Abstract
Myofibroblasts are important mediators of fibrogenesis; thus blocking fibroblast-to-myofibroblast differentiation (FMD) may be an effective strategy to treat pulmonary fibrosis (PF). Previously, we reported that histone deacetylase 4 (HDAC4) activity is necessary for transforming growth factor-β1 (TGF-β1)-induced human lung FMD. Here, we show that TGF-β1 increases NADPH oxidase 4 (NOX4) mRNA and protein expression in normal human lung fibroblasts (NHLFs) and causes nuclear export of HDAC4. Application of the NOX family inhibitor diphenyleneiodonium chloride reduces TGF-β1-induced HDAC4 nuclear export, expression of the myofibroblast marker α-smooth muscle actin (α-SMA), and α-SMA fiber formation. Inhibition of HDAC4 nucleus-to-cytoplasm translocation using leptomycin B (LMB) had little effect on α-SMA expression but blocked α-SMA fiber formation. A coimmunoprecipitation assay showed that HDAC4 associates with α-SMA. Moreover, LMB abolishes TGF-β1-induced α-SMA fiber formation and cell contraction. Relevant to human pulmonary fibrosis, idiopathic PF specimens showed significantly higher NOX4 RNA expression and scant HDAC4 staining within nuclei of fibroblast foci myofibroblasts. Taken together, these results indicate that reactive oxygen species promote TGF-β1-mediated myofibroblast differentiation and HDAC4 nuclear export. The physical association of HDAC4 with α-SMA suggests that HDAC4 has a role in regulating the α-SMA cytoskeleton arrangement.
Keywords: myofibroblasts, HDAC4, reactive oxygen species, NOX4, α-smooth muscle actin, IPF
idiopathic pulmonary fibrosis (IPF) is a progressive lung disorder of unknown etiology. The myofibroblast, characterized by the expression of α-smooth muscle actin (α-SMA) and increased contractile ability, is a key effecter for fibrogenesis. Understanding the molecular mechanisms involved with fibroblast-to-myofibroblast differentiation (FMD) may provide possible targets for clinical treatment of pulmonary fibrosis.
Our previous work demonstrated that histone deacetylase 4 (HDAC4) activity is required for transforming growth factor-β1 (TGF-β1)-induced lung FMD (12). HDAC4 is a member of class IIa HDACs and possesses a long NH2-terminal regulatory region that harbors binding regions for myocyte enhancer factor-2 (MEF2), 14-3-3, calcium-responsive phosphorylation sites, and a nuclear localization signal (35). Its COOH terminus contains a deacetylase domain and a nuclear export signal. The phosphorylation of serines in its NH2 terminus results in the binding of protein 14-3-3 that cooperates with chromosome region maintenance 1 (CRM1)/exportin 1 to redistribute HDAC4 to the cytoplasm (36).
Independent of phosphorylation, reduction and oxidation (REDOX) has been recently uncovered as a novel regulatory mechanism for subcellular distribution of HDAC4 (1). In cardiomyocytes, HDAC4 undergoes oxidation at cysteines 667 and 669, permitting the formation of intramolecular disulfide bonds in response to reactive oxygen species (ROS), which results in the nuclear export of HDAC4, followed by MEF2-mediated cardiac hypertrophy. Importantly, REDOX modifications can potentially override phosphorylation in regard to HDAC4 localization because thioredoxin-1 (Trx1), which interferes with HDAC4 oxidation and the formation of intramolecular disulfide bonds, results in attenuation of nuclear export of phosphorylation-mimicking mutations in HDAC4. Moreover, a recent report and our studies demonstrate that HDAC4 is required for TGF-β1-induced myofibroblast differentiation by skin and lung fibroblasts, respectively (9, 12). Understanding how TGF-β1 regulates HDAC4 during FMD requires further characterization.
The NADPH oxidase (NOX) family has seven members that are involved in differentiation, among which NOX4 expression was reported in human lung fibroblasts (7, 30). NOX enzymes function in transferring electrons from NADPH to oxygen to produce superoxide. Unlike other NOXs, the activity of NOX4 does not require p47phox, NOXO1, or p67phox, although NOX4 does require p22phox (24). NOX4 is constitutively active and prefers NADPH over NADH as the electron donor (29). Importantly, antioxidants such as glutathione abrogate TGF-β1-induced collagen accumulation in NIH/3T3 cells (6, 22). Pertinent to lung fibrogenesis, TGF-β1-induced NOX4 expression is SMAD3 dependent, and NOX4 is overexpressed in the fibroblast foci of IPF specimens (14).
NOX4 is required for TGF-β1-induced differentiation of human cardiac fibroblasts into myofibroblasts (6), and TGF-β1 stimulates ROS production by activating NOX enzymes in human lung fibroblasts (31). The above findings are relevant because increased oxidative stress has been detected in IPF patients (4, 5, 18, 20, 25, 33). Here, we confirm that TGF-β1 promotes NOX4-derived ROS production and additionally show that TGF-β1 causes HDAC4 translocation to the cytoplasm through a mechanism that requires the generation of ROS. In addition, we present new evidence that HDAC4 associates with α-SMA and modulates fiber formation.
MATERIALS AND METHODS
Cell culture.
Normal human lung fibroblasts (NHLFs) were purchased from Lonza (Basel, Switzerland) and cultured in full growth medium FGM-2. Subculture was conducted per the manufacturer’s protocol.
Immunoblot analysis.
HDAC4 antibodies were purchased from Abcam (Cambridge, MA) and Sigma-Aldrich (St. Louis, MO). Anti-α-SMA was obtained from Sigma-Aldrich. The NOX4 antibody was kindly provided by Dr. John R. Hoidal (University of Utah). β-Actin and α-tubulin antibodies were purchased from Cell Signaling Technology (Danvers, MA).
Equal quantities of protein (20 μg) were separated using 4–12% SDS-PAGE gels (Invitrogen, Waltham, MA) and then transferred to PVDF membranes (Invitrogen) using a standard protocol. Immunoblots were developed by using either the ECL Plus kit (GE Healthcare Life Sciences, Pittsburgh, PA) or a fluorescent-labeled secondary antibody.
Nuclear, cytosolic, and membrane fractionation.
Nuclear, cytosolic, and membrane fractions were separated from equal number of cells using the Qproteome Cell Compartment Kit (Qiagen, Hilden, Germany). The fractions were redissolved in one-third of the volume of 4× SDS buffer. An equal volume for each sample was subjected to SDS-PAGE and immunoblotted as described above.
Analysis of gene expression using quantitative real-time RT-PCR.
