Abstract
Purpose
To investigate the impact of radioembolization with yttrium-90 resin microspheres on the regulation of angiogenesis through observation of serial changes in a spectrum of angiogenic markers and other cytokines after therapy.
Materials and Methods
This prospective pilot study enrolled 22 patients with liver-dominant disease deriving from biopsy-proven hepatocellular carcinoma (HCC) (n = 7) or metastatic colorectal carcinoma (mCRC) (n = 15). Circulating angiogenic markers were measured from serum samples drawn at baseline and at time points after therapy ranging from 6 hours to 120 days. Using multiplex enzyme-linked immunosorbent assay, several classic angiogenesis factors (vascular endothelial growth factor [VEGF], angiopoietin-2 [Ang-2], basic fibroblast growth factor [bFGF], platelet-derived growth factor subunit BB [PDGF-BB], thrombospondin-1 [Tsp-1]) and nonclassic factors (follistatin, leptin, interleukin [IL]-8) were evaluated.
Results
Increases in cytokine levels ≥ 50% over baseline were observed in more than half of all patients studied for many cytokines, including classic angiogenic factors such as VEGF, Ang-2, and Tsp-1 as well as nonclassic factors IL-8 and follistatin (range, 36% – 82% for all cytokines). Baseline cytokine levels in patients with overall survival (OS) ≤ 6 months differed significantly from patients with longer survival for Ang-2 (P = .033) and IL-8 (P = .041). Patients with OS ≤ 6 months exhibited transient increases in VEGF and PDGF-BB after therapy compared with patients with OS > 6 months.
Conclusions
Radioembolization is associated with early transient increases in many angiogenic cytokines. In this small sample size, some of these changes were associated with worse OS. This research has important implications for future studies of radioembolization with antiangiogenic therapy performed during and after the procedure.
Despite advances in chemotherapy for unresectable hepatocellular carcinoma (HCC) and hepatic colorectal metastases, most patients experience progression of disease, and alternative regional hepatic therapies are considered (1,2). Yttrium-90 (90Y) radioembolization has emerged as an important locoregional therapy for primary and metastatic cancers of the liver and is approved in the United States for the treatment of HCC and metastatic colorectal carcinoma (mCRC) (3). 90Y radioembolization takes advantage of the dominant blood supply to hepatic neoplasms, which derives from the hepatic artery, whereas normal liver tissue receives its dominant supply from the portal vein. This differential blood supply promotes the delivery of relatively large radiation doses (≥ 300 Gy) to the tumor with relative sparing of normal liver parenchyma (4,5). 90Y radioembolization is being rapidly adopted for both HCC and mCRC worldwide and is being prospectively evaluated in combination with first-line systemic chemotherapy in mCRC (6).
Although there have been encouraging reports of tumor response in many patients, some patients experience early failure, either locally or at distant metastatic sites (7). Because both radiation and embolization are known to promote angiogenesis (8,9), we hypothesized that upregulation of proangiogenic cytokines or decrease in cytokines inhibiting angiogenesis may influence disease response after radioembolization. This hypothesis is supported in previous observations of cytokine response after transcatheter arterial chemoembolization of HCC (10). In this pilot study, we investigated the relationship between 90Y radioembolization resin microsphere therapy and serum angiogenic cytokine profiles in patients with HCC and mCRC.
MATERIALS AND METHODS
This prospective study was performed according to a protocol approved by the institutional review board. The study design included recruitment of all patients with either HCC or mCRC with liver-limited disease who were eligible for 90Y radioembolization and who gave written informed consent to evaluation during the 3-year study period beginning in 2009. Radioembolization was performed using 90Y resin microspheres (SIR-Spheres; SIRTeX Medical Limited, Lane Cove, Australia).
Evaluation before and after the Procedure
Patients were considered eligible for treatment of meta-static disease to the liver if there was liver-dominant disease deriving from primary tumors outside of the liver, adequate hepatic reserve, Eastern Cooperative Oncology Group performance status of 0 – 2, ineligibility for surgical resection, and failure or complications with first-line and second-line chemotherapy. Patients with HCC who were not candidates for surgical resection or ablative therapy (Barcelona Clinic Liver Cancer classification stage B and C) were also eligible for treatment with radioembolization. Consensus evaluation by physicians from medical oncology, surgical oncology, radiation oncology, and interventional radiology was required before deciding if the patient qualified for 90Y radio-embolization. Patients were included in the present investigation if they received whole-liver, sequential bilobar, or unilobar 90Y radioembolization treatment.
There were 46 patients screened for enrollment, and 22 patients were entered into the protocol (Table 1). Patients screened but not enrolled were either unwilling or unable to return at designated follow-up intervals. The seven patients with HCC were either Barcelona Clinic Liver Cancer stage B (n = 5) or C (n = 2, each having single-site bone metastasis). All patients were chemotherapy-naïve before treatment, and no patient received chemotherapy within 30 days after completion of treatment. All patients with colorectal carcinoma (15 of 15) had liver-dominant disease with only 1 patient with extrahepatic disease (single-site bone metastasis). All patients had failed at least two lines of chemotherapy, and some had failed five lines. Of the patients with mCRC, 73% (11 of 15) received bevacizumab, 33% (5 of 15) received cetuximab, and 6.7% (1 of 15) received sorafenib before, but not within 60 days of, treatment. No patient received antiangiogenic agents within 60 days before or after treatment.
