Abstract
Muscarinic M1/M4 stimulation can reduce abuse-related effects of cocaine and may represent avenues for treating cocaine addiction. Muscarinic antagonists can mimic and enhance effects of cocaine, including discriminative stimulus (SD) effects, but the receptor subtypes mediating those effects are not known. A better understanding of the complex cocaine/muscarinic interactions is needed to evaluate and develop potential muscarinic-based medications. Here, knockout mice lacking M1, M2, or M4 receptors (M1-/-, M2-/-, M4-/-), as well as control wild-type mice and outbred Swiss-Webster mice, were trained to discriminate 10 mg/kg cocaine from saline. Muscarinic receptor antagonists with no subtype selectivity (scopolamine), or preferential affinity at the M1, M2, or M4 subtype (telenzepine, trihexyphenidyl; methoctramine, AQ-RA 741; tropicamide) were tested alone and in combination with cocaine. In intact animals, antagonists with high affinity at M1/M4 receptors partially substituted for cocaine and increased the SD effect of cocaine, while M2-preferring antagonists did not substitute, and reduced the SD effect of cocaine. The cocaine-like effects of scopolamine were absent in M1-/- mice. The cocaine SD attenuating effects of methoctramine were absent in M2-/- mice and almost absent in M1-/- mice. The findings indicate that the cocaine-like SD effects of muscarinic antagonists are primarily mediated through M1 receptors, with a minor contribution of M4 receptors. The data also support our previous findings that stimulation of M1 receptors and M4 receptors can each attenuate the SD effect of cocaine, and show that this can also be achieved by blocking M2 autoreceptors, likely via increased acetylcholine release.
Keywords: Muscarinic receptor, cholinergic, cocaine, knockout mouse, drug discrimination, M1, M2, M4
1. Introduction
There are currently no approved or widely effective medications to curb addiction to psychostimulant drugs like cocaine. Manipulations targeting muscarinic cholinergic systems can modulate abuse-related effects of cocaine, and muscarinic receptors are thus emerging as potential targets for medications development in cocaine addiction [1]. We have previously shown that agonists or positive allosteric modulators that selectively stimulate either the M1 or the M4 muscarinic receptor subtypes attenuate the discriminative stimulus (SD) 1effects and reinforcing effects of cocaine in rats and mice ([2-5]; [6]). Studies in knockout mice that lack M1 receptors (M1-/-), M4 receptors (M4-/-), or both receptors, suggested that both receptor subtypes mediate the attenuation of cocaine's SD effects, while other receptors, likely M3, mediate the rate-suppressing side effects that were observed with less selective ligands [2, 5]. Conversely, muscarinic receptor antagonists increased the SD effect of cocaine, and can produce cocaine-appropriate responding when substituted for cocaine [2, 7-9].
Based on rat studies that used intracranial infusions or lesions, striatal areas appear to be central to mediating both the SD effects of cocaine [10, 11], and the modulation of the cocaine SD effect by muscarinic receptor ligands [12-15]. Striatal tissues express predominantly the M1 and M4 muscarinic receptor subtypes, and lower densities of the M2 subtype: M1 receptors mostly postsynaptically, M4 receptors both pre-and postsynaptically, and M2 receptors mostly as presynaptic inhibitory autoreceptors [16-20]. M2 and M4 receptors modulate the tonic acetylcholine release by striatal cholinergic interneurons, which in turn modulates striatal dopamine release via a nicotinic receptor-dependent mechanism [21-25]. Postsynaptically, M1 and M4 receptors modulate the excitability and activity patterns of GABAergic medium spiny neurons, the striatum's main output neurons. M1 and M4 receptor activation has long been known to produce functional dopamine antagonism, but more recent studies are showing the reciprocal modulation of striatal dopamine and acetylcholine release to be quite complex (for review, see [26-29]).
Here, we used subtype-preferring muscarinic receptor antagonists and knockout mice lacking M1, M2, or M4 receptors to investigate the contributions of specific muscarinic receptor subtypes in the muscarinic/cocaine interaction in the drug discrimination assay. Scopolamine is a non-selective muscarinic antagonist with comparable affinities at the M1 and M3-M5 subtypes and a marginally lower affinity at the M2 subtype [30-32]. Telenzepine is a moderately M1-preferring antagonist, and trihexyphenidyl has about equal affinity at M1 and M4 receptors, with modest selectivity over M2, M3, and M5 subtypes [30, 33, 34]. Tropicamide was reported to be a modestly M4-preferring antagonist with comparable affinities across M1-M3 subtypes [35]. Methoctramine and AQ-RA 741 are M2-preferring antagonists, with methoctramine generally showing a more selective profile than AQ-RA 741 [33, 36-39]. Methoctramine binds at both the orthosteric site and an allosteric site on M2 receptors, but the latter only at high concentrations less likely to be relevant in vivo [40, 41]. Each antagonist was either shown to be fully brain penetrant, or has been inferred to penetrate the central nervous system based on producing effects known to be centrally mediated, following systemic administration [32, 42-44].
2. Materials and Methods
2.1 Animals
Male Swiss-Webster, C57BL/6NTac, M1-/-, M2-/- and M4-/- mice were acquired from Taconic Farms (Germantown, NY) at 4-8 weeks of age, the knockout mice with the permission of Dr. Jürgen Wess. M1-/-, M2-/- and M4-/- mice were generated as described previously [45-47] and backcrossed 11 generations to C57BL/6NTac females. Age- and sex-matched wild-type C57BL/6NTac (Taconic Farms) mice served as controls. Mice were acclimated to the housing facilities for at least 7 days before training began, at no earlier than 7 weeks of age. Mice were kept in a 12-h light/dark cycle, group housed up to four per cage. Experiments were conducted during the light phase of the circadian cycle. Water was accessible ad libitum and food (rodent diet 5001; PMI Feeds, Inc., St. Louis, MO) was provided daily after training/testing sessions, 4 g/mouse/day. Rodent “treats”, nesting material, and exercise/nesting devices were provided for enrichment. Some mice had been tested previously with muscarinic receptor ligands (M1 agonist and/or M4 positive allosteric modulator) before the tests reported here. All procedures were approved by the McLean Hospital Institutional Animal Care and Use Committee and were carried out in accordance with the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research and US laws.
2.2 Training and evaluation in cocaine discrimination
Operant-conditioning chambers and the experimental procedure were as previously described [2]. In brief, each chamber contained two nose-poke holes each equipped with a photocell and a cue light, and a cup into which liquid food was delivered from a syringe pump. Mice were trained to discriminate 10 mg/kg cocaine from saline (i.p.), reinforced with Vanilla-flavored Ensure nutrition drink. 30 reinforcers were available per 20-min session. Mice were trained initially under a FR 1 schedule, then, the ratio was gradually increased to FR 10, with increasing pretreatment time spent in the chamber rather than home cage. Eventually, sessions were preceded by the 10-min pretreatment period in the chamber, during which all lights were off, and responding had no scheduled consequences. Cocaine and saline were presented in pseudorandom order across daily training sessions, typically five days/week, and mice were counterbalanced with cocaine trained on the left or right nose-poke. Stable discrimination was defined as at least 7 of 8 consecutive sessions satisfying the following criteria: 1) ≥10 reinforcers earned per session, 2) ≥80% correct responses for the first reinforcer, and 3) ≥90% correct total responses.
