Abstract
Objective
To examine motor unit characteristics (size and firing rate) associated with aging.
Design
Cross-sectional, observational.
Setting
Community.
Participants
Baltimore Longitudinal Study of Aging participants (N = 102), aged 22.2 to 94.1 years, were studied.
Interventions
Not applicable.
Main Outcome Measures
Surface-represented motor unit size and firing rate were collected from the vastus medialis during knee extension at 10%, 20%, 30%, and 50% of each subject’s maximum isometric voluntary contraction (MVC).
Results
MVC declined with older age (P <.0001). Adjusting for differences in MVC, both firing rate and motor unit size per newton force generated began to increase in the 6th decade of life. Motor unit size increased per newton force to a greater extent than firing rate. Those over the age of 75 years also activated significantly larger motor units per unit force (P = .04). Relative to force generated, the average firing rate began increasing at 57.8 ± 3.4 years and between 50.2 and 56.4 years (±4y) for motor unit size.
Conclusions
The size of motor units and firing rates used to achieve a given force changes with age, particularly after middle age. Whether these changes precede, follow, or occur concurrent to age-related modifications in muscle structure and contractile properties or sarcopenia is not known.
Keywords: Aging, Muscles, Rehabilitation
Loss of muscle mass and strength in late life1 contribute to increased disability2,3 and also increase the risk of mortality in older persons.4,5 This age-related loss begins by the 4th or 5th decade and is accompanied by a decline in muscle power, movement speed, and reaction times.6,7 Age-associated changes in the peripheral nerve have also been described8,9 and together with independently observed muscle composition changes10,11 may contribute to the process that leads to sarcopenia. Furthermore, how these functional and anatomic changes relate to motor unit control and muscle force generation have not been well studied.
We previously applied a technique to elucidate motor unit activation characteristics of the vastus medialis muscle through EMG signals simultaneously acquired through a surface electrode sEMG and an intramuscular needle electrode during submaximal, progressive muscle activation during knee extension. In these studies, we observed the orderly recruitment of motor units during forceful contraction, with a subsequent increase in firing rate at higher contraction levels (over 30% MVC).12–18 The current study sought to determine whether motor unit size and firing rate change with aging during submaximal isometric muscle contractions. This information may provide insight into the neuronal contributions to age-associated sarcopenia.
METHODS
Design
The BLSA is a study of normative human aging active since 1958. This study was conducted in accordance with the ethical principles embodied in the Declaration of Helsinki.
Subjects
Since its inception, more than 3000 volunteers have participated in the BLSA. A subset of 102 BLSA volunteers eligible for this study consented to participate coincidently with their regularly scheduled BLSA visit between 1997 and 2002. Eligible participants were representative of the adult lifespan and capable of safely participating in the testing protocol.
Measures
Strength measurement
All strength measurements were performed on a Kin-Com 125E dynamometera that was externally calibrated before each measurement by hanging known weights from the load cell. Before testing, subjects were introduced to machinery and coached through a single practice trial before the measurement trials and engaged in a 3-minute warm-up performed by using a bicycle ergometer. Testing followed a standardized protocol12,15 developed in keeping with exercise physiology approach to studying force development. The maximal isometric contractions were performed with a 10-second rest period between each effort. The average of the two best trials was used as MVC. We did not observe a difference regardless of which strength representation we used in the analysis, the average of the 2 best trials, the average of all trials, and the best of the 3 trials.
Motor unit measurements
The methods for assessing motor units are described in detail by Conwit et al.12,13,15 For these studies, the compound muscle action potential was obtained by supramaximal stimulation of the femoral nerve and recording over the VM muscle. To obtain the largest amplitude response with the shortest rise time, the active surface electrode was positioned over the motor point. A concentric needle electrode was inserted into the VM near the active surface electrode and positioned to maximize the rise time of S-MUAP generated during low level contractions. Subjects increased the level of isometric contraction up to a specified percentage of MVC and maintained that level for 20 to 30 seconds during which time the surface and needle EMG signals were collected. Generally, from 1 to 5 S-MUAPs could be reliably recorded with each contraction. The position of the intramuscular electrode was adjusted and additional contractions performed until at least 15 high-quality S-MUAPs had been collected at each effort level at 10%, 20%, 30%, and 50% relative to each subject’s maximum effort (% effort). During the contractions, subjects were provided with visual feedback of the level of force generated. The simultaneously detected concentric needle and S-EMG signals acquired during each contraction used bandpasses of 10 Hz to 10 kHz and 5 Hz to 1000 Hz and sampling rates of 25 kHz and 2.5 kHz, respectively. The concentric needle EMG signal was decomposed by using algorithms developed by Stashuk.19 Decomposition of the composite electrical signal detected with the intramuscular electrode made it possible to distinguish between the electrical signals generated by different motor units, from which the mean motor unit firing rates could be derived.20 In addition, the intramuscularly detected signals generated by separate motor units were used to derive the sizes and shapes of the associated S-MUAPs by using spike-triggered averaging.19 The area (an estimate of motor unit size) of the S-MUAP and the mFR of the motor unit were calculated for each of the approximately 15 motor units sampled.