Table 1.
Patient Characteristics
| Number | Percent | |
|---|---|---|
| Gender | ||
| Male | 15 | 68% |
| Female | 7 | 32% |
| Age (median) | ||
| All | 62.5 y | |
| mCRC | 63 y (range, 36–83 y) | |
| HCC | 57 y (range, 51–80 y) | |
| Primary diagnosis | ||
| mCRC | 15 | 68% |
| HCC | 7 | 32% |
| Survival | ||
| > 6 mo | 14 | 64% |
| ≤ 6 mo | 8 | 36% |
| Radioembolization treatment protocol | ||
| Sequential bilobar | 14 total (10 CRC, 4 HCC) | 64% (71% CRC, 29% HCC) |
| Unilobar | 4 total (3 CRC, 1 HCC) | 18% (75% CRC, 25% HCC) |
| Whole-liver | 1 total (1 CRC, 0 HCC) | 5% (100% CRC, 0% HCC) |
| Retreatment | 3 total (1 CRC, 2 HCC) | 14% (33% CRC, 67% HCC) |
| Percent of liver occupied by tumor (median) | ||
| All | 31% | |
| mCRC | 32% | |
| HCC | 30% | |
| Estimated tumor volume (median) | ||
| All | 580 mL | |
| mCRC | 589 mL | |
| HCC | 520 mL | |
CRC = colorectal carcinoma, HCC = hepatocellular carcinoma, mCRC = metastatic colorectal carcinoma.
The type of therapy administered to each patient (unilobar, sequential bilobar, or whole-liver) was determined based on tumor location or arterial anatomy on a per-case basis (Table 1). The median number of treatment sessions per patient was two, with a median delivered dose of 36.6 Gy per treatment session. Median baseline bilirubin level was 0.7 mg/dL (95% confidence interval, 0.6 – 0.8 mg/dL).
All patients underwent standard of care imaging and laboratory testing before and after radioembolization, as described previously (11). Patients underwent baseline triphasic computed tomography (CT) imaging and positron emission tomography/CT imaging (patients with mCRC only) within 3 weeks of treatment, followed by imaging after treatment at 3-month intervals for the first 12 – 24 months. All imaging studies were assessed unblinded by a vascular interventional radiologist with > 20 years of experience in oncology imaging and 6 years of experience in radioembolization. Laboratory evaluation, including serum bilirubin, aspartate amino-transferase, alanine aminotransferase, alkaline phosphatase, white blood cell and platelet counts, and tumor markers as appropriate (ie, alpha fetoprotein for HCC and carcinoembryonic antigen for mCRC), was obtained at baseline; 6 hours; 3, 14, 30, 60, and 90 days; and subsequently at intervals similar to the imaging protocol. All blood draws after baseline, 6 hours, and day 3 time points were scheduled ± 3 days around the specified time interval to account for avoidance of weekends or holidays or for patient convenience.
Additional evaluation before the procedure included angiographic optimization of the hepatic vasculature with coil embolization of arterial branches providing extrahepatic perfusion. All patients underwent infusion of 99m-technetium-labeled macroaggregated albumin into the hepatic artery to determine arteriovenous lung shunt fraction and for appropriate dose adjustment. Detailed descriptions of indications and contraindications for radioembolization treatment and evaluation before the procedure have been reported previously (12 – 14).
Radiation Treatment Planning
Triphasic contrast-enhanced CT images reconstructed at 1.25-mm and 5-mm slice thickness were used for treatment planning. Both liver and tumor volumes were contoured manually by the radiation oncology team based on contrast-enhanced CT and positron emission tomography/CT scans obtained before the procedure, were reviewed with the interventional radiologist, and were calculated for each patient on the Eclipse treatment planning system (Varian Medical Systems, Inc, Palo Alto, California). The body surface area method was used for dose calculation (12). The dose delivered to the liver was calculated with the following equation:
where LSF is the lung shunt fraction, and R is the residual activity in the dose vial connecting tubes and delivery catheter as measured by the ionization chamber (12).
Besides dose modifications based on arteriovenous lung shunt fraction, dose was also modified based on prior chemotherapy administration (12). At the time of treatment, the determined dose was delivered into the proper hepatic (whole-liver treatment) or left hepatic or right hepatic (sequential bilobar treatment) artery based on anatomic considerations.
