Abstract
Asthma is a common disorder characterized, in part, by airway smooth muscle (ASM) hyperresponsiveness. Transient receptor potential vanilloid 1 (TRPV1) is a nonselective cation channel expressed on airway nerve fibers that modulates afferent signals, resulting in cough, and potentially bronchoconstriction. In the present study, the TRPV1 transcript was detected by RT-PCR in primary cultured human ASM cells, and the TRPV1 protein was detected in ASM of human trachea by immunohistochemistry. Proximity ligation assays suggest that TRPV1 is expressed in the sarcoplasmic reticulum membrane of human ASM cells in close association with sarco/endoplasmic reticulum Ca2+-ATPase-2. In guinea pig tracheal ring organ bath experiments, the TRPV1 agonist capsaicin led to ASM contraction, but this contraction was significantly attenuated by the sodium channel inhibitor bupivacaine (n = 4, P < 0.05) and the neurokinin-2 receptor antagonist GR-159897 (n = 4, P < 0.05), suggesting that this contraction is neutrally mediated. However, pretreatment of guinea pig and human ASM in organ bath experiments with the TRPV1 antagonist capsazepine inhibited the maintenance phase of an acetylcholine-induced contraction (n = 4, P < 0.01 for both species). Similarly, capsazepine inhibited methacholine-induced contraction of peripheral airways in mouse precision-cut lung slice (PCLS) experiments (n = 4–5, P < 0.05). Although capsazepine did not inhibit store-operated calcium entry in mouse ASM cells in PCLS (n = 4–7, P = nonsignificant), it did inhibit calcium oscillations (n = 3, P < 0.001). These studies suggest that TRPV1 is expressed on ASM, including the SR, but that ASM TRPV1 activation does not play a significant role in initiation of ASM contraction. However, capsazepine does inhibit maintenance of contraction, likely by inhibiting calcium oscillations.
Keywords: capsazepine, asthma, organ bath, precision-cut lung slice, calcium oscillations
reactive airway diseases, such as asthma, remain a significant clinical challenge worldwide. Airway smooth muscle (ASM) hyperresponsiveness, hypertrophy, and hyperplasia are all known to contribute to asthma pathophysiology (9), and all of these processes are modulated by intracellular calcium concentrations (22).
The transient receptor potential (TRP) family of channels is one of several groups of Ca2+ permeant channels that contributes to intracellular Ca2+ regulation in a variety of cells types, including ASM (8, 15, 16, 21). While there is an increasing understanding of the roles that TRP channels play in airway physiological and pathophysiological processes, much is still unclear. For example, it is known that TRP vanilloid 1 [TRPV1; formerly vanilloid receptor 1 (VR1)], a channel that has been extensively studied in nociceptive neurons and is the receptor for the hot chili pepper component capsaicin (4, 20), is expressed on the plasma membrane of airway nerve fibers and mediates cough and neurogenic bronchoconstriction (16, 20). In fact, a clinical study showed that a loss-of-function variant of TRPV1, resulting from a single nucleotide polymorphism, was associated with lower rates of wheezing and cough (3). However, there has been little investigation into the potential expression of TRPV1 directly on human ASM and what role these channels may play in physiological and pathophysiological processes of asthma and airway hyperresponsiveness. Notably, a previous study demonstrated that capsazepine, a TRPV1 antagonist, relaxed peripheral human airways contracted with a variety of agents in organ bath experiments. However, the mechanism of this relaxation is unclear, and other TRPV1 antagonists failed to mimic this relaxation effect of capsazepine (28).
In addition to being a potential source of Ca2+ flux that potentiates ASM contraction, TRPV1 channels on ASM cells may play other important roles in asthma. Both the TRPV1 agonist capsaicin and the antagonist capsazepine have been shown to modulate store-operated calcium entry (SOCE) in PC12 cells (6). In ASM cells, SOCE is required for the maintenance of sufficient intracellular calcium to sustain calcium oscillations (5) and contraction (13, 17, 31). Therefore, modulation of this process may be of therapeutic benefit in asthma. The aim of this study was to determine if TRPV1 is expressed in ASM and, if confirmed, describe the role that TRPV1 ligands play in modulating ASM tone. Finally, experiments were conducted to determine the role for TRPV1 antagonist, capsazepine, on calcium oscillations and SOCE, two critical mechanisms of ASM contraction.
MATERIALS AND METHODS
Reagents.
Capsaicin, capsazepine, tetrodotoxin, SKF96365, and GR159897 were obtained from Tocris Bioscience (Bristol, UK). Bupivacaine was obtained from Hospira (Lake Forest, IL). GSK 7975A was obtained from Aobious (Gloucester, MA). Oregon Green 488 BAPTA-1 AM was obtained from Life Technologies (Grand Island, NY). Ryanodine was obtained from Abcam (Cambridge, MA). All other reagents were obtained from Sigma-Aldrich (St. Louis, MO).
Human tracheal tissue and primary ASM cell culture.
De-identified healthy human tracheal samples were obtained from discarded trimmings of lung transplantation donor organs at the time of surgery at Columbia University Medical Center. This tissue was deemed to be nonhuman research material by the Columbia University Medical Center Institutional Review Board. Primary human ASM cells were prepared from these samples as described previously (14, 33) and grown at 37°C and 5% CO2 in DMEM/F-12 media (Life Technologies) with 10% fetal bovine serum and 1× antibiotics/antimycotics. All studies were conducted on cells of fewer than four passages.
RT-PCR.
Total RNA was isolated from cultured primary human ASM cells, immortalized human ASM cells (gift of Dr. William Gerthoffer, University of South Alabama), and intact human tracheal ASM tissue using Trizol Regent (Life Technologies). cDNA was subsequently synthesized using SuperScript VILO cDNA synthesis reagents (Life Technologies). PCR was then performed on cDNA samples to assess for the presence of the TRPV1 transcript using primers designed to flank large genomic introns to distinguish mRNA from genomic-derived PCR products (forward primer: ACCCTGAGCTTCTCCCTGCGGTCAA; reverse primer: CAGAGACCCTGAAAACTGTCGCAGATAAACTT). RNA from whole human brain served as a positive control, and a sample devoid of input cDNA (water blanks) served as a negative control.
Human airway immunohistochemistry.
Human tracheal samples were fixed in 4% paraformaldehyde (4°C overnight) and then incubated in 30% sucrose in phosphate-buffered saline (PBS) for an additional 24 h before cryostat sectioning (6 μm). The sections were washed in PBS, incubated with 0.1% Triton X-100 for 10 min, blocked with 15% goat serum, and then incubated overnight at 4°C in primary antisera. The primary antibody used was anti-TRPV1 (anti-VR1, rabbit, polyclonal, 1:800 dilution in PBS, Abcam, Cambridge, UK; ab74855). The validity of this antibody was previously established using TRPV1 transgenic mice (35). The secondary antibodies consisted of FITC-conjugated goat anti-rabbit IgG (1:400 dilution; Life Technologies). Rhodamine phalloidin conjugate (1:40; Life Technologies) was used to stain the filamentous actin of ASM. Nuclear staining was achieved by using mounting medium premixed with DAPI (Vector, Burlingame, CA). Negative controls were performed on serial sections by omitting primary antibody. Samples were viewed under confocal microscopy (Nikon Eclipse), and images were acquired with NIS software version 4.10.
