Abstract
Background
Individuals with type 2 diabetes (T2D) are at greater risk of bone fractures than those without diabetes. Certain oral diabetic medications may further increase the risk of fracture. Dipeptidyl peptidase-IV (DPP-IV) inhibitors are incretin-based therapies that are being increasingly used for the management of T2D. It has been hypothesized that these agents may reduce fracture risk in those with T2D. In this study, we used a mouse model of T2D to examine the effects of the DPP-IV inhibitor, MK-0626, on bone.
Methods
Male wild type (WT) and diabetic muscle-lysine-arginine (MKR) mice were treated with MK-0626, pioglitazone, alendronate or vehicle. The effects of treatment with MK-0626 on bone microarchitecture and turnover were compared with treatment with pioglitazone, alendronate and vehicle. Osteoblast differentiation was determined by alkaline phosphatase staining of bone marrow cells from WT and MKR mice after treatment with pioglitazone, MK-0626 or phosphate buffered saline.
Results
We found that MK-0626 had neutral effects on cortical and trabecular bone in diabetic mice. Pioglitazone had detrimental effects on the trabecular bone of WT but not of diabetic mice. Alendronate caused improvements in cortical and trabecular bone architecture in diabetic and WT mice. MK-0626 did not alter osteoblast differentiation, but pioglitazone impaired osteoblast differentiation in vitro.
Conclusions
Overall, the DPP-IV inhibitor, MK-0626, had no adverse effects on bone in an animal model of T2D or directly on osteoblasts in culture. These findings are reassuring as DPP-IV inhibitors are being widely used to treat patients with T2D who are already at an increased risk of fractures.
Keywords: diabetes, bone, DPPIV inhibitors, fractures
Introduction
Men and women with type 2 diabetes (T2D) have a greater risk of hip fracture and fracture at other sites (particularly peripheral sites) than those without T2D [1–4]. Although the risk of fracture is increased, many studies report that bone mineral density (BMD) is not necessarily lower in those with T2D who develop fractures, and therefore, BMD does not always reflect bone fragility in individuals with T2D [5–7]. This has led to the opinion that bone quality is the key to determining fracture risk. The cause for the increased fracture risk is still largely unknown, but alterations in insulin-like growth factors, insulin resistance, adipokines and advanced glycation end-products (AGEs), as well as the adverse effects of diabetes medications, such as certain thiazolidinediones, are some of the potential factors that affect bone fragility in those with T2D [8].
Incretin-based therapies have become an important class of agents for the treatment of T2D. After ingestion of a meal, glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) are secreted from the L and K cells of the intestine, respectively. GLP-1 and GIP participate in the regulation of glucose homeostasis by increasing insulin secretion and inhibiting glucagon secretion. The incretin response to an oral mixed meal is impaired in individuals with T2D [9]; therefore, medications to improve the incretin response have been developed. These agents include incretin-mimetics (GLP-1 receptor agonists) and inhibitors of the protease dipeptidyl peptidase-IV (DPP-IV) that inactivates GLP-1 and GIP, along with other peptides such as GLP-2 and Peptide YY. It has been hypothesized that incretin-based therapies could be protective against bone fractures, and indeed, a recent meta-analysis of clinical trials found a reduced risk of fractures in patients taking DPP-IV inhibitors, compared with placebo or other diabetes medications [10]. However, fractures were not a primary end point in any of the studies; they were only reported as adverse events [10]. Saxagliptin has been reported in some studies to be associated with an increased risk of bone fractures, although other studies have shown no effect [11,12]. The DPP-IV inhibitor vildagliptin was not found to alter serum concentrations of cross-linked C-terminal telopeptide in patients with T2D [13]. To date, no large clinical trials have primarily investigated the effect of treatment with DPP-IV inhibitors on fracture rates. Therefore, the effects of DPP-IV inhibitors on bone metabolism and fractures in those with T2D remain uncertain.
There are numerous potential direct and indirect mechanisms through which incretin-based therapies, such as DPP-IV inhibitors, may affect bone. Previous rodent and in vitro studies have reported that GLP-1, GIP, GLP-2 and Peptide YY may all have direct effects on receptors on osteoblasts and/or osteoclasts, resulting in decreased bone resorption [14–21]. GLP-1 also has indirect effects on bone in rodents and increases calcitonin secretion from the thyroid C cells reducing bone resorption, whereas Peptide YY may activate the parasympathetic nervous system through its action on the hypothalamus [14–21]. GLP-1 and GIP have been previously shown to reduce the AGE receptor and may therefore have a protective role against AGEs [22]. AGEs have been implicated as one of the factors that potentially causes the bone quality impairment seen in T2D [22,23]. The protease DPP-IV is expressed on the surface of many cells [24]. DPP-IV was recently found to be expressed by mineralizing osteoblasts in mice and was regulated by the androgen receptor in these cells: inactivation of androgen receptor led to increased expression of DPP-IV among other genes and was associated with increased bone resorption [25]. The reason for this discrepancy remains unexplained. Therefore, DPP-IV inhibitors may have positive or negative effects on bone by directly inhibiting DPP-IV activity on osteoblasts or may exert positive indirect effects on bone by preventing the degradation of GLP-1, GIP, GLP-2 and Peptide YY or reducing the effects of AGEs.
Although, one previous study [26] examined the effects of sitagliptin in the setting of high fat diet-induced obesity and insulin resistance, DPP-IV inhibitors have not been examined in an animal model of T2D, where bone quality is already impaired [27]. In this study, we used the MKR mouse model of T2D. These mice have severe insulin resistance and hyperglycaemia due to over expression of a tyrosine kinase dead insulin-like growth factor-1 receptor in skeletal muscle [28]. The MKR mouse is known to have decreased cortical bone area, bone volume and trabecular number and thickness, associated with increases in osteoclast activity [27]. We hypothesized that the DPP-IV inhibitor MK-0626 would improve bone quality in this mouse model of T2D. In addition, we wished to examine the direct effects of inhibiting DPP-IV on osteoblastogenesis in vitro. We compared the effect of MK-0626 in vivo with alendronate that is reported to reduce fractures in patients with T2D and pioglitazone that has been reported to increase fracture risk in those with T2D [29–31].
Materials and methods
Animals
The generation and characterization of the MKR mice and their bone phenotype have been previously described [27,28]. All animal studies were approved by the Mount Sinai School of Medicine Institutional Animal Care and Use Committee. Mice were housed in The Mount Sinai School of Medicine Center for Comparative Medicine and Surgery, Association for Assessment and Accreditation of Laboratory Animal Care International and Office of Laboratory Animal Welfare accredited facility, where animal care and maintenance were provided. Mice were kept on a 12-h light/dark cycle and had free access to diet and fresh water. All MKR and WT mice used in these studies were male, on the Friend virus b/NIH (FVB/N) background and were 8—16 weeks of age. Mice were injected with calcein (15 mg/kg) 14 and 2 days prior to euthanasia.
