Abstract
Previous studies have shown that reduced levels of the adipocyte fatty acid binding protein (FABP)4 (AFABP/aP2), result in metabolic improvement including potentiated insulin sensitivity and attenuated atherosclerosis. Mechanistically, pharmacologic or genetic inhibition of FABP4 in macrophages upregulates UCP2, attenuates reactive oxygen species (ROS) production, polarizes cells toward the anti-inflammatory M2 state, and reduces leukotriene (LT) secretion. At the protein level, FABP4 stabilizes LTA4 toward chemical hydrolysis, thereby potentiating inflammatory LTC4 synthesis. Herein, we extend the FABP4-LT axis and demonstrate that genetic knockout of FABP4 reduces expression of the major macrophage LT receptor, LTB4 receptor 1 (BLT1R), via a ROS-dependent mechanism. Consistent with inflammation driving BLT1R expression, M1 polarized macrophages express increased levels of BLT1R relative to M2 polarized macrophages and treatment with proinflammatory lipopolysaccharide increased BLT1R mRNA and protein expression. In FABP4 knockout macrophages, silencing of UCP2, increased ROS levels and led to increased expression of BLT1R mRNA. Similarly, addition of exogenous H2O2 upregulated BLT1R expression, whereas the addition of a ROS scavenger, N-acetyl cysteine, decreased BLT1R levels. As compared with WT macrophages, LTB4-BLT1R-dependent JAK2-phosphorylation was reduced in FABP4 knockout macrophages. In summary, these results indicate that FABP4 regulates the expression of BLT1R and its downstream signaling via control of oxidative stress in macrophages.
Keywords: macrophages, leukotrienes, inflammation, lipids, lipid mediators, signal transduction, leukotriene B4 receptor 1, adipocyte myelin P2
The prevalence of obesity and its associated pathologies, including insulin resistance, have increased in the past several decades (1–4). Numerous studies have implicated adipose tissue-recruited immune cells as a critical component of insulin resistance and significant attention has been focused on macrophages, whose malleable nature allows for alternative states of activation dependent on the local environment (5, 6). With the development of obesity, adipose tissue acquires a chronic low-grade proinflammatory state, along with increased reactive oxygen species (ROS), dysfunctional mitochondria, and whole body insulin resistance (7–9).
Leukotrienes (LTs) are lipid mediators derived from arachidonic acid via the actions of the 5-lipoxygenase pathway (10–13). LTA4 hydrolase activity results in the production of LTB4, whereas LTC4 synthase initiates the glutathione conjugation of LTA4 to produce LTC4, LTD4, and LTE4 (14, 15). These lipids bind with high affinity to a subfamily of G protein-coupled receptors, thus initiating signal cascades to direct functional consequences, typically of a proinflammatory nature (16–18). Lipopolysaccharide (LPS), zymosan, and calcium ionophores have all been demonstrated to increase LTB4 production and secretion in multiple cell types, including neutrophils and macrophages (19, 20). LTB4 binds with high affinity to the LTB4 receptor 1 (BLT1R), a member of the G protein-coupled receptor family (16, 21, 22). A second LTB4 receptor, BLT2R, binds LTB4 with a 20-fold higher Kd (21, 22). Several other lipoxygenase products have been demonstrated to bind to BLT2R, with the thromboxane synthase product, 12-HHT, having a 10-fold higher affinity than LTB4; thus 12-HHT is thought to be an endogenous ligand (22, 23). LTB4 is involved in host defense following infection in a number of ways, including increasing the recruitment of immune cells via increased chemotaxis, calcium mobilization, and altered gene expression, ultimately leading to an inflammatory state (13, 24, 25). Additionally, LTB4 stimulates neutrophil secretion of proteins, including lysozyme and matrix metalloproteinases (26, 27). In macrophages, LTB4 has been demonstrated to increase proliferation and plays a role in phagocytosis (28–30). Chronic LTB4 proinflammatory signaling has been implicated in several pathological states including atherosclerosis, asthma, arthritis and cancer (16, 24, 31–33).
On a high-fat diet, murine adipose tissue accumulates M1-activated macrophages, thereby producing an inflammatory state (34–36). However, BLT1R knockout mice demonstrate an anti-inflammatory adipose phenotype and are resistant to pathologies associated with a high-fat diet. Without BLT1R, adipose tissue accumulates M2 macrophages with a corresponding decrease in M1 macrophages. This ultimately results in decreased circulating proinflammatory chemokines and cytokines and reduced insulin resistance (37, 38). Additionally, BLT1R has been implicated in the insulin resistance derived by adipose tissue B2 cells (39). BLT1R deletion also protects from the progression of atherosclerosis in an ApoE-deficient mouse (37). In addition to the BLT1R, Olefsky and colleagues have demonstrated that high-fat diet-induced obesity results in increased LTB4 production in metabolic tissues, including adipose, liver, and muscle (38).
Fatty acid binding proteins (FABPs) are small soluble fatty acid carriers and comprise a family of nine members (40). FABP4 (aP2/AFABP) plays a significant role in lipid metabolism; in the absence of FABP4, lipolysis is reduced (41–43). Furthermore in the obese state, genetic deletion of FABP4 improves metabolism, resulting in reduced insulin resistance, atherosclerosis, and asthma (44–49). More specifically, FABP4-null macrophages have been shown to exert an anti-inflammatory phenotype, most closely resembling an M2 state. Interestingly, members of the FABP family, including FABP4, have been implicated in stabilizing short-lived LT intermediates, by binding the unstable epoxide containing LTA4, functionally protecting it from water hydrolysis to the inactive 5,6- or 5,12-diHETEs (50, 51). Herein, we report that in macrophages, the inflammatory stimuli LPSs, as well as ROS, upregulate BLT1R expression in a FABP4-dependent manner. Furthermore, we demonstrate that in macrophages lacking FABP4, the LTB4-induced signal cascade is dramatically reduced.
RESEARCH DESIGN AND METHODS
Cell culture
RAW264.7 macrophages were maintained in DMEM (Invitrogen) with 10% FBS. WT, FABP4/aP2 knockout (AKO), shGFP control, and shUCP2 knockdown AKO macrophages were maintained in RPMI 1640 (Invitrogen) with 5% FBS. Concentrations and times of treatment of LPS, H2O2, N-acetyl cysteine, or LTB4 are listed in each figure legend.
