Abstract
Hepatic mitochondrial function, APOC-III, and LPL are potential targets for triglyceride (TG)-lowering drugs. After 3 weeks of dietary treatment with the compound 2-(tridec-12-yn-1-ylthio)acetic acid (1-triple TTA), the hepatic mitochondrial FA oxidation increased more than 5-fold in male Wistar rats. Gene expression analysis in liver showed significant downregulation of APOC-III and upregulation of LPL and the VLDL receptor. This led to lower hepatic (53%) and plasma (73%) TG levels. Concomitantly, liver-specific biomarkers related to mitochondrial biogenesis and function (mitochondrial DNA, citrate synthase activity, and cytochrome c and TFAM gene expression) were elevated. Interestingly, 1-triple TTA lowered plasma acetylcarnitine levels, whereas the concentration of β-hydroxybutyrate was increased. The hepatic energy state was reduced in 1-triple TTA-treated rats, as reflected by increased AMP/ATP and decreased ATP/ADP ratios, whereas the energy state remained unchanged in muscle and heart. The 1-triple TTA administration induced gene expression of uncoupling protein (UCP)2 and UCP3 in liver. In conclusion, the 1-triple TTA-mediated clearance of blood TG may result from lowered APOC-III production, increased hepatic LPL gene expression, mitochondrial FA oxidation, and (re)uptake of VLDL facilitating drainage of FAs to the liver for β-oxidation and production of ketone bodies as extrahepatic fuel. The possibility that UCP2 and UCP3 mediate a moderate degree of mitochondrial uncoupling should be considered.
Keywords: 2-(tridec-12-yn-1-ylthio)acetic acid, lipids, lipoproteins, metabolic syndrome, mitochondria, nonalcoholic fatty liver disease, fatty acid metabolism, lipids/oxidation, fatty acid, triglyceride, uncoupling protein, apolipoprotein C-III
CVD is the leading cause of death worldwide, especially in developed countries (1, 2). Meta-analyses have shown that low HDL-cholesterol and high triglyceride (TG) levels in blood are strongly associated with increased CVD risk (3, 4). Recently, TG-rich lipoproteins were found to causally influence risk of coronary heart disease in individuals with favorable HDL-cholesterol (5). Thus, it is important to consider TG in addition to the traditional risk factors, cholesterol and LDL cholesterol (6–8). TG is transported in chylomicrons (CHYLs) and VLDL from the intestine and liver, respectively, to peripheral tissues where LPL hydrolyzes TG to glycerol and FAs for subsequent uptake. Several lines of evidence have implicated APOC-II and APOC-III in the regulation of CHYL and VLDL metabolism, as LPL uses APOC-II as a cofactor and is inhibited by APOC-III (9). Overexpression of APOC-III in transgenic mice causes hypertriglyceridemia (10) and has also been suggested to be a characteristic feature of hypertriglyceridemia in human patients (11). APOC-III deficiency protects against metabolic disease in mice (12) and it has been proposed that levels of VLDL and APOC-III are better prognostic markers of CVD than plasma TG (13). Accordingly, APOC-III and LPL are among the potential targets for lipid-lowering drugs.
The liver is important for regulating lipid homeostasis and metabolism. CHYL and VLDL remnants are taken up by the liver and, under normolipidemic conditions, TG is either oxidized or secreted as VLDL. However, excessive accumulation of hepatic fat unrelated to alcohol consumption, hepatic steatosis, or nonalcoholic fatty liver disease is the most frequent liver disease in industrialized countries (14). Moreover, the development of this disease often parallels that of insulin resistance and is associated with obesity, metabolic syndrome, dyslipidemia, and type 2 diabetes mellitus (15), and has been linked to mitochondrial malfunction (16). Mitochondria are active in β-oxidation of FAs and, in situations with excess uptake of lipids by the liver, an adaptive increase in mitochondrial oxidative capacity is required. Under these conditions, mitochondrial dysfunction will compromise metabolic performance and may lead to elevated production of reactive oxygen species (17). Hence, mitochondrial adaptations are important to protect against harmful effects of oxidative stress, inflammation, and intrahepatic accumulation of lipids (16, 18).
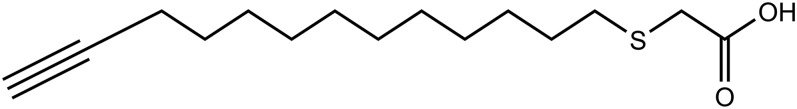
Given that mitochondrial function and mitochondrial FA oxidation capacity seem to regulate TG levels both in liver and plasma, we have synthesized FA analogs as tetradecylthioacetic acid (TTA), targeting mitochondria (19, 20), which were subsequently shown to interfere with APOC-III gene expression (20). Notably, TTA is blocked for β-oxdation due to the sulfur atom, but can be degraded from the ω-end. Therefore, 2-(tridec-12-yn-1-ylthio)acetic acid (C15H26O2S) (1-triple TTA) is of special interest; in addition to the sulfur atom at the β position, a triple bond is introduced at the ω-1 position. These alterations make the compound resistant to both β-oxidation and ω-oxidation (Fig. 1); thus, 1-triple TTA might be a more potent drug than TTA. In this study, we aimed to test how 1-triple TTA influences mitochondrial function and plasma and hepatic TG levels, as well as hepatic ApoC-III mRNA levels.
Fig. 1.
Skeletal structure of 1-triple TTA.
MATERIALS AND METHODS
Animal study
The animal study was conducted according to the Guidelines for the Care and Use of Experimental Animals and in accordance with the Norwegian legislation and regulations governing experiments using live animals. The Norwegian State Board of Biological Experiments with Living Animals approved the protocol (permit number 2015-7367). All efforts were made to optimize the animal environment and minimize suffering. Male Wistar rats, 5 weeks old, were purchased from Taconic (Ejby, Denmark). Upon arrival, the rats were randomized using Research Randomizer (21), labeled, and placed in open cages, four in each cage, where they were allowed to acclimatize to their surroundings for 1 week. During the acclimatization and experimental period, the rats had unrestricted access to chow and tap water. The rats were kept in a 12 h light/dark cycle at a constant temperature (22 ± 2°C) and a relative humidity of 55% (±5%). Upon the start of the experiment, the rats were block randomized to their respective interventions (21). During the experiment, there were two rats in each cage separated with a divider that let them have sniffing contact. The control group (n = 8) received 0.5 ml 0.5% methylcellulose (Hospital Pharmacy, Bergen, Norway) daily and the experimental group (n = 5) received 0.02 g 1-triple TTA (Synthetica AS, Oslo, Norway) dissolved in 0.5 ml 0.5% methylcellulose daily. Methylcellulose was given orally by gavage. All animals were weighed daily and feed intake was determined weekly.
At euthanization, rats were anesthetized by inhalation of 5% isoflurane (Schering-Plough, Kent, UK). EDTA-blood was collected by cardiac puncture and immediately placed on ice. The samples were centrifuged and plasma was stored at −80°C prior to analysis. Heart, liver, and epididymal white adipose tissue were collected and weighed. Fresh samples from liver were prepared for β-oxidation analysis (see below). The remaining parts of the liver, heart, and samples from adipose and muscle tissues were immediately snap-frozen in liquid nitrogen and stored at −80°C until further analysis.
Quantification of plasma and liver lipids
Liver lipids were extracted from frozen samples according to Bligh and Dyer (22), evaporated under nitrogen, and redissolved in isopropanol before analysis. Lipids from liver and plasma were measured enzymatically on a Hitachi 917 system (Roche Diagnostics GmbH, Mannheim, Germany) using the TG kit from Roche Diagnostics (GPO-PAP, 11730711) and NEFA FS kit from Diagnostic Systems GmbH (Holzheim, Germany).
Quantification of carnitine metabolites and ketone body in plasma
L-carnitine, trimethyllysine, γ-butyrobetaine, and acetylcarnitine were analyzed in plasma by HPLC-MS/MS, as described by Vernez, Wenk, and Krahenbuhl (23) with some modifications (24). The ketone body, β-hydroxybutyrate, was analyzed in plasma by colorimetric assay kit from Cayman Chemical Company (item number 700190; Ann Arbor, MI).
Measurement of high energy phosphates by HPLC
Freeze-clamped biopsies were taken from the apex of the heart and liver, as well as skeletal muscle, and stored in liquid nitrogen. The sample preparation and HPLC measurement of ATP, ADP, AMP, and phosphocreatine (for heart and muscle samples), as well as hypoxanthine and xanthine, were performed as previously described (25–27). A piece of frozen tissue (50–100 mg) was homogenized with a micro-dismembrator and deproteinized with 500 μl of 0.4 mol/l perchloric acid. After centrifugation (12,000 g), 200 μl of the acid extract were neutralized with 20–25 μl of 2 mol/l potassium carbonate (4°C). The supernatant (20 μl injection volume) obtained after centrifugation was used for HPLC analysis. The pellets of the acid extract were dissolved in 1 ml of 0.1 mol/l sodium hydroxide and further diluted 1:10 with physiologic saline for protein determination (BCA protein assay; Pierce). High energy phosphates (ATP, ADP, AMP, and phosphocreatine for heart and muscle samples), hypoxanthine, and xanthine were measured by HPLC, as previously described (25–27). In brief, separation was performed on a Hypersil ODS column (5 μm, 250 × 4 mm internal diameter) using an L-2200 autosampler, two L-2130 HTA pumps, and a L-2450 diode array detector (all VWR Hitachi). Detector signals (absorbance at 214 and 254 nm) were recorded and the program EZchrom Elite (VWR) was used for data requisition and analysis. Energy charge was calculated employing the following formula: energy charge = (ATP + 0.5 ADP)/(AMP + ADP + ATP).
