology contributes to respiratory insufficiency in Pompe disease. Using an established mouse model of Pompe disease, the Gaa−/− mouse, we compared histology, pulmonary mechanics, airway smooth muscle (ASM) function, and calcium signaling between Gaa−/− and age-matched wild-type (WT) mice. Lysosomal glycogen accumulation was observed in the smooth muscle of both the bronchi and the trachea in Gaa−/− but not WT mice. Furthermore, Gaa−/− mice had hyporesponsive airway resistance and bronchial ring contraction to the bronchoconstrictive agents methacholine (MCh) and potassium chloride (KCl) and to a bronchodilator (albuterol). Finally, calcium signaling during bronchiolar smooth muscle contraction was impaired in Gaa−/− mice indicating impaired extracellular calcium influx. We conclude that GAA enzyme deficiency leads to glycogen accumulation in the trachea and bronchi and impairs the ability of lower ASM to regulate calcium and respond appropriately to bronchodilator or constrictors. Accordingly, ASM dysfunction may contribute to respiratory impairments in Pompe disease.
Keywords: Pompe disease, airway smooth muscle, glycogen storing disease, pulmonary mechanics
pompe disease is an autosomal recessive disease caused by a mutation in the gene encoding the acid α-glucosidase (GAA) enzyme, an enzyme responsible for hydrolyzing lysosomal glycogen. Deficiency of GAA results in lysosomal glycogen accumulation and disruption of cellular architecture throughout the body including skeletal muscle, heart, central nervous system (CNS), vascular smooth muscle, and smooth muscle of the bladder, eye, and gastrointestinal system (6, 12, 26, 30, 44, 55, 62). Respiratory insufficiency and ventilator dependence are common complications of this disease (11, 18, 33, 46, 58). Respiratory failure appears to reflect dysfunction in both skeletal muscles (18, 38, 40) and motor neurons (11, 58). The only Food and Drug Administration-approved therapy available to Pompe patients is enzyme replacement therapy (ERT), which prolongs ventilator-free survival by one-third in early onset patients (6). However, up to 75% of patients on ERT therapy eventually require mechanical ventilation (7), which reflects the inability of ERT to effectively treat the entire motor unit (7, 18, 23, 41). As survival is increasing, lower airway weakness or collapse (tracheomalacia and bronchomalacia) has been unmasked in patients on ERT (60, 66). This tracheomalacia and bronchomalacia result in floppy airways that obstruct air flow and exacerbate respiratory insufficiency (28, 29, 66).
Upper airway obstruction in Pompe disease is classically thought to occur due to biomechanical problems associated with macroglossia (33) as well as a possible contribution of impaired neural inputs to airway muscles (16, 33). However, very little is known regarding the lower airways because bronchoscopies are not regularly performed in Pompe patients (66). Tracheomalacia and bronchomalacia have been identified and described in patients with declining respiratory function despite ERT or diaphragm pacing (60, 66). Interestingly, declining respiratory status can be improved when an airway stent is used to treat the airway malacia (66). In other lysosomal storage disorders such as mucopolysaccharidosis types I, II, and VI, both tracheomalacia and bronchomalacia can lead to complete major airway collapse and placement of airway stents is important for maintaining airway patency (29). In the current study, we characterized the impact of GAA enzyme deficiency on lower airway architecture and smooth muscle structure and function. With the use of a Pompe mouse model, the Gaa−/− mouse, our primary aim was to determine if GAA deficiency leads to glycogen accumulation in airway smooth muscle (ASM), abnormal pulmonary mechanics, and dysfunctional smooth muscle contraction. Our secondary aim was to determine if GAA deficiency disrupted calcium signaling during ASM contraction or relaxation. Collectively, the results provide new insights regarding the impact of GAA deficiency on smooth muscle pathology and highlight the importance of evaluating and treating the lower airway pathology in patients with Pompe disease.
METHODS
Animals.
All experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Massachusetts Medical School. B6;129-GaaTm1Rabn/J mice were obtained from Jackson Laboratory (Bar Harbor, ME) and bred in house. Age-matched 6- to 7-mo-old wild-type (WT) and Gaa−/− littermates were used in this study. Before enrollment in the study, littermates were genotyped using standard PCR methods to confirm lack of the GAA gene (38, 54). A 131-bp product indicated the WT allele and a 251-bp product confirmed the mutant allele in the Gaa−/− mouse as per the Jackson Laboratory protocol.
Histology.
Airway sections were embedded in epoxy resin or in OCT. For the epoxy resin-embedded tissue, tracheal and bronchial cross sections 3-mm long were immersion fixed in 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.3) at 4°C overnight, postfixed in 1% osmium tetroxide, and embedded into epon-araldite resin mixture as previously described (37). Polymerized blocks were trimmed and cut at 1 and 2 µm on a Leica Ultracut E ultramicrotome and mounted on charged slides. Tissues embedded in OCT and fresh frozen were sectioned at 10-µm thick and placed directly on to slides. To evaluate histological glycogen load in the trachealis smooth muscle and adjacent tissues, we adopted the periodic acid Schiff (PAS)-staining technique successfully used by Lee et al. (33) with some modification. Briefly, tissues were incubated in 1% periodic acid for 10 min at 60°C, rinsed with tap water for 1 min, and then immediately stained with Schiff’s reagent (Sigma-Aldrich, St. Louis, MO) at room temperature for 10 min, followed by a tap water rinse until the water ran clear. Plastic sections were then counterstained in 1% toluidine blue with 1% borax added at 60°C for 20 s. Sections were washed with distilled water and placed on the hot plate at 65°C. Once the slides were dried completely, they were coverslipped with a Permount mounting medium (Fisher Scientific, Hampton, NH) without dehydration in ethanol and the use of xylene. Fresh frozen sections were PAS stained according to standard techniques (33) and counterstained with hematoxylin. Images were taken on a LeicaDM5500B fluorescence microscope. Ultrathin sections were cut with Leica Ultramicrotome to 70 nm (or 700 Å), placed on golden grids, stained with uranyl acetate and lead citrate, and viewed under CM10 Phillips transmission electron microscope.
Pulmonary mechanics.
Pulmonary mechanics at baseline and in response to incremental doses of methacholine (MCh) were performed using forced oscillometry (FlexiVent system; SCIREQ, Montreal, Canada) in WT and Gaa−/− mice. Forced oscillometry was performed in tracheotomized and anesthetized mice. Mice were anesthetized with an intraperitoneal injection of a ketamine (90 mg/kg; Animal Health International) and xylazine (4.5 mg/kg; Propharma) mixture. Following an adequate plane of anesthesia (loss of withdrawal to toe pinch), tracheotomy was performed, and a precalibrated cannula was introduced into the trachea. The mouse was then placed on a computer controlled piston-ventilator FlexiVent system (SCIREQ) and ventilated at a tidal volume of 10 ml/kg, with a rate of 150 breaths/min and a positive end expiratory pressure of 3 mmHg. Neuromuscular blockade with pancuronium bromide (2.5 mg/kg; Hospira, Lake Forest, IL) was given to prevent spontaneous respiratory effort. Respiratory mechanics were obtained and calculated using FlexiWare software (SCIREQ) as previously described (42, 56). In brief, measurements were obtained by analyzing pressure and volume signals acquired in reaction to predefined, small amplitude, oscillatory airflow waveforms (perturbations) applied to the subject’s airways. After an initial mechanical scan protocol, animals were subjected to incremental doses (3.125, 6.25, 12.5, 25, 50, and 100 mg/ml) of nebulized MCh. Respiratory system resistance (Rrs) was obtained by assessing the mouse’s response to a single frequency forced oscillation maneuver (42). Resistance of central airways (Rn) and small airway and tissue resistance (G) were measured using broadband low-frequency forced oscillation maneuvers over a range of frequencies (Quick Prime-3) (42). These maneuvers were performed every 15 s for a total of 12 automated measurements per dose. Between each challenge, the mouse’s lungs were subjected to two deep lung inflations. For albuterol studies, following completion of the nebulized MCh doses, animals were nebulized with 2.5 mg/3 ml R-albuterol and the above 12 automated measurements were obtained. The mouse lungs were then subjected to 2 deep lung inflations, and another 12 measurements were obtained to ensure that there was adequate time given for the effects of albuterol to take place. To ensure that the animal was alive during these in vivo pulmonary mechanical scans and dose response, the heartbeat was visualized between each dose during the deep inflation and at the end of the study.