Bone marrow-derived macrophage isolation
Bone marrow-derived macrophages (BMDMs) were isolated from 10-week-old female C57BL/6J mice as indicated in Ying et al. (52). Cells were plated and maintained in Iscove’s modified Dulbecco’s medium (Invitrogen) with 10% FBS and 10 ng/ml macrophage colony-stimulating factor (M-CSF) for 1 week to allow maturation of the cells into naïve macrophages. Polarization of cells to either M1 or M2 activation states occurred by treatment with either LPS (1 μg/ml)/IFNγ (10 ng/ml) or IL-4 (10 ng/ml)/IL-13 (10 ng/ml) for 48 h, respectively. All experimental procedures using animals were reviewed and approved by the University of Minnesota Institutional Animal Care and Use Committee.
Quantitative RT-PCR
Total RNA was isolated using TRIzol reagent (Invitrogen) and cDNA synthesis was performed using iScript according to the manufacturer’s protocol (Bio-Rad). Quantitative (q)RT-PCR amplification utilized a Bio-Rad CFX 96 real-time system with a SYBR green Supermix (Bio-Rad). Transcription factor II E (TFIIE) was used as the internal control to normalize expression. Primer sequences used are (forward; reverse): Arginase, (AACACGGCAGTGGCTTTAACC; GGTTTTCATCTGGCGCATTC); BLT1R, (GGCATCTGGGTGGTGTCTTTTC; TGCTCTTTGTTGGGATAGTTCG); CD206, (CTCGTGGATCTCCGTGACAC; GCAAATGGAGCCGTCTGTGC); CXCL10, (CGTGTTGAGATCATTGCCAC; TTAAGGAGCCCTTTTAGACC); IL-1β, (AAATACCTGTGGCCTTGGGC; CTTGGGATCCACACTCTCCAG); iNOS, (AGCGAGTTGTGGATTGTCC; TCTCTGCCTATCCGTCTCG); LCN2, (GGGAGTGCTGGCCAAATAAG; TGCCACTCCATCTTTCCTGTT); PPARγ, (GCCATTGAGTGCCGAGTC; TGTGGATCCGGCAGTTAA); TFIIE, (CAAGGCTTTAGGGGACCAGATAC; CATCCATTGACTCCACAGTGACAC).
Immunoblot analysis
Cells were lysed with RIPA buffer supplemented with protease inhibitors (Calbiochem). Fifty micrograms of protein from each sample were separated by SDS-PAGE and transferred to a PVDF membrane. After blocking with Odyssey blocking buffer (Li-Cor Biosciences), membranes were incubated with primary antibody overnight at 4°C. Membranes were washed and incubated with secondary antibody conjugated to Li-Cor IRDye for 1 h and visualized using Odyssey infrared imaging (Li-Cor Biosciences). The primary antibodies used were anti-BLT1R (Cayman Chemical) and anti-actin (Sigma).
Immunoprecipitation
For JAK2 phosphorylation experiments, following LTB4 treatments, JAK2 antibody (Cell Signaling) was added to cell lysates and incubated overnight at 4°C. Subsequently protein A-agarose was added and after 2 h of rocking at 4°C, centrifuged and the pellet was washed three times. The immunoprecipitants were denatured by boiling and run on SDS-PAGE. Immunoblots utilized antibodies to phospho-tyrosine (4G10; Millipore) and total JAK2 (Cell Signaling).
Statistical analysis
All data in this work are expressed as ±SEM. Statistical significance was determined using an unpaired two-tailed Student t-test (*P < 0.05).
RESULTS
Genetic or pharmacologic depletion of FABP4 reduces BLT1R expression
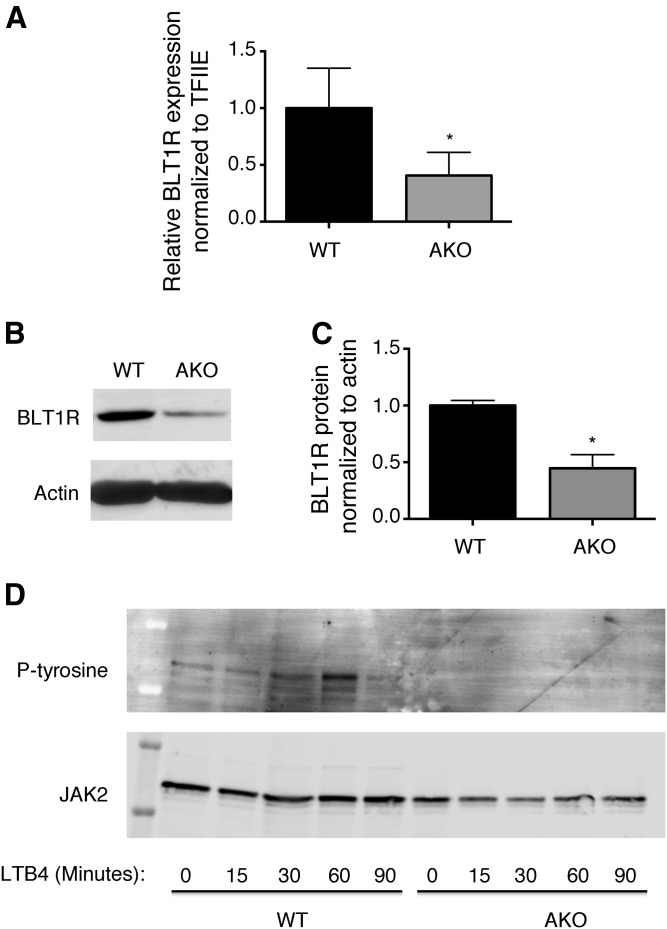
Previously, genetic knockout or pharmacologic inhibition of FABP4 has been shown to reduce inflammation and improve insulin sensitivity (44, 53). This inhibition of FABP4 results in accumulation of intracellular fatty acids in both adipocytes and macrophages (41, 42, 54). Recently we demonstrated that monounsaturated fatty acids, particularly C16:1 palmitoleic acid and C18:1 oleic acid, drive the upregulation of UCP2 in macrophages resulting in reduced ROS and proinflammatory cytokine production (54). Because the LTB4 pathway is intimately involved in promoting inflammation, we evaluated the expression of the receptor, BLT1R, in macrophages generated from C57BL/6J (WT) or FABP4 knockout mice (AKO). FABP4 knockout macrophages expressed approximately 50% the BLT1R mRNA levels of WT (Fig. 1A) with BLT1R protein levels similarly reduced in FABP4 knockout macrophages (Fig. 1B, C).
Fig. 1.