Serologic Testing of Angiogenic Cytokines
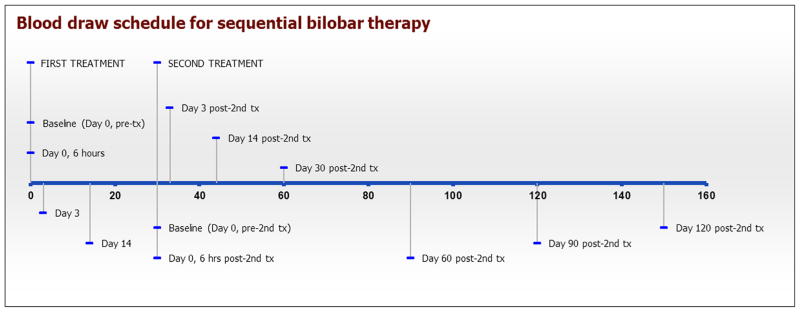
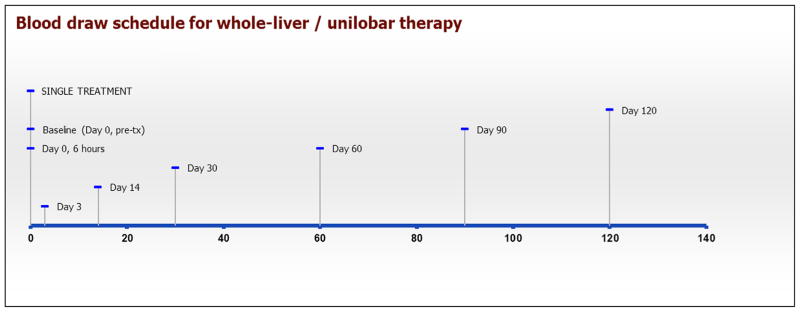
At the time of routine follow-up evaluation and laboratory testing, additional blood was drawn for testing of angiogenic markers according to the schedule illustrated in timeline format in Figures 1 and 2. At the specified time points, 4 mL of blood was collected into a serum tube; serum samples were prepared and stored at −80°C until time of analysis.
Figure 1.
Blood draw schematic for cytokine assessments in single lobar or whole-liver radioembolization treatment plan. Tx = treatment. (Available in color online at www.jvir.org.)
Figure 2.
Blood draw schematic for cytokine assessments in sequential bilobar radioembolization treatment plan. Tx = treatment. (Available in color online www.jvir.org.)
Human angiogenesis markers and cytokine levels were measured from serum samples according to the manufacturer’s instructions using the magnetic bead-based immunoassay multiplex arrays Human Angiogenesis 9-Plex and Human Cytokine 27-Plex (BioPlex; Bio-Rad, Hercules, California). Human acidic fibroblast growth factor (aFGF) and human thrombospondin-1 (Tsp-1) levels were measured separately by individual enzyme-linked immunosorbent assay kits (R&D Systems, Minneapolis, Minnesota). Bio-Rad reports the intraassay coefficient of variance as 15% and the interassay coefficient of variance as 25%. There were 109 samples analyzed in three independent experiments. In all three experiments, the samples were assayed in duplicates and averaged.
In all patients analyzed, the patients served as their own control. The baseline (day 0), 6-hour, day 3, and day 14 data were compared in the same experiment using the same standard curves. For patients treated with sequential bilobar treatment, the new baseline (day 0 from the second treatment), 6-hour, day 3, day 14, and day 30 samples were run in the same experiment using the same standard curve. We also compared the data for three proteins that overlapped between the 27-Plex and 9-Plex, and there was relatively good correlation (Table E1 [available online at www.jvir.org]).
Human Angiogenesis 9-Plex contained the following markers: angiopoietin-2 (Ang-2), follistatin, granulocyte colony-stimulating factor (G-CSF), hepatocyte growth factor (HGF), interleukin (IL)-8, leptin, platelet-derived growth factor subunit BB (PDGF-BB), platelet endothelial cell adhesion molecule (PECAM-1), and vascular endothelial growth factor (VEGF). Human Cytokine 27-Plex contained the following markers: IL-1β, IL-1ra, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12 (p70), IL-13, IL-15, IL-17, basic fibroblast growth factor (bFGF), eotaxin, G-CSF, granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon (IFN)-γ, interferon-inducible protein (IP)-10, monocyte chemo-tactic protein-1, macrophage chemotactic and activating factor (MCAF), macrophage inflammatory protein (MIP)-1α, MIP-1β, PDGF-BB, regulated on activation, normal T-cell expressed and secreted (RANTES), tumor necrosis factor (TNF)-α, and VEGF. Additional individual enzyme-linked immunosorbent assay kits include aFGF and Tsp-1.
Outcome Measures and Statistics
Overall survival (OS) was computed as the number of days from initial radioembolization treatment to date of death. For patients still surviving at the time of analysis (n = 6), a common close date was used to assess OS. Statistical analysis was performed using the statistical software packages SAS (SAS Institute, Cary, North Carolina) and SPSS (SPSS, Inc, Chicago, Illinois). Cox proportional hazards survival analyses of survival times of these patients for each of several biomarkers (VEGF, PDGF-BB, Ang-2, and Tsp-1) were performed. For each biomarker, three analyses were conducted, using as predictors the biomarker level at 6 hours, day 3, and day 12. In each analysis, only a single predictor was used. In addition, we stratified the patients by median survival of 6 months into short survival (n = 8) versus long survival (n = 14). Comparative median statistics using nonparametric Mann-Whitney U tests were employed to assess differences between the two groups, with a minimum of three in-range samples in each group. All tests used a P value of .05 as the cutoff for statistical significance.