Total RNA was extracted from IPF and control lung biopsy specimens using the RNeasy Plus Mini Kit (Qiagen) per the manufacturer’s protocol. One microgram of RNA was used for reverse transcription using the iScript kit (Qiagen) according to the manufacturer’s instructions. Quantitative real-time PCR was employed to measure RNA expression using iQ SYBR Green Supermix (Bio-Rad, Hercules, CA). NOX4 primers were purchased from Qiagen. RNA quantification was normalized to the housekeeping genes 36B4 or 18S.
Transient transfection with siRNA.
Small interfering NOX4 RNA (NOX4 siRNA) was synthesized by Integrated DNA Technologies (Coralville, IA). The NOX4 siRNA used in this study has been described by Dikalov et al. (8). The sequences used for the sense and antisense were 5′-ACUGAGGUACAGCUGGAUGUU-3′ and 5′-CAUCCAGCUGUACCUCAGUUU-3′, respectively. siRNAs were transiently transfected into NHLFs by electroporation as described by Guo et al. (12), and the knockdown effect was confirmed by RT-PCR.
Immunofluorescence.
Immunofluorescence was conducted as previously described (12). Briefly, NHLFs were seeded into 8-well slides at 2 × 104 cells per well. When the wells were 80% confluent, cells were serum-starved for 16 h and then treated with TGF-β1 with or without TSA, diphenyleneiodonium chloride (DPI), or leptomycin B (LMB). Cells were fixed in 4% paraformaldehyde and stained using the specified primary antibodies.
Detection of ROS.
NHLFs were seeded into 6-well plates at 2 × 105 cells per well. The cells were serum-starved for 16 h when they were ~80% confluent and then treated with various reagents as described in the figure legends. The 2′,7′-dichlorofluorescein diacetate (DCFH-DA) was purchased from Sigma-Aldrich and is a cell membrane-penetrable chemical that changes to a fluorescent compound in response to ROS. One milliliter of 30 μM DCFH-DA in HBSS with Ca2+ and Mg2+ was added to each well at the end of each treatment period and then incubated at 37°C for 30 min. Cells were washed twice with HBSS with Ca2+ and Mg2+ and then lysed in 500 μl of 1 N NaOH on a shaker for 5 min. One hundred microliters was used to read the fluorescent signal using a FLUOstar plate reader. When determining the effects of DPI on ROS level, NHLFs were exposed to DPI for 1 h before TGF-β1 treatment.
BIAM labeling of HDAC4.
Biotinylated iodoacetamide (BIAM) labeling of HDAC4 to probe disulfide bonds in HDAC4 protein was performed as described by Ying et al. (37). Briefly, cell lysate protein concentration was adjusted to contain equal amounts of HDAC4 (“inputs”). After labeling with BIAM at pH 6.5, lysates were incubated with streptavidin-Sepharose beads overnight. After release from beads with Laemmli buffer containing 5 mol/l urea and 2% SDS, protein was separated by SDS-PAGE and blotted for HDAC4 with anti-HDAC4 antibody.
Type I collagen gel contraction assay.
The contraction assay was done as previously described. Twelve-well cell culture plates were precoated with 5% BSA-PBS coating solution overnight. On the next day, rat tail type I collagen (BD Biosciences, Bedford, MA) was prepared and mixed with NHLFs according to the manufacturer’s instructions. Briefly, NHLFs in fibroblast basal medium (FBM-2) with 0.2% BSA were added to type I collagen at a final concentration of 2 × 105 cells per milliliter. FBM-2 with 1% BSA and 1 N NaOH was added to the collagen mixture to make a final concentration of 0.2% BSA and 2 mg/ml collagen. The 5% BSA-PBS coating solution was aspirated, and plates were washed twice with PBS. Eight hundred microliters of the cell-gel mixture was added to each well, and the plates were kept in a 37°C incubator for 30 min before treatment with TGF-β1, DPI, and LMB. Gel contraction was assessed as the ratio of the gel surface area to the area of the well.
HDAC4 immunohistochemistry.
The Dako LSAB2 K0673 kit (Agilent Technologies, Santa Clara, CA) was used following the manufacturer’s protocol. Anti-HDAC4 (Sigma-Aldrich) antibody was diluted in TBS with 5% goat serum at a 1:100 dilution, and 100 μl of diluted antibody was applied to each sample.
Statistics.
Graphs demonstrate the mean and standard error derived from at least two independent experiments performed with triplicates. The Student’s t-test was used throughout to test for statistical significance.
RESULTS
TGF-β1 stimulates HDAC4 nucleus-to-cytoplasm translocation.
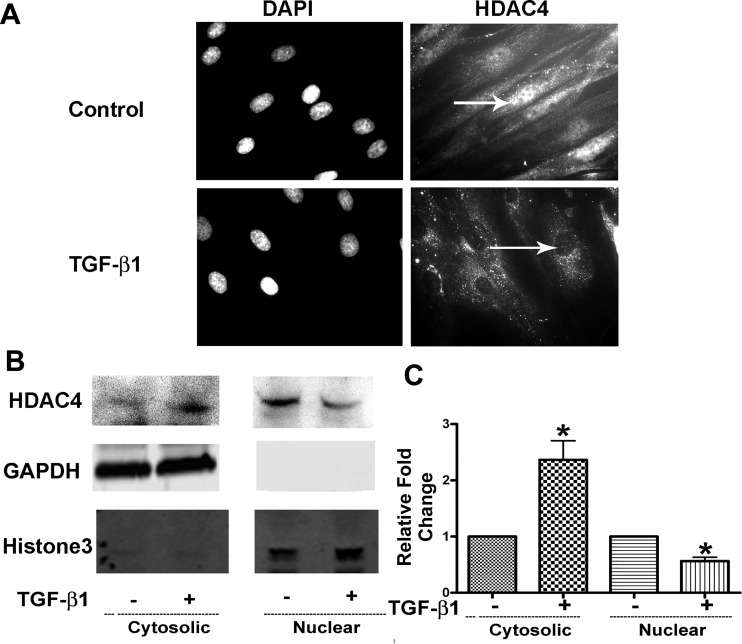
HDAC4 plays a critical role in lung and dermal fibroblast-myofibroblast differentiation (FMD). Nucleus-to-cytoplasm shuttling is a feature that differentiates class I from class IIa HDACs. To comprehend further the effect of TGF-β1 on HDAC4 during TGF-β1-mediated FMD, we treated NHLFs with TGF-β1 for 12 h and evaluated the location of HDAC4 in NHLFs using immunostaining. HDAC4 localized to both the cytoplasm and nucleus in untreated NHLFs, but the nucleus demonstrated stronger HDAC4 staining compared with the cytoplasm. In contrast, TGF-β1-treated cells showed negligible staining for HDAC4 within nuclei (Fig. 1A).
Fig. 1.