FABP4 regulates BLT1R expression and activity. A: BLT1R mRNA expression normalized to TFIIE in stable macrophage cell lines from WT C57BL/6J (black bars) and genetic knockout of FABP4/aP2 (AKO) (gray bars) mice. B: BLT1R and actin protein expression in WT and AKO macrophages. C: Quantification of BLT1R protein levels normalized to actin. D: WT and FABP4/aP2 (AKO) macrophages were treated with 100 nM LTB4 for the indicated times. Following JAK2 immunoprecipitation, immunoblot analysis was conducted using phospho-tyrosine and total JAK2 antibodies. Data were analyzed by Student’s t-test; *P < 0.05.
Signaling by LTB4 is compromised in FABP4 knockout macrophages
Because the expression of BLT1R is reduced in FABP4 knockout macrophages, we next determined whether this translated into a functional effect on signaling. LTB4 has been demonstrated to activate the JAK2 pathway in macrophages via coupling to GαI, which inhibits the known JAK2 inhibitor, SOCS1 (55). Therefore, we tested whether the LTB4-dependent activation of JAK2 differed in FABP4 knockout as compared with control macrophages. As expected, a time course of LTB4 treatment resulted in phosphorylation of JAK2 in WT macrophages; however this was dramatically blunted in FABP4 knockout macrophages (Fig. 1D).
Macrophage polarization affects BLT1R expression
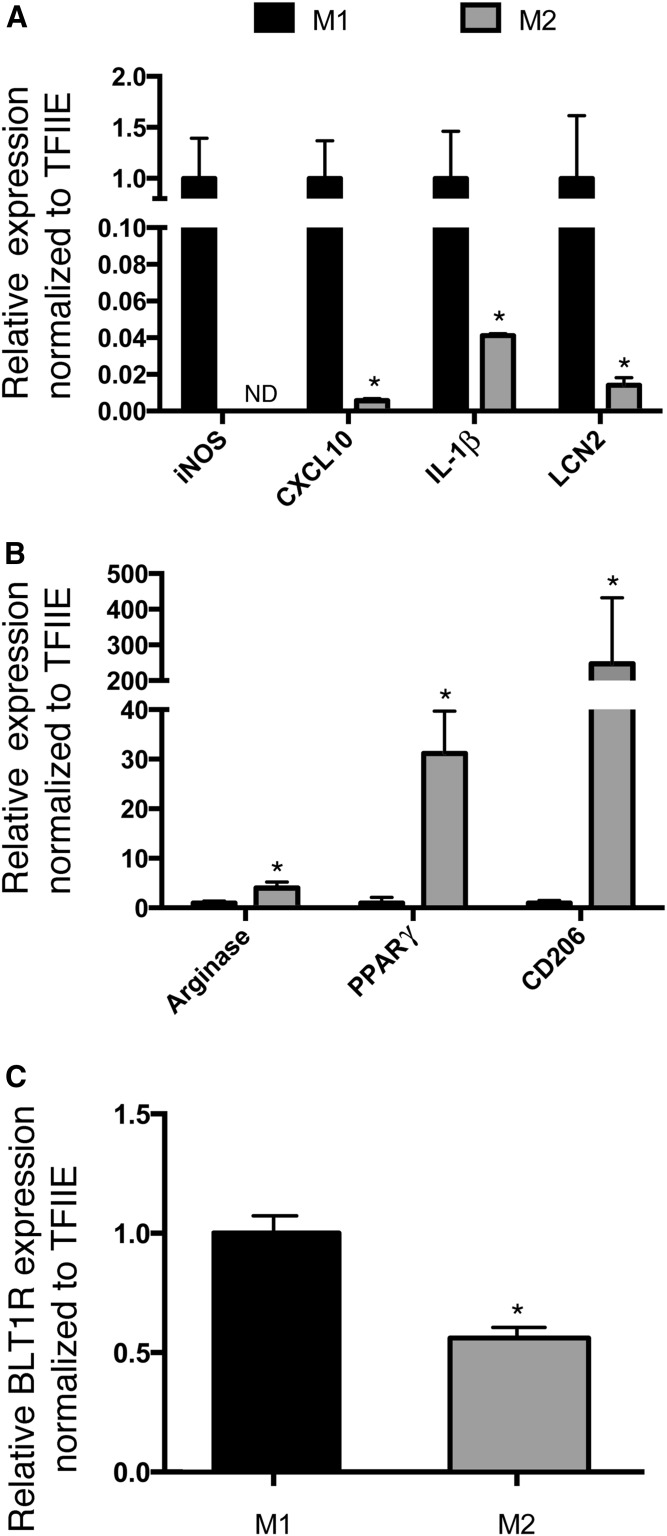
Macrophages can be activated by a variety of autocrine or paracrine factors to yield either a classical inflammatory M1 state or an alternatively activated anti-inflammatory M2 state (35, 56, 57). To determine whether expression of BLT1R is altered depending on the activation state, we utilized BMDMs activated to the M1 proinflammatory state using LPS and IFNγ, or to the M2 anti-inflammatory state using IL-4 and IL-13. To evaluate macrophage polarization, we quantified the expression of several genes known to be markers of M1 or M2 macrophages (58). As shown in Fig. 2A, LPS and IFNγ resulted in robust expression of the M1 markers, iNOS, CXCL10, IL-1β, and LCN2, while reducing expression of the M2 markers, arginase, PPARγ, and CD206 (Fig. 2B). In contrast, IL-4 and IL-13 increased the M2 markers while reducing the expression of the M1 markers. Consistent with an inflammatory state regulating the expression of BLT1R, M1 macrophages expressed higher BLT1R mRNA levels than macrophages in the M2 state (Fig. 2C).
Fig. 2.
Macrophage polarization regulates BLT1R expression. Cultured BMDMs from C57BL/6J mice were activated to the M1 state with LPS (1 μg/ml)/IFNγ (10 ng/ml) (black bars) or the M2 state with IL-4 (10 ng/ml)/IL-13 (10 ng/ml) (gray bars) for 48 h and gene expression evaluated by quantitative RT-PCR. A: Expression of genes representing M1-polarized macrophage markers. B: Expression of genes representing M2-polarized macrophage markers. C: Relative expression of BLT1R in the M1- and M2-activated macrophages. All expression results were normalized to TFIIE. Data were analyzed by Student’s t-test; *P < 0.05.