RESULTS
Changes in Angiogenic Cytokines after Therapy
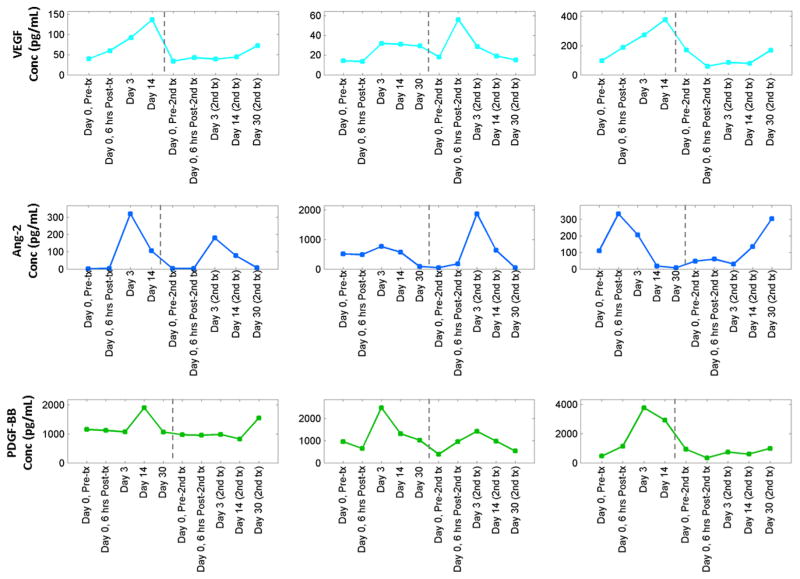
We observed an increase in angiogenic cytokine levels, defined as an increase (spike) of ≥ 50% from the baseline value, that coincided with radioembolization therapy for both classic and nonclassic angiogenic cytokines. Examples of these observed spikes are illustrated in Figure 3. Not all patients experienced such spikes; some patients demonstrated progressive declines in cytokine levels after treatment. Comparison with curves for patients with minimal changes in cytokine levels is demonstrated in Figure E1 (available online at www.jvir.org). Among patients who underwent sequential bilobar treatments and exhibited perturbations or spikes after therapy, some were apparent after the first treatment (eg, Fig 3, VEGF [first row], first and third plots), some were apparent after bimodal treatment (after both first and second treatments; eg, Fig 3, Ang-2 [second row], first and third plots), and others were apparent only after the second treatment (eg, Fig 3, second plots shown for VEGF and Ang-2). Observed patterns are presented in detail in Table 2. In more than half of all patients studied, cytokines including both classic angiogenic factors (eg, VEGF, Ang-2, and Tsp-1) and nonclassic factors (eg, IL-8 and follistatin) demonstrated a spike in response to therapy (range, 36% – 82%).
Figure 3.
Examples of spikes in circulating angiogenic cytokines after radioembolization therapy. First row illustrates representative changes in VEGF from three patients after treatment, second row represents Ang-2, and third row represents PDGF-BB. Points correspond to samples drawn at specific time points after first and second radioembolization treatments. Note the bimodal Ang-2 patterns after therapy for the first and third patients. Also note the strong spike in PDGF-BB after the first treatment for the third patient. Conc = concentration, Tx = treatment. (Available in color online at www.jvir.org.)
Table 2.
Distribution of Observed Spikes in Circulating Cytokine Levels after Treatment
| Cytokine Group | Cytokine | Percent with ≥ 50% Spike after Treatment | Percent with No Major Spike after Treatment | Percent Undetermined |
|---|---|---|---|---|
| Classic angiogenic factors | VEGF | 59 | 32 | 9 |
| Ang-2 | 59 | 23 | 18 | |
| bFGF | 64 | 9 | 27 | |
| PDGF-BB | 55 | 41 | 5 | |
| Tsp-1 | 77 | 23 | 0 | |
| Nonclassic angiogenic factors | IL-8 | 77 | 23 | 0 |
| Leptin | 50 | 45 | 5 | |
| Follistatin | 82 | 14 | 5 | |
| HGF | 55 | 41 | 5 | |
| PECAM-1 | 36 | 59 | 5 |
Note. Spikes were identified by at least a 50% increase over baseline levels at any time point after treatment.
Ang-2 = angiopoietin-2, bFGF = basic fibroblast growth factor, HGF = hepatocyte growth factor, IL-8 = interleukin-8, PDGF-BB = platelet-derived growth factor subunit BB, PECAM-1 = platelet endothelial cell adhesion molecule, Tsp-1 = thrombospondin-1, VEGF = vascular endothelial growth factor.
Survival Comparisons over Time
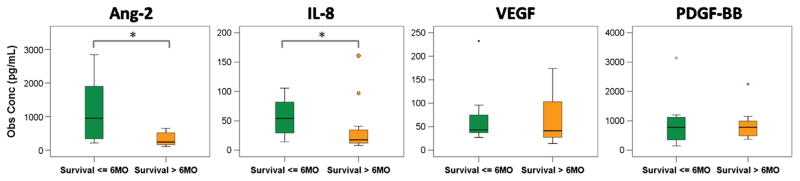
We next examined the relationship between the increases in angiogenic cytokine levels after radioembolization and OS. Median OS for all patients was 8 months, with a 1-year survival rate of 41.25%. Patients were stratified into two survival groups according to 6-month survival (n = 8 surviving ≤ 6 mo; n = 14 surviving > 6 mo). Baseline (before treatment) cytokine levels were significantly greater in the shorter survival group (≤ 6 mo) for Ang-2 (P = .033) and IL-8 (P = .041) but not for VEGF (P = .43) or PDGF-BB (P = .91) (Fig 4).
Figure 4.