TGF-β1 promotes nuclear export of HDAC4. NHLFs were treated with TGF-β1 (1 ng/ml) for 12 h. A: immunostaining of HDAC4. Arrows indicate nuclei. DAPI, 4′,6′-diamidino-2-phenylindole. B: HDAC4 subcellular distribution in cytosolic and nuclear fractions was analyzed using immunoblot. C: densitometry measurements of triplicates for B. *P < 0.05 vs. control.
To confirm the HDAC4 immunostaining results, the distribution of HDAC4 within nuclear and cytoplasmic compartments was assessed in protein fractions using immunoblot. Results indicated that untreated NHLFs had stronger HDAC4 expression within nuclei and weaker HDAC4 signal in the cytoplasmic fraction when compared with TGF-β1-treated NHLFs (Fig. 1, B and C), which is consistent with the immunostaining results. Taken together, the results confirmed HDAC4 nuclear export during TGF-β1-induced FMD.
ROS inhibition blocks FMD.
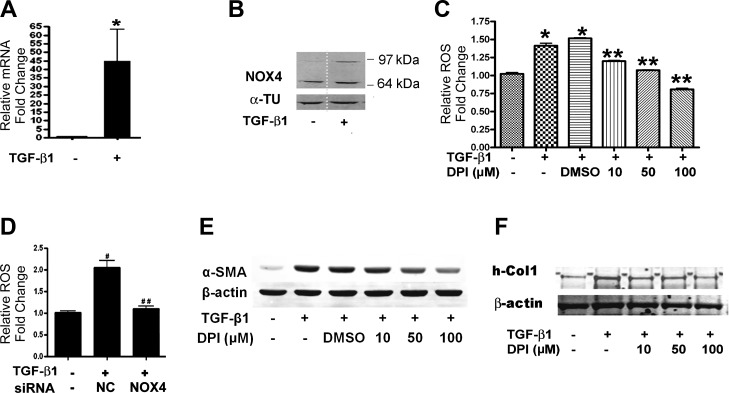
TGF-β1 is a potent fibrogenic factor that stimulates FMD characterized by increased α-SMA expression and contractile ability (12). NOX4 plays an important role in promoting cardiac fibroblast-to-myofibroblast differentiation (6). We observed that some cells started to show myofibroblast morphology as early as 12 h after TGF-β1 treatment, so we analyzed NOX4 mRNA and protein expression in response to TGF-β1 at 9 and 12 h, respectively. Results showed that NOX4 expression is ~45-fold higher than control after 9 h of TGF-β1 treatment (Fig. 2A). All NOX family members are transmembrane proteins (2), so membrane fractions were isolated and used to test NOX4 protein expression using immunoblot. The predicted band size for NOX4 is 65 kDa, but it also has been shown there is a larger NOX4 band (15), which is considered to be a posttranslational modification of NOX4 (2, 30). Results revealed increased expression of the large band of NOX4 in TGF-β1-treated NHLFs (Fig. 2B). TGF-β1 increased ROS production in NHLFs by ~50% (Fig. 2C). Importantly, cotreatment with the NOX family-specific inhibitor DPI reduced ROS production and diminished expression of the myofibroblast marker α-SMA in a dose-dependent manner (Fig. 2, C and E). NOX4 knockdown using siRNA reduced ROS production to the level of untreated control cells (Fig. 2D). Furthermore, pretreatment with the ROS scavenger DPI repressed TGF-β1-induced collagen expression (Fig. 2F). Taken together, these results indicate that TGF-β1-induced ROS production is NOX4-dependent and contributes to FMD.
Fig. 2.
TGF-β1 stimulates NOX4-derived ROS to augment α-SMA expression in NHLFs. A: NOX4 expression was analyzed using quantitative real-time PCR 9 h after TGF-β1 treatment. B: NOX4 expression in the membrane fraction was analyzed using immunoblot. α-TU, α-tubulin. C: ROS were measured using the DCFH-DA method after TGF-β1 treatment for 12 h with or without DPI (*P < 0.05 vs. control, **P < 0.05 vs. TGF-β1 + DMSO). D: NHLFs were transfected with NOX4 siRNA or nontarget control siRNA (NC) for 48 h and then treated with TGF-β1 for 12 h. ROS were measured using the DCFH-DA method (#P < 0.05 vs. control, ##P < 0.05 vs. NC + DMSO). E: NHLFs were pretreated with DPI for 1 h followed by treatment with TGF-β1 for 12 h. α-SMA expression was assessed using immunoblot. F: immunoblot for type I human collagen 1 (h-Col1). Immunoblot results represent at least 2 independent assays. RT-PCR represents 3 independent assays.
NOX inhibition abrogates TGF-β1-mediated HDAC4 nuclear export.
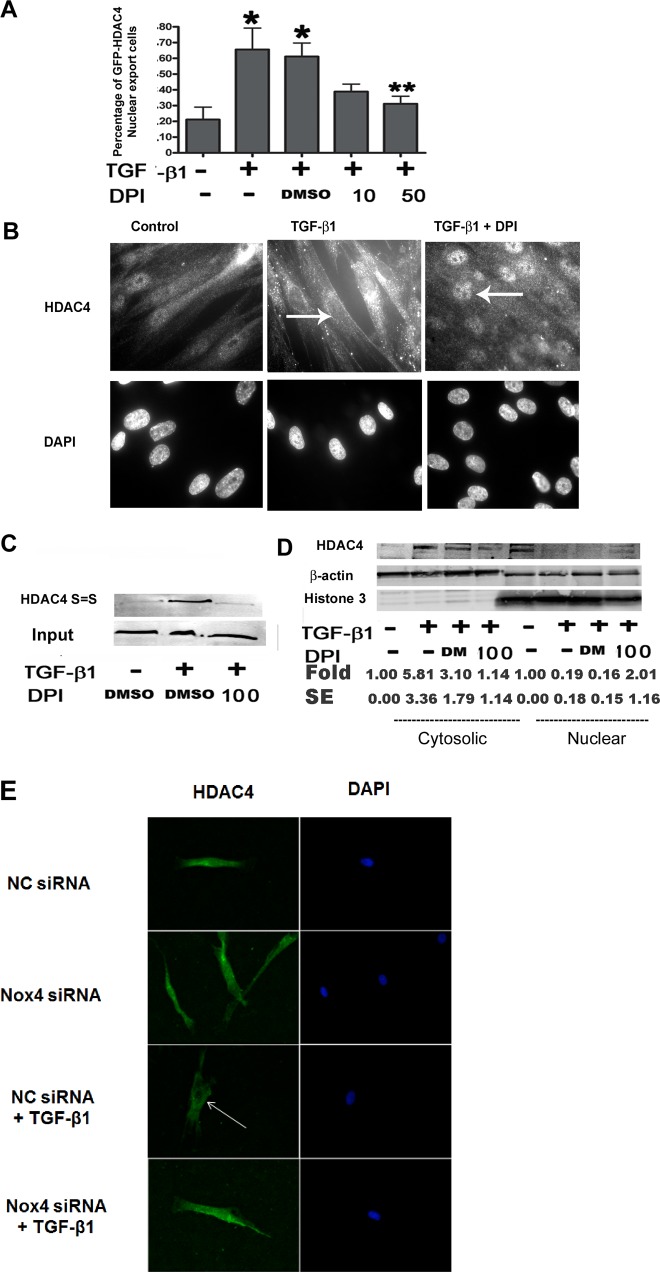
