Abstract
Among the LDL receptor (LDLR) family members, the roles of LDLR-related protein (LRP)1 in the pathogenesis of Alzheimer’s disease (AD), especially late-onset AD, have been the most studied by genetic, neuropathological, and biomarker analyses (clinical studies) or cellular and animal model systems (preclinical studies) over the last 25 years. Although there are some conflicting reports, accumulating evidence from preclinical studies indicates that LRP1 not only regulates the metabolism of amyloid-β peptides (Aβs) in the brain and periphery, but also maintains brain homeostasis, impairment of which likely contributes to AD development in Aβ-independent manners. Several preclinical studies have also demonstrated an involvement of LRP1 in regulating the pathogenic role of apoE, whose gene is the strongest genetic risk factor for AD. Nonetheless, evidence from clinical studies is not sufficient to conclude how LRP1 contributes to AD development. Thus, despite very promising results from preclinical studies, the role of LRP1 in AD pathogenesis remains to be further clarified. In this review, we discuss the potential mechanisms underlying how LRP1 affects AD pathogenesis through Aβ-dependent and -independent pathways by reviewing both clinical and preclinical studies. We also discuss potential therapeutic strategies for AD by targeting LRP1.
Keywords: low density lipoprotein receptor-related protein 1, gene expression, receptors/lipoprotein, transport
The LDL receptor (LDLR) family members are cell surface receptors sharing structural and functional similarities, which include LDLR, LDLR-related protein (LRP)1, LRP1B, megalin/LRP2, VLDL receptor (VLDLR), apoE receptor 2 (apoER2), sortilin-related receptor (SorLA/LR11), LRP5, and LRP6. These receptors have distinct physiological or pathophysiolgical roles, likely due to differences in their affinity to ligands, protein stability, endocytosis rate, signaling potency, cellular localization, and tissue expression pattern. While these receptors bind diverse extracellular ligands, some of them recognize key molecules in the pathogenesis of Alzheimer’s disease (AD), such as apoE and amyloid-β peptides (Aβs) for cellular trafficking and/or signaling pathways. Given that the ε4 allele of the APOE gene (APOE4) is the strongest genetic risk factor for AD among the three human alleles (ε2, ε3, and ε4), the potential contributions of LDLR family members to AD development have attracted great interest by many laboratories around the world for over two decades (1, 2).
Cumulative evidence from preclinical studies using cellular or animal studies indicates that LDLR family members can contribute to AD pathogenesis through modulating Aβ accumulation or neurodegenerative processes. However, as there are currently no complete cellular or animal models that can recapitulate all pathological features of AD, parallel understanding between cellular/animal studies and human studies is necessary to define the specific role of any molecule in AD pathogenesis. Herein, among the LDLR family members, we comprehensively discuss the roles of LRP1 in AD pathogenesis by reviewing both preclinical studies using cellular/animal models, and clinical studies analyzing human samples/cohorts. We will specifically review current knowledge regarding: 1) LRP1 expression during AD progression, 2) contribution of LRP1 to Aβ metabolism, 3) the role of LRP1 in brain homeostasis, and 4) apoE-LRP1 pathways in AD pathogenesis. Finally, we will also discuss potential therapeutic strategies targeting LRP1 to prevent or treat AD.
STRUCTURAL AND FUNCTIONAL FEATURES
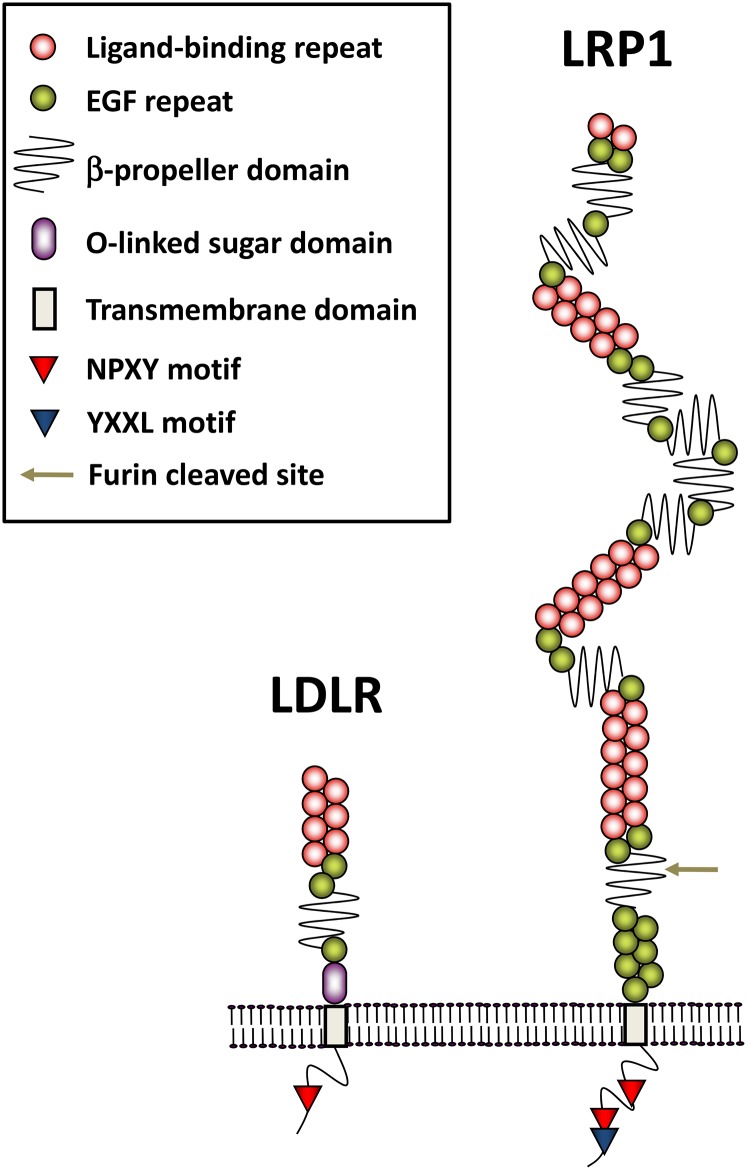
LRP1 was first identified in 1988 from mouse lymphocyte and human liver cDNA libraries by screening for homologous sequences of the apoE-binding domain within LDLR (3). LRP1 is a very large type I transmembrane protein compared with LDLR (Fig. 1). It encodes a 4,525 amino acid protein, composed of a large 515 kDa N-terminal extracellular subunit and an 85 kDa C-terminal transmembrane subunit, which are noncovalently associated with one another (4). The two subunits of LRP1 are generated by furin cleavage in the trans-Golgi complex before being transported to the cell surface (4, 5). The extracellular domain has four ligand binding domains (I–IV) with 2, 8, 10, and 11 cysteine-rich complement-type repeats, respectively, where domains II and IV are the major binding regions for almost all ligands in the presence of calcium (6, 7). Most LDLR family members have NPXY motif(s) in their cytoplasmic tails, which serve as a signal for endocytosis through clathrin-coated pits (8). Moreover, LRP1 has a YXXL motif adjacent to the second NPXY motif, enabling rapid endocytosis together with the proximal di-leucine motif (9). Cysteine-rich EGF repeats and a YWTD β-propeller domain in the extracellular portion facilitate dissociation of ligands in the endocytic vesicles (10). The efficient delivery of LRP1 to the cell surface requires receptor-associated protein (RAP), a molecular chaperone of 39 kDa in size (11, 12). RAP associates with LRP1 in the early secretary pathway, thereby preventing premature binding of LRP1 with other ligands. Dissociation from RAP occurs at the lower pH encountered in the late secretory pathway (13). RAP is a unique ligand for LRP1 in terms of universally inhibiting all known ligand interactions with LRP1 (13).
Fig. 1.
Schematic diagram of domain structure for LRP1 and LDLR. The large extracellular domain of LRP1 has four ligand binding domains (I–IV) with 2, 8, 10, and 11 cysteine-rich complement-type repeats, respectively, while that of LDLR has one ligand binding domain with seven such repeats. The extracellular domain of LRP1 is cleaved by furin, and attached with the C-terminal domain via a noncovalent association. Cysteine-rich EGF repeat and YWTD β-propeller domain in the extracellular portion facilitate dissociation of ligands within the endocytic pathway under acidic conditions. In the cytoplasmic tail, in addition to the two NPXY motifs, LRP1 has an YXXL motif adjacent to the second NPXY motif, enabling rapid endocytosis.
Over 40 proteins, including Aβ, apoE, and activated α2-macroglobulin, are reported to be ligands for LRP1 (14). However, it is controversial whether Aβ directly binds to these domains of LRP1; though Deane et al. (15) demonstrated that domains II and IV bind with Aβs (especially monomeric Aβ40) in vitro, such results were not confirmed in a similar assay by Yamada et al. (16). LRP1 might facilitate Aβ uptake into cells through cooperating with other Aβ-binding proteins, such as heparan sulfate proteoglycan (17, 18). Oligomeric or aggregated Aβ is also a poor ligand for LRP1 (15, 19). Of note, the binding of apoE to LRP1 appears to be affected by the conformational and lipidation status of apoE (1).
In addition to controlling ligand metabolism, LRP1 can regulate signaling pathways or functional activations by coupling with other cell surface receptors or proteins, such as the platelet-derived growth factor (20, 21) and the N-methyl-D-aspartate (NMDA) receptor (22, 23). LRP1 can also be cleaved by β-secretase (24, 25) or α-secretase (26) and then γ-secretase (27). The resulting intracellular domain of LRP1 serves as a transcription regulator, affecting expression of several genes, including interferon-γ, similar to amyloid precursor protein (APP) (28–31), while secreted soluble forms of LRP1 (sLRP1) can affect Aβ metabolism (32, 33) as discussed later.
LRP1 is abundantly expressed in diverse tissues, including liver, brain, and vasculatures. Conventional deletion of the LRP1 gene in mice led to early embryonic lethality, which is a remarkable difference when compared with nonlethal LDLR knockout mice (34). Conditional knockouts of LRP1 in hepatocytes, macrophages, adipocytes, or vascular smooth muscle cells in mouse models have supported critical roles of LRP1 in the development and homeostasis of each tissue by regulating the metabolism of ligands, as well as the strength of signaling pathways. Thus, disturbances of LRP1 functions likely contribute to disease development, such as atherosclerosis and cancer, which have been reviewed elsewhere and are beyond the aim of this review (10, 35). As described later, conditional deletion of LRP1 in neurons in mouse models showed several features of neurodegenerative diseases relevant to AD.
CLINICAL EVIDENCE FROM GENETIC, NEUROPATHOLOGICAL, AND BIOMARKER STUDIES
An initial finding from neuropathological studies was the detection of LRP1 and its multiple ligands, including apoE and α2-macroglubulin, on senile plaques in the brains from AD patients, suggesting an involvement of LRP1 in the accumulation of Aβ (36, 37). Following this leading neuropathological finding, several genetic studies have been performed to address the association of LRP1 gene variants with AD risk. Though initial intriguing observations were that a silent polymorphism in the exon 3 at codon 766 (C766T) of LRP1 were under-represented in late-onset AD (38, 39), following results were conflicting (40, 41). A meta-analysis in 2005 combining 4,668 AD patients and 4,473 controls from 18 case-control studies failed to detect a significant effect of the LRP1 C766T polymorphism on AD risk. The obtained results were not affected by AD onset (early- and late-onset) or APOE4 status (42). Also, no significant effects were observed in a recent meta-analysis of four genome-wide association study data sets consisting of 17,008 AD cases and 37,154 controls (43). Nonetheless, another study showed that the effects of the LRP1 C766T polymorphism were especially obvious in the presence of tau intron 9 rs2471738 polymorphism (odds ratio = 6.20, P = 0.005) (44). In addition, by introducing the amyloid imaging 11C-Pittsburgh compound B technique, which enables antemortem detection of Aβ accumulation in the brain through positron emission tomography (45), the LRP1 C667T polymorphism was moderately, but significantly, associated with global amyloid deposition in AD patients (n = 72, P = 0.046) (46). Also, a neuropathological study observed a significant association between the LRP1 C766T polymorphism and cerebral amyloid angiopathy (CAA), where Aβ accumulates around blood vessels, especially in the smooth muscle cell layers; the polymorphism was more frequent in AD patients without capillary CAA, compared with AD cases with capillary CAA and control individuals (47). Other studies showed that not only LRP1 C667T but also LRP1 haplotypes influence the risk for AD or the preclinical stage of AD (48, 49). Together, these genetic studies suggest that influences of LRP1 on AD risk may differ depending on the status of Aβ pathology, CAA formation, and tauopathy, although further studies are needed.