Bronchial ring isometric contraction bioassay.
For the force measurements, the left and right extrapulmonary bronchi from WT and Gaa−/− mice were dissected from the lung, and the surrounding tissues were cut off carefully in ice-cold Krebs solution containing the following (in mM): 118.07 NaCl, 4.69 KCl, 2.52 CaCl2, 1.16 MgSO4, 1.01 NaH2PO4, 25 NaHCO3, and 11.1 glucose. Bronchial rings of ~2.5 mm in length were cut and attached to mounting support pins that were connected to force transducers, and changes in their force were recorded with a multiwire myograph system (610-M; Danish Myo Technology). The bronchial rings were kept in oxygenated Krebs buffer (95% O2-5% CO2) at 37°C. After a 20-min equilibrium, a 2-mN stretch was applied to the rings three times with a 5-min interval. KCl (60 mM) was then used to stimulate the rings twice with a 10-min interval before the response to incremental doses of MCh was assessed.
Isolation of single ASM cells.
Smooth muscle strips were prepared from the trachea and main bronchi of Gaa−/− and WT mice and the ASM cells were isolated as previously described (67). Briefly, the trachea and main bronchi were quickly removed and placed in ice-cold dissociation medium (DM) containing the following (in mM): 136 NaCl, 5.36 KCl, 0.44 KH2PO4, 4.16 NaHCO3, 0.34 Na2HPO4, 5 MgCl2, 20 mM HEPES, and 10 glucose, pH 7.1. The trachea was dissected from the connective tissue carefully and incubated in the MgCl2-free DM with 30 U/ml papain, 0.2 mM dithiothreitol, and 0.02 mM EDTA at room temperature for 30 min. The tissues were then transferred to a second digestion solution (MgCl2-free DM) containing 3 U/ml collagenase 1A, 1 mg/ml bovine serum albumin, and 0.1 mg/ml deoxyribonuclease I at 34°C for 6, 4, and 4 min in sequence. The tissue pieces were gently triturated using a fire-polished glass pipette to generate a cell suspension. The dispersed cells were maintained in the DM solution containing 1 mM Ca2+ at 4°C for later use in the same day. Drops of the cell suspension were transferred to a glass-bottomed chamber and allowed to adhere to the bottom of the chamber for 10 min before being recorded.
Measurement of global intracellular calcium concentration.
To monitor global intracellular calcium concentration ([Ca2+]i), fluo-3 fluorescence was measured using a custom-built wide-field digital imaging system (69). Fresh isolated cells were incubated in DM solution containing 1 μM fluo-3-AM for 1 h at room temperature. The cells were then placed on the glass-bottomed chamber, and the medium was changed to bath solution containing the following (in mM): 130 NaCl, 5.5 KCl, 1 MgCl2, 10 HEPES, 2.2 mM CaCl2, and 5 glucose, pH 7.4. For the calcium depletion studies, the medium was changed to a calcium-free bath solution containing the following (in mM): 130 NaCl, 5.5 KCl, 1 MgCl2, 10 HEPES, 0.05 EGTA, and 5 glucose, pH 7.4. The camera was interfaced to a custom-made inverted microscope, and the cells were imaged at the speed of 1 Hz using a ×20 Nikon 1.3 NA. The 488-nm line of an argon ion laser provided fluorescence excitation, with a shutter to control exposure duration, and emission of the Ca2+ indicator was monitored at wavelengths >500 nm. A custom-designed software package was used for subsequent image processing and analysis running on a Linux/PC workstation. [Ca2+]i was represented as ΔF/F0 × 100, with F calculated by integrating fluo-3 fluorescence over entire cells for global [Ca2+]i after background correction with areas free of cells.
Statistics.
All data are reported as means ± SE. All statistical analyses were performed using Prism software (version 7; Graphpad, La Jolla, CA). Significance for dose responses was determined by two-way repeated-measures ANOVA. The Bonferroni post hoc analysis was used for multiple pair-wise comparisons and comparisons with baseline. In the ex vivo KCl studies and global calcium measurements, peak and sustained responses between Gaa−/− and WT mice were assessed using the Students t-test. Significance was considered at a P < 0.05.
RESULTS
Glycogen accumulates in Gaa−/− tracheal and bronchial smooth muscle.
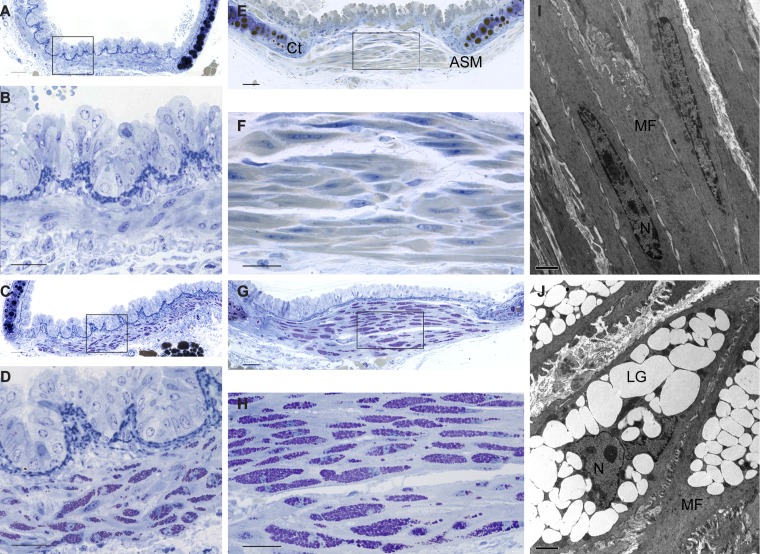
Periodic acid Schiff (PAS) staining was used to identify glycogen in the ASM. Semithin plastic sections from WT mice revealed a normal spindle-shaped smooth bronchial muscle cells with a central elongated nucleus (Fig. 1, A and B). In sharp contrast, Gaa−/− bronchial smooth muscle cells showed cytoplasmic inclusions filled with PAS-positive material, consistent with lysosomal glycogen accumulation (Fig. 1, C and D). Similarly, within the trachealis muscle, WT ASM had no indication of pathology (Fig. 1, E and F), whereas Gaa−/− trachealis smooth muscle had extensive cytoplasmic vacuolization filled with PAS-positive material (Fig. 1, G and H). In fact, the Gaa−/− mouse smooth muscle cytoplasm is virtually filled with glycogen-laden lysosomes with a resultant disruption in cellular architecture including displacement of the nuclei to the edge of the cell (Fig. 1, D and H). Electron microscopy of the trachealis smooth muscle confirmed disruption of Gaa−/− fibers and enlarged lysosomes (Fig. 1J), features that were absent in WT mice (Fig. 1I). Glycogen accumulation in Gaa−/− tissues has an evenly stippled texture imparted by small granules of low electron density.