Once criteria were met, mice were tested with saline, 0.32, 1.0, 3.2, 10, and 18 mg/kg cocaine to generate dose-effect functions. The non subtype-selective antagonist scopolamine (0.032–10 mg/kg i.p.), the M1/M4-preferring antagonists telenzepine (3.2-56 mg/kg s.c.) and trihexyphenidyl (0.032– 32 mg/kg i.p.), the M4-preferring antagonist tropicamide (0.1–10 mg/kg s.c.), and the M2-preferring antagonists AQ-RA 741 and methoctramine (0.1–3.2 mg/kg s.c.) were each tested alone (i.e., as “substitution” for cocaine stimulus). In addition, pretreatment/combination tests were conducted, in which cocaine doses were tested in combination with scopolamine (0.32 mg/kg i.p., administered with cocaine as a single injection), tropicamide (0.1 mg/kg s.c.), AQ-RA 741 (0.1–1 mg/kg s.c.), and methoctramine (0.0032–1.0 mg/kg s.c.). Pretreatment times before cocaine injection (when tested in combination) or before session start (when tested alone as substitution) were: telenzepine, 10 min; methoctramine, 15 min; all other drugs, immediately before. Doses were tested within-subjects in a counterbalanced sequence. At least one training session was interspersed between each test session, and tests were only performed when mice satisfied discrimination criteria. If responding was suppressed to less than 10 responses in a session, the quantity of behavior was considered insufficient to evaluate response selection and the percentage of drug-appropriate responding (DAR) was not included in the data set.
2.3 Drugs
Cocaine hydrochloride was supplied by the National Institute on Drug Abuse (National Institutes of Health, Bethesda, MD), Scopolamine hydrobromide, telenzepine dihydrochloride hydrate, trihexyphenidyl hydrochloride, tropicamide, and methoctramine hydrate were purchased from Sigma-Aldrich (St. Louis, MO). AQ-RA 741 was purchased from R&D Systems, Inc (formerly Tocris, Ellisville, MO). Cocaine, scopolamine, telenzepine, and AQ-RA 741 were dissolved in 0.9% saline, methoctramine, in sterile water. Trihexyphenidyl was dissolved by gentle heating in sterile water. Tropicamide was dissolved in ethanol and diluted to ≤1% ethanol in sterile water. All drug doses refer to the weights of the respective salts. Vehicles, route of administration, pretreatment times, and initial dose ranges were selected based on published reports, and adjusted empirically in initial studies [2, 44, 48-51].
2.4 Data analysis
The %DAR for the whole session and total rates of responding (i.e., in both holes) are presented. Comparable effects were observed in %DAR for the first reinforcer, unless stated otherwise. Repeated measures ANOVA were performed with dose of pretreatment drug and/or cocaine or dose of substitution drug, as variables, on %DAR and response rate. For knockout strain studies, ANOVA were performed with genotype as between-subjects variable and drug doses/pretreatments as repeated-measures variables. Occasionally, responding was eliminated or suppressed to the point that no reinforcers were earned during the session; in those cases, no %DAR was calculated for that mouse (i.e., missing value). Data are reported as group means with standard error of the mean. Significance level was set at P<0.05; statistical software was Stata/SE for Mac.
3. Results
3.1 “Cocaine-like” muscarinic receptor antagonists in Swiss-Webster mice
In outbred Swiss-Webster mice, the M1/M4-preferring antagonists telenzepine and trihexyphenidyl, and the M4-preferring antagonist tropicamide, each produced some cocaine-appropriate responding, with peak averages between 38% and 61% (Fig. 1). DAR was related to antagonist dose for telenzepine [F(3,21)=4.07, P<0.05], trihexyphenidyl [F(3,39)=3.68, P<0.05], and tropicamide [F(5,39)=3.82, P<0.01]. Trihexyphenidyl decreased rates of responding at the highest dose tested (main effect [F(3,39)=14.3, P<0.0001]; 32mg/kg vs. vehicle P<0.001). Tropicamide and telenzepine did not affect rates of responding significantly.
Figure 1.
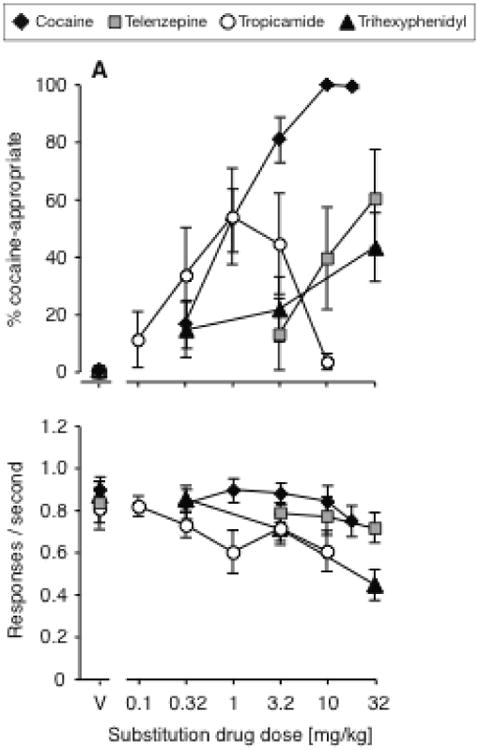
Dose-effect functions for cocaine and muscarinic M1/M4 receptor -preferring antagonists in Swiss-Webster mice, tested as substitutions for the training dose of 10 mg/kg cocaine: telenzepine (N=8), tropicamide (N=9), trihexyphenidyl (N=14), and cocaine in the same cohort (N=19). Data are %DAR (top) and responses per second (bottom), as a function of substitution drug dose.
When telenzepine (3.2 mg/kg) and tropicamide (0.1 mg/kg) were tested in combination with cocaine, each antagonist produced a small shift of the cocaine dose-effect function to the left (Fig. 2), but only the effect of tropicamide reached statistical significance by ANOVA [F(1,63)=10.6, P<0.01]. The effect of cocaine dose on response allocation was always highly significant (P<0.0001). In two mice, telenzepine profoundly suppressed responding at the highest doses (despite no to minimal effects on rates of responding in other mice), which resulted in missing values and consequently reduced statistical power. As an alternative analysis method less affected by missing values, potencies of cocaine were calculated by interpolation in each mouse with and without telenzepine, which confirmed a leftward shift (1.34 mg/kg, 95% confidence interval 0.68 – 2.65 vs. 0.39 [0.23 – 0.65] mg/kg, P<0.01 by paired-sample t-test). Telenzepine produced a small decrease in rates of responding regardless of the cocaine dose [F(1,62)=6.90, P<0.05], while tropicamide did not affect rates.
Figure 2.
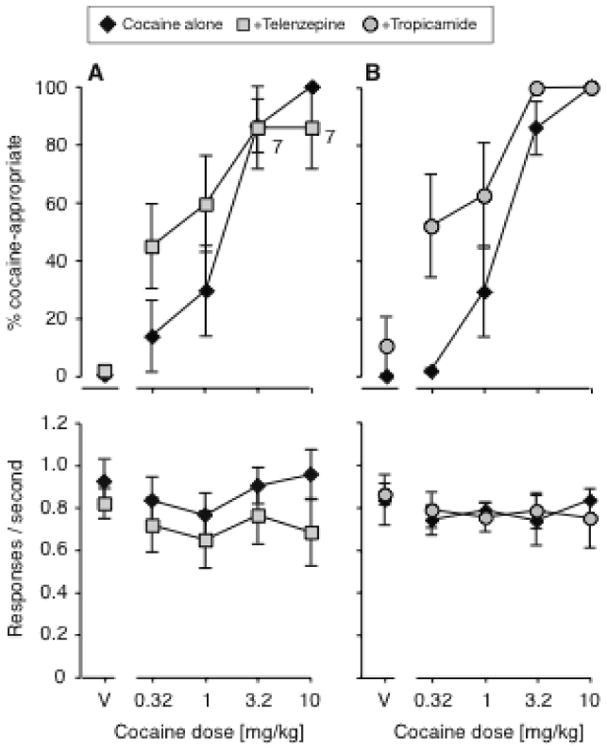
Effects of adding 3.2 mg/kg telenzepine (A, N=9) or 0.1 mg/kg tropicamide (B, N=8) to cocaine in Swiss-Webster mice. Data are %DAR (top) and responses per second (bottom), as a function of cocaine dose. In the top panel, exceptions to the group sizes are indicated on the figure when some mice failed to respond at a given dose.