In addition to LRP1 gene polymorphisms, many studies using human postmortem brain samples have focused on understanding how LRP1 levels are modulated during the AD pathogenesis (Table 1). Some groups reported that LRP1 mRNA levels are increased in the temporal cortex and hippocampus of AD patients or demented individuals (50, 51) although there is a conflicting report showing decreased levels of LRP1 mRNA expression in the frontal cortex of AD patients (52). Because LRP1 is expressed in diverse cells, including neurons, astrocytes, and vasculatures in the brain, LRP1 levels may be differently altered depending on cell types in AD (53, 54). Indeed, it was reported that LRP1 levels were decreased in neurons, but increased in vasculatures or astrocytes proximate to amyloid plaques in AD brains (53–55). Kang et al. (56) demonstrated that LRP1 levels in the frontal cortex were lower in AD patients compared with age-matched controls when analyzed by Western blotting. An age-dependent reduction of LRP1 was also detected in normal individuals in the same study. Further, they reported an increased LRP1 level in LRP1 C667T carriers (56). However, Qiu et al. (57) observed increased levels of LRP1 in the frontal cortical area, though they used a relatively small number of subjects and antibodies detecting the extracellular domain of LRP1 rather than its C-terminal domain used in the former study. Also, Causevic et al. (58) did not observe an association between LRP1 levels and either dementia status or LRP1 C766T polymorphism, despite examining similar brain areas in a similar number of subjects. On the other hand, our recent studies using ELISA measurements found a significant reduction of LRP1 levels (C-terminal domain) in several cortical areas of AD patients, which were positively correlated with synaptic proteins, including the scaffold protein, postsynaptic density protein 95, at the postsynaptic sites (59). Thus, there is a possibility that the reduction of LRP1 levels in AD brains is caused by neuronal loss as the disease progresses, because neuronal LRP1 accounts for more than 50% of its protein amount in the brain (60). It is of note that oxidized LRP1 was increased in the hippocampus of AD patients, suggesting that posttranslational modifications need to be considered to address the functional change of LRP1 in AD (61).
TABLE 1.
Findings of LRP1 protein or mRNA change from studies analyzing clinical samples related to AD
| Study | Objects | Methods | Cohorts | Number of Subjects | Observations | Notes |
| Rebeck et al. (36) | LRP1 protein in the temporal cortex (BA20) | Immunohistochemistry | Control and AD patients | n = 35–39/group | Immunoreactivity of N-terminal domain of LRP1 mostly existed in neurons in controls, while astrocytes and senile plaques were also stained in AD. No effects of APOE isoforms | Fixed in paraformaldehyde-lysine-periodate. Cryosection. Mouse monoclonal 8G1 Ab (179) |
| Thal, Schober, and Birkenmeier (180) | LRP1 protein in the hippocampus and posterior cortex (BA17) | Immunohistochemistry | Control and AD patients | n = 7–16/group | Immunoreactivity of N-terminal extracellular domain of LRP1 existed in reactive astrocytes surrounding core plaques in AD and some neurons, while that of C-terminal transmembrane domain existed in core plaques | Mouse monoclonal Ab against N-terminal extracellular domain of LRP1 (IIF6), and C-terminal domain of LRP1 (II4/8). Formalin fixed. Paraffin section |
| Kang et al. (56) | LRP1 protein levels in the midfrontal cortex | Western blot | Control and AD patients | n = 39/group | LRP1 protein levels were decreased at old age, and further decreased in AD patients. C667T polymorphism was associated with the lower levels of LRP1 | Normalized by actin or synaptophysin. Polyclonal Ab against C-terminal domain of LRP1 (181) |
| Shibata et al. (78) | LRP1 protein in the frontal cortex (BA10) | Immunohistochemistry | Control and AD patients | n = 3/group | Decreased immunoreactivity of LRP1 in brain vasculature | R777 Ab for extracellular domain of LRP1 (182). Formalin fixed section or frozen section. No quantification of the data |
| Qiu et al. (57) | LRP1 protein levels in the frontal cortex, and sLRP1 levels in CSF | Western blot | Control, other CNS disease, and AD patients | n = 7–9/group (brain), and n = 9–16/group (CSF) | Increased LRP1 protein levels in brain, and sLRP1 levels in CSF of AD patients, compared with controls, and other CNS diseases | R777 Ab for extracellular domain of LRP1. Normalized by total protein levels |
| Arélin et al. (55) | LRP1 protein in the brain (area not described) | Immunohistochemistry | Controls and AD patients | n = 3–5/group | LRP1 immunoreactivity was only present in cored plaques among plaques. LRP1 immunoreactivity existed in neurons and reactive astrocytes around plaques | Ab for extracellular domain of LRP1 (182), and C-terminal domain of LRP1 (179). Fixed in paraformaldehyde-lysine-periodate. Cryosection |
| Causevic et al. (58) | LRP1 protein levels in the frontal cortex (BA 8/9) | Western blot | Control and AD patients | n = 20–38/group | LRP1 protein levels were not different between controls and AD. LRP1 levels were not associated with cognitive scores, and C667T polymorphism | 1,704 Ab for C-terminal domain of LRP1 (183). Normalized by GAPDH levels |
| Donahue et al. (54) | LRP1 protein levels in the hippocampus | Immunohistochemistry and Western blot | Control and AD patients | n = 6/group | LRP1 protein levels in microvasculature were increased in AD, but LRP1 protein levels in neurons were decreased in AD | Rabbit polyclonal antibody (Orbigen Inc.). Normalized by total protein levels. Paraformaldehyde fixed. Cryosection |
| Matsui et al. (50) | LRP1 mRNA levels in the temporal cortex | RT-qPCR | Control and AD patients | n = 21–27/group | LRP1 mRNA levels were increased in AD | |
| Sultana, Banks, and Butterfield (63) | LRP1 protein levels in the hippocampus | Western blot | Control and MCI | n = 6/group | LRP1 protein levels were decreased in MCI | Ab from Santa Cruz Biotechnology. Normalized by total protein levels |
| Owen et al. (61) | LRP1 and oxidized LRP1 in the hippocampus | Western blot and immunoprecipitation | Control and AD patients | n = 9/group | Increased levels of the lipid peroxidation product 4-hydroxy-2-nonenal (HNE) bound to LRP1 in AD | Rabbit anti-LRP1 Ab (30) |
| Sagare et al. (65) | Oxidized sLRP1 in the plasma | ELISA and enzymatic assay | Control, MCI-AD converters, AD patients | n = 14/group | Oxidized sLRP1 levels were increased in MCI-AD and AD, and its increase correlated with CSF tau/Aβ42 ratio and MMSE reduction | ELISA (coating: recombinant RAP, detection: 8G1 Ab) |
| Akram et al. (51) | LRP1 mRNA levels in the inferior temporal cortex and hippocampus | RT-qPCR | Subjects with different CDR and AD pathology | n = 88 (temporal cortex) and 73 (hippocampus) | LRP1 mRNA levels were associated with CDR, Aβ, and tau pathology | Only samples with RIN > 5.5 were included |
| Ruzali, Kehoe, and Love (53) | LRP1 mRNA and protein levels | RT-qPCR (mRNA) and dot blot (protein) | Controls and AD patients | n = 19–23/group | LRP1 mRNA levels were increased in meningeal vessels, but not cortex or choroid plexus, in AD and in association with APOE ε4 | Mouse anti-LRP1 Ab (ab28320, Abcam) |
| Shinohara and colleagues (59, 117) | LRP1 protein levels in 12 brain areas | ELISA | Control, pathological aging, sporadic AD, and familial AD | n = 10–19/group | Cortical LRP1 protein levels were decreased in AD patients, and the regional distribution of LRP1 in nondemented controls was associated with that of Aβ accumulation | ELISA (coating: 6F8 antibody, detection: 5A6 antibody) Normalized by total protein levels |
| Provias and Jeynes (184) | LRP1 protein in the capillary vessels of temporal cortex | Immunohistochemistry | Control and AD patients | n = 15/group | LRP1 positive capillaries were not different between controls and AD, but positively associated with plaque burden in AD | Mouse monoclonal Ab (Biodesign International, ME). No description how to fix the tissue. Paraffin section |
| Yamanaka et al. (52) | LRP1 mRNA levels in the superior frontal cortex | RT-qPCR | Controls and AD patients | n = 8–15/group | LRP1 mRNA levels were decreased in AD | No detail description of the procedure, and samples |
BA, Brodmann area; CDR, clinical dementia rating; MMSE, mini-mental state examination.
Importantly, it remains to be clarified whether LRP1 levels change in the preclinical stage of AD. As Aβ accumulation almost reaches a plateau at the preclinical stage of AD (62), functional changes of LRP1 during the early stage of AD might be more relevant to brain Aβ accumulation. In this regard, Sultana, Banks, and Butterfield (63) analyzed postmortem brains from subjects with mild cognitive impairment (MCI), which would correspond to the early stage of AD, and observed a significant decrease of LRP1 levels in the hippocampus of MCI subjects compared with controls. Controversially, compared with normal aging, we failed to see a significant change in LRP1 levels in the cortical areas of pathological aging, which is neuropathologically characterized by the moderate to marked Aβ deposition in nondemented elderly people and, thus, often referred to be comparable to the early stage of AD (64), although we did observe a trend toward increased LRP1 levels in them (59). An interesting finding was made by Sagare et al. (65). They measured the sLRP1 in plasma of MCI individuals who converted to AD later (65). They previously reported that sLRP1 can accelerate Aβ clearance through peripheral “sink” activity, which will be described in the next section (32, 66). In their study, they observed an increase in the plasma of MCI individuals of oxidized sLRP1, which showed extremely low affinity for Aβ, suggesting that Aβ clearance from the brain through peripheral sink activity of sLRP1 is impaired at the early stage of AD (65). Despite these findings, most of the studies employed small cohort size, thus it remains to be clarified whether LRP1 levels and functions are changed prior to AD onset. Additional studies in larger cohorts are warranted to determine whether the quantitative and qualitative changes of LRP1 and its derivatives are causative phenotypes for AD.
LRP1 AND Aβ METABOLISM: PRECLINICAL EVIDENCE FROM CELLULAR AND ANIMAL STUDIES
As Aβ deposition plays a central role in initiating the pathogenesis of AD, it is important to explore how LRP1 is involved in Aβ metabolism. Accordingly, many interesting observations have been reported using cellular or animal models where LRP1 amount or function was biologically or genetically manipulated.
Initial efforts were devoted to addressing the effects of LRP1 on APP trafficking and processing to Aβ by using cellular models, as it was known that LRP1 catabolizes tissue factor pathway inhibitor, and this protein shares the Kunitz protease inhibitor domain with the longer forms of APP (67–70). Blocking LRP1 function by RAP increased cell surface APP and reduced Aβ production in H4 cells stably expressing human APP (69). Consistently, overexpressing a mini-receptor form of LRP1 containing the ligand-binding domain IV reduced cell surface APP in Chinese hamster ovary cells (71). However, other studies showed that overexpression of the LRP1 C-terminal transmembrane domain can reduce Aβ production by competing with APP for processing through β-secretase and γ-secretase in neuronal cell lines (24, 27). These conflicting results might be due to differences in experimental conditions, such as cell types, expression status of LRP1 or APP, or ways that LRP1 function was manipulated. Nonetheless, these results imply that LRP1 has the ability to modulate APP processing and subsequent Aβ generation.
LRP1 is a fast endocytic receptor mediating the trafficking and lysosomal degradation of an array of ligands. By focusing on such a function of LRP1, it has also been intensely studied regarding how LRP1 modulates the cellular uptake, trafficking, degradation and/or aggregation of Aβ. An initial finding was that activated α2-macroglobulin, an LRP1 ligand, strongly binds Aβ and promotes Aβ uptake into the glioblastoma cells or cortical neurons, which was blocked by RAP (72, 73). Our recent study using cellular-specific LRP1 knockout mice in the background of APP/PS1, which overexpress a mutant form of APP (KM670/671NL) and presenilin-1 (ΔE9) (74), demonstrated the receptor-mediated clearance of Aβ through LRP1 in neurons and astrocytes (75, 76). Aside from the brain parenchymal cells, vascular cells were also reported to internalize Aβ through LRP1 (77). By injecting [125I]Aβ40 into rodents, several interesting observations regarding the role of vascular LRP1 in Aβ metabolism were reported. One group showed the existence of an active transport system of Aβ from brain to plasma at the blood-brain barrier (BBB), which was blocked by LRP1 ligands and anti-LRP1 antibody (15, 78), while another group failed to show the existence of such a system at the BBB (79). Although it has been debated whether internalized Aβ in the endothelial cells would move into the lysosome for degradation, or to the other side of cells for transcytosis (16, 79–82), several other groups have also reported LRP1-mediated Aβ clearance in animal models (83, 84). In particular, a recent animal model study nicely demonstrated the existence of the LRP1-mediated Aβ transport system at the BBB using a conditional mouse model deleting LRP1 in endothelial cells (84). MEOX2 homeobox gene controls LRP1 expression at the BBB, and might link to neurovascular dysfunction in AD (85), while another group reported that haploinsufficiency of the MEOX2 gene did not affect plaque deposition in APP/PS1 mice (86). Of note, the choroid plexus, which forms the blood-cerebrospinal fluid (CSF) barrier, can actively eliminate Aβ from CSF through LRP1, forming a critical pathway regulating Aβ levels in CSF (87). The important roles of LRP1 in vascular mural cells (vascular smooth muscle cells and pericytes) were also reported. Serum response factor and myocardin regulate LRP1 expression in smooth muscle cells and control cerebrovascular clearance of Aβ (88). Our group also has demonstrated that conditional deletion of LRP1 in vascular smooth muscle cells increases Aβ deposition as plaques and CAA in APP/PS1 model (89). Pericytes, which regulate several neurovascular functions, including BBB integrity and cerebral blood flow, can clear Aβ through LRP1, and loss of pericytes exaggerated Aβ accumulation in Tg2576 mice, which overexpress a mutant form of APP (KM670/671NL) under the hamster prion promoter (90, 91). LRP1 in vascular mural cells might promote lysosomal degradation of Aβ or control the perivascular drainage pathway of Aβ (92).