To determine if TRPV1 is expressed on the sarcoplasmic reticulum (SR) of human ASM cells, a proximity ligation assay (Duolink, Invitrogen) was performed to determine whether TRPV1 is present in close proximity (within 40 nm) of the sarcoplasmic/endoplasmic reticulum calcium-ATPase-2 (SERCA2, an SR marker). Briefly, primary human ASM cells were cultured on eight-well chamber slides, fixed with 4% paraformaldehyde for 5 min, and blocked for 60 min with 10% goat serum. The slides were then incubated with primary antibodies overnight at 4°C (rabbit anti-human TRPV1, 1:800, and mouse anti-human SERCA2, 1:200, in PBS, Abcam, Cambridge, UK; ab2861). The validity of the SERCA2 antibody has been previously established in SERCA2 transgenic mice (27). The proximity ligation assay was then performed as per instructions using anti-rabbit and anti-mouse probes. Negative controls were completed by omitting each primary antibody individually and together, and all studies were done in triplicate (3 separate human ASM cell donors).
Guinea pig tracheal rings experiments.
Guinea pig animal protocols were approved by the Columbia University Animal Care and Use Committee. Male Hartley guinea pigs (∼400 g) were anesthetized with intraperitoneal pentobarbital (100 mg/kg). Guinea pig closed tracheal rings consisting of two cartilaginous rings were isolated, denuded of epithelium, suspended in water-jacketed (37°C) organ baths (Radnoti Glass Technology, Monrovia, CA), and attached to a FT-03 force transducer (Grass Telefactor, West Warwick, RI), which was coupled to a computer via BioPac hardware and Acqknowledge 7.3.3 software (Biopac Systems, Goleta, CA). The baths contained Krebs-Henseleit (KH) buffer (in mM: NaCl 118, KCl 5.6, CaCl2 0.5, MgSO4 0.24, NaH2PO4 1.3, NaHCO3 25, glucose 5.6, indomethacin 0.01 to inhibit prostaglandin synthesis, pH 7.4) and were bubbled with 95% oxygen and 5% carbon dioxide. Tissues were allowed to equilibrate at 1 g isotonic force for 1 h with fresh KH buffer changes every 15 min before experiments.
Tracheal rings were then subjected to two cycles of increasing cumulative concentrations of acetylcholine (0.1 μM to 0.1 mM) to determine the EC50 concentrations of acetylcholine for each individual ring. To avoid bias between treatment groups, tissue with similar acetylcholine EC50 values were randomly assigned to treatments within individual experiments. Following six KH buffer changes, tissues were allowed to stabilize at isotonic resting tension (∼1.0 g).
In one set of experiments, tracheal rings where then exposed to either inhibitors of nerve conduction (tetrodotoxin 1 µM or bupivacaine 200 µM), a neurokinin 2 (NK-2) receptor inhibitor (GR 159798; 20 µM), or vehicle for 15 min, then contracted with 10 µM capsaicin. Peak contraction force (as a fraction of peak 0.1 mM acetylcholine-induced force accessed before capsaicin-induced contraction) was measured and compared between groups.
In a separate set of experiments, tracheal rings were treated with 10 µM capsaicin or vehicle, and then all rings were treated 70 min later with a subsequent 10 µM capsaicin exposure to determine whether capsaicin could induce a repeated contraction.
Finally, in a third set of experiments, guinea pig tracheal rings were pretreated with 100 µM capsazepine vs. vehicle for 15 min and contracted with an EC50 concentration of acetylcholine. Contraction force 15 min later, as a percentage of peak contraction force, was compared between groups. The buffers for these set of experiments contained 1 µM tetrodotoxin and 10 µM pyrilamine to inhibit potential nerve- and histamine-mediated contributions to the contraction.
Human tracheal ASM strip experiments.
Smooth muscle tissue obtained from human trachea samples was carefully dissected into strips, denuded of epithelium, and suspended in organ baths as described for guinea pig experiments, with the exception that the resting tension was increased to 1.5 g. The strips were then subjected to three cycles of increasing cumulative concentrations of acetylcholine to determine the EC50 concentrations of acetylcholine required for each individual strip. The strips were then treated with 100 µM capsazepine vs. vehicle for 15 min (tissues with similar acetylcholine EC50 values were randomly assigned to treatments within individual experiments) and contracted with an EC50 concentration of acetylcholine. Contraction force at 60 min (as a fraction of force at 10 min) was compared between the groups to determine whether capsazepine inhibited the maintenance of human ASM contraction.
Mouse precision-cut lung slice experiments.
All mouse experiments were approved by the University of Massachusetts Medical College Animal Care and Use Committee. Animals were randomly assigned to treatment groups. Hanks’ balanced salt solution (HBSS) was supplemented with 20 mM HEPES buffer (sHBSS) and adjusted to pH 7.4. Hanks’ zero-calcium solution (0-Ca2+ sHBSS) was prepared by supplementing HBSS without Ca2+ with 20 mM HEPES, 0.9 mM MgSO4, and 1 mM Na2H2-EGTA. A detailed precision-cut lung slice (PCLS) protocol has been published previously (5, 25). Briefly, female BALB/c mice (8–12 wk old) were euthanized, their thoracic cavities opened, and their lungs inflated with warm 1.8% agarose. After allowing the agarose to cool, the lungs were removed and sectioned into ~180-µm thick slices with a vibratome (VF-300; Precisionary Instruments, Greenville, NC). All experiments were performed at 37°C with constant perfusion using a custom-made, temperature-controlled microscope enclosure, as described previously (1). To measure airway contraction, PCLS were placed on a cover-glass that was mounted in a custom-made Plexiglas support and held down by a 200-µm nylon mesh with a hole aligned over a selected airway. A perfusion chamber was created by placing another smaller cover-glass on the top of the nylon mesh and sealing the edges with silicone grease. An inverted microscope (Diaphot; Nikon, Tokyo, Japan; or IX71; Olympus, Tokyo, Japan) with a ×10 objective was used to monitor airway area changes in response to superfused compounds. Phase-contrast images were collected at a rate of one image per 2 s with a CCD camera, which was controlled by custom-programmed software based on Video Savant 4 (IO Industries, Montreal, Canada). The change in lumen area of a selected airway was analyzed using ImageJ (National Institutes of Health, Bethesda, MD), as described previously (32). To summarize experimental compound-induced relaxation (%), we initially normalized the contractile state of an airway (contracted with 400 nM methacholine, measured at the mid-time point at 30 min) to its own initial contraction (measured at 15 min) by the formula: %relaxation (R) = (area30 min − area15 min)/(100 – area15 min). We then normalized the compound-induced relaxation to the amount of relaxation that occurred in the control response by the formula: %normalized relaxation = (Rcompound – Rcontrol)/(100 – Rcontrol).
To monitor ASM intracellular calcium concentrations, PCLS were loaded with sHBSS containing 20 µM Oregon Green 488 BAPTA-1-AM, 0.1% Pluronic F-127 and 200 µM sulfobromophthalein in the dark at 30°C for 1 h, followed by sHBSS containing 200 µM sulfobromophthalein for an additional 30 min at room temperature to allow the deesterification of Oregon Green. The intracellular calcium-mediated fluorescence of ASM cells in PCLS was recorded with a custom-built, video-rate scanning, two-photon laser microscope (1, 25). Fluorescent images were recorded at a rate of 15 images per second using custom-written image acquisition software based on Video Savant. The fluorescence intensity in a region of interest (8 × 10 pixels) within an ASM cell, with respect to time, was determined using custom-written software. Relative fluorescence intensity was expressed as Ft/F0: ratio of the fluorescence intensity at a particular time (Ft) normalized to the initial time (F0).
To quantify SOCE in PCLS, a previously validated “SR-permeabilization” protocol was utilized (1). Briefly, Oregon Green-loaded PCLS were simultaneously exposed to 20 mM caffeine and 25 µM ryanodine for 5 min, followed by a thorough washout with sHBSS before the experiments. This treatment gates ryanodine receptors of the SR in the open state, and thereby induces the depletion of the SR calcium store. This, in turn, leads to a persistent calcium influx via SOCE to elevate the intracellular calcium concentration. After experimental compound exposure in the presence of 0-Ca2+ sHBSS, the effect of these compounds on SOCE is quantified by measuring the degree of increase in calcium-mediated fluorescence on reexposure of PCLS to 1.3 mM extracellular Ca2+ concentration, presented as a percentage of baseline fluorescence in 1.3 mM extracellular Ca2+ concentration) by the formula: %Ca2+ increase (CI) = [(AIin presence of drug (19-21 min) − AIzero calcium (14-17 min))/(AIsHBSS (8-10 min) – AIzero calcium (14-17 min))], where AI is average intensity.