The data-collection protocol used was designed to elucidate the properties of active motor units sampled at fixed force levels that are within the range normally used for usual activities. By using this method, we previously showed that sampling 15 motor units at force levels corresponding to 10% and 20% of isometric knee extensor maximal voluntary contraction gave a test-retest coefficient of variation of approximately 10%, with test-retest correlations between trials above 0.65 for most comparisons.12
Dual-photon x-ray absorptiometry
Body fatness was assessed by dual-photon x-ray absorptiometry on a Prodigy scanner with body composition software.b
Data Analysis
Data were analyzed to assess motor unit properties of those units that were sampled under the assumption that they were a random sample from all active units within the muscle.12 Subject characteristics and univariate analyses are presented as means and standard deviations. Mixed-effects models were constructed to analyze motor unit characteristics in relationship to force and force2 with a random effect for subject and force. Height and weight were added to the model but subsequently removed because they did not significantly contribute to the fit. Data were also analyzed by using the S-MUAP area and motor unit firing rates relative to MVC (N). Plots were created to show the analytic models for S-MUAP area and motor unit firing rates per unit force (MVC [N]) by percent effort. All S-MUAP area/force and motor unit firing rates/force measurements across all force levels were plotted against age with a smoothing regression line.
RESULTS
The characteristics of the subjects are summarized by age group in table 1. Participants were 22.2 to 94.1 years of age. Body weight and body mass index were significantly lower in older age groups (P <.05). Mean strength (MVC) was highest in the less than 40 years of age group and decreased progressively in older age groups with the 75 year and older age group exhibiting the lowest MVC (P <.0001).
Table 1.
Participant Characteristics
| Variable | 20–39 Years (n 15) |
40–59 Years (n 34) |
60–75 Years (n 30) |
75 Years (n 23) |
P |
|---|---|---|---|---|---|
| N (% women) | 6 (40) | 13 (38) | 10 (33) | 8 (35) | 0.960 |
| Weight | 80.1 (14.9) | 81.4 (14.1) | 80.2 (12.7) | 68.3 (13.2) | 0.003 |
| Height | 175.3 (9.2) | 172.0 (10.3) | 172.2 (7.7) | 168.1 (11.5) | 0.160 |
| BMI | 25.9 (3.3) | 27.5 (3.7) | 26.9 (3.2) | 24.1 (3.4) | 0.003 |
| % Body fat | 30.9 (8.5) | 32.1 (6.0) | 34.3 (6.1) | 30.3 (8.2) | 0.300 |
| MVC (N) | 605 (203) | 586 (158) | 470 (119) | 331 (94) | 0.000 |
NOTE: Values are mean ±SD or as otherwise indicated.
Abbreviation: BMI, body mass index.
Age-associated changes in the ratio of S-MUAP area to force (N) as an indicator of activated motor unit size relative to the force being generated during knee extension at the specified effort levels are shown in figure 1. The average S-MUAP area of motor units activated per unit force for the 4 study groups was significantly larger in the 75 year and older age groups across all effort levels compared with the less than 40 years of age group (P = .04), whereas the age groups younger than age 75 years were quite similar. In addition, the S-MUAP area/force relationship changed differently in all groups as the percent effort increased. In contrast to the less than 40 years of age group that maintained motor unit size to force ratio across effort levels, motor unit size per unit force decreased as effort increased for all other age groups (P <.0001).
Fig 1.
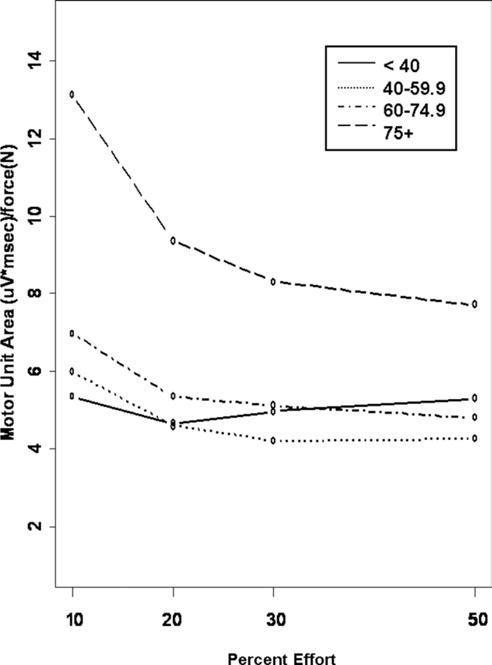
Effort-associated changes in average S-MUAP area per unit force by age group. The age groups are depicted as follows: less than 40-year-old group (solid line), 40- to 59-year-old group (dashed line), 60- to 75-year old group (dotted line), and greater than 75-year-old group (dashed and dotted line).