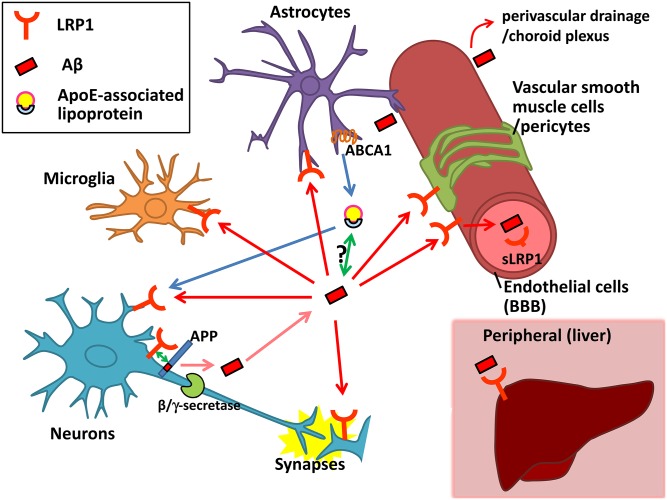
It is noteworthy that LRP1 in peripheral tissues could influence Aβ metabolism in the brain. It has been hypothesized that a dynamic equilibrium exists between Aβ pools in the periphery and those in the brain (peripheral sink hypothesis) (66, 93, 94). Large portions of Aβ in plasma is cleared through the liver (95, 96), and LRP1 is a major receptor for Aβ in the liver (97). Thus, upregulating LRP1 in the liver could accelerate the elimination of peripherally circulating Aβ, allowing brain Aβ sinks into the blood through the BBB. Consistently, modulating LRP1 function in the liver through pharmacological or nonpharmacological approaches was associated with reduced Aβ accumulation in mouse brain (98, 99). In addition, circulating sLRP1 in plasma may also help Aβ clearance to reduce Aβ accumulation in the brains of Tg2576 mice, which would be consistent with the peripheral sink hypothesis (32, 65). However, such a hypothesis has been questioned by several studies, including recent ones showing that reduction of plasma Aβ by neprilysin, an Aβ degrading enzyme (100), did not affect brain Aβ levels in mice and monkeys (101–103). Also, the function of sLRP1 remains to be further clarified; whether it binds directly with Aβ (16, 17) and whether it interferes with the normal function of LRP1. The latter point might be more important in a subset of AD patients whose BBB integrity is disturbed (104) and, thus, circulating sLRP1 might enter the brain. Cumulatively, LRP1 expressed in neurons (17, 73, 75), astrocytes (19, 76, 105), microglia (106), endothelial cells (16, 78, 84), vascular smooth muscle cells or pericytes (88–90, 107), choroid plexus (87, 108), plasma (32), and liver (97–99) was reported to be involved in Aβ cellular uptake and/or clearance (Fig. 2). Further studies would be warranted to address the relative contributions of LRP1 in different cell types and tissues to brain Aβ metabolism. Additional aspects regarding LRP1-mediated Aβ clearance systems and their potential mechanism have been discussed in detail in our previous review (2, 109).
Fig. 2.
Proposed LRP1-related pathways for Aβ production, clearance, and accumulation and their relationship with apoE. Aβ production from APP in neurons might be influenced by LRP1 through its interaction with APP or competition with the β/γ-secretase cleavage of APP. Once Aβ is produced and secreted into the extracellular space in the brain, LRP1 is involved in cellular uptake of Aβ in neurons, microglia, astrocytes, vascular smooth muscle cells, pericytes, endothelial cells, and the choroid plexus. It might be context-dependent whether internalized Aβ is degraded in lysosomes or accumulated in the cells provoking cellular toxicity. A portion of Aβ may be transported through LRP1 at the BBB or pulled out by sink activity of soluble LRP1 into the blood. LRP1 in the liver might also help clear Aβ from the blood, possibly leading to the elimination of Aβ from the brain. apoE, which is mainly produced and secreted from astrocytes in the brain, is lipidated by ABCA1 to supply cholesterol/lipids to neurons and other cells through LRP1. apoE isoforms likely affect LRP1-mediated Aβ metabolism by directly interacting with Aβ or competing with Aβ for receptor binding.
Importantly, it remains to be further clarified whether Aβ metabolism mediated by LRP1 would benefit Aβ clearance or exacerbate Aβ accumulation during AD pathogenesis. Indeed, although complete neuronal deletion of LRP1 exaggerates Aβ accumulation (75), we also observed that functional enhancement of the LRP1 mini-receptor, mLRP2, in neurons increased soluble Aβ and intraneuronal Aβ accumulation in PDAPP mouse models (110, 111). As lysosomal function can be perturbed by the excess amount of aggregated Aβ and aging-related stress, which manifests in AD (112, 113), the LRP1-mediated Aβ uptake pathway could lead to either clearance or accumulation of Aβ. Indeed, mice deficient of vitamin E synthesis enzyme showed increased levels of LRP1 in brain vasculature, but impaired Aβ clearance from the brain, although they also exhibited decreased levels of insulin-degrading enzyme, an Aβ degrading enzyme (98, 114). Of note, although LRP1 can protect against Aβ toxicity (115), internalized Aβ through LRP1 also could provoke cellular toxicity and lethality, which might promote the neurodegenerative pathway in AD (107, 116). In addition to the trend of increased LRP1 levels in the presumable early stage of AD, as mentioned before, we observed significant regional associations between Aβ, synaptic markers, and LRP1 in nondemented individuals and sporadic AD patients, which would provide important insights into the pathogenesis of Aβ accumulation (59, 117). Taken together, these results suggest that LRP1 could play a role in promoting Aβ accumulation and its toxicity in AD pathogenesis. Further studies are needed to define whether LRP1 helps Aβ clearance or exacerbates Aβ accumulation during AD pathogenesis.
LRP1 AND Aβ-INDEPENDENT PATHWAYS IN AD PATHOGENESIS
LRP1, which is distributed in the post synapses of neurons, interacts directly or indirectly with several synaptic proteins, including postsynaptic density protein 95 (22), NMDA receptor subunit, NMDAR1 (118) and NMDAR2A (22, 23), and α-amino-3-hydroxy-5-methyl-4-isoxazole propionate receptor subunit, GluA1 (119, 120), thereby regulating their trafficking, turnover, and functions. Of note, LRP1 was also shown to regulate insulin signaling and glucose uptake in mouse brains by coupling with the insulin receptor β and reducing the levels of glucose transporters, GLUT3 and GLUT4, in neurons (121). Moreover, LRP1 interacts with leptin/leptin receptor complex in the hypothalamus to regulate its signaling pathway. Deletion of neuronal LRP1 resulted in increased food intake and decreased energy consumption causing obesity and diabetic phenotypes (60). Thus, the deletion of LRP1 in neurons has been shown to affect multiple pathways not directly related to Aβ metabolism: NMDA or α-amino-3-hydroxy-5-methyl-4-isoxazole propionate signaling, lipid metabolism, leptin signaling, glucose metabolism, insulin signaling, and anti-apoptotic signaling that lead to neuroinflammation, motor dysfunction, and cognitive function in mice (22, 60, 118, 119, 121, 122).
These findings clearly indicate that LRP1 plays very important roles in neuronal function, and its perturbation contributes to neurodegeneration. Some of these features indeed might be a trigger for AD pathogenesis. For example, the reduction of sulfatide levels by LRP1 deletion (118) was observed in the brains of early stage of AD cases (123). Dysfunction of NMDA neurotransmission might contribute to the pathogenesis of AD (124). Increasing evidence demonstrates that diabetes is associated with increased risk for AD through Aβ-dependent and independent mechanisms; impaired insulin/insulin-like growth factors signaling and a compromised glucose-energy balance in the brain are predicted to impact cognitive performance during aging and AD (125). Given that hyperglycemia decreases brain LRP levels (121), it is possible that neuronal LRP1 plays a role in diabetes-related AD pathogenesis. Leptin signaling, which is regulated by LRP1, might also contribute to AD pathogenesis (126).
However, because LRP1 deletion also leads to phenotypes uncommon to AD, it remains to be further clarified whether reduction of LRP1 levels in AD brains could be interpreted as a driving event or a downstream event in the neurodegenerative processes. Also, few studies exist addressing the effects of LRP1 on tau accumulation, which is another important pathological feature of AD (127). Thus, in future animal studies, it should be further clarified whether manipulation of LRP1 expression and function in specific cell types, such as neurons, glia cells, or vascular cells, could lead to cognitive dysfunction, and whether those effects could be reminiscent of neurodegenerative processes of AD, including tau accumulation. Age- or disease stage-specific manipulation would also be helpful to understand the role of LRP1 in AD development and progression.
LRP1 AND apoE IN AD PATHOGENESIS
As APOE4 is the strongest genetic risk factor for AD and apoE is a ligand for LRP1, there is an urgent need to define how apoE mediates AD pathogenesis, and whether LRP1 is involved in apoE-mediated AD pathogenesis (128, 129). Results from genetic studies are conflicting, making it difficult to conclude whether APOE and LRP1 interact with AD risk from the perspective of genetics. Although apoE promotes Aβ accumulation in an apoE-isoform-dependent manner (E4 > E3 > E2) (109), recent clinical and preclinical studies, including ours, demonstrated that apoE isoform also influences cognitive performance and AD development in an Aβ-independent manner (129–133). Thus, in this section, we discuss both Aβ-dependent and Aβ-independent effects of apoE isoform on AD pathogenesis with a specific focus on LRP1-related pathways (Fig. 2).
In vitro experiments have shown that apoE3 produced by human embryonic kidney 293 cells forms apoE3-Aβ complex more than apoE4, and protects against Aβ toxicity in primary neurons, which was blocked by the LRP1 antagonist, RAP (134). While treatments with conditioned medium from apoE2- and apoE3-expressing cells, but not apoE4-expressing cells, facilitated Aβ uptake into Chinese hamster ovary cells, the effects were diminished by anti-LRP1 antibody, suggesting that apoE2 and apoE3, but not apoE4, promote Aβ clearance from the extracellular space through LRP1 (135). Consistently, other studies also revealed that the apoE-LRP1 pathway mediates the synaptic uptake and astrocytic degradation of Aβ (136, 137). When the Aβ-apoE complex was injected into mouse brains, apoE2- and apoE3-bound Aβ was cleared through both LRP1 and VLDLR at the BBB. Interestingly, the rapid clearance of free Aβ from LRP1 is likely redirected to the slower clearance through VLDLR when Aβ is bound to apoE4 (138), although another group observed the apoE isoform-independent effects on the inhibition of Aβ clearance (139). Controversially, several other observations reported that LRP1 rather potentiates apoE-mediated Aβ accumulation (140–142). Whereas these studies generally showed the importance of LRP1 in mediating the metabolism of the apoE-Aβ complex, there is also a report showing that apoE competes with Aβ for interaction with LRP1, resulting in suppressed Aβ clearance (105). These discrepancies might be caused by potentially different properties of Aβ and apoE isoforms (e.g., amount, aggregation, and lipidation status), and different brain areas where Aβ, apoE, and LRP1 interact during the progression of Aβ deposition in AD. In addition to Aβ clearance, LRP1 likely modifies apoE-induced APP processing under certain conditions; the treatment with lipid-poor apoE4 increases Aβ production compared with apoE3 in neuronal cells depending on LRP1 (143).
Regarding the Aβ-independent pathways, an initial study showed that apoE3 purified from human plasma and CSF or reconstituted apoE3 particles, but not apoE4, increases neurite extension in neuronal cells, and this effect was blocked by LRP1 antagonist, RAP, or anti-LRP1 antibody (144–146). A similar phenomenon was also observed using human recombinant apoE; neurite outgrowth was promoted in the presence of apoE3 through LRP1, but suppressed by apoE4, in cultured mouse cortical neurons (147). While astrocytes were shown to promote synaptogenesis, cholesterol carried by apoE-containing lipoprotein particles appears to be essential (148, 149). Of note, the beneficial effect was inhibited in the presence of RAP (148, 149). Consistently, the treatment with a retinoid X receptor agonist, bexarotene, which stimulates apoE production and its lipidation, significantly increased synaptic proteins through neuronal LRP1 in aged mice (150). Furthermore, mouse apoE and human apoE3, but not apoE4, also contribute to several signaling pathways through LRP1 in neurons, including activation of p44/42 MAPK (151), protein kinase C (PKC)δ (152), and PKCε (153), and inactivation of glycogen synthase kinase 3β (GSK3β) (152). Thus, the supplies of cholesterol/lipids to neurons through the apoE-LRP1 pathway and/or subsequent regulation of specific signaling pathways might be critical in maintaining neuronal homeostasis, which likely depends on apoE isoforms. Because apoE4 induces nuclear import of histone deacetylases through LRP1 in neuronal cells resulting in reduced brain-derived neurotrophic factor expression (154), the apoE4-LRP1 axis might facilitate apoE4-related toxic effects.
In addition to neurons, the apoE-LRP1 pathway plays a critical role in BBB integrity. LRP1 is abundantly expressed in pericytes at the BBB, where apoE prevents the activation of the proinflammatory cyclophilin A-nuclear factor κ-light-chain-enhancer of activated B cells-matrix metallopeptidase 9 (CypA-NFκB-MMP9) pathway through LRP1 (155). A study using an in vitro BBB model also showed that apoE3 induces PKCη activation and phosphorylation of a tight junction protein, occludin, in endothelial cells to preserve BBB integrity, which was blocked by RAP and anti-LRP1 antibody (156). However, both reports demonstrated that those protective effects are deficient in apoE4, which is potentially associated with BBB dysfunction (155, 156). apoE3 also inhibits pericyte mobility through LRP1, while apoE4 does not have such effects (157).