Statistical analysis.
Each experimental permutation included intraexperimental controls. All experiments in which quantitative analyses were performed were repeated in at least triplicate. Where appropriate, two-tailed Student’s t-tests or one-way ANOVA with Bonferroni posttest comparisons were employed using Prism 4.0 software (GraphPad, San Diego, CA). Data are presented as means ± SE; P < 0.05 was considered significant. For organ bath experiment sample size calculations, changes in contractile force of 30% or more were considered biologically significant, and a standard deviation of 15% was assumed based on extensive previous experiments in this model. For PCLS experiments, changes in airway area or calcium dynamics of 30% were considered biologically significant, and a standard deviation of 10% was assumed based on previous studies by our laboratory with these preparations (power of 0.80 and α = 0.05).
RESULTS
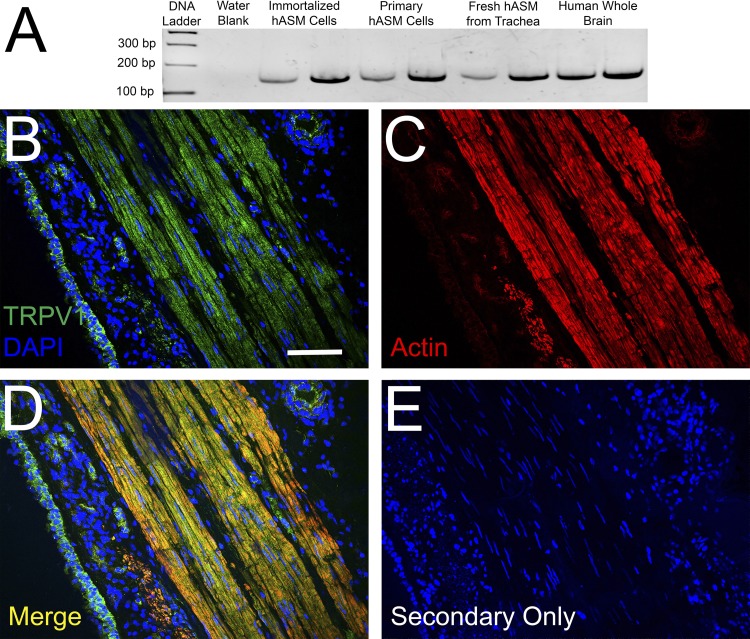
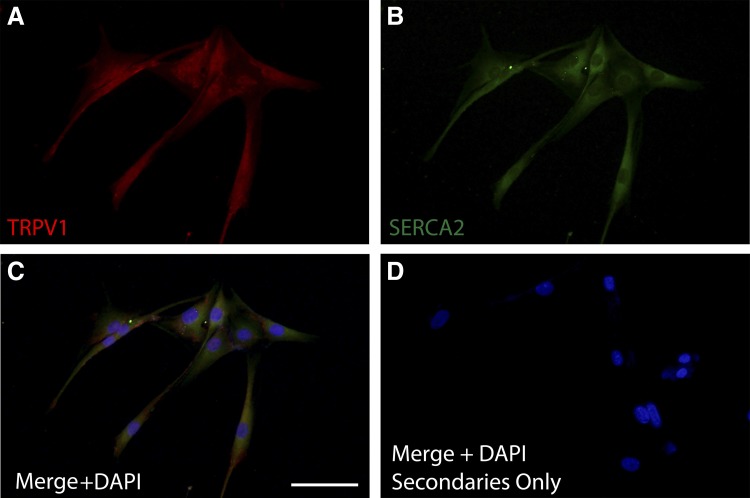
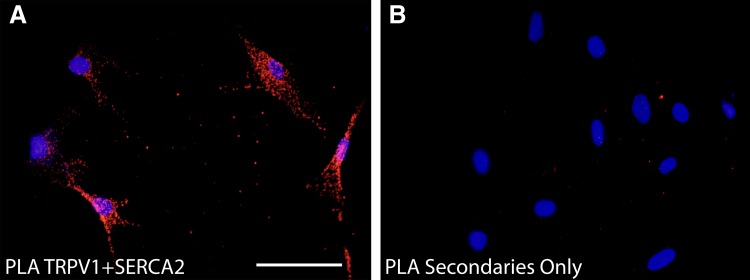
RT-PCR analyses identified the TRPV1 transcript in immortalized human ASM cells, primary human ASM cells, and fresh ASM tissue obtained from human trachea (Fig. 1A). Furthermore, TRPV1 protein was evident on immunohistochemical analysis of human trachea (Fig. 1B) and was colocalized with actin (Fig. 1C; merged image in Fig. 1D), suggesting expression in ASM. Immunocytochemical analysis demonstrates TRPV1 expression in primary human ASM cells (Fig. 2A), along with SERCA2 (Fig. 2B), a SR membrane-associated protein. To gain additional evidence for the expression of TRPV1 at the SR membrane, we performed a proximity ligation assay with the SR-specific protein SERCA2. This assay revealed positive red fluorescent punctate staining, demonstrating that the TRPV1 and SERCA2 proteins are within 40 nm of each other (Fig. 3A), suggesting TRPV1 is also expressed in the SR membrane. Negative controls performed with both primary antibodies omitted (Fig. 3B) or each primary antibody individually omitted (data not shown) demonstrated an absence of a red fluorescent signal.
Fig. 1.
Detection of transient receptor potential channel V1 (TRPV1) mRNA and protein in human ASM (hASM). A: RT-PCR analysis of TRPV1 expression in multiple preparations of hASM cells and tissue. RT-PCR sample devoid of cDNA (water blank) served as negative control, and whole human brain served as positive control. B: immunohistochemical analysis of TRPV1 in human tracheal airway tissue with DAPI nuclear staining. C: actin. D: merging of TRPV1 and actin. E: staining control. Bar: 35 µm.
Fig. 2.
Primary human ASM cell representative immunocytochemistry demonstrating TRPV1 colocalization with SERCA2. TRPV1 (red; A) and SERCA2 (green, sarcoplasmic reticulum marker; B) are detected in human ASM cells. C: the merged image (×20) demonstrates TRPV1 and SERCA2 expression in the perinuclear region, showing TRPV1 expression on sarcoplasmic reticulum. D: staining control. Bar: 100 µm; n = 3.
Fig. 3.
Proximity ligation assay (PLA) demonstrating TRPV1 and SERCA2 colocalization in primary human ASM cells. A: positive (red dots) PLA (PLA; Duolink, Invitrogen) in primary human ASM cells performed using primary antibodies against TRPV1 and SERCA2. This demonstrates that the proteins exist within 40 nm of each other, suggesting that TRPV1 expression is in the SR membrane. B: negative control with primary antibodies omitted. Bar: 100 µm; n = 3.