Boxplots showing baseline comparisons across survival groups for several angiogenic cytokines. *P < .05. Obs Conc = observed concentration. (Available in color online at www.jvir.org.)
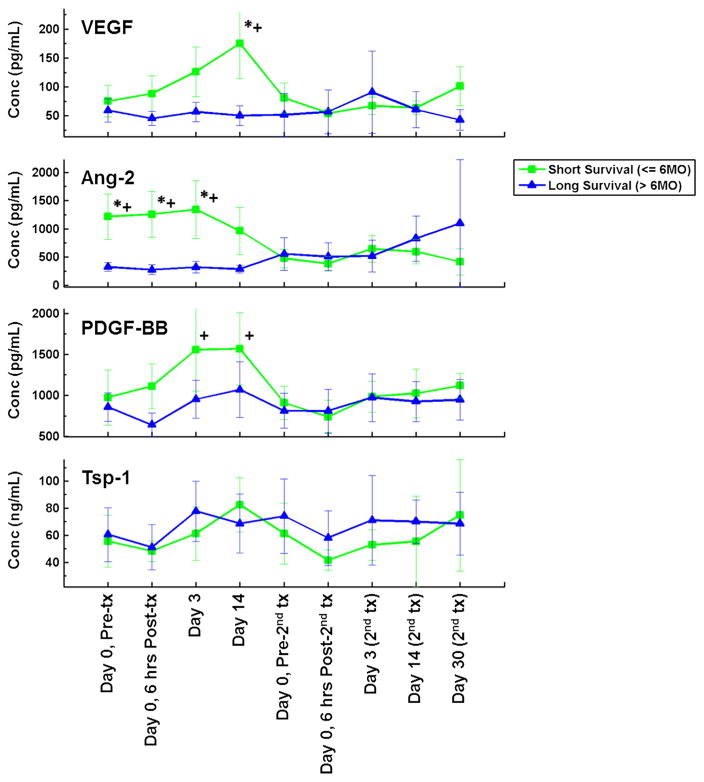
We next compared the variations in angiogenic cytokine levels over time stratified by OS for VEGF, Ang-2, PDGF-BB, and Tsp-1 using the Mann-Whitney test. We found that patients within the shorter survival category experienced statistically significant increases in VEGF, Ang-2, and PDGF-BB at various early time points (6 h, day 3, and day 14) before decreasing in the later time points essentially to merge with the curves of the patients in the longer survival category (Fig 5). We also performed Cox proportional hazard survival analyses of survival times of these patients for each angiogenic cytokine focusing on the time points of 6 hours, day 3, and day 14. These results yielded complementary results (to the Mann-Whitney test) in that for VEGF, Ang-2, and PDGF-BB several early time points yielded significantly different survival times. VEGF was found to be significant at day 14 only, whereas Ang-2 was significant for the 6-hour, day 3, and day 14 time points. PDGF-BB was significant at the day 3 and day 14 time points. Tsp-1 level fluctuations in patients appeared to be unrelated to the timing of treatment. No difference was found between the shorter and longer survival groups (as shown in Fig 5, bottom row). We conclude that for patients who experienced transient increases in VEGF, Ang-2, and PDGF-BB after radioembolization, the transient increases correlated with shorter OS.
Figure 5.
Variations in circulating cytokine levels after radioembolization therapy for classic angiogenesis factors VEGF, Ang-2, PDGF-BB, and Tsp-1 after therapy for two survival groups. Short survival (≤ 6 mo) is shown in green with square symbols; long survival ( > 6 mo) is shown in blue with triangular symbols. Time points marked with an asterisk differed significantly between survival groups on Mann-Whitney U comparisons; time points marked with a plus sign demonstrated significant differences on Cox proportional hazards analysis. Conc = concentration, Tx = treatment. (Available in color online at www.jvir.org.)
DISCUSSION
90Y hepatic radioembolization, a regional form of brachytherapy, has been approved for the treatment of both HCC and mCRC and is used off-label for various other cancers, either primary or metastatic to the liver (3). The 90Y-bearing microspheres used in this study have diameters ranging from 20 – 60 μm and activity of 50 Bq per sphere, with a 3-Gbq vial (81 mCi) of SIR-Spheres containing 40 – 80 million microspheres. 90Y radioembolization with resin microspheres delivers both a radiation and an embolic effect (12). Our observations with respect to angiogenic cytokine levels may be specific to resin micro-sphere radioembolization.
Although many patients with both primary and meta-static cancer to the liver experience prolonged local control after hepatic radioembolization, others experience early failure, either locally or at distant metastatic sites (7). Angiogenesis is an integral component in tumor growth and spread that is driven by a complex interplay between proangiogenic and antiangiogenic cytokines (15). Circulating VEGF, the most frequently studied angiogenic cytokine, strongly correlates with tumor expression of VEGF (16). VEGF levels are reported to be associated with poor outcomes in both HCC and mCRC. In HCC, circulating VEGF further correlates with stage of disease, tumor burden, presence of intrahepatic and extrahepatic metastasis, vascular invasion, postoperative recurrence, response to treatment, progression-free survival, and OS (17,18). Previous work has demonstrated the potential for both radiation and embolization to upregulate angio-genesis in vitro and in vivo (19,20). It is plausible that an angiogenic response to radioembolization may influence therapeutic outcomes.