BLT1R expression is regulated by LPS in macrophages
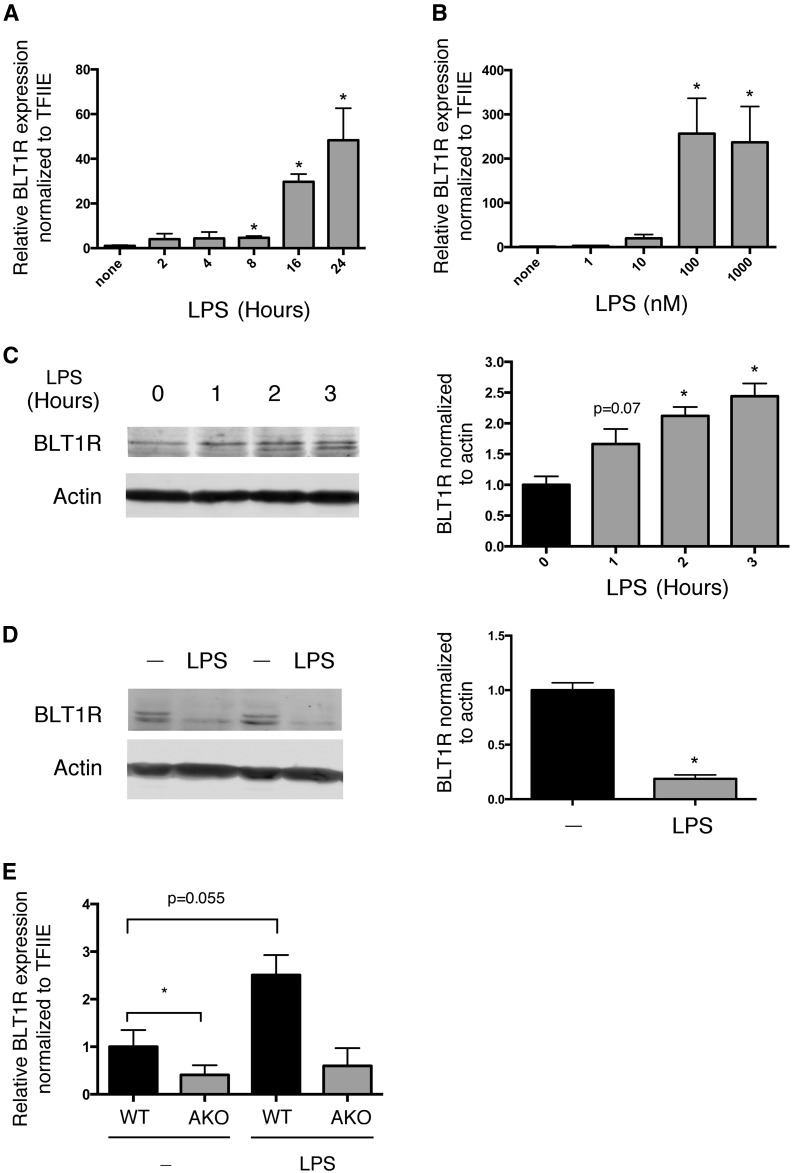
The regulatory effect of inflammatory signals such as LPS on BLT1R expression varies greatly dependent on the specific cell type (59–61). Therefore, we tested the effect of LPS on BLT1R in the murine macrophage cell line, RAW264.7. LPS exerted a time- and dose-dependent increase in BLT1R mRNA (Fig. 3A, B) in RAW264.7 cells. Interestingly, short-term exposure to LPS increased BLT1R protein levels (Fig. 3C). However, longer LPS exposure resulted in a decrease in BLT1R protein (Fig. 3D). This result might be due to receptor desensitization that includes internalization and degradation or perhaps some other mechanism. Because AKO macrophages express lower basal levels of BLT1R, we evaluated the effect of LPS treatment on BLT1R expression in AKO cells. Figure 3E demonstrates that although LPS increased BLT1R mRNA expression in WT macrophages, LPS had no effect on AKO macrophages, consistent with the reduced levels of ROS and inflammation in this cell line (54, 62, 63).
Fig. 3.
LPS regulates BLT1R expression. A: Time course (2–24 h) of BLT1R mRNA expression in RAW264.7 macrophages treated with LPS (100 ng/ml). B: Expression of BLT1R mRNA in response to a 24 h concentration course of LPS. C: Expression of BLT1R protein in response to short-term LPS (100 ng/ml) treatment (0–3 h). D: Expression of BLT1R protein in response to 24 h of LPS (100 ng/ml) treatment. E: BLT1R mRNA expression in WT and AKO macrophages under basal conditions and in response to LPS (100 ng/ml) treatment for 24 h. Data were analyzed by Student’s t-test. *P < 0.05 as compared with no treatment (A–D). *P < 0.05 AKO compared with WT (E).
Regulation of BLT1R expression by UCP2 and ROS
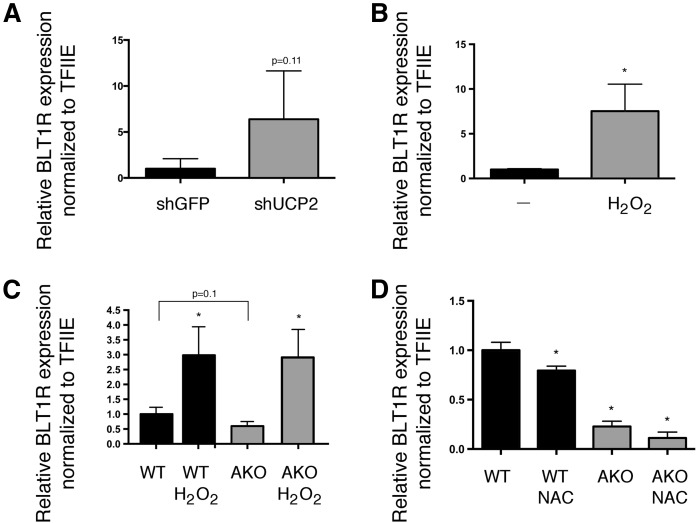
We have previously demonstrated that knockout or inhibition of FABP4 leads to decreased inflammation and ROS due to the upregulation of UCP2 (54). To determine whether downregulation of BLT1R in AKO cells was UCP2-dependent, we silenced UCP2 expression (approximately 80%) in AKO macrophages using stable shRNA (54). As shown in Fig. 4A, loss of UCP2 in AKO cells resulted in increased expression of BLT1R mRNA. Although there is some controversy as to whether UCP2 has uncoupling activity, it is generally agreed that UCP2 expression is negatively correlated with ROS (64, 65). We too have provided evidence supporting this correlation, because we measured increased ROS levels when UCP2 was silenced in RAW264.7 macrophages (52). To determine whether ROS could independently regulate BLT1R, Raw264.7 macrophages were treated with hydrogen peroxide and BLT1R mRNA levels were evaluated. As shown in Fig. 4B, BLT1R mRNA levels were robustly increased following H2O2 treatment of RAW264.7 macrophages. Furthermore, if reduced oxidative stress in AKO macrophages was responsible for the decreased BLT1R expression, exogenous H2O2 should increase BLT1R in both WT and AKO macrophages. For this, we isolated and treated BMDMs from C57BL/6J (WT) and AKO mice, and treated both with exogenous H2O2. Figure 3C shows that basal expression of BLT1R in AKO is trending down, similar to our stable macrophage cell lines, but exogenous H2O2 results in a similar increase in BLT1R expression in both cell types. Finally, we tested whether the general ROS scavenger, N-acetyl cysteine, could decrease expression of BLT1R in WT and AKO macrophages. Consistent with the model of ROS being a primary regulator of BLT1R, mRNA levels of BLT1R decreased in both WT and AKO following treatment with N-acetyl cysteine (Fig. 4D).