3.2 “Cocaine-like” muscarinic receptor antagonists in muscarinic receptor knockout mice
Wild-type mice and knockout mice lacking M1, M2, or M4 receptors acquired cocaine discrimination after on average 89.3±5.4, 85.5±5.2, 96.3±5.8, and 129.9±10.5 sessions, respectively. The M4-/- mice required significantly longer training to meet criteria relative to wild-type mice (χ2=13.1, P<0.001). The genotypes did not differ in their cocaine dose-effect functions (Supplemental Fig. 1).
To test the hypothesis that M1 and/or M4 receptors mediated the cocaine-like discriminative stimulus effect of the non subtype-selective muscarinic antagonist scopolamine, scopolamine was tested as substitution in each knockout strain. Tests were aborted in the M2-/- strain due to unexpected toxicity, as the first few M2-/- mice tested with doses of scopolamine above 0.32 mg/kg died within a day. Thus, dose-effect functions for scopolamine substitution were obtained in wild-type mice, M1-/- mice, and M4-/- mice (Fig. 3). DAR was related to both genotype [F(2,71)=3.54, P<0.05] and scopolamine dose [F(4,71)=8.47, P<0.0001]. Scopolamine produced partial substitution, and significant effects on DAR, in the wild-type mice [F(4,25)=7.28, P<0.001] and in the M4-/- mice [F(4,24)=3.41, P<0.05], but not in the M1-/- mice. Rates of responding were also affected differentially (genotype effect [F(2,74)=6.41, P<0.01], scopolamine effect [F(4,74)=6.08, P<0.001].
Figure 3.
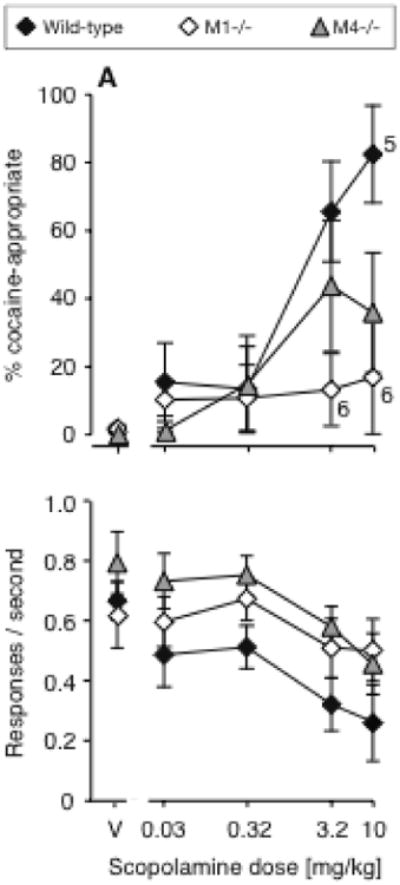
Dose-effect functions for the non subtype-selective antagonist scopolamine in wild-type mice (WT, N=8), M1-/- mice (N=7), and M4-/- mice (N=7), tested as substitutions for the training dose of 10 mg/kg cocaine. No data in the M2-/- mice due to toxicity. Data are %DAR (top) and responses per second (bottom), as a function of scopolamine dose. In the top panel, exceptions to the group sizes are indicated on the figure when some mice failed to respond at a given dose.
A low dose of scopolamine can shift the cocaine discrimination dose-effect function to the left in intact mice [2]. To test the hypothesis that M1 receptors also mediated the potentiation of the cocaine discriminative stimulus effect by scopolamine, we determined cocaine dose-effect functions with and without 0.32 mg/kg scopolamine in wild-type mice, M1-/- mice, M2-/- mice, and M4-/- mice. We observed a qualitative difference between strains, in that scopolamine produced the expected shifts to the left in the wild-type mice, M2-/- mice, and M4-/- mice, but produced a shift to the right in the M1-/- mice (Fig. 4). A three-way ANOVA with the factors genotype, cocaine, and scopolamine confirmed a significant genotype by scopolamine interaction [F(3,237)=6.11, P<0.0001], and follow-up simple effects confirmed a significant effect of scopolamine in each knockout line (P<0.05 to P<0.001). No evidence of toxicity was observed in the M2-/- mice with this dosing. The effect of cocaine dose on DAR was significant in all pretreatment/combinations tests (P<0.0001).
Figure 4.
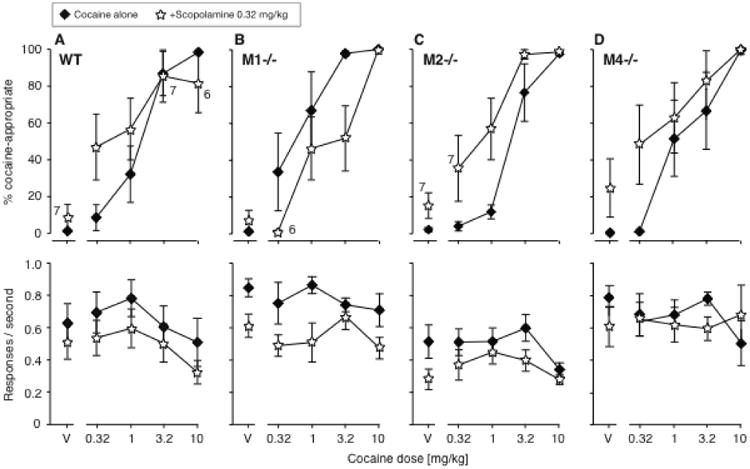
Effects of adding 0.32 mg/kg scopolamine to cocaine in wild-type mice (A, N=8), M1-/- mice (B, N=6), M2-/- mice (C, N=6), and M4-/- mice (D, N=8). Note the qualitatively different effect in the M1-/- mice. Data are %DAR (top) and responses per second (bottom), as a function of cocaine dose. In the top panel, exceptions to the group sizes are indicated on the figure when some mice failed to respond at a given dose.
3.3 “Cocaine-attenuating” muscarinic receptor antagonists in Swiss-Webster mice
The M2-preferring antagonist methoctramine produced little cocaine-appropriate responding per se, with a maximal DAR of 35% at the highest dose (main effect [F(4,26)=3.43, P<0.05]; 3.2mg/kg vs. vehicle P<0.05), (Fig. 5A). Methoctramine was tested over a range of doses, up to doses that produced rate-decreasing effects [F(4,28)=7.21, P<0.001].
Figure 5.
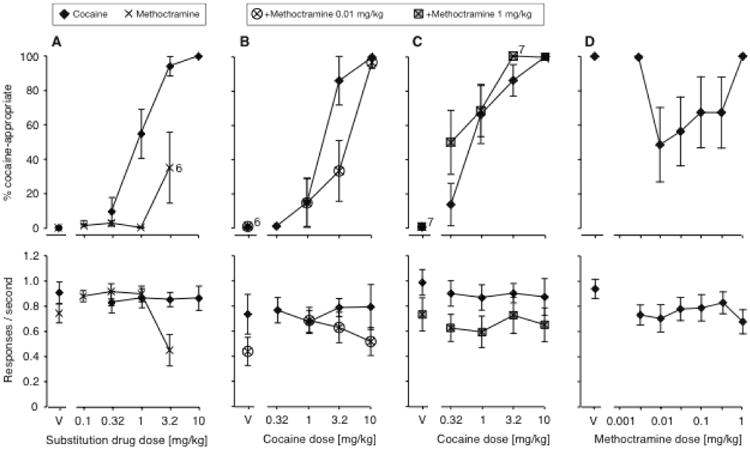
A: Dose-effect functions for cocaine (N=12) and the muscarinic M2 receptor-preferring antagonist methoctramine (N=8) in Swiss-Webster mice, tested as substitutions for the training dose of 10 mg/kg cocaine. B: Effect of adding a 0.01 mg/kg methoctramine to a range of cocaine doses (N=7). C: Effect of adding 1 mg/kg methoctramine to a range of cocaine doses (N=8). D: Dose-response relationship of adding methoctramine to 3.2 mg/kg cocaine in Swiss-Webster mice (N=6). Data are %DAR (top) and responses per second (bottom), as a function of cocaine dose or methoctramine dose. In the top panel, exceptions to the group sizes are indicated on the figure when some mice failed to respond at a given dose.
