Abstract
The oncogenic transcription factor c-Jun plays an important role in cell proliferation, transformation and differentiation. All identified c-Jun-interacting proteins are localized to the nucleus or cytoplasm and function in their intact forms. Here we show that the pleckstrin homology domain-containing protein CKIP-1 (casein kinase 2-interacting protein-1) functions as a plasma membrane-bound AP-1 regulator. During apoptosis, CKIP-1 is cleaved by caspase-3 and translocated to the cytoplasm and then to the nucleus. C-terminal fragments of cleaved CKIP-1 strongly repress AP-1 activity. Importantly, CKIP-1 overexpression promotes apoptosis by forming a positive feedback loop between CKIP-1 and caspase-3. RNA interference of CKIP-1 or overexpression of c-Jun attenuates the sensitivity to apoptosis, indicating a novel role of CKIP-1 in apoptosis. CKIP-1 is the first case of a c-Jun-interacting protein that regulates AP-1 activity via caspase-3-dependent cleavage and translocation.
Keywords: AP-1, apoptosis, caspase-3, CKIP-1
Introduction
c-Jun, the core component of the AP-1 family of transcription factors (Shaulian and Karin, 2001, 2002), is regulated by post-translational modifications, for example, phosphorylation, acetylation and ubiquitination by interacting proteins (Karin et al, 1997; Nateri et al, 2004; Wertz et al, 2004). More than 60 proteins, including bZIP factors, non-bZIP DNA-binding proteins, coactivators that do not bind DNA directly and structural components of the nucleus, have been demonstrated to interact with c-Jun (Chinenov and Kerppola, 2001).
Unlike other transcription factors, such as STATs, NF-κB, Notch and tubby, which are localized outside of the nucleus (cytoplasmic or plasma membrane-associated) until particular signaling events trigger their nuclear translocation, c-Jun/AP-1 family members are nuclear proteins constitutively bound to DNA at most times (Brivanlou and Darnell, 2002). All of the identified c-Jun-interacting proteins are localized to the nucleus or cytoplasm and function in their intact forms. For example, Smad and JAB1 translocate from the cytoplasm to the nucleus, where they bind to and regulate c-Jun/AP-1 activity (Chinenov and Kerppola, 2001). However, whether plasma membrane-associated proteins could transduce signals directly from the cell surface to the nuclear c-Jun/AP-1 is unknown. We have evaluated if the plasma membrane pleckstrin homology (PH) domain-containing protein CKIP-1 (casein kinase 2-interacting protein-1) would play such a role.
CKIP-1 contains a C-terminal putative c-Jun-interacting region comprising 72 amino acids (aa 338–409, named C-term1), which was first isolated in a two-hybrid screen as an interacting partner of the c-Jun leucine zipper (Chevray and Nathans, 1992), implying CKIP-1 as a novel c-Jun/AP-1 regulator. Full-length CKIP-1 was identified in a two-hybrid screen as an interacting partner of the CK2 catalytic subunit and shown to include an N-terminal PH domain (Bosc et al, 2000). CKIP-1 is implicated in phosphatidylinositol 3-kinase-regulated muscle differentiation (Safi et al, 2004) as well as in the recruitment of CK2 to the plasma membrane (Olsten et al, 2004).
CKIP-1 might mediate a novel regulatory mechanism for AP-1 activity. The C-term1 domain of CKIP-1 possesses a leucine zipper motif (aa 352–380) predicted to be capable of forming coiled coils. However, CKIP-1 lacks either an adjacent basic region typical of bZIP proteins to the leucine zipper or any other DNA-binding motifs, indicating that CKIP-1 is not a transcription factor and distinct from other c-Jun bZIP region-binding proteins. Indeed, proteins with both a PH domain and a leucine zipper motif have been rarely observed, that is, only in proteins such as AFAP-110, β-Pix, APPL and Vav (Romero and Fischer, 1996; Mitsuuchi et al, 1999; Baisden et al, 2001; Kim et al, 2001). None of these proteins have been functionally related to AP-1. Hence, the PH domain of CKIP-1 predicts plasma membrane localization, whereas the leucine zipper may retain CKIP-1 within the nucleus.
Here we propose that CKIP-1 functions as a plasma membrane-bound AP-1 regulator that translocates to the nucleus. We provide evidence that CKIP-1 has the capacity to interact with c-Jun and JunD, but not JunB, c-Fos or ATF2, in the yeast-two hybrid system. Although full-length CKIP-1 is localized to the plasma membrane in mammalian cells, we observed that the CKIP-1-C-term1 domain localizes to the nucleus, where it is colocalized and can be co-immunoprecipitated with c-Jun. We further demonstrated that the C-term1 domain and another truncated CKIP-1 construct named C-term2 (aa 308–409) are potent negative regulators of AP-1 activity. Furthermore, C-term1 and C-term2 fragments could be released during apoptosis via caspase-3-dependent cleavage. The cleavage of CKIP-1 promotes apoptosis via amplified caspase-3 activation. Knockdown of CKIP-1 by RNA interference (RNAi) or overexpression of c-Jun attenuates the sensitivity to apoptotic stimuli, indicating a novel role of CKIP-1 in apoptosis. Therefore, CKIP-1 represses AP-1 activity via caspase-3-dependent cleavage and translocation, and this repression contributes to the execution of apoptosis.