Mitochondrial FA oxidation and enzyme activities
Post-nuclear fractions from liver, muscle, and heart were prepared (28). Palmitoyl-CoA oxidation was measured in the postnuclear fraction from fresh liver as acid-soluble products, as described by (29). The following enzyme activities were measured in the postnuclear fraction from frozen liver tissue: carnitine O-palmitoyltransferase (CPT)-II (EC: 2.3.1.21) (30), mitochondrial HMG-CoA synthase (EC: 2.3.3.10) (31), citrate synthase (EC: 2.3.3.1) (32, 33), FAS (EC: 2.3.1.85) (34), acetyl-CoA carboxylase (EC: 6.4.1.2) (35), malonyl-CoA decarboxylase (EC: 4.1.1.9) (36), and β-ketothiolase (EC: 2.3.1.16) (37). The 3-oxoacid CoA-transferase (EC: 2.8.3.5) activity was measured in frozen heart and muscle tissue (38, 39).
The amount of proteins was measured by Bio-Rad protein assay kit (Bio-Rad Laboratories, Hercules, CA).
Western blot: nonphosphorylated and phosphorylated AMP-activated protein kinase
Amounts of AMP-activated protein kinase (AMPK) and phosphorylated AMPK (AMPK-P) were analyzed by Western blot. Proteins from frozen liver samples were extracted using a nuclear extract kit (Active Motif, Carlsbad, CA). The amount of protein was determined by Bio-Rad DC™ protein assay reagents. Twenty micrograms of proteins, Laemmli 1× sample buffer (Bio-Rad), and 10 mM DTT were run on Mini-protean TGX Precast gels 4–20% (Bio-Rad), blotted on a PVDF membrane using iBlot gel transfer stack (Invitrogen, Carlsbad, CA), blocked in TBS (pH 8.0) with 3% nonfat milk (catalog number T8793; Sigma-Aldrich), and washed in TBS with Tween20 (Sigma-Aldrich). Blots were incubated with rabbit-anti-AMPK [1:500 or 1:1,000 (catalog number 2532, lot 19)] or rabbit-anti-AMPK-P [1:1,000 (catalog number 2535, lot 14)] and β-actin [1:1,000 (catalog number 4970, lot 7; Cell Signaling Technology, Danvers, MA)], overnight at 4°C, and anti-rabbit IgG HRP-linked secondary Ab [1:1,000 (catalog number 7074, lot 24; Cell Signaling) for 1 h at room temperature and developed with Supersignal™ ELISA Femto substrate for 1 min and analyzed using the FluorChem HD2 program (Alpha Innotech, Kasendorf, Germany). AMPK and AMPK-P were normalized to β-actin.
Transmission electron microscopy
Frozen liver samples were fixed in cold 0.1 M Na-cacodylate buffer (pH 7.4) containing 2.5% glutaraldehyde 4°C, overnight, and prepared as previously described (40).
Hepatic gene expression analysis
Tissue samples (20 mg frozen liver) were homogenized in RNeasy lysis buffer from Qiagen (catalog number 79216; Hilden, Germany) with 1% β-mercaptoethanol using Tissuelyser II (Qiagen) for 2 × 2 min at 25 Hz and total cellular RNA was further purified using the RNeasy mini kit (Qiagen), including DNase digestion. cDNA was produced from 500 ng RNA using the high-capacity cDNA reverse transcription kits (Applied Biosystems, Waltham, MA). RT-PCR was performed on Sarstedt 384-well Multiply-PCR plates (Sarstedt Inc., Newton, NC,) using an ABI Prism 7900HT sequence detection system from Applied Biosystems with the software, SDS 2.3. mRNA levels of genes of interest were detected in 1× Taqman buffer by using the following probes and primers listed in alphabetical order: acetyl-CoA carboxylase (Acaca) (Rn00573474_m1); medium-chain acyl-CoA dehydrogenase (Acadm) (Rn00566390_m1); long-chain acyl-CoA dehydrogenase (Acadl) (Rn00563121_m1); very-long-chain acyl-CoA dehydrogenase (Acadvl) (Rn00563649_m1); 4-N-trimethylaminobutyraldehyde dehydrogenase (Aldh9a1) (Rn01491039_m1); ApoB (Rn01499054_m1); ApoC-II (Rn01764530_g1); ApoC-III (Rn00560743); ATP synthase H+ transporting mitochondrial F1 complex γ polypeptide 1 (Atp5c1) (Rn01487287_m1); ATP synthase H+ transporting mitochondrial F0 complex subunit F2 (Atp5j2) (Rn01409509_g1); γ-butyrobetaine hydroxylase (Bbox1) (Rn00575255_m1); cluster of differentiation 36 (Cd36) (Rn00580728_m1); Cpt1a (Rn00580702_m1); Cpt2 (Rn00563995_m1); carnitine O-acetyltransferase (Crat) (Rn01758585_m1); somatic cytochrome c (Cycs) (Rn00820639_g1); mitochondrial 2,4 dienoyl-CoA reductase 1 (Decr1) (Rn00589420_m1); diglyceride acyltransferase (Dgat)1 (Rn00584870_m1); Dgat2 (Rn01506787_m1); FA binding protein 1 (Fabp1) (Rn00664587_m1); Fasn (Rn00569117_m1); mitochondrial glycerol-3-phosphate dehydrogenase 2 (Gpd2) (Rn00562472_m1); hydroxyacyl-CoA dehydrogenase/3-ketoacyl-CoA thiolase/enoyl-CoA hydratase (trifunctional protein) α subunit (Hadha) (Rn00590828_m1); hydroxyacyl-CoA dehydrogenase/3-ketoacyl-CoA thiolase/enoyl-CoA hydratase (trifunctional protein) β subunit (Hadhb) (Rn00592435_m1); mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase 2 (Hmgcs2) (Rn00597339_m1); Lpl (Rn00561482_m1); microsomal TG transfer protein (large subunit) (Mttp) (Rn01522970_m1); NADH dehydrogenase (ubiquinone) 1α subcomplex 9 (Ndufa9) (Rn01462923_m1); Pparα (Rn00566193_m1); Pparδ (Rn00565707_m1); Pparγ (Rn00440945_m1); PPARγ coactivator 1α (Ppargc1a) (Rn00580241_m1); protein kinase AMP-activated α1 catalytic subunit (Prkaa1) (Rn00569558_m1); protein kinase AMP-activated α2 catalytic subunit (Prkaa2) (Rn00576935_m1); Slc25a20 (Rn00588652_m1); solute carrier family 22 (organic cation/carnitine transporter) member 5 (Slc22a5) (Rn01471177_m1); mitochondrial transcription factor A (Tfam) (Rn00580051_m1); Nε-trimethyllysine hydroxylase (Tmlhe) (Rn00591314_m1); uncoupling protein (Ucp)2 (Rn01754856_m1); Ucp3 (Rn00565874_m1); VLDL receptor (Vldlr) (Rn00565784_m1). A standard curve using either an appropriate cDNA sample or universal rat reference RNA was performed for each probe. The 18S (RT-CKFT-18S) was run for every probe. Data presented are normalized to 18S.
Mitochondrial DNA quantification
Total DNA was isolated from liver, heart, and muscle tissue using DNAeasy blood and tissue kit and Allprep DNA/RNA Mini kit (both from Qiagen) and analyzed as described previously (41, 42). A specific primer/probe set for mitochondrial DNA (mtDNA), mitochondrially encoded NADH dehydrogenase 1 (Rn03296764_s1), was purchased from Applied Biosystems and nuclear DNA (nDNA) primer 18S (RT-CKFT-18S) from Eurogentec. Quantitative PCR was carried out as above and the mtDNA:nDNA ratio was calculated.
Statistical analysis
Data were analyzed using Prism software (Graph-Pad Software; San Diego, CA) to determine statistical significance. The results are shown as mean ± SD of 5–8 rats per group. mRNA levels are shown as relative levels compared with control mean with SD, except for Ucp3. Student’s t-test was used to evaluate statistical differences between the intervention and control. Pearson’s correlation coefficients were used when comparing two independent variables. P <0.05 was considered statistically significant.
RESULTS
1-triple TTA decreases free carnitine and acetylcarnitine in plasma
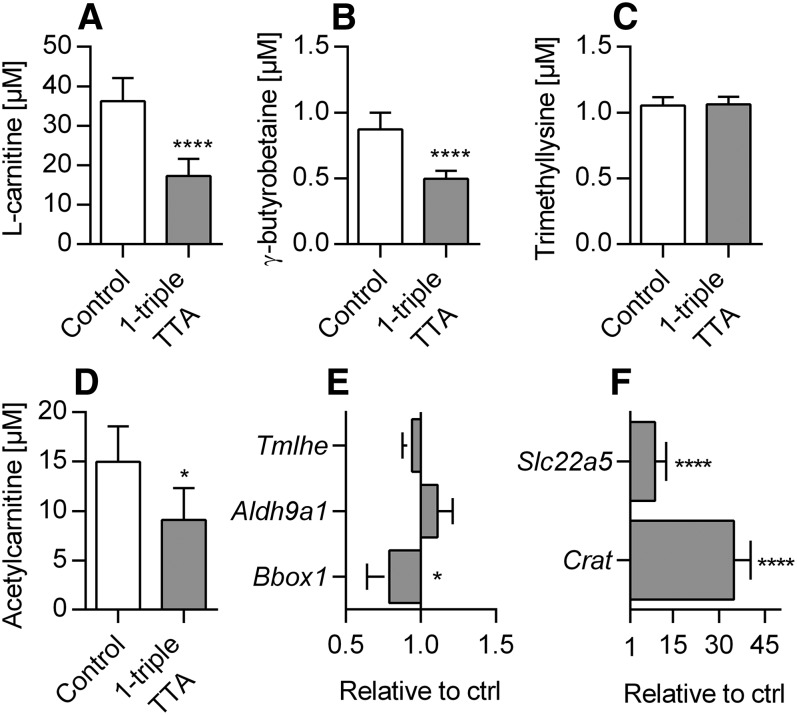
Carnitine is involved in the generation of metabolic energy from long-chain FAs by mediating their transport across the mitochondrial membrane. The levels of plasma carnitine and its precursor, γ-butyrobetaine, were significantly decreased by 1-triple TTA administration, whereas trimethyllysine remained constant (Fig. 2). The short-chain acetylcarnitine level was also decreased (Fig. 2D). The liver is an important site of carnitine production; therefore, we also evaluated whether 1-triple TTA changed the hepatic gene expression of proteins related to carnitine biosynthesis. The mRNA levels of Tmlhe and Aldh9a1 remained constant, whereas Bbox1 was significantly reduced (Fig. 2E). Interestingly, the mRNA level of Crat (a mitochondrial matrix enzyme that catalyzes the interconversion of acetyl-CoA and acetylcarnitine) and the carnitine transporter, Slc22a5, were significantly increased by 1-triple TTA compared with controls (Fig. 2F). These results suggest that 1-triple TTA downregulates the levels of carnitine and short-chained acylcarnitines by reduced biosynthesis of carnitine and/or increased consumption.
