Abstract
Disseminated toxoplasmosis is uncommon in both immunocompetent and immunocompromised hosts with gastrointestinal involvement being rarely described. We report a case of disseminated gastrointestinal toxoplasmosis in an immunocompromised man who presented with one month of diarrhea and abdominal pain. Imaging showed thickening of the ascending colon and cecum. Esophagogastroduodenoscopy and colonoscopy biopsies revealed Toxoplasma gondii, confirmed by immunostain. Symptoms completely resolved following treatment with pyrimethamine, sulfadiazine, and leucovorin. This case highlights the importance of including toxoplasmosis in the differential diagnosis of any immunocompromised individual presenting with gastrointestinal symptoms.
1. Introduction
Gastrointestinal toxoplasmosis is a rare manifestation of a relatively common disease. Disseminated Toxoplasma gondii must be considered in the differential diagnosis of any immunocompromised individual presenting with nonspecific gastrointestinal symptoms, particularly if from or traveling from a region with high T. gondii seropositivity. A biopsy is necessary for definitive diagnosis.
2. Case Report
A 54-year-old man who recently immigrated from El-Salvador with a past medical history of Human Immunodeficiency Virus (HIV) presented with one-month progressive diarrhea, abdominal pain, subjective fevers, and 30-pound weight loss. There were no neurologic complaints. He was immunocompromised with an absolute CD4 count of 6 cells/μL after being off antiretroviral therapy for the past 10 years. He had been working full time as a car mechanic and reported having multiple indoor pet cats throughout his life. The patient was cachectic and frail appearing but with an otherwise unremarkable physical exam. Laboratory showed a HIV viral load of >130,000 copies/mL with Toxoplasma IgG > 700 IU/mL. Laboratory was unremarkable otherwise with Toxoplasma IgM < 3 AU/mL, no fecal ova or parasites observed, negative cryptococcal antigen, negative enteric cultures, and a nonreactive rapid plasma reagin. CT of the chest, abdomen, and pelvis demonstrated diffuse circumferential colonic wall thickening with prominent mesenteric lymph nodes (Figure 1). Bidirectional endoscopy was performed with esophagogastroduodenoscopy showing large ulcerations in the gastric cardia and fundus. Severe inflammation and ulcerations in the right colon and cecum were noted on colonoscopy (Figure 2). Biopsies were taken from both the gastric and colonic ulcerations. Histologic examination revealed markedly active gastritis and colitis with ulceration and ischemic changes (Figure 3). Many free tachyzoites and encysted forms were identified in the lamina propria as well as the cytoplasm of epithelial, endothelial, and smooth muscle cells (Figures 3, 4, and 5). Immunohistochemistry (polyclonal rabbit anti-Toxoplasma, 1 : 200; Dako Corporation, Carpinteria, Calif) confirmed the diagnosis of toxoplasmosis (Figure 5). Follow-up CT head demonstrated a 1.6 cm peripherally enhancing lesion consistent with toxoplasmosis. There was subtle surrounding vasogenic edema without midline shift. The patient was diagnosed with disseminated toxoplasmosis and treatment was initiated with pyrimethamine, sulfadiazine, and leucovorin with subsequent resolution of his gastrointestinal and intracranial toxoplasmosis.
Figure 1.
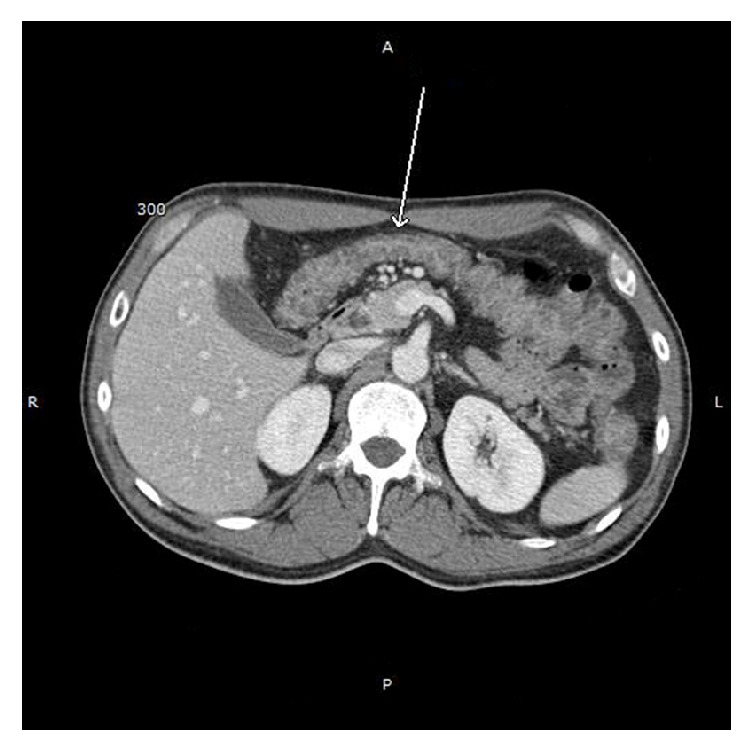
Abdominal computed tomography showing diffuse circumferential thickening of the colonic wall. The arrows refer to circumferential thickening of the transverse colon.