We then tested methoctramine in combination with cocaine, testing for a shift in the cocaine dose-effect function at two doses of methoctramine, and testing a range of methoctramine doses in combination with 3.2 mg/kg cocaine. As opposed to scopolamine and the M1/M4 receptor-preferring antagonists telenzepine, trihexyphenidyl, and tropicamide, methoctramine moderately attenuated the cocaine discriminative stimulus effect in Swiss-Webster mice: administration of 0.01 mg/kg methoctramine produced a small shift of the dose-effect function to the right (Fig. 5B; methoctramine effect [F(1,53)=4.34, P<0.05], cocaine by methoctramine interaction [F(4,53)=2.67, P<0.05]. At 1.0 mg/kg, methoctramine had no signignificant effect on DAR (in fact showed a trend to shift the cocaine curve to the left), and moderately decreased rates of responding ([F(1,63)=17.6, P=0.0001; Fig. 5C). This “biphasic” effect was also apparent when testing a range of methoctramine doses with 3.2 mg/kg cocaine, in which doses from 0.01 to 0.32 mg/kg methoctramine decreased DAR (main effect of methoctramine dose [F(6,30)=3.28, P<0.05]), but 1 mg/kg had no effect (Fig. 5D).
In an effort to replicate those findings, the putative M2-preferring antagonist AQ-RA 741 was also tested. AQ-RA 741 alone produced no cocaine-appropriate responding in a range of doses previously shown to be active in vivo (no effect of dose on DAR or rate; see Supplemental Fig. 2A). However, the selectivity of AQ-RA 741 is modest, and the usefulness of those data is limited by the fact that higher, rate-suppressing doses were not tested, out of concerns for potential toxicity. Administration of 1.0 mg/kg AQ-RA 741 produced a small shift of the cocaine dose-effect function to the right (Supplemental Fig. 2B), although the effect only reached statistical significance for the first reinforcer (AQ-RA 741 dose [F(1,62)=4.45, P<0.05], AQ-RA 741 by cocaine interaction [F(4,62)=2.98, P<0.05]), but not for total-session responding. A range of AQ-RA 741 doses were tested with 3.2 mg/kg cocaine (Supplemental Fig. 2C). Again, AQ-RA 741 produced moderate decreases in DAR that was significant for first-reinforcer responding [F(3,15)=4.43, P<0.05] (total session analysis: P=0.06). Rates of responding were not significantly affected.
3.4 “Cocaine-attenuating” muscarinic receptor antagonists in muscarinic receptor knockout mice
Because methoctramine has relatively low selectivity for the M2 subtype, we wanted to test the hypothesis that effects on cocaine discrimination were mediated through M2 receptors. To this end, we tested 0.032 mg/kg methoctramine as pretreatment to cocaine in wild-type mice, M1-/- mice, M2-/- mice, and M4-/- mice (Fig. 6). A three-way ANOVA with the factors genotype, cocaine dose and methoctramine dose confirmed a significant genotype by methoctramine interaction [F(3,205)=2.97, P<0.05]. As hypothesized, methoctramine had no effect in the M2-/- mice (Fig. 6C), while the moderate rightward shift observed in the Swiss-Webster mice was confirmed in the wild-type mice (P=0.001; Fig. 6A). The effect appeared intact in the M4-/- mice (P<0.01; Fig. 6D), but was diminished in the M1-/- mice, in which the shift was not significant (Fig. 6B).
Figure 6.
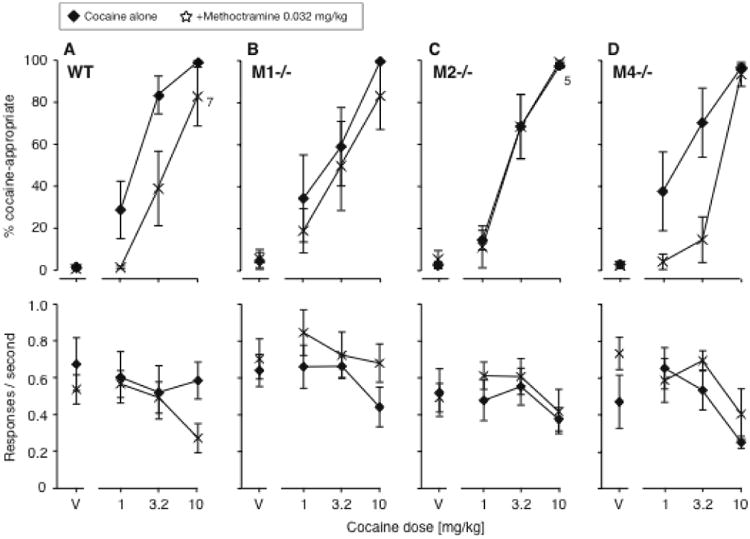
Effects of adding 0.032 mg/kg methoctramine to cocaine in wild-type mice (A, N=8), M1-/- mice (B, N=6), M2-/- mice (C, N=6), and M4-/- mice (D, N=6). Data are %DAR (top) and responses per second (bottom), as a function of cocaine dose. In the top panel, exceptions to the group sizes are indicated on the figure when some mice failed to respond at a given dose.
4. Discussion
Muscarinic antagonists can partially mimic and increase the SD effects of cocaine, but the muscarinic receptor subtypes involved in these effects are not known. We tested muscarinic receptor antagonists with no subtype selectivity, or moderately preferential affinity at the M1, M2 or M4 subtype, in wild-type mice, M1-/- mice, M2-/- mice and M4-/- mice trained to discriminate 10 mg/kg cocaine from saline. We found that the non-selective antagonist scopolamine or antagonists with relatively higher affinity at M1/M4 receptor subtypes produced some cocaine-appropriate responding per se, and produced leftward shifts in the cocaine SD effect curve in intact animals. Experiments in the knockout mice further indicated that M1 receptors, rather than M4 receptors, mediate the cocaine-like SD effects of scopolamine. In contrast, antagonists with relatively higher affinity at the M2 receptor subtype attenuated the cocaine SD effect. Experiments in knockout mice also supported the notion that M2 receptors mediate the cocaine SD attenuating effects of methoctramine.
4.1 Cocaine-like effects of non-selective and M1/M4-preferring antagonists in intact mice
We previously showed that scopolamine produced cocaine-appropriate responding in Swiss-Webster mice (partial substitution with an inverted U-shaped curved), and that a sub-threshold dose of scopolamine shifted the cocaine dose-effect curve to the left [2]. Methylscopolamine, which has poor brain penetration, was less potent than scopolamine in producing these effects, indicating a centrally mediated effect [2]. This is in agreement with earlier studies in rats, in which the non subtype-selective muscarinic antagonists atropine and scopolamine produced leftward shifts in cocaine's SD effects [7, 8]. Potentiation of psychostimulant effects by muscarinic receptor blockade has been observed consistently in mice, rats, and non-human primates across a range of endpoints, including locomotor activity, stereotypies, and intravenous self-administration [8, 52-55]. Isobolographic analyses confirmed that the effects of cocaine-scopolamine combinations were more than additive both in drug discrimination in mice, and in locomotor activity in rats, implying that muscarinic receptor antagonists and cocaine produce their effects through different brain pathways [2, 56]. The interaction likely involves dopamine release, as systemic administration of muscarinic antagonists induces striatal dopamine release in humans and rats, and potentiates cocaine-induced dopamine increases [34, 57, 58].
