Abstract
Purpose
Delayed treatment of congenital hypothyroidism (CH) is a common cause of mental retardation. The aim of the present study was to evaluate intellectual outcomes in preschool children with treated CH.
Methods
We retrospectively reviewed the clinical records of 43 children (age range: 13 to 60 days of life; 22 girls and 21 boys) diagnosed with CH. Children aged 5 to 7 years were examined using the Korean Wechsler Intelligence Scale for Children or the Korean Wechsler Preschool and Primary Scale of Intelligence.
Results
The patients started treatment between 13 and 60 days of age. The mean intelligence quotient (IQ) of patients tested at age 5 to 7 years was 103.14±11.68 (IQ range: 76–126). None had intellectual disability (defined as an IQ <70). Twenty-one subjects were treated with a low dose (6.0–9.9 µg/kg/day) and 22 with a high dose of levothyroxine (10.0–16.0 µg/kg/day). There was no significant difference in the mean full-scale IQ (FSIQ), verbal IQ (VIQ), and performance IQ (PIQ) scores between the 2 groups. FSIQ, PIQ, and VIQ scores were not significantly correlated with initial dose of L-T4, initial fT4, age at treatment in multivariate analysis.
Conclusion
IQ scores of subjects with early treated CH diagnosed through a neonatal screening test were within normal range, regardless of etiology, thyroid function, initial dose of levothyroxine, and age at start of treatment.
Keywords: Congenital, Hypothyroidism, Intellectual disability, Synthyroid
Introduction
Congenital hypothyroidism (CH) is one of the most common causes of preventable mental retardation, with an overall incidence of 1:3,000–1:4,0001). Thyroid hormone plays a critical role in normal brain development and function, so early diagnosis and appropriate L-thyroxine (L-T4) treatment is very important to the neurological prognosis in CH patients2).
Several studies have evaluated the factors that affect the intellectual outcome in CH. Some reported that a high dose of L-T4 and rapid thyroid-stimulating hormone (TSH) normalization were associated with an improvement in the intelligence quotient (IQ) score compared with low-dose treatment, whereas others suggested that the timing of treatment was the most important factor3,4,5,6). Maintaining a high level of T4 by administration of a high dose of L-T4 may increase the risk of craniosynostosis and neurological symptoms, and did not affect cognitive outcomes at 11 years of age7).
We therefore aimed to evaluate intellectual outcomes in preschool children with treated CH. We also investigated the association between IQ scores and several factors influencing neurodevelopment.
Materials and methods
1. Subjects
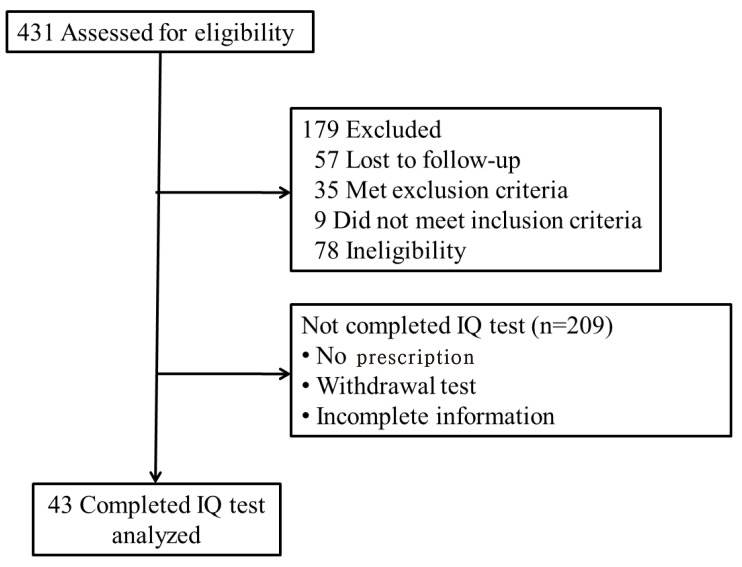
We retrospectively reviewed the clinical data of 431 children diagnosed with CH from January 2003 to March 2015 by pediatric endocrinologists at the pediatric clinic of Ajou University Hospital. Of 431 children with CH, 43 who underwent IQ testing were included in this study (Fig. 1). All patients fulfilled the following inclusion criteria: CH newly diagnosed by a pediatric endocrinologist, and low serum free thyroxine (fT4), triiodothyronine (T3), or abnormal TSH (>10 IU/L) levels regardless of the presence of maternal thyroid autoimmune antibodies. Children at increased risk of developmental problems such as extreme prematurity, birth asphyxia, congenital malformation, and chromosomal abnormalities such as Down syndrome were excluded. Subjects with central hypothyroidism of hypothalamic or pituitary origin were also excluded.