Though these studies showed Aβ-independent effects of apoE on synaptic degeneration or vascular dysfunction through LRP1, it remains to be clarified whether and how these effects contribute to AD pathogenesis. Indeed, we recently observed that apoE2 protects against cognitive decline during aging, while apoE4 exaggerates it independent of amyloid accumulation. Of note, in our animal study, these effects also appeared to be independent of synaptic degeneration and neuroinflammation (133). Thus, future efforts should be devoted to clinical studies addressing the importance of these Aβ-independent effects of apoE through LRP1 during AD pathogenesis or cognitive decline during aging.
LRP1 AS A THERAPEUTIC TARGET FOR AD
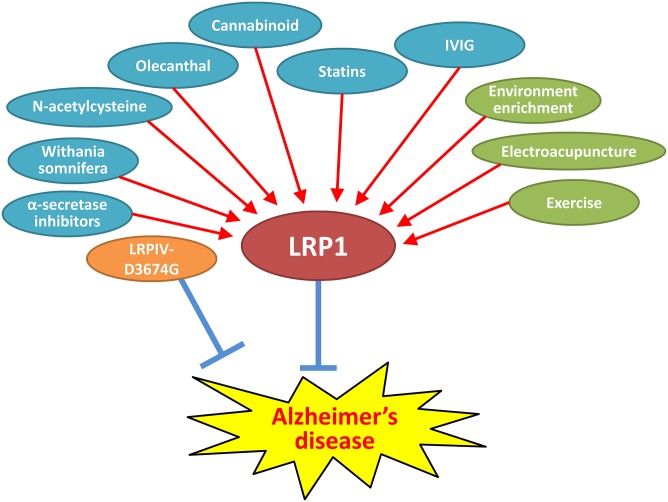
Several pharmaceutical and nonpharmaceutical methods were reported to modulate LRP1 function and benefit AD-related pathogenesis in animal models (Fig. 3). Although some of them have multiple targets in addition to LRP1, their translation into clinical trials could provide opportunities to understand how LRP1 is involved in AD pathogenesis in humans.
Fig. 3.
Potential therapeutics for AD targeting LRP1. Several pharmacological approaches [cyan colored: statins, Withania somnifera, oleocanthal, cannabinoid, intravenous immunoglobulin (IVIG), and α-secretase inhibitors] and several nonpharmacological approaches (green colored: environmental enrichment, electroacupuncture, and exercise) might be promising therapeutic strategies for AD by enhancing LRP1-mediated Aβ clearance pathways. The treatment with LRPIV-D3674G mimicking soluble LRP1 may also facilitate Aβ elimination from brain through sink activity. Because the role of LRP1 in AD pathogenesis is likely context-dependent, further preclinical studies are desired to define the potential impacts of LRP1-targeted therapy on AD-related phenotypes prior to moving to clinical trials.
Environmental enrichment conditions could ameliorate cognitive function and amyloid pathology in amyloid AD mouse models (158, 159). The receptor for advanced glycation end products was reported to mediate Aβ influx into the brain across the BBB (160). In TgCRND8 mice, which overexpress a double mutant form of APP (KM670/671NL + V717F) under the control of the hamster prion promoter (161), environmental enrichment likely counteracts the vascular dysfunction by increasing angiogenesis accompanied by upregulation of LRP1, apoE, and α2-macroglobulin, as well as downregulation of the receptor for advanced glycation end products, such that Aβ clearance is improved (162). Brain electroacupuncture also reduced hippocampal Aβ deposition and restored cognitive impairment in APP V717I transgenic mice, which was associated with an increase of hippocampal LRP1 (163). In addition, exercise ameliorated brain Aβ deposition and cognitive decline in APP transgenic mice concomitant with upregulations of LRP1 as well as neprilysin, insulin-degrading enzyme, matrix metallopeptidase 9, HSP70 (the 70 kDa heat shock proteins), or brain-derived neurotrophic factor (164, 165).
Several epidemiological studies indicate that HMG-CoA reductase inhibitors or statins, which are used to treat hypercholesterolemia, can reduce the risk of AD, in particular if they are given from middle age, although therapeutic effects of statins for AD patients appears to be moderate if any, which might be similar to anti-Aβ therapy (166). Interestingly, its oral administration has been demonstrated to promote Aβ clearance from the brain by increasing cerebrovascular LRP1 in wild-type mice and APP transgenic mice, which is likely mediated by the isoprenoid pathway (167). In wild-type mice or TgSwDI mice, which overexpress a triple mutant form of APP (KM670/671NL + E693Q + D694N) under the control of the mouse Thy1 promoter (168), oleocanthal, a phenolic secoiridoid component of extra virgin olive oil, upregulated p-glycoprotein and LRP1 at the BBB, resulting in enhanced Aβ clearance and reduction of neuroinflammation (169, 170). In vitro and in vivo experiments showed that cannabinoid treatment also enhanced Aβ transit at the BBB, associated with increased LRP1 levels in the brain and plasma (171). Oxidative and inflammatory stress might be involved in the pathogenesis of AD (172–175). Interestingly, antioxidant N-acetylcysteine protected against inflammation-induced dysfunction of Aβ clearance by LRP1 at the BBB (176). In addition, the administration of an α-secretase ADAM10 inhibitor, GI254023X, could enhance Aβ clearance through the BBB by reducing LRP1 shedding (177). Thus, pharmacological approaches to increase LRP1 in the cerebrovasculature may be promising strategies for AD therapy. However, increasing LRP1 alone may not be sufficient and cofactors are likely involved in the mechanisms (17). This might explain why mice deficient in vitamin E synthesis enzyme showed disturbed Aβ clearance from the brain despite increased LRP1 levels at brain vasculatures (98).
Interestingly, statin treatment also can upregulate LRP1 in the liver as well as brain (178). Oral administration of an Indian traditional drug, semi-purified extract of the root, Withania somnifera, increased liver LRP1 and ameliorated Aβ pathology in APP/PS1 mice likely by facilitating Aβ clearance across the BBB (99). Although further studies are needed, these results suggest that increasing liver LRP1 is potentially beneficial against brain Aβ accumulation, likely consistent with the peripheral sink hypothesis. Circulating sLRP1 may also be involved in the mechanism (32, 65). Of note, a therapeutic effect of modified sLRP1-like peptide, LRPIV-D3674G, which has a higher binding affinity to Aβ, but less binding affinity to other LRP1 ligands than wild-type sLRP1, was reported; subcutaneous administration of LRPIV-D3674G reduced Aβ40 and Aβ42 levels in the hippocampus, cortex, and CSF by 60–80%, and improved vascular and neuronal functions in Tg2576 mice (33). Thus, LRPIV-D3674G treatment can be a novel candidate for AD therapy directly targeting LRP1 function.
Although these treatments to increase LRP1 in brain, cerebrovasculature, liver, and plasma might be promising strategies for AD therapy, the effects should be carefully evaluated because LRP1 may exacerbate Aβ production/accumulation and enhance the cellular toxicity under some conditions, as described above. Further studies to address their impacts on more diverse endpoints through different intervention periods using multiple animal models would be desired to define potential LRP1-targeted therapy for AD. In addition, combination treatment with Aβ immunotherapy should also be explored as an alternative approach because intravenous injection of anti-Aβ antibody likely enhances Aβ clearance through LRP1 at the choroid plexus in APP transgenic mice (108).
SUMMARY AND PERSPECTIVE
Accumulating evidence through in vitro and in vivo studies has demonstrated that LRP1 critically regulates Aβ metabolism and brain homeostasis through multiple pathways in both apoE isoform-dependent and -independent manners. However, there are several discrepancies in those observations. It also remains to be clarified how much these pathways contribute to AD pathogenesis, because most of the findings were from cellular and animal models. Thus, further efforts should be devoted to comprehensively address: 1) the potential change of LRP1 levels/functions across specific stages of AD, especially at the preclinical stage, in human postmortem and antemortem cohorts; and 2) the potentially different roles of LRP1 depending on specific context, including cell types, ages, disease stages, and apoE isoforms. Clarifying these aspects should provide novel insights, enabling us to develop effective LRP1-targetted therapies for AD.
Acknowledgments
The authors thank Ms. Mary D. Davis for the careful reading of this manuscript.
Footnotes
Abbreviations:
- Aβ
- amyloid-β peptide
- AD
- Alzheimer’s disease
- APP
- amyloid precursor protein
- BBB
- blood-brain barrier
- CAA
- cerebral amyloid angiopathy
- CSF
- cerebrospinal fluid
- LDLR
- LDL receptor
- LRP
- LDL receptor-related protein
- MCI
- mild cognitive impairment
- NMDA
- N-methyl-D-aspartate
- sLRP1
- soluble form of LRP1
- PKC
- protein kinase C
- RAP
- receptor-associated protein
- VLDLR
- VLDL receptor
This work was supported by National Institutes of Health Grants P50AG016574, RF1AG051504, R01AG027924, R01AG035355, R01AG046205, and P01NS074969 (to G.B.), a grant from the Cure Alzheimer’s Fund (to G.B.), and fellowship supports from the Japan Heart Foundation, Naito Foundation, Mayo Clinic Alzheimer’s Disease Research Center, BrightFocus Foundation, and the National Alzheimer’s Coordinating Center (NACC) junior investigator award (to M.S.).