Here, M1 and/or M4-preferring antagonists produced some cocaine-appropriate responding, comparable to results obtained previously with scopolamine [2]. Previous investigations similarly found that the moderately M1-preferring antagonists telenzepine and trihexyphenidyl potentiated the locomotor stimulant and/or SD effects of cocaine in rats [9, 34]. Tropicamide was described as somewhat M4-preferring, although recent data suggest that is a relatively nonspecific muscarinic antagonist [35, 59]. Effects of M4 receptor blockade on cocaine's SD effects have not been reported previously, but our findings are consistent with a report that tropicamide increased or prolonged cocaine-induced stereotypies in rats [52]. The notion that blocking either M1 receptors or M4 receptors increases effects of cocaine is also consistent with our findings that, conversely, pharmacological stimulation of M1 and/or M4 receptors attenuated the SD effects and reinforcing effects of cocaine in rats and mice ([2-5]; [6]). Taken together, this first data set suggests that M1 and/or M4 receptor blockade at least partly mediate the effects of scopolamine in the cocaine discrimination assay.
4.2 Cocaine-like effects of muscarinic antagonists in muscarinic receptor knockout mice
Brain-penetrant muscarinic receptor antagonists with a high degree of selectivity for each of the five receptor subtypes are still being developed, and we therefore used a combination of subtype-preferring ligands and receptor knockout mice to determine the contributions of individual receptor subtypes to the above effects. Cocaine produced comparable SD dose-effect functions in all four strains – wild-type, M1-/-, M2-/-, and M4-/-. In contrast, substitution of scopolamine for cocaine revealed striking differences: scopolamine produced over 80% cocaine-appropriate responding in the wild-type mice, but no appreciable cocaine-appropriate responding in the M1-/- mice in the same dose range. Partial substitution was observed in the M4-/- mice. Although we cannot exclude that higher doses of scopolamine could have produced some cocaine-appropriate responding in the knockout mice, these findings indicate that M1 receptors are necessary for scopolamine to produce cocaine-SD like effects, while M4 receptors may contribute partially to the effect.
When we tested scopolamine in combination with cocaine, the M1-/- mice again showed a qualitatively different effect: whereas scopolamine produced leftward shifts in the cocaine dose-effect function in wild-type mice, M2-/- mice, and M4-/- mice, M1-/- mice showed a rightward shift. Thus, the cocaine-potentiating effect of scopolamine appears to be dependent upon blockade of M1 receptors.
These findings illustrate the opposing modulatory effects of different muscarinic receptor subtypes on striatal dopaminergic transmission (for review, see [28]; Thomsen et al. 2017 under review). In the absence of M1 receptors, scopolamine would block M2-M5 receptors, and the fact that scopolamine produced a rightward shift, not leftward shift, in M1-/- mice, suggests that blocking M4 receptors alone is not sufficient to potentiate the effects of cocaine. Rather, antagonism of M2 receptors and the resulting increase in acetylcholine tone appears to produce sufficient stimulation of M4 receptors to attenuate cocaine's effects, consistent with the effect of an M4 positive allosteric modulator in the same assay [6]. It is possible that effects at M5 receptors play a role as well [66, 67]. M1-/- mice (but not M2-/- mice or M4-/- mice) showed plasma levels of scopolamine roughly twice as high as the wild-type controls 30 min after an intraperitoneal injection of 1 mg/kg, as well as a trend for higher brain levels [32]. However, given that scopolamine produced cocaine-like effects up to at least 10 mg/kg in wild-type mice, it seems unlikely that this slightly higher blood level would account for the complete reversal of effect observed here between the wild-type mice and the M1-/- mice.
The scopolamine-induced leftward shift in cocaine SD was preserved or if anything larger in the M2-/- mice and M4-/- mice relative to wild-type mice. Loss of inhibitory autoreceptors may be expected to lead to increased extracellular levels of acetylcholine, and/or reduced effect of scopolamine on acetylcholine release. Indeed, these effects were observed in M2-/- mice, M4-/- mice, and M2-/-M4-/- double knockout mice [25, 68]. Thus, loss of scopolamine-increased acetylcholine tone (which would tend to attenuate the cocaine SD effect) would unmask the M1 receptor-mediated potentiation of the cocaine SD effect. These data suggest that M1 receptors, not M4 receptors, play the major role in scopolamine-potentiated cocaine SD effects. Studies using M1-/- mice, M4-/- mice, and double M1-/-M4-/-mice similarly indicated that M1 receptors, not M4 receptors, mediated the disruption of prepulse inhibition of the startle response by scopolamine, another effect that is shared between muscarinic receptor antagonists and psychomotor stimulant drugs [69].
4.3 Cocaine-attenuating effects of M2-preferring antagonists
In contrast to the M1/M4 receptors, we did not anticipate that blockade of the (primarily presynaptic) M2 receptors would produce cocaine-like effects in the drug discrimination assay. Indeed, neither methoctramine nor AQ-RA 741 substituted for cocaine in intact mice, with the caveat that neither ligand is highly selective for the M2 receptor, and that neither ligand was tested at doses that produced strong behavioral suppression, due to expected risks of toxicity. To evaluate the effects of methoctramine in combination with cocaine, we therefore tested a wide range of doses (two and a half log units), including doses that may bind M2 receptors preferentially, and doses that may have off-target effects. This produced a biphasic dose-effect function, from an ineffective, sub-threshold dose, over a range of doses that decreased cocaine-appropriate responding, to a complete reversal of effect this at the highest dose. A biphasic dose-effect function of methoctramine has been observed previously, similarly attributed to recruitment of non-M2 receptors [60]. M2 receptor antagonists reduced the SD effects of 3.2 mg/kg cocaine by up to 50% in Swiss-Webster mice, an effect similar to that produced by M1/M4 agonists [2, 5]. When tested against a range of cocaine doses, a small rightward shift was obtained.
Brain M2 receptors are mainly inhibitory autoreceptors [61, 62]. M2 receptors serve as autoreceptors throughout the brain with the probable exception of the nucleus accumbens, while M4 receptors appear to serve this function only in the striatum [18, 21, 23, 25]. Consistent with this function, in vitro and in vivo studies have shown that M2-preferring antagonists increase acetylcholine in dorsal striatum, hippocampus, and cortex [39, 60, 63-65]. We therefore hypothesize that the M2 antagonists attenuated the cocaine SD effect indirectly, through stimulation of brain M1 and/or M4 receptors by endogenous acetylcholine. Nicotinic receptors, which also modulate dopamine release in the dorsal and ventral striatum, could also be involved [23]. A complete lack of effect of methoctramine in the M2-/- mice supports the interpretation that methoctramine attenuated the cocaine SD effect in intact mice via antagonism of M2 receptors. Although rat studies indicated that the nucleus accumbens is important in mediating the SD effects of cocaine [10, 11], the above results suggest that the effect of methoctramine is likely mediated at least partially in dorsal striatum (and/or other brain regions) rather than the nucleus accumbens, because M4 receptors, not M2 receptors, serve as autoreceptors in the accumbens [23]. While methoctramine produced a rightward shift of the cocaine curve in wild-type mice and in the M4-/- mice, the effect was strongly attenuated in the M1-/- mice. This is perhaps surprising in the light of the effects of scopolamine in the knockout mice, but may suggest that stimulation of postsynaptic M1 receptors plays a more important role relative to M4 receptors in attenuating the cocaine SD.
4.4 Conclusions
Taken together, the present findings suggest that cocaine-like SD effects of muscarinic antagonists are primarily mediated through muscarinic M1 receptors, with a minor contribution of M4 receptors. The data are in agreement with the notion that stimulation of M1 receptors and M4 receptors can each attenuate the SD effect of cocaine, not only through direct pharmacological stimulation as shown previously ([2, 5]; [6]), but also through increased cholinergic tone due to blockade of M2 autoreceptors. Finally, the findings also suggest that the cocaine-modulating effects of muscarinic receptor manipulations are likely not mediated entirely in the nucleus accumbens, but involve dorsal striatum or other brain regions as well.
Supplementary Material
Highlights.
