Abstract
There are several anomalies of the middle cerebral artery (MCA) in humans, such as accessory MCA, duplicated MCA, fenestration of MCA, and duplicated origin of MCA. Recently, unfused or twig-like MCA, which indicates MCA trunk occlusion with collateral plexiform arterial network, have been reported. During the embryonic stage, MCA is thought to generate from plexiform arterial twigs arising from the anterior cerebral artery, and these twigs form the definitive MCA by fusion and regression at the end of the development stage. Any interruption during the fusion of the arterial twigs may result in MCA anomalies, and the unfused or twig-like MCA, especially, is hypothesized to be the persistent primitive arterial twigs. Clinically, it is challenging to differentiate the unfused or twig-like MCA from unilateral moyamoya disease, in which stenotic change begins at the MCA. The knowledge of the anomalies of the MCA is important to perform a safe surgical or endovascular intervention.
Keywords: middle cerebral artery, anatomy, embryology, anomaly
Introduction
Middle cerebral artery (MCA) is the largest and most complex of cerebral arteries, because the cerebral neocortex has significantly developed in humans. MCA is a recent phylogenetic acquisition and can be considered as a collateral branch of the anterior cerebral artery (ACA).1) Anomalies of the MCA occur less frequently than anomalies of the other major intracranial arteries. MCA anomalies such as accessory MCA, duplicated MCA, duplicated origin of MCA and fenestration of MCA might be found in humans.2–7) Recently, several reports describing unfused or twig-like MCA, in which the MCA trunk has been occluded with the collateral plexiform arterial network have been published.8–12) Their clinical features and pathogenesis are not yet known.
In this article, we elucidate the anatomical variation of MCA from the embryological perspective and discuss the relationship between twig-like MCA and moyamoya disease (MMD).
Embryology of the MCA
Padget’s detailed description of the development of cranial arteries, published in 1948, provides useful information about the embryology of MCA.13)
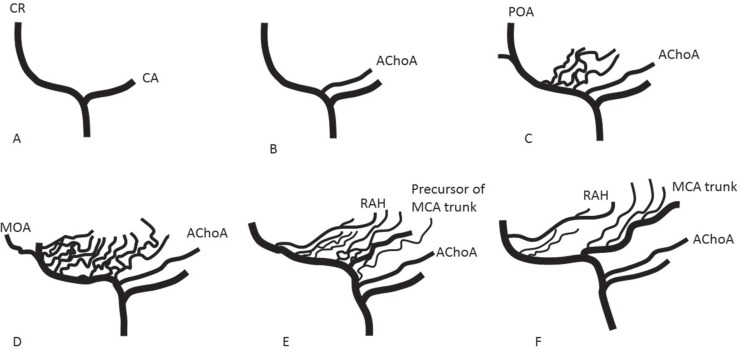
When the embryo is 28–30 days old (crown-rump length 4–5 mm), internal carotid artery (ICA) divides into a cranial and a caudal branch (Fig. 1A). The ACA is a continuation of the cranial branch, and MCA is one of its collaterals. The caudal branch later develops into a posterior communicating artery. When the embryo is 31–33 days old (7–12 mm), the primary cranial branch of ICA gives rise to several branches. The largest of these is the primitive anterior choroidal artery (Fig. 1B). At 34–36 days of the embryonal stage (12–14 mm), the anterior choroidal artery becomes quite prominent. Just distal to the anterior choroidal artery, multiple plexiform arterial twigs appear, which later develop into lateral striate arteries and the trunk of MCA by fusion and regression (Fig. 1C). The terminal end of the cranial division of the carotid constitutes the primitive olfactory artery (POA). It has two branches, a primary branch to the nasal fossa, and a secondary branch passing more medially to the emerging olfactory nerve root. The latter is the medial olfactory artery, which joins with the artery from the contralateral side, in the mid-line region of the future anterior communicating artery. By 39–41 days of the embryo (16–18 mm), the MCA becomes a prominent stem, with with plexiform arteries, which enter the lateral part of anterior perforated substance, for supply to the striatum. At 43–45 days of the embryo (20–24 mm), the POA dwindles. On the other hand, larger derivatives of the POA extend laterally, to enter the mesial part of the anterior substance, for supplying the basal ganglia (Fig. 1D). They are the recurrent artery of Heubner (RAH) and medial striate arteries, which may have potential anastomosis with lateral striate groups (Fig. 1E). Finally, at 40 mm stage of the embryo, the MCA attains approximate adult configuration (Fig. 1F).