Figure 2.
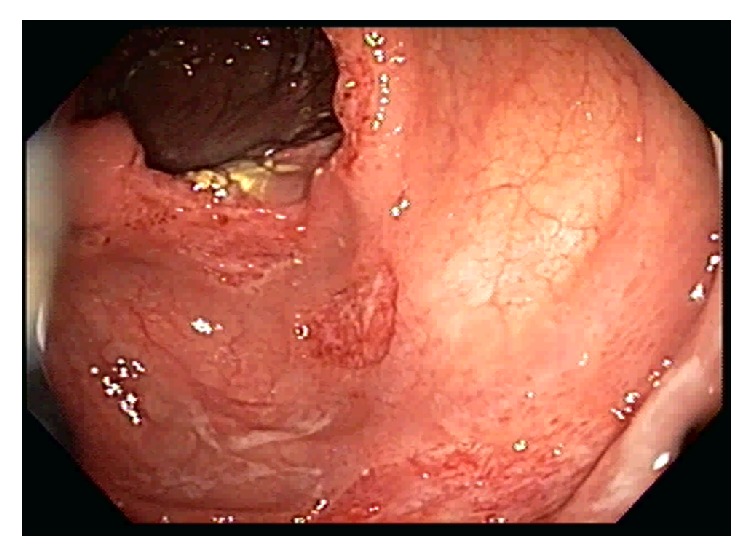
Colonoscopy with ulcerations in the cecum and ascending colon.
Figure 3.
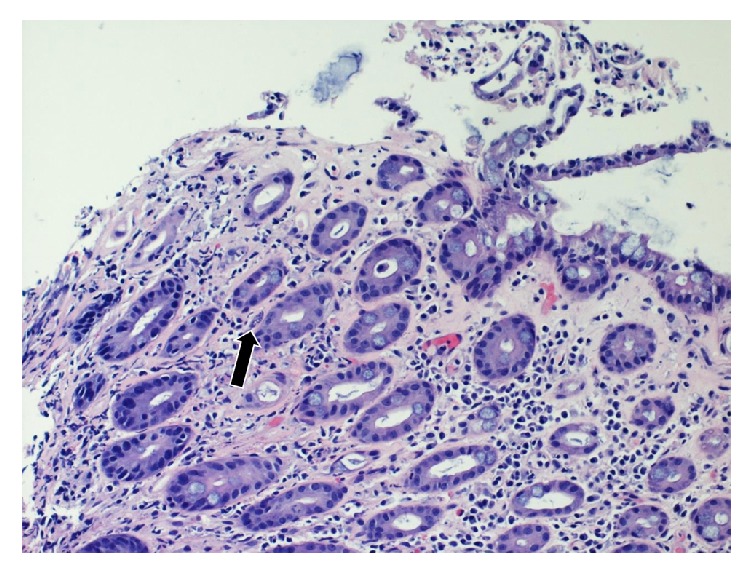
Colonic ulcer biopsy demonstrating ischemic morphology with hyalinization of the lamina propria and atrophic crypts. Small cystic forms are evident. The arrows refer to toxoplasmosis cyst present within the colonic ulcer biopsy.
Figure 4.