Fig. 2.
Plasma concentrations of carnitines and hepatic mRNA levels of carnitine metabolism in Wistar male rats given 100 mg/kg 1-triple TTA daily for 3 weeks compared with controls. A: Plasma L-carnitine. B: Plasma γ-butyrobetaine. C: Plasma trimethyllysine. D: Plasma acetylcarnitine. E: Hepatic mRNA levels of enzymes involved in carnitine synthesis, Tmlhe, Aldh9a1, and Bbox1, relative to control. F: Hepatic mRNA levels of the carnitine transporter, Slc22a5, and Crat relative to control. Values are shown as mean ± SD (n = 5–8). Significant difference compared with controls was determined with Student’s t-test (*P ≤ 0.05, ****P ≤ 0.0001).
1-triple TTA changes energy state and mitochondrial function in liver
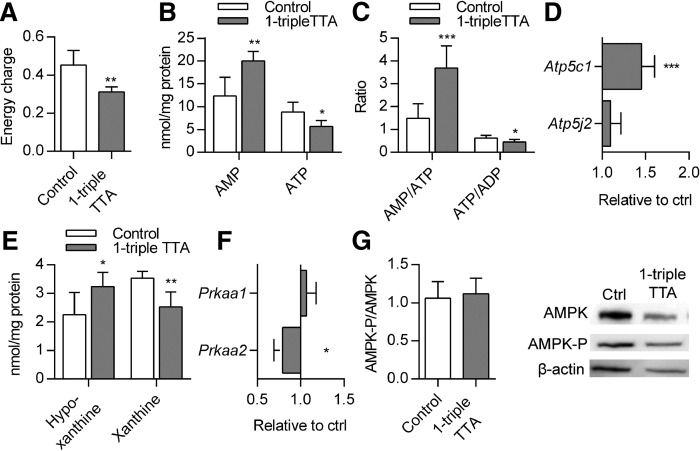
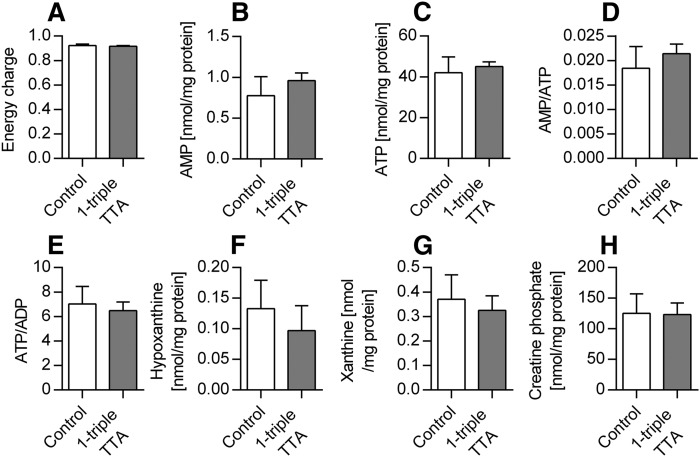
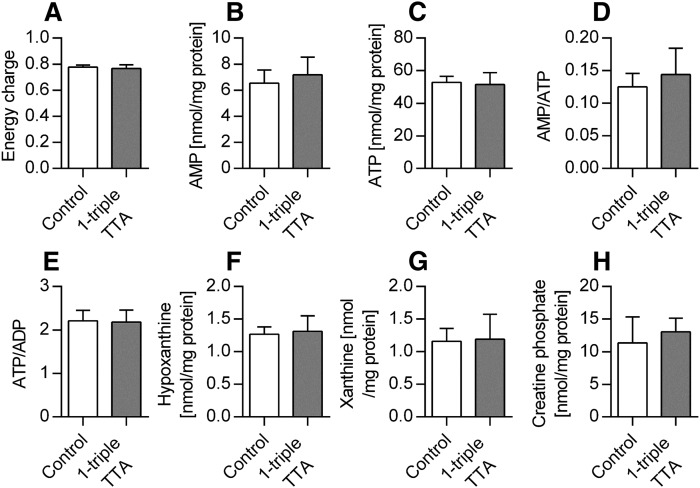
The ability of 1-triple TTA to influence the carnitine and acetylcarnitine plasma levels prompted us to investigate the hepatic energy status, as well as mitochondrial biogenesis and function. The 1-triple TTA treatment significantly decreased energy charge (Fig. 3A) and ATP concentrations (Fig. 3B) of the liver compared with controls. The decreased energy charge was also reflected by significantly increased AMP concentrations (Fig. 3B), significantly increased AMP/ATP ratio, and significantly decreased ATP/ADP ratio (Fig. 3C) compared with controls. The hepatic ADP level was not changed by 1-triple-TTA (data not shown). In accordance with the decreased hepatic ATP concentrations, the mRNA level of the F0 hepatic ATP synthase subunit, Atp5c1, was significantly increased after 1-triple TTA administration, whereas the mRNA level of an F1 subunit, Atp5j2, remained constant (Fig. 3D). Furthermore, the hepatic content of the adenine degradation products, xanthine and hypoxanthine, was altered. Hypoxanthine levels were significantly increased, whereas xanthine levels were significantly decreased after 1-triple TTA administration (Fig. 3E). It is noteworthy that the hepatic mRNA level of the catalytic subunit of AMPK, Prkaa1, was unchanged, whereas the catalytic subunit, Prkaa2, of AMPK was significantly decreased after 1-triple TTA treatment (Fig. 3F). No difference in AMPK-P compared with nonphosphorylated AMPK was observed (Fig. 3G). The effect of 1-triple TTA on tissue energy status was found to be specific for liver because no changes were seen in samples from muscle (Fig. 4) and heart (Fig. 5) . Also, the creatine phosphate levels were unchanged in these two tissues compared with control (Figs. 4H, 5H).
Fig. 3.
Hepatic energy parameters in Wistar male rats given 100 mg/kg 1-triple TTA for 3 weeks compared with controls. A: Energy charge {[(ATP) + 1/2(ADP)]/[(ATP) + (ADP) + (AMP)]}. B: Adenosine mono- and triphosphates (AMP, ATP). C: Ratio of AMP/ATP and ATP/ADP. D: Hepatic mRNA levels of genes of ATP synthase, Atp5c1 and Atp5j2, relative to control. E: Purine degradation products, hypoxanthine and xanthine. F: Hepatic mRNA levels of AMPK catalytic subunits, Prkaa1 and Prkaa2, are shown relative to control. G: Quantitative results of the ratio between AMPK-P and AMPK related to β-actin in liver from Western blot and representative bonds. Protein levels were quantified by normalizing to one bond on each membrane. Electrophoresis and the Western blot procedure were repeated four times. Values are shown as mean ± SD (n = 5–8 if not otherwise stated). Significant difference compared with controls was determined with Student’s t-test (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001).
Fig. 4.
Muscle energy parameters in Wistar male rats given 100 mg/kg 1-triple TTA for 3 weeks compared with controls. A: Energy charge {[(ATP) + 1/2(ADP)]/[(ATP) + (ADP) + (AMP)]}. B: AMP. C: ATP. D: Ratio of AMP/ATP. E: Ratio of ATP/ADP. F: Purine degradation product, hypoxanthine. G: Purine degradation product, xanthine. H: Creatine phosphate. Values are shown as mean ± SD (n = 5–7). Significant difference compared with controls was determined with Student’s t-test.
Fig. 5.
Heart energy parameters in Wistar male rats given 100 mg/kg 1-triple TTA for 3 weeks compared with controls. A: Energy charge {[(ATP) + 1/2(ADP)]/[(ATP) + (ADP) + (AMP)]}. B: AMP. C: ATP. D: Ratio AMP/ATP. E: Ratio ATP/ADP. F: Purine degradation product, hypoxanthine. G: Purine degradation product, xanthine. H: Creatine phosphate. Values are shown as mean ± SD (n = 5–8). Significant difference compared with controls was determined with Student’s t-test.