Treatments
Wild type and MKR mice were treated from 8 to 16 weeks of age with control diet containing 2.2 IU/g of vitamin D3 (PicoLab Rodent Diet 20, #5053, Brentwood, MO, USA), the DPP-IV inhibitor, MK-0626, provided by Merck Co. (Whitehouse Station, NJ, USA), 4 g/kg supplemented chow or the thiazolidinedione, pioglitazone (Takeda Pharmaceuticals, USA Inc. Deerfield IL, USA), 0.28 g/kg supplemented chow (Research Diets, New Brunswick, NJ, USA) [26] or the bisphosphonate alendronate by intraperitoneal injection (0.04 mg/kg twice weekly) [32,33] or the equivalent volume of phosphate buffered saline (PBS) injection.
Body weights, body composition, blood glucose and plasma dipeptidyl peptidase-IV
Body weights were measured prior to commencing treatment and weekly thereafter. Body composition analysis was performed using the EchoMRI 3-in-1 NMR system (Echo Medical Systems, Houston, TX, USA), at 8, 12 and 16 weeks of age. Fed blood glucose was measured from the tail vein biweekly using a Contour Glucometer (Bayer Healthcare, Tarrytown, NY, USA). The glucose tolerance test was performed after 8 weeks of treatment and an 8-h fast. Mice were injected with 20% glucose (2 g/kg body weight) by intraperitoneal injection. Glucose was measured at time 0 (before glucose injection) and 15, 30, 60 and 120 min after injection. Plasma DPP-IV concentrations were measured using a mouse DPP-IV ELISA kit (Sigma-Aldrich, St. Louis, MO, USA).
Assessment of bone morphology and turnover
Cortical bone morphology at the mid-femoral diaphysis and trabecular bone volume fraction and microarchitecture in the excised distal femoral metaphysis were assessed as previously described [34,35]. Femora were reconstructed at a 6 μm voxel resolution. For trabecular bone regions, we assessed the bone volume fraction (BV/TV, %), trabecular thickness (Tb.Th, μm), trabecular number (Tb.N) and trabecular spacing (Tb.Sp, μm). For cortical bone at the femoral midshaft, we measured the average total cross-sectional area inside the periosteal envelope (Tt.Ar, mm2), the cortical bone and medullary area within this same envelope (Ct.Ar, mm2 and Ma.Ar, mm2, respectively), the relative cortical area (RCA; Ct.Ar/Tt.Ar, %), the average cortical thickness (Ct.Th, μm) and the polar moment of inertia (Jo). All regions of analysis were standardized according to anatomical landmarks.
Histomorphometry was performed on femora fixed in 10% neutral buffered formalin, embedded in polymethylmethacrylate. Tartrate-resistant acid phosphatase (TRAP) staining was performed by incubating slides in a TRAP staining solution (0.05 M sodium acetate, 0.025 M sodium tartrate, 0.125 mg/ml Fast Red Violet LB Salt and 0.125 mg/ml Naphtol AS-MX phosphate) for 180 min and then washing with water. For determination of osteoblast number, toluidine blue staining was performed by incubating slides in a solution of 0.02% toluidine blue for 30 min and then washing with water. All measurements were performed using an OsteoMeasure system (Osteometrics, Atlanta, GA, USA) in accordance with standard protocols. Sections were imaged using a digital camera attached to a visible light/fluorescence microscope (Zeiss Axioplan2, Zeiss AxioVision, Thornwood, NY).
Marrow-derived osteoblast culture
Bone marrow (BM) cells were flushed out from femurs using a 26-gauge needle and collected in an α-modified minimum essential medium (αMEM). Cells were cultured in αMEM supplemented with 10% heat-inactivated foetal bovine serum with β-glycerolphosphate (10 mm) and ascorbic acid (50 mm). Cells were treated with 20 μM of pioglitazone, MK-0626 or PBS every other day. The number of alkaline phosphatase positive colonies was determined using a para-nitrophenol phosphate-based colorimetric assay at 14, 21 and 28 days (Sigma-Aldrich, St. Louis, MO, USA).
Gene expression
Total RNAwas extracted from osteoblast-like cells after 28 days in culture with PBS, pioglitazone or MK-0626 treatments using TRIzol reagent and reverse transcribed according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA, USA). Real time-polymerase chain reaction (PCR) was performed using the QuantiTect SYBR green PCR kit (QIAGEN, Valencia, CA, USA) in ABI PRISM 7900HT sequence detection systems (Applied Biosystems, Foster City, CA, USA). Primers were selected using the web site www.genome.wi.mit.edu/cgi-bin/primer/primer3.cgi or published primer sequences. The primers are listed in Supplementary Table 1; for each gene, a single sample was assayed three times, and gene expression was normalized to beta actin.
Statistics
Means and standard error of the mean (SEM) for each bone trait were determined separately for each strain and treatment. Results are expressed as means ± SEM, unless otherwise stated. Significant differences in mean trait values among ages and genotype were identified using two-way ANOVA and Holm—Sidak post-hoc tests (p <0.05) (SPSS, IBM, Armonk, NY, USA).