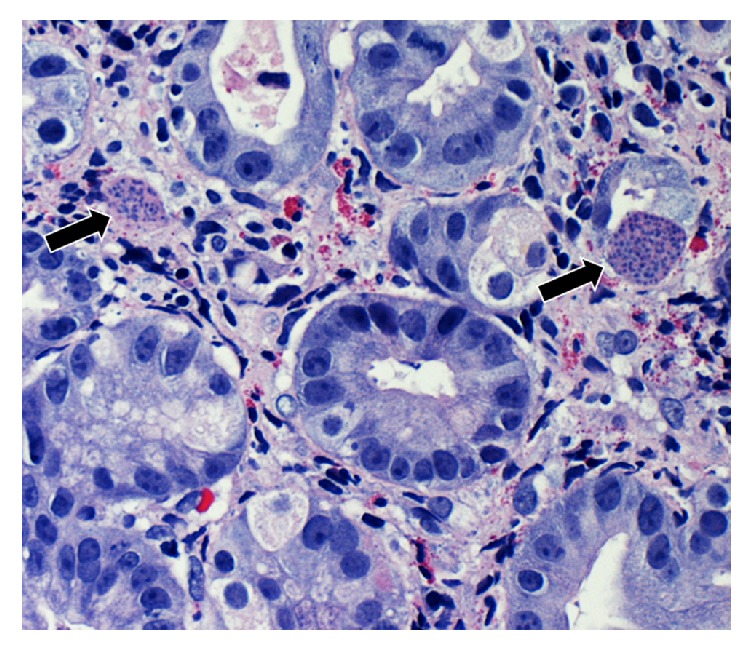
Gastric biopsy specimen showing large cystic forms present in the glandular epithelium and stroma within a background of eosinophils. The arrows refer to toxoplasmosis cyst present within the gastric biopsy.
Figure 5.
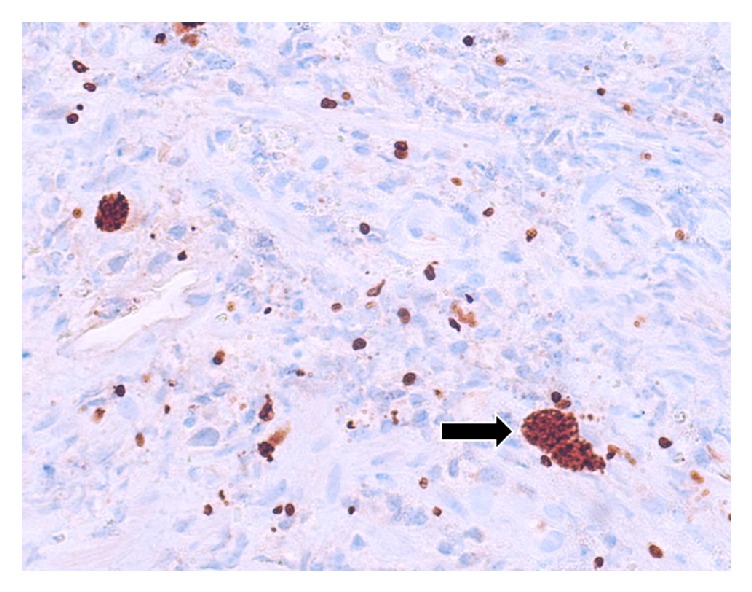
Pathologic specimen with confirmation of T. gondii by immunohistochemistry. Cystic forms are present alongside dispersed tachyzoites. The arrows refer to toxoplasmosis cyst highlighted by immunohistochemistry.
3. Discussion
T. gondii is an obligate intracellular parasite with felines serving as the definitive host. T. gondii can take several forms including oocysts containing sporozoites, tachyzoites, and bradyzoites contained within tissue cysts. Oral ingestion is the primary route of adult infection through consumption of either raw meat containing cysts or water contaminated with the oocysts of infected cat feces [1]. Primary infection is asymptomatic in the vast majority of immunocompetent patients [2]. Infection becomes latent in the form of tissue cysts and reactivation is rare. In immunocompromised individuals however, reactivation of latent infection is the most common cause of symptomatic toxoplasmosis. Patients with Acquired Immunodeficiency Syndrome (AIDS) and a CD4 count < 100 cells/μL who are T. gondii seropositive without prophylaxis have a 30% chance of developing reactivated toxoplasmosis [3]. The most common site of reactivation is the central nervous system followed by the eye, myocardium, skeletal muscle, lungs, bone marrow, and peripheral blood [2, 4]. Extracranial toxoplasmosis occurs in less than 2% of all immunocompromised individuals with reactivation [5, 6].
Although the gastrointestinal tract serves as the most common port of entry into the adult human, gastrointestinal manifestations of toxoplasmosis are rare, occurring in only 6–20% of patients with disseminated disease (defined as affecting >1 organ system) [1, 4, 7–9]. The reason for this paradoxical sparing remains unclear. Antemortem diagnosis is rare with only a handful of cases ever described [10]. When gastrointestinal toxoplasmosis has been identified, the most common underlying risk factor has been severe immunosuppression, with a CD4 count < 60 cells/μL [4, 5, 11]. Previous studies have found between 6.5% and 9.7% of all AIDS patients to have evidence of gastrointestinal toxoplasmosis at time of autopsy [9, 12]. Disseminated toxoplasmosis is often difficult to diagnose due to its nonspecific symptoms and requisite immunohistochemical techniques. For this reason, gastrointestinal toxoplasmosis is often identified postmortem leading to a likely underestimation of its prevalence [13]. Symptoms of gastrointestinal toxoplasmosis include diarrhea, abdominal pain, nausea, vomiting, anorexia, and ascites [14]. Complete or partial involvement of the gastrointestinal tract may be present [15]. If toxoplasmosis is suspected, biopsies are necessary as disseminated toxoplasmosis is almost invariably fatal if left untreated [11, 16]. The most common endoscopic findings of gastrointestinal toxoplasmosis are thickened gastric folds, ulcerative lesions, and nonspecific inflammation [4, 14, 17, 18].