Fig. 4.
UCP2 and ROS regulate BLT1R expression. Macrophage expression of BLT1R mRNA normalized to TFIIE mRNA. A: shRNA knockdown of GFP (control) and UCP2 in AKO macrophages. B: RAW264.7 macrophages treated with hydrogen peroxide (100 μM, 6 h). C: BMDMs from WT and AKO mice treated with hydrogen peroxide (100 μM, 6 h). D: WT and FABP4/aP2-null (AKO) macrophages treated with or without N-acetyl cysteine (10 mM) for 24 h. Data were analyzed by Student’s t-test (*P < 0.05). *P < 0.05 as compared with WT (C, D).
DISCUSSION
Herein, we demonstrate that the expression of macrophage BLT1R is upregulated by LPS and ROS, and that FABP4 knockout (AKO) macrophages have significantly blunted BLT1R expression and activity. Previous studies have demonstrated that the reduced proinflammatory activity in the FABP4 knockout macrophages is due in large part to increased UCP2 expression and, in the present study, we extend that analysis to link UCP2 expression and ROS to the expression of BLT1R (40, 62, 63). Increased expression of UCP2 in FABP4 knockout macrophages reduces inflammatory cytokine synthesis and secretion (54). Moreover, FABP4 protein stabilizes LTA4 to chemical degradation and stimulates LT synthesis and secretion. These findings are consistent with our current data demonstrating the inverse relationship of elevated BLT1R expression and reduced UCP2 expression.
FABP4 knockout mice are protected from chronic inflammation, insulin resistance, atherosclerosis, and experimental autoimmune encephalomyelitis (44–49, 66). The LTB4-BLT1R axis has been demonstrated to play a significant role in promoting each of these conditions (16, 24, 37, 67, 68) and has been identified as a potential therapeutic target (69). Furthermore, the phenotype of the BLT1R knockout mice shares similarities to that of the FABP4 knockout mice. These include increased alternative activation (M2) of macrophages, decreased MCP-1 expression, and JNK activation, as well as decreased liver triglycerides (37). Additionally, inhibition of BLT1R in the leptin-deficient obesity model, ob/ob, increased the amount of M2 adipose tissue macrophages and decreased M1 macrophages (38). Consistent with this, inhibition of BLT1R also led to decreased expression and circulation of proinflammatory cytokines (37, 55). Therefore, the reduction of BLT1R in the FABP4-null macrophages may underlie the reduced susceptibility to metabolic diseases in the FABP4-null mice.
Upon ligand binding, G protein-coupled receptors typically become desensitized, internalized, and degraded (70, 71) and this regulatory mechanism has been demonstrated for LTB4-BLT1R in an atypical phosphorylation-independent manner via β-arrestin association (72). In this study, LPS treatment resulted in a short-term increase in protein expression of BLT1R; however, chronic LPS treatment resulted in decreased BLT1R protein levels (Fig. 3C, D). The apparent discordance between BLT1R mRNA and protein expression may be linked to desensitization and subsequent degradation of the receptor protein. However, preliminary work thus far has not supported this assertion (data not shown). Thus the mechanism that leads to the dramatic decrease in LTB4 signaling in the FABP4-null macrophages, in addition to the reduction of the receptor, is not currently known. Additional work is necessary to determine the mechanism underlying this observation.
The LTB4 precursor, LTA4, has been shown to be highly unstable due to the water hydrolysis of its epoxide ring, eliminating its biological activity (73). The half-life of LTA4 is markedly increased by members of the FABP family, including FABP4 (50), and pharmacologic inhibition of FABP4 in macrophages markedly reduces LTC4 secretion (74). As such, FABP4 regulates LT signaling on multiple levels. First, it directly affects the stability and secretion of LTs and, second, it indirectly regulates BLT1R via an UCP2-dependent ROS-based mechanism. Together these effects significantly influence the activity of LTB4/BLT1R proinflammatory signaling and suggest that decreased LTB4 signaling in FABP4 knockout mice would contribute to the anti-inflammatory phenotype of the animals.
Acknowledgments
The authors would like to thank the members of the Bernlohr laboratory for helpful discussions during the study and preparation of the manuscript. The support of the Minnesota Supercomputing Institute is gratefully acknowledged.