ROS have been implicated in enhancing HDAC4 nucleus-to-cytoplasm translocation by promoting cysteine 667 (Cys-667) and Cys-669 disulfide bond formation (1). To test whether ROS have a causal role in HDAC4 nucleus-to-cytoplasm translocation in NHLFs, we assessed whether an ROS scavenger could inhibit HDAC4 translocation. The pcDNA3-HDAC4-GFP plasmid was transferred into NHLFs by electroporation. After serum starvation, NHLFs were stimulated with TGF-β1 alone or combined with DPI for 12 h. Results showed that ~80% of NHLFs displayed nuclear HDAC4-GFP localization in the control group but in only 34% of cells when treated with TGF-β1 alone and 39% when TGF-β1 was administered with DMSO. Treatment with the ROS scavenger DPI at 50 μM significantly enhanced the HDAC4-GFP nuclear localization when compared with TGF-β1 with DMSO group (69 vs. 39%, respectively; P = 0.039; Fig. 3A). Changes in endogenous HDAC4 localization were also assessed using immunostaining. Results showed that there was an absence of HDAC4 staining signal in nuclei of TGF-β1-treated cells, however, a significant increase in the HDAC4 nuclear signal was detected when TGF-β1-treated cells were cotreated with DPI (Fig. 3B). The immunostaining results were further confirmed via immunoblot of cytosolic and nuclear fractions (Fig. 3D).
Fig. 3.
Inhibition of ROS reduces HDAC4 nuclear export. A: NHLFs were transfected with GFP-HDAC4-expressing plasmids using electroporation and then treated with TGF-β1 ± DPI for 24 h. Green fluorescent protein (GFP)-positive cells were counted for each treatment. The results represent the percentage of cells lacking nuclear GFP (*P < 0.05 vs. control, **P < 0.05 vs. TGF-β1 + DMSO). B: immunostaining of endogenous HDAC4 in NHLFs. NHLFs were treated with DPI for 2 h before TGF-β1 treatment for 12 h. C: immunoblot analysis of HDAC4 disulfide bond formation. The upper blot, “HDAC4 S=S,” represents BIAM HDAC4 (BIAM-labeled HDAC4 disulfide bonds). The lower blot, “Input,” indicates total HDAC4. D: immunoblot analysis showing HDAC4 distribution within cytoplasm and nuclear compartments. E: immunostaining of HDAC4 localization. NHLFs were pretreated with nontargeting control (NC) siRNA or NOX4 siRNA for 48 h and then treated with or without TGF-β1 for 24 h. Results represent at least 2 independent assays. Arrows indicate nuclei. DM, DMSO.
In addition, we observed increased intramolecular HDAC4 disulfide bond formation after TGF-β1 treatment. In contrast, cotreatment with TGF-β1 and DPI reduced the appearance of the HDAC4 disulfide bond (Fig. 3C).
Furthermore, NOX4 knockdown using siRNA prevented TGF-β1-induced nuclear export of HDAC4 (Fig. 3E). Taken together, we demonstrated that NOX4-dependent ROS production is required for HDAC4 nuclear export during TGF-β1-induced FMD.
HDAC4 interacts physically with α-SMA.
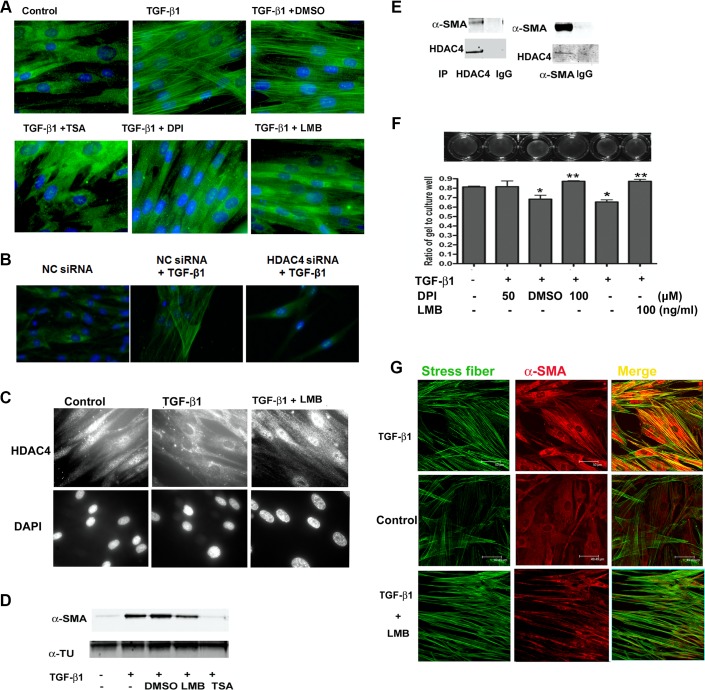
The binding of CRM1 is critical for HDAC4 nuclear exportation in both phosphorylation- and REDOX-mediated nuclear exporting models (26, 36). The nonselective HDAC inhibitor trichostatin A (TSA) and the ROS scavenger DPI inhibit α-SMA expression at the protein level (Fig. 2 and Ref. 12), and keeping with this observation, both DPI and TSA abolished TGF-β1-induced α-SMA fiber formation (Fig. 4A). HDAC4 knockdown using siRNA also repressed the TGF-β1-induced α-SMA fiber formation (Fig. 4B). Pretreatment of NHLFs with the CRM1-specific inhibitor leptomycin B (LMB) blocked TGF-β1-induced HDAC4 nuclear export (Fig. 4C) and the formation of α-SMA fibers (Fig. 4A). However, LMB had little effect on TGF-β1-induced α-SMA expression at the protein level (Fig. 4D). These results suggest that HDAC4 contributes to α-SMA cytoskeleton arrangement not only in regulating its expression at the transcriptional level, but also in fiber assembly after translation.
Fig. 4.