Primary infection with T. gondii initially gives an IgM immune response which is followed by a T. gondii specific IgG response. These IgG antibodies last for life, resulting in lifelong seropositivity [19]. Seropositivity rates vary with geography and age with older individuals and those living in tropical climates more likely to be seropositive [1, 20]. With particular relevance to this case report, one study in El-Salvador found a 97% seropositivity rate by the 6th decade of life with an overall population seropositivity of 59% [21]. This is in stark contrast with a 12.4% overall seropositivity rate in the United States [22]. Although it remains impossible to say whether this particular case was primary versus reactivated toxoplasmosis, given the patients age, immigration history, and immunocompromised state, it is likely he was T. gondii seropositive at time of immigration arguing for reactivation over primary infection.
Unfortunately, data on treatment of extracerebral toxoplasmosis remains lacking. At current, disseminated treatment consists of the standard regimens typically used for cerebral toxoplasmosis. In all patients with diagnosed extracranial toxoplasmosis, even in the absence of neurologic symptoms, occult intracranial toxoplasmosis must be ruled out as up to 41% may have central nervous system involvement [5].
In summary, gastrointestinal toxoplasmosis is a rare manifestation of a relatively common disease. As demonstrated by this case report, disseminated T. gondii must be considered in the differential diagnosis of any immunocompromised individual presenting with nonspecific gastrointestinal symptoms, particularly if from or traveling from a region with high T. gondii seropositivity. A biopsy is necessary for definitive diagnosis.
Consent
Informed consent was obtained for this case report and can be provided upon request.
Disclosure
Zhouwen Tang is now an advanced endoscopy fellow at UT Southwestern Medical Center, Dallas, TX, United States.
Conflicts of Interest
The authors report no conflicts of interest, financial or otherwise, regarding the publication of this paper.
Authors' Contributions
Matthew Glover wrote the manuscript and is the article guarantor. Zhouwen Tang and Robert Sealock revised and edited the manuscript and provided endoscopic imaging. Shilpa Jain revised and edited the manuscript and provided pathologic imaging.
References
- 1.Montoya J. G., Liesenfeld O. Toxoplasmosis. The Lancet. 2004;363(9425):1965–1976. doi: 10.1016/S0140-6736(04)16412-X. [DOI] [PubMed] [Google Scholar]
- 2.Montoya J. G. Laboratory diagnosis of Toxoplasma gondii infection and toxoplasmosis. Journal of Infectious Diseases. 2002;185(1):S73–S82. doi: 10.1086/338827. [DOI] [PubMed] [Google Scholar]
- 3.Gandhi R. T. Toxoplasmosis in HIV-infected patients. In: Post T. W., editor. UpToDate. Waltham, MA, USA: 2016. [Google Scholar]
- 4.Merzianu M., Gorelick S. M., Paje V., Kotler D. P., Sian C. Gastric toxoplasmosis as the presentation of acquired immunodeficiency syndrome. Archives of Pathology & Laboratory Medicine. 2005;129(4):e87–90. doi: 10.5858/2005-129-e87-GTATPO. [DOI] [PubMed] [Google Scholar]
- 5.Rabaud C., May T., Amiel C., et al. Extracerebral toxoplasmosis in patients infected with HIV: a french national survey. Medicine (Baltimore) 1994;73(6):306–314. doi: 10.1097/00005792-199411000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Belanger F., Derouin F., Grangeot-Keros L., Meyer L. Incidence and risk factors of toxoplasmosis in a cohort of human immunodeficiency virus-infected patients: 1988-1995. Clinical Infectious Diseases. 1999;28(3):575–581. doi: 10.1086/515147. [DOI] [PubMed] [Google Scholar]
- 7.Garcia L. W., Hemphill R. B., Marasco W. A., Ciano P. S. Acquired immunodeficiency syndrome with disseminated toxoplasmosis presenting as an acute pulmonary and gastrointestinal illness. Archives of Pathology and Laboratory Medicine. 1991;115(5):459–463. [PubMed] [Google Scholar]