In support of this notion, several studies have documented transient increases in angiogenic cytokine levels shortly after transarterial chemoembolization for patients with HCC and have correlated these changes with worse oncologic outcomes (10,18). These reports were limited to either one (VEGF (10)) or three (VEGF, bFGF, and urokinase-type plasminogen activator (18)) cytokines, whereas in this study we took a more comprehensive strategy to studying this relationship through the investigation of classic, nonclassic, and nonangiogenic cytokines.
After radioembolization, changes from baseline were noted in serum levels of classic angiogenic cytokines, including Ang-2, PDGF-BB, VEGF, and IL-8. However, the magnitude and temporal sequence of changes in cytokine expression varied among patients. These observations support appreciation that radioembolization is associated with significant increases in many angiogenic cytokines. The variety of cytokines in which we observed changes is more reflective of the complex biologic process of angiogenesis that has emerged since the identification of the dominant genes, such as VEGF and FGF, in the 1990s (21,22).
We found that Ang-2 levels were higher at baseline and increased after treatment in a large number of patients in this cohort. Ang-2 is a ligand for the Tie-2 receptor on endothelial cells and serves as a positive regulator of tumor angiogenesis (23). Circulating Ang-2 levels have been shown to serve as a surrogate marker for tumor progression and metastasis, and blockade of Ang-2 represses metastasis (24). Mechanistic research in tumor angiogenesis indicates that the Ang/Tie system works together with the VEGF system to break down and remodel tumor blood vessels (25). For these reasons, Tie-2 receptor inhibitors have been investigated; some reports indicate increased efficacy compared with VEGF inhibition alone (26). Our results suggest that consideration needs to be given to Ang-2/Tie-2 inhibition in patients undergoing radioembolization. One possibility might be the multi – tyrosine kinase inhibitor regorafenib. Regorafenib and its active metabolites inhibit multiple membrane-bound and intracellular kinases that are involved in normal cellular functions and pathologic processes, including kinases in the RET, vascular endothelial growth factor receptor (VEGFR) 1, VEGFR2, VEGFR3, KIT, platelet-derived growth factor receptor (PDGFR)-α, PDGFR-β, fibroblast growth factor receptor (FGFR) 1, FGFR2, Tie-2, DDR2, Trk2A, Eph2A, RAF-1, BRAF, BRAFV600E, SAPK2, PTK5, and Abl pathways. Regorafenib was reported more recently in the CORRECT trial to show activity as monotherapy in patients with chemotherapy-refractory mCRC (27). The patients in this trial were very similar to the types of patients undergoing radioembolization in the present study. Another possibility would be cabozantinib (XL184), an agent approved by the U.S. Food and Drug Administration that is another multi – tyrosine kinase inhibitor that targets c-Met, VEGFR2, and Tie-2 receptors. Specific Tie-2 inhibitors are currently in clinical trials, such as AMG-386, which is an angiopoietin peptibody (peptide [Fc]fragment of an angiopoietin antibody) currently in a phase III randomized trial of paclitaxel plus AMG-386 in metastatic ovarian cancer (NCT01204749) (28).
An essential part of tumor angiogenesis is the recruitment of pericytes to nascent endothelial tubes to allow for their maturation into functional blood vessels. This is the role of PDGF (29). PDGF-BB is an isoform of PDGF that binds heparan sulfate proteoglycans in the extracellular matrix and helps localize it at the surface of endothelial cells. This localization enhances its ability to recruit pericytes (30). We found that PDGF-BB levels transiently increased on 90Y radioembolization to a greater degree in patients surviving ≤ 6 months compared with patients who survived > 6 months. Although this trend in this cohort was not significant, it may be with a larger sample size.
IL-8 belongs to the chemokine family and has been shown to be a macrophage-derived mediator of angio-genesis (31). IL-8 has been evaluated as a biomarker of angiogenesis, and high levels of IL-8 have demonstrated a correlation with increased progression risk in a study of patients with non – small cell lung cancer who were treated with systemic cytotoxic chemotherapy and a VEGFR inhibitor (32). In this study, IL-8 levels at baseline were significantly greater in the group of patients with shorter survival; this suggests that IL-8 may also be useful as a biomarker to predict outcome in any type of hepatic embolization therapy.
As in the studies of Shim et al (10) and Sergio et al (18), we found a potential correlation between increases in VEGF, Ang-2, and PDGF-BB and worse oncologic outcomes, suggesting not only that these cytokines may be useful as biomarkers of prognosis after treatment but also that these changes may be clinically relevant. When patients were separated into long and short survival groups, there were trends for separation of groups by level of cytokine expression, and similar to previous studies, the significant increases were transient. Collectively, these cytokines may influence survival in patients undergoing radioembolization either by promoting angiogenesis or by decreasing the effectiveness of radiation.
Our study may have important therapeutic implications because it is has been suggested that patients might benefit from antiangiogenic therapy as an adjunct to transarterial chemoembolization in HCC (10,18,33). Our results in patients receiving radioembolization support this concept, but the broader spectrum of angiogenic cytokines we have reported complicate the choice of antiangiogenic agent and make the case for using multi – tyrosine kinase inhibitors that would inhibit not only the VEGFR but also Tie-2 receptor, FGFR, and PDGFR. The temporal nature of the changes we observed should be considered in the timing of administration of anti-angiogenic strategies used as adjuvant therapy in chemo-embolization or radioembolization. Our results support giving consideration to administering antiangiogenic therapy before regional embolic therapy.