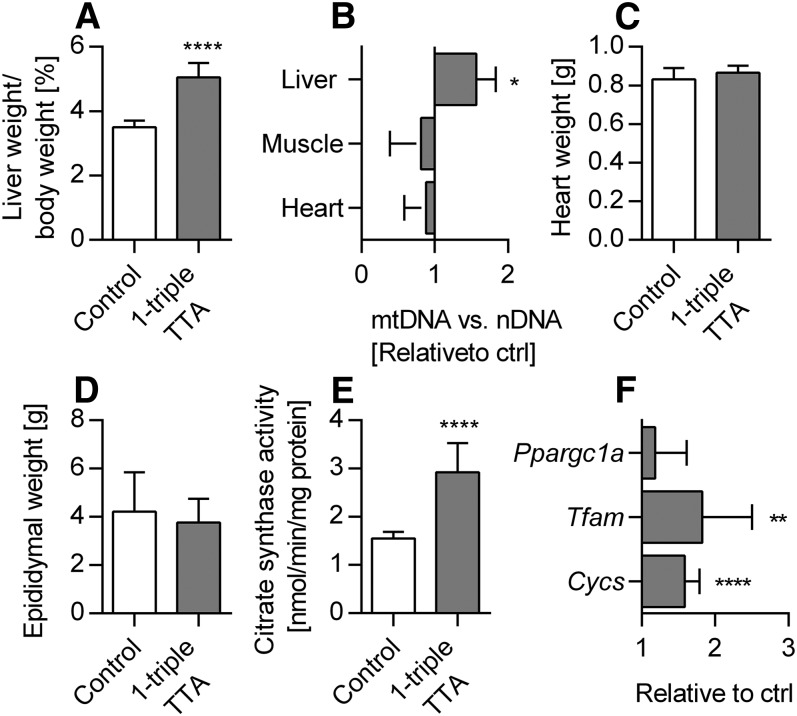
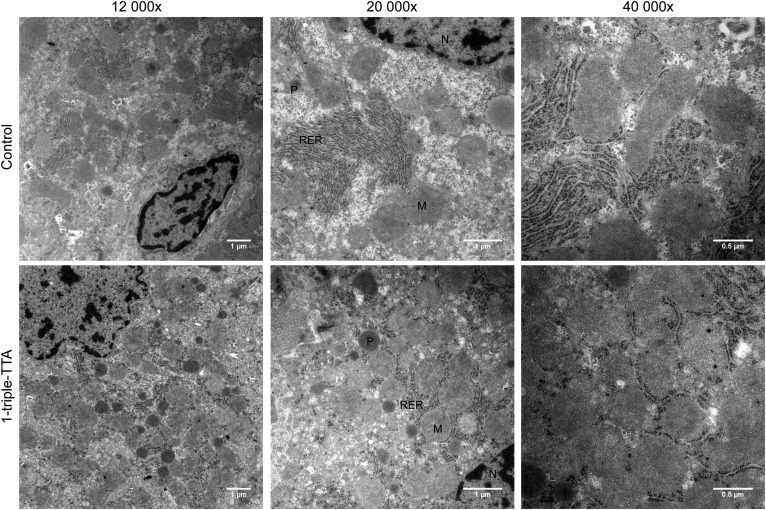
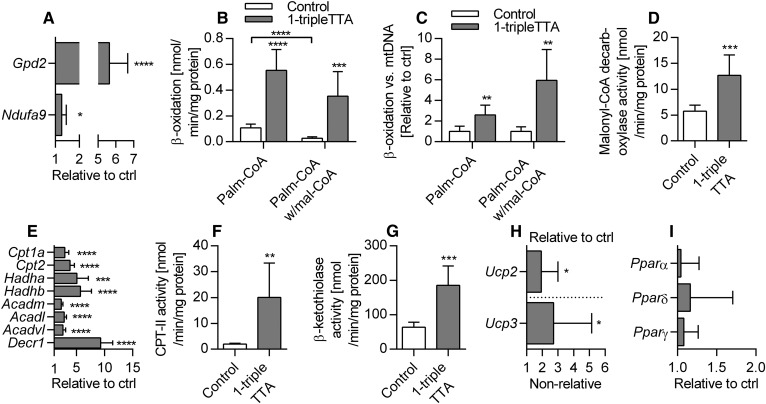
Because 1-triple TTA administration affected energy parameters in a tissue-specific manner, we next evaluated the effects on liver mitochondrial function and biogenesis. The liver index was increased by 1-triple TTA (Fig. 6A) and a significantly higher level of mtDNA relative to nDNA was found in the livers of 1-triple TTA fed rats, but not in heart and skeletal muscle (Fig. 6B). The weights of heart (Fig. 6C) and epididymal white adipose tissue (Fig. 6D) were unchanged by 1-triple TTA administration. Induction of mitochondrial biogenesis in the livers of 1-triple TTA-treated rats was further supported by the increase of the mitochondrial biomarkers citrate synthase activity (Fig. 6E), cytochrome c (Cycs) gene expression, and TFAM gene expression, although Ppargc1a mRNA was not increased (Fig. 6F). Citrate synthase activity was unchanged when related to mtDNA (data not shown), demonstrating that its increase (Fig. 6E) was linked to mitochondrial proliferation. Transmission electron microscopy (TEM) was performed on liver tissue samples from control and 1-triple-TTA-treated rats and subcellular morphology was compared. Although the samples were prepared from snap-frozen biopsies, the structures were generally intact and differences could be observed between controls and 1-triple TTA-treated rats (Fig. 7). In control samples, rough endoplasmic reticulum (RER) had the classical appearance, with ribosomes attached to a folded ER membrane, often forming a stack at specific localizations in the cytoplasm. In the 1-triple TTA-treated rats, the RER structures did not tend to form stacks and, therefore, had a more delocalized appearance, often between or around mitochondrial organelles. Mitochondria with classic spherical morphology predominated in both groups. In these samples, the number of both mitochondria and peroxisomes appeared to be somewhat increased in the 1-triple-TTA-treated group compared with control. To explore the implications of these findings, liver energy metabolism was investigated in more detail. The mRNA level of mitochondrial Gpd2 and a subunit of NADH dehydrogenase, Ndufa9, was increased by 1-triple TTA administration (Fig. 8A). The findings, so far, suggested that 1-triple TTA mediates a general induction of enzymes involved in mitochondrial energy production. As mitochondrial FA oxidation serves to fuel oxidative phosphorylation, we next investigated to determine whether 1-triple TTA caused increased oxidation of palmitoyl-CoA in liver. Confirming this hypothesis, 1-triple TTA was found to increase hepatic FA oxidation, calculated in relation to protein (Fig. 8B) and mtDNA (Fig. 8C). The 1-triple TTA was also found to reduce the inhibitory effect of malonyl-CoA on FA oxidation (Fig. 8B, C). Increased β-oxidation was accompanied by an increased activity of malonyl-CoA decarboxylase (Fig. 8D). In line with these data, we observed, in the livers of rats treated with 1-triple TTA, a significant increase in mRNA level of Cpt1a and Cpt2, α and β subunits of the mitochondrial trifunctional protein (Hadha and Hadhb), Acadm, Acadl, Acadvl, and Decr1, an enzyme involved in β-oxidation of polyunsaturated FAs (Fig. 8E). This was associated with increased activity of CPT-II (Fig. 8F) and β-ketothiolase of the trifunctional protein (Fig. 8G), as well as higher gene expression of UCP2 and UCP3 (Fig. 8H). Several of these genes are known to be regulated by the PPAR transcription factor family, suggesting that 1-triple TTA causes PPAR activation. The gene expression of the three isoforms of PPAR remained constant after 1-triple TTA administration (Fig. 8I).
Fig. 6.
Organ weights and mitochondrial biomarkers in Wistar male rats given 100 mg/kg 1-triple TTA for 3 weeks compared with controls. A: Liver index [(liver weight/body weight) × 100]. B: mtDNA (NADH dehydrogenase 1) compared with nDNA (18S) relative to control. C: Heart weight. D: Epididymal WAT weight. E. Hepatic citrate synthase activity. F. Hepatic mRNA levels of PGC-1α (Ppargc1a) mitochondrial transcription factor A (Tfam) and cytochrome c (Cycs). Values are shown as mean ± SD (n = 4–8). Significant difference compared with controls was determined with Student’s t-test (*P ≤ 0.05, **P ≤ 0.0001 ****P ≤ 0.0001).
Fig. 7.
TEM of liver from male Wistar rats. Representative samples are shown from control (upper row) and 1-triple TTA-treated rats (100 mg 1-triple TTA/kg body weight for 21 days; second row). The magnification used for image acquisition is indicated (12,000×, 20,000×, and 40,000×). M, mitochondria; N, nucleus; P, peroxisomes.
Fig. 8.
Mitochondrial activity and biomarkers in Wistar male rats given 100 mg/kg 1-triple TTA for 3 weeks compared with controls. A: Hepatic mRNA levels of mitochondrial biomarkers, mitochondrial Gpd2 and NADH dehydrogenase (ubiquinone) 1α subcomplex 9 (Ndufa9), shown relative to control. B: Hepatic palmitoyl-CoA oxidation with or without malonyl-CoA. C: Hepatic palmitoyl-CoA oxidation with or without malonyl-CoA, related to mtDNA, set as relative to control. D: Hepatic malonyl-CoA decarboxylase activity. E: Hepatic mRNA levels of enzymes involved in β-oxidation: Cp1a and Cpt2; hydroxyacyl-CoA dehydrogenase/β-ketothiolase/enoyl-CoA hydratase (trifunctional protein) α and β subunit (Hadha, Hadhb); Acadm, Acadl, Acadvl; and mitochondrial Decr1 shown relative to the control. F: Hepatic CPT-II activity. G: Hepatic β-ketothiolase activity. H: Hepatic mRNA levels of Ucp2 and Ucp3. Ucp2 is relative to control. Ucp3 is only normalized to 18S. I: Hepatic mRNA levels of PPARα, PPARδ, and PPARγ shown as relative to the control. Values are shown as mean ± SD (n = 5–8). Significant difference was determined with Student’s t-test (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001).