REFERENCES
- 1.Bu G. 2009. Apolipoprotein E and its receptors in Alzheimer’s disease: pathways, pathogenesis and therapy. Nat. Rev. Neurosci. 10: 333–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kanekiyo T., and Bu G.. 2014. The low-density lipoprotein receptor-related protein 1 and amyloid-β clearance in Alzheimer’s disease. Front. Aging Neurosci. 6: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herz J., Hamann U., Rogne S., Myklebost O., Gausepohl H., and Stanley K. K.. 1988. Surface location and high affinity for calcium of a 500-kd liver membrane protein closely related to the LDL-receptor suggest a physiological role as lipoprotein receptor. EMBO J. 7: 4119–4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herz J., Kowal R. C., Goldstein J. L., and Brown M. S.. 1990. Proteolytic processing of the 600 kd low density lipoprotein receptor-related protein (LRP) occurs in a trans-Golgi compartment. EMBO J. 9: 1769–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Willnow T. E., Moehring J. M., Inocencio N. M., Moehring T. J., and Herz J.. 1996. The low-density-lipoprotein receptor-related protein (LRP) is processed by furin in vivo and in vitro. Biochem. J. 313: 71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neels J. G., van Den Berg B. M., Lookene A., Olivecrona G., Pannekoek H., and van Zonneveld A. J.. 1999. The second and fourth cluster of class A cysteine-rich repeats of the low density lipoprotein receptor-related protein share ligand-binding properties. J. Biol. Chem. 274: 31305–31311. [DOI] [PubMed] [Google Scholar]
- 7.Obermoeller-McCormick L. M., Li Y., Osaka H., FitzGerald D. J., Schwartz A. L., and Bu G.. 2001. Dissection of receptor folding and ligand-binding property with functional minireceptors of LDL receptor-related protein. J. Cell Sci. 114: 899–908. [DOI] [PubMed] [Google Scholar]
- 8.Chen W. J., Goldstein J. L., and Brown M. S.. 1990. NPXY, a sequence often found in cytoplasmic tails, is required for coated pit-mediated internalization of the low density lipoprotein receptor. J. Biol. Chem. 265: 3116–3123. [PubMed] [Google Scholar]
- 9.Li Y., Paz Marzolo M., van Kerkhof P., Strous G. J., and Bu G.. 2000. The YXXL motif, but not the two NPXY motifs, serves as the dominant endocytosis signal for low density lipoprotein receptor-related protein. J. Biol. Chem. 275: 17187–17194. [DOI] [PubMed] [Google Scholar]
- 10.Lillis A. P., Van Duyn L. B., Murphy-Ullrich J. E., and Strickland D. K.. 2008. LDL receptor-related protein 1: unique tissue-specific functions revealed by selective gene knockout studies. Physiol. Rev. 88: 887–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Willnow T. E., Armstrong S. A., Hammer R. E., and Herz J.. 1995. Functional expression of low density lipoprotein receptor-related protein is controlled by receptor-associated protein in vivo. Proc. Natl. Acad. Sci. USA. 92: 4537–4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Willnow T. E., Rohlmann A., Horton J., Otani H., Braun J. R., Hammer R. E., and Herz J.. 1996. RAP, a specialized chaperone, prevents ligand-induced ER retention and degradation of LDL receptor-related endocytic receptors. EMBO J. 15: 2632–2639. [PMC free article] [PubMed] [Google Scholar]
- 13.Bu G., Geuze H. J., Strous G. J., and Schwartz A. L.. 1995. 39 kDa receptor-associated protein is an ER resident protein and molecular chaperone for LDL receptor-related protein. EMBO J. 14: 2269–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zlokovic B. V., Deane R., Sagare A. P., Bell R. D., and Winkler E. A.. 2010. Low-density lipoprotein receptor-related protein-1: a serial clearance homeostatic mechanism controlling Alzheimer’s amyloid beta-peptide elimination from the brain. J. Neurochem. 115: 1077–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deane R., Wu Z., Sagare A., Davis J., Du Yan S., Hamm K., Xu F., Parisi M., LaRue B., Hu H. W., et al. 2004. LRP/amyloid beta-peptide interaction mediates differential brain efflux of Abeta isoforms. Neuron. 43: 333–344. [DOI] [PubMed] [Google Scholar]
- 16.Yamada K., Hashimoto T., Yabuki C., Nagae Y., Tachikawa M., Strickland D. K., Liu Q., Bu G., Basak J. M., Holtzman D. M., et al. 2008. The low density lipoprotein receptor-related protein 1 mediates uptake of amyloid beta peptides in an in vitro model of the blood-brain barrier cells. J. Biol. Chem. 283: 34554–34562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanekiyo T., Zhang J., Liu Q., Liu C. C., Zhang L., and Bu G.. 2011. Heparan sulphate proteoglycan and the low-density lipoprotein receptor-related protein 1 constitute major pathways for neuronal amyloid-beta uptake. J. Neurosci. 31: 1644–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu Y., Zhao J., Atagi Y., Nielsen H. M., Liu C. C., Zheng H., Shinohara M., Kanekiyo T., and Bu G.. 2016. Apolipoprotein E lipoprotein particles inhibit amyloid-beta uptake through cell surface heparan sulphate proteoglycan. Mol. Neurodegener. 11: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y., Cheng D., Cheng R., Zhu X., Wan T., Liu J., and Zhang R.. 2014. Mechanisms of U87 astrocytoma cell uptake and trafficking of monomeric versus protofibril Alzheimer’s disease amyloid-beta proteins. PLoS One. 9: e99939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muratoglu S. C., Mikhailenko I., Newton C., Migliorini M., and Strickland D. K.. 2010. Low density lipoprotein receptor-related protein 1 (LRP1) forms a signaling complex with platelet-derived growth factor receptor-beta in endosomes and regulates activation of the MAPK pathway. J. Biol. Chem. 285: 14308–14317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Craig J., Mikhailenko I., Noyes N., Migliorini M., and Strickland D. K.. 2013. The LDL receptor-related protein 1 (LRP1) regulates the PDGF signaling pathway by binding the protein phosphatase SHP-2 and modulating SHP-2- mediated PDGF signaling events. PLoS One. 8: e70432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.May P., Rohlmann A., Bock H. H., Zurhove K., Marth J. D., Schomburg E. D., Noebels J. L., Beffert U., Sweatt J. D., Weeber E. J., et al. 2004. Neuronal LRP1 functionally associates with postsynaptic proteins and is required for normal motor function in mice. Mol. Cell. Biol. 24: 8872–8883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maier W., Bednorz M., Meister S., Roebroek A., Weggen S., Schmitt U., and Pietrzik C. U.. 2013. LRP1 is critical for the surface distribution and internalization of the NR2B NMDA receptor subtype. Mol. Neurodegener. 8: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.von Einem B., Schwanzar D., Rehn F., Beyer A. S., Weber P., Wagner M., Schneckenburger H., and von Arnim C. A.. 2010. The role of low-density receptor-related protein 1 (LRP1) as a competitive substrate of the amyloid precursor protein (APP) for BACE1. Exp. Neurol. 225: 85–93. [DOI] [PubMed] [Google Scholar]
- 25.von Arnim C. A., Kinoshita A., Peltan I. D., Tangredi M. M., Herl L., Lee B. M., Spoelgen R., Hshieh T. T., Ranganathan S., Battey F. D., et al. 2005. The low density lipoprotein receptor-related protein (LRP) is a novel beta-secretase (BACE1) substrate. J. Biol. Chem. 280: 17777–17785. [DOI] [PubMed] [Google Scholar]
- 26.Liu Q., Zhang J., Tran H., Verbeek M. M., Reiss K., Estus S., and Bu G.. 2009. LRP1 shedding in human brain: roles of ADAM10 and ADAM17. Mol. Neurodegener. 4: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lleó A., Waldron E., von Arnim C. A., Herl L., Tangredi M. M., Peltan I. D., Strickland D. K., Koo E. H., Hyman B. T., Pietrzik C. U., et al. 2005. Low density lipoprotein receptor-related protein (LRP) interacts with presenilin 1 and is a competitive substrate of the amyloid precursor protein (APP) for gamma-secretase. J. Biol. Chem. 280: 27303–27309. [DOI] [PubMed] [Google Scholar]
- 28.Zurhove K., Nakajima C., Herz J., Bock H. H., and May P.. 2008. Gamma-secretase limits the inflammatory response through the processing of LRP1. Sci. Signal. 1: ra15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spuch C., Ortolano S., and Navarro C.. 2012. LRP-1 and LRP-2 receptors function in the membrane neuron. Trafficking mechanisms and proteolytic processing in Alzheimer’s disease. Front. Physiol. 3: 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Q., Zerbinatti C. V., Zhang J., Hoe H. S., Wang B., Cole S. L., Herz J., Muglia L., and Bu G.. 2007. Amyloid precursor protein regulates brain apolipoprotein E and cholesterol metabolism through lipoprotein receptor LRP1. Neuron. 56: 66–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kinoshita A., Shah T., Tangredi M. M., Strickland D. K., and Hyman B. T.. 2003. The intracellular domain of the low density lipoprotein receptor-related protein modulates transactivation mediated by amyloid precursor protein and Fe65. J. Biol. Chem. 278: 41182–41188. [DOI] [PubMed] [Google Scholar]
- 32.Sagare A., Deane R., Bell R. D., Johnson B., Hamm K., Pendu R., Marky A., Lenting P. J., Wu Z., Zarcone T., et al. 2007. Clearance of amyloid-beta by circulating lipoprotein receptors. Nat. Med. 13: 1029–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sagare A. P., Bell R. D., Srivastava A., Sengillo J. D., Singh I., Nishida Y., Chow N., and Zlokovic B. V.. 2013. A lipoprotein receptor cluster IV mutant preferentially binds amyloid-beta and regulates its clearance from the mouse brain. J. Biol. Chem. 288: 15154–15166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herz J., Clouthier D. E., and Hammer R. E.. 1992. LDL receptor-related protein internalizes and degrades uPA-PAI-1 complexes and is essential for embryo implantation. Cell. 71: 411–421. [DOI] [PubMed] [Google Scholar]
- 35.Gonias S. L., and Campana W. M.. 2014. LDL receptor-related protein-1: a regulator of inflammation in atherosclerosis, cancer, and injury to the nervous system. Am. J. Pathol. 184: 18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rebeck G. W., Reiter J. S., Strickland D. K., and Hyman B. T.. 1993. Apolipoprotein E in sporadic Alzheimer’s disease: allelic variation and receptor interactions. Neuron. 11: 575–580. [DOI] [PubMed] [Google Scholar]
- 37.Rebeck G. W., Harr S. D., Strickland D. K., and Hyman B. T.. 1995. Multiple, diverse senile plaque-associated proteins are ligands of an apolipoprotein E receptor, the alpha 2-macroglobulin receptor/low-density-lipoprotein receptor-related protein. Ann. Neurol. 37: 211–217. [DOI] [PubMed] [Google Scholar]
- 38.Kang D. E., Saitoh T., Chen X., Xia Y., Masliah E., Hansen L. A., Thomas R. G., Thal L. J., and Katzman R.. 1997. Genetic association of the low-density lipoprotein receptor-related protein gene (LRP), an apolipoprotein E receptor, with late-onset Alzheimer’s disease. Neurology. 49: 56–61. [DOI] [PubMed] [Google Scholar]
- 39.Lambert J. C., Wavrant-De Vrieze F., Amouyel P., and Chartier-Harlin M. C.. 1998. Association at LRP gene locus with sporadic late-onset Alzheimer’s disease. Lancet. 351: 1787–1788. [DOI] [PubMed] [Google Scholar]
- 40.Woodward R., Singleton A. B., Gibson A. M., Edwardson J. A., and Morris C. M.. 1998. LRP gene and late-onset Alzheimer’s disease. Lancet. 352: 239–240. [DOI] [PubMed] [Google Scholar]
- 41.Beffert U., Arguin C., and Poirier J.. 1999. The polymorphism in exon 3 of the low density lipoprotein receptor-related protein gene is weakly associated with Alzheimer’s disease. Neurosci. Lett. 259: 29–32. [DOI] [PubMed] [Google Scholar]
- 42.Pritchard A., Harris J., Pritchard C. W., St Clair D., Lemmon H., Lambert J. C., Chartier-Harlin M. C., Hayes A., Thaker U., Iwatsubo T., et al. 2005. Association study and meta-analysis of low-density lipoprotein receptor related protein in Alzheimer’s disease. Neurosci. Lett. 382: 221–226. [DOI] [PubMed] [Google Scholar]
- 43.Lambert J. C., Ibrahim-Verbaas C. A., Harold D., Naj A. C., Sims R., Bellenguez C., DeStefano A. L., Bis J. C., Beecham G. W., Grenier-Boley B., et al. 2013. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat. Genet. 45: 1452–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vázquez-Higuera J. L., Mateo I., Sánchez-Juan P., Rodríguez-Rodríguez E., Pozueta A., Infante J., Berciano J., and Combarros O.. 2009. Genetic interaction between tau and the apolipoprotein E receptor LRP1 increases Alzheimer’s disease risk. Dement. Geriatr. Cogn. Disord. 28: 116–120. [DOI] [PubMed] [Google Scholar]
- 45.Klunk W. E., Engler H., Nordberg A., Wang Y., Blomqvist G., Holt D. P., Bergstrom M., Savitcheva I., Huang G. F., Estrada S., et al. 2004. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann. Neurol. 55: 306–319. [DOI] [PubMed] [Google Scholar]
- 46.Grimmer T., Goldhardt O., Guo L. H., Yousefi B. H., Forster S., Drzezga A., Sorg C., Alexopoulos P., Forstl H., Kurz A., et al. 2014. LRP-1 polymorphism is associated with global and regional amyloid load in Alzheimer’s disease in humans in-vivo. Neuroimage Clin. 4: 411–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thal D. R., Papassotiropoulos A., Saido T. C., Griffin W. S., Mrak R. E., Kolsch H., Del Tredici K., Attems J., and Ghebremedhin E.. 2010. Capillary cerebral amyloid angiopathy identifies a distinct APOE epsilon4-associated subtype of sporadic Alzheimer’s disease. Acta Neuropathol. 120: 169–183. [DOI] [PubMed] [Google Scholar]