Fig. 1. Flow diagram of subjects who were diagnosed with congenital hypothyroidism. IQ, intelligence quotient.
2. Study design
Children with a positive neonatal screening test were subjected to confirmatory diagnostic tests (serum fT4, T3, and TSH). All children confirmed to have CH immediately started oral L-T4. TSH and thyroid hormone concentrations were monitored every 2–4 weeks for an initial 3 months, followed by every 3–6 months.
At about 3 years of age, L-T4 was stopped. Four weeks later, thyroid function tests and thyroid imaging including ultrasonography and Tc-99m scintigraphy were performed. Imaging tests enabled identification of dysgenesis (agenesis, ectopic thyroid, and hypoplasia). If the thyroid function tests remained normal without L-T4, patients were diagnosed with transient congenital hypothyroidism (TCH), and L-T4 was no longer administered. Otherwise, they were diagnosed with permanent congenital hypothyroidism (PCH) and received continuous hormone replacement therapy. Normal or dyshormonogenesis was defined as requiring treatment reintroduction, while a normally shaped thyroid gland was verified with ultrasound or scan.
Patients with CH were examined at age 5 to 7 years with the Korean Wechsler Intelligence Scale for Children-third edition (K-WISC-III, for those 6–16 years of age)8,9) or the Korean Wechsler Preschool and Primary Scale of Intelligence-third edition (K-WIPSSI-III, for those 4–6 years of age)10). The K-WISC-III consists of a full-scale IQ (FSIQ), verbal IQ (VIQ, 6 of the subtests: information, digit span, vocabulary, arithmetic, comprehension, and similarities) and performance IQ (PIQ, 7 subtests: picture completion, picture arrangement, block design, object assembly, coding, mazes, and symbol search). IQ was classified as follows: ≤69 is extremely low, 70–79 is borderline, 80–89 is low average, 90–109 is average, 110–119 is high average, 120–129 is superior and ≥130 is very superior11).
3. Laboratory measurements
Radioimmunoassay (RIA) methods (Beckman Coulter, Fullerton, CA, USA) were used to determine serum fT4, T3, and TSH concentrations. The analytical sensitivity of RIA was 0.03 ng/dL for fT4, 16.9 ng/dL for T3, and 0.04 µIU/mL for TSH. The reference normal ranges were 0.64–1.72 ng/dL for fT4, 76.2–190.1 ng/dL for T3, and 0.15–5.00 µIU/mL for TSH. The intraassay coefficients of variation (CVs) were 3.1%–10.29% for fT4, 3.7%–-5.3% for T3, and 3.0%–3.7% for TSH; the inter-assay CVs were 2.5%–7.5% for fT4, 4.4%–7.6% for T3, and 2.8%–8.6% for TSH.