Results
Body weight and body composition were maintained in the diabetic MKR mice treated with MK-0626
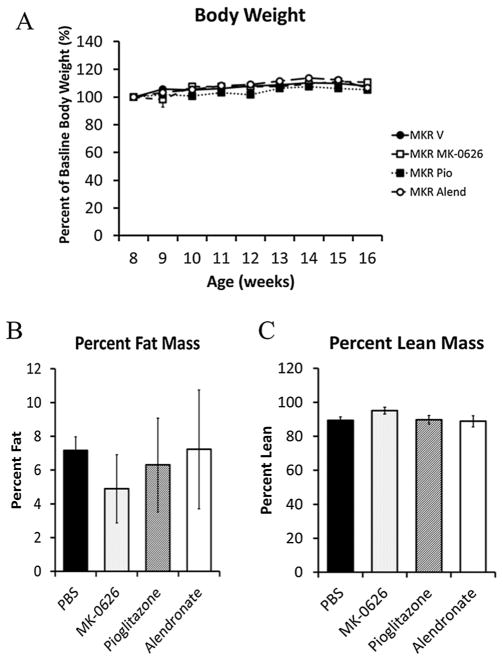
As body weight and fat content are known to affect the architecture and turnover of bone [36,37], we first examined whether MK-0626, pioglitazone or alendronate led to changes in body composition in the MKR mice. Body weight in the diabetic MKR mice was maintained throughout the 8-week treatment period in the MK-0626, pioglitazone, alendronate and vehicle-treated groups (Figure 1A). Although the pioglitazone group had significantly higher body weights at baseline and throughout the treatment period than the other groups, there was no difference in the percent of body weight gained in any group compared with the MKR vehicle-treated group (MKR vehicle 8.4 ± 2.0%, MKR MK-0626 8.9 ± 3.3%, MKR alendronate 6.9 ± 2.3% and MKR pioglitazone 5.2 ± 1.0%). The WT vehicle-treated mice had higher body weight than the MKR vehicle-treated mice throughout the study (WT 29.4 ± 0.6 g vs MKR 24.6 ± 0.4 g at age 16 weeks, p <0.05), consistent with our previous studies [27,28]. All WT mice treated with MK-0626, pioglitazone and alendronate maintained their body weight throughout the treatment period, and there was no significant difference between treatment groups (data not shown). Fat mass and lean mass remained unchanged in the MKR mice after 8 weeks of treatment with MK-0626, pioglitazone or alendronate compared with vehicle (Figure 1B and C). Although the WT mice had higher percentage body fat (11.36 ± 1.07%) than the MKR mice (7.17 ± 0.80%), no differences were observed in the body composition in any of the WT treatment groups after 8 weeks of treatment (data not shown).
Figure 1.
Weekly body weights of the MKR mice treated with vehicle, pioglitazone (Pio), MK-0626 or alendronate (Alend) revealed that there was no significant difference in percent of body weight gained in any treatment group compared with the vehicle-treated mice (1A). NMR body composition analysis revealed no difference in percent fat mass (1B) or percent lean mass (1C) in any of the MKR treatment groups after 8 weeks of treatment (n = 9–21 per group). Graphs represent means for each group, and error bars indicate standard error of the mean
Dipeptidyl peptidase-IV inhibition did not lead to changes in glycemia in the MKR mice
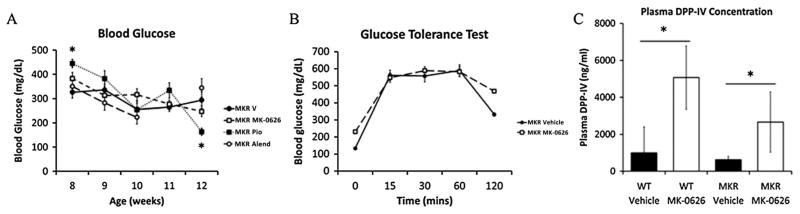
One of the potential mechanisms through which diabetes may affect bone fragility is through AGE/RAGE interaction [22], therefore, we wished to determine whether any of the medications significantly reduced blood glucose levels in the MKR mice. Consistent with the reports of others [38], MK-0626 did not affect fed or fasting blood glucose levels in the MKR mice compared with control mice after 8 weeks of treatment (Figure 2A). Glucose intolerance remained in the MK-0626-treated MKR mice after a 2 g/kg glucose tolerance test (Figure 2B). The pioglitazone-treated MKR mice had higher baseline glucose levels and significantly lower glucose after 8 weeks of treatment, compared with vehicle-treated MKR mice (Figure 2A). No significant differences in glucose level were detected in the MK-0626 or alendronate-treated MKR mice, compared with vehicle-treated mice at the end of 8 weeks of treatment (Figure 2A). Although the blood glucose levels in the WT vehicle-treated mice were consistently lower than those of the MKR mice (WT 149.9 ± 7.8 mg/dL vs MKR 293.8 ± 34.1 mg/dL, p <0.05 at 16 weeks of age), no differences in blood glucose levels were observed with any treatment in the WT mice (data not shown). As MK-0626 led to no significant alterations in glucose levels, we measured plasma DPP-IV levels in the WT and MKR mice treated with vehicle and MK-0626 to ensure that MK-0626 was exerting expected biological actions. Treatment with MK-0626 significantly increased total plasma DPP-IV levels in WT and MKR mice, compared with control-treated mice (Figure 2C). This finding was consistent with previously published studies on the effects of DPP-IV inhibitors [39]. Thus, the lack of change in body weight, body composition and glucose levels in the MKR mice with MK-0626 treatment allowed us to study the effects of DPP-IV inhibition on the skeletal system of the MKR mice in the absence of any body composition or metabolic changes.
Figure 2.
Random blood glucose levels in the MKR mice at baseline (8 weeks) and biweekly during the treatment period (Figure 2A). The MKR pioglitazone-treated group had higher glucose levels than the vehicle-treated group at baseline (* is the p value <0.05), but no differences were detected between groups for the remainder of the treatment period (n = 9–21 per group). A glucose tolerance test was performed with 2 g/kg glucose in the MKR vehicle and MK-0626-treated group. No statistically significant difference in glucose tolerance was detected between the MKR vehicle and MK-0626 groups (n = 4–11 per group). Plasma dipeptidyl peptidase-IV (DPP-IV) concentrations were found to be significantly elevated in the WT and MKR mice treated with MK-0626 compared with vehicle-treated mice (Figure 2C). (* is the p value <0.05, n = 6 per group). Graphs represent means for each group, and error bars indicate standard error of the mean
MK-0626 did not alter the bone microarchitecture or turnover in the hyperglycemic MKR mice
Consistent with our previous findings [27], in the 16 weeks old MKR vehicle-treated mice, microCT analyses of cortical bone demonstrated reduced cortical area (Ct. Ar. 0.68 ± 0.04 mm2) compared with the WT vehicle-treated mice (0.83 ± 0.04 mm2, p value <0.05), whereas trabecular bone analyses revealed decreased BV/TV (MKR vehicle 7.16 ± 1.7% vs WT vehicle 13.33 ± 3.3%, p value <0.05), Tb.Th. (MKR vehicle 0.043 ± 0.002 μm vs WT vehicle 0.047 ± 0.00 μm, p value <0.05) and Tb. N. (MKR vehicle 1.64 ± 0.38/mm vs WT vehicle 2.81 ± 0.60/mm, p value <0.05), with increased Tb.Sp. (MKR vehicle 0.25 ± 0.03 μm vs WT vehicle 0.19 ± 0.02 μm, p value <0.05). MK-0626 treatment for 8 weeks led to no differences in cortical or trabecular bone parameters (Tables 1 and 2), whereas alendronate treatment led to greater trabecular number and decreased trabecular spacing in both MKR mice (Table 2) and in the WT mice (data not shown). Histomorphometry revealed no changes in bone formation rate, osteoclast or osteo-blast number or mineralized surface per bone surface in the MK-0626-treated MKR mice compared with the control-treated mice (Table 3). Pioglitazone led to no changes in cortical or trabecular bone architecture in the MKR mice (Tables 1 and 2); however, pioglitazone-treated WT mice had significantly lower Ct. Ar. (0.77 ± 0.03 mm2, p value 0.05), Tb. N. (2.2 ± 0.22/mm, p value <0.05) and increased Tb Sp. (0.22 ± 0.02 μm, p value <0.05) compared with the vehicle-treated WT mice. Therefore, although pioglitazone had detrimental effects on the trabecular bone of WT mice, in the hyperglycemic MKR mice, pioglitazone led to no further deterioration in the trabecular bone. Alendronate treatment led to improvements in cortical and trabecular architecture of the bone in the MKR mice. However, despite the increase in bone volume and improvements in cortical and trabecular architecture, histomorphometry revealed a significant increase in osteoclast number (Oc.N/BS), osteoclast perimeter (Oc. S/BS) and erosion perimeter (ES/BS) (Table 3). Overall, treatment with MK-0626 had neutral effects on bone in the MKR mice, pioglitazone led to no further deterioration in bone in the MKR mice, whereas alendronate led to improvements in the bone architecture, despite the increased parameters of bone resorption on histomorphometry.