Fig. 1.
Glycogen accumulation in airway smooth muscle of Gaa−/− mice. A–H: representative plastic-embedded 2-µm cross sections stained using the periodic acid Schiff (PAS) method (purple) and counterstained with toluidine blue. Shown are illustratrations of wild-type (WT) bronchi (A and B) and trachea (E and F) (B and F are enlargements of A and E, respectively). Shown are Gaa−/− bronchi (C and D) and trachea (G and H) (D and H are enlargements of C and G, respectively). I and J: low-magnification electron micrographs of airway smooth muscle of WT (I) and Gaa−/− (J) mouse trachealis muscle cells. C, cartilage; ASM, airway smooth muscle cells; MF, myofilaments; LG, lysosomal glycogen; N, nuclei. Scale bar = 50 µm (A, C, E, and G), 25 µm (B, D, F, and H), and 2 µm (I and J).
Gaa−/− smooth muscle contractility is impaired both in vivo and ex vivo.
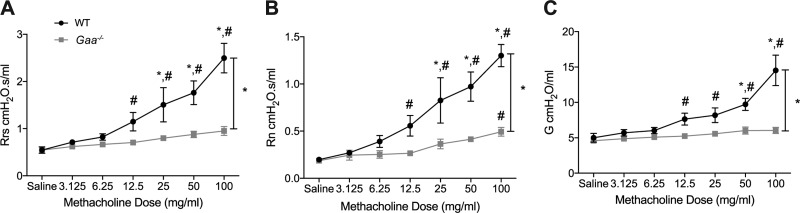
The bronchoconstrictive agent MCh results in airway contraction and increased respiratory, airway, and tissue resistance (63). Gaa−/− and WT mice were administered increasing doses of MCh via a nebulizer, and in vivo lung mechanics including total respiratory system resistance, central airway resistance, and tissue resistance were assessed. Total respiratory system resistance in Gaa−/− was reduced at higher doses of MCh compared with WT controls (P = 0.0036; Fig. 2A). This decreased resistance indicates that Gaa−/− mouse airways are hyporesponsive to MCh. A similar finding was present for central airway resistance with reduced values in Gaa−/− mice at high doses of MCh (P = 0.0028 vs. WT; Fig. 2B). Finally, tissue resistance was also significantly different in Gaa−/− and WT mice (P = 0.0018; Fig. 2C).
Fig. 2.
Airways of Gaa−/− mice are hyporesponsive to methacholine (MCh) in vivo. Forced oscillometry measures of respiratory and airway resistance in anesthetized and mechanically ventilated Gaa−/− and WT mice are shown. Measurements were taken following incremental doses of nebulized MCh from 3.123 to 100 mg/ml. A: overall respiratory resistance (Rrs) in WT (n = 4) and Gaa−/− (n = 6) mice. Initially, during saline administration no differences in overall resistance were appreciated between WT and Gaa−/− mice, but with increasing doses, the resistance showed a more robust increase in WT mice compared with Gaa−/− mice. B: Gaa−/− mice have a central airway resistance (Rn) that is hyporesponsive to higher doses of MCh in compared with WT mice. C: finally, tissue damping (G), which is reflection of the smallest airways and tissue resistance, was also hyporesponsive to MCh in Gaa−/− mice. *P ≤ 0.05, pair-wise comparison; #P ≤ 0.05, baseline comparison.
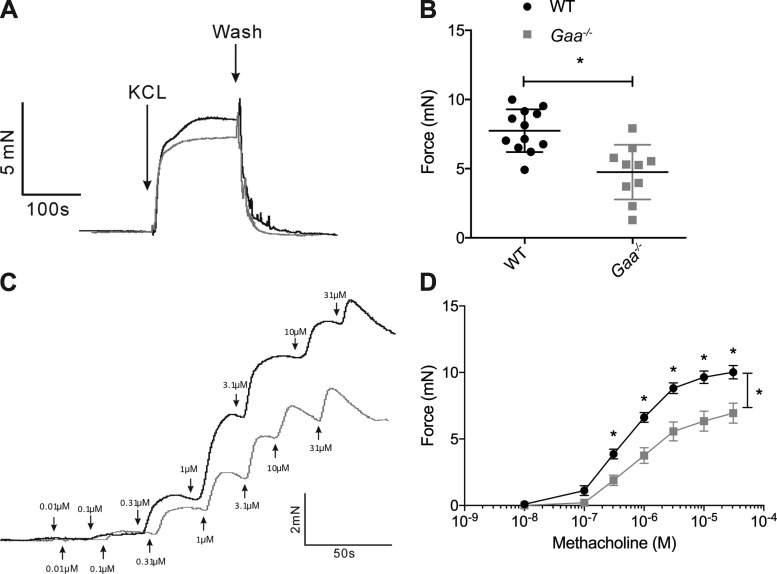
Next, Gaa−/− and WT bronchial smooth muscle contractility was measured ex vivo using a bronchial ring isometric contraction bioassay. Contractile force was assessed following exposure to MCh and potassium chloride (KCl). The force generated by Gaa−/− bronchi upon MCh exposure was substantially reduced as compared with WT bronchi (P = 0.0001; Fig. 3, A and B). Similarly, the bronchial contraction of Gaa−/− mice to a single administration of KCl was significantly reduced compared with WT controls (P = 0.007; Fig. 3, C and D).
Fig. 3.
Gaa−/− bronchial rings have reduced contractile force upon exposure to MCh and potassium chloride (KCl). A and B: representative traces (A) and summary data (B) confirm that KCl induced bronchial ring contraction is more robust in WT (n = 12) than in Gaa−/− (n = 9) bronchi. C: representative traces of MCh-induced bronchial ring force of contraction at incremental increases in concentrations in WT (n = 11) and Gaa−/− (n = 9) mice. D: bronchial ring contraction in response to increasing doses of MCh confirmed that Gaa−/− bronchi had a reduced force of contraction compared with WT bronchi. *P ≤ 0.05, pair-wise comparison.
Impaired responses to R-albuterol in Gaa−/− mice both ex vivo and in vivo.
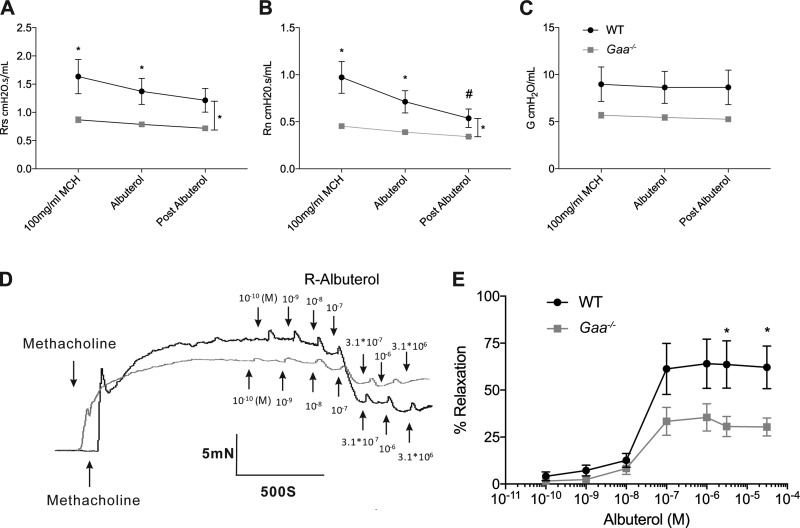
To assess the ability of the smooth muscle to respond to bronchodilators, Gaa−/− and WT ASM relaxation was assessed in response to the β2-adrenergic receptor agonist R-albuterol. In vivo airway resistance in response to albuterol delivered via the nebulizer was significantly different between Gaa−/− and WT mice (Fig. 4, A and B). Minimal changes in Gaa−/− respiratory system resistance occurred in response to albuterol and ~5-min later when another set of forced oscillation maneuvers and measurements was obtained (P = 0.0262; Fig. 4A). Furthermore, the central airway resistance minimally decreased when Gaa−/− mice were exposed to albuterol (P = 0.0159 vs. WT, Fig. 4B). R-albuterol had little effect on tissue resistance (P = 0.0749; Fig. 4C) of either Gaa−/− or WT mice.