4. Statistical analysis
The SPSS ver. 21.0 (SPSS Institute., Chicago, IL, USA) was used for statistical analyses of the data. Values were summarized as means±standard deviation (SD). For classification of clinical parameters according to initial L-T4 dose (low: 6.0–9.9 µg/kg/day or high: 10.0–16.0 µg/kg/day)12), Student t-test was performed for 2 groups with normal distribution. Student t-test was also used to evaluate differences in intellectual outcomes between TCH and PCH. Pearson correlation analysis was performed to evaluate clinical variables, including gestational age, birth weight, age at diagnosis, initial dose of L-T4, initial fT4, and pretreatment TSH, that could affect the FSIQ, PIQ, and VIQ. Clinical variables and IQ scores were compared according to etiology of CH using analysis of variance with Tukey test. For any significant association with IQ score, multiple regression analysis was performed with initial dose of L-T4 (low dose and high dose group), initial fT4, and age at treatment as independent variables. Statistical significance was defined as P<0.05. Results are presented as mean±SD unless stated otherwise.
Results
1. Demographics and clinical data
Of the 43 children included in this study, 22 (51.2%) were female and 21 (48.8%) were male patiens (Table 1). Mean gestational age was 39.03±1.59 weeks (range, 36–43 weeks). Treatment with L-T4 was started after a mean of 27.3±11.1 days (range, 13–60 days) at a mean initial dose of 9.44±3.11 µg/kg/day. K-WISC-III for intellectual outcome was performed at a mean age of 5.9 years (range, 5–7.6 years). The mean IQ score was 103.14±11.68; the mean VIQ score was 99.02±12.01 and the mean PIQ score was 104.81±15.91. An IQ score above average was measured in 38 patients (range, 90–126); 5 patients had an IQ score below 90 (range, 76–89). None had intellectual disability (defined as IQ <70).
Table 1. Comparison of demographic and clinical features in female and male patients with congenital hypothyroidism.
| Variable | Total (n=43) | Female (n=22) | Male (n=21) |
|---|---|---|---|
| Gestational age (wk) | 39.03±1.59 | 39.25±1.24 | 38.86±1.82 |
| Birth weight (g) | 3,293.72±391.61 | 3,377.22±297.38 | 3,222.14±452.29 |
| Age at treatment (day) | 27.30±11.10 | 26.64±10.25 | 28.00±12.14 |
| Weight at treatment (kg) | 4.28±0.95 | 4.24±0.71 | 4.33±1.16 |
| Pretreatment fT4 (ng/dL) | 0.72±0.49 | 0.73±0.49 | 0.71±0.45 |
| Pretreatment TSH (μIU/mL) | 59.18±76.76 | 67.76±95.86 | 50.20±50.60 |
| Initial dosage (μg/kg/day) | 9.44±3.11 | 9.16±2.54 | 9.69±3.67 |
| Age at IQ test (yr) | 5.90±0.65 | 6.01±0.60 | 5.79±0.70 |
| Full-scale IQ | 103.14±11.68 | 101.64±9.66 | 104.71±13.53 |
| Verbal IQ | 99.02±12.01 | 97.05±11.74 | 101.10±12.22 |
| Performance IQ | 104.81±15.91 | 102.14±15.80 | 107.62±15.92 |
| Early treatmenta) | 31 (72.0) | 17 (77.3) | 14 (66.7) |
| Low fT4b) | 22 (51.2) | 11 (50) | 11 (52.4) |
| Type of CH | |||
| Normal or dyshormonogenesis | 26 (60.5) | 10 (45.5) | 16 (76.2) |
| Agenesis | 5 (11.6) | 3 (13.6) | 2 (9.5) |
| Hypoplasia | 5 (11.6) | 2 (9.1) | 3 (14.3) |
| Ectopic | 7 (16.3) | 7 (31.8) | 0 (0) |
Values are presented as mean±standard deviation or number (%).
fT4, free thyroxine; TSH, thyroid-stimulating hormone; IQ, intelligence quotient.
a)Early treatment means that the age at treatment was earlier than 4 weeks. b)Low fT4 means that fT4 levels were lower than 0.64 ng/mL.