Footnotes
Abbreviations:
- AKO
- FABP4/aP2 knockout
- BLT1R
- leukotriene B4 receptor 1
- BMDM
- bone marrow-derived macrophage
- FABP
- fatty acid binding protein
- LPS
- lipopolysaccharide
- LT
- leukotriene
- ROS
- reactive oxygen species
- TFIIE
- transcription factor II E
This work was supported by National Institutes of Health Grant R01 DK053189 to D.A.B. and the Minnesota Nutrition and Obesity Center (National Institutes of Health Grant P30 DK050456). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Ogden C. L., Carroll M. D., Kit B. K., and Flegal K. M.. 2014. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 311: 806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flegal K. M., Kruszon-Moran D., Carroll M. D., Fryar C. D., and Ogden C. L.. 2016. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 315: 2284–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arroyo-Johnson C., and Mincey K. D.. 2016. Obesity epidemiology worldwide. Gastroenterol. Clin. North Am. 45: 571–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shoelson S. E., Herrero L., and Naaz A.. 2007. Obesity, inflammation, and insulin resistance. Gastroenterology. 132: 2169–2180. [DOI] [PubMed] [Google Scholar]
- 5.Schäffler A., and Scholmerich J.. 2010. Innate immunity and adipose tissue biology. Trends Immunol. 31: 228–235. [DOI] [PubMed] [Google Scholar]
- 6.Boutens L., and Stienstra R.. 2016. Adipose tissue macrophages: going off track during obesity. Diabetologia. 59: 879–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DiSpirito J. R., and Mathis D.. 2015. Immunological contributions to adipose tissue homeostasis. Semin. Immunol. 27: 315–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han C. Y. 2016. Roles of reactive oxygen species on insulin resistance in adipose tissue. Diabetes Metab. J. 40: 272–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boudina S., and Graham T. E.. 2014. Mitochondrial function/dysfunction in white adipose tissue. Exp. Physiol. 99: 1168–1178. [DOI] [PubMed] [Google Scholar]
- 10.Rådmark O., and Samuelsson B.. 2010. Regulation of the activity of 5-lipoxygenase, a key enzyme in leukotriene biosynthesis. Biochem. Biophys. Res. Commun. 396: 105–110. [DOI] [PubMed] [Google Scholar]
- 11.Anwar Y., Sabir J. S. M., Qureshi M. I., and Saini K. S.. 2014. 5-lipoxygenase: a promising drug target against inflammatory diseases-biochemical and pharmacological regulation. Curr. Drug Targets. 15: 410–422. [DOI] [PubMed] [Google Scholar]
- 12.Haeggström J. Z., and Funk C. D.. 2011. Lipoxygenase and leukotriene pathways: biochemistry, biology, and roles in disease. Chem. Rev. 111: 5866–5898. [DOI] [PubMed] [Google Scholar]
- 13.Bennett M., and Gilroy D. W.. 2016. Lipid mediators in inflammation. Microbiol. Spectr. 4: doi:10.1128/microbiolspec.MCHD-0035-2016. [DOI] [PubMed] [Google Scholar]
- 14.Samuelsson B. 1983. Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation. Science. 220: 568–575. [DOI] [PubMed] [Google Scholar]
- 15.Yokomizo T., Izumi T., and Shimizu T.. 2001. Leukotriene B4: metabolism and signal transduction. Arch. Biochem. Biophys. 385: 231–241. [DOI] [PubMed] [Google Scholar]
- 16.Bäck M., Powell W. S., Dahlén S-E., Drazen J. M., Evans J. F., Serhan C. N., Shimizu T., Yokomizo T., and Rovati G. E.. 2014. Update on leukotriene, lipoxin and oxoeicosanoid receptors: IUPHAR Review 7. Br. J. Pharmacol. 171: 3551–3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shimizu T. 2009. Lipid mediators in health and disease: enzymes and receptors as therapeutic targets for the regulation of immunity and inflammation. Annu. Rev. Pharmacol. Toxicol. 49: 123–150. [DOI] [PubMed] [Google Scholar]
- 18.Cornejo-García J. A., Perkins J. R., Jurado-Escobar R., García-Martín E., Agúndez J. A., Viguera E., Pérez-Sánchez N., and Blanca-López N.. 2016. Pharmacogenomics of prostaglandin and leukotriene receptors. Front. Pharmacol. 7: 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin T. R., Altman L. C., Albert R. K., and Henderson W. R.. 1984. Leukotriene B4 production by the human alveolar macrophage: a potential mechanism for amplifying inflammation in the lung. Am. Rev. Respir. Dis. 129: 106–111. [DOI] [PubMed] [Google Scholar]
- 20.Rankin J. A., Sylvester I., Smith S., Yoshimura T., and Leonard E. J.. 1990. Macrophages cultured in vitro release leukotriene B4 and neutrophil attractant/activation protein (interleukin 8) sequentially in response to stimulation with lipopolysaccharide and zymosan. J. Clin. Invest. 86: 1556–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yokomizo T. 2011. Leukotriene B4 receptors: novel roles in immunological regulations. Adv. Enzyme Regul. 51: 59–64. [DOI] [PubMed] [Google Scholar]
- 22.Yokomizo T. 2015. Two distinct leukotriene B4 receptors, BLT1 and BLT2. J. Biochem. 157: 65–71. [DOI] [PubMed] [Google Scholar]
- 23.Okuno T., Iizuka Y., Okazaki H., Yokomizo T., Taguchi R., and Shimizu T.. 2008. 12(S)-Hydroxyheptadeca-5Z, 8E, 10E-trienoic acid is a natural ligand for leukotriene B4 receptor 2. J. Exp. Med. 205: 759–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Subbarao K., Jala V. R., Mathis S., Suttles J., Zacharias W., Ahamed J., Ali H., Tseng M. T., and Haribabu B.. 2004. Role of leukotriene B4 receptors in the development of atherosclerosis: potential mechanisms. Arterioscler. Thromb. Vasc. Biol. 24: 369–375. [DOI] [PubMed] [Google Scholar]
- 25.Afonso P. V., Janka-Junttila M., Lee Y. J., McCann C. P., Oliver C. M., Aamer K. A., Losert W., Cicerone M. T., and Parent C. A.. 2012. LTB4 is a signal-relay molecule during neutrophil chemotaxis. Dev. Cell. 22: 1079–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hafstrom I., Palmblad J., Malmsten C. L., Rådmark O., and Samuelsson B.. 1981. Leukotriene B4–a stereospecific stimulator for release of lysosomal enzymes from neutrophils. FEBS Lett. 130: 146–148. [DOI] [PubMed] [Google Scholar]
- 27.Flamand L., Tremblay M. J., and Borgeat P.. 2007. Leukotriene B4 triggers the in vitro and in vivo release of potent antimicrobial agents. J. Immunol. 178: 8036–8045. [DOI] [PubMed] [Google Scholar]