Fig. 4.
Airways and bronchial rings of Gaa−/− mice have an attenuated response to albuterol. A–C: after MCh dosage response, R-albuterol was nebulized and measurement cycle was taken, and then an additional measurement was taken postalbuterol. A and B: Gaa−/− mice have a decreased response to albuterol in vivo compared with WT controls as evidenced by a lack of response in overall pulmonary resistance (Rrs; A) and central airway resistance (Rn; B). WT (n = 4) and Gaa−/− (n = 5) mice. C: tissue resistance (G) did not respond to R-albuterol in WT or Gaa−/− animals. D and E: ex vivo Gaa−/− bronchial rings have a blunted response to the bronchodilator effects of R-albuterol compared with WT bronchial rings. D: representative tracing of bronchial ring force as a response to R-albuterol administration following MCh-induced contraction. E: summary data of R-albuterol inhibitory effects on MCh-induced tone as percent relaxation. The values were normalized to the max effect of MCh on smooth muscle tone in both WT (n = 6) and Gaa−/− (n = 7) mice. *P ≤ 0.05, pair-wise comparison. #P ≤ 0.05, baseline comparison.
Ex vivo, cumulative doses of R-albuterol were administered to relax the bronchial rings of WT and Gaa−/− mice (Fig. 4, D and E). Incremental doses of R-albuterol were administered once a stable plateau of contraction force was observed with MCh. The initial relaxation of the smooth muscle to R-albuterol occurred at 10−9 M and reached the maximum effect at 10−7 M in both Gaa−/− and WT tissues (Fig. 4, D and E). Relaxation was blunted at doses of R-albuterol greater than 10−7 in Gaa−/− tissues, and this response was significantly different than WT (P < 0.0346; Fig. 4E).
Calcium signaling is disrupted in Gaa−/− mice ASM cells.
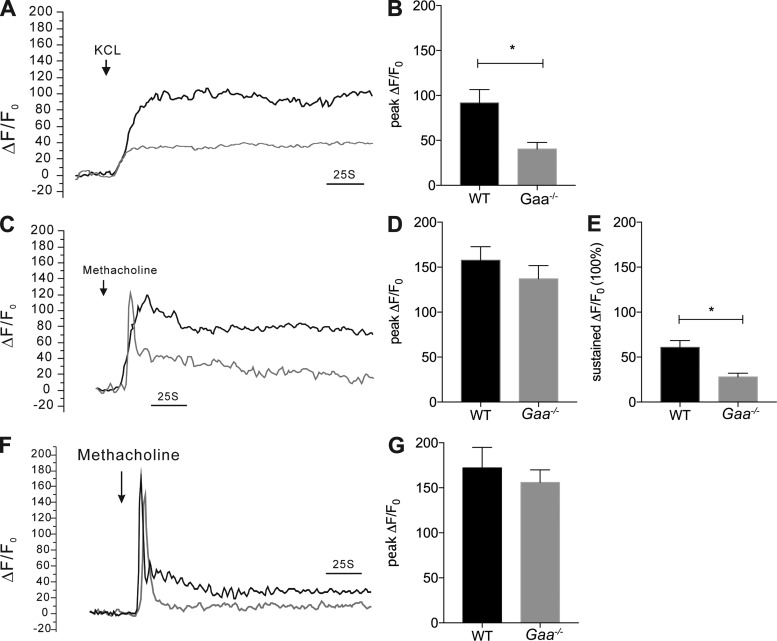
The intracellular calcium concentration ([Ca2+]i) plays a critical role in triggering and maintaining smooth muscle contraction. Since smooth muscle contraction was less robust in Gaa−/− mice compared with WT controls, we hypothesized that Ca2+ signaling in response to KCl and MCh would be different between the two strains. To test this, we compared the changes in [Ca2+]i induced by KCl and MCh in isolated single ASM cells. KCl induces membrane depolarization that leads to an influx of Ca2+ into the cells via voltage-dependent Ca2+ channels and ASM contraction (3, 13, 57). In WT smooth muscle cells, KCl induced a sustained increase in [Ca2+]i (Fig. 5, A and B) whereas Gaa−/− cells had a minimal increase in [Ca2+]i (P = 0.0059; Fig. 5, A and B; P = 0.0059). Next, we assessed the response of the ASM cells to MCh. MCh induces a biphasic [Ca2+]i response: an initial peak [Ca2+]i followed by a sustained [Ca2+]i phase. The initial peak [Ca2+]i occurs when Ca2+ is released from intracellular sarcoplasmic reticulum stores by activating the inositol 1,4,5-triphosphate receptor (47) whereas the sustained phase occurs by an influx of extracellular Ca2+ (47). When exposed to MCh, Gaa−/− mice ASM cells had a similar [Ca2+]i peak to WT mice (Fig. 5, C and D; P = 0.3323). In contrast, the MCh-induced sustained [Ca2+]i phase was significantly lower in the Gaa−/− mice ASM cells compared with the WT mice (Fig. 5, C and E; P = 0.0002). These results again suggest that Ca2+ influx is impaired in ASM cells from Gaa−/− mice.
Fig. 5.
Deficient extracellular calcium influx in response to KCl and MCh in Gaa−/− mouse airway smooth muscle cells. A and B: the change in calcium global intracellular calcium concentration ([Ca2+]i) (ΔF/F0) induced by 60 mM KCl is more robust in WT (n = 25 cells) compared with Gaa−/− (20 cells) smooth muscle cells (A: representative trace; B: mean data). C–E: in response to MCh, the initial calcium release in WT and Gaa−/− animals is similar (C and D), but the sustained response is more robust in WT animals (n = 46 cells) compared with Gaa−/− (48 cells) airway smooth muscle cells (C and E). F and G: change in [Ca2+]i (represented as ΔF/F0) was raised by MCh (10−4 M) in Ca2+-free solution in WT (n = 23 cells) and Gaa−/− (n = 26 cells) animals and confirms that the initial rise in in [Ca2+]i is due to intracellular Ca2+ release. The peak value was measured as the peak component of calcium signal, and the average values of last 50 s were measured as the sustained component. *P ≤ 0.05.
Lastly, we confirmed that the initial rise in calcium during MCh stimulation was due to intracellular calcium release and that intracellular calcium release was normal in Gaa−/− ASM cells. ASM cells from WT and Gaa−/− mice were placed in a calcium-free environment, and changes in [Ca2+]i were assessed upon exposure to MCh. Cells from both strains produced only monophasic transients in response to MCh, as would be expected if MCh only releases Ca2+ from intracellular stores (21, 24). No difference in the peak [Ca2+]i between Gaa−/− and WT mice was detected (Fig. 5, F and G; P = 0.5411) suggesting intracellular Ca2+ release is not impaired in Gaa−/− mice. These results confirm that internal Ca2+ release appears unaffected in ASM cells from Gaa−/− mice despite the abundant glycogen accumulation.