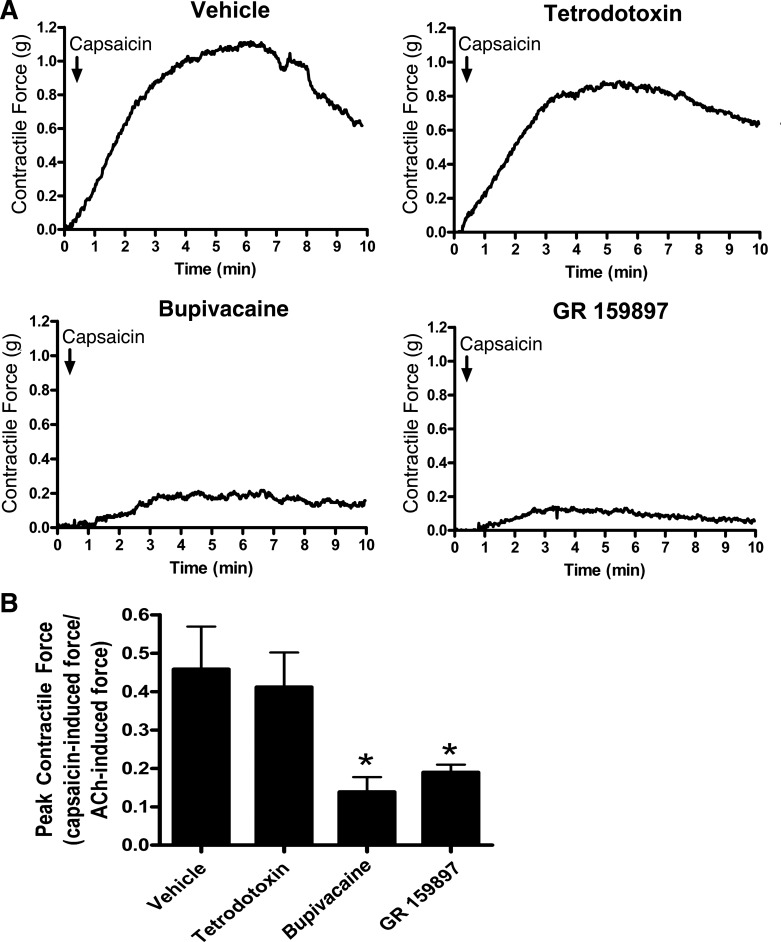
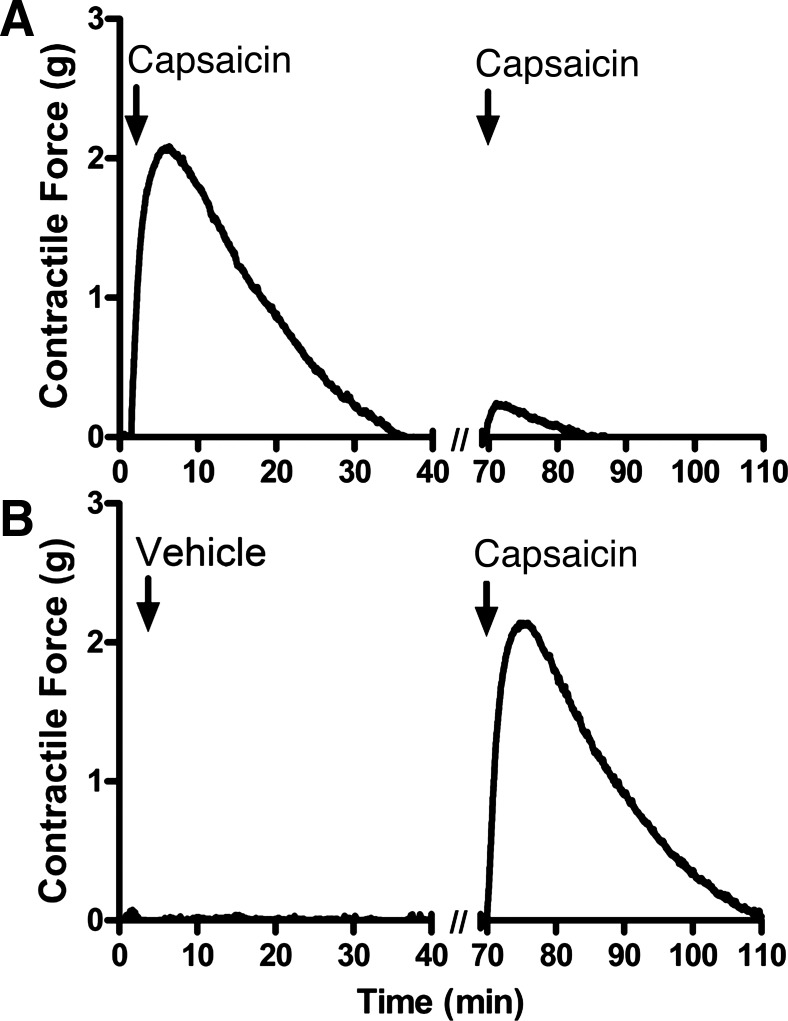
The TRPV1 agonist capsaicin induced contraction of guinea pig tracheal rings (Fig. 4A demonstrates representative organ bath contractile force tracings; Fig. 4B demonstrates quantification of these contractile forces). However, this contraction was significantly inhibited by the Na+-channel inhibitor bupivacaine and the NK-2 receptor antagonist GR-159897. This finding suggests that capsaicin-mediated contraction is likely neutrally mediated, potentially via release of tachykinins from endogenous C-fiber excitatory nonadrenergic, noncholinergic (NANC) nerves in the airway (Fig. 4B; n = 4, P < 0.05 for both compounds by ANOVA with Bonferroni post hoc correction) (30). In contrast, tetrodotoxin, another Na+-channel inhibitor, did not significantly inhibit capsaicin-induced tracheal ring contraction (n = 4, P = nonsignificant), consistent with a previous study demonstrating that capsaicin-sensitive airway nerve fibers have a significantly higher percentage of tetrodotoxin-insensitive Na+-channel fibers compared with airway nerve fibers that are unresponsive to capsaicin (19). Furthermore, repeated exposure of tracheal rings to capsaicin exhibited tachyphylaxis (Fig. 5A) compared with untreated rings serving as time controls (Fig. 5B), suggesting a depletion of neurotransmitters from NANC nerves (11).
Fig. 4.
Capsaicin-mediated contraction of guinea pig tracheal rings. A: representative tracings of 10 µM capsaicin-induced contraction of tracheal rings in the presence of 1 µM tetrodotoxin, 100 µM Na+-channel inhibitor bupivacaine, 20 µM NK-2 receptor antagonist GR-159897, or vehicle. B: quantification of capsaicin-induced contraction force as a fraction of 100 µM acetylcholine-induced force. Pretreatment with bupivacaine and GR-159897 significantly inhibited contraction force. Values are means ± SE; n = 4 for all groups. *P < 0.05 compared with untreated controls by ANOVA with Bonferroni post hoc correction.
Fig. 5.
Contraction force tracings of guinea pig tracheal rings with repeated exposure to capsaicin. A: contraction force tracing of tracheal ring receiving two successive exposures to 10 µM capsaicin. B: contraction force tracing of tracheal ring receiving vehicle followed by 10 µM capsaicin. A second exposure to capsaicin shows tachyphylaxis compared with the first, suggesting a “depletable” mechanism of contraction consistent with the activation of nonadrenergic, noncholinergic (NANC) nerves.