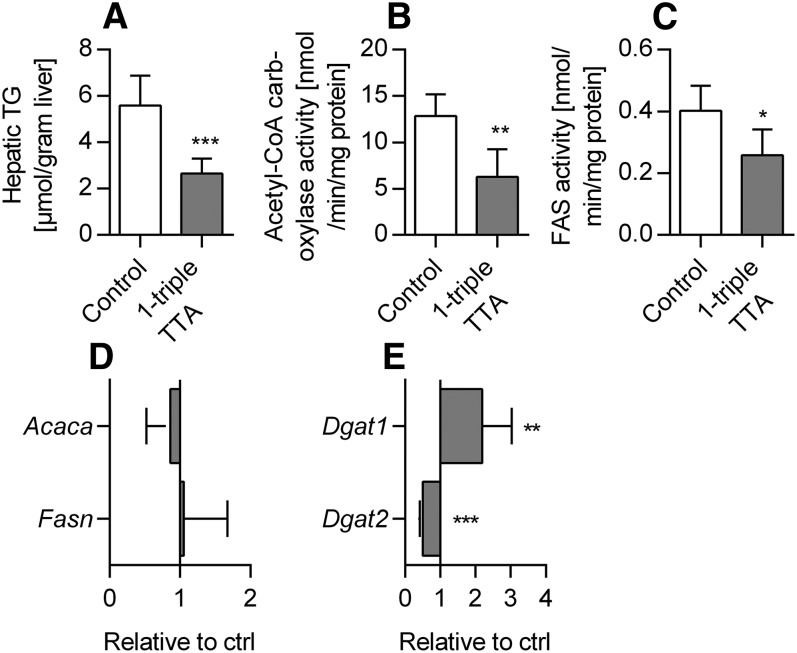
1-triple TTA lowers TG accumulation in liver
Given the relevance of mitochondrial FA oxidation in regulation of both liver and plasma TG, we next wondered how 1-triple TTA affected TG metabolism in rat liver. The hepatic TG level was reduced by 53% and was accompanied by a reduction in the activities of acetyl-CoA carboxylase and FAS (Fig. 9A–C). However, the gene expressions (Acaca and Fasn) were not changed (Fig. 9D). It is noteworthy that a negative correlation of liver TG and palmitoyl-CoA oxidation, as well as a positive correlation between liver TG and acetyl-CoA carboxylase activity, was observed (Table 1). Moreover, the mRNA level of Dgat1 was increased by 1-triple TTA, whereas Dgat2 was decreased (Fig. 9E).
Fig. 9.
Hepatic lipid levels, enzyme activities, and mRNA levels related to lipogenesis and plasma insulin in Wistar male rats given 100 mg/kg 1-triple TTA for three weeks compared with controls. A: Hepatic TG content. B: Hepatic acetyl-CoA carboxylase activity. C: Hepatic FAS activity. D: Hepatic mRNA levels of acetyl-CoA carboxylase (Acaca) and Fasn, shown as relative to control. E: Hepatic mRNA levels of Dgat1 and Dgat2 shown as relative to control. Values are shown as mean ± SD (n = 5–8). Significant difference was determined with Student’s t-test (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001).
Table 1.
Pearson correlation coefficients
| Parameters | Palmitoyl-CoA Oxidation | FAS Activity | Acetyl-CoA Carboxylase Activity | mRNA ApoC-III |
| Plasma TG | −0.7456a | 0.8263b | 0.6547c | 0.6499c |
| Hepatic TG | −0.7921a | 0.6742c | ||
| mRNA ApoC-III | −0.8873d |
Pearson correlation coefficients of mitochondrial β-oxidation and TG in liver, mitochondrial β-oxidation and TG in plasma, FAS activity and TG in liver, hepatic FAS activity and TG in plasma and mitochondrial β-oxidation and hepatic mRNA ApoC-III in Wistar male rats fed 100 mg/kg 1-triple TTA for 3 weeks. Values are shown as correlation coefficients (r), (n = 5–8). Significance of coefficients are shown.
P ≤ 0.01.
P ≤ 0.001.
P ≤ 0.05.
P ≤ 0.0001.
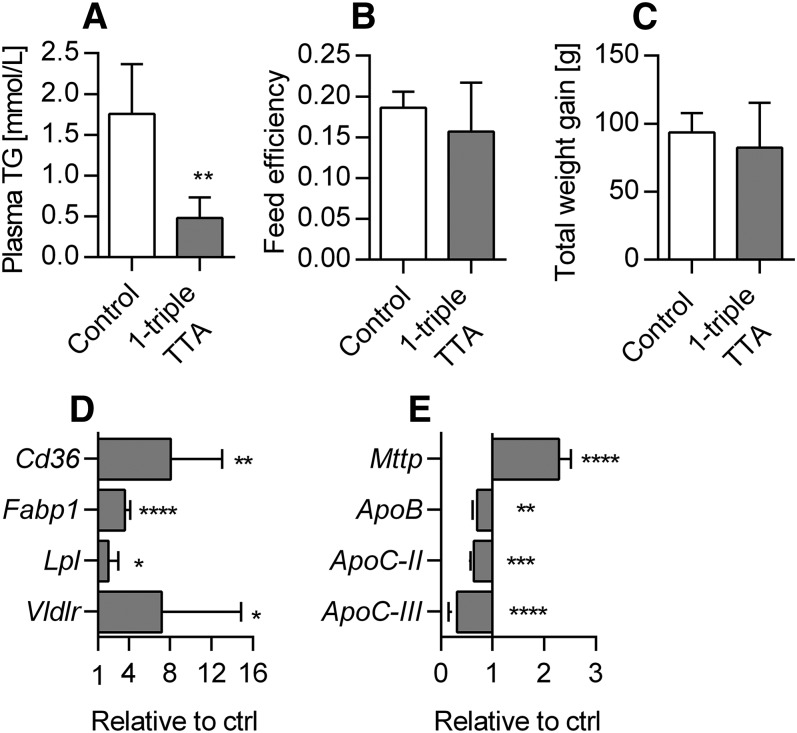
1-triple TTA lowers plasma TG concentration
The plasma TG level was reduced by more than 70% in 1-triple TTA-treated rats compared with controls (Fig. 10A), which was not caused by changes in feed intake (data not shown) or feed efficiency, as this remained constant (Fig. 10B). No significant reduction in body weight was observed during the 3 week study (Fig. 10C). Because the blood TG level is determined by a delicate balance between hepatic TG synthesis and secretion, as well as plasma clearance, we were prompted to test whether 1-triple TTA could affect hepatic genes related to FA uptake and secretion. The mRNA levels of Cd36 and Fabp1 were highly significantly increased (Fig. 10D), and the mRNA levels of ApoB and Mttp (i.e., subunit of MTP) were decreased and increased, respectively, by 1-triple TTA administration compared with controls (Fig. 10E). The gene expression of hepatic LPL was also significantly increased (Fig. 10D), whereas the mRNA levels of ApoC-II and, especially, ApoC-III were decreased compared with controls (Fig. 10E). This was associated with an increased mRNA level of Vldlr (Fig. 10D). It is noteworthy that this resulted in a negative correlation between mitochondrial FA oxidation and the ApoC-III mRNA level and a positive correlation between ApoC-III and plasma TG (Table 1). Moreover, the plasma TG concentration correlated negatively with palmitoyl-CoA oxidation and positively with acetyl-CoA carboxylase and FAS activity (Table 1). Thus the 1-triple TTA-mediated clearance of blood TG seems to partly be a result of lowered APOC-III with a subsequent induction of LPL and (re)uptake of FAs from VLDL. Whether 1-triple TTA also decreased hepatic VLDL secretion should be considered because APOB gene expression was decreased.
Fig. 10.
Plasma levels and enzyme activities in muscle and heart tissue in Wistar male rats given 100 mg/kg 1-triple TTA for 3 weeks compared with controls. A: Plasma TG concentration. B: Feed conversion efficiency [change in body weight (g)/feed intake (g)]. C: Total body weight gain. D: Hepatic mRNA levels of the fatty acid translocase, Cd36, Fabp1, Vldlr, and Lpl shown as relative to control. E: Hepatic mRNA levels of Mttp, ApoB, ApoC-II, and ApoC-III. Values are shown as means and SD (n = 5–8). Significant difference compared with controls was determined with Student’s t-test (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001).
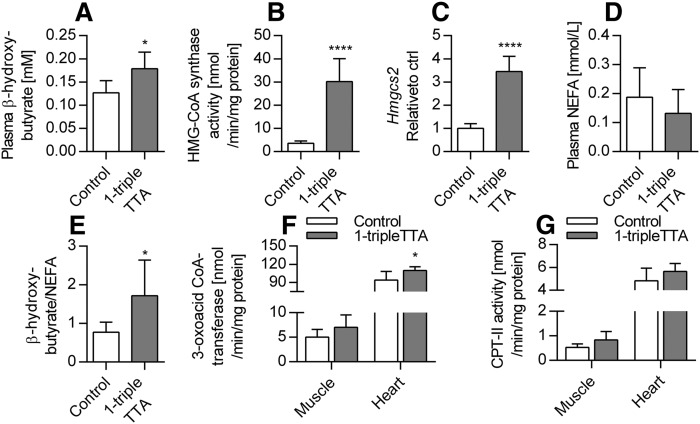
1-triple TTA increases plasma ketone bodies
The liver is able to produce ketone bodies from acetyl-CoA, which may be used as an alternative fuel by other tissues. It is of interest that 1-triple TTA significantly increased the plasma level of β-hydroxybutyrate accompanied by increased hepatic activity and mRNA level of the mitochondrial Hmgcs2 (Fig. 11A–C). Accordingly, because the concentration of NEFAs was unchanged (Fig. 10D), a significant increase in the β-hydroxybutyrate/NEFA ratio was found in 1-triple TTA-treated rats compared with controls (Fig. 11E). The ketone bodies were taken up by extrahepatic tissue, such as skeletal and cardiac muscle, where they were converted to CoA derivatives for oxidative metabolism. It is noteworthy that the activity of oxalate-CoA transferase, an enzyme involved in the oxidation process of β-hydroxybutyrate, was significantly increased in heart, but not muscle, while CPT-II activity was marginally increased in muscle, but not heart (Fig. 11F, G). Hence, in the livers of 1-triple TTA-treated animals, the increased mitochondrial FA oxidation appears to fuel the formation of ketone bodies, which may be an efficient source of energy for other tissues.
Fig. 11.