HDAC4 associates with α-SMA, and inhibition of ROS reduces myofibroblast contractility. A: immunostaining of α-SMA fibers. NHLFs were pretreated with DMSO, TSA, or LMB for 2 h and then treated with TGF-β1 for 24 h. B: immunostaining of α-SMA fibers. NHLFs were transfected with HDAC4 siRNA or nontarget control siRNA (NC) for 48 h and then treated with TGF-β1 for 24 h. C: immunostaining of endogenous HDAC4 localization. NHLFs were pretreated with LMB for 2 h and then treated with TGF-β1 for 12 h. D: immunoblot analysis of α-SMA protein expression in NHLFs treated with TGF-β1, with or without LMB or TSA. E: coimmunoprecipitation (IP) of endogenous HDAC4 with α-SMA. NHLFs were stimulated with TGF-β1 for 24 h. F: NHLF collagen gel contraction assay. The ratio of the area of gel to culture well was used to express the contractile ability (*P < 0.05 vs. control, **P < 0.05 vs. DMSO). G: colocalization of stress fibers (phalloidin staining) and α-SMA. Results represent at least 2 independent assays.
To determine the potential connection between HDAC4 and α-SMA cytoskeleton assembly, we investigated whether HDAC4 physically interacts with α-SMA. Endogenous HDAC4 and α-SMA were immunoprecipitated with specific antibodies after TGF-β1 treatment for 24 h. Immunoblot analysis of the immunoprecipitates revealed that HDAC4 associates with α-SMA (Fig. 4E).
To test the functional consequence of blocking HDAC4 nuclear export, we used a collagen gel contraction assay. TGF-β1 treatment enhanced the contractile ability of NHLFs as shown by increased collagen gel contraction, whereas the addition of either LMB or DPI reduced the contractile ability of the cells to the control level (Fig. 4F).
α-SMA increases rat myofibroblast contraction via recruitment into β-cytoplasmic actin stress fibers (10, 16). As expected, immunostaining for α-SMA showed that TGF-β1 not only enhances α-SMA protein expression, but also promotes its recruitment into stress fibers. In contrast, the nuclear export inhibitor LMB does not affect β-cytoplasmic actin stress fibers but does block α-SMA recruitment into stress fibers (Fig. 4G). Taken together, these results indicate that HDAC4 export from nuclear to cytoplasm during FMD contributes to stress fiber assembly and myofibroblast contraction.
NOX4 and HDAC4 expression in IPF lung biopsy specimens.
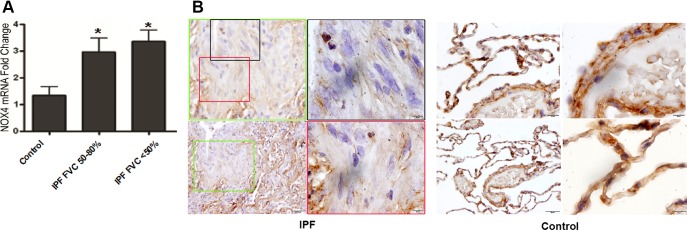
To determine whether NOX4 may contribute to the increased ROS production observed in IPF lung, total RNA was isolated from IPF lung biopsy specimens, and quantitative RT-PCR was used to measure the NOX4 mRNA expression. Results showed that NOX4 mRNA expression was significantly higher in IPF lung biopsy specimens than that of the control group (Fig. 5A).
Fig. 5.
Expressions of NOX4 and HDAC4 in IPF lung biopsy specimens. A: total RNA was isolated from lung biopsy specimens of IPF patients [n = 10; 3 patients with forced vital capacity (FVC) of 50–80% predicted and 7 patients with FVC of <50% predicted] or controls [very mild chronic obstructive pulmonary disease; COPD; Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage 1; n = 11]. NOX 4 mRNA expression was measured using real-time PCR and normalized to 18S. *P < 0.05 vs. control. B: immunohistochemistry localizing HDAC4 in IPF lung biopsy specimens. Scale bars are for the panoramic view 100 μm (bottom left), middle magnification 20 μm (top left), and high magnification 10 μm (top right and bottom right).
We also assessed HDAC4 localization in myofibroblasts within fibrotic foci. IPF and control lung biopsy specimens were stained for HDAC4 using immunohistochemistry. Results showed that HDAC4 majorly localized in nuclei of alveolar and airway epithelial cells in IPF lung biopsy specimens, but there was a paucity of nuclear HDAC4 staining within the myofibroblasts in the fibrotic foci (Fig. 5B). Taken together, these results support changes in subcellular distribution of HDAC4 concomitant with increase in NOX4 expression as a feature of fibrotic foci in IPF lungs.
DISCUSSION
Although extensive studies have been published, the molecular mechanisms involved in FMD are still not completely understood. In this study, we confirmed that TGF-β1 stimulates NOX4-related ROS production in NHLFs. The novel findings are as follows: 1) TGF-β1 induces HDAC4 nucleus-to-cytoplasm translocation; and 2) blocking ROS production using DPI inhibits endogenous and exotically expressed HDAC4 nuclear export, diminishes α-SMA expression, and suppresses type I collagen expression. We also demonstrated HDAC4 associates with α-SMA and that this feature is important for fiber formation and cell contraction. IPF lung biopsy specimens had threefold higher NOX4 expression compared with controls, and elongated nuclei in fibrotic foci demonstrate an absence of HDAC4 staining, lending some clinical relevance to our in vitro observations.
HDAC4 belongs to the class-IIa family of histone deacetylases that can be shuttled between the nucleus and cytoplasm. HDAC4 manifests distinct distribution in cytoplasm or nucleus depending on cell type, the stage of cell development, and even physiological condition. The phosphorylation of serines in the NH2 terminus of HDAC4 in the nucleus by calcium/calmodulin-dependent protein kinase results in the binding of the chaperone protein 14-3-3, which cooperates with CRM1/exportin 1 to redistribute HDAC4 to the cytoplasm (21, 36). However, extracellular signal-regulated kinases 1 and 2 (ERK1 and -2)-induced HDAC4 phosphorylation promotes its nuclear localization in NIH/3T3 fibroblasts and C2C12 myoblasts (39). We found that HDAC4 was located in both the cytoplasm and nuclei of lung fibroblasts, but the major expression resided within the nucleus under normal conditions. After TGF-β1 stimulation, the majority of HDAC4 was found in the cytoplasm (Fig. 1). The change in HDAC4 localization could result from either inhibition of nuclear import or by promoting nuclear export. The blocking of CRM1 using LMB increased HDAC4 nuclear distribution (Fig. 4C), which confirms that TGF-β1 modulates HDAC4 nuclear export.