DISCUSSION
Several important results emerge from this histological and physiological evaluation of the Gaa−/− mouse airways and ASM cells. First, GAA enzyme deficiency results in a significant accumulation of glycogen in tracheal and bronchial smooth muscle with resultant disruption of the cytoplasmic structure. Second, airway mechanics and smooth muscle contractile function are impaired in Gaa−/− mice in response to bronchoconstrictor or bronchodilator compounds. Finally, GAA enzyme deficiency is associated with altered calcium signaling dynamics in ASM. Collectively, these results indicate that ASM dysfunction is likely to contribute to respiratory-related impairments in Pompe disease.
Smooth muscle in Pompe disease.
Progressive respiratory insufficiency is a known complication of Pompe disease. Although glycogen accumulation in respiratory skeletal muscles and motor neurons has been well described in Pompe disease patients (11, 18, 26, 30) and mice (11, 17, 18, 33), ASM involvement has not previously been systematically examined. Smooth muscle pathology in other organ systems and vascular smooth muscle has been described in Pompe disease or relevant animal models (26, 30, 44, 48, 55, 61, 62). In Pompe patients, accumulation of glycogen in vascular smooth muscle is known to result in weakness of the arterial walls and formation of aneurysms (15, 27, 64). Patients with Pompe disease have cerebrovascular aneurysms, dilation of the carotid arteries with resultant carotid artery dissection (65), and dilation of the basilar arteries (5). In addition, there have been reports of aortic artery aneurysms and dissection of the aortic artery (15). Involvement of nonvascular smooth muscle has also been reported in patients with Pompe disease. For example, lysosomal glycogen accumulation occurs in the smooth muscle of the urinary bladder (26), which could explain patient reports of increased urinary incontinence (43). In addition, involvement of the smooth muscle of the gastrointestinal tract and smooth muscle of the iris has been reported in autopsies of patients with Pompe disease (26, 48, 50). Similarly, our study found extensive glycogen accumulation in the smooth muscle of the trachea and bronchi. Based on these histological findings (Fig. 1), it appears that GAA deficiency and the associated accumulation of glycogen in tracheal smooth muscle produce contractile dysfunction, and accordingly this may contribute to the tracheal and bronchial weakness, or malacia, seen in Pompe patients (60, 66).
Blunted response to bronchoconstriction and bronchodilation.
In the current study, smooth muscle physiology was assessed both in vivo and ex vivo, and the data show airway and bronchial ring hyporesponsiveness to bronchoconstricting and bronchodilating agonists. Although the exact mechanisms are unknown, we speculate that the disrupted smooth muscle architecture (e.g., Fig. 1) and even more importantly abnormal calcium signaling (e.g., Fig. 5) play prominent roles. In Pompe disease, the prevailing hypothesis regarding the mechanisms of skeletal muscle weakness is that myofibers filled with excessive glycogen have little space to expand and the associated mechanical stress during contraction results in lysosomal rupture and further muscle damage (22). However, recent studies indicate that the mechanism(s) of muscle weakness in Pompe disease are much more complicated (35). For example, autophagic accumulation and alterations calcium signaling both appear to play a vital role in skeletal muscle weakness (35). Autophagic disruption occurs because lysosomes play an important role in autophagy by fusing with autophagosomes and recycling and breaking down the autophagic debris. In skeletal muscle, impaired autophagy and GAA enzyme deficiency are associated with abnormal calcium homeostasis and increased calcium signaling (35). Impaired calcium signaling also appears to play a role in the impaired in vivo and ex vivo response to bronchodilating and constricting agonists (e.g., Fig. 5).
ASM GAA deficiency and calcium signal disruption.
ASM contraction is induced by an increase in intracellular calcium concentration (39, 45, 49, 51). Bronchoconstrictive agonists such as MCh increase the intracellular calcium concentration by calcium release from the sarcoplasmic reticulum through activation of the inositol 1,4,5-triphosphate receptor. To sustain ASM contraction, sustained calcium influx is necessary to replenish calcium and maintain calcium homeostasis. Several pathways are involved in calcium entry including voltage-dependent Ca2+ channels (VDCCs), receptor-operated calcium channels, and store-operated calcium (SOC) channels (21, 24, 68). Here, we found that in Gaa−/− ASM cells the initial release of Ca2+ was comparable to that of WT mice, which implies normal Ca2+ release from the sarcoplasmic reticulum. However, the ability to sustain contraction was significantly blunted. This inability to sustain the contraction is consistent with a defect in an influx of calcium. This could be due to involvement of any of the pathways necessary for calcium influx, including the VDCC or SOC channels. Indeed, when we used KCl to depolarize and contract the ASM, the Gaa−/− mice airways had a blunted response, which may indicate a defect in VDCC calcium influx, since KCl is known to activate VDCCs by depolarizing the membrane (25). However, this does not rule out the involvement of SOC entry.
In vivo and ex vivo studies of the airways and bronchial rings using bronchodilators also resulted in a blunted response to smooth muscle bronchodilators (β2-adrenergic receptor agonists or β2-agonists) in Gaa−/− mice. β2-Agonists activate cAMP and inhibit the sarcoplasmic reticulum calcium release and calcium influx through SOC channels and VDCCs. β2-Agonists also enhance calcium efflux in ASM cells (10, 20, 53). In the GAA-deficient ASM cells, if VDCC activity is reduced or SOC channels are disrupted, the effects of β2-agonists would be attenuated. This may account for the lack of response to β2-agonists in Gaa−/− mice as revealed in this study, in keeping with findings that a rise in cAMP upon β2-adrenergic receptor activation inhibits VDCCs directly or indirectly in ASM cells (31, 32). Since cAMP can inhibit the SOC entry in ASM cells (1, 20, 52), it would be of great interest to study the potential role of this calcium entry pathway in mediating the defect in β2-agonist induced airway relaxation in Gaa−/− mice.
Interestingly, calcium homeostasis is also disrupted in other lysosomal storage diseases with increased cytosolic calcium and disrupted reuptake of calcium into the endoplasmic reticulum in some (4) and decreased cytosolic calcium in others (36). Furthermore, Raben et al. (54) discovered defective calcium homeostasis in Pompe disease skeletal muscle. Unlike our findings in ASM, they discovered an increase in calcium entry in skeletal muscle resulting in a surge of cytosolic calcium levels (34, 35). In addition, they noted that the expression of some VDCCs was upregulated in skeletal muscle (35). It is important to note that skeletal muscles express Cav1.1 while smooth muscles mainly the Cav1.2 to mediate calcium influx and these two VDCCs could be regulated differently in GAA deficiency. VDCC regulation and function have not been characterized in ASM cells from Gaa−/− mice and are beyond the scope of this study. Further studies are also needed to elucidate the impact of GAA enzyme deficiency in ASM on other Ca2+ influx mechanisms such as transient receptor potential channels and SOC channels (2, 9).
Conclusion and clinical significance.