- 48.Bian L., Yang J. D., Guo T. W., Duan Y., Qin W., Sun Y., Feng G. Y., and He L.. 2005. Association study of the A2M and LRP1 Genes with Alzheimer disease in the Han Chinese. Biol. Psychiatry. 58: 731–737. [DOI] [PubMed] [Google Scholar]
- 49.Shi Y. M., Zhou H., Zhang Z. J., Yu H., Bai F., Yuan Y. G., Deng L. L., and Jia J. P.. 2009. Association of the LRP1 gene and cognitive performance with amnestic mild cognitive impairment in elderly Chinese. Int. Psychogeriatr. 21: 1072–1080. [DOI] [PubMed] [Google Scholar]
- 50.Matsui T., Ingelsson M., Fukumoto H., Ramasamy K., Kowa H., Frosch M. P., Irizarry M. C., and Hyman B. T.. 2007. Expression of APP pathway mRNAs and proteins in Alzheimer’s disease. Brain Res. 1161: 116–123. [DOI] [PubMed] [Google Scholar]
- 51.Akram A., Schmeidler J., Katsel P., Hof P. R., and Haroutunian V.. 2012. Association of ApoE and LRP mRNA levels with dementia and AD neuropathology. Neurobiol. Aging. 33: 628.e1–628.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamanaka Y., Faghihi M. A., Magistri M., Alvarez-Garcia O., Lotz M., and Wahlestedt C.. 2015. Antisense RNA controls LRP1 Sense transcript expression through interaction with a chromatin-associated protein, HMGB2. Cell Rep. 11: 967–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ruzali W. A., Kehoe P. G., and Love S.. 2012. LRP1 expression in cerebral cortex, choroid plexus and meningeal blood vessels: relationship to cerebral amyloid angiopathy and APOE status. Neurosci. Lett. 525: 123–128. [DOI] [PubMed] [Google Scholar]
- 54.Donahue J. E., Flaherty S. L., Johanson C. E., Duncan J. A. III, Silverberg G. D., Miller M. C., Tavares R., Yang W., Wu Q., Sabo E., et al. 2006. RAGE, LRP-1, and amyloid-beta protein in Alzheimer’s disease. Acta Neuropathol. 112: 405–415. [DOI] [PubMed] [Google Scholar]
- 55.Arélin K., Kinoshita A., Whelan C. M., Irizarry M. C., Rebeck G. W., Strickland D. K., and Hyman B. T.. 2002. LRP and senile plaques in Alzheimer’s disease: colocalization with apolipoprotein E and with activated astrocytes. Brain Res. Mol. Brain Res. 104: 38–46. [DOI] [PubMed] [Google Scholar]
- 56.Kang D. E., Pietrzik C. U., Baum L., Chevallier N., Merriam D. E., Kounnas M. Z., Wagner S. L., Troncoso J. C., Kawas C. H., Katzman R., et al. 2000. Modulation of amyloid beta-protein clearance and Alzheimer’s disease susceptibility by the LDL receptor-related protein pathway. J. Clin. Invest. 106: 1159–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qiu Z., Strickland D. K., Hyman B. T., and Rebeck G. W.. 2001. Elevation of LDL receptor-related protein levels via ligand interactions in Alzheimer disease and in vitro. J. Neuropathol. Exp. Neurol. 60: 430–440. [DOI] [PubMed] [Google Scholar]
- 58.Causevic M., Ramoz N., Haroutunian V., Davis K. L., and Buxbaum J. D.. 2003. Lack of association between the levels of the low-density lipoprotein receptor-related protein (LRP) and either Alzheimer dementia or LRP exon 3 genotype. J. Neuropathol. Exp. Neurol. 62: 999–1005. [DOI] [PubMed] [Google Scholar]
- 59.Shinohara M., Fujioka S., Murray M. E., Wojtas A., Baker M., Rovelet-Lecrux A., Rademakers R., Das P., Parisi J. E., Graff-Radford N. R., et al. 2014. Regional distribution of synaptic markers and APP correlate with distinct clinicopathological features in sporadic and familial Alzheimer’s disease. Brain. 137: 1533–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu Q., Zhang J., Zerbinatti C., Zhan Y., Kolber B. J., Herz J., Muglia L. J., and Bu G.. 2011. Lipoprotein receptor LRP1 regulates leptin signaling and energy homeostasis in the adult central nervous system. PLoS Biol. 9: e1000575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Owen J. B., Sultana R., Aluise C. D., Erickson M. A., Price T. O., Bu G., Banks W. A., and Butterfield D. A.. 2010. Oxidative modification to LDL receptor-related protein 1 in hippocampus from subjects with Alzheimer disease: implications for Abeta accumulation in AD brain. Free Radic. Biol. Med. 49: 1798–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jack C. R. Jr., Knopman D. S., Jagust W. J., Shaw L. M., Aisen P. S., Weiner M. W., Petersen R. C., and Trojanowski J. Q.. 2010. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 9: 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sultana R., Banks W. A., and Butterfield D. A.. 2010. Decreased levels of PSD95 and two associated proteins and increased levels of BCl2 and caspase 3 in hippocampus from subjects with amnestic mild cognitive impairment: insights into their potential roles for loss of synapses and memory, accumulation of Abeta, and neurodegeneration in a prodromal stage of Alzheimer’s disease. J. Neurosci. Res. 88: 469–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dickson D. W., Crystal H. A., Mattiace L. A., Masur D. M., Blau A. D., Davies P., Yen S. H., and Aronson M. K.. 1992. Identification of normal and pathological aging in prospectively studied nondemented elderly humans. Neurobiol. Aging. 13: 179–189. [DOI] [PubMed] [Google Scholar]
- 65.Sagare A. P., Deane R., Zetterberg H., Wallin A., Blennow K., and Zlokovic B. V.. 2011. Impaired lipoprotein receptor-mediated peripheral binding of plasma amyloid-beta is an early biomarker for mild cognitive impairment preceding Alzheimer’s disease. J. Alzheimers Dis. 24: 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.DeMattos R. B., Bales K. R., Cummins D. J., Dodart J. C., Paul S. M., and Holtzman D. M.. 2001. Peripheral anti-A beta antibody alters CNS and plasma A beta clearance and decreases brain A beta burden in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA. 98: 8850–8855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kounnas M. Z., Moir R. D., Rebeck G. W., Bush A. I., Argraves W. S., Tanzi R. E., Hyman B. T., and Strickland D. K.. 1995. LDL receptor-related protein, a multifunctional ApoE receptor, binds secreted beta-amyloid precursor protein and mediates its degradation. Cell. 82: 331–340. [DOI] [PubMed] [Google Scholar]
- 68.Knauer M. F., Orlando R. A., and Glabe C. G.. 1996. Cell surface APP751 forms complexes with protease nexin 2 ligands and is internalized via the low density lipoprotein receptor-related protein (LRP). Brain Res. 740: 6–14. [DOI] [PubMed] [Google Scholar]
- 69.Ulery P. G., Beers J., Mikhailenko I., Tanzi R. E., Rebeck G. W., Hyman B. T., and Strickland D. K.. 2000. Modulation of beta-amyloid precursor protein processing by the low density lipoprotein receptor-related protein (LRP). Evidence that LRP contributes to the pathogenesis of Alzheimer’s disease. J. Biol. Chem. 275: 7410–7415. [DOI] [PubMed] [Google Scholar]
- 70.Rebeck G. W., Moir R. D., Mui S., Strickland D. K., Tanzi R. E., and Hyman B. T.. 2001. Association of membrane-bound amyloid precursor protein APP with the apolipoprotein E receptor LRP. Brain Res. Mol. Brain Res. 87: 238–245. [DOI] [PubMed] [Google Scholar]
- 71.Cam J. A., Zerbinatti C. V., Li Y., and Bu G.. 2005. Rapid endocytosis of the low density lipoprotein receptor-related protein modulates cell surface distribution and processing of the beta-amyloid precursor protein. J. Biol. Chem. 280: 15464–15470. [DOI] [PubMed] [Google Scholar]
- 72.Narita M., Holtzman D. M., Schwartz A. L., and Bu G.. 1997. Alpha2-macroglobulin complexes with and mediates the endocytosis of beta-amyloid peptide via cell surface low-density lipoprotein receptor-related protein. J. Neurochem. 69: 1904–1911. [DOI] [PubMed] [Google Scholar]
- 73.Qiu Z., Strickland D. K., Hyman B. T., and Rebeck G. W.. 1999. Alpha2-macroglobulin enhances the clearance of endogenous soluble beta-amyloid peptide via low-density lipoprotein receptor-related protein in cortical neurons. J. Neurochem. 73: 1393–1398. [DOI] [PubMed] [Google Scholar]
- 74.Jankowsky J. L., Fadale D. J., Anderson J., Xu G. M., Gonzales V., Jenkins N. A., Copeland N. G., Lee M. K., Younkin L. H., Wagner S. L., et al. 2004. Mutant presenilins specifically elevate the levels of the 42 residue beta-amyloid peptide in vivo: evidence for augmentation of a 42-specific gamma secretase. Hum. Mol. Genet. 13: 159–170. [DOI] [PubMed] [Google Scholar]
- 75.Kanekiyo T., Cirrito J. R., Liu C. C., Shinohara M., Li J., Schuler D. R., Holtzman D. M., and Bu G.. 2013. Neuronal clearance of amyloid-beta by endocytic receptor LRP1. J. Neurosci. 33: 19276–19283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu C. C., Hu J., Zhao N., Wang J., Na W., Cirrito J. R., Kanekiyo T., Holtzman D. M., and Bu G.. 2017. Astrocytic LRP1 mediates brain Aβ clearance and impacts amyloid deposition. J. Neurosci. 37: 4023–4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Urmoneit B., Prikulis I., Wihl G., D’Urso D., Frank R., Heeren J., Beisiegel U., and Prior R.. 1997. Cerebrovascular smooth muscle cells internalize Alzheimer amyloid beta protein via a lipoprotein pathway: implications for cerebral amyloid angiopathy. Lab. Invest. 77: 157–166. [PubMed] [Google Scholar]
- 78.Shibata M., Yamada S., Kumar S. R., Calero M., Bading J., Frangione B., Holtzman D. M., Miller C. A., Strickland D. K., Ghiso J., et al. 2000. Clearance of Alzheimer’s amyloid-β1-40 peptide from brain by LDL receptor–related protein-1 at the blood-brain barrier. J. Clin. Invest. 106: 1489–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ito S., Ueno T., Ohtsuki S., and Terasaki T.. 2010. Lack of brain-to-blood efflux transport activity of low-density lipoprotein receptor-related protein-1 (LRP-1) for amyloid-beta peptide(1-40) in mouse: involvement of an LRP-1-independent pathway. J. Neurochem. 113: 1356–1363. [DOI] [PubMed] [Google Scholar]
- 80.Pflanzner T., Janko M. C., Andre-Dohmen B., Reuss S., Weggen S., Roebroek A. J., Kuhlmann C. R., and Pietrzik C. U.. 2011. LRP1 mediates bidirectional transcytosis of amyloid-beta across the blood-brain barrier. Neurobiol. Aging. 32: 2323.e1–2323.e11. [DOI] [PubMed] [Google Scholar]
- 81.Nazer B., Hong S., and Selkoe D. J.. 2008. LRP promotes endocytosis and degradation, but not transcytosis, of the amyloid-beta peptide in a blood-brain barrier in vitro model. Neurobiol. Dis. 30: 94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Candela P., Saint-Pol J., Kuntz M., Boucau M. C., Lamartiniere Y., Gosselet F., and Fenart L.. 2015. In vitro discrimination of the role of LRP1 at the BBB cellular level: focus on brain capillary endothelial cells and brain pericytes. Brain Res. 1594: 15–26. [DOI] [PubMed] [Google Scholar]
- 83.Jaeger L. B., Dohgu S., Hwang M. C., Farr S. A., Murphy M. P., Fleegal-DeMotta M. A., Lynch J. L., Robinson S. M., Niehoff M. L., Johnson S. N., et al. 2009. Testing the neurovascular hypothesis of Alzheimer’s disease: LRP-1 antisense reduces blood-brain barrier clearance, increases brain levels of amyloid-beta protein, and impairs cognition. J. Alzheimers Dis. 17: 553–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Storck S. E., Meister S., Nahrath J., Meissner J. N., Schubert N., Di Spiezio A., Baches S., Vandenbroucke R. E., Bouter Y., Prikulis I., et al. 2016. Endothelial LRP1 transports amyloid-beta(1-42) across the blood-brain barrier. J. Clin. Invest. 126: 123–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wu Z., Guo H., Chow N., Sallstrom J., Bell R. D., Deane R., Brooks A. I., Kanagala S., Rubio A., Sagare A., et al. 2005. Role of the MEOX2 homeobox gene in neurovascular dysfunction in Alzheimer disease. Nat. Med. 11: 959–965. [DOI] [PubMed] [Google Scholar]
- 86.Soto I., Grabowska W. A., Onos K. D., Graham L. C., Jackson H. M., Simeone S. N., and Howell G. R.. 2016. Meox2 haploinsufficiency increases neuronal cell loss in a mouse model of Alzheimer’s disease. Neurobiol. Aging. 42: 50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fujiyoshi M., Tachikawa M., Ohtsuki S., Ito S., Uchida Y., Akanuma S., Kamiie J., Hashimoto T., Hosoya K., Iwatsubo T., et al. 2011. Amyloid-beta peptide(1-40) elimination from cerebrospinal fluid involves low-density lipoprotein receptor-related protein 1 at the blood-cerebrospinal fluid barrier. J. Neurochem. 118: 407–415. [DOI] [PubMed] [Google Scholar]
- 88.Bell R. D., Deane R., Chow N., Long X., Sagare A., Singh I., Streb J. W., Guo H., Rubio A., Van Nostrand W., et al. 2009. SRF and myocardin regulate LRP-mediated amyloid-beta clearance in brain vascular cells. Nat. Cell Biol. 11: 143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kanekiyo T., Liu C. C., Shinohara M., Li J., and Bu G.. 2012. LRP1 in brain vascular smooth muscle cells mediates local clearance of Alzheimer’s amyloid-beta. J. Neurosci. 32: 16458–16465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sagare A. P., Bell R. D., Zhao Z., Ma Q., Winkler E. A., Ramanathan A., and Zlokovic B. V.. 2013. Pericyte loss influences Alzheimer-like neurodegeneration in mice. Nat. Commun. 4: 2932. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 91.Hsiao K., Chapman P., Nilsen S., Eckman C., Harigaya Y., Younkin S., Yang F., and Cole G.. 1996. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 274: 99–102. [DOI] [PubMed] [Google Scholar]