NOX4 is a member of the NADPH oxidase family of enzymes that catalyze the reduction of O2 to ROS. NOX4-derived ROS are required for TGF-β1-induced human fetal lung mesenchymal cells to differentiate into myofibroblasts (14). Here, we demonstrate that NOX4-derived ROS are also necessary for TGF-β1-mediated lung FMD. The inhibition of ROS by DPI ameliorated FMD (Fig. 2). Consistent with the in vitro studies, we also showed that IPF lung biopsy specimens have threefold higher NOX4 RNA expression (Fig. 5A). Genetic or pharmacological targeting of NOX4 abrogates fibrogenesis in bleomycin and fluorescein isothiocyanate mouse lung injury models (14). Our results are in line with other experimental reports suggesting the potential of ROS inhibitors as a promising therapeutic strategy against pulmonary fibrosis.
NOX4 resides within nuclei and focal adhesions in vascular smooth muscle cells, and overexpression of GFP-labeled NOX4 colocalizes with the endoplasmic reticulum marker in human umbilical vein endothelial cells (15, 32). The predicted band size for NOX4 is 65 kDa, but it also has been shown there is a larger NOX4 band (15). The larger band is considered to be a posttranslational modification of NOX4 but is not N-glycosylation because of the lack of sensitivity to N-glycosidase F, which would not exclude the possibilities of other kinds glycosylation (2, 30). Our antibody picks up both the large and small NOX4 bands, but only the larger band appeared in TGF-β1-treated samples (Fig. 2B). These results suggested that TGF-β1 not only stimulates NOX4 RNA and protein expression, but also may activate a posttranslational modification pathway. The functional difference between the two NOX4 bands requires further investigation.
The NOX inhibitor DPI did not fully prevent the expression of α-SMA and HDAC4 nuclear export in response to TGF-β1, which could reflect that ROS act in parallel with other pathways to control HDAC4 localization and FMD. There are several binding sites for SMAD proteins on the α-SMA promoter, and Akt phosphorylation is required for the expression of α-SMA (12). HDAC4 nuclear export is regulated by phosphorylation and oxidative stress as well (21, 36, 39). Nuclear export of HDAC4 may also be a response to changes of the physiological environment or intracellular physiology because we observed HDAC4 nuclear export when NHLFs were overtrypsinized (data not shown).
Recent studies showed that HDACs exert much broader biological activities than the exclusive control of gene transcription. For example, HDAC activities are involved in regulating cell contractile ability. HDAC8 associates with the α-SMA cytoskeleton to regulate the capacity of smooth muscle cells to contract (34). HDAC4 binds to cardiac sarcomas to regulate myofilament contractile activity by deacetylating MLP, a sensor of cardiac mechanical stretch (13). Here, we show that TGF-β1 stimulates HDAC4 nucleus-to-cytoplasm translocation during FMD and associates with α-SMA (Figs. 1, 3B, and 4C). Consistent with the study in cardiac cells, inhibiting HDAC4 nuclear export using LMB blocks TGF-β1-induced contractile ability (Fig. 4C). HDAC4 exportation from the nucleus is also required for the transformation of vascular smooth muscle cells from quiescent to activated (11). Thus HDAC4 has cytoplasmic activity in addition to its coregulatory role involving other HDACs in the nucleus. In contrast to class I HDACs, the transcriptional corepressor function of HDAC4 is linked to a weak but measurable intrinsic capability of hydrolyzing acetyllysines in vitro. Most of the enzymatic activity associated with HDAC4 ectopically expressed in mammalian cells is due to endogenous class I HDACs presented in class IIa immunocomplexes, and the catalytic domain of HDAC4 is required for association with class I HDACs (3). These unique features of HDAC4 impose obstacles to identify and characterize biological substrates of HDAC4.
The intramolecular disulfide bond formation between Cys-667 and Cys-669 in HDAC4 favors its nuclear exportation (1). Cotreatment of TGF-β1 with the ROS scavenger DPI not only ameliorated intracellular ROS to control levels (Fig. 2C), but also inhibited HDAC4-GFP and endogenous nuclear exportation (Fig. 3, A and B). In line with this result, IL-1β stimulates HDAC4 nuclear exportation and increases NOX-derived ROS in human tracheal smooth muscle cells, which supports a relationship between HDAC4 nuclear exportation and increased levels of ROS (23). DPI treatment decreases α-SMA expression (Fig. 2E), which is consistent with cardiac fibroblasts where DPI has been shown to inhibit myofibroblast differentiation (6). Moreover, lung and dermal fibroblasts lacking HDAC4 lose their ability to differentiate into myofibroblasts in response to TGF-β1 (12). In contrast to the consensus of the function of nuclear HDAC4 as a transcriptional corepressor, our results support that HDAC4 has at least one substrate in the cytoplasm that is important for FMD.
It is noteworthy that HDAC4 coimmunoprecipitates with α-SMA and HDAC4 exhibits prominent cytoplasmic localization after TGF-β1 stimulation. However, unlike α-SMA, HDAC4 immunostaining does not appear to localize to fibers within the cytoplasm (Figs. 1 and 3), and the cytoskeleton cell fraction failed to show HDAC4 expression by immunoblot (data not shown). α-SMA can be acetylated at one of its lysine residues (38), which suggests that α-SMA can be recognized and deacetylated by HDACs; however, the functional significance of this has not been explored. Our findings suggest that HDAC4 may play a role for α-SMA cytoskeleton assembly.
Disulfide bond formation is a critical protein posttranslational modification with the capacity to alter protein structure and function. Endoplasmic reticulum and mitochondria intermembrane spaces are favorable environments for disulfide bond-catalyzing enzymes in eukaryotes (28). It remains unanswered how HDAC4 forms disulfide bonds within nuclei where disulfide-catalyzing enzymes have not been found. However, H2O2-mediated disulfide bond formation in the absence of enzymes in vitro has also been reported (27), which supports the possibility that increased ROS are enough to promote HDAC4 disulfide bond formation.
Our data afford a novel understanding of how ROS affect HDAC4 localization and subsequent FMD and provide a mechanism addressing how ROS inhibitors may modulate lung fibrogenesis. This study also extends recent studies showing that HDAC4 is dysregulated in IPF and is likely to contribute to its pathogenesis (17, 19). Further studies to investigate the effects of modulating HDAC4 expression in pulmonary fibrosis models are ongoing in our laboratory.
GRANTS
This study was supported by the National Heart, Lung, and Blood Institute Grants R01-HL-083901/083480 (to J. A. Lasky) and the Wetmore Foundation Grant 553860G1 (to J. A. Lasky). This work was also supported in part by 1-U54-GM-104940 (to S. Saito) from the National Institute of General Medical Sciences, which funds the Louisiana Clinical & Translational Science Center.
DISCLAIMERS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the authors.