The motor unit firing rates to force (N) ratio, an indicator of active motor units during knee extension at specified effort levels relative to MVC, was higher in the 60- to 75-year-old and 75 year and older groups but not the 40- to 60-year-old group compared with the less than 40 years of age group across all effort levels (fig 2) (P <.01). The motor unit firing rates to force ratio changed differently as percent effort increased in the 60- to 75-year-old and 75 year and older groups compared with the less than 40 years of age group (P <.0001).
Fig 2.
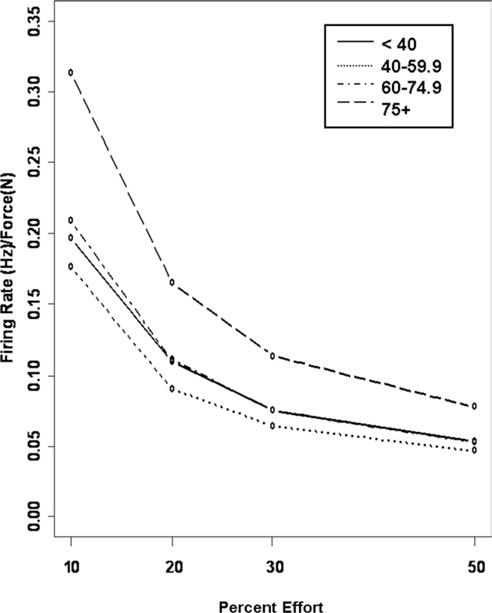
Effort-associated changes in (mFR) by age group. The age groups are depicted as follows: less than 40-year-old group (solid line), 40- to 59-year-old group (dashed line), 60- to 75-year-old group (dotted line), and greater than 75-year-old group (dashed and dotted line).
DISCUSSION
This study shows that age is associated with an increase in the size of the motor unit and a decline in motor unit firing rate during sustained quadriceps muscle activation at effort levels relevant to daily mobility tasks. These changes were particularly notable in the oldest subjects who used larger units per unit force and higher firing rates per unit force than younger subjects at low force levels (10% of MVC), whereas no discernable difference was observed in middle age. These observations suggest that the altered motor unit activation appears later in life than the age at which sarcopenia develops.
Age-related strength decreases have been reported as beginning by the 4th or 5th decade. Consistent with this, we observed that maximum knee extensor strength was progressively lower from the youngest to oldest age groups. The rationale for reporting motor unit activation properties relative to force generated is to take into consideration the changes in force known to occur with aging. By examining muscle motor unit activation using data that were normalized by strength and percent effort, we showed that, compared with younger adults, older adults use different motor unit activation strategies to generate the same amount of force during knee extension and also showed that these changes were most prominent at older ages. Our observation of larger motor units associated with older age is consistent with that reported by McComas.18 In contrast, Kamen and Knight’s observations of lower motor unit discharge rates in older compared with younger individuals21,22 refute our report, the discordance of which might be explained by methodologic differences between the 2 studies. Specifically, we report motor unit firing rates averaged over the entire contraction at lower force levels in contrast to Kamen and Knight’s observations of individual motor unit discharge rates at 50% and 100% MVC by using intramuscular needle electrodes.
A critical question is whether the age-associated changes in motor unit activation observed in this study play a causal role in the anatomic and functional changes in muscle with age or merely represents mechanisms of compensation and adaptation aimed at conserving musculoskeletal performance. The pattern of motor unit activation observed in the youngest age group, with recruitment of progressively larger units and increases in firing rate at higher force levels, is consistent with the Henneman’s size principle.23 As more motor units are recruited and/or as motor unit firing rates increase, the force of contraction increases. In contrast, in older adults, motor unit size and firing rate are already higher at low force levels with a progressive recruitment of larger units as force increases.