Hepatic ketone body production and muscle and cardiac enzyme activities in Wistar male rats given 100 mg/kg 1-triple TTA for 3 weeks compared with controls. A: Mitochondrial HMG-CoA synthase activity in liver. B: Hepatic mRNA level of mitochondrial Hmgcs2. C: NEFA plasma concentration. D: Plasma concentration of the ketone body, β-hydroxybutyrate. E: Ratio of β-hydroxybutyrate and NEFA. F: Oxalate CoA-transferase activity in muscle and heart tissue. G: CPT-II activity in muscle and heart tissue. Values are shown as means and SD (n = 5–8). Significant difference compared with controls was determined with Student’s t-test (*P ≤ 0.05, ****P ≤ 0.0001).
DISCUSSION
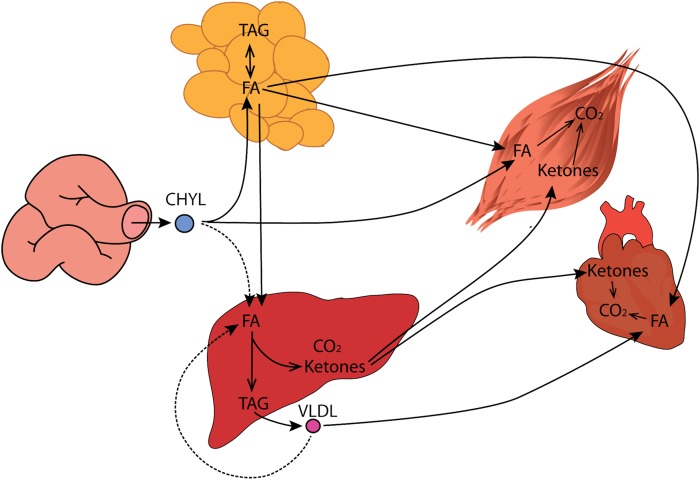
This work demonstrates that the mitochondrial targeted compound, 1-triple TTA, leads to enhanced hepatic FA oxidation and has the potential to protect against TG accumulation in liver and plasma. The observed increase in mitochondrial FA oxidation capacity and ketogenesis in liver can be explained by induction of mitochondrial biogenesis accompanied by increased oxidative phosphorylation and/or metabolism. These findings are associated with altered energy state parameters in a tissue-specific manner and possibly with moderate uncoupling, as hepatic mRNA levels of Ucp2 and Ucp3 were increased. The decreased hepatic TG content in 1-triple TTA-treated rats was most likely related to increased FA oxidation, decreased lipogenesis, and reduced TG synthesis. The plasma TG was also reduced by 1-triple TTA treatment and this was accompanied by an increased level of ketone bodies in plasma. The 1-triple TTA-mediated clearance of blood TG may result from lowered APOC-III levels, subsequent increased hepatic LPL, mitochondrial FA oxidation, and (re)uptake of FAs from VLDL, thereby draining FAs from blood to the liver for β-oxidation and production of ketone bodies for extrahepatic fuel (Fig. 12). Under these conditions, we also hypothesize that TG-rich lipoproteins, including CHYLs, will be drained from intestine to liver.
Fig. 12.
Scheme of the drainage hypothesis provoked by 1-triple TTA. Due to increased mitochondrial function, TG is drained from the blood toward the liver for catabolism leading to production of ketone bodies for extrahepatic fuel. The dashed arrows demonstrate a theory that 1-triple TTA may induce uptake of TG from the intestine as well. TAG, triacylglycerol.
CHYLs formed in the enterocytes of the small intestine are transported into the main circulation through the ductus thoracicus and bypass the portal circulation and liver. The TG-enriched CHYLs are hydrolyzed in adipose tissue and in muscle by LPL attached to the capillary walls. Therefore, TG from CHYLs is normally not taken up in the liver to a great extent, as LPL is not expressed in this tissue (43). However, some of the NEFAs formed by LPL enter the circulation and reach the liver through the hepatic artery. It is known that regulation of MTP is rather complex (44), but it is usually correlated with plasma TG levels (45). Although further studies are needed to elucidate the influence of 1-triple TTA on lipoprotein assembly and secretion, the data presented suggest that 1-triple TTA does not lower TG levels by inhibition at the transcriptional level. Further, 1-triple TTA decreased the hepatic mRNA level of ApoC-III, but increased the hepatic gene expression of LPL and the VLDL receptor. Hence, a hypothesis is presented in Fig. 12 that both CHYLs and VLDLs may directly be taken up by the liver and, thus, establish a direct route of FA transport from the intestine to the liver and/or reabsorption of VLDL formed in the liver itself (Fig. 12). However, further studies are needed to elucidate this hypothesis. The uptake of NEFA in the liver is relatively small in relation to total NEFA turnover. Therefore, the liver has little immediate influence on blood NEFA levels; but after 1-triple TTA treatment, stimulated FA oxidation and ketogenesis in the liver will drain FAs from blood.
It is known that PPARα is more prominent in liver; hence, it indicates that the liver-specific function of 1-triple TTA is due to PPARα activation. Further, increased in vitro palmitoyl-CoA oxidation along with increased enzyme activities and mRNA levels of enzymes involved in mitochondrial β-oxidation (Fig. 8) are well-known PPAR responsive effects/genes (46). The gene expression of PPAR isoforms was not altered by 1-triple TTA. Nevertheless, 1-triple TTA increased mitochondrial FA β-oxidation and liver size, a well-known effect of PPARα activation in rodents (47). PPAR-induced FA oxidation has previously been associated with increased L-carnitine and acetylcarnitine in plasma (47, 48), and with increased mRNA levels of Bbox1 and Slc22a5 (47, 49); while, in this study, hepatic Bbox1 mRNA and plasma L-carnitine and acetylcarnitine were decreased. In contrast, mRNA levels of Crat and the carnitine transporter, Slc22a5, were increased as a consequence of 1-triple TTA treatment (Fig. 2). These results imply an efficient carnitine turnover sufficient for the observed increase in palmitoyl-CoA oxidation, partly unrelated to PPAR activation. The increase in mitochondrial β-oxidation and the decrease in plasma acetylcarnitine were accompanied by increased HMG-CoA synthase activity and plasma level of ketone bodies. This indicates efficient removal of the final β-oxidation product, acetyl-CoA, by its conversion to ketone bodies. Extrahepatic tissues from 1-triple TTA-treated rats may preferably use ketone bodies as fuel, because the activity of the enzyme involved in ketone body utilization, oxalate CoA-transferase, was increased in 1-triple TTA-treated rats, while CPT-II activity was decreased in heart and vice versa in muscle.
Plasma TG was significantly reduced in 1-triple TTA-treated rats accompanied by reduced hepatic mRNA levels of ApoB, suggesting reduction of VLDL secretion. We found a positive correlation between hepatic ApoC-III mRNA and plasma TG, a negative correlation between ApoC-III mRNA and hepatic palmitoyl-CoA oxidation, and a negative correlation between palmitoyl-CoA oxidation and plasma TG levels (Table 1). This is in agreement with previous findings that increased hepatic mitochondrial FA oxidation is involved in the regulation of plasma TG (50). In addition, it is hypothesized that plasma APOC-III could be negatively associated with stimulated mitochondrial FA oxidation. The hepatic TG concentration was also reduced by 1-triple-TTA, accompanied by increased mRNA levels of Cd36 and Fabp1, decreased activities of acetyl-CoA carboxylase and FAS, and reduced gene expression of DGAT2. Assuming that FA flux pathways are associated with changes in mRNA levels, FAs are efficiently oxidized and chain-shortened in mitochondria due to an increased flux (see above) to the liver resulting in a decrease of hepatic TG. This observed decrease in TG may also be partly due to decreased lipogenesis and subsequently reduced TG biosynthesis, as it is evident that DGAT2 is responsible for the majority of TG synthesis (51). Both DGAT1 and DGAT2 reside in the ER, in which DGAT2 also colocalizes with mitochondria (52) and lipid droplets (53). Whether 1-triple TTA reduces mitochondria-associated TG biosynthesis should be considered.
Mitochondrial function and biogenesis depend on regulatory coordination between nuclear and mitochondrial genomes (54, 55). TFAM is an essential factor for mtDNA replication and expression (56). The increase of hepatic Tfam, Cycs, Ndufa9 (Figs. 6, 8), Atp5c1, and Atp5j2 (Fig. 3) mRNA levels accompanied by increased mtDNA and citrate synthase activity (Fig. 6F), as well as more mitochondria observed by TEM (Fig. 7), indicate that 1-triple TTA administration induces mitochondrial biogenesis even though gene expression of PGC-1α (Ppargc1a) was not affected (Fig. 6F). Examination of morphology studies also showed changes in subcellular organization, which have been found to be associated with induction of a hypertrophic response involving mitochondrial biogenesis (40). Although some aspects of the intracellular morphology appeared less organized in samples from 1-triple TTA-treated rats, possibly due to hypertrophy, there were no signs of cellular toxicity. β-Oxidation was increased by 1-triple TTA compared with control, both in relation to milligrams of protein and in relation to mtDNA. This implies that, although 1-triple TTA leads to mitochondrial proliferation, the inducement of mitochondrial activity is partly independent of this proliferation. Induction of mitochondrial biogenesis may increase the capacities of mitochondrial respiration and FA oxidation to support ATP production. Reducing equivalents (electrons) from FA oxidation enter the respiratory chain at the level of complex I and coenzyme Q. Mitochondrial glycerol-3-phosphate dehydrogenase is a peripheral component of the glycerophosphate shuttle, in which electrons from cytosolic NADH can enter the mitochondrial respiratory chain at the level of coenzyme Q. This enzyme, which was strongly upregulated in the liver after 1-triple TTA administration (Fig. 8), may function as an important link between mitochondrial and cytosolic energy pathways and also influence the cellular redox state (57). Increased gene expression of UCP2 and UCP3 suggested that respiratory uncoupling contributes to decreased ATP yield, and may therefore explain the decreased energy charge. Similar observations were made previously after treatment with the modified FA, TTA (19), where proton electrochemical potential was lowered without alteration of ΔpH. Moreover, an increase in AMP is known to activate AMPK, which is an energy sensor, serving to support ATP production under conditions of energy depletion (58). The hepatic mRNA levels of two genes encoding the catalytic subunit of AMPK were either unchanged or decreased (Fig. 3) and no change in the AMPK phosphorylation level was found with 1-triple-TTA treatment. The degradation product of adenine, hypoxanthine, was increased, whereas xanthine was decreased compared with controls. This indicates that there is a partial inhibition of xanthinoxidase with 1-triple-TTA treatment in the liver and, as a consequence, reduced superoxide production by the enzyme.