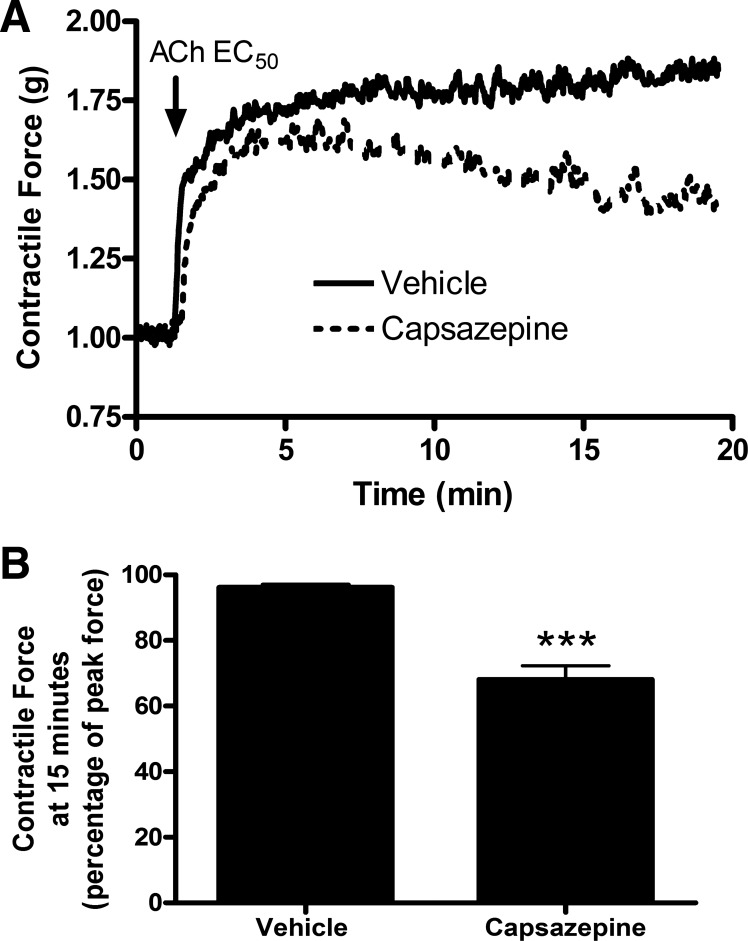
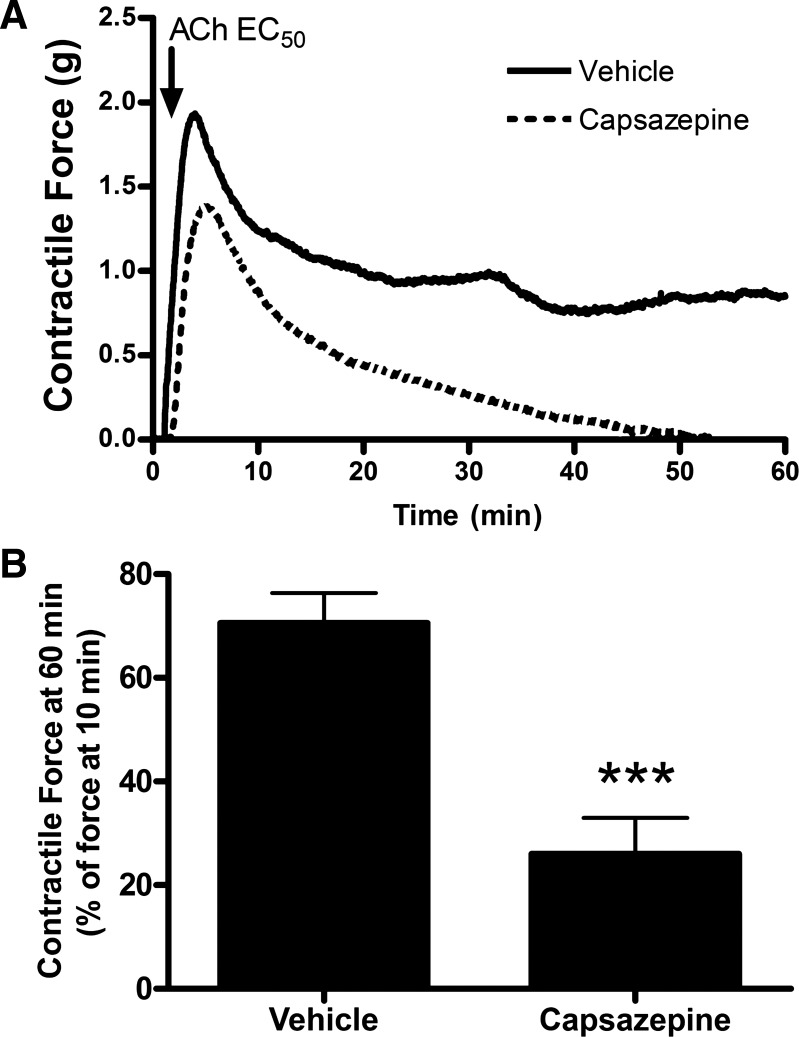
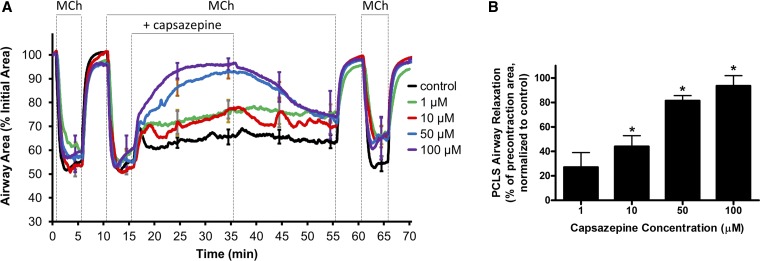
As the TRPV1 agonist capsaicin led to ASM contraction via neural mechanisms, organ bath studies were undertaken to confirm previous studies demonstrating that the TRPV1 antagonist capsazepine mediated relaxation of ASM contracted with Gq-protein coupled receptor agonists (28). Capsazepine pretreatment of guinea pig and human ASM samples significantly inhibited the maintenance of acetylcholine-induced ASM contraction. Figure 6A demonstrates representative organ bath contractile force tracings in guinea pig tracheal rings, and Fig. 6B demonstrates quantification of these forces (n = 4 animals, P < 0.001 by Student’s t-test). Figure 7A demonstrates representative organ bath contractile force tracings of human tracheal ASM strips, and Fig. 7B demonstrates quantification of these forces; (n = 4 human donors, P < 0.001 by Student’s t-test). To explore the effects of capsazepine on peripheral airways, we performed mouse PCLS experiments. Similar to what was seen in the central ASM from guinea pig and human, capsazepine treatment inhibited the maintenance of methacholine-induced airway narrowing in mouse peripheral lung slices in a dose-dependent manner. Figure 8A demonstrates mouse PCLS airway diameters, and capsazepine-mediated relaxation is quantified in Fig. 8B (n = 3 animals; P < 0.05 for 10, 50, and 100 µM capsazepine by ANOVA with Bonferroni post hoc correction). Thus capsazepine-mediated relaxation of muscarinic ASM contraction is demonstrated in three species and in central and peripheral lung tissues.
Fig. 6.
Capsazepine-mediated attenuation of acetylcholine-induced contraction of guinea pig tracheal rings. A: representative tracings of guinea pig tracheal ring contractile force receiving pretreatment with 100 µM capsazepine vs. vehicle. All rings were contracted with an EC50 concentration of acetylcholine calculated for each individual ring. B: quantification of contractile force 15 min after acetylcholine exposure (as a percentage of peak force). Capsazepine pretreatment significantly attenuated the maintenance of contractile force in guinea pig ASM. Values are means ± SE; n = 4 animals. ***P < 0.001 by Student’s t-test.
Fig. 7.
Capsazepine-mediated attenuation of acetylcholine-induced contraction of human ASM in organ bath preparations. A: representative tracings of human ASM strip contraction force receiving pretreatment with 100 µM capsazepine vs. vehicle. B: quantification of contractile force 60 min after acetylcholine exposure (as a percentage of force at 10 min). Capsazepine pretreatment significantly attenuates the maintenance of contractile force in human ASM. Values are means ± SE; n = 4 human donors. ***P < 0.001 by Student’s t-test.
Fig. 8.
Capsazepine-mediated inhibition of methacholine-induced airway contraction in mouse precision-cut peripheral lung slice. A: combined tracings of the area of peripheral airways (normalized to baseline area) in mouse precision-cut lung slice (PCLS) preparations. The airways were contracted with 400 nM methacholine (MCh) and subsequently exposed to a concentration range of capsazepine. B: capsazepine led to a significant relaxation (increase in airway area; measured at 30 min) of the MCh-induced airway contraction in a dose-dependent manner. Values are means ± SE; n = 3. *P < 0.05 compared with untreated controls by ANOVA with Bonferroni post hoc correction.