AUTHOR CONTRIBUTIONS
W.G., S.S., and J.A.L. conceived and designed research; W.G., S.S., C.G.S., Y.Z., R.E.G.R., and F.L. performed experiments; W.G., S.S., C.G.S., Y.Z., B.S., and F.L. analyzed data; W.G., S.S., C.G.S., Y.Z., B.S., F.L., and J.A.L. interpreted results of experiments; W.G., S.S., and R.E.G.R. prepared figures; W.G., S.S., and J.A.L. drafted manuscript; W.G., S.S., C.G.S., and J.A.L. edited and revised manuscript; W.G., S.S., C.G.S., Y.Z., R.E.G.R., B.S., F.L., and J.A.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Tony Kouzarides (University of Cambridge) for providing the pcDNA3-HDAC4-GFP plasmid. We also thank Dr. John R. Hoidal (University of Utah) for generously providing the NOX4 antibody.
REFERENCES
- 1.Ago T, Liu T, Zhai P, Chen W, Li H, Molkentin JD, Vatner SF, Sadoshima J. A redox-dependent pathway for regulating class II HDACs and cardiac hypertrophy. Cell 133: 978–993, 2008. doi: 10.1016/j.cell.2008.04.041. [DOI] [PubMed] [Google Scholar]
- 2.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87: 245–313, 2007. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 3.Bottomley MJ, Lo Surdo P, Di Giovine P, Cirillo A, Scarpelli R, Ferrigno F, Jones P, Neddermann P, De Francesco R, Steinkühler C, Gallinari P, Carfí A. Structural and functional analysis of the human HDAC4 catalytic domain reveals a regulatory structural zinc-binding domain. J Biol Chem 283: 26694–26704, 2008. doi: 10.1074/jbc.M803514200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cantin AM, Hubbard RC, Crystal RG. Glutathione deficiency in the epithelial lining fluid of the lower respiratory tract in idiopathic pulmonary fibrosis. Am Rev Respir Dis 139: 370–372, 1989. doi: 10.1164/ajrccm/139.2.370. [DOI] [PubMed] [Google Scholar]
- 5.Cantin AM, North SL, Fells GA, Hubbard RC, Crystal RG. Oxidant-mediated epithelial cell injury in idiopathic pulmonary fibrosis. J Clin Invest 79: 1665–1673, 1987. doi: 10.1172/JCI113005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cucoranu I, Clempus R, Dikalova A, Phelan PJ, Ariyan S, Dikalov S, Sorescu D. NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ Res 97: 900–907, 2005. doi: 10.1161/01.RES.0000187457.24338.3D. [DOI] [PubMed] [Google Scholar]
- 7.Dhaunsi GS, Paintlia MK, Kaur J, Turner RB. NADPH oxidase in human lung fibroblasts. J Biomed Sci 11: 617–622, 2004. doi: 10.1007/BF02256127. [DOI] [PubMed] [Google Scholar]
- 8.Dikalov SI, Dikalova AE, Bikineyeva AT, Schmidt HH, Harrison DG, Griendling KK. Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free Radic Biol Med 45: 1340–1351, 2008. doi: 10.1016/j.freeradbiomed.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glenisson W, Castronovo V, Waltregny D. Histone deacetylase 4 is required for TGFbeta1-induced myofibroblastic differentiation. Biochim Biophys Acta 1773: 1572–1582, 2007. doi: 10.1016/j.bbamcr.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 10.Goffin JM, Pittet P, Csucs G, Lussi JW, Meister JJ, Hinz B. Focal adhesion size controls tension-dependent recruitment of alpha-smooth muscle actin to stress fibers. J Cell Biol 172: 259–268, 2006. doi: 10.1083/jcb.200506179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gordon JW, Pagiatakis C, Salma J, Du M, Andreucci JJ, Zhao J, Hou G, Perry RL, Dan Q, Courtman D, Bendeck MP, McDermott JC. Protein kinase A-regulated assembly of a MEF2·HDAC4 repressor complex controls c-Jun expression in vascular smooth muscle cells. J Biol Chem 284: 19027–19042, 2009. doi: 10.1074/jbc.M109.000539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo W, Shan B, Klingsberg RC, Qin X, Lasky JA. Abrogation of TGF-beta1-induced fibroblast-myofibroblast differentiation by histone deacetylase inhibition. Am J Physiol Lung Cell Mol Physiol 297: L864–L870, 2009. doi: 10.1152/ajplung.00128.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta MP, Samant SA, Smith SH, Shroff SG. HDAC4 and PCAF bind to cardiac sarcomeres and play a role in regulating myofilament contractile activity. J Biol Chem 283: 10135–10146, 2008. doi: 10.1074/jbc.M710277200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hecker L, Vittal R, Jones T, Jagirdar R, Luckhardt TR, Horowitz JC, Pennathur S, Martinez FJ, Thannickal VJ. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med 15: 1077–1081, 2009. doi: 10.1038/nm.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hilenski LL, Clempus RE, Quinn MT, Lambeth JD, Griendling KK. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 24: 677–683, 2004. doi: 10.1161/01.ATV.0000112024.13727.2c. [DOI] [PubMed] [Google Scholar]
- 16.Hinz B, Dugina V, Ballestrem C, Wehrle-Haller B, Chaponnier C. Alpha-smooth muscle actin is crucial for focal adhesion maturation in myofibroblasts. Mol Biol Cell 14: 2508–2519, 2003. doi: 10.1091/mbc.E02-11-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khalil W, Xia H, Bodempudi V, Kahm J, Hergert P, Smith K, Peterson M, Parker M, Herrera J, Bitterman PB, Henke CA. Pathologic regulation of collagen I by an aberrant protein phosphatase 2A/histone deacetylase C4/microRNA-29 signal axis in idiopathic pulmonary fibrosis fibroblasts. Am J Respir Cell Mol Biol 53: 391–399, 2015. doi: 10.1165/rcmb.2014-0150OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kinnula VL, Fattman CL, Tan RJ, Oury TD. Oxidative stress in pulmonary fibrosis: a possible role for redox modulatory therapy. Am J Respir Crit Care Med 172: 417–422, 2005. doi: 10.1164/rccm.200501-017PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korfei M, Skwarna S, Henneke I, MacKenzie B, Klymenko O, Saito S, Ruppert C, von der Beck D, Mahavadi P, Klepetko W, Bellusci S, Crestani B, Pullamsetti SS, Fink L, Seeger W, Krämer OH, Guenther A. Aberrant expression and activity of histone deacetylases in sporadic idiopathic pulmonary fibrosis. Thorax 70: 1022–1032, 2015. doi: 10.1136/thoraxjnl-2014-206411. [DOI] [PubMed] [Google Scholar]