There are several limitations to our study. The major limitation is sample size, which compromises the likelihood of reaching statistical significance. Care must be exercised in interpreting the importance of our observations given the limitations of using cytokine levels as predictors of response to therapy (34). However, there is experimental evidence that both radiation and embolization efficacy can be enhanced by the addition of antiangiogenic agents to chemoradiation protocols (8,20). Identification of specific proangiogenic pathways provides the opportunity for targeted selection of appropriate antiangiogenic agents from an ever-increasing armamentarium—the goal of precision medicine.
Supplementary Material
Acknowledgments
This study was supported by a research grant from SIRTeX Medical, Inc. (Wilmington, Massachusetts), under study principal investigator J.L.N.
The authors wish to acknowledge the support of the Biospecimen Repository Service at the Rutgers Cancer Institute of New Jersey, under the direction of Julie Friedman.
ABBREVIATIONS
- aFGF
acidic fibroblast growth factor
- Ang-2
angiopoietin-2
- bFGF
basic fibroblast growth factor
- Conc
concentration
- FGFR
fibroblast growth factor receptor
- G-CSF
granulocyte colony-stimulating factor
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- HCC
hepatocellular carcinoma
- HGF
hepatocyte growth factor
- IFN
interferon
- IL
interleukin
- IP
interferon-inducible protein
- MCAF
macrophage chemotactic and activating factor
- mCRC
metastatic colorectal carcinoma
- MIP
macrophage inflammatory protein
- OS
overall survival
- PDGF
platelet-derived growth factor
- PDGF-BB
platelet-derived growth factor subunit BB
- PDGFR
platelet-derived growth factor receptor
- PECAM-1
platelet endothelial cell adhesion molecule
- RANTES
regulated on activation, normal T-cell expressed and secreted
- TNF
tumor necrosis factor
- Tsp-1
thrombospondin 1
- Tx
treatment
- VEGF
vascular endothelial growth factor
- VEGFR
vascular endothelial growth factor receptor
- 90Y
yttrium-90
Footnotes
None of the other authors have identified a conflict of interest.
Figure E1 and Table E1 are available online at www.jvir.org.
References
- 1.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepato-cellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg RM, Sargent DJ, Morton RF, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22:23–30. doi: 10.1200/JCO.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 3.Kennedy AS, Salem R. Radioembolization (yttrium-90 microspheres) for primary and metastatic hepatic malignancies. Cancer J. 2010;16:163–175. doi: 10.1097/PPO.0b013e3181d7e8cf. [DOI] [PubMed] [Google Scholar]
- 4.Welsh JS, Kennedy AS, Thomadsen B. Selective internal radiation therapy (SIRT) for liver metastases secondary to colorectal adenocarcinoma. Int J Radiat Oncol Biol Phys. 2006;66:S62–S73. doi: 10.1016/j.ijrobp.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 5.Kennedy AS, Nutting C, Coldwell D, Gaiser J, Drachenberg C. Pathologic response and microdosimetry of (90)Y microspheres in man: review of four explanted whole livers. Int J Radiat Oncol Biol Phys. 2004;60:1552–1563. doi: 10.1016/j.ijrobp.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Gibbs P, van Hazel G. FOLFOX plus SIR-SPHERES MICROSPHERES versus FOLFOX alone in patients with liver mets from primary colorectal cancer (SIRFLOX) Bethesda, MD: National Library of Medicine; 2008–2013. ([cited 2013 Oct 7]. http://clinicaltrials.gov/show/NCT00724503. NLM Identifier: NCT00724503.) [Google Scholar]
- 7.Schonewolf CA, Patel B, Gensure RH, et al. Patterns of failure in colorectal patients with liver metastases after yttrium-90 radioembolization. Am J Clin Oncol. doi: 10.1097/COC.0b013e318277d610. In press available online December 27, 2012. [DOI] [PubMed] [Google Scholar]
- 8.Gorski DH, Beckett MA, Jaskowiak NT, et al. Blockage of the vascular endothelial growth factor stress response increases the antitumor effects of ionizing radiation. Cancer Res. 1999;59:3374–3378. [PubMed] [Google Scholar]
- 9.Korse CM, Bonfrer JM, Prevoo W, Baas P, Taal BG. Increase of angiogenic growth factors after hepatic artery embolization in patients with neuroendocrine tumours. Tumour Biol. 2011;32:647–652. doi: 10.1007/s13277-011-0164-7. [DOI] [PubMed] [Google Scholar]
- 10.Shim JH, Park J-W, Kim JH, et al. Association between increment of serum VEGF level and prognosis after transcatheter arterial chemo-embolization in hepatocellular carcinoma patients. Cancer Sci. 2008;99:2037–2044. doi: 10.1111/j.1349-7006.2008.00909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nosher JL, Ohman-Strickland PA, Jabbour S, Narra V, Nosher B. Changes in liver and spleen volumes and liver function after radio-embolization with yttrium-90 resin microspheres. J Vasc Interv Radiol. 2011;22:1706–1713. doi: 10.1016/j.jvir.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 12.Salem R, Thurston KG. Radioembolization with 90yttrium micro-spheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies. Part 1: technical and methodologic considerations. J Vasc Interv Radiol. 2006;17:1251–1278. doi: 10.1097/01.RVI.0000233785.75257.9A. [DOI] [PubMed] [Google Scholar]