- 8.Gleason T. H., Hamlin W. B. Disseminated toxoplasmosis in the compromised host: a report of five cases. Archives of Internal Medicine. 1974;134(6):1059–1062. doi: 10.1001/archinte.1974.00320240093011. [DOI] [PubMed] [Google Scholar]
- 9.Jautzke G., Sell M., Thalmann U., et al. Extracerebral toxoplasmosis in AIDS: histological and immunohistological findings based on 80 autopsy cases. Pathology Research and Practice. 1993;189(4):428–436. doi: 10.1016/S0344-0338(11)80331-6. [DOI] [PubMed] [Google Scholar]
- 10.Mackowiak P. A., Asmal M., Factor R. E., Walensky R. P. An HIV-infected man with an upset stomach. Clinical Infectious Diseases. 2008;47(7):979–980. doi: 10.1086/591695. [DOI] [PubMed] [Google Scholar]
- 11.Williams S. L., Burton E. C. Disseminated toxoplasmosis in a patient with undiagnosed AIDS. Proceedings (Baylor University Medical Center) 2009;22(1):20–22. doi: 10.1080/08998280.2009.11928463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guimarães L. C., Silva A. C. A. L., Micheletti A. M. R., Moura E. N. M., Silva-Vergara M. L., Adad S. J. Morphological changes in the digestive system of 93 human immunodeficiency virus positive patients: An autopsy study. Revista do Instituto de Medicina Tropical de Sao Paulo. 2012;54(2):89–93. doi: 10.1590/S0036-46652012000200006. [DOI] [PubMed] [Google Scholar]
- 13.Hofman P., Bernard E., Michiels J. F., Thyss A., Le Fichoux Y., Loubiere R. Extracerebral toxoplasmosis in the acquired immunodeficiency syndrome (AIDS) Pathology Research and Practice. 1993;189(8):894–901. doi: 10.1016/S0344-0338(11)81101-5. [DOI] [PubMed] [Google Scholar]
- 14.Ganji M., Tan A., Maitar M. I., Weldon-Linne C. M., Weisenberg E., Rhone D. P. Gastric toxoplasmosis in a patient with acquired immunodeficiency syndrome: a case report and review of the literature. Archives of Pathology and Laboratory Medicine. 2003;127(6):732–734. doi: 10.5858/2003-127-732-GTIAPW. [DOI] [PubMed] [Google Scholar]
- 15.Tahmasbi M., Al Diffalha S., Strosberg C. M., Sandin R., Ghayouri M. Duodenal toxoplasmosis in a bone marrow transplant patient: a rare case report. Human Pathology: Case Reports. 2015 doi: 10.1016/j.ehpc.2016.03.006. [DOI] [Google Scholar]
- 16.Yang M., Perez D. Disseminated toxoplasmosis as a cause of diarrhea. Southern Medical Journal. 1995;88(8):860–861. doi: 10.1097/00007611-199508000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Alpert L., Miller M., Alpert E., Satin R., Lamoureux E., Trudel L. Gastric toxoplasmosis in acquired immunodeficiency syndrome: Antemortem diagnosis with histopathologic characterization. Gastroenterology. 1996;110(1):258–264. doi: 10.1053/gast.1996.v110.pm8536865. [DOI] [PubMed] [Google Scholar]
- 18.Lowe D., Hessler R., Lee J., Schade R., Chaudhary A. Toxoplasma colitis in a patient with acquired immune deficiency syndrome. Gastrointestinal Endoscopy. 2006;63(2):341–342. doi: 10.1016/j.gie.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Yohanes T., Debalke S., Zemene E. Latent toxoplasma gondii infection and associated risk factors among HIV-infected individuals at Arba Minch Hospital, south Ethiopia. AIDS Research and Treatment. 2014;2014 doi: 10.1155/2014/652941.652941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akuffo H., Linder E., Wahlgren M., Ljungström I. Parasites of the Colder Climates. CRC Press; 2002. [DOI] [Google Scholar]
- 21.Remington J. S., Efron B., Cavanaugh E., Simon H. J., Trejos A. Studies on toxoplasmosis in El Savador prevalence and incidence of toxoplasmosis as measured by the Sabin-Feldman dye test. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1970;64(2):252–267. doi: 10.1016/0035-9203(70)90132-X. [DOI] [PubMed] [Google Scholar]
- 22.Jones J. L., Kruszon-Moran D., Rivera H. N., Price C., Wilkins P. P. Toxoplasma gondii seroprevalence in the United States 2009-2010 and comparison with the past two decades. American Journal of Tropical Medicine and Hygiene. 2014;90(6):1135–1139. doi: 10.4269/ajtmh.14-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]