This study confirms that ASM dysfunction occurs in the absence of GAA enzyme activity. Respiratory insufficiency is a hallmark of Pompe disease and patients on ERT often require respiratory support. Several prior reports have led to the suggestion that respiratory failure, despite ongoing ERT, represents the inability of ERT to treat the entire respiratory motor unit (i.e., both skeletal myofibers and associated motor neurons) (7, 11, 14, 18). Here we show that ASM is profoundly affected in Gaa−/− mice and accordingly the contribution of airway dysfunction to respiratory insufficiency in Pompe disease becomes an important consideration. Our study is the first to describe the impact of GAA enzyme deficiency on lower airway mechanics and ASM calcium signaling and highlights the importance of evaluating patients with declining respiratory function for airway malacia. The current findings also highlight the importance of addressing ASM pathology in preclinical therapeutic studies for respiratory insufficiency in Pompe disease. Adeno-associated virus gene therapy approaches targeting the diaphragm have advanced to stage I/II clinical trials in Pompe patients (59), and future work should evaluate the impact of adeno-associated virus therapies on ASM.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants K08-HD-077040–01A1 and 1R21-NS-098131-01 and Parker B. Francis Fellowship (to M. K. ElMallah) and NIH Grants R01-HL-117104 (to R. ZhuGe) and 2R01-HD-052682-06A1 (to D. D. Fuller and B. J. Byrne).
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the author(s).
AUTHOR CONTRIBUTIONS
A.M.K., L.X., R.Z., and M.K.E. conceived and designed research; A.M.K., D.L., M.Z., L.X., J.S., and M.K.E. performed experiments; A.M.K., D.L., M.Z., L.X., J.S., and M.K.E. analyzed data; A.M.K., D.L., M.Z., J.S., K.B., B.J.B., D.D.F., R.Z., and M.K.E. interpreted results of experiments; A.M.K., D.L., M.Z., and M.K.E. prepared figures; A.M.K. drafted manuscript; A.M.K., D.L., M.Z., J.S., D.D.F., R.Z., and M.K.E. edited and revised manuscript; A.M.K., D.L., M.Z., L.X., J.S., K.B., B.J.B., D.D.F., R.Z., and M.K.E. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Kaitlyn Desrochers for technical support and the University of Massachusetts Electron Microscopy Core.
REFERENCES
- 1.Ay B, Iyanoye A, Sieck GC, Prakash YS, Pabelick CM. Cyclic nucleotide regulation of store-operated Ca2+ influx in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 290: L278–L283, 2006. doi: 10.1152/ajplung.00188.2005. [DOI] [PubMed] [Google Scholar]
- 2.Ay B, Prakash YS, Pabelick CM, Sieck GC. Store-operated Ca2+ entry in porcine airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 286: L909–L917, 2004. doi: 10.1152/ajplung.00317.2003. [DOI] [PubMed] [Google Scholar]
- 3.Bai Y, Zhang M, Sanderson MJ. Contractility and Ca2+ signaling of smooth muscle cells in different generations of mouse airways. Am J Respir Cell Mol Biol 36: 122–130, 2007. doi: 10.1165/rcmb.2006-0036OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ballabio A, Gieselmann V. Lysosomal disorders: from storage to cellular damage. Biochim Biophys Acta 1793: 684–696, 2009. doi: 10.1016/j.bbamcr.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Braunsdorf WE. Fusiform aneurysm of basilar artery and ectatic internal carotid arteries associated with glycogenosis type 2 (Pompe’s disease). Neurosurgery 21: 748–749, 1987. doi: 10.1227/00006123-198711000-00030. [DOI] [PubMed] [Google Scholar]
- 6.Byrne BJ, Falk DJ, Pacak CA, Nayak S, Herzog RW, Elder ME, Collins SW, Conlon TJ, Clement N, Cleaver BD, Cloutier DA, Porvasnik SL, Islam S, Elmallah MK, Martin A, Smith BK, Fuller DD, Lawson LA, Mah CS. Pompe disease gene therapy. Hum Mol Genet 20, R1: R61–R68, 2011. doi: 10.1093/hmg/ddr174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byrne BJ, Kishnani PS, Case LE, Merlini L, Müller-Felber W, Prasad S, van der Ploeg A. Pompe disease: design, methodology, and early findings from the Pompe Registry. Mol Genet Metab 103: 1–11, 2011. doi: 10.1016/j.ymgme.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Chen J, Sanderson MJ. Store-operated calcium entry is required for sustained contraction and Ca(2+) oscillations of airway smooth muscle. J Physiol 2016 Jul 11. [Epub ahead of print]. doi: 10.1113/JP272694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delmotte P, Sanderson MJ. Effects of formoterol on contraction and Ca2+ signaling of mouse airway smooth muscle cells. Am J Respir Cell Mol Biol 42: 373–381, 2010. doi: 10.1165/rcmb.2008-0403OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeRuisseau LR, Fuller DD, Qiu K, DeRuisseau KC, Donnelly WH Jr, Mah C, Reier PJ, Byrne BJ. Neural deficits contribute to respiratory insufficiency in Pompe disease. Proc Natl Acad Sci USA 106: 9419–9424, 2009. doi: 10.1073/pnas.0902534106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DiMauro S, Stern LZ, Mehler M, Nagle RB, Payne C. Adult-onset acid maltase deficiency: a postmortem study. Muscle Nerve 1: 27–36, 1978. doi: 10.1002/mus.880010105. [DOI] [PubMed] [Google Scholar]
- 13.Du W, McMahon TJ, Zhang ZS, Stiber JA, Meissner G, Eu JP. Excitation-contraction coupling in airway smooth muscle. J Biol Chem 281: 30143–30151, 2006. doi: 10.1074/jbc.M606541200. [DOI] [PubMed] [Google Scholar]
- 14.Dubrovsky A, Corderi J, Lin M, Kishnani PS, Jones HN. Expanding the phenotype of late-onset Pompe disease: tongue weakness: a new clinical observation. Muscle Nerve 44: 897–901, 2011. doi: 10.1002/mus.22202. [DOI] [PubMed] [Google Scholar]
- 15.El-Gharbawy AH, Bhat G, Murillo JE, Thurberg BL, Kampmann C, Mengel KE, Kishnani PS. Expanding the clinical spectrum of late-onset Pompe disease: dilated arteriopathy involving the thoracic aorta, a novel vascular phenotype uncovered. Mol Genet Metab 103: 362–366, 2011. doi: 10.1016/j.ymgme.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 16.ElMallah MK, Federico RA, Sandhu MS, Lane MA, Reier PJ, Byrne BJ, Fuller DD. Persistent correction of hypoglossal motoneuron enzyme deficiency following gene delivery to the tongue in a mouse model of Pompe disease. Soc Neurosci 2012. [Google Scholar]
- 17.ElMallah MK, Falk DJ, Nayak S, Federico RA, Sandhu MS, Poirier A, Byrne BJ, Fuller DD. Sustained correction of motoneuron histopathology following intramuscular delivery of AAV in Pompe mice. Mol Ther 22: 702–712, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuller DD, ElMallah MK, Smith BK, Corti M, Lawson LA, Falk DJ, Byrne BJ. The respiratory neuromuscular system in Pompe disease. Respir Physiol Neurobiol 189: 241–249, 2013. doi: 10.1016/j.resp.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuller DD, Falk DJ, Lawson L, Martin D, Smith B, Byrne BJ. The importance of targeting the central nervous system (CNS) for treatment of respiratory insufficiency in Pompe disease. World Lysosomal Meeting, 2013. [Google Scholar]