In conclusion, increased hepatic mitochondrial FA oxidation was found to explain the TG-lowering effects observed in 1-triple TTA-treated rats. This mechanism was further supported by upregulation of mitochondrial biogenesis and the oxidative machinery, and remodeling of the lipoprotein profile. Further, the data indicate that 1-triple TTA causes a pseudo-fasted state in rat liver, with a reduced yield of ATP caused by what we assume to be a moderate degree of respiratory uncoupling. Further studies are required to evaluate the potential use of 1-triple TTA to target pathogenic mechanisms in disorders such as the metabolic syndrome, nonalcoholic fatty liver disease, and CVD.
Acknowledgments
The authors thank Kari Helland Mortensen, Anders Lund, and Mari-Ann Jørstad Davidsen for animal assistance and Kari Williams, Randi Sandvik, Svein Krüger, and Liv Kristine Øysæd for technical expertise. TEM was performed using the infrastructure of the Molecular Imaging Center, Department of Biomedicine, University of Bergen. The authors are thankful to Prof. Reidar Myklebust for help with evaluating subcellular morphology.
Footnotes
Abbreviations:
- Acadl
- long-chain acyl-CoA dehydrogenase
- Acadm
- medium-chain acyl-CoA dehydrogenase
- Acadvl
- very-long-chain acyl-CoA dehydrogenase
- Aldh9a1
- 4-N-trimethylaminobutyraldehyde dehydrogenase
- AMPK
- AMP-activated protein kinase
- AMPK-P
- phosphorylated AMP-activated protein kinase
- Bbox1
- γ-butyrobetaine hydroxylase
- CHYL
- chylomicron
- CPT-II
- carnitine O-palmitoyltransferase
- Crat
- carnitine O-acetyltransferase
- Cycs
- somatic cytochrome c
- Decr1
- 2,4-dienoyl-CoA reductase
- DGAT
- diglyceride acyltransferase
- ER
- endoplasmic reticulum
- Fabp1
- FA binding protein 1
- Gpd2
- glycerol-3-phosphate dehydrogenase 2
- Hmgcs2
- HMG-CoA synthase 2
- mtDNA
- mitochondrial DNA
- Mttp
- microsomal triglyceride transfer protein
- nDNA
- nuclear DNA
- RER
- rough endoplasmic reticulum
- TEM
- transmission electron microscopy
- TG
- triglyceride
- Tmlhe
- Nε-trimethyllysine hydroxylase
- 1-triple TTA
- 2-(tridec-12-yn-1-ylthio)acetic acid
- TTA
- tetradecylthioacetic acid
- UCP
- uncoupling protein
- Vldlr
- VLDL receptor
This work was supported by Bergen Research Foundation, Western Norway Regional Health Authority Grant 911945, and the Norwegian Research Council.
REFERENCES
- 1.Nichols M., Townsend N., Luengo-Fernandez R., Leal J., Gray A., Scarborough P., and Rayner M.. 2012. . European Heart Network, Brussels, Belgium. [Google Scholar]
- 2.World Health Organization. 2016. Cardiovascular diseases (CVDs). Accessed December 4, 2016, at http://www.who.int/mediacentre/factsheets/fs317/en/. [Google Scholar]
- 3.Galassi A., Reynolds K., and He J.. 2006. Metabolic syndrome and risk of cardiovascular disease: a meta-analysis. Am. J. Med. 119: 812–819. [DOI] [PubMed] [Google Scholar]
- 4.Mottillo S., Filion K. B., Genest J., Joseph L., Pilote L., Poirier P., Rinfret S., Schiffrin E. L., and Eisenberg M. J.. 2010. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J. Am. Coll. Cardiol. 56: 1113–1132. [DOI] [PubMed] [Google Scholar]
- 5.Egeland G. M., Igland J., Sulo G., Nygard O., Ebbing M., and Tell G. S.. 2015. Non-fasting triglycerides predict incident acute myocardial infarction among those with favourable HDL-cholesterol: Cohort Norway. Eur. J. Prev. Cardiol. 22: 872–881. [DOI] [PubMed] [Google Scholar]
- 6.Brunzell J. D., and Hokanson J. E.. 1999. Low-density and high-density lipoprotein subspecies and risk for premature coronary artery disease. Am. J. Med. 107: 16S–18S. [DOI] [PubMed] [Google Scholar]
- 7.Wadhera R. K., Steen D. L., Khan I., Giugliano R. P., and Foody J. M.. 2016. A review of low-density lipoprotein cholesterol, treatment strategies, and its impact on cardiovascular disease morbidity and mortality. J. Clin. Lipidol. 10: 472–489. [DOI] [PubMed] [Google Scholar]
- 8.Huang P. L. 2009. A comprehensive definition for metabolic syndrome. Dis. Model. Mech. 2: 231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jong M. C., Hofker M. H., and Havekes L. M.. 1999. Role of ApoCs in lipoprotein metabolism: functional differences between ApoC1, ApoC2, and ApoC3. Arterioscler. Thromb. Vasc. Biol. 19: 472–484. [DOI] [PubMed] [Google Scholar]
- 10.Ito Y., Azrolan N., O’Connell A., Walsh A., and Breslow J. L.. 1990. Hypertriglyceridemia as a result of human apo CIII gene expression in transgenic mice. Science. 249: 790–793. [DOI] [PubMed] [Google Scholar]
- 11.Cohn J. S., Tremblay M., Batal R., Jacques H., Rodriguez C., Steiner G., Mamer O., and Davignon J.. 2004. Increased apoC-III production is a characteristic feature of patients with hypertriglyceridemia. Atherosclerosis. 177: 137–145. [DOI] [PubMed] [Google Scholar]
- 12.Maeda N., Li H., Lee D., Oliver P., Quarfordt S. H., and Osada J.. 1994. Targeted disruption of the apolipoprotein C-III gene in mice results in hypotriglyceridemia and protection from postprandial hypertriglyceridemia. J. Biol. Chem. 269: 23610–23616. [PubMed] [Google Scholar]
- 13.Sacks F. M., Alaupovic P., Moye L. A., Cole T. G., Sussex B., Stampfer M. J., Pfeffer M. A., and Braunwald E.. 2000. VLDL, apolipoproteins B, CIII, and E, and risk of recurrent coronary events in the Cholesterol and Recurrent Events (CARE) trial. Circulation. 102: 1886–1892. [DOI] [PubMed] [Google Scholar]
- 14.Bedogni G., and Bellentani S.. 2004. Fatty liver: how frequent is it and why? Ann. Hepatol. 3: 63–65. [PubMed] [Google Scholar]
- 15.Paschos P., and Paletas K.. 2009. Non alcoholic fatty liver disease and metabolic syndrome. Hippokratia. 13: 9–19. [PMC free article] [PubMed] [Google Scholar]
- 16.Wei Y., Rector R. S., Thyfault J. P., and Ibdah J. A.. 2008. Nonalcoholic fatty liver disease and mitochondrial dysfunction. World J. Gastroenterol. 14: 193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tronstad K. J., Nooteboom M., Nilsson L. I., Nikolaisen J., Sokolewicz M., Grefte S., Pettersen I. K., Dyrstad S., Hoel F., Willems P. H., et al. . 2014. Regulation and quantification of cellular mitochondrial morphology and content. Curr. Pharm. Des. 20: 5634–5652. [DOI] [PubMed] [Google Scholar]
- 18.Paradies G., Paradies V., Ruggiero F. M., and Petrosillo G.. 2014. Oxidative stress, cardiolipin and mitochondrial dysfunction in nonalcoholic fatty liver disease. World J. Gastroenterol. 20: 14205–14218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grav H. J., Tronstad K. J., Gudbrandsen O. A., Berge K., Fladmark K. E., Martinsen T. C., Waldum H., Wergedahl H., and Berge R. K.. 2003. Changed energy state and increased mitochondrial beta-oxidation rate in liver of rats associated with lowered proton electrochemical potential and stimulated uncoupling protein 2 (UCP-2) expression: evidence for peroxisome proliferator-activated receptor-alpha independent induction of UCP-2 expression. J. Biol. Chem. 278: 30525–30533. [DOI] [PubMed] [Google Scholar]
- 20.Raspé E., Madsen L., Lefebvre A. M., Leitersdorf I., Gelman L., Peinado-Onsurbe J., Dallongeville J., Fruchart J. C., Berge R., and Staels B.. 1999. Modulation of rat liver apolipoprotein gene expression and serum lipid levels by tetradecylthioacetic acid (TTA) via PPARalpha activation. J. Lipid Res. 40: 2099–2110. [PubMed] [Google Scholar]
- 21.Urbaniak G. C. , and S. Plous. 2013. Research Randomizer. Accessed March 9, 2015, at https://www.randomizer.org/. [Google Scholar]
- 22.Bligh E. G., and Dyer W. J.. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37: 911–917. [DOI] [PubMed] [Google Scholar]
- 23.Vernez L., Wenk M., and Krahenbuhl S.. 2004. Determination of carnitine and acylcarnitines in plasma by high-performance liquid chromatography/electrospray ionization ion trap tandem mass spectrometry. Rapid Commun. Mass Spectrom. 18: 1233–1238. [DOI] [PubMed] [Google Scholar]
- 24.Vigerust N. F., Bohov P., Bjorndal B., Seifert R., Nygard O., Svardal A., Glintborg D., Berge R. K., and Gaster M.. 2012. Free carnitine and acylcarnitines in obese patients with polycystic ovary syndrome and effects of pioglitazone treatment. Fertil. Steril. 98: 1620–1626.e1. [DOI] [PubMed] [Google Scholar]
- 25.Fürst W., and Hallström S.. 1992. Simultaneous determination of myocardial nucleotides, nucleosides, purine bases and creatine phosphate by ion-pair high-performance liquid chromatography. J. Chromatogr. 578: 39–44. [DOI] [PubMed] [Google Scholar]