2. Effects of clinical and laboratory variables on the IQ of patients with CH
Initially, 21 subjects were treated with a low dose (6.0–9.9 µg/kg/day) and 22 were treated with a high dose (10.0–16.0 µg/kg/day) of L-T4. Initial fT4 levels were significantly different between the low- and high-dose groups. However, at the first follow-up, TSH and serum fT4 levels were not significantly different. There was no statistically significant difference in the mean FSIQ, VIQ, and PIQ scores between the 2 groups (Table 2).
Table 2. Comparison of intellectual outcomes in congenital hypothyroidism patients stratified by starting dose of levothyroxine.
| Variable | L-T4 low dose group (6–9.9 µg/kg/day) (n=21) | L-T4 high dose group (10–16 µg/kg/day) (n=22) | P-value |
|---|---|---|---|
| Age at treatment (day) | 31.38±13.31 | 23.41±6.71 | 0.002 |
| Weight at treatment (kg) | 4.53±1.13 | 4.01±0.62 | 0.005 |
| Pretreatment fT4 (ng/dL) | 0.98±0.39 | 0.46±0.33 | 0.021 |
| Pretreatment TSH (µIU/mL) | 28.27±17.44 | 88.69±98.12 | 0.001 |
| Age at IQ test (yr) | 5.85±0.62 | 5.94±0.68 | 0.843 |
| Full-scale IQ | 106.14±11.10 | 100.27±11.72 | 0.406 |
| Verbal IQ | 100.23±14.15 | 97.86±9.73 | 0.158 |
| Performance IQ | 106.57±17.87 | 103.13±14.00 | 0.787 |
Values are presented as mean±standard deviation.
L-T4, levothyroxine; fT4, free thyroxine; TSH, thyroid-stimulating hormone; IQ, intelligence quotient.
In this study, 31 cases started treatment prior to 4 weeks of age and 12 started treatment after 4 weeks of age. The mean FSIQ, VIQ, and PIQ scores were not significantly different between the patients who began therapy prior to 4 weeks of age and those who began therapy after 4 weeks of age (data not shown).
TCH or PCH was diagnosed in 46.5% (20 of 43) and 53.5% (23 of 43), respectively. The mean serum TSH level at diagnosis was lower in children with TCH compared to those with PCH (33.15±22.72 µIU/mL vs. 81.82±98.24 µIU/mL, respectively, P=0.005). However, no significant differences were found in the means of all IQ scores between children with TCH and PCH (Table 3).
Table 3. Characteristics of patients with transient and permanent congenital hypothyroidism.
| Variable | TCH (n=20) | PCH (n=23) | P-value |
|---|---|---|---|
| Age at treatment (day) | 29.20±14.17 | 25.65±7.46 | 0.003 |
| Weight at treatment (kg) | 4.27±1.12 | 4.28±0.80 | 0.076 |
| Pretreatment fT4 (ng/dL) | 0.86±0.38 | 0.59±0.46 | 0.546 |
| Pretreatment TSH (µIU/mL) | 33.15±22.72 | 81.82±98.24 | 0.005 |
| Initial dosage (µg/kg/day) | 8.66±3.15 | 10.10±2.98 | 0.846 |
| Age at IQ test (yr) | 5.84±0.63 | 5.95±0.67 | 0.933 |
| Full-scale IQ | 105.05±11.22 | 101.47±12.04 | 0.909 |
| Verbal IQ | 100.10±11.55 | 98.08±12.56 | 0.848 |
| Performance IQ | 109.50±13.69 | 100.73±16.86 | 0.610 |
Values are presented as mean±standard deviation.
TCH, transient congenital hypothyroidism; PCH, permanent congenital hypothyroidism; fT4, free thyroxine; TSH, thyroid-stimulating hormone; IQ, intelligence quotient.
Further analysis of etiology was determined by thyroid scan or ultrasound: normal or dyshormonogenesis (n=26), agenesis (n=5), hypoplasia (n=5), and ectopic (n=7). IQ scores did not differ significantly according to etiology.