- 20.Gao YD, Zou JJ, Zheng JW, Shang M, Chen X, Geng S, Yang J. Promoting effects of IL-13 on Ca2+ release and store-operated Ca2+ entry in airway smooth muscle cells. Pulm Pharmacol Ther 23: 182–189, 2010. doi: 10.1016/j.pupt.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Gosens R, Zaagsma J, Meurs H, Halayko AJ. Muscarinic receptor signaling in the pathophysiology of asthma and COPD. Respir Res 7: 73, 2006. doi: 10.1186/1465-9921-7-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Griffin JL. Infantile acid maltase deficiency. I. Muscle fiber destruction after lysosomal rupture. Virchows Arch B Cell Pathol Incl Mol Pathol 45: 23–36, 1984. doi: 10.1007/BF02889849. [DOI] [PubMed] [Google Scholar]
- 23.Haley SM, Fragala MA, Skrinar AM. Pompe disease and physical disability. Dev Med Child Neurol 45: 618–623, 2003. doi: 10.1111/j.1469-8749.2003.tb00966.x. [DOI] [PubMed] [Google Scholar]
- 24.Hirota K, Hashiba E, Yoshioka H, Kabara S, Matsuki A. Effects of three different L-type Ca2+ entry blockers on airway constriction induced by muscarinic receptor stimulation. Br J Anaesth 90: 671–675, 2003. doi: 10.1093/bja/aeg118. [DOI] [PubMed] [Google Scholar]
- 25.Hirota S, Pertens E, Janssen LJ. The reverse mode of the Na+/Ca2+ exchanger provides a source of Ca2+ for store refilling following agonist-induced Ca2+ mobilization. Am J Physiol Lung Cell Mol Physiol 292: L438–L447, 2007. doi: 10.1152/ajplung.00222.2006. [DOI] [PubMed] [Google Scholar]
- 26.Hobson-Webb LD, Proia AD, Thurberg BL, Banugaria S, Prater SN, Kishnani PS. Autopsy findings in late-onset Pompe disease: a case report and systematic review of the literature. Mol Genet Metab 106: 462–469, 2012. doi: 10.1016/j.ymgme.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 27.Huded V, Bohra V, Prajapati J, DeSouza R, Ramankutty R. Stroke in young-dilative arteriopathy: a clue to late-onset Pompe’s disease? J Stroke Cerebrovasc Dis 25: e50–e52, 2016. doi: 10.1016/j.jstrokecerebrovasdis.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 28.Hysinger EB, Panitch HB. Paediatric tracheomalacia. Paediatr Respir Rev 17: 9–15, 2016. [DOI] [PubMed] [Google Scholar]
- 29.Kampmann C, Wiethoff CM, Huth RG, Staatz G, Mengel E, Beck M, Gehring S, Mewes T, Abu-Tair T. Management of life-threatening tracheal stenosis and tracheomalacia in patients with mucopolysaccharidoses. JIMD Rep 33: 33–39, 2016. doi: 10.1007/8904_2016_578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kretzschmar HA, Wagner H, Hübner G, Danek A, Witt TN, Mehraein P. Aneurysms and vacuolar degeneration of cerebral arteries in late-onset acid maltase deficiency. J Neurol Sci 98: 169–183, 1990. doi: 10.1016/0022-510X(90)90258-O. [DOI] [PubMed] [Google Scholar]
- 31.Kume H, Fukunaga K, Oguma T. Research and development of bronchodilators for asthma and COPD with a focus on G protein/KCa channel linkage and β2-adrenergic intrinsic efficacy. Pharmacol Ther 156: 75–89, 2015. doi: 10.1016/j.pharmthera.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 32.Kume H, Ishikawa T, Oguma T, Ito S, Shimokata K, Kotlikoff MI. Involvement of Ca2+ mobilization in tachyphylaxis to beta-adrenergic receptors in trachealis. Am J Respir Cell Mol Biol 29: 359–366, 2003. doi: 10.1165/rcmb.2002-0197OC. [DOI] [PubMed] [Google Scholar]
- 33.Lee KZ, Qiu K, Sandhu MS, Elmallah MK, Falk DJ, Lane MA, Reier PJ, Byrne BJ, Fuller DD. Hypoglossal neuropathology and respiratory activity in pompe mice. Front Physiol 2: 31, 2011. doi: 10.3389/fphys.2011.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lim JA, Kakhlon O, Li L, Myerowitz R, Raben N. Pompe disease: shared and unshared features of lysosomal storage disorders. Rare Dis 3: e1068978, 2015. doi: 10.1080/21675511.2015.1068978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lim JA, Li L, Kakhlon O, Myerowitz R, Raben N. Defects in calcium homeostasis and mitochondria can be reversed in Pompe disease. Autophagy 11: 385–402, 2015. doi: 10.1080/15548627.2015.1009779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lloyd-Evans E, Platt FM. Lysosomal Ca(2+) homeostasis: role in pathogenesis of lysosomal storage diseases. Cell Calcium 50: 200–205, 2011. doi: 10.1016/j.ceca.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 37.Lynch CM, Johnson J, Vaccaro C, Thurberg BL. High-resolution light microscopy (HRLM) and digital analysis of Pompe disease pathology. J Histochem Cytochem 53: 63–73, 2005. doi: 10.1177/002215540505300108. [DOI] [PubMed] [Google Scholar]
- 38.Mah C, Pacak CA, Cresawn KO, Deruisseau LR, Germain S, Lewis MA, Cloutier DA, Fuller DD, Byrne BJ. Physiological correction of Pompe disease by systemic delivery of adeno-associated virus serotype 1 vectors. Mol Ther 15: 501–507, 2007. doi: 10.1038/sj.mt.6300100. [DOI] [PubMed] [Google Scholar]
- 39.Mahn K, Ojo OO, Chadwick G, Aaronson PI, Ward JP, Lee TH. Ca(2+) homeostasis and structural and functional remodelling of airway smooth muscle in asthma. Thorax 65: 547–552, 2010. doi: 10.1136/thx.2009.129296. [DOI] [PubMed] [Google Scholar]
- 40.Margolis ML, Howlett P, Goldberg R, Eftychiadis A, Levine S. Obstructive sleep apnea syndrome in acid maltase deficiency. Chest 105: 947–949, 1994. doi: 10.1378/chest.105.3.947. [DOI] [PubMed] [Google Scholar]
- 41.Marsden D. Infantile onset Pompe disease: a report of physician narratives from an epidemiologic study. Genet Med 7: 147–150, 2005. doi: 10.1097/01.GIM.0000154301.76619.5C. [DOI] [PubMed] [Google Scholar]
- 42.McGovern TK, Robichaud A, Fereydoonzad L, Schuessler TF, Martin JG. Evaluation of respiratory system mechanics in mice using the forced oscillation technique. J Vis Exp 52: e50172, 2013. doi: 10.1165/rcmb.2014-0374OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McNamara ER, Austin S, Case L, Wiener JS, Peterson AC, Kishnani PS. Expanding our understanding of lower urinary tract symptoms and incontinence in adults with pompe disease. JIMD Rep 20: 5–10, 2015. doi: 10.1007/8904_2014_381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miyamoto Y, Etoh Y, Joh R, Noda K, Ohya I, Morimatsu M. Adult-onset acid maltase deficiency in siblings. Acta Pathol Jpn 35: 1533–1542, 1985. [DOI] [PubMed] [Google Scholar]
- 45.Montaño LM, Bazán-Perkins B. Resting calcium influx in airway smooth muscle. Can J Physiol Pharmacol 83: 717–723, 2005. doi: 10.1139/y05-063. [DOI] [PubMed] [Google Scholar]
- 46.Muller CW, Jones HN, O’Grady G, Suarez AH, Heller JH, Kishnani PS. Language and speech function in children with infantile Pompe disease. J Pediatr Neurol 7: 147–156, 2009. [Google Scholar]