Fig. 1.
Schematic representation of the formation of internal cerebral artery (ICA), anterior cerebral artery (ACA), and middle cerebral artery (MCA). A. ICA divides into a cranial branch (CR) and a caudal branch (CA). B. Anterior choroidal artery (AChoA) arises from the CR. C. Multiple plexiform arterial twigs appear just distal to the ACoA. The terminal end of CR constitutes the primitive olfactory artery (POA). D. The POA dwindles. Medial olfactory artery (MOA), which will constitute the ACA, branches out from the POA. E. Multiple plexiform arterial twigs evolve into recurrent artery of Heubner (RAH), medial and lateral striate arteries, and a single MCA trunk. F. Adult configuration of cerebral arteries of anterior circulation.
Anomalies of the MCA
The MCA has several anomalies, although they occur less frequently than the variations of other major cranial arteries. The MCA normally arises from the ICA as a single artery. In some cases, two MCAs arise from the ICA, such as accessory MCA or duplicated MCA.2–4) In MCA fenestration, MCA originates as a single trunk, but there is a presence of a fenestration.7) The term, duplicated origin of the MCA indicates an anomaly wherein, two MCA arise separately from the terminal segment of ICA and fuse to form an arterial ring.6) In the twig-like MCA anomaly, there is no MCA trunk, but a twig-like arterial network instead.8–12)
Double origin of MCA (Accessory MCA, duplicated MCA, and duplicated origin of MCA) and fenestration of MCA
The term accessory MCA was first used by Crompton in 1962, to describe a vessel that passes into the Sylvian fissure along with the MCA;2) the term included both, the duplication and accessory MCA as currently defined. In 1973, Teal et al. proposed that the term accessory MCA be restricted to the anomalous artery that arises from the ACA;3) and the branch arising from ICA be termed as duplication of MCA. Today, Teal’s classification is widely accepted. The incidence of accessory MCA in angiographic and anatomical studies is reported to be 0.3–4.0% and that of duplicated MCA 0.2–2.9%.14)
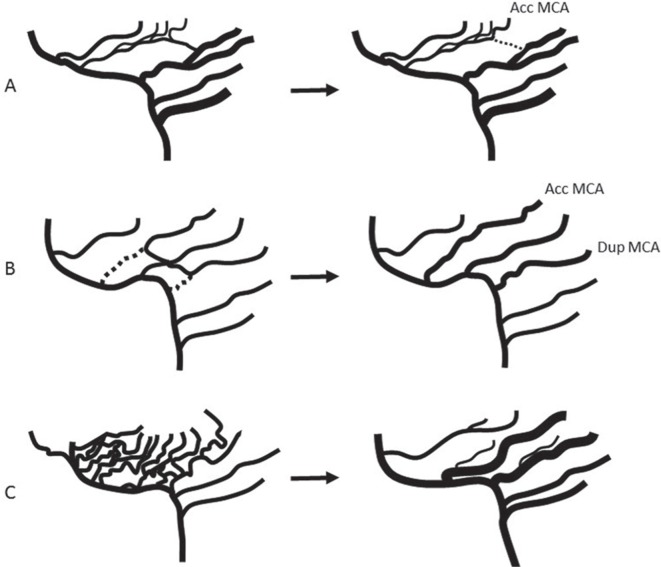
There are several explanations regarding the embryologic origins of accessory and duplicated MCA. Handa et al. suggested that the accessory MCA represents hypertrophy of the RAH;15) however, the accessory MCA and RAH may coexist, and this hypothesis was rejected. Takahashi et al. proposed that the accessory MCA could be considered as a persistent form of anastomotic channels between medial striate arteries from the ACA and lateral striate arteries from the MCA (Fig. 2A).5) Komiyama et al. suggested that the development of accessory and/or duplicated MCA is an anomalously early ramification of the early branches of MCA, because they have a consistent cortical supply through the accessory and duplicated MCA to the anterior frontal lobe and anterior temporal lobe, respectively (Fig. 2B).14)
Fig. 2.
Formation of accessory MCA. A. left: Anastomosis between medial and lateral striate arteries can exist. If communication between MCA trunk and frontal branch of MCA is interrupted (dotted line), accessory (Acc) MCA will be formed. B. If anterior temporal artery arises directly from ICA, it is called duplicated (Dup) MCA. If orbitofrontal artery arises directly from ACA, it is called accessory MCA. C. Multiple plexiform arterial twigs evolve into a single main trunk and lateral striate arteries by fusion and regression. Failure of fusion can form double MCA trunks.