3. Factors related to intellectual outcomes in CH children at preschool age
Pearson correlation analyses were firstly performed to investigate correlation between several factors (gestational age, birth weight, initial fT4, pretreatment TSH, age at diagnosis, and initial dose of L-T4) and each IQ scores (FSIQ, PIQ, and VIQ), respectively. Only the initial fT4 level was significantly and positively associated with the FSIQ score (r=0.287, P=0.045) (Table 4).
Table 4. Pearson correlation analysis showing the effects of clinical variables on intellectual outcome.
| Variable | Full-scale IQ | Verbal IQ | Performance IQ | |||
|---|---|---|---|---|---|---|
| r | P-value | r | P-value | r | P-value | |
| Pretreatment fT4 (ng/dL) | 0.287 | 0.045 | 0.231 | 0.087 | 0.175 | 0.153 |
| Pretreatment TSH (µIU/mL) | −0.200 | 0.121 | −0.110 | 0.261 | −0.098 | 0.285 |
| Age at treatment (day) | −0.024 | 0.446 | −0.198 | 0.123 | 0.173 | 0.157 |
| Initial dosage (µg/kg/day) | −0.199 | 0.123 | −0.045 | 0.398 | −0.153 | 0.187 |
IQ, intelligence quotient; fT4, free thyroxine; TSH, thyroid-stimulating hormone.
When multivariate regression analysis was performed to identify independent determinants of FSIQ, PIQ, and VIQ scores, respectively, none of initial dose of L-T4 (low dose and high dose group), initial fT4, and age at treatment was not statistically significant determinant (data not shown).
Discussion
In this study, most subjects with CH had normal neurodevelopmental outcomes, and IQ is considered not to be directly related to the clinical variables or to treatment factors based on our results. Initial fT4 level was associated with the FSIQ score, however, no independent determinants for FSIQ, PIQ, and VIQ scores was identified as a result of multivariate regression analysis. There have been a lot of studies on the intellectual outcome in congenital hypothyroidism.
After the onset of NST programs, IQ scores have been improved due to earlier detection and treatment13). Dimitropoulos et al.14) reported that mean IQ scores were within the normal ranges in 63 CH patients at age of 14 years. Another study reported that IQ scores were not significantly different between CH patients and controls7). In Korean study, all patients with CH also had normal range of IQ scores15).
The current treatment guidelines recommend a relatively high starting dose (10–15 µg/kg/day) to achieve rapid TSH normalization and improve intellectual outcomes1,16). Although most studies suggested that a high initial dose of L-T4 resulted in greater improvements in intellectual outcomes compared to a low dose5,17), conflicting results regarding the starting dose of L-T4 have been reported. Bongers-Schokking et al.18) reported that IQ scores did not differ significantly between a low-dose (≤9.5 µg/kg/day) and high-dose group (>9.5 µg/kg/day) when treatment was started prior to 13 days of age. Moreover, a recent study suggested that overtreatment associated with high initial doses of L-T4 during the first 2 years leads to worse neurologic outcomes at 11 years of age7). A recent review of studies of the effects of different starting L-T4 doses on intellectual outcomes suggested that evidence is lacking on whether a high dose is more beneficial than a low dose of L-T4 in the treatment of CH19). In our study, intellectual outcomes were not significantly different according to initial L-T4 dose. These differences could be due to differences in sample size, study design, and intelligence test methods.