Muscarinic antagonists produce cocaine-like effects mainly via M1 receptors
M2-preferring antagonists attenuate cocaine effects
M2 antagonist effects are likely mediated by M1/M4 receptors via increased acetylcholine
Acknowledgments
This research was supported by grant DA027825 from the National Institutes on Drug Abuse (MT). LJ was supported an Undergraduate Research Opportunity Program fellowship from the Howard Hughes Medical Institute (principal mentor: Dr. S. Barak Caine, Harvard Medical School and Boston University). MT was supported by funds from Psychiatric Center Copenhagen while preparing most of the manuscript. We thank professor Jürgen Wess (National Institutes of Diabetes and Digestive and Kidney Diseases) for providing the muscarinic receptor knockout mice. We thank Jeffrey Wessell, Mark Fusunyan, Charlotte Bavley, and Rachel Hart for technical assistance. The sponsors had no role in study design, data collection, analysis, or interpretation, manuscript preparation, or in the decision to publish.
Footnotes
The authors declare that they have no conflict of interest.
Abbreviations: SD discriminative stimulus, DAR: drug-appropriate responding
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kruse AC, Kobilka BK, Gautam D, Sexton PM, Christopoulos A, Wess J. Muscarinicacetylcholine receptors: novel opportunities for drug development. Nat Rev Drug Discov. 2014;13(7):549–60. doi: 10.1038/nrd4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomsen M, Conn PJ, Lindsley C, Wess J, Boon JY, Fulton BS, Fink-Jensen A, Caine SB. Attenuation of cocaine's reinforcing and discriminative stimulus effects via muscarinic M1 acetylcholine receptor stimulation. The Journal of pharmacology and experimental therapeutics. 2010;332(3):959–69. doi: 10.1124/jpet.109.162057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dencker D, Weikop P, Sorensen G, Woldbye DP, Wortwein G, Wess J, Fink-Jensen A. An allosteric enhancer of M(4) muscarinic acetylcholine receptor function inhibits behavioral and neurochemical effects of cocaine. Psychopharmacology (Berl) 2012;224(2):277–87. doi: 10.1007/s00213-012-2751-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomsen M, Fulton BS, Caine SB. Acute and chronic effects of the M1/M4-preferring muscarinic agonist xanomeline on cocaine vs. food choice in rats. Psychopharmacology (Berl) 2014;231(3):469–79. doi: 10.1007/s00213-013-3256-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomsen M, Lindsley CW, Conn PJ, Wessell JE, Fulton BS, Wess J, Caine SB. Contribution of both M1 and M4 receptors to muscarinic agonist-mediated attenuation of the cocaine discriminative stimulus in mice. Psychopharmacology (Berl) 2012;220(4):673–85. doi: 10.1007/s00213-011-2516-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dall CW, Weikop P, Dencker D, Molander AC, örtwein GW, Conn PJ, Fink-Jensen A, Thomsen M. Muscarinic receptor M4 positive allosteric modulators attenuate central effects of cocaine. Drug and Alcohol Dependence accepted for publication. 2017 doi: 10.1016/j.drugalcdep.2017.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Acri JB, Siedleck BK, Witkin JM. Effects of benztropine on behavioral and toxic effects of cocaine: comparison with atropine and the selective dopamine uptake inhibitor 1-[2-(diphenylmethoxy)ethyl]-4-(3-phenyl-propyl)-piperazine. The Journal of pharmacology and experimental therapeutics. 1996;277(1):198–206. [PubMed] [Google Scholar]
- 8.Katz JL, Izenwasser S, Kline RH, Allen AC, Newman AH. Novel 3alpha-diphenylmethoxytropane analogs: selective dopamine uptake inhibitors with behavioral effects distinct from those of cocaine. The Journal of pharmacology and experimental therapeutics. 1999;288(1):302–15. [PubMed] [Google Scholar]
- 9.Tanda G, Katz JL. Muscarinic preferential M(1) receptor antagonists enhance the discriminative-stimulus effects of cocaine in rats. Pharmacol Biochem Behav. 2007;87(4):400–4. doi: 10.1016/j.pbb.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Callahan PM, De La Garza R, 2nd, Cunningham KA. Discriminative stimulus properties of cocaine: modulation by dopamine D1 receptors in the nucleus accumbens. Psychopharmacology (Berl) 1994;115(1-2):110–4. doi: 10.1007/BF02244759. [DOI] [PubMed] [Google Scholar]
- 11.Wood DM, Emmett-Oglesby MW. Mediation in the nucleus accumbens of the discriminative stimulus produced by cocaine. Pharmacol Biochem Behav. 1989;33(2):453–7. doi: 10.1016/0091-3057(89)90529-7. [DOI] [PubMed] [Google Scholar]
- 12.Hikida T, Kaneko S, Isobe T, Kitabatake Y, Watanabe D, Pastan I, Nakanishi S. Increased sensitivity to cocaine by cholinergic cell ablation in nucleus accumbens. Proc Natl Acad Sci U S A. 2001;98(23):13351–4. doi: 10.1073/pnas.231488998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hikida T, Kitabatake Y, Pastan I, Nakanishi S. Acetylcholine enhancement in the nucleus accumbens prevents addictive behaviors of cocaine and morphine. Proc Natl Acad Sci U S A. 2003;100(10):6169–73. doi: 10.1073/pnas.0631749100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mark GP, Kinney AE, Grubb MC, Zhu X, Finn DA, Mader SL, Berger SP, Bechtholt AJ. Injection of oxotremorine in nucleus accumbens shell reduces cocaine but not food self-administration in rats. Brain Res. 2006;1123(1):51–9. doi: 10.1016/j.brainres.2006.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith JE, Co C, Yin X, Sizemore GM, Liguori A, Johnson WE, 3rd, Martin TJ. Involvement of cholinergic neuronal systems in intravenous cocaine self-administration. Neurosci Biobehav Rev. 2004;27(8):841–50. doi: 10.1016/j.neubiorev.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Bernard V, Normand E, Bloch B. Phenotypical characterization of the rat striatal neurons expressing muscarinic receptor genes. J Neurosci. 1992;12(9):3591–600. doi: 10.1523/JNEUROSCI.12-09-03591.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hersch SM, Gutekunst CA, Rees HD, Heilman CJ, Levey AI. Distribution of M1-M4 muscarinic receptor proteins in the rat striatum: light and electron microscopic immunocytochemistry using subtype-specific antibodies. J Neurosci. 1994;14z(5 Pt 2):3351–63. doi: 10.1523/JNEUROSCI.14-05-03351.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hersch SM, Levey AI. Diverse pre- and post-synaptic expression of M1-M4 muscarinic receptor proteins in neurons and afferents in the rat neostriatum. Life Sci. 1995;56(11-12):931–8. doi: 10.1016/0024-3205(95)00030-a. [DOI] [PubMed] [Google Scholar]
- 19.Smiley JF, Levey AI, Mesulam MM. M2 muscarinic receptor immunolocalization in cholinergic cells of the monkey basal forebrain and striatum. Neuroscience. 1999;90(3):803–14. doi: 10.1016/s0306-4522(98)00527-2. [DOI] [PubMed] [Google Scholar]