- 92.Kanekiyo T., and Bu G.. 2014. The low-density lipoprotein receptor-related protein 1 and amyloid-beta clearance in Alzheimer’s disease. Front. Aging Neurosci. 6: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Matsuoka Y., Saito M., LaFrancois J., Saito M., Gaynor K., Olm V., Wang L., Casey E., Lu Y., Shiratori C., et al. 2003. Novel therapeutic approach for the treatment of Alzheimer’s disease by peripheral administration of agents with an affinity to beta-amyloid. J. Neurosci. 23: 29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vasilevko V., Xu F., Previti M. L., Van Nostrand W. E., and Cribbs D. H.. 2007. Experimental investigation of antibody-mediated clearance mechanisms of amyloid-beta in CNS of Tg-SwDI transgenic mice. J. Neurosci. 27: 13376–13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hone E., Martins I. J., Fonte J., and Martins R. N.. 2003. Apolipoprotein E influences amyloid-beta clearance from the murine periphery. J. Alzheimers Dis. 5: 1–8. [DOI] [PubMed] [Google Scholar]
- 96.Ghiso J., Shayo M., Calero M., Ng D., Tomidokoro Y., Gandy S., Rostagno A., and Frangione B.. 2004. Systemic catabolism of Alzheimer’s Abeta40 and Abeta42. J. Biol. Chem. 279: 45897–45908. [DOI] [PubMed] [Google Scholar]
- 97.Tamaki C., Ohtsuki S., Iwatsubo T., Hashimoto T., Yamada K., Yabuki C., and Terasaki T.. 2006. Major involvement of low-density lipoprotein receptor-related protein 1 in the clearance of plasma free amyloid beta-peptide by the liver. Pharm. Res. 23: 1407–1416. [DOI] [PubMed] [Google Scholar]
- 98.Nishida Y., Ito S., Ohtsuki S., Yamamoto N., Takahashi T., Iwata N., Jishage K., Yamada H., Sasaguri H., Yokota S., et al. 2009. Depletion of vitamin E increases amyloid beta accumulation by decreasing its clearances from brain and blood in a mouse model of Alzheimer disease. J. Biol. Chem. 284: 33400–33408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sehgal N., Gupta A., Valli R. K., Joshi S. D., Mills J. T., Hamel E., Khanna P., Jain S. C., Thakur S. S., and Ravindranath V.. 2012. Withania somnifera reverses Alzheimer’s disease pathology by enhancing low-density lipoprotein receptor-related protein in liver. Proc. Natl. Acad. Sci. USA. 109: 3510–3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Iwata N., Tsubuki S., Takaki Y., Shirotani K., Lu B., Gerard N. P., Gerard C., Hama E., Lee H. J., and Saido T. C.. 2001. Metabolic regulation of brain Abeta by neprilysin. Science. 292: 1550–1552. [DOI] [PubMed] [Google Scholar]
- 101.Levites Y., Smithson L. A., Price R. W., Dakin R. S., Yuan B., Sierks M. R., Kim J., McGowan E., Reed D. K., Rosenberry T. L., et al. 2006. Insights into the mechanisms of action of anti-Abeta antibodies in Alzheimer’s disease mouse models. FASEB J. 20: 2576–2578. [DOI] [PubMed] [Google Scholar]
- 102.Henderson S. J., Andersson C., Narwal R., Janson J., Goldschmidt T. J., Appelkvist P., Bogstedt A., Steffen A. C., Haupts U., Tebbe J., et al. 2014. Sustained peripheral depletion of amyloid-beta with a novel form of neprilysin does not affect central levels of amyloid-beta. Brain. 137: 553–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Walker J. R., Pacoma R., Watson J., Ou W., Alves J., Mason D. E., Peters E. C., Urbina H. D., Welzel G., Althage A., et al. 2013. Enhanced proteolytic clearance of plasma Abeta by peripherally administered neprilysin does not result in reduced levels of brain Abeta in mice. J. Neurosci. 33: 2457–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bowman G. L., Kaye J. A., Moore M., Waichunas D., Carlson N. E., and Quinn J. F.. 2007. Blood–brain barrier impairment in Alzheimer disease: stability and functional significance. Neurology. 68: 1809–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Verghese P. B., Castellano J. M., Garai K., Wang Y., Jiang H., Shah A., Bu G., Frieden C., and Holtzman D. M.. 2013. ApoE influences amyloid-beta (Abeta) clearance despite minimal apoE/Abeta association in physiological conditions. Proc. Natl. Acad. Sci. USA. 110: E1807–E1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kitazawa M., Hsu H. W., and Medeiros R.. 2016. Copper exposure perturbs brain inflammatory responses and impairs clearance of amyloid-beta. Toxicol. Sci. 152: 194–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wilhelmus M. M. M., Otte-Höller I., van Triel J. J. J., Veerhuis R., Maat-Schieman M. L. C., Bu G., de Waal R. M. W., and Verbeek M. M.. 2007. Lipoprotein receptor-related protein-1 mediates amyloid-β-mediated cell death of cerebrovascular cells. Am. J. Pathol. 171: 1989–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gu H., Zhong Z., Jiang W., Du E., Dodel R., Liu J., Farlow M. R., Zheng W., and Du Y.. 2014. The role of choroid plexus in IVIG-induced beta-amyloid clearance. Neuroscience. 270: 168–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kanekiyo T., Xu H., and Bu G.. 2014. ApoE and Abeta in Alzheimer’s disease: accidental encounters or partners? Neuron. 81: 740–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zerbinatti C. V., Wozniak D. F., Cirrito J., Cam J. A., Osaka H., Bales K. R., Zhuo M., Paul S. M., Holtzman D. M., and Bu G.. 2004. Increased soluble amyloid-beta peptide and memory deficits in amyloid model mice overexpressing the low-density lipoprotein receptor-related protein. Proc. Natl. Acad. Sci. USA. 101: 1075–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zerbinatti C. V., Wahrle S. E., Kim H., Cam J. A., Bales K., Paul S. M., Holtzman D. M., and Bu G.. 2006. Apolipoprotein E and low density lipoprotein receptor-related protein facilitate intraneuronal Abeta42 accumulation in amyloid model mice. J. Biol. Chem. 281: 36180–36186. [DOI] [PubMed] [Google Scholar]
- 112.Nixon R. A., and Cataldo A. M.. 2006. Lysosomal system pathways: genes to neurodegeneration in Alzheimer’s disease. J. Alzheimers Dis. 9: 277–289. [DOI] [PubMed] [Google Scholar]
- 113.Hu X., Crick S. L., Bu G., Frieden C., Pappu R. V., and Lee J. M.. 2009. Amyloid seeds formed by cellular uptake, concentration, and aggregation of the amyloid-beta peptide. Proc. Natl. Acad. Sci. USA. 106: 20324–20329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Qiu W. Q., Walsh D. M., Ye Z., Vekrellis K., Zhang J., Podlisny M. B., Rosner M. R., Safavi A., Hersh L. B., and Selkoe D. J.. 1998. Insulin-degrading enzyme regulates extracellular levels of amyloid β-protein by degradation. J. Biol. Chem. 273: 32730–32738. [DOI] [PubMed] [Google Scholar]
- 115.Van Uden E., Sagara Y., Van Uden J., Orlando R., Mallory M., Rockenstein E., and Masliah E.. 2000. A protective role of the low density lipoprotein receptor-related protein against amyloid β-protein toxicity. J. Biol. Chem. 275: 30525–30530. [DOI] [PubMed] [Google Scholar]
- 116.Rushworth J. V., Griffiths H. H., Watt N. T., and Hooper N. M.. 2013. Prion protein-mediated toxicity of amyloid-beta oligomers requires lipid rafts and the transmembrane LRP1. J. Biol. Chem. 288: 8935–8951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shinohara M., Petersen R. C., Dickson D. W., and Bu G.. 2013. Brain regional correlation of amyloid-beta with synapses and apolipoprotein E in non-demented individuals: potential mechanisms underlying regional vulnerability to amyloid-beta accumulation. Acta Neuropathol. 125: 535–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liu Q., Trotter J., Zhang J., Peters M. M., Cheng H., Bao J., Han X., Weeber E. J., and Bu G.. 2010. Neuronal LRP1 knockout in adult mice leads to impaired brain lipid metabolism and progressive, age-dependent synapse loss and neurodegeneration. J. Neurosci. 30: 17068–17078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nakajima C., Kulik A., Frotscher M., Herz J., Schafer M., Bock H. H., and May P.. 2013. Low density lipoprotein receptor-related protein 1 (LRP1) modulates N-methyl-D-aspartate (NMDA) receptor-dependent intracellular signaling and NMDA-induced regulation of postsynaptic protein complexes. J. Biol. Chem. 288: 21909–21923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gan M., Jiang P., McLean P., Kanekiyo T., and Bu G.. 2014. Low-density lipoprotein receptor-related protein 1 (LRP1) regulates the stability and function of GluA1 alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptor in neurons. PLoS One. 9: e113237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu C. C., Hu J., Tsai C. W., Yue M., Melrose H. L., Kanekiyo T., and Bu G.. 2015. Neuronal LRP1 regulates glucose metabolism and insulin signaling in the brain. J. Neurosci. 35: 5851–5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fuentealba R. A., Liu Q., Kanekiyo T., Zhang J., and Bu G.. 2009. Low density lipoprotein receptor-related protein 1 promotes anti-apoptotic signaling in neurons by activating Akt survival pathway. J. Biol. Chem. 284: 34045–34053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cheng H., Wang M., Li J-L., Cairns N. J., and Han X.. 2013. Specific changes of sulfatide levels in individuals with pre-clinical Alzheimer’s disease: an early event in disease pathogenesis. J. Neurochem. 127: 733–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lin C. H., Huang Y. J., Lin C. J., Lane H. Y., and Tsai G. E.. 2014. NMDA neurotransmission dysfunction in mild cognitive impairment and Alzheimer’s disease. Curr. Pharm. Des. 20: 5169–5179. [DOI] [PubMed] [Google Scholar]
- 125.Sato N., and Morishita R.. 2014. Brain alterations and clinical symptoms of dementia in diabetes: abeta/tau-dependent and independent mechanisms. Front. Endocrinol. (Lausanne). 5: 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Marwarha G., and Ghribi O.. 2012. Leptin signaling and Alzheimer’s disease. Am. J. Neurodegener. Dis. 1: 245–265. [PMC free article] [PubMed] [Google Scholar]
- 127.Ballatore C., Lee V. M. Y., and Trojanowski J. Q.. 2007. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat. Rev. Neurosci. 8: 663–672. [DOI] [PubMed] [Google Scholar]
- 128.Bales K. R., Liu F., Wu S., Lin S., Koger D., DeLong C., Hansen J. C., Sullivan P. M., and Paul S. M.. 2009. Human APOE isoform-dependent effects on brain beta-amyloid levels in PDAPP transgenic mice. J. Neurosci. 29: 6771–6779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liu C. C., Kanekiyo T., Xu H., and Bu G.. 2013. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat. Rev. Neurol. 9: 106–118. [Erratum. 2013. Nat. Rev. Neurol.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Berlau D. J., Corrada M. M., Head E., and Kawas C. H.. 2009. APOE epsilon2 is associated with intact cognition but increased Alzheimer pathology in the oldest old. Neurology. 72: 829–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Andrews-Zwilling Y., Bien-Ly N., Xu Q., Li G., Bernardo A., Yoon S. Y., Zwilling D., Yan T. X., Chen L., and Huang Y.. 2010. Apolipoprotein E4 causes age- and Tau-dependent impairment of GABAergic interneurons, leading to learning and memory deficits in mice. J. Neurosci. 30: 13707–13717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rodriguez G. A., Burns M. P., Weeber E. J., and Rebeck G. W.. 2013. Young APOE4 targeted replacement mice exhibit poor spatial learning and memory, with reduced dendritic spine density in the medial entorhinal cortex. Learn. Mem. 20: 256–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shinohara M., Kanekiyo T., Yang L., Linthicum D., Fu Y., Price L., Frisch-Daiello J. L., Han X., Fryer J. D., and Bu G.. 2016. APOE2 eases cognitive decline during aging: clinical and preclinical evaluations. Ann. Neurol. 2: 24628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jordán J., Galindo M. F., Miller R. J., Reardon C. A., Getz G. S., and LaDu M. J.. 1998. Isoform-specific effect of apolipoprotein E on cell survival and β-amyloid-induced toxicity in rat hippocampal pyramidal neuronal cultures. J. Neurosci. 18: 195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yang D. S., Small D. H., Seydel U., Smith J. D., Hallmayer J., Gandy S. E., and Martins R. N.. 1999. Apolipoprotein E promotes the binding and uptake of β-amyloid into Chinese hamster ovary cells in an isoform-specific manner. Neuroscience. 90: 1217–1226. [DOI] [PubMed] [Google Scholar]
- 136.Gylys K. H., Fein J. A., Tan A. M., and Cole G. M.. 2003. Apolipoprotein E enhances uptake of soluble but not aggregated amyloid-beta protein into synaptic terminals. J. Neurochem. 84: 1442–1451. [DOI] [PubMed] [Google Scholar]
- 137.Koistinaho M., Lin S., Wu X., Esterman M., Koger D., Hanson J., Higgs R., Liu F., Malkani S., Bales K. R., et al. 2004. Apolipoprotein E promotes astrocyte colocalization and degradation of deposited amyloid-beta peptides. Nat. Med. 10: 719–726. [DOI] [PubMed] [Google Scholar]
- 138.Deane R., Sagare A., Hamm K., Parisi M., Lane S., Finn M. B., Holtzman D. M., and Zlokovic B. V.. 2008. apoE isoform-specific disruption of amyloid beta peptide clearance from mouse brain. J. Clin. Invest. 118: 4002–4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ito S., Ohtsuki S., Kamiie J., Nezu Y., and Terasaki T.. 2007. Cerebral clearance of human amyloid-beta peptide (1–40) across the blood-brain barrier is reduced by self-aggregation and formation of low-density lipoprotein receptor-related protein-1 ligand complexes. J. Neurochem. 103: 2482–2490. [DOI] [PubMed] [Google Scholar]