- 28.Nieves D., and Moreno J. J.. 2006. Role of 5-lipoxygenase pathway in the regulation of RAW 264.7 macrophage proliferation. Biochem. Pharmacol. 72: 1022–1030. [DOI] [PubMed] [Google Scholar]
- 29.Sun R., Ba X., Cui L., Xue Y., and Zeng X.. 2009. Leukotriene B4 regulates proliferation and differentiation of cultured rat myoblasts via the BLT1 pathway. Mol. Cells. 27: 403–408. [DOI] [PubMed] [Google Scholar]
- 30.Okamoto F., Saeki K., Sumimoto H., Yamasaki S., and Yokomizo T.. 2010. Leukotriene B4 augments and restores Fc gammaRs-dependent phagocytosis in macrophages. J. Biol. Chem. 285: 41113–41121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu M., and Yokomizo T.. 2015. The role of leukotrienes in allergic diseases. Allergol. Int. 64: 17–26. [DOI] [PubMed] [Google Scholar]
- 32.Mathis S. P., Jala V. R., Lee D. M., and Haribabu B.. 2010. Nonredundant roles for leukotriene B4 receptors BLT1 and BLT2 in inflammatory arthritis. J. Immunol. 185: 3049–3056. [DOI] [PubMed] [Google Scholar]
- 33.Yokota Y., Inoue H., Matsumura Y., Nabeta H., Narusawa M., Watanabe A., Sakamoto C., Hijikata Y., Iga-Murahashi M., Takayama K., et al. 2012. Absence of LTB4/BLT1 axis facilitates generation of mouse GM-CSF-induced long-lasting antitumor immunologic memory by enhancing innate and adaptive immune systems. Blood. 120: 3444–3454. [DOI] [PubMed] [Google Scholar]
- 34.Lee J. 2013. Adipose tissue macrophages in the development of obesity-induced inflammation, insulin resistance and type 2 diabetes. Arch. Pharm. Res. 36: 208–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kraakman M. J., Murphy A. J., Jandeleit-Dahm K., and Kammoun H. L.. 2014. Macrophage polarization in obesity and type 2 diabetes: weighing down our understanding of macrophage function? Front. Immunol. 5: 470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olefsky J. M., and Glass C. K.. 2010. Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 72: 219–246. [DOI] [PubMed] [Google Scholar]
- 37.Spite M., Hellmann J., Tang Y., Mathis S. P., Kosuri M., Bhatnagar A., Jala V. R., and Haribabu B.. 2011. Deficiency of the leukotriene B4 receptor, BLT-1, protects against systemic insulin resistance in diet-induced obesity. J. Immunol. 187: 1942–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li P., Oh D. Y., Bandyopadhyay G., Lagakos W. S., Talukdar S., Osborn O., Johnson A., Chung H., Mayoral R., Maris M., et al. 2015. LTB4 promotes insulin resistance in obese mice by acting on macrophages, hepatocytes and myocytes. Nat. Med. 21: 239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ying W., Wollam J., Ofrecio J. M., Bandyopadhyay G., El Ouarrat D., Lee Y. S., Oh D. Y., Li P., Osborn O., and Olefsky J. M.. 2017. Adipose tissue B2 cells promote insulin resistance through leukotriene LTB4/LTB4R1 signaling. J. Clin. Invest. 127: 1019–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hotamisligil G. S., and Bernlohr D. A.. 2015. Metabolic functions of FABPs—mechanisms and therapeutic implications. Nat. Rev. Endocrinol. 11: 592–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scheja L., Makowski L., Uysal K. T., Wiesbrock S. M., Shimshek D. R., Meyers D. S., Morgan M., Parker R. A., and Hotamisligil G. S.. 1999. Altered insulin secretion associated with reduced lipolytic efficiency in aP2−/− mice. Diabetes. 48: 1987–1994. [DOI] [PubMed] [Google Scholar]
- 42.Coe N. R., Simpson M. A., and Bernlohr D. A.. 1999. Targeted disruption of the adipocyte lipid-binding protein (aP2 protein) gene impairs fat cell lipolysis and increases cellular fatty acid levels. J. Lipid Res. 40: 967–972. [PubMed] [Google Scholar]
- 43.Baar R. A., Dingfelder C. S., Smith L. A., Bernlohr D. A., Wu C., Lange A. J., and Parks E. J.. 2005. Investigation of in vivo fatty acid metabolism in AFABP/aP2(-/-) mice. Am. J. Physiol. Endocrinol. Metab. 288: E187–E193. [DOI] [PubMed] [Google Scholar]
- 44.Hotamisligil G. S., Johnson R. S., Distel R. J., Ellis R., Papaioannou V. E., and Spiegelman B. M.. 1996. Uncoupling of obesity from insulin resistance through a targeted mutation in aP2, the adipocyte fatty acid binding protein. Science. 274: 1377–1379. [DOI] [PubMed] [Google Scholar]
- 45.Makowski L., Boord J. B., Maeda K., Babaev V. R., Uysal K. T., Morgan M. A., Parker R. A., Suttles J., Fazio S., Hotamisligil G. S., et al. 2001. Lack of macrophage fatty-acid-binding protein aP2 protects mice deficient in apolipoprotein E against atherosclerosis. Nat. Med. 7: 699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boord J. B. 2002. Adipocyte fatty acid-binding protein, aP2, alters late atherosclerotic lesion formation in severe hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 22: 1686–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shum B. O. V., Mackay C. R., Görgün C. Z., Frost M. J., Kumar R. K., Hotamisligil G. S., and Rolph M. S.. 2006. The adipocyte fatty acid-binding protein aP2 is required in allergic airway inflammation. J. Clin. Invest. 116: 2183–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Erbay E., Babaev V. R., Mayers J. R., Makowski L., Charles K. N., Snitow M. E., Fazio S., Wiest M. M., Watkins S. M., Linton M. F., et al. 2009. Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat. Med. 15: 1383–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ghelfi E., Yu C-W., Elmasri H., Terwelp M., Lee C. G., Bhandari V., Comhair S. A., Erzurum S. C., Hotamisligil G. S., Elias J. A., et al. 2013. Fatty acid binding protein 4 regulates VEGF-induced airway angiogenesis and inflammation in a transgenic mouse model: implications for asthma. Am. J. Pathol. 182: 1425–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zimmer J. S. D., Dyckes D. F., Bernlohr D. A., and Murphy R. C.. 2004. Fatty acid binding proteins stabilize leukotriene A4: competition with arachidonic acid but not other lipoxygenase products. J. Lipid Res. 45: 2138–2144. [DOI] [PubMed] [Google Scholar]
- 51.Dickinson Zimmer J. S., Voelker D. R., Bernlohr D. A., and Murphy R. C.. 2004. Stabilization of leukotriene A4 by epithelial fatty acid-binding protein in the rat basophilic leukemia cell. J. Biol. Chem. 279: 7420–7426. [DOI] [PubMed] [Google Scholar]