Table 1.
Cortical bone analysis of the femoral mid-diaphysis from diabetic MKR mice after 8 weeks of treatment assessed by microCT
| Vehicle (n = 20) | MK-0626 (n = 13) | Pioglitazone (n = 12) | Alendronate (n = 7) | |
|---|---|---|---|---|
| Tt.Ar mm2 | 1.24 ± 0.19 | 1.13 ± 0.07 | 1.38 ± 0.25 | 1.20 ± 0.12 |
| Ct.Ar mm2 | 0.68 ± 0.04 | 0.66 ± 0.04 | 0.67 ± 0.05 | 0.72 ± 0.04 * |
| Ct.Th μm | 0.16 ± 0.00 | 0.16 ± 0.00 | 0.16 ± 0.00 | 0.17 ± 0.01 * |
| Ma.Ar mm2 | 0.56 ± 0.17 | 0.46 ± 0.04 | 0.70 ± 0.22 | 0.49 ± 0.09 |
| RCA (Ct.Ar/Tt.Ar) | 0.55 ± 0.06 | 0.58 ± 0.01 | 0.50 ± 0.07 | 0.59 ± 0.04 |
| J0 (mm4) | 0.24 ± 0.03 | 0.24 ± 0.02 | 0.25 ± 0.04 | 0.25 ± 0.02 |
Results are expressed as mean values ± SEM.
Tt.Ar, mm2, total cross-sectional area inside the periosteal envelope; Ct.Ar, mm2, cortical bone area; CtTh, μm, average cortical thickness; Ma Ar mm2, medullary area, RCA, Ct.Ar/Tt.Ar, %, the bone area fraction; J0, the polar moment of inertia.
p value <0.05 between MKR vehicle and MKR alendronate-treated groups.
Table 2.
Trabecular bone analyses of the femoral distal metaphyses from the diabetic MKR mice after 8 weeks of treatment
| Vehicle (n = 20) | MK-0626 (n = 13) | Pioglitazone (n = 12) | Alendronate (n = 7) | |
|---|---|---|---|---|
| BV/TV (%) | 7.16 ± 1.7 | 6.58 ± 1.60 | 8.7 ± 1.64 | 10.79 ± 1.07 * |
| Tb.Th, μm | 0.043 ± 0.002 | 0.044 ± 0.002 | 0.044 ± 0.000 | 0.041 ± 0.001 |
| Tb.N/mm | 1.64 ± 0.379 | 1.45 ± 0.32 | 1.96 ± 0.34 | 2.59 ± 0.26 * |
| Tb.Sp, μm | 0.25 ± 0.034 | 0.28 ± 0.027 | 0.24 ± 0.015 | 0.19 ± 0.014* |
BV/TV, %, bone volume per total volume; Tb.Th, μm, trabecular thickness; Tb.N, mm−1, trabecular number; Tb.Sp, μm, trabecular separation. Results are expressed as mean values ± SEM.
p value <0.05 between MKR vehicle and MKR alendronate-treated groups.
Table 3.
Histomorphometry analysis of the endosteal surface of femora from the diabetic MKR mice after 8 weeks of treatment
| Vehicle (n = 9) | MK-0626 (n = 8) | Pioglitazone (n = 5) | Alendronate (n = 6) | |
|---|---|---|---|---|
| BFR μm/day | 0.37 ± 0.22 | 0.29 ± 0.17 | 0.32 ± 0.06 | 0.15 ± 0.18 |
| MAR μm/day | 0.69 ± 0.25 | 0.75 ± 0.16 | 0.58 ± 0.11 | 0.38 ± 0.28 |
| MS/BS% | 53.82 ± 18.21 | 37.03 ± 17.58 | 55.37 ± 6.4 | 33.02 ± 17.77 |
| ES/BS% | 6.13 ± 2.11 | 6.40 ± 1.41 | 3.04 ± 0.86 | 9.5 ± 1.95 * |
| Ob.N/BS/mm | 13.41 ± 1.85 | 14.68 ± 5.08 | 15.18 ± 3.33 | 11.76 ± 3.45 |
| Oc.N/BS/mm | 1.81 ± 0.47 | 1.96 ± 0.44 | 1.03 ± 0.27 * | 2.93 ± 0.52 * |
BFR, μm/day, bone formation rate; MAR, μm/day, mineral apposition rate; MS/BS, %, mineralization surface/bone surface; ES/BS, %, erosion surface/bone surface; Ob.N/BS, mm−1, osteoblast number/bone surface; Oc. N/BS, mm−1,osteoclast number/bone perimeter. Results are expressed as mean values ± SEM.
p value <0.05 between MKR vehicle and other treatment group.