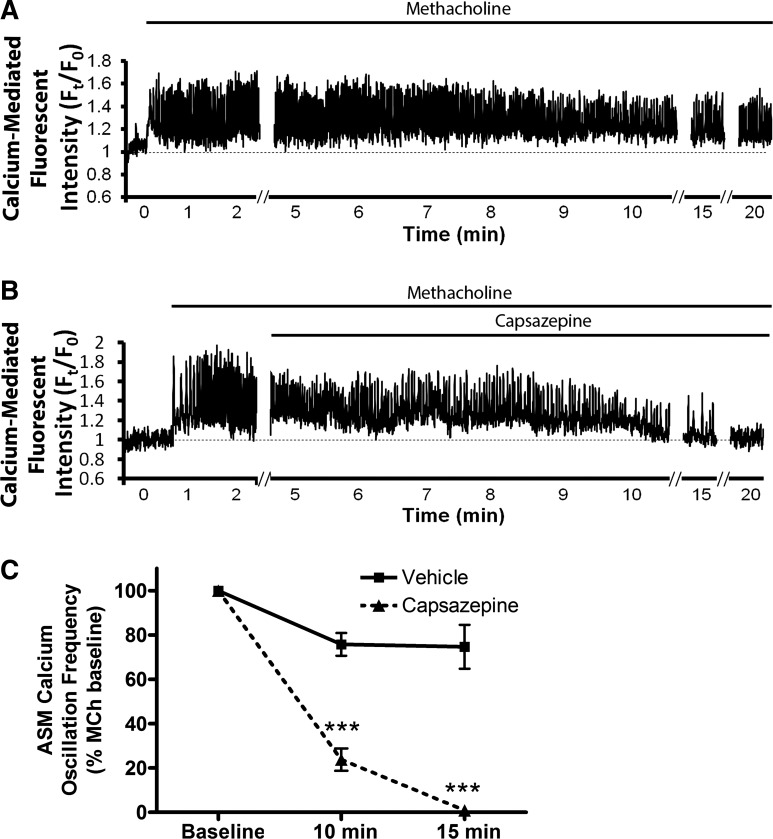
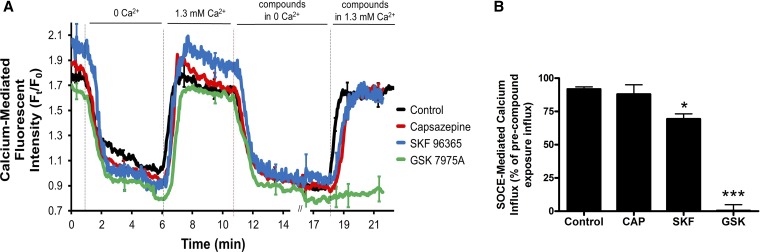
To explore the mechanism of this relaxation, we conducted ASM calcium imaging experiments, again in mouse PCLS. Capsazepine significantly inhibited methacholine-induced ASM calcium oscillations after 15 min. Figure 9A is a representative tracing of ASM calcium oscillations induced by 400 nM methacholine, and Fig. 9B is a representative tracing demonstrating inhibition of ASM calcium oscillations by 100 µM capsazepine. This inhibition is quantified in Fig. 9C (n = 3 animals, P < 0.001 by ANOVA with Bonferroni post hoc correct). Given that capsazepine has been demonstrated to inhibit SOCE in PC12 cells (6) and SOCE is required to maintain calcium oscillations in ASM (5), studies were conducted to determine the effect of capsazepine on ASM SOCE using the PCLS “SR permeabilization” technique, which induces SOCE by locking SR ryanodine receptors in the open state with caffeine and ryanodine, equalizing SR and cytosolic calcium concentrations (5). These studies demonstrated no capsazepine-mediated SOCE inhibition. Figure 10A demonstrates tracings of calcium-mediated fluorescent intensity in the setting of “SR permeabilization”; fluorescent intensity upon reintroduction of 1.3 mM extracellular calcium in the presence of the indicated compounds is quantified in Fig. 10B as a representation of SOCE (n = 3 animals, P = nonsignificant; purported SOCE inhibitor, GSK 7975A, served as a positive control, n = 3 animals and P < 0.001 by ANOVA with Bonferroni post hoc correction).
Fig. 9.
Capsazepine-mediated inhibition of methacholine-induced calcium oscillations in ASM cells in mouse PCLS. A: mouse PCLS preparations were loaded with a calcium indicator dye (Oregon Green-BAPTA) and imaged in real-time using a custom two-photon confocal microscope and software. A: exposure of the slices to methacholine (MCh; 400 nM) led to sustained, rapid calcium oscillations over a 20-min recording period (representative tracing). B: 100 µM capsazepine significantly inhibited these MCh-induced oscillations. C: quantification of oscillation frequency demonstrates capsazepine led to a near abolishment of oscillations by 15 min. Values are means ± SE; n = 3. ***P < 0.01 compared with untreated controls by ANOVA with Bonferroni post hoc correction.
Fig. 10.
Absence of capsazepine effect on store-operated calcium entry (SOCE) in ASM cells in mouse PCLS. Mouse peripheral lung slices loaded with an intracellular calcium indicator dye were exposed to caffeine (20 mM) and ryanodine (25 μM) simultaneously to “lock” the ryanodine receptor of sarcoplasmic reticulum (SR) in the open state, to irreversibly deplete the SR of calcium, and to induce SOCE in the presence of zero-calcium external buffer. The slices were then reexposed to 1.3 mM Ca2+ extracellular buffer, and the resultant increase in ASM calcium-mediated fluorescence was considered a measure of SOCE. GSK 7575A, 100 µM, a SOCE inhibitor, significantly inhibited SOCE in this assay; however, 100 µM capsazepine did not. This suggests SOCE inhibition is not the mechanism by which capsazepine inhibits calcium oscillations in ASM. A: combined Ca2+-mediated fluorescent tracings. B: quantification of SOCE-mediate calcium influx. Values are means ± SE; n = 3. ***P < 0.001 and *P < 0.05 compared with untreated controls by ANOVA with Bonferroni post hoc correction.
DISCUSSION
The primary findings of the present study are that the TRPV1 receptor is expressed on human ASM cells, including expression in the SR, and that the TRPV1 antagonist capsazepine impairs muscarinic receptor-mediated contraction force maintenance of ex vivo ASM from multiple species via inhibition of calcium oscillations.
TRPV1, a nonspecific cation channel that is permeant to calcium, has been well studied in airway nerves, where it mediates cough and neurogenic bronchoconstriction (16, 20). This study demonstrates that TRPV1 activation with capsaicin produces ASM contraction, which is mediated by neuronal mechanisms, likely tachykinin release from NANC nerves, and is not due to a direct effect on ASM (2, 10). The results of this present study are consistent with previous findings, as the Na+ channel inhibitor, bupivacaine, and the NK2 receptor antagonist, GR-159897, significantly inhibited capsaicin-induced contraction in guinea pig tracheal rings, consistent with capsaicin-induced activation of TRPV1 receptors on NANC nerves. Repetitive addition of capsaicin to guinea pig tracheal rings exhibited tachyphylaxis, which is also consistent with capsaicin-induced depletion of neurotransmitters from NANC nerves (11). Interestingly, the Na+-channel inhibitor bupivacaine inhibited the nerve-mediated contractile effect of capsaicin, but tetrodotoxin, a widely used Na+-channel inhibitor, did not (Fig. 2). This reinforces findings in a previous report showing that capsaicin-sensitive airway nerve fibers express many tetrodotoxin-insensitive Na+ channels (19).