- 52.Ying W., Cheruku P. S., Bazer F. W., Safe S. H., and Zhou B.. 2013. Investigation of macrophage polarization using bone marrow derived macrophages. J. Vis. Exp. 76: e50323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hertzel A. V., Hellberg K., Reynolds J. M., Kruse A. C., Juhlmann B. E., Smith A. J., Sanders M. A., Ohlendorf D. H., Suttles J., and Bernlohr D. A.. 2009. Identification and characterization of a small molecule inhibitor of fatty acid binding proteins. J. Med. Chem. 52: 6024–6031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu H., Hertzel A. V., Steen K. A., Wang Q., Suttles J., and Bernlohr D. A.. 2015. Uncoupling lipid metabolism from inflammation through fatty acid binding protein-dependent expression of UCP2. Mol. Cell. Biol. 35: 1055–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Serezani C. H., Lewis C., Jancar S., and Peters-Golden M.. 2011. Leukotriene B4 amplifies NF-κB activation in mouse macrophages by reducing SOCS1 inhibition of MyD88 expression. J. Clin. Invest. 121: 671–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Classen A., Lloberas J., and Celada A.. 2009. Macrophage activation: classical versus alternative. Methods Mol. Biol. 531: 29–43. [DOI] [PubMed] [Google Scholar]
- 57.Gordon S., and Martinez F. O.. 2010. Alternative activation of macrophages: mechanism and functions. Immunity. 32: 593–604. [DOI] [PubMed] [Google Scholar]
- 58.Jablonski K. A., Amici S. A., Webb L. M., Ruiz-Rosado Jde. D., Popovich P. G., Partida-Sanchez S., and Guerau-de-Arellano M.. 2015. Novel markers to delineate murine M1 and M2 macrophages. PLoS One. 10: e0145342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goldman D. W., Enkel H., Gifford L. A., Chenoweth D. E., and Rosenbaum J. T.. 1986. Lipopolysaccharide modulates receptors for leukotriene B4, C5a, and formyl-methionyl-leucyl-phenylalanine on rabbit polymorphonuclear leukocytes. J. Immunol. 137: 1971–1976. [PubMed] [Google Scholar]
- 60.Pettersson A., Sabirsh A., Bristulf J., Kidd-Ljunggren K., Ljungberg B., Owman C., and Karlsson U.. 2005. Pro- and anti-inflammatory substances modulate expression of the leukotriene B4 receptor, BLT1, in human monocytes. J. Leukoc. Biol. 77: 1018–1025. [DOI] [PubMed] [Google Scholar]
- 61.Bäck M., Bu D-X., Bränström R., Sheikine Y., Yan Z-Q., and Hansson G. K.. 2005. Leukotriene B4 signaling through NF-kappaB-dependent BLT1 receptors on vascular smooth muscle cells in atherosclerosis and intimal hyperplasia. Proc. Natl. Acad. Sci. USA. 102: 17501–17506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Makowski L., Brittingham K. C., Reynolds J. M., Suttles J., and Hotamisligil G. S.. 2005. The fatty acid-binding protein, aP2, coordinates macrophage cholesterol trafficking and inflammatory activity. Macrophage expression of aP2 impacts peroxisome proliferator-activated receptor gamma and IkappaB kinase activities. J. Biol. Chem. 280: 12888–12895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu H., Hertzel A. V., Steen K. A., and Bernlohr D. A.. 2016. Loss of fatty acid binding protein 4/aP2 reduces macrophage inflammation through activation of SIRT3. Mol. Endocrinol. 30: 325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Krauss S., Zhang C-Y., and Lowell B. B.. 2005. The mitochondrial uncoupling-protein homologues. Nat. Rev. Mol. Cell Biol. 6: 248–261. [DOI] [PubMed] [Google Scholar]
- 65.Donadelli M., Dando I., Fiorini C., and Palmieri M.. 2014. UCP2, a mitochondrial protein regulated at multiple levels. Cell. Mol. Life Sci. 71: 1171–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reynolds J. M., Liu Q., Brittingham K. C., Liu Y., Gruenthal M., Görgün C. Z., Hotamisligil G. S., Stout R. D., and Suttles J.. 2007. Deficiency of fatty acid-binding proteins in mice confers protection from development of experimental autoimmune encephalomyelitis. J. Immunol. 179: 313–321. [DOI] [PubMed] [Google Scholar]
- 67.Gladue R. P., Carroll L. A., Milici A. J., Scampoli D. N., Stukenbrok H. A., Pettipher E. R., Salter E. D., Contillo L., and Showell H. J.. 1996. Inhibition of leukotriene B4-receptor interaction suppresses eosinophil infiltration and disease pathology in a murine model of experimental allergic encephalomyelitis. J. Exp. Med. 183: 1893–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kihara Y., Yokomizo T., Kunita A., Morishita Y., Fukayama M., Ishii S., and Shimizu T.. 2010. The leukotriene B4 receptor, BLT1, is required for the induction of experimental autoimmune encephalomyelitis. Biochem. Biophys. Res. Commun. 394: 673–678. [DOI] [PubMed] [Google Scholar]
- 69.Filgueiras L. R., Serezani C. H., and Jancar S.. 2015. Leukotriene B4 as a potential therapeutic target for the treatment of metabolic disorders. Front. Immunol. 6: 515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ferguson S. S. 2001. Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol. Rev. 53: 1–24. [PubMed] [Google Scholar]
- 71.Walther C., and Ferguson S. S. G.. 2013. Arrestins: role in the desensitization, sequestration, and vesicular trafficking of G protein-coupled receptors. Prog. Mol. Biol. Transl. Sci. 118: 93–113. [DOI] [PubMed] [Google Scholar]
- 72.Chen Z., Gaudreau R., Le Gouill C., Rola-Pleszczynski M., and Stanková J.. 2004. Agonist-induced internalization of leukotriene B(4) receptor 1 requires G-protein-coupled receptor kinase 2 but not arrestins. Mol. Pharmacol. 66: 377–386. [DOI] [PubMed] [Google Scholar]
- 73.Borgeat P., and Samuelsson B.. 1979. Arachidonic acid metabolism in polymorphonuclear leukocytes: unstable intermediate in formation of dihydroxy acids. Proc. Natl. Acad. Sci. USA. 76: 3213–3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Long E. K., Hellberg K., Foncea R., Hertzel A. V., Suttles J., and Bernlohr D. A.. 2013. Fatty acids induce leukotriene C4 synthesis in macrophages in a fatty acid binding protein-dependent manner. Biochim. Biophys. Acta. 1831: 1199–1207. [PubMed] [Google Scholar]