MK-0626 had no direct effects on osteoblast differentiation in vitro
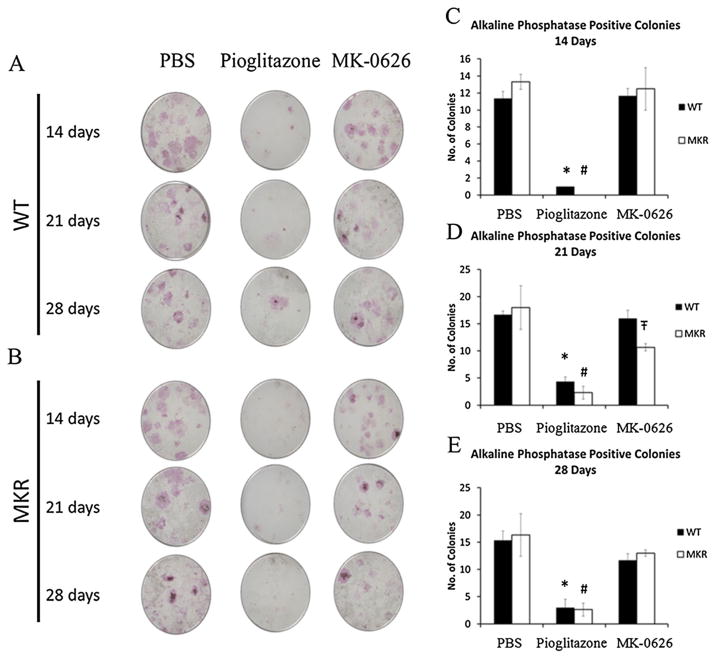
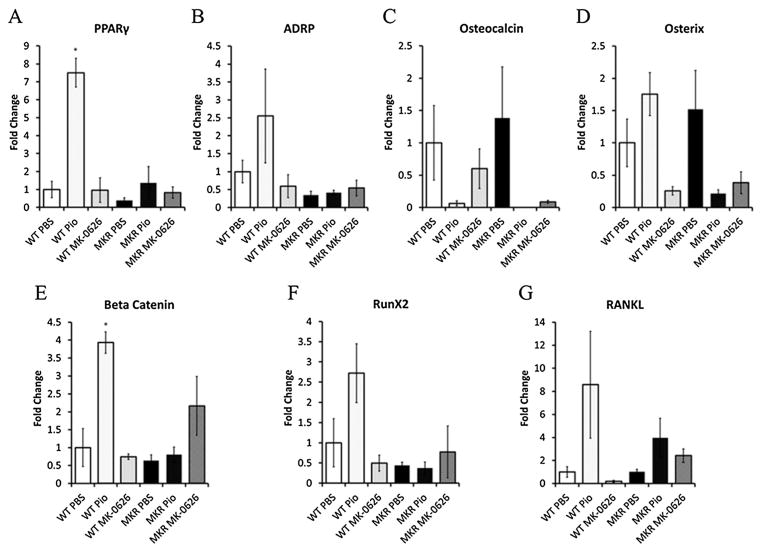
A recent study demonstrated that genetic inactivation of DPP-IV had no effect on osteoclast number [26]. We therefore wished to determine whether MK-0626 had direct effects on osteoblast differentiation, compared with PBS and pioglitazone treatment. Primary BM cultures were performed on BM cells from the long bones of adult MKR and WT mice. After isolation, the cells were treated for 14, 21 and 28 days with PBS, pioglitazone or MK-0626. Alkaline phosphatase staining revealed that MK-0626 treatment was associated with no difference in osteoblast colony number in MKR-derived or WT-derived marrow cells (Figure 3A–E). On the other hand, pioglitazone treatment led to a significant decrease in the number of alkaline phosphatase positive colonies at each time point (Figure 3A–E), consistent with the previous studies on human BM stromal cells [40]. Therefore, while pioglitazone did not lead to significant changes in the cortical or trabecular architecture of the MKR mouse bone in vivo, it had direct effects on osteoblastogenesis in vitro, whereas MK-0626 had neutral effects on the bone of the diabetic mouse in vivo and in vitro. Consistent with these findings, no changes in adipocyte differentiation genes, Wnt signalling genes or osteoblast markers were observed in WT-derived or MKR-derived cells treated with MK-0626 for 28 days (Figure 4). However, pioglitazone treatment for 28 days led to increased expression of adipocyte markers PPARγ and adipocyte differentiation related protein (ADRP) in cells from the WT but not the MKR mice (Figure 4A and B). The changes in ADRP did not reach statistical significance. Pioglitazone treatment also led to non-significant decreases in the expression of osteocalcin (Figure 4C) and no significant change in osterix expression in the cells from both WT and MKR mice. In vitro treatment with pioglitazone led to significantly increased expression of β-catenin and non-significant increases in Runx2 and RANKL in the cells derived from WT mice compared with PBS-treated cells, but no difference was observed in the cells from MKR mice treated with PBS or pioglitazone (Figure 4E and F). These results demonstrate that directly inhibiting DPP-IV in BM stromal cells derived from WT and diabetic mice does not affect osteoblast differentiation.
Figure 3.
Representative images of alkaline phosphatase staining of WT (3A)-derived and MKR (3B)-derived bone marrow stromal cells after treatment for 14, 21 or 28 days with PBS, pioglitazone or the dipeptidyl peptidase-IV inhibitor MK-0626. The alkaline phosphatase staining experiments were performed in triplicate. Figure 3C–E show the quantification of the number of alkaline phosphatase positive colonies (>20 cells per colony). Graphs represent means for each group, and error bars indicate standard error of the mean. * is the statistically significant difference between pioglitazone treatment and phosphate buffered saline (PBS) treatment of wild type (WT) cells, and # is the statistically significant difference between MKR pioglitazone and PBS treatment of WT cells. Ŧ is the statistically significant difference between MK-0626-treated WT-derived and MKR-derived cells
Figure 4.