Study Limitations
The following limitations are acknowledged. The first limitation relates to the study sample. The relatively small sample size limits our ability to detect subtle differences in motor unit activation between age groups. Also, by considering the less than 40-year-old individuals as the comparison group, we were unable to delineate events that might precede the development of sarcopenia. The second limitation relates to the testing paradigm used. We acknowledge that we are studying one of the muscles that contribute to knee extension force and assume that the muscle studied (vastus medialis) contributes uniformly to knee force generation across individuals and also over time. Additionally, although participants were provided a visual representation of the target force and were coached to maintain that target force to minimize variability and maintain force consistency, the equipment used did not allow simultaneous recording of EMG signals together with force output. Finally, the testing paradigm used does not allow for the evaluation of muscle fatigue. The third limitation is that of the signal decomposition program used. Our previous work15 strongly suggested that at high force levels, the decomposition software aimed at picking up electrical signals associated with the smallest and lowest threshold motor units became less reliable. Thus, more low-threshold motor units appeared to drop out as the intensity of the contraction increased because it becomes more difficult to extract their signals from the increasingly complex interference pattern at the detection site. Additionally, the EMG signal decomposition method provides mean values of motor unit firing rates over the epoch providing the best surface representation. We refer to this as mFR (motor unit firing rates), whereas others refer to this as motor unit discharge rates. The motor unit firing rates estimates we observed are consistent with average discharge rates reported by others using different collection and analysis techniques.21,22 Despite this consistency, we were not able to assess firing rate variability for individual motor units as others have.21,22 We acknowledge that changes in motor unit firing rate variability might represent an important physiologic consequence of the aging process that cannot be fully assessed by using current techniques. Finally, although we observed differences in mFR between age groups, the absolute difference between groups was on the order of less than 1Hz and therefore of questionable clinical significance (P <.007).
Despite these limitations, our study has several strengths. This study uniquely shows age-associated changes in motor unit–activation strategies in the context of the force generated by the vastus medialis during knee extension. The data were collected while forces were generated similar to those that are engaged during ambulation and other activities of daily living and were analyzed relative to each subject’s maximum force. This study also yields valuable motor unit activation data in a muscle group that is often affected by late-life chronic diseases and is critical to walking ability and mobility function in general. Finally, it is plausible that the motor unit activation strategies used by older adults might contribute to the lower force generated. The effects of muscle fatigue, muscle compositional changes (ie, fiber type), and physical inactivity on age-associated changes in motor unit–activation properties need to be assessed in future studies. Most importantly, future studies are required to discern whether age-associated changes in motor unit activation might arise from the same process that leads to sarcopenia or alternatively may represent neural compensation for sarcopenia.
CONCLUSIONS
We conclude that motor unit–activation properties change with age and become apparent at an age that is either coincident with or following the age at which strength and muscle mass begin to decline. Whether the observed changes contribute to, follow in response to, or develop coincident to the loss of strength and muscle mass known to occur with aging have yet to be determined.
Acknowledgments
Supported by the Intramural Research Program of the National Institutes of Health, National Institute on Aging.
List of Abbreviations
- BLSA
Baltimore Longitudinal Study of Aging
- EMG
electromyography
- mFR
mean firing rate
- MVC
maximum voluntary contraction
- sEMG
surface electromyography
- S-MUAP
surface-detected motor unit action potential
- VM
vastus medialis
Footnotes
Suppliers
Kin-Com 125E dynamometer; Chattecx, Chattanooga, TN.
General Electric, Lunar, Madison, WI.
No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit on the authors or on any organization with which the authors are associated.