Additionally, the present studies show that the TRPV1 antagonist, capsazepine, inhibits a nonneuronal mechanism of force maintenance by inhibiting intracellular calcium oscillations, but not as a result of impaired SOCE. Our results suggest that this inhibition was due to a direct effect of capsazepine on ASM, since these studies were performed with exogenous activation of ASM muscarinic receptors in the absence of neural stimulation. Sustained ASM contraction requires an elevated intracellular calcium concentration produced in part by continuous Ca2+ waves, called Ca2+ oscillations, and the frequency of these oscillations determines the magnitude of contraction (25). Following M3 muscarinic receptor activation of the Gq protein and phospholipase Cβ-mediated inositol-1,4,5-trisphosphate (IP3) formation, calcium is initially released from the SR and then taken back into the SR via SERCA to generate calcium cycling and subsequent oscillations. We demonstrated that capsazepine inhibits these oscillations (Fig. 9), likely accounting for its impaired force maintenance and subsequent relaxation of ASM.
Recent studies demonstrated that SOCE is necessary to maintain calcium oscillations in ASM cells (5). SOCE is calcium influx that results from depletion of SR stores. Briefly, stromal interaction molecule 1 in the SR membrane senses low SR calcium concentrations following SR calcium release, oligomerizes, and interacts with Orai1 in the plasma membrane to form calcium entry channels (12, 23, 24, 34, 37). A channel related to TRPV1, the TRP cation channel, subfamily C, member 3, also contributes to SOCE in ASM cells (36). It is thought that the majority of the SR calcium release that contributes to calcium oscillations occurs via IP3 receptor Ca2+ channels, particularly after Gq-protein coupled receptor activation, and the majority of the re-uptake results from SR Ca2+ transport ATPases (SERCA) (5, 26). However, other efflux pathways exist, including ryanodine receptors (7, 18). Capsazepine reportedly inhibits SOCE in PC12 cells in vitro (6). Thus we hypothesized that capsazepine was inhibiting oscillations and contraction by inhibiting SOCE in ASM. However, PCLS experiments presented here show that capsazepine does not inhibit SOCE in ASM. This suggests that at least one additional mechanism in addition to SOCE contributes to the generation of calcium oscillations in ASM, and that this is a target of capsazepine. Further studies are needed to determine this mechanism, as well as additional roles for TRPV1 in ASM. Given the data presented here showing TRPV1 is expressed in the SR membrane in ASM cells (Fig. 2), perhaps the TRPV1 receptor interacts with ryanodine receptors or another novel calcium efflux pathway, such as the IP3 receptor. However, previous reports demonstrating that non-capsazepinoid TRPV1 inhibitors fail to relax ASM argue against this, as the lack of relaxation suggests that oscillations were not inhibited (28). Another possibility is an “off-target” effect of capsazepine, such as inhibition of the IP3 receptor.
Establishing the clinical relevance of these studies will require more investigation. However, it is interesting to note that capsazepine did not significantly inhibit the initiation of ASM calcium oscillations or contraction; however, it did inhibit the maintenance of oscillations and contraction. This would suggest that agents acting similarly to capsazepine may be useful as rescue medications during an acute asthma attack. Given the increasing concern for tolerance to β2-adrenoceptor agonists (29), new classes of acute bronchodilators are desperately needed.
These studies demonstrate that the TRPV1 receptor is expressed on human ASM cells, including expression on the SR, and that capsazepine inhibits calcium oscillations in ASM, leading to inhibition of contraction force maintenance. As the rates of asthma and number of asthma exacerbations continue to increase worldwide, it is imperative to investigate novel mechanisms of ASM force modulation to better understand this complex disease and to develop new therapeutic targets.
GRANTS
This study was funded by National Institutes of Health Grants R01 GM-065281 (G. T. Yocum, C W. Emala), NIH R01 HL-122340 (C. W. Emala), and R01 HL-103405 (M. J. Sanderson); Stony Wold-Herbert Fund (G. T. Yocum); and Foundation for Anesthesia Education and Research (G. T. Yocum).
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the authors.
AUTHOR CONTRIBUTIONS
G.T.Y., J.C., C.H.C., E.A.T., Y.Z., D.X., X.W.F., M.J.S., and C.W.E. conceived and designed research; G.T.Y., J.C., C.H.C., E.A.T., Y.Z., D.X., X.W.F., and C.W.E. performed experiments; G.T.Y., J.C., C.H.C., E.A.T., Y.Z., D.X., X.W.F., M.J.S., and C.W.E. analyzed data; G.T.Y., J.C., C.H.C., E.A.T., D.X., M.J.S., and C.W.E. interpreted results of experiments; G.T.Y. and X.W.F. prepared figures; G.T.Y. drafted manuscript; G.T.Y., J.C., C.H.C., E.A.T., Y.Z., D.X., X.W.F., and C.W.E. edited and revised manuscript; G.T.Y., J.C., C.H.C., E.A.T., Y.Z., D.X., X.W.F., and C.W.E. approved final version of manuscript.
ACKNOWLEDGMENTS
Present address of E. A. Townsend: University of Wisconsin, Madison, WI 53706.
REFERENCES
- 1.Bai Y, Sanderson MJ. Modulation of the Ca2+ sensitivity of airway smooth muscle cells in murine lung slices. Am J Physiol Lung Cell Mol Physiol 291: L208–L221, 2006. doi: 10.1152/ajplung.00494.2005. [DOI] [PubMed] [Google Scholar]
- 2.Belvisi MG, Miura M, Stretton D, Barnes PJ. Capsazepine as a selective antagonist of capsaicin-induced activation of C-fibres in guinea-pig bronchi. Eur J Pharmacol 215: 341–344, 1992. doi: 10.1016/0014-2999(92)90054-8. [DOI] [PubMed] [Google Scholar]
- 3.Cantero-Recasens G, Gonzalez JR, Fandos C, Duran-Tauleria E, Smit LA, Kauffmann F, Antó JM, Valverde MA. Loss of function of transient receptor potential vanilloid 1 (TRPV1) genetic variant is associated with lower risk of active childhood asthma. J Biol Chem 285: 27532–27535, 2010. doi: 10.1074/jbc.C110.159491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389: 816–824, 1997. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Sanderson MJ. Store-operated calcium entry is required for sustained contraction and Ca(2+) oscillations of airway smooth muscle. J Physiol. In press. doi: 10.1113/JP272694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi SY, Kim KT. Capsaicin inhibits phospholipase C-mediated Ca(2+) increase by blocking thapsigargin-sensitive store-operated Ca(2+) entry in PC12 cells. J Pharmacol Exp Ther 291: 107–114, 1999. [PubMed] [Google Scholar]
- 7.Croisier H, Tan X, Chen J, Sneyd J, Sanderson MJ, Brook BS. Ryanodine receptor sensitization results in abnormal calcium signaling in airway smooth muscle cells. Am J Respir Cell Mol Biol 53: 703–711, 2015. doi: 10.1165/rcmb.2014-0386OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dietrich A, Chubanov V, Kalwa H, Rost BR, Gudermann T. Cation channels of the transient receptor potential superfamily: their role in physiological and pathophysiological processes of smooth muscle cells. Pharmacol Ther 112: 744–760, 2006. doi: 10.1016/j.pharmthera.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 9.Elias JA, Zhu Z, Chupp G, Homer RJ. Airway remodeling in asthma. J Clin Invest 104: 1001–1006, 1999. doi: 10.1172/JCI8124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellis JL, Undem BJ. Inhibition by capsazepine of resiniferatoxin- and capsaicin-induced contractions of guinea pig trachea. J Pharmacol Exp Ther 268: 85–89, 1994. [PubMed] [Google Scholar]