MK-0626 treatment led to no changes in the gene expression in bone marrow cells derived from wild type (WT) or MKR mice. Gene expression analysis was performed by extracting total RNA from WT-derived or MKR-derived bone marrow stromal cells after 28 days of treatment with phosphate buffered saline (PBS), pioglitazone (PIO) or MK-0626 as described in the materials and methods. Experimental conditions were performed in triplicate, and gene expression for each sample was analysed in triplicate. MK-0626 led to no significant changes in gene expression compared with PBS treatment. Pioglitazone treatment led to an increase in PPARγ (4A) and a non-significant increase in the expression of adipocyte differentiation genes in cells derived from WT mice (4B). A decrease in osteocalcin (4C), with non-significant decrease in osterix expression (4D) was observed in cells from MKR mice treated with pioglitazone. Significant increases in expression of beta catenin (4E) and non-significant increases in Runx2 (4 F) and RANKL (4G) were observed in the pioglitazone-treated cells derived from the WT mice. Graphs represent means for each group, corrected to the WT saline treated group, and error bars indicate standard error of the mean.* is the p value <0.05 versus WT PBS-treated cells
Discussion
This study demonstrates that in an animal model of T2D, with known defects in bone microarchitecture, the DPP-IV inhibitor MK-0626 had neutral effects on bone morphology and turnover. Although our results are contrary to our hypothesis that MK-0626 treatment would improve the bone quality in the MKR mice, these findings are reassuring, given that incretin therapy is being increasingly used for the treatment of T2D. The lack of improvement in the cortical and trabecular bone of the MKR mice treated with MK-0626 treatment may be related to the lack of improvement in glucose control. Our previous studies have shown that the defects in skeletal integrity in the MKR mice develop at 8 and 16 weeks of age, at which time hyperglycaemia has developed [27]. Hyperglycaemia and AGE/RAGE signalling are hypothesized as one mechanism through which diabetes increases bone fragility [22]. A previous study on the MKR mice demonstrated that short-term (3 weeks) administration of the DPP-IV inhibitor MK-0626 (3 mg/kg body weight) by oral gavage did not alter fasting glucose, insulin or glucagon levels in the MKR mice [38]. Although these doses have been previously demonstrated to inhibit DPP-IV by 90% and enhance GLP-1 secretion by sevenfold in C57BL/six mice [41], the MKR mice are known to have elevated fasting GLP-1 levels (1.7-fold greater than WT mice) and have impaired GLP-1 secretion compared with WT mice following an oral glucose challenge [42]. Therefore, in the MKR mouse, the glucose lowering abilities of MK-0626 may be impaired because of the high basal levels of GLP-1 and the lack of change in glucose levels and insulin levels in response to MK-0626. An animal study on high fat diet fed C57BL/six mice examined the effects of the DPP-IV inhibitor sitagliptin on bone architecture. No significant differences in bone architecture were observed in male mice or female mice post-oophorectomy treated with sitagliptin or vehicle [26]. The authors reported that sitagliptin treatment led to improvements in glucose tolerance but did not alter fasting glucose or haemoglobin A1c (HbA1c), therefore, perhaps, the lack of significant change with DPP-IV inhibition was again because glycemia and incretin levels were not significantly altered [26]. In the same publication, they found that the genetic inactivation of DPP-IV led to detrimental effects on bone mechanics of oophorectomy-DPP-IV−/− mice. Although the majority of analyses in the male and female DPP-IV−/− mice revealed no significant differences, a decrease in Tb.Th. and mineral apposition rate in male DPP-IV−/− mice was observed. No significant effects on osteoclast staining were observed [26]. Furthermore, previous studies have suggested that DPP-IV is regulated by the androgen receptor (AR) in mineralizing osteoblasts, and targeted deletion of the AR in osteoblasts and osteocytes leads to increased expression of DPP-IV and may be a mechanism involved in the increase in bone resorption that occurs in animals with osteoblast-targeted AR inactivation [25]. We found no differences in the number of osteoblast colonies or on the expression of genes involved in osteoblastogenesis in the BM cells derived from WT or MKR mice treated with DPP-IV inhibitor. The results of our studies along with those on the genetic inactivation of DPP-IV in vivo suggest that the potential beneficial effects of DPP-IV inhibitors on bone are mediated through indirect mechanisms and not through the direct effects of inhibiting DPP-IV in osteoblasts.
The WT mice treated with pioglitazone demonstrated bone findings typical of thiazoledinedione treatment [43–45], with decreased bone volume, cortical area, trabecular thickness and number, compared with the vehicle-treated group. The lack of further deterioration in bone architecture in the pioglitazone-treated MKR mice is likely because of the already defective cortical and trabecular bone architecture in these mice, which may be due to uncontrolled hyperglycaemia in the MKR mice. It is possible that longer duration of treatment may have further worsened the bone architecture in the MKR mice or alternatively that the detrimental effects of pioglitazone on bone may be overshadowed by the effects of improved glucose control on bone.
In women with T2D, treatment with alendronate has been shown to improve BMD relative to placebo and prevent fractures to a similar extent as in women without T2D [29,46,47]. Consistent with these findings, in the hyperglycemic MKR mice, alendronate improved cortical and trabecular bone architecture after 8 weeks of treatment. Histomorphometry revealed that alendronate increased the number of osteoclasts in both the MKR and WT mice, although this finding was more pronounced in the MKR mice. Although the mechanism of action of bisphosphonates is to inhibit bone resorption caused by osteoclasts, our seemingly paradoxical findings on bone volume and osteoclast number have been previously described in animal studies by other groups [48–50]. Human studies have also revealed that after 3 years of bisphosphonate use, the number of osteoclasts observed in bone biopsies was increased 2.6-fold in those on 10 mg daily of alendronate, compared with the control group [51]. Both human and animal studies have also revealed an increase in the number of giant nucleated osteoclasts, the significance of which remains to be determined [48,51].
Overall, our study reveals that in an animal model of T2D, with uncontrolled hyperglycaemia, 8 weeks of treatment with DPP-IV inhibitor MK-0626 had neutral effects on bone architecture, bone turnover, osteoclast and osteoblast number in vivo. Directly inhibiting DPP-IV in BM-derived cells did not affect osteoblastogenesis in vitro. These findings are consistent with those in an animal model of insulin resistance and a model of genetic inactivation of DPP-IV with normal glucose tolerance [26]. Our findings that inhibiting DPP-IV did not lead to any detrimental effects on bone in vivo in the setting of T2D or on osteoblasts in vitro are reassuring for physicians and patients with T2D who already have an increased risk of bone fractures.
Supplementary Material
Acknowledgments
This study was supported by a grant from Merck and Co, New Jersey, USA to DLR.
Footnotes
Conflicts of interest
None declared.
Supporting information
Additional supporting information may be found in the online version of this article at the publisher’s web site.