Results
CKIP-1 interacts with c-Jun in vitro and in vivo
We first confirmed the interaction in the yeast two-hybrid system. C-term1 of CKIP-1 was sufficient and was required for binding to c-Jun. The region of c-Jun required for interaction with CKIP-1 was mapped to the leucine zipper domain, whereas the adjacent basic region was not required (Figure 1A). C-term1 specifically interacts with c-Jun and JunD, but not JunB, c-Fos and ATF2 (Figure 1B). It is of interest that C-term1 was capable of self-interaction with CKIP-1, probably mediated by the leucine zipper region (aa 352–380) (Figure 1B). The interaction with c-Jun was also confirmed by in vitro GST pull-down assays, in which in vitro-translated CKIP-1 protein specifically bound to GST-c-Jun but not to GST alone (Figure 1C).
Figure 1.
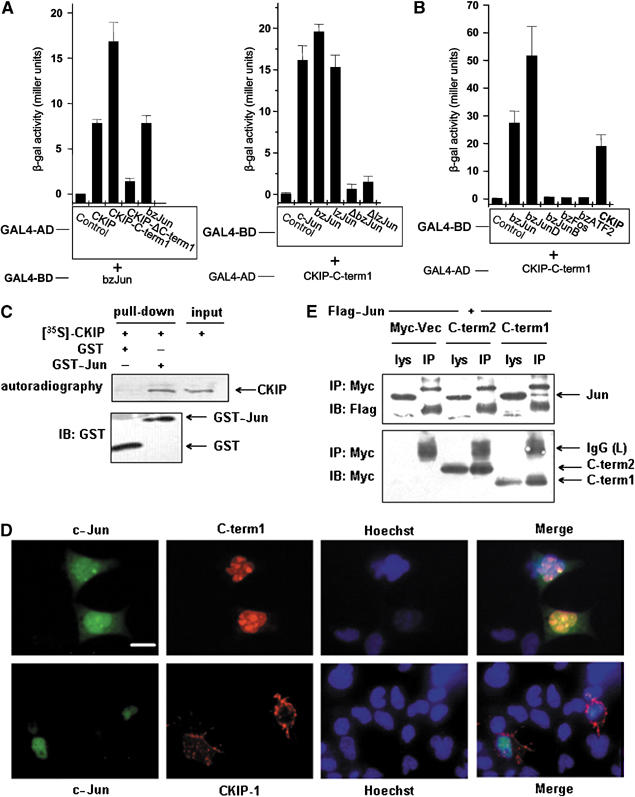
CKIP-1 interacts with c-Jun in vitro and in vivo. (A) To examine the interactions between CKIP-1 and c-Jun, cDNAs encoding full-length CKIP-1 or deletion mutants (C-term1, ΔC-term1) were expressed in yeast as fusions with the DNA activation domain (AD) of GAL4, using the plasmid pGADT7. Constructs encoding GAL4 DNA-binding domain fusions of c-Jun or deletion mutants (bzJun 247–331, lzJun 277–331, ΔbzJun 1–246, ΔlzJun 1–276) were cotransformed along with CKIP-1 constructs. The binding activity was quantified by a liquid β-galactosidase assay (n=3, mean±s.d. indicated on the histograms). (B) C-term1 specially interacts with c-Jun and JunD, but not JunB, c-Fos and ATF2 among the AP-1 family (n=3, mean±s.d.). (C) In vitro-translated 35S-labeled CKIP-1 proteins and purified GST or GST-c-Jun proteins expressed in E. coli BL21 were used in pull-down assays. Proteins bound to GST fusion proteins were eluted from beads using Laemmli sample buffer. CKIP-1 proteins were detected by autoradiography (upper panel), and CKIP-1 input was loaded as a control (lane 3). GST fusion proteins were detected on immunoblots. (D) c-Jun was colocalized with C-term1 within the nucleus, but not with the plasma membrane-associated full-length CKIP-1. GFP-c-Jun and RFP-C-term1 or RFP-CKIP-1 were cotransfected into COS7 cells and visualized after 24 h. Scale bar, 10 μm. (E) Flag-c-Jun was coexpressed in COS7 cells together with Myc-tagged C-term2, C-term1 or vector alone