- 20.Weiner DM, Levey AI, Brann MR. Expression of muscarinic acetylcholine and dopamine receptor mRNAs in rat basal ganglia. Proc Natl Acad Sci U S A. 1990;87(18):7050–4. doi: 10.1073/pnas.87.18.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonsi P, Martella G, Cuomo D, Platania P, Sciamanna G, Bernardi G, Wess J, Pisani A. Loss of muscarinic autoreceptor function impairs long-term depression but not long-term potentiation in the striatum. J Neurosci. 2008;28(24):6258–63. doi: 10.1523/JNEUROSCI.1678-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cachope R, Mateo Y, Mathur BN, Irving J, Wang HL, Morales M, Lovinger DM, Cheer JF. Selective activation of cholinergic interneurons enhances accumbal phasic dopamine release: setting the tone for reward processing. Cell reports. 2012;2(1):33–41. doi: 10.1016/j.celrep.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Threlfell S, Clements MA, Khodai T, Pienaar IS, Exley R, Wess J, Cragg SJ. Striatal muscarinic receptors promote activity dependence of dopamine transmission via distinct receptor subtypes on cholinergic interneurons in ventral versus dorsal striatum. J Neurosci. 2010;30(9):3398–408. doi: 10.1523/JNEUROSCI.5620-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Threlfell S, Lalic T, Platt NJ, Jennings KA, Deisseroth K, Cragg SJ. Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron. 2012;75(1):58–64. doi: 10.1016/j.neuron.2012.04.038. [DOI] [PubMed] [Google Scholar]
- 25.Zhang W, Basile AS, Gomeza J, Volpicelli LA, Levey AI, Wess J. Characterization of central inhibitory muscarinic autoreceptors by the use of muscarinic acetylcholine receptor knockout mice. J Neurosci. 2002;22(5):1709–17. doi: 10.1523/JNEUROSCI.22-05-01709.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Exley R, Cragg SJ. Presynaptic nicotinic receptors: a dynamic and diverse cholinergic filter of striatal dopamine neurotransmission. Br J Pharmacol. 2008;153(1):S283–97. doi: 10.1038/sj.bjp.0707510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldberg JA, Ding JB, Surmeier DJ. Muscarinic modulation of striatal function and circuitry. Handbook of experimental pharmacology. 2012;(208):223–41. doi: 10.1007/978-3-642-23274-9_10. [DOI] [PubMed] [Google Scholar]
- 28.Oldenburg IA, Ding JB. Cholinergic modulation of synaptic integration and dendritic excitability in the striatum. Curr Opin Neurobiol. 2011;21(3):425–32. doi: 10.1016/j.conb.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Surmeier DJ, Graybiel AM. A feud that wasn't: acetylcholine evokes dopamine release in the striatum. Neuron. 2012;75(1):1–3. doi: 10.1016/j.neuron.2012.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bolden C, Cusack B, Richelson E. Antagonism by antimuscarinic and neuroleptic compounds at the five cloned human muscarinic cholinergic receptors expressed in Chinese hamster ovary cells. The Journal of pharmacology and experimental therapeutics. 1992;260(2):576–80. [PubMed] [Google Scholar]
- 31.Huang F, Buchwald P, Browne CE, Farag HH, Wu WM, Ji F, Hochhaus G, Bodor N. Receptor binding studies of soft anticholinergic agents. AAPS PharmSci. 2001;3(4):E30. doi: 10.1208/ps030430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Witkin JM, Overshiner C, Li X, Catlow JT, Wishart GN, Schober DA, Heinz BA, Nikolayev A, Tolstikov VV, Anderson WH, Higgs RE, Kuo MS, Felder CC. M1 and M2 muscarinic receptor subtypes regulate antidepressant-like effects of the rapidly acting antidepressant scopolamine, The Journal of pharmacology and experimental therapeutics. 2014;351(2):448–56. doi: 10.1124/jpet.114.216804. [DOI] [PubMed] [Google Scholar]
- 33.Dorje F, Wess J, Lambrecht G, Tacke R, Mutschler E, Brann MR. Antagonist binding profiles of five cloned human muscarinic receptor subtypes. The Journal of pharmacology and experimental therapeutics. 1991;256(2):727–33. [PubMed] [Google Scholar]
- 34.Tanda G, Ebbs AL, Kopajtic TA, Elias LM, Campbell BL, Newman AH, Katz JL. Effects of muscarinic M1 receptor blockade on cocaine-induced elevations of brain dopamine levels and locomotor behavior in rats. The Journal of pharmacology and experimental therapeutics. 2007;321(1):334–44. doi: 10.1124/jpet.106.118067. [DOI] [PubMed] [Google Scholar]
- 35.Lazareno S, Farries T, Birdsall NJ. Pharmacological characterization of guanine nucleotide exchange reactions in membranes from CHO cells stably transfected with human muscarinic receptors M1-M4. Life Sci. 1993;52(5-6):449–56. doi: 10.1016/0024-3205(93)90301-i. [DOI] [PubMed] [Google Scholar]
- 36.Doods HN, Quirion R, Mihm G, Engel W, Rudolf K, Entzeroth M, Schiavi GB, Ladinsky H, Bechtel WD, Ensinger HA, et al. Therapeutic potential of CNS-active M2 antagonists: novel structures and pharmacology. Life Sci. 1993;52(5-6):497–503. doi: 10.1016/0024-3205(93)90307-o. [DOI] [PubMed] [Google Scholar]
- 37.Eglen RM, Nahorski SR. The muscarinic M(5) receptor: a silent or emerging subtype? Br J Pharmacol. 2000;130(1):13–21. doi: 10.1038/sj.bjp.0703276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gillberg PG, Sundquist S, Nilvebrant L. Comparison of the in vitro and in vivo profiles of tolterodine with those of subtype-selective muscarinic receptor antagonists. Eur J Pharmacol. 1998;349(2-3):285–92. doi: 10.1016/s0014-2999(98)00214-3. [DOI] [PubMed] [Google Scholar]
- 39.Tumiatti V, Minarini A, Milelli A, Rosini M, Buccioni M, Marucci G, Ghelardini C, Bellucci C, Melchiorre C. Structure-activity relationships of methoctramine-related polyamines as muscarinic antagonist: effect of replacing the inner polymethylene chain with cyclic moieties. Bioorg Med Chem. 2007;15(6):2312–21. doi: 10.1016/j.bmc.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 40.Giraldo E, Micheletti R, Montagna E, Giachetti A, Vigano MA, Ladinsky H, Melchiorre C. Binding and functional characterization of the cardioselective muscarinic antagonist methoctramine. The Journal of pharmacology and experimental therapeutics. 1988;244(3):1016–20. [PubMed] [Google Scholar]
- 41.Jakubik J, Zimcik P, Randakova A, Fuksova K, El-Fakahany EE, Dolezal V. Molecular mechanisms of methoctramine binding and selectivity at muscarinic acetylcholine receptors. Mol Pharmacol. 2014;86(2):180–92. doi: 10.1124/mol.114.093310. [DOI] [PubMed] [Google Scholar]
- 42.Bymaster FP, Heath I, Hendrix JC, Shannon HE. Comparative behavioral and neurochemical activities of cholinergic antagonists in rats. The Journal of pharmacology and experimental therapeutics. 1993;267(1):16–24. [PubMed] [Google Scholar]
- 43.Freedman SB, Harley EA, Patel S. Direct measurement of muscarinic agents in the central nervous system of mice using ex vivo binding. Eur J Pharmacol. 1989;174(2-3):253–60. doi: 10.1016/0014-2999(89)90317-8. [DOI] [PubMed] [Google Scholar]