In addition to the starting dose of L-T4, several factors, such as age at onset of treatment, pretreatment TSH, and birth weight, could affect intellectual outcomes in CH20). In this study, univariate analyses showed a significant difference in initial fT4 according to FSIQ, but multiple regression analyses showed that initial fT4 was not independently associated with IQ scores. None of factors influencing neurodevelopment were associated with intellectual outcomes in preschool age children. Kim and Lee15) reported that IQ scores were not significantly different in groups according to sex, etiology, or age at diagnosis in 45 Korean children with CH, similar to our study. Tillotson et al.21) reported that subjects with serum T4 levels of less than 3.3 µg/dL (42.8 nmol/L) had a mean IQ score 10.3 points lower than those with serum T4 levels greater than 3.3 µg/dL (42.8 nmol/L). In a Taiwanese study, IQ scores were significantly higher in children with higher initial serum T4 levels (>2 µg/dL) at the time of diagnosis of CH compared to those in children with lower initial T4 levels (106±16 vs. 95±19, P<0.05)22). Additional studies found a correlation between timing of treatment initiation and intellectual outcome. Hulse23) and MacFaul and Grant24) showed that children with CH usually had normal growth and intelligence if treated before age 6 weeks.
This study had several limitations. The population size was small, and may not be adequate for accurate comparison of IQ scores. This was a retrospective study based on hospital charts and did not include a control group. In addition, socioeconomic status (SES) based on parental occupation and maternal education, which may affect intellectual outcomes, was not evaluated. A larger, randomized controlled clinical trial is needed to determine the optimum dose of L-T4 that improves neurologic outcomes.
In conclusion, children with early treated CH diagnosed through a neonatal screening test might have a normal range of IQ scores regardless of etiology, thyroid function, the starting dose of L-T4, or age at treatment.
Footnotes
Conflict of interest: No potential conflict of interest relevant to this article was reported.
References
- 1.American Academy of Pediatrics; Section on Endocrinology and Committee on Genetics, American Thyroid Association; Public Health Committee, Lawson Wilkins Pediatric Endocrine Society. Rose SR, Brown RS, Foley T, et al. Update of newborn screening and therapy for congenital hypothyroidism. Pediatrics. 2006;117:2290–2303. doi: 10.1542/peds.2006-0915. [DOI] [PubMed] [Google Scholar]
- 2.Rovet JF. The role of thyroid hormones for brain development and cognitive function. Endocr Dev. 2014;26:26–43. doi: 10.1159/000363153. [DOI] [PubMed] [Google Scholar]
- 3.Kreisner E, Schermann L, Camargo-Neto E, Gross JL. Predictors of intellectual outcome in a cohort of Brazilian children with congenital hypothyroidism. Clin Endocrinol (Oxf) 2004;60:250–255. doi: 10.1046/j.1365-2265.2004.01974.x. [DOI] [PubMed] [Google Scholar]
- 4.Boileau P, Bain P, Rives S, Toublanc JE. Earlier onset of treatment or increment in LT4 dose in screened congenital hypothyroidism: which as the more important factor for IQ at 7 years? Horm Res. 2004;61:228–233. doi: 10.1159/000076597. [DOI] [PubMed] [Google Scholar]
- 5.Salerno M, Militerni R, Bravaccio C, Micillo M, Capalbo D, Di MS, et al. Effect of different starting doses of levothyroxine on growth and intellectual outcome at four years of age in congenital hypothyroidism. Thyroid. 2002;12:45–52. doi: 10.1089/105072502753451968. [DOI] [PubMed] [Google Scholar]
- 6.Huo K, Zhang Z, Zhao D, Li H, Wang J, Wang X, et al. Risk factors for neurodevelopmental deficits in congenital hypothyroidism after early substitution treatment. Endocr J. 2011;58:355–361. doi: 10.1507/endocrj.k10e-384. [DOI] [PubMed] [Google Scholar]