References
- 1.Metter EJ, Lynch N, Conwit R, Lindle R, Tobin J, Hurley B. Muscle quality and age: cross-sectional and longitudinal comparisons. J Gerontol A Biol Sci Med Sci. 1999;54:B207–18. doi: 10.1093/gerona/54.5.b207. [DOI] [PubMed] [Google Scholar]
- 2.Ferrucci L, Penninx BW, Volpato S, et al. Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. J Am Geriatr Soc. 2002;50:1947–54. doi: 10.1046/j.1532-5415.2002.50605.x. [DOI] [PubMed] [Google Scholar]
- 3.Lauretani F, Russo CR, Bandinelli S, et al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol. 2003;95:1851–60. doi: 10.1152/japplphysiol.00246.2003. [DOI] [PubMed] [Google Scholar]
- 4.Metter EJ, Talbot LA, Schrager M, Conwit R. Skeletal muscle strength as a predictor of all-cause mortality in healthy men. J Gerontol A Biol Sci Med Sci. 2002;57:B359–65. doi: 10.1093/gerona/57.10.b359. [DOI] [PubMed] [Google Scholar]
- 5.Metter EJ, Talbot LA, Schrager M, Conwit RA. Arm-cranking muscle power and arm isometric muscle strength are independent predictors of all-cause mortality in men. J Appl Physiol. 2004;96:814–21. doi: 10.1152/japplphysiol.00370.2003. [DOI] [PubMed] [Google Scholar]
- 6.Metter EJ, Conwit R, Tobin J, Fozard JL. Age-associated loss of power and strength in the upper extremities in women and men. J Gerontol A Biol Sci Med Sci. 1997;52:B267–76. doi: 10.1093/gerona/52a.5.b267. [DOI] [PubMed] [Google Scholar]
- 7.Lynch NA, Metter EJ, Lindle RS, et al. Muscle quality. I. Age-associated differences between arm and leg muscle groups. J Appl Physiol. 1999;86:188–94. doi: 10.1152/jappl.1999.86.1.188. [DOI] [PubMed] [Google Scholar]
- 8.Roubenoff R. Sarcopenia: a major modifiable cause of frailty in the elderly. J Nutr Health Aging. 2000;4:140–2. [PubMed] [Google Scholar]
- 9.Metter EJ, Conwit R, Metter B, Pacheco T, Tobin J. The relationship of peripheral motor nerve conduction velocity to age-associated loss of grip strength. Aging (Milano) 1998;10:471–8. doi: 10.1007/BF03340161. [DOI] [PubMed] [Google Scholar]
- 10.Goodpaster BH, Thaete FL, Kelley DE. Composition of skeletal muscle evaluated with computed tomography. Ann N Y Acad Sci. 2000;904:18–24. doi: 10.1111/j.1749-6632.2000.tb06416.x. [DOI] [PubMed] [Google Scholar]
- 11.Visser M, Goodpaster BH, Kritchevsky SB, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60:324–33. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- 12.Conwit RA, Ling S, Roth S, et al. The relationship between ciliary neurotrophic factor (CNTF) genotype and motor unit physiology: preliminary studies. BMC Physiol. 2005;5:15. doi: 10.1186/1472-6793-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conwit RA, Stashuk D, Tracy B, McHugh M, Brown WF, Metter EJ. The relationship of motor unit size, firing rate and force. Clin Neurophysiol. 1999;110:1270–5. doi: 10.1016/s1388-2457(99)00054-1. [DOI] [PubMed] [Google Scholar]
- 14.Conwit RA, Tracy B, Cowl A, et al. Firing rate analysis using decompostion-enhanced spike triggered averaging in the quadriceps femoris. Muscle Nerve. 1998;21:1338–40. doi: 10.1002/(sici)1097-4598(199810)21:10<1338::aid-mus17>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 15.Conwit RA, Tracy B, Jamison C, et al. Decomposition-enhanced spike-triggered averaging: contraction level effects. Muscle Nerve. 1997;20:976–82. doi: 10.1002/(sici)1097-4598(199708)20:8<976::aid-mus7>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 16.Doherty T, Simmons Z, O’Connell B, et al. Methods for estimating the numbers of motor units in human muscles. J Clin Neurophysiol. 1995;12:565–84. doi: 10.1097/00004691-199511000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Doherty TJ. Effects of short-term training on physiologic properties of human motor units. Can J Appl Physiol. 2000;25:194–203. doi: 10.1139/h00-015. [DOI] [PubMed] [Google Scholar]
- 18.McComas AJ. Motor unit estimation: anxieties and achievements. Muscle Nerve. 1995;18:369–79. doi: 10.1002/mus.880180402. [DOI] [PubMed] [Google Scholar]
- 19.Stashuk DW. Decomposition and quantitative analysis of clinical electromyographic signals. Med Eng Phys. 1999;21:389–404. doi: 10.1016/s1350-4533(99)00064-8. [DOI] [PubMed] [Google Scholar]
- 20.Stashuk D, Qu Y. Robust method for estimating motor unit firing-pattern statistics. Med Biol Eng Comput. 1996;34:50–7. doi: 10.1007/BF02637022. [DOI] [PubMed] [Google Scholar]
- 21.Kamen G. Aging, resistance training, and motor unit discharge behavior. Can J Appl Physiol. 2005;30:341–51. doi: 10.1139/h05-126. [DOI] [PubMed] [Google Scholar]
- 22.Kamen G, Knight CA. Training-related adaptations in motor unit discharge rate in young and older adults. J Gerontol A Biol Sci Med Sci. 2004;59:1334–8. doi: 10.1093/gerona/59.12.1334. [DOI] [PubMed] [Google Scholar]
- 23.Henneman E, Olson CB. Relations between structure and function in the design of skeletal muscles. J Neurophysiol. 1965;28:581–98. doi: 10.1152/jn.1965.28.3.581. [DOI] [PubMed] [Google Scholar]