- 44.Howell G, 3rd, West L, Jenkins C, Lineberry B, Yokum D, Rockhold R. In vivo antimuscarinic actions of the third generation antihistaminergic agent, desloratadine. BMC Pharmacol. 2005;5:13. doi: 10.1186/1471-2210-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gomeza J, Shannon H, Kostenis E, Felder C, Zhang L, Brodkin J, Grinberg A, Sheng H, Wess J. Pronounced pharmacologic deficits in M2 muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci U S A. 1999;96(4):1692–7. doi: 10.1073/pnas.96.4.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gomeza J, Zhang L, Kostenis E, Felder C, Bymaster F, Brodkin J, Shannon H, Xia B, Deng C, Wess J. Enhancement of D1 dopamine receptor-mediated locomotor stimulation in M(4) muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci U S A. 1999;96(18):10483–8. doi: 10.1073/pnas.96.18.10483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miyakawa T, Yamada M, Duttaroy A, Wess J. Hyperactivity and intact hippocampus-dependent learning in mice lacking the M1 muscarinic acetylcholine receptor. J Neurosci. 2001;21(14):5239–50. doi: 10.1523/JNEUROSCI.21-14-05239.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Betz AJ, McLaughlin PJ, Burgos M, Weber SM, Salamone JD. The muscarinic receptor antagonist tropicamide suppresses tremulous jaw movements in a rodent model of parkinsonian tremor: possible role of M4 receptors. Psychopharmacology (Berl) 2007;194(3):347–59. doi: 10.1007/s00213-007-0844-6. [DOI] [PubMed] [Google Scholar]
- 49.Knuepfer MM. Muscarinic cholinergic and beta-adrenergic contribution to hindquarters vasodilation and cardiac responses to cocaine. The Journal of pharmacology and experimental therapeutics. 2003;306(2):515–22. doi: 10.1124/jpet.103.051177. [DOI] [PubMed] [Google Scholar]
- 50.Li Z, Bonhaus DW, Huang M, Prus AJ, Dai J, Meltzer HY. AC260584 (4-[3-(4-butylpiperidin-1-yl)-propyl]-7-fluoro-4H-benzo1,[4]oxazin-3-one), a selective muscarinic M1 receptor agonist, increases acetylcholine and dopamine release in rat medial prefrontal cortex and hippocampus. Eur J Pharmacol. 2007;572(2-3):129–37. doi: 10.1016/j.ejphar.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 51.Thomsen M, Wortwein G, Fink-Jensen A, Woldbye DP, Wess J, Caine SB. Decreased prepulse inhibition and increased sensitivity to muscarinic, but not dopaminergic drugs in M5 muscarinic acetylcholine receptor knockout mice, Psychopharmacology (Berl) 2007;192(1):97–110. doi: 10.1007/s00213-006-0682-y. [DOI] [PubMed] [Google Scholar]
- 52.Aliane V, Perez S, Bohren Y, Deniau JM, Kemel ML. Key role of striatal cholinergic interneurons in processes leading to arrest of motor stereotypies. Brain: a journal of neurology. 2011;134(Pt 1):110–8. doi: 10.1093/brain/awq285. [DOI] [PubMed] [Google Scholar]
- 53.Galambos E, Pfeifer AK, Gyorgy L, Molnar J. Study on the excitation induced by amphetamine, cocaine and alpha-methyltryptamine. Psychopharmacologia. 1967;11(2):122–9. doi: 10.1007/BF00401248. [DOI] [PubMed] [Google Scholar]
- 54.Itzhak Y, Martin JL. Scopolamine inhibits cocaine-conditioned but not unconditioned stimulant effects in mice. Psychopharmacology (Berl) 2000;152(2):216–23. doi: 10.1007/s002130000537. [DOI] [PubMed] [Google Scholar]
- 55.Wilson MC, Schuster CR. Cholinergic influence on intravenous cocaine self-administration by rhesus monkeys. Pharmacol Biochem Behav. 1973;1(6):643–9. doi: 10.1016/0091-3057(73)90027-0. [DOI] [PubMed] [Google Scholar]
- 56.Thomsen M. Locomotor activating effects of cocaine and scopolamine combinations in rats: isobolographic analysis. Behav Pharmacol. 2014;25(4):259–66. doi: 10.1097/FBP.0000000000000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chapman CA, Yeomans JS, Blaha CD, Blackburn JR. Increased striatal dopamine efflux follows scopolamine administered systemically or to the tegmental pedunculopontine nucleus. Neuroscience. 1997;76(1):177–86. doi: 10.1016/s0306-4522(96)00358-2. [DOI] [PubMed] [Google Scholar]
- 58.Dewey SL, Smith GS, Logan J, Brodie JD, Simkowitz P, MacGregor RR, Fowler JS, Volkow ND, Wolf AP. Effects of central cholinergic blockade on striatal dopamine release measured with positron emission tomography in normal human subjects. Proc Natl Acad Sci U S A. 1993;90(24):11816–20. doi: 10.1073/pnas.90.24.11816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Croy CH, Chan WY, Castetter AM, Watt ML, Quets AT, Felder CC. Characterization of PCS1055 a novel muscarinic M4 receptor antagonist. Eur J Pharmacol. 2016;782:70–6. doi: 10.1016/j.ejphar.2016.04.022. [DOI] [PubMed] [Google Scholar]
- 60.Stillman MJ, Shukitt-Hale B, Galli RL, Levy A, Lieberman HR. Effects of M2 antagonists on in vivo hippocampal acetylcholine levels. Brain Res Bull. 1996;41(4):221–6. doi: 10.1016/s0361-9230(96)00180-3. [DOI] [PubMed] [Google Scholar]
- 61.Alcantara AA, Mrzljak L, Jakab RL, Levey AI, Hersch SM, Goldman-Rakic PS. Muscarinic M1 and M2 receptor proteins in local circuit and projection neurons of the primate striatum: anatomical evidence for cholinergic modulation of glutamatergic prefronto-striatal pathways. J Comp Neurol. 2001;434(4):445–60. doi: 10.1002/cne.1186. [DOI] [PubMed] [Google Scholar]
- 62.Stoll C, Eltze M, Lambrecht G, Zentner J, Feuerstein TJ, Jackisch R. Functional characterization of muscarinic autoreceptors in rat and human neocortex. J Neurochem. 2009;110(3):837–47. doi: 10.1111/j.1471-4159.2009.06193.x. [DOI] [PubMed] [Google Scholar]
- 63.De Klippel N, Sarre S, Ebinger G, Michotte Y. Effect of M1- and M2-muscarinic drugs on striatal dopamine release and metabolism: an in vivo microdialysis study comparing normal and 6-hydroxydopamine-lesioned rats. Brain Res. 1993;630(1-2):57–64. doi: 10.1016/0006-8993(93)90642-z. [DOI] [PubMed] [Google Scholar]
- 64.Quirion R, Richard J, Wilson A. Muscarinic and nicotinic modulation of cortical acetylcholine release monitored by in vivo microdialysis in freely moving adult rats. Synapse. 1994;17(2):92–100. doi: 10.1002/syn.890170205. [DOI] [PubMed] [Google Scholar]
- 65.Stillman MJ, Shukitt-Hale B, Kong RM, Levy A, Lieberman HR. Elevation of hippocampal extracellular acetylcholine levels by methoctramine. Brain Res Bull. 1993;32(4):385–9. doi: 10.1016/0361-9230(93)90204-o. [DOI] [PubMed] [Google Scholar]
- 66.Fink-Jensen A, Fedorova I, Wortwein G, Woldbye DP, Rasmussen T, Thomsen M, Bolwig TG, Knitowski KM, McKinzie DL, Yamada M, Wess J, Basile A. Role for M5 muscarinic acetylcholine receptors in cocaine addiction. J Neurosci Res. 2003;74(1):91–6. doi: 10.1002/jnr.10728. [DOI] [PubMed] [Google Scholar]
- 67.Thomsen M, Woldbye DP, Wortwein G, Fink-Jensen A, Wess J, Caine SB. Reduced cocaine self-administration in muscarinic M5 acetylcholine receptor-deficient mice. J Neurosci. 2005;25(36):8141–9. doi: 10.1523/JNEUROSCI.2077-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tzavara ET, Bymaster FP, Felder CC, Wade M, Gomeza J, Wess J, McKinzie DL, Nomikos GG. Dysregulated hippocampal acetylcholine neurotransmission and impaired cognition in M2, M4 and M2/M4 muscarinic receptor knockout mice. Mol Psychiatry. 2003;8(7):673–9. doi: 10.1038/sj.mp.4001270. [DOI] [PubMed] [Google Scholar]
- 69.Thomsen M, Wess J, Fulton BS, Fink-Jensen A, Caine SB. Modulation of prepulse inhibition through both M(1) and M (4) muscarinic receptors in mice. Psychopharmacology (Berl) 2010;208(3):401–16. doi: 10.1007/s00213-009-1740-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