- 11.Ellis JL, Undem BJ. Non-adrenergic, non-cholinergic contractions in the electrically field stimulated guinea-pig trachea. Br J Pharmacol 101: 875–880, 1990. doi: 10.1111/j.1476-5381.1990.tb14174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 441: 179–185, 2006. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 13.Flores-Soto E, Reyes-García J, Sommer B, Montaño LM. Sarcoplasmic reticulum Ca(2+) refilling is determined by L-type Ca(2+) and store operated Ca(2+) channels in guinea pig airway smooth muscle. Eur J Pharmacol 721: 21–28, 2013. doi: 10.1016/j.ejphar.2013.09.060. [DOI] [PubMed] [Google Scholar]
- 14.Gallos G, Yim P, Chang S, Zhang Y, Xu D, Cook JM, Gerthoffer WT, Emala CW Sr. Targeting the restricted α-subunit repertoire of airway smooth muscle GABAA receptors augments airway smooth muscle relaxation. Am J Physiol Lung Cell Mol Physiol 302: L248–L256, 2012. doi: 10.1152/ajplung.00131.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gosling M, Poll C, Li S. TRP channels in airway smooth muscle as therapeutic targets. Naunyn Schmiedebergs Arch Pharmacol 371: 277–284, 2005. doi: 10.1007/s00210-005-1058-2. [DOI] [PubMed] [Google Scholar]
- 16.Grace MS, Baxter M, Dubuis E, Birrell MA, Belvisi MG. Transient receptor potential (TRP) channels in the airway: role in airway disease. Br J Pharmacol 171: 2593–2607, 2014. doi: 10.1111/bph.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Helli PB, Pertens E, Janssen LJ. Cyclopiazonic acid activates a Ca2+-permeable, nonselective cation conductance in porcine and bovine tracheal smooth muscle. J Appl Physiol (1985) 99: 1759–1768, 2005. doi: 10.1152/japplphysiol.00242.2005. [DOI] [PubMed] [Google Scholar]
- 18.Kannan MS, Prakash YS, Brenner T, Mickelson JR, Sieck GC. Role of ryanodine receptor channels in Ca2+ oscillations of porcine tracheal smooth muscle. Am J Physiol 272: L659–L664, 1997. [DOI] [PubMed] [Google Scholar]
- 19.Kwong K, Lee LY. Prostaglandin E2 potentiates a TTX-resistant sodium current in rat capsaicin-sensitive vagal pulmonary sensory neurones. J Physiol 564: 437–450, 2005. doi: 10.1113/jphysiol.2004.078725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McAlexander MA, Taylor-Clark T. The role of transient receptor potential channels in respiratory symptoms and pathophysiology. Adv Exp Med Biol 704: 969–986, 2011. doi: 10.1007/978-94-007-0265-3_50. [DOI] [PubMed] [Google Scholar]
- 21.Moran MM, McAlexander MA, Bíró T, Szallasi A. Transient receptor potential channels as therapeutic targets. Nat Rev Drug Discov 10: 601–620, 2011. doi: 10.1038/nrd3456. [DOI] [PubMed] [Google Scholar]
- 22.Panettieri RA., Jr Cellular and molecular mechanisms regulating airway smooth muscle proliferation and cell adhesion molecule expression. Am J Respir Crit Care Med 158, Suppl 2: S133–S140, 1998. doi: 10.1164/ajrccm.158.supplement_2.13tac900. [DOI] [PubMed] [Google Scholar]
- 23.Peel SE, Liu B, Hall IP. A key role for STIM1 in store operated calcium channel activation in airway smooth muscle. Respir Res 7: 119, 2006. doi: 10.1186/1465-9921-7-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peel SE, Liu B, Hall IP. ORAI and store-operated calcium influx in human airway smooth muscle cells. Am J Respir Cell Mol Biol 38: 744–749, 2008. doi: 10.1165/rcmb.2007-0395OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perez JF, Sanderson MJ. The frequency of calcium oscillations induced by 5-HT, ACH, and KCl determine the contraction of smooth muscle cells of intrapulmonary bronchioles. J Gen Physiol 125: 535–553, 2005. doi: 10.1085/jgp.200409216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prakash YS, Sathish V, Thompson MA, Pabelick CM, Sieck GC. Asthma and sarcoplasmic reticulum Ca2+ reuptake in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 297: L794, 2009. doi: 10.1152/ajplung.00237.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sjåland C, Lunde PK, Swift F, Munkvik M, Ericsson M, Lunde M, Boye S, Christensen G, Ellingsen Ø, Sejersted OM, Andersson KB. Slowed relaxation and preserved maximal force in soleus muscles of mice with targeted disruption of the Serca2 gene in skeletal muscle. J Physiol 589: 6139–6155, 2011. doi: 10.1113/jphysiol.2011.211987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skogvall S, Berglund M, Dalence-Guzmán MF, Svensson K, Jönsson P, Persson CG, Sterner O. Effects of capsazepine on human small airway responsiveness unravel a novel class of bronchorelaxants. Pulm Pharmacol Ther 20: 273–280, 2007. doi: 10.1016/j.pupt.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 29.Spitzer WO, Suissa S, Ernst P, Horwitz RI, Habbick B, Cockcroft D, Boivin JF, McNutt M, Buist AS, Rebuck AS. The use of beta-agonists and the risk of death and near death from asthma. N Engl J Med 326: 501–506, 1992. doi: 10.1056/NEJM199202203260801. [DOI] [PubMed] [Google Scholar]
- 30.Stretton D. Non-adrenergic, non-cholinergic neural control of the airways. Clin Exp Pharmacol Physiol 18: 675–684, 1991. doi: 10.1111/j.1440-1681.1991.tb01380.x. [DOI] [PubMed] [Google Scholar]
- 31.Sweeney M, McDaniel SS, Platoshyn O, Zhang S, Yu Y, Lapp BR, Zhao Y, Thistlethwaite PA, Yuan JX. Role of capacitative Ca2+ entry in bronchial contraction and remodeling. J Appl Physiol (1985) 92: 1594–1602, 2002. doi: 10.1152/japplphysiol.00722.2001. [DOI] [PubMed] [Google Scholar]
- 32.Tan X, Sanderson MJ. Bitter tasting compounds dilate airways by inhibiting airway smooth muscle calcium oscillations and calcium sensitivity. Br J Pharmacol 171: 646–662, 2014. doi: 10.1111/bph.12460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Townsend EA, Thompson MA, Pabelick CM, Prakash YS. Rapid effects of estrogen on intracellular Ca2+ regulation in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 298: L521–L530, 2010. doi: 10.1152/ajplung.00287.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, Kinet JP. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science 312: 1220–1223, 2006. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Q, Zhang Y, Li D, Zhang Y, Tang B, Li G, Yang Y, Yang D. Transgenic overexpression of transient receptor potential vanilloid subtype 1 attenuates isoproterenol-induced myocardial fibrosis in mice. Int J Mol Med 38: 601–609, 2016. doi: 10.3892/ijmm.2016.2648. [DOI] [PubMed] [Google Scholar]
- 36.White TA, Xue A, Chini EN, Thompson M, Sieck GC, Wylam ME. Role of transient receptor potential C3 in TNF-alpha-enhanced calcium influx in human airway myocytes. Am J Respir Cell Mol Biol 35: 243–251, 2006. doi: 10.1165/rcmb.2006-0003OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang SL, Yeromin AV, Zhang XH, Yu Y, Safrina O, Penna A, Roos J, Stauderman KA, Cahalan MD. Genome-wide RNAi screen of Ca(2+) influx identifies genes that regulate Ca(2+) release-activated Ca(2+) channel activity. Proc Natl Acad Sci USA 103: 9357–9362, 2006. doi: 10.1073/pnas.0603161103. [DOI] [PMC free article] [PubMed] [Google Scholar]