References
- 1.Janghorbani M, Van Dam RM, Willett WC, Hu FB. Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am J Epidemiol. 2007;166:495–505. doi: 10.1093/aje/kwm106. [DOI] [PubMed] [Google Scholar]
- 2.Moseley KF. Type 2 diabetes and bone fractures. Curr Opin Endocrinol Diabetes Obes. 2012;19:128–135. doi: 10.1097/MED.0b013e328350a6e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamann C, Kirschner S, Gunther KP, Hofbauer LC. Bone, sweet bone--osteoporotic fractures in diabetes mellitus. Nat Rev Endocrinol. 2012;8:297–305. doi: 10.1038/nrendo.2011.233. [DOI] [PubMed] [Google Scholar]
- 4.Gorman E, Chudyk AM, Madden KM, Ashe MC. Bone health and type 2 diabetes mellitus a systematic review. Physiother Can. 2011;63:8–20. doi: 10.3138/ptc.2010-23bh. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wakasugi M, Wakao R, Tawata M, Gan N, Koizumi K, Onaya T. Bone mineral density measured by dual energy x-ray absorptiometry in patients with non-insulin-dependent diabetes mellitus. Bone. 1993;14:29–33. doi: 10.1016/8756-3282(93)90252-6. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz AV, Sellmeyer DE, Ensrud KE, et al. Older women with diabetes have an increased risk of fracture a prospective study. J Clin Endocrinol Metab. 2001;86:32–38. doi: 10.1210/jcem.86.1.7139. [DOI] [PubMed] [Google Scholar]
- 7.Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes--a meta-analysis. Osteoporos Int. 2007;18:427–444. doi: 10.1007/s00198-006-0253-4. [DOI] [PubMed] [Google Scholar]
- 8.Epstein S, Leroith D. Diabetes and fragility fractures - a burgeoning epidemic? Bone. 2008;43:3–6. doi: 10.1016/j.bone.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 9.Vilsboll T, Krarup T, Deacon CF, Madsbad S, Holst JJ. Reduced postprandial concentrations of intact biologically active glucagon-like peptide 1 in type 2 diabetic patients. Diabetes. 2001;50:609–613. doi: 10.2337/diabetes.50.3.609. [DOI] [PubMed] [Google Scholar]
- 10.Monami M, Dicembrini I, Antenore A, Mannucci E. Dipeptidyl peptidase-4 inhibitors and bone fractures a meta-analysis of randomized clinical trials. Diabetes Care. 2011;34:2474–2476. doi: 10.2337/dc11-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saxagliptin No more effective than other gliptins, but a high potential for drug interactions. Prescrire Int. 2011;20:33–37. [PubMed] [Google Scholar]
- 12.Yang W, Pan CY, Tou C, Zhao J, Gause-Nilsson I. Efficacy and safety of saxagliptin added to metformin in Asian people with type 2 diabetes mellitus a randomized controlled trial. Diabetes Res Clin Pract. 2011;94:217–224. doi: 10.1016/j.diabres.2011.07.035. [DOI] [PubMed] [Google Scholar]
- 13.Bunck MC, Poelma M, Eekhoff EM, et al. Effects of vildagliptin on postprandial markers of bone resorption and calcium homeostasis in recently diagnosed, well-controlled type 2 diabetes patients. J Diabetes. 2012;4:181–185. doi: 10.1111/j.1753-0407.2011.00168.x. [DOI] [PubMed] [Google Scholar]
- 14.Pacheco-Pantoja EL, Ranganath LR, Gallagher JA, Wilson PJ, Fraser WD. Receptors and effects of gut hormones in three osteoblastic cell lines. BMC Physiol. 2011;11:12. doi: 10.1186/1472-6793-11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bollag RJ, Zhong Q, Phillips P, et al. Osteoblast-derived cells express functional glucose-dependent insulinotropic peptide receptors. Endocrinology. 2000;141:1228–1235. doi: 10.1210/endo.141.3.7366. [DOI] [PubMed] [Google Scholar]
- 16.Sanz C, Vazquez P, Blazquez C, Barrio PA, del Alvarez MM, Blazquez E. Signaling and biological effects of glucagon-like peptide 1 on the differentiation of mesenchymal stem cells from human bone marrow. Am J Physiol Endocrinol Metab. 2010;298:E634–643. doi: 10.1152/ajpendo.00460.2009. [DOI] [PubMed] [Google Scholar]
- 17.Nuche-Berenguer B, Portal-Nunez S, Moreno P, et al. Presence of a functional receptor for GLP-1 in osteoblastic cells, independent of the cAMP-linked GLP-1 receptor. J Cell Physiol. 2010;225:585–592. doi: 10.1002/jcp.22243. [DOI] [PubMed] [Google Scholar]
- 18.Nuche-Berenguer B, Moreno P, Portal-Nunez S, Dapia S, Esbrit P, Villanueva-Penacarrillo ML. Exendin-4 exerts osteogenic actions in insulin-resistant and type 2 diabetic states. Regul Pept. 2010;159:61–66. doi: 10.1016/j.regpep.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 19.Nuche-Berenguer B, Lozano D, Gutierrez-Rojas I, et al. GLP-1 and exendin-4 can reverse hyperlipidic-related osteopenia. J Endocrinol. 2011;209:203–210. doi: 10.1530/JOE-11-0015. [DOI] [PubMed] [Google Scholar]
- 20.Ding KH, Shi XM, Zhong Q, et al. Impact of glucose-dependent insulinotropic peptide on age-induced bone loss. J Bone Miner Res. 2008;23:536–543. doi: 10.1359/JBMR.071202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wortley KE, Garcia K, Okamoto H, et al. Peptide YY regulates bone turnover in rodents. Gastroenterology. 2007;133:1534–1543. doi: 10.1053/j.gastro.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 22.Yamagishi S. Role of advanced glycationendproducts (AGEs) inosteoporosis in diabetes. Curr Drug Targets. 2011;12:2096–2102. doi: 10.2174/138945011798829456. [DOI] [PubMed] [Google Scholar]
- 23.Yamagishi S, Matsui T. Pleiotropic effects of glucagon-like peptide-1 (GLP-1)-based therapies on vascular complications in diabetes. Curr Pharm Des. 2011;17:4379–4385. doi: 10.2174/138161211798999456. [DOI] [PubMed] [Google Scholar]
- 24.Boonacker E, Van Noorden CJ. The multifunctional or moonlighting protein CD26/DPPIV. Eur J Cell Biol. 2003;82:53–73. doi: 10.1078/0171-9335-00302. S0171-9335(04)70277-0 [pii] [DOI] [PubMed] [Google Scholar]
- 25.Russell PK, Clarke MV, Skinner JP, Pang TP, Zajac JD, Davey RA. Identification of gene pathways altered by deletion of the androgen receptor specifically in mineralizing osteoblasts and osteocytes in mice. J Mol Endocrinol. 2012;49:1–10. doi: 10.1530/JME-12-0014. [DOI] [PubMed] [Google Scholar]