- 47.Pelaia G, Renda T, Gallelli L, Vatrella A, Busceti MT, Agati S, Caputi M, Cazzola M, Maselli R, Marsico SA. Molecular mechanisms underlying airway smooth muscle contraction and proliferation: implications for asthma. Respir Med 102: 1173–1181, 2008. doi: 10.1016/j.rmed.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 48.Pena LD, Proia AD, Kishnani PS. Postmortem findings and clinical correlates in individuals with infantile-onset pompe disease. JIMD Rep 23: 45–54, 2015. doi: 10.1007/8904_2015_426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perez-Zoghbi JF, Karner C, Ito S, Shepherd M, Alrashdan Y, Sanderson MJ. Ion channel regulation of intracellular calcium and airway smooth muscle function. Pulm Pharmacol Ther 22: 388–397, 2009. doi: 10.1016/j.pupt.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prakalapakorn SG, Proia AD, Yanovitch TL, DeArmey S, Mendelsohn NJ, Aleck KA, Kishnani PS. Ocular and histologic findings in a series of children with infantile pompe disease treated with enzyme replacement therapy. J Pediatr Ophthalmol Strabismus 51: 355–362, 2014. doi: 10.3928/01913913-20140813-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prakash YS. Airway smooth muscle in airway reactivity and remodeling: what have we learned? Am J Physiol Lung Cell Mol Physiol 305: L912–L933, 2013. doi: 10.1152/ajplung.00259.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prakash YS, Iyanoye A, Ay B, Sieck GC, Pabelick CM. Store-operated Ca2+ influx in airway smooth muscle: interactions between volatile anesthetic and cyclic nucleotide effects. Anesthesiology 105: 976–983, 2006. doi: 10.1097/00000542-200611000-00019. [DOI] [PubMed] [Google Scholar]
- 53.Prakash YS, van der Heijden HF, Kannan MS, Sieck GC. Effects of salbutamol on intracellular calcium oscillations in porcine airway smooth muscle. J Appl Physiol (1985) 82: 1836–1843, 1997. [DOI] [PubMed] [Google Scholar]
- 54.Raben N, Nagaraju K, Lee E, Kessler P, Byrne B, Lee L, LaMarca M, King C, Ward J, Sauer B, Plotz P. Targeted disruption of the acid alpha-glucosidase gene in mice causes an illness with critical features of both infantile and adult human doi: 10.1074/jbc.273.30.19086. [DOI] [PubMed] [Google Scholar]
- 55.Ravaglia S, Repetto A, De Filippi P, Danesino C, Leen WG, Groen WB, Vos M, Cruysberg JR, van Doorn PA, van Engelen BG. Ptosis as a feature of late-onset glycogenosis type II. Neurology 69: 116, 2007. doi: 10.1212/01.wnl.0000270101.95790.fb. [DOI] [PubMed] [Google Scholar]
- 56.Robichaud A, Fereydoonzad L, Schuessler TF. Delivered dose estimate to standardize airway hyperresponsiveness assessment in mice. Am J Physiol Lung Cell Mol Physiol 308: L837–L846, 2015. doi: 10.1152/ajplung.00343.2014. [DOI] [PubMed] [Google Scholar]
- 57.Sanderson MJ, Delmotte P, Bai Y, Perez-Zogbhi JF. Regulation of airway smooth muscle cell contractility by Ca signaling and sensitivity. Proc Am Thorac Soc 5: 23–31, 2008. doi: 10.1513/pats.200704-050VS. [DOI] [PubMed] [Google Scholar]
- 58.Sidman RL, Taksir T, Fidler J, Zhao M, Dodge JC, Passini MA, Raben N, Thurberg BL, Cheng SH, Shihabuddin LS. Temporal neuropathologic and behavioral phenotype of 6neo/6neo Pompe disease mice. J Neuropathol Exp Neurol 67: 803–818, 2008. doi: 10.1097/NEN.0b013e3181815994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith BK, Collins SW, Conlon TJ, Mah CS, Lawson LA, Martin AD, Fuller DD, Cleaver BD, Clément N, Phillips D, Islam S, Dobjia N, Byrne BJ. Phase I/II trial of adeno-associated virus-mediated alpha-glucosidase gene therapy to the diaphragm for chronic respiratory failure in Pompe disease: initial safety and ventilatory outcomes. Hum Gene Ther 24: 630–640, 2013. doi: 10.1089/hum.2012.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smith BK, Fuller DD, Martin AD, Lottenberg L, Islam S, Lawson LA, Onders RP, Byrne BJ. Diaphragm pacing as a rehabilitative tool for ventilator-dependent Pompe disease: case series. Phys Ther, 2016. doi: 10.2522/ptj.20150122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thurberg BL, Lynch Maloney C, Vaccaro C, Afonso K, Tsai AC, Bossen E, Kishnani PS, O’Callaghan M. Characterization of pre- and post-treatment pathology after enzyme replacement therapy for Pompe disease. Lab Invest 86: 1208–1220, 2006. doi: 10.1038/labinvest.3700484. [DOI] [PubMed] [Google Scholar]
- 62.van der Walt JD, Swash M, Leake J, Cox EL. The pattern of involvement of adult-onset acid maltase deficiency at autopsy. Muscle Nerve 10: 272–281, 1987. doi: 10.1002/mus.880100311. [DOI] [PubMed] [Google Scholar]
- 63.Vanoirbeek JA, Rinaldi M, De Vooght V, Haenen S, Bobic S, Gayan-Ramirez G, Hoet PH, Verbeken E, Decramer M, Nemery B, Janssens W. Noninvasive and invasive pulmonary function in mouse models of obstructive and restrictive respiratory diseases. Am J Respir Cell Mol Biol 42: 96–104, 2010. doi: 10.1165/rcmb.2008-0487OC. [DOI] [PubMed] [Google Scholar]
- 64.Wens SC, Kuperus E, Mattace-Raso FU, Kruijshaar ME, Brusse E, van Montfort KC, de Boer MS, Sijbrands EJ, van der Ploeg AT, van Doorn PA. Increased aortic stiffness and blood pressure in non-classic Pompe disease. J Inherit Metab Dis 37: 391–397, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Winkel LP, Hagemans ML, van Doorn PA, Loonen MC, Hop WJ, Reuser AJ, van der Ploeg AT. The natural course of non-classic Pompe’s disease; a review of 225 published cases. J Neurol 252: 875–884, 2005. doi: 10.1007/s00415-005-0922-9. [DOI] [PubMed] [Google Scholar]
- 66.Yang CF, Niu DM, Jeng MJ, Lee YS, Taso PC, Soong WJ. Late-onset Pompe disease with left-sided bronchomalacia. Respir Care 60: e26–e29, 2015. doi: 10.4187/respcare.03419. [DOI] [PubMed] [Google Scholar]
- 67.Zhang CH, Li Y, Zhao W, Lifshitz LM, Li H, Harfe BD, Zhu MS, ZhuGe R. The transmembrane protein 16A Ca(2+)-activated Cl- channel in airway smooth muscle contributes to airway hyperresponsiveness. Am J Respir Crit Care Med 187: 374–381, 2013. doi: 10.1164/rccm.201207-1303OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang CH, Lifshitz LM, Uy KF, Ikebe M, Fogarty KE, ZhuGe R. The cellular and molecular basis of bitter tastant-induced bronchodilation. PLoS Biol 11: e1001501, 2013. doi: 10.1371/journal.pbio.1001501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.ZhuGe R, Fogarty KE, Tuft RA, Lifshitz LM, Sayar K, Walsh JV Jr. Dynamics of signaling between Ca(2+) sparks and Ca(2+)- activated K(+) channels studied with a novel image-based method for direct intracellular measurement of ryanodine receptor Ca(2+) current. J Gen Physiol 116: 845–864, 2000. doi: 10.1085/jgp.116.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]