- 13.Salem R, Thurston KG. Radioembolization with 90yttrium micro-spheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies. Part 2: special topics. J Vasc Interv Radiol. 2006;17:1425–1439. doi: 10.1097/01.RVI.0000235779.88652.53. [DOI] [PubMed] [Google Scholar]
- 14.Salem R, Lewandowski RJ, Gates VL, et al. Research reporting standards for radioembolization of hepatic malignancies. J Vasc Interv Radiol. 2011;22:265–278. doi: 10.1016/j.jvir.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 16.Poon RT, Lau CP, Cheung ST, Yu WC, Fan ST. Quantitative correlation of serum levels and tumor expression of vascular endothelial growth factor in patients with hepatocellular carcinoma. Cancer Res. 2003;63:3121–3126. [PubMed] [Google Scholar]
- 17.Xiong ZP, Yang SR, Liang ZY, et al. Association between vascular endothelial growth factor and metastasis after transcatheter arterial chemoembolization in patients with hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2004;3:386–390. [PubMed] [Google Scholar]
- 18.Sergio A, Cristofori C, Cardin R, et al. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol. 2008;103:914–921. doi: 10.1111/j.1572-0241.2007.01712.x. [DOI] [PubMed] [Google Scholar]
- 19.Poon RT, Ng IO, Lau C, et al. Serum vascular endothelial growth factor predicts venous invasion in hepatocellular carcinoma: a prospective study. Ann Surg. 2001;233:227–235. doi: 10.1097/00000658-200102000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gadaleta CD, Ranieri G. Trans-arterial chemoembolization as a therapy for liver tumours: new clinical developments and suggestions for combination with angiogenesis inhibitors. Crit Rev Oncol Hematol. 2011;80:40–53. doi: 10.1016/j.critrevonc.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Chung AS, Lee J, Ferrara N. Targeting the tumour vasculature: insights from physiological angiogenesis. Nat Rev Cancer. 2010;10:505–514. doi: 10.1038/nrc2868. [DOI] [PubMed] [Google Scholar]
- 22.Weis SM, Cheresh DA. Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med. 2011;17:1359–1370. doi: 10.1038/nm.2537. [DOI] [PubMed] [Google Scholar]
- 23.Fagiani E, Christofori G. Angiopoietins in angiogenesis. Cancer Lett. 2013;328:18–26. doi: 10.1016/j.canlet.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 24.Holopainen T, Saharinen P, D’Amico G, et al. Effects of angiopoietin-2-blocking antibody on endothelial cell-cell junctions and lung metastasis. J Natl Cancer Inst. 2012;104:461–475. doi: 10.1093/jnci/djs009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lobov IB, Brooks PC, Lang RA. Angiopoietin-2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo. Proc Natl Acad Sci U S A. 2002;99:11205–11210. doi: 10.1073/pnas.172161899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koh YJ, Kim H-Z, Hwang S-I, et al. Double antiangiogenic protein, DAAP, targeting VEGF-A and angiopoietins in tumor angiogenesis, metastasis, and vascular leakage. Cancer Cell. 2010;18:171–184. doi: 10.1016/j.ccr.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–312. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 28.Amgen . A phase 3, randomized, double-blind trial of weekly paclitaxel plus AMG 386 or placebo in women with recurrent partially platinum sensitive or resistant epithelial ovarian, primary peritoneal or fallopian tube cancers. Bethesda, MD: National Library of Medicine; 2013. [cited 2013 Oct 7]. Available at: http://clinicaltrials.gov/show/NCT01204749. NLM Identifier: NCT01204749. [Google Scholar]
- 29.Hellström M, Gerhardt H, Kalén M, et al. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol. 2001;153:543–553. doi: 10.1083/jcb.153.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindblom P, Gerhardt H, Liebner S, et al. Endothelial PDGF-B retention is required for proper investment of pericytes in the microvessel wall. Genes Dev. 2003;17:1835–1840. doi: 10.1101/gad.266803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koch AE, Polverini PJ, Kunkel SL, et al. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992;258:1798–1801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- 32.Hanrahan EO, Lin HY, Kim ES, et al. Distinct patterns of cytokine and angiogenic factor modulation and markers of benefit for vandetanib and/or chemotherapy in patients with non-small-cell lung cancer. J Clin Oncol. 2010;28:193–201. doi: 10.1200/JCO.2009.22.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weintraub JL, Salem R. Treatment of hepatocellular carcinoma combining sorafenib and transarterial locoregional therapy: state of the science. J Vasc Interv Radiol. 2013;24:1123–1134. doi: 10.1016/j.jvir.2013.01.494. [DOI] [PubMed] [Google Scholar]
- 34.Hegde PS, Jubb AM, Chen D, et al. Predictive impact of circulating vascular endothelial growth factor in four phase III trials evaluating bevacizumab. Clin Cancer Res. 2013;19:929–937. doi: 10.1158/1078-0432.CCR-12-2535. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.