- 26.Kyle KA, Willett TL, Baggio LL, Drucker DJ, Grynpas MD. Differential effects of PPAR-{gamma} activation versus chemical or genetic reduction of DPP-4 activity on bone quality in mice. Endocrinology. 2011;152:457–467. doi: 10.1210/en.2010-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawashima Y, Fritton JC, Yakar S, et al. Type 2 diabetic mice demonstrate slender long bones with increased fragility secondary to increased osteoclastogenesis. Bone. 2009;44:648–655. doi: 10.1016/j.bone.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernandez AM, Kim JK, Yakar S, et al. Functional inactivation of the IGF-I and insulin receptors in skeletal muscle causes type 2 diabetes. Genes Dev. 2001;15:1926–1934. doi: 10.1101/gad.908001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keegan TH, Schwartz AV, Bauer DC, Sellmeyer DE, Kelsey JL. Effect of alendronate on bone mineral density and biochemical markers of bone turnover in type 2 diabetic women: the fracture intervention trial. Diabetes Care. 2004;27:1547–1553. doi: 10.2337/diacare.27.7.1547. [DOI] [PubMed] [Google Scholar]
- 30.Colhoun HM, Livingstone SJ, Looker HC, et al. Hospitalised hip fracture risk with rosiglitazone and pioglitazone use compared with other glucose-lowering drugs. Diabetologia. 2012;55:2929–2937. doi: 10.1007/s00125-012-2668-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leslie WD, Rubin MR, Schwartz AV, Kanis JA. Type 2 diabetes and bone. J Bone Miner Res. 2012;27:2231–2237. doi: 10.1002/jbmr.1759. [DOI] [PubMed] [Google Scholar]
- 32.Sato M, Grasser W, Endo N, et al. Bisphosphonate action. Alendronate localization in rat bone and effects on osteoclast ultrastructure. J Clin Invest. 1991;88:2095–2105. doi: 10.1172/JCI115539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Evans KD, Lau ST, Oberbauer AM, Martin RB. Alendronate affects long bone length and growth plate morphology in the oim mouse model for osteogenesis imperfecta. Bone. 2003;32:268–274. doi: 10.1016/s8756-3282(02)00974-2. [DOI] [PubMed] [Google Scholar]
- 34.Courtland HW, Elis S, Wu Y, et al. Serum IGF-1 affects skeletal acquisition in a temporal and compartment-specific manner. PLoS One. 2011;6:e14762. doi: 10.1371/journal.pone.0014762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Courtland HW, Sun H, Beth-On M, et al. Growth hormone mediates pubertal skeletal development independent of hepatic IGF-1 production. J Bone Miner Res. 2011;26:761–768. doi: 10.1002/jbmr.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patsch JM, Kiefer FW, Varga P, et al. Increased bone resorption and impaired bone microarchitecture in short-term and extended high-fat diet-induced obesity. Metabolism. 2011;60:243–249. doi: 10.1016/j.metabol.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iwaniec UT, Dube MG, Boghossian S, et al. Body mass influences cortical bone mass independent of leptin signaling. Bone. 2009;44:404–412. doi: 10.1016/j.bone.2008.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhattacharjee A, Allister EM, Wheeler MB. MK-626 a dipeptidyl peptidase-4 inhibitor, does not improve the hyperglycemia or hyperinsulinemia of nonobese diabetic MKR mice. Can J Physiol Pharmacol. 2012;90:663–668. doi: 10.1139/y2012-039. [DOI] [PubMed] [Google Scholar]
- 39.Makdissi A, Ghanim H, Vora M, et al. Sitagliptin exerts an antinflammatory action. J Clin Endocrinol Metab. 2012;97:3333–3341. doi: 10.1210/jc.2012-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beck GR, Jr, Khazai NB, Bouloux GF, et al. The effects of thiazolidinediones on human bone marrow stromal cell differentiation in vitro and in thiazolidinedione-treated patients with type 2 diabetes. Transl Res. 2012 doi: 10.1016/j.trsl.2012.08.006. S1931-5244(12)00300-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Edmondson SD, Mastracchio A, Mathvink RJ, et al. (2S,3S)-3-Amino-4-(3,3-difluoropyrrolidin-1-yl)-N,N-dimethyl-4-oxo-2-(4-[1,2,4]tr iazolo[1,5-a]-pyridin-6-ylphenyl)butanamide a selective alpha-amino amide dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. J Med Chem. 2006;49:3614–3627. doi: 10.1021/jm060015t. [DOI] [PubMed] [Google Scholar]
- 42.Lim GE, Huang GJ, Flora N, LeRoith D, Rhodes CJ, Brubaker PL. Insulin regulates glucagon-like peptide-1 secretion from the enteroendocrine L cell. Endocrinology. 2009;150:580–591. doi: 10.1210/en.2008-0726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Syversen U, Stunes AK, Gustafsson BI, et al. Different skeletal effects of the peroxisome proliferator activated receptor (PPAR)alpha agonist fenofibrate and the PPARgamma agonist pioglitazone. BMC Endocr Disord. 2009;9:10. doi: 10.1186/1472-6823-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rzonca SO, Suva LJ, Gaddy D, Montague DC, Lecka-Czernik B. Bone is a target for theantidiabetic compound rosiglitazone. Endocrinology. 2004;145:401–406. doi: 10.1210/en.2003-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lazarenko OP, Rzonca SO, Hogue WR, Swain FL, Suva LJ, Lecka-Czernik B. Rosiglitazone induces decreases in bone mass and strength that are reminiscent of aged bone. Endocrinology. 2007;148:2669–2680. doi: 10.1210/en.2006-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iwamoto J, Sato Y, Uzawa M, Takeda T, Matsumoto H. Three-year experience with alendronate treatment in postmenopausal osteoporotic Japanese women with or without type 2 diabetes. Diabetes Res Clin Pract. 2011;93:166–173. doi: 10.1016/j.diabres.2011.03.033. [DOI] [PubMed] [Google Scholar]
- 47.Vestergaard P, Rejnmark L, Mosekilde L. Are antiresorptive drugs effective against fractures in patients with diabetes? Calcif Tissue Int. 2011;88:209–214. doi: 10.1007/s00223-010-9450-4. [DOI] [PubMed] [Google Scholar]
- 48.Escudero ND, Mandalunis PM. Influence of bisphosphonate treatment on medullary macrophages and osteoclasts: an experimental study. Bone Marrow Res. 2012;2012:526236. doi: 10.1155/2012/526236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Escudero ND, Lacave M, Ubios AM, Mandalunis PM. Effect of monosodium olpadronate on osteoclasts and megakaryocytes: an in vivo study. J Musculoskelet Neuronal Interact. 2009;9:109–120. [PubMed] [Google Scholar]
- 50.Ralte S, Khatri K, Nagar M. Short-term effects of zoledronate on the histomorphology of osteoclast in young albino rats. Ann Anat. 2011;193:509–515. doi: 10.1016/j.aanat.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 51.Weinstein RS, Roberson PK, Manolagas SC. Giant osteoclast formation and long-term oral bisphosphonate therapy. N Engl J Med. 2009:360. doi: 10.1056/NEJMoa0802633. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.