- 20.Kuwano K, Nakashima N, Inoshima I, Hagimoto N, Fujita M, Yoshimi M, Maeyama T, Hamada N, Watanabe K, Hara N. Oxidative stress in lung epithelial cells from patients with idiopathic interstitial pneumonias. Eur Respir J 21: 232–240, 2003. doi: 10.1183/09031936.03.00063203. [DOI] [PubMed] [Google Scholar]
- 21.Little GH, Bai Y, Williams T, Poizat C. Nuclear calcium/calmodulin-dependent protein kinase IIdelta preferentially transmits signals to histone deacetylase 4 in cardiac cells. J Biol Chem 282: 7219–7231, 2007. doi: 10.1074/jbc.M604281200. [DOI] [PubMed] [Google Scholar]
- 22.Liu RM, Liu Y, Forman HJ, Olman M, Tarpey MM. Glutathione regulates transforming growth factor-beta-stimulated collagen production in fibroblasts. Am J Physiol Lung Cell Mol Physiol 286: L121–L128, 2003. doi: 10.1152/ajplung.00231.2003. [DOI] [PubMed] [Google Scholar]
- 23.Luo SF, Chang CC, Lee IT, Lee CW, Lin WN, Lin CC, Yang CM. Activation of ROS/NF-kappaB and Ca2+/CaM kinase II are necessary for VCAM-1 induction in IL-1beta-treated human tracheal smooth muscle cells. Toxicol Appl Pharmacol 237: 8–21, 2009. doi: 10.1016/j.taap.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 24.Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal 18: 69–82, 2006. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 25.Montuschi P, Toni GC, Paredi P, Pantelidis P, du Bois RM, Kharitonov SA, Barnes PJ. 8-Isoprostane as a biomarker of oxidative stress in interstitial lung diseases. Am J Respir Crit Care Med 158: 1524–1527, 1998. doi: 10.1164/ajrccm.158.5.9803102. [DOI] [PubMed] [Google Scholar]
- 26.Oka S, Ago T, Kitazono T, Zablocki D, Sadoshima J. The role of redox modulation of class II histone deacetylases in mediating pathological cardiac hypertrophy. J Mol Med (Berl) 87: 785–791, 2009. doi: 10.1007/s00109-009-0471-2. [DOI] [PubMed] [Google Scholar]
- 27.Paoli P, Giannoni E, Pescitelli R, Camici G, Manao G, Ramponi G. Hydrogen peroxide triggers the formation of a disulfide dimer of muscle acylphosphatase and modifies some functional properties of the enzyme. J Biol Chem 276: 41862–41869, 2001. doi: 10.1074/jbc.M106886200. [DOI] [PubMed] [Google Scholar]
- 28.Riemer J, Bulleid N, Herrmann JM. Disulfide formation in the ER and mitochondria: two solutions to a common process. Science 324: 1284–1287, 2009. doi: 10.1126/science.1170653. [DOI] [PubMed] [Google Scholar]
- 29.Serrander L, Cartier L, Bedard K, Banfi B, Lardy B, Plastre O, Sienkiewicz A, Fórró L, Schlegel W, Krause KH. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem J 406: 105–114, 2007. doi: 10.1042/BJ20061903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shiose A, Kuroda J, Tsuruya K, Hirai M, Hirakata H, Naito S, Hattori M, Sakaki Y, Sumimoto H. A novel superoxide-producing NAD(P)H oxidase in kidney. J Biol Chem 276: 1417–1423, 2001. doi: 10.1074/jbc.M007597200. [DOI] [PubMed] [Google Scholar]
- 31.Thannickal VJ, Fanburg BL. Activation of an H2O2-generating NADH oxidase in human lung fibroblasts by transforming growth factor beta 1. J Biol Chem 270: 30334–30338, 1995. doi: 10.1074/jbc.270.51.30334. [DOI] [PubMed] [Google Scholar]
- 32.Van Buul JD, Fernandez-Borja M, Anthony EC, Hordijk PL. Expression and localization of NOX2 and NOX4 in primary human endothelial cells. Antioxid Redox Signal 7: 308–317, 2005. doi: 10.1089/ars.2005.7.308. [DOI] [PubMed] [Google Scholar]
- 33.Waghray M, Cui Z, Horowitz JC, Subramanian IM, Martinez FJ, Toews GB, Thannickal VJ. Hydrogen peroxide is a diffusible paracrine signal for the induction of epithelial cell death by activated myofibroblasts. FASEB J 19: 854–856, 2005. doi: 10.1096/fj.04-2882fje. [DOI] [PubMed] [Google Scholar]
- 34.Waltregny D, Glénisson W, Tran SL, North BJ, Verdin E, Colige A, Castronovo V. Histone deacetylase HDAC8 associates with smooth muscle alpha-actin and is essential for smooth muscle cell contractility. FASEB J 19: 966–968, 2005. doi: 10.1096/fj.04-2303fje. [DOI] [PubMed] [Google Scholar]
- 35.Wang AH, Grégoire S, Zika E, Xiao L, Li CS, Li H, Wright KL, Ting JP, Yang XJ. Identification of the ankyrin repeat proteins ANKRA and RFXANK as novel partners of class IIa histone deacetylases. J Biol Chem 280: 29117–29127, 2005. doi: 10.1074/jbc.M500295200. [DOI] [PubMed] [Google Scholar]
- 36.Wang AH, Yang XJ. Histone deacetylase 4 possesses intrinsic nuclear import and export signals. Mol Cell Biol 21: 5992–6005, 2001. doi: 10.1128/MCB.21.17.5992-6005.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ying J, Tong X, Pimentel DR, Weisbrod RM, Trucillo MP, Adachi T, Cohen RA. Cysteine-674 of the sarco/endoplasmic reticulum calcium ATPase is required for the inhibition of cell migration by nitric oxide. Arterioscler Thromb Vasc Biol 27: 783–790, 2007. doi: 10.1161/01.ATV.0000258413.72747.23. [DOI] [PubMed] [Google Scholar]
- 38.Zhao S, Xu W, Jiang W, Yu W, Lin Y, Zhang T, Yao J, Zhou L, Zeng Y, Li H, Li Y, Shi J, An W, Hancock SM, He F, Qin L, Chin J, Yang P, Chen X, Lei Q, Xiong Y, Guan KL. Regulation of cellular metabolism by protein lysine acetylation. Science 327: 1000–1004, 2010. doi: 10.1126/science.1179689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou X, Richon VM, Wang AH, Yang XJ, Rifkind RA, Marks PA. Histone deacetylase 4 associates with extracellular signal-regulated kinases 1 and 2, and its cellular localization is regulated by oncogenic Ras. Proc Natl Acad Sci USA 97: 14329–14333, 2000. doi: 10.1073/pnas.250494697. [DOI] [PMC free article] [PubMed] [Google Scholar]