In a 12–14 mm embryo, multiple plexiform arterial twigs appear just distal to the anterior choroidal artery, which then evolve into a single main trunk and lateral striate arteries by fusion and regression (Fig. 2C). The failure of fusion may result in two MCA trunks. Usually, the larger trunk is defined as main MCA trunk. If the smaller trunk arises distal to the main MCA, it is defined as an accessory MCA. Conversely, if the smaller trunk arises proximal to the main MCA, it is defined as a duplicated MCA. If the size of both MCA trunks is equal, it is difficult to define the main trunk (Fig. 3). Lasjaunius et al. considered the proximal trunk of double MCA as the main MCA, which should have only cortical branches without any perforating arteries.1) However, the duplicated MCA may have perforating arteries.
Fig. 3.
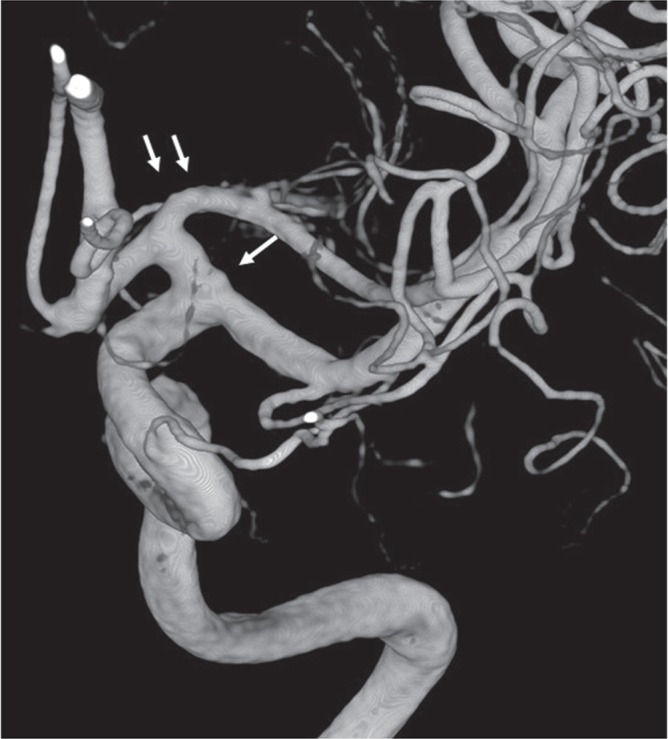
Double MCA trunks. If the point of single arrow is considered as the ICA terminal, distal MCA trunk is called accessory MCA. On the other hand, a point of double arrows as ICA terminal, proximal MCA trunk is called duplicated MCA.
Uchino et al. reported four cases of duplicated origin of the MCA, which represented a prevalence of 0.11%.6) They speculated that the duplicated origin of the MCA is formed by distal fusion of the duplicated MCA or accessory MCA.
MCA fenestration is rare. Selective cerebral angiographic prevalence has been reported to be 0.17–0.43%.16,17) Gailloud et al. reported a high frequency of associated early branching of the temporopolar artery, from the inferior limb of a fenestration segment of the MCA. They hypothesized that MCA fenestration results when the early branching of temporopolar artery causes failure in fusion of the primitive arterial network of MCA.7)
Twig-like MCA
Double origin of the MCA and fenestration of MCA, may form due to the failure in fusion of primitive arterial network of the MCA. However, the pathogenesis of the unfused/twig-like MCA is not yet well known.
In 2005, Cekirge et al. reported one case of an occluded MCA trunk with collateral plexiform arterial network and named this variation as embryonic unfused MCA,9) and Liu et al. reported two cases and named them as twig-like MCA.8) In 2012, Seo et al. reported the largest series with 15 cases and named the finding as aplastic or twig-like MCA.10) Recently, Shin et al. reported four cases,11) and Akkan et al. reported six cases.12) Common angiographical features of these cases were as follows; 1) a steno-occlusive lesion can be seen on the unilateral MCA, 2) plexiform arterial network exists instead of the main MCA trunk, 3) perforators arise from the plexiform arterial network, and 4) cortical branches beyond the occluded MCA trunk have anterograde flow with normal configuration. Although almost half the cases presented with intracranial hemorrhage, few cases were found incidentally. The authors speculated that unfused/twig-like MCA might be the persistent fetal arterial network of primitive MCAs formed due to regression interference.
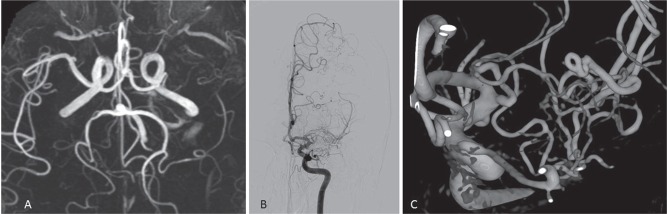
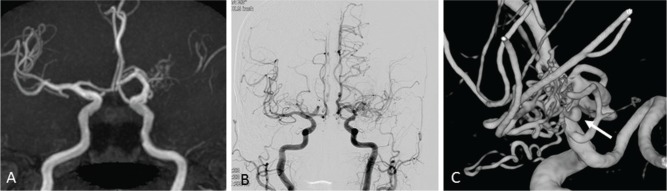
At our institute, we have come across two cases of twig-like MCAs. Case 1: A 56-year-old healthy man underwent Magnetic resonance (MR) imaging of the brain, which indicated left MCA occlusion (Fig. 4A). Conventional and three-dimensional (3D) rotational angiography (RA) revealed a twig-like MCA (Figs. 4B and 4C). As he was asymptomatic, he did not undergo any surgical intervention. Follow-up MR angiography at 35 months did not reveal any changes. Case 2: A 77-year-old woman presented with subarachnoid hemorrhage (SAH). Although MR angiography performed 7 years earlier had detected an abnormality on her left MCA (Fig. 5A), she underwent no surgical intervention at that time, since she was asymptomatic. Conventional angiography and 3D-RA at this time revealed an aneurysm in a twig-like MCA (Figs. 5B and 5C).
Fig. 4.
Case 1. A. MR angiography shows left MCA occlusion. B. Conventional angiography shows twig-like MCA. C. 3D-rotational angiography clearly shows plexiform arterial twigs.
Fig. 5.
Case 2. A. MR angiography shows left MCA occlusion with plexiform arterial twig. B. Conventional angiography shows twig-like MCA. C. 3D-rotational angiography clearly shows plexiform arterial twigs and an aneurysm in the twig (arrow).
The most important differential diagnosis of the twig-like MCA is moyamoya disease (MMD).18) MMD is a progressive steno-occlusive disease, which typically affects bilateral supraclinoid ICAs while sparing the posterior circulation.19) Although previous diagnostic criteria for MMD required bilateral findings,20) the revised edition presented by Japan Intractable Diseases Information Center in 2015 does not necessarily require bilateral involvement.21) In fact, Kawano et al. reported seventeen (27%) of 64 patients with unilateral occlusive disease of the circle of Willis, who developed bilateral lesions during a period of 1–7 years.22) Smith and Scott reported that 10 (30%) of 33 patients progressed to bilateral lesions during a period 1–16 years.23) Recently, a patient with MMD in whom the stenotic change initiated in a unilateral MCA trunk, but progressed to bilateral lesions, has been reported.24) Thus, it is extremely difficult to distinguish a twig-like MCA from unilateral MMD. In 15 cases series of twig-like MCA reported by Seo,10) four patients underwent follow-up catheter angiography, and two patients underwent CT or MR angiography. During a mean 31-month follow-up period, no patients developed bilateral lesions.
Cerebral arteries of patients with MMD, include ICA, ACA, and MCA, formed normally at birth.19) The cerebral arteries are gradually occluded near the terminal portion of ICA. Kuroda et al. demonstrated the specific shrinkage of the involved arteries in MMD by using 3D constructive interference in steady state (CISS) and direct surgical inspection.25) On the other hand, a twig-like MCA should already have been formed at birth, if the hypothesis that the twig-like MCA is the persistent fetal arterial network, is correct. If an infant with a twig-like MCA is found, the hypothesis is persuasive. However, the youngest patient reported with a twig-like MCA is a 10-year-old girl mentioned by Seo et al.10) At this time, there is no definitive evidence that supports the hypothesis that the twig-like MCA is the persistent fetal arterial network.
Although the pathogenesis of MMD is still unclear, the strong association of genetic variant RNF213 c.14576G > A with definitive MMD among the East Asian populations has been reported recently.26,27) How the RNF213 abnormality leads to MMD is still unknown. Miyawaki et al. reported that RNF213 variant was found in 73% of definitive MMD patients, compared to 1.8% in the normal control group.28) Interestingly, they also found the RNF213 variant in 50% of unilateral MMD patients. As mentioned above, since it is difficult to distinguish a twig-like MCA from unilateral MMD, there might be two types of patients in the unilateral MMD group. One group might be “definitive” MMD, in which the lesion develops bilaterally. Another group of patients might be those with twig-like MCA, who are asymptomatic. Analysis of the RNF213 variant may help in the differential diagnosis of twig-like MCA from MMD.
Conclusion
MCA anomalies occur less frequently than anomalies of other major intracranial arteries. Accessory MCA, duplicated MCA, duplicated origin of MCA, and fenestration of MCA may form due to the failure in fusion of the primitive arterial network. Although these anomalies are asymptomatic, and do not require intervention, knowledge of the anomalous configuration of the MCA is essential in neurosurgical and neuro-interventional practice. Whether the twig-like MCA is the persistent fetal network of the primitive MCA is still unclear. Analysis of the RNF213 variant in patients with twig-like MCA will identify the pathogenesis of the twig-like MCA, and make the differential diagnosis from MMD possible.
Acknowledgments
The author would like to express my special thanks to Dr. Masaki Komiyama, Department of Neuro-Intervention, Osaka City General Hospital, and, Prof. Mitsutoshi Nakada, Department of Neurosurgery, Kanazawa University Hospital, for their insightful suggestions and comments
Footnotes
Conflicts of Interest Disclosure
The author has no conflicts of interest with regard to this manuscript. The author has registered online Self-reported COI Disclosure Statement Forms.
References
- 1).Lasjaunias P, Berenstein A, ter Brugge KG: Intradural arteries, Surgical Neuroangiography, ed 2 Vol 1 New York, Springer; 2001, pp. 479–630 [Google Scholar]
- 2).Crompton MR: The pathology of ruptured middle-cerebral aneurysms with special reference to the differences between the sexes. Lancet 2: 421–425, 1962 [DOI] [PubMed] [Google Scholar]
- 3).Teal JS, Rumbaugh CL, Bergeron RT, Segall HD: Anomalies of the middle cerebral artery: accessory artery, duplication, and early bifurcation. Am J Roentgenol 118: 567–575, 1973 [DOI] [PubMed] [Google Scholar]
- 4).Abanou A, Lasjaunias P, Manelfe C, Lopez-Ibor L: The accessory middle cerebral artery (AMCA). Diagnostic and therapeutic consequences. Anat Clin 6: 305–309, 1984 [DOI] [PubMed] [Google Scholar]
- 5).Takahashi S, Hoshino F, Uemura K, Takahashi A, Sakamoto K: Accessory middle cerebral artery: is it a variant form of the recurrent artery of Heubner? AJNR Am J Neuroradiol 10: 563–568, 1989 [PMC free article] [PubMed] [Google Scholar]
- 6).Uchino A, Saito N, Okada Y, Nakajima R: Duplicate origin and fenestration of the middle cerebral artery on MR angiography. Surg Radiol Anat 34: 401–404, 2012 [DOI] [PubMed] [Google Scholar]
- 7).Gailloud P, Albayram S, Fasel JH, Beauchamp NJ, Murphy KJ: Angiographic and embryologic considerations in five cases of middle cerebral artery fenestration. AJNR Am J Neuroradiol 23: 585–587, 2002 [PMC free article] [PubMed] [Google Scholar]
- 8).Liu HM, Lai DM, Tu YK, Wang YH: Aneurysms in twig-like middle cerebral artery. Cerebrovasc Dis 20: 1–5, 2005 [DOI] [PubMed] [Google Scholar]
- 9).Cekirge HS, Peynircioglu B, Saatci I: Endovascular treatment of an “anterior cerebral artery” aneurysm in a patient with “embryonic unfused middle cerebral artery” anomaly: a case report. Neuroradiology 47: 690–694, 2005 [DOI] [PubMed] [Google Scholar]
- 10).Seo BS, Lee YS, Lee HG, Lee JH, Ryu KY, Kang DG: Clinical and radiological features of patients with aplastic or twiglike middle cerebral arteries. Neurosurgery 70: 1472–1480, 2012 [DOI] [PubMed] [Google Scholar]
- 11).Shin HS, Lee SH, Ryu CW, Koh JS: Flow-related intracranial aneurysms associated with unfused arterial twigs relevant to different vascular anomalies: embryologic and hemodynamic considerations. Acta Neurochir (Wien) 156: 1637–1646, 2014 [DOI] [PubMed] [Google Scholar]
- 12).Akkan K, Ucar M, Kilic K, Celtikci E, Ilgit E, Onal B: Unfused or twig-like middle cerebral artery. Eur J Radiol 84: 2013–2018, 2015 [DOI] [PubMed] [Google Scholar]
- 13).Padget DH: The development of the cranial arteries in the human embryo. Contrib Embryol 212: 207–261, 1948 [Google Scholar]
- 14).Komiyama M, Nakajima H, Nishikawa M, Yasui T: Middle cerebral artery variations: duplicated and accessory arteries. AJNR Am J Neuroradiol 19: 45–49, 1998 [PMC free article] [PubMed] [Google Scholar]
- 15).Handa J, Shimizu Y, Matsuda M, Handa H: The accessory middle cerebral artery: report of further two cases. Clin Radiol 21: 415–416, 1970 [DOI] [PubMed] [Google Scholar]
- 16).Ito J, Maeda H, Inoue K, Onishi Y: Fenestration of the middle cerebral artery. Neuroradiology 28: 37–39, 1977 [DOI] [PubMed] [Google Scholar]
- 17).Uchino A, Kato A, Takase Y, Kudo S: Middle cerebral artery variations detected by magnetic resonance angiography. Eur Radiol 10: 560–563, 2000 [DOI] [PubMed] [Google Scholar]
- 18).Suzuki J, Takaku A: Cerebrovascular “moyamoya” disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol 20: 288–299, 1969 [DOI] [PubMed] [Google Scholar]
- 19).Kuroda S, Houkin K: Moyamoya disease: current concepts and future perspectives. Lancet Neurol 7: 1056–1066, 2008 [DOI] [PubMed] [Google Scholar]
- 20).Research Committee on the Pathology and Treatment of Spontaneous Occlusion of the Circle of Willis Health Labour Sciences Research Grant for Research on Measures for Infractable Diseases : Guidelines for diagnosis and treatment of moyamoya disease (spontaneous occlusion of the circle of Willis). Neurol Med Chir (Tokyo) 52: 245–266, 2012 [DOI] [PubMed] [Google Scholar]
- 21).Moyamoya disease (Japanese guideline): http://www.nanbyou.or.jp/entry/209 Access 2017.2.10
- 22).Kawano T, Fukui M, Hashimoto N, Yonekawa Y: Follow-up study of patients with “unilateral” moyamoya disease. Neurol Med Chir (Tokyo) 34: 744–747, 1994 [DOI] [PubMed] [Google Scholar]
- 23).Smith ER, Scott RM: Progression of disease in unilateral moyamoya syndrome. Neurosurg Focus 24: E17, 2008 [DOI] [PubMed] [Google Scholar]
- 24).Akamatsu Y, Fujimura M, Uenohara H, Shimizu H, Tominaga T: Development of moyamoya disease in pregnancy and puerperium: case report. Neurol Med Chir (Tokyo) 54: 824–826, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Kuroda S, Kashiwazaki D, Akioka N, Koh M, Hori E, Nishikata M, Umemura K, Horie Y, Noguchi K, Kuwayama N: Specific Shrinkage of Carotid Forks in Moyamoya Disease: A Novel Key Finding for Diagnosis. Neurol Med Chir (Tokyo) 55: 796–804, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Kamada F, Aoki Y, Narisawa A, Abe Y, Komatsuzaki S, Kikuchi A, Kanno J, Niihori T, Ono M, Ishii N, Owada Y, Fujimura M, Mashimo Y, Suzuki Y, Hata A, Tsuchiya S, Tominaga T, Matsubara Y, Kure S: A genome-wide association study identifies RNF213 as the first Moyamoya disease gene. J Hum Genet 56: 34–40, 2011 [DOI] [PubMed] [Google Scholar]
- 27).Liu W, Morito D, Takashima S, Mineharu Y, Kobayashi H, Hitomi T, Hashikata H, Matsuura N, Yamazaki S, Toyoda A, Kikuta K, Takagi Y, Harada KH, Fujiyama A, Herzig R, Krischek B, Zou L, Kim JE, Kitakaze M, Miyamoto S, Nagata K, Hashimoto N, Koizumi A: Identification of RNF213 as a susceptibility gene for moyamoya disease and its possible role in vascular development. PLoS ONE 6: e22542, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Miyawaki S, Imai H, Shimizu M, Yagi S, Ono H, Mukasa A, Nakatomi H, Shimizu T, Saito N: Genetic variant RNF213 c.14576G > A in various phenotypes of intracranial major artery stenosis/occlusion. Stroke 44: 2894–2897, 2013 [DOI] [PubMed] [Google Scholar]