- 26.Hallström S., Gasser H., Neumayer C., Fügl A., Nanobashvili J., Jakubowski A., Huk I., Schlag G., and Malinski T.. 2002. S-nitroso human serum albumin treatment reduces ischemia/reperfusion injury in skeletal muscle via nitric oxide release. Circulation. 105: 3032–3038. [DOI] [PubMed] [Google Scholar]
- 27.Pelzmann B., Hallstrom S., Schaffer P., Lang P., Nadlinger K., Birkmayer G. D., Vrecko K., Reibnegger G., and Koidl B.. 2003. NADH supplementation decreases pinacidil-primed I K ATP in ventricular cardiomyocytes by increasing intracellular ATP. Br. J. Pharmacol. 139: 749–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berge R. K., Flatmark T., and Osmundsen H.. 1984. Enhancement of long-chain acyl-CoA hydrolase activity in peroxisomes and mitochondria of rat liver by peroxisomal proliferators. Eur. J. Biochem. 141: 637–644. [DOI] [PubMed] [Google Scholar]
- 29.Willumsen N., Hexeberg S., Skorve J., Lundquist M., and Berge R. K.. 1993. Docosahexaenoic acid shows no triglyceride-lowering effects but increases the peroxisomal fatty acid oxidation in liver of rats. J. Lipid Res. 34: 13–22. [PubMed] [Google Scholar]
- 30.Bremer J. 1981. The effect of fasting on the activity of liver carnitine palmitoyltransferase and its inhibition by malonyl-CoA. Biochim. Biophys. Acta. 665: 628–631. [DOI] [PubMed] [Google Scholar]
- 31.Clinkenbeard K. D., Reed W. D., Mooney R. A., and Lane M. D.. 1975. Intracellular localization of the 3-hydroxy-3-methylglutaryl coenzme A cycle enzymes in liver. Separate cytoplasmic and mitochondrial 3-hydroxy-3-methylglutaryl coenzyme A generating systems for cholesterogenesis and ketogenesis. J. Biol. Chem. 250: 3108–3116. [PubMed] [Google Scholar]
- 32.Stadlmann S., Renner K., Pollheimer J., Moser P. L., Zeimet A. G., Offner F. A., and Gnaiger E.. 2006. Preserved coupling of oxidative phosphorylation but decreased mitochondrial respiratory capacity in IL-1beta-treated human peritoneal mesothelial cells. Cell Biochem. Biophys. 44: 179–186. [DOI] [PubMed] [Google Scholar]
- 33.Srere P. A., Gonen L., and Brazil H.. 1963. The citrate condensing enzyme of pigeon breast muscle and moth flight muscle. Acta Chem. Scand. 17: S129–S134. [Google Scholar]
- 34.Roncari D. A. 1981. Fatty acid synthase from human liver. Methods Enzymol. 71: 73–79. [DOI] [PubMed] [Google Scholar]
- 35.Tanabe T., Nakanishi S., Hashimoto T., Ogiwara H., Nikawa J., and Numa S.. 1981. Acetyl-CoA carboxylase from rat liver. Methods Enzymol. 71: 5–16. [DOI] [PubMed] [Google Scholar]
- 36.Kim Y. S., and Kolattukudy P. E.. 1978. Malonyl-CoA decarboxylase from the uropygial gland of waterfowl: purification, properties, immunological comparison, and role in regulating the synthesis of multimethyl-branched fatty acids. Arch. Biochem. Biophys. 190: 585–597. [DOI] [PubMed] [Google Scholar]
- 37.Raaka B. M., and Lowenstein J. M.. 1979. Inhibition of fatty acid oxidation by 2-bromooctanoate. Evidence for the enzymatic formation of 2-bromo-3-ketooctanoyl coenzyme A and the inhibition of 3-ketothiolase. J. Biol. Chem. 254: 6755–6762. [PubMed] [Google Scholar]
- 38.Stern J. R., Coon M. J., Del Campillo A., and Schneider M. C.. 1956. Enzymes of fatty acid metabolism. IV. Preparation and properties of coenzyme A transferase. J. Biol. Chem. 221: 15–31. [PubMed] [Google Scholar]
- 39.Williamson D. H., Bates M. W., Page M. A., and Krebs H. A.. 1971. Activities of enzymes involved in acetoacetate utilization in adult mammalian tissues. Biochem. J. 121: 41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hagland H. R., Nilsson L. I., Burri L., Nikolaisen J., Berge R. K., and Tronstad K. J.. 2013. Induction of mitochondrial biogenesis and respiration is associated with mTOR regulation in hepatocytes of rats treated with the pan-PPAR activator tetradecylthioacetic acid (TTA). Biochem. Biophys. Res. Commun. 430: 573–578. [DOI] [PubMed] [Google Scholar]
- 41.He L., Chinnery P. F., Durham S. E., Blakely E. L., Wardell T. M., Borthwick G. M., Taylor R. W., and Turnbull D. M.. 2002. Detection and quantification of mitochondrial DNA deletions in individual cells by real-time PCR. Nucleic Acids Res. 30: e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hodneland Nilsson L. I., Nitschke Pettersen I. K., Nikolaisen J., Micklem D., Avsnes Dale H., Vatne Rosland G., Lorens J., and Tronstad K. J.. 2015. A new live-cell reporter strategy to simultaneously monitor mitochondrial biogenesis and morphology. Sci. Rep. 5: 17217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao W. S., Hu S. L., Yu K., Wang H., Wang W., Loor J., and Luo J.. 2014. Lipoprotein lipase, tissue expression and effects on genes related to fatty acid synthesis in goat mammary epithelial cells. Int. J. Mol. Sci. 15: 22757–22771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hussain M. M., Nijstad N., and Franceschini L.. 2011. Regulation of microsomal triglyceride transfer protein. Clin. Lipidol. 6: 293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pan X., and Hussain M. M.. 2007. Diurnal regulation of microsomal triglyceride transfer protein and plasma lipid levels. J. Biol. Chem. 282: 24707–24719. [DOI] [PubMed] [Google Scholar]
- 46.Tyagi S., Gupta P., Saini A. S., Kaushal C., and Sharma S.. 2011. The peroxisome proliferator-activated receptor: a family of nuclear receptors role in various diseases. J. Adv. Pharm. Technol. Res. 2: 236–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burri L., Thoresen G. H., and Berge R. K.. 2010. The role of PPARalpha activation in liver and muscle. PPAR Res. 2010: 542359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Burri L., Bjorndal B., Wergedahl H., Berge K., Bohov P., Svardal A., and Berge R. K.. 2011. Tetradecylthioacetic acid increases hepatic mitochondrial beta-oxidation and alters fatty acid composition in a mouse model of chronic inflammation. Lipids. 46: 679–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Makowski L., Noland R. C., Koves T. R., Xing W., Ilkayeva O. R., Muehlbauer M. J., Stevens R. D., and Muoio D. M.. 2009. Metabolic profiling of PPARalpha-/- mice reveals defects in carnitine and amino acid homeostasis that are partially reversed by oral carnitine supplementation. FASEB J. 23: 586–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Berge R. K., Tronstad K. J., Berge K., Rost T. H., Wergedahl H., Gudbrandsen O. A., and Skorve J.. 2005. The metabolic syndrome and the hepatic fatty acid drainage hypothesis. Biochimie. 87: 15–20. [DOI] [PubMed] [Google Scholar]
- 51.Stone S. J., Myers H. M., Watkins S. M., Brown B. E., Feingold K. R., Elias P. M., and Farese R. V. Jr.. 2004. Lipopenia and skin barrier abnormalities in DGAT2-deficient mice. J. Biol. Chem. 279: 11767–11776. [DOI] [PubMed] [Google Scholar]
- 52.Stone S. J., Levin M. C., Zhou P., Han J., Walther T. C., and Farese R. V. Jr.. 2009. The endoplasmic reticulum enzyme DGAT2 is found in mitochondria-associated membranes and has a mitochondrial targeting signal that promotes its association with mitochondria. J. Biol. Chem. 284: 5352–5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kuerschner L., Moessinger C., and Thiele C.. 2008. Imaging of lipid biosynthesis: how a neutral lipid enters lipid droplets. Traffic. 9: 338–352. [DOI] [PubMed] [Google Scholar]
- 54.Dominy J. E., and Puigserver P.. 2013. Mitochondrial biogenesis through activation of nuclear signaling proteins. Cold Spring Harb. Perspect. Biol. 5: a015008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Taanman J. W. 1999. The mitochondrial genome: structure, transcription, translation and replication. Biochim. Biophys. Acta. 1410: 103–123. [DOI] [PubMed] [Google Scholar]
- 56.Garstka H. L., Schmitt W. E., Schultz J., Sogl B., Silakowski B., Perez-Martos A., Montoya J., and Wiesner R. J.. 2003. Import of mitochondrial transcription factor A (TFAM) into rat liver mitochondria stimulates transcription of mitochondrial DNA. Nucleic Acids Res. 31: 5039–5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mráček T., Drahota Z., and Houštěk J.. 2013. The function and the role of the mitochondrial glycerol-3-phosphate dehydrogenase in mammalian tissues. Biochim. Biophys. Acta. 1827: 401–410. [DOI] [PubMed] [Google Scholar]
- 58.Hardie D. G. 2014. AMPK–sensing energy while talking to other signaling pathways. Cell Metab. 20: 939–952. [DOI] [PMC free article] [PubMed] [Google Scholar]