- 140.Rolyan H., Feike A. C., Upadhaya A. R., Waha A., Van Dooren T., Haass C., Birkenmeier G., Pietrzik C. U., Van Leuven F., and Thal D. R.. 2011. Amyloid-beta protein modulates the perivascular clearance of neuronal apolipoprotein E in mouse models of Alzheimer’s disease. J. Neural Transm. 118: 699–712. [DOI] [PubMed] [Google Scholar]
- 141.Ruzali W. A., Kehoe P. G., and Love S.. 2013. Influence of LRP-1 and apolipoprotein E on amyloid-beta uptake and toxicity to cerebrovascular smooth muscle cells. J. Alzheimers Dis. 33: 95–110. [DOI] [PubMed] [Google Scholar]
- 142.Gilat-Frenkel M., Boehm-Cagan A., Liraz O., Xian X., Herz J., and Michaelson D. M.. 2014. Involvement of the Apoer2 and Lrp1 receptors in mediating the pathological effects of ApoE4 in vivo. Curr. Alzheimer Res. 11: 549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ye S., Huang Y., Mullendorff K., Dong L., Giedt G., Meng E. C., Cohen F. E., Kuntz I. D., Weisgraber K. H., and Mahley R. W.. 2005. Apolipoprotein (apo) E4 enhances amyloid beta peptide production in cultured neuronal cells: apoE structure as a potential therapeutic target. Proc. Natl. Acad. Sci. USA. 102: 18700–18705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Holtzman D. M., Pitas R. E., Kilbridge J., Nathan B., Mahley R. W., Bu G., and Schwartz A. L.. 1995. Low density lipoprotein receptor-related protein mediates apolipoprotein E-dependent neurite outgrowth in a central nervous system-derived neuronal cell line. Proc. Natl. Acad. Sci. USA. 92: 9480–9484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bellosta S., Nathan B. P., Orth M., Dong L. M., Mahley R. W., and Pitas R. E.. 1995. Stable expression and secretion of apolipoproteins E3 and E4 in mouse neuroblastoma cells produces differential effects on neurite outgrowth. J. Biol. Chem. 270: 27063–27071. [DOI] [PubMed] [Google Scholar]
- 146.Fagan A. M., Bu G., Sun Y., Daugherty A., and Holtzman D. M.. 1996. Apolipoprotein E-containing high density lipoprotein promotes neurite outgrowth and is a ligand for the low density lipoprotein receptor-related protein. J. Biol. Chem. 271: 30121–30125. [DOI] [PubMed] [Google Scholar]
- 147.Nathan B. P., Jiang Y., Wong G. K., Shen F., Brewer G. J., and Struble R. G.. 2002. Apolipoprotein E4 inhibits, and apolipoprotein E3 promotes neurite outgrowth in cultured adult mouse cortical neurons through the low-density lipoprotein receptor-related protein. Brain Res. 928: 96–105. [DOI] [PubMed] [Google Scholar]
- 148.Mauch D. H., Nagler K., Schumacher S., Goritz C., Muller E. C., Otto A., and Pfrieger F. W.. 2001. CNS synaptogenesis promoted by glia-derived cholesterol. Science. 294: 1354–1357. [DOI] [PubMed] [Google Scholar]
- 149.Hayashi H., Campenot R. B., Vance D. E., and Vance J. E.. 2004. Glial lipoproteins stimulate axon growth of central nervous system neurons in compartmented cultures. J. Biol. Chem. 279: 14009–14015. [DOI] [PubMed] [Google Scholar]
- 150.Tachibana M., Shinohara M., Yamazaki Y., Liu C. C., Rogers J., Bu G., and Kanekiyo T.. 2016. Rescuing effects of RXR agonist bexarotene on aging-related synapse loss depend on neuronal LRP1. Exp. Neurol. 277: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Qiu Z., Hyman B. T., and Rebeck G. W.. 2004. Apolipoprotein E receptors mediate neurite outgrowth through activation of p44/42 mitogen-activated protein kinase in primary neurons. J. Biol. Chem. 279: 34948–34956. [DOI] [PubMed] [Google Scholar]
- 152.Hayashi H., Campenot R. B., Vance D. E., and Vance J. E.. 2007. Apolipoprotein E-containing lipoproteins protect neurons from apoptosis via a signaling pathway involving low-density lipoprotein receptor-related protein-1. J. Neurosci. 27: 1933–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sen A., Alkon D. L., and Nelson T. J.. 2012. Apolipoprotein E3 (ApoE3) but not ApoE4 protects against synaptic loss through increased expression of protein kinase C epsilon. J. Biol. Chem. 287: 15947–15958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sen A., Nelson T. J., and Alkon D. L.. 2015. ApoE4 and Abeta oligomers reduce BDNF expression via HDAC nuclear translocation. J. Neurosci. 35: 7538–7551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bell R. D., Winkler E. A., Singh I., Sagare A. P., Deane R., Wu Z., Holtzman D. M., Betsholtz C., Armulik A., Sallstrom J., et al. 2012. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature. 485: 512–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Nishitsuji K., Hosono T., Nakamura T., Bu G., and Michikawa M.. 2011. Apolipoprotein E regulates the integrity of tight junctions in an isoform-dependent manner in an in vitro blood-brain barrier model. J. Biol. Chem. 286: 17536–17542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Casey C. S., Atagi Y., Yamazaki Y., Shinohara M., Tachibana M., Fu Y., Bu G., and Kanekiyo T.. 2015. Apolipoprotein E inhibits cerebrovascular pericyte mobility through a RhoA protein-mediated pathway. J. Biol. Chem. 290: 14208–14217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lazarov O., Robinson J., Tang Y. P., Hairston I. S., Korade-Mirnics Z., Lee V. M., Hersh L. B., Sapolsky R. M., Mirnics K., and Sisodia S. S.. 2005. Environmental enrichment reduces Abeta levels and amyloid deposition in transgenic mice. Cell. 120: 701–713. [DOI] [PubMed] [Google Scholar]
- 159.Jankowsky J. L., Melnikova T., Fadale D. J., Xu G. M., Slunt H. H., Gonzales V., Younkin L. H., Younkin S. G., Borchelt D. R., and Savonenko A. V.. 2005. Environmental enrichment mitigates cognitive deficits in a mouse model of Alzheimer’s disease. J. Neurosci. 25: 5217–5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Deane R., Du Yan S., Submamaryan R. K., LaRue B., Jovanovic S., Hogg E., Welch D., Manness L., Lin C., Yu J., et al. 2003. RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat. Med. 9: 907–913. [DOI] [PubMed] [Google Scholar]
- 161.Chishti M. A., Yang D. S., Janus C., Phinney A. L., Horne P., Pearson J., Strome R., Zuker N., Loukides J., French J., et al. 2001. Early-onset amyloid deposition and cognitive deficits in transgenic mice expressing a double mutant form of amyloid precursor protein 695. J. Biol. Chem. 276: 21562–21570. [DOI] [PubMed] [Google Scholar]
- 162.Herring A., Yasin H., Ambree O., Sachser N., Paulus W., and Keyvani K.. 2008. Environmental enrichment counteracts Alzheimer’s neurovascular dysfunction in TgCRND8 mice. Brain Pathol. 18: 32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wang X., Miao Y., Abulizi J., Li F., Mo Y., Xue W., and Li Z.. 2016. Improvement of electroacupuncture on APP/PS1 transgenic mice in spatial learning and memory probably due to expression of Abeta and LRP1 in hippocampus. Evid. Based Complement. Alternat. Med. 2016: 7603975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lin T. W., Shih Y. H., Chen S. J., Lien C. H., Chang C. Y., Huang T. Y., Chen S. H., Jen C. J., and Kuo Y. M.. 2015. Running exercise delays neurodegeneration in amygdala and hippocampus of Alzheimer’s disease (APP/PS1) transgenic mice. Neurobiol. Learn. Mem. 118: 189–197. [DOI] [PubMed] [Google Scholar]
- 165.Moore K. M., Girens R. E., Larson S. K., Jones M. R., Restivo J. L., Holtzman D. M., Cirrito J. R., Yuede C. M., Zimmerman S. D., and Timson B. F.. 2016. A spectrum of exercise training reduces soluble Abeta in a dose-dependent manner in a mouse model of Alzheimer’s disease. Neurobiol. Dis. 85: 218–224. [DOI] [PubMed] [Google Scholar]
- 166.Shinohara M., Sato N., Shimamura M., Kurinami H., Hamasaki T., Chatterjee A., Rakugi H., and Morishita R.. 2014. Possible modification of Alzheimer’s disease by statins in midlife: interactions with genetic and non-genetic risk factors. Front. Aging Neurosci. 6: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Shinohara M., Sato N., Kurinami H., Takeuchi D., Takeda S., Shimamura M., Yamashita T., Uchiyama Y., Rakugi H., and Morishita R.. 2010. Reduction of brain beta-amyloid (Abeta) by fluvastatin, a hydroxymethylglutaryl-CoA reductase inhibitor, through increase in degradation of amyloid precursor protein C-terminal fragments (APP-CTFs) and Abeta clearance. J. Biol. Chem. 285: 22091–22102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Davis J., Xu F., Deane R., Romanov G., Previti M. L., Zeigler K., Zlokovic B. V., and Van Nostrand W. E.. 2004. Early-onset and robust cerebral microvascular accumulation of amyloid beta-protein in transgenic mice expressing low levels of a vasculotropic Dutch/Iowa mutant form of amyloid beta-protein precursor. J. Biol. Chem. 279: 20296–20306. [DOI] [PubMed] [Google Scholar]
- 169.Abuznait A. H., Qosa H., Busnena B. A., El Sayed K. A., and Kaddoumi A.. 2013. Olive-oil-derived oleocanthal enhances beta-amyloid clearance as a potential neuroprotective mechanism against Alzheimer’s disease: in vitro and in vivo studies. ACS Chem. Neurosci. 4: 973–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Qosa H., Batarseh Y. S., Mohyeldin M. M., El Sayed K. A., Keller J. N., and Kaddoumi A.. 2015. Oleocanthal enhances amyloid-beta clearance from the brains of TgSwDI mice and in vitro across a human blood-brain barrier model. ACS Chem. Neurosci. 6: 1849–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bachmeier C., Beaulieu-Abdelahad D., Mullan M., and Paris D.. 2013. Role of the cannabinoid system in the transit of beta-amyloid across the blood-brain barrier. Mol. Cell. Neurosci. 56: 255–262. [DOI] [PubMed] [Google Scholar]
- 172.Zhu X., Lee H. G., Perry G., and Smith M. A.. 2007. Alzheimer disease, the two-hit hypothesis: an update. Biochim. Biophys. Acta. 1772: 494–502. [DOI] [PubMed] [Google Scholar]
- 173.Bennett S., Grant M. M., and Aldred S.. 2009. Oxidative stress in vascular dementia and Alzheimer’s disease: a common pathology. J. Alzheimers Dis. 17: 245–257. [DOI] [PubMed] [Google Scholar]
- 174.Heneka M. T., O’Banion M. K., Terwel D., and Kummer M. P.. 2010. Neuroinflammatory processes in Alzheimer’s disease. J. Neural Transm. (Vienna). 117: 919–947. [DOI] [PubMed] [Google Scholar]
- 175.Malik M., Parikh I., Vasquez J. B., Smith C., Tai L., Bu G., LaDu M. J., Fardo D. W., Rebeck G. W., and Estus S.. 2015. Genetics ignite focus on microglial inflammation in Alzheimer’s disease. Mol. Neurodegener. 10: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Erickson M. A., Hansen K., and Banks W. A.. 2012. Inflammation-induced dysfunction of the low-density lipoprotein receptor-related protein-1 at the blood-brain barrier: protection by the antioxidant N-acetylcysteine. Brain Behav. Immun. 26: 1085–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Shackleton B., Crawford F., and Bachmeier C.. 2016. Inhibition of ADAM10 promotes the clearance of Aβ across the BBB by reducing LRP1 ectodomain shedding. Fluids Barriers CNS. 13: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Moon J. H., Kang S. B., Park J. S., Lee B. W., Kang E. S., Ahn C. W., Lee H. C., and Cha B. S.. 2011. Up-regulation of hepatic low-density lipoprotein receptor-related protein 1: a possible novel mechanism of antiatherogenic activity of hydroxymethylglutaryl-coenzyme A reductase inhibitor Atorvastatin and hepatic LRP1 expression. Metabolism. 60: 930–940. [DOI] [PubMed] [Google Scholar]
- 179.Strickland D. K., Ashcom J. D., Williams S., Burgess W. H., Migliorini M., and Argraves W. S.. 1990. Sequence identity between the alpha 2-macroglobulin receptor and low density lipoprotein receptor-related protein suggests that this molecule is a multifunctional receptor. J. Biol. Chem. 265: 17401–17404. [PubMed] [Google Scholar]
- 180.Thal D. R., Schober R., and Birkenmeier G.. 1997. The subunits of alpha2-macroglobulin receptor/low density lipoprotein receptor-related protein, native and transformed alpha2-macroglobulin and interleukin 6 in Alzheimer’s disease. Brain Res. 777: 223–227. [DOI] [PubMed] [Google Scholar]
- 181.Czekay R. P., Orlando R. A., Woodward L., Adamson E. D., and Farquhar M. G.. 1995. The expression of megalin (gp330) and LRP diverges during F9 cell differentiation. J. Cell Sci. 108: 1433–1441. [DOI] [PubMed] [Google Scholar]
- 182.Kounnas M. Z., Morris R. E., Thompson M. R., FitzGerald D. J., Strickland D. K., and Saelinger C. B.. 1992. The alpha 2-macroglobulin receptor/low density lipoprotein receptor-related protein binds and internalizes Pseudomonas exotoxin A. J. Biol. Chem. 267: 12420–12423. [PubMed] [Google Scholar]
- 183.Pietrzik C. U., Busse T., Merriam D. E., Weggen S., and Koo E. H.. 2002. The cytoplasmic domain of the LDL receptor-related protein regulates multiple steps in APP processing. EMBO J. 21: 5691–5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Provias J., and Jeynes B.. 2014. The role of the blood-brain barrier in the pathogenesis of senile plaques in Alzheimer’s disease. Int. J. Alzheimers Dis. 2014: 191863. [DOI] [PMC free article] [PubMed] [Google Scholar]