- 7.Bongers-Schokking JJ, Resing WC, de Rijke YB, de Ridder MA, de Muinck Keizer-Schrama SM. Cognitive development in congenital hypothyroidism: is overtreatment a greater threat than undertreatment. J Clin Endocrinol Metab. 2013;98:4499–4506. doi: 10.1210/jc.2013-2175. [DOI] [PubMed] [Google Scholar]
- 8.Kwak K, Park H, Kim C. The manual for the Korean WISC-III. Seoul: Special Education Publishing Co.; 2002. [Google Scholar]
- 9.Kwak K, Park H, Kim C. A study for the standardization of Korean WISC-3 (1) Korean J Dev Psychol. 2002;15:19–33. [Google Scholar]
- 10.Park H, Kwak K, Park K. Korean-Wechsler preschool and primary scale of intelligence. Seoul: Special Education Publishing Co.; 1995. [Google Scholar]
- 11.Wechsler D. Wechsler preschool and primary scale of intelligence. San Antonio (TX): Psychological Corp.; 1989. [Google Scholar]
- 12.Tuhan H, Abaci A, Cicek G, Anik A, Catli G, Demir K, et al. Levothyroxine replacement in primary congenital hypothyroidism: the higher the initial dose the higher the rate of overtreatment. J Pediatr Endocrinol Metab. 2016;29:133–138. doi: 10.1515/jpem-2015-0047. [DOI] [PubMed] [Google Scholar]
- 13.Rastogi MV, LaFranchi SH. Congenital hypothyroidism. Orphanet J Rare Dis. 2010;5:17. doi: 10.1186/1750-1172-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dimitropoulos A, Molinari L, Etter K, Torresani T, Lang-Muritano M, Jenni OG, et al. Children with congenital hypothyroidism: long-term intellectual outcome after early high-dose treatment. Pediatr Res. 2009;65:242–248. doi: 10.1203/PDR.0b013e31818d2030. [DOI] [PubMed] [Google Scholar]
- 15.Kim S, Lee DH. Intellectual outcome in children with congenital hypothyroidism treated from early life. J Korean Soc Pediatr Endocrinol. 2006;11:45–49. [Google Scholar]
- 16.Léger J, Olivieri A, Donaldson M, Torresani T, Krude H, van Vliet G, et al. European Society for Paediatric Endocrinology consensus guidelines on screening, diagnosis, and management of congenital hypothyroidism. Horm Res Paediatr. 2014;81:80–103. doi: 10.1159/000358198. [DOI] [PubMed] [Google Scholar]
- 17.Rovet JF, Ehrlich RM. Long-term effects of L-thyroxine therapy for congenital hypothyroidism. J Pediatr. 1995;126:380–386. doi: 10.1016/s0022-3476(95)70452-3. [DOI] [PubMed] [Google Scholar]
- 18.Bongers-Schokking JJ, de Muinck Keizer-Schrama SM. Influence of timing and dose of thyroid hormone replacement on mental, psychomotor, and behavioral development in children with congenital hypothyroidism. J Pediatr. 2005;147:768–774. doi: 10.1016/j.jpeds.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 19.Ng SM, Anand D, Weindling AM. High versus low dose of initial thyroid hormone replacement for congenital hypothyroidism. Cochrane Database Syst Rev. 2009;(1):CD006972. doi: 10.1002/14651858.CD006972.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LaFranchi SH, Austin J. How should we be treating children with congenital hypothyroidism? J Pediatr Endocrinol Metab. 2007;20:559–578. doi: 10.1515/jpem.2007.20.5.559. [DOI] [PubMed] [Google Scholar]
- 21.Tillotson SL, Fuggle PW, Smith I, Ades AE, Grant DB. Relation between biochemical severity and intelligence in early treated congenital hypothyroidism: a threshold effect. BMJ. 1994;309:440–445. doi: 10.1136/bmj.309.6952.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsiao PH, Chiu YN, Tsai WY, Su SC, Lee JS, Soong WT. Intellectual outcome of patients with congenital hypothyroidism detected by neonatal screening. J Formos Med Assoc. 2001;100:40–44. [PubMed] [Google Scholar]
- 23.Hulse JA. Outcome for congenital hypothyroidism. Arch Dis Child. 1984;59:23–29. doi: 10.1136/adc.59.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacFaul R, Grant DB. Early detection of congenital hypothyroidism. Arch Dis Child. 1977;52:87–88. doi: 10.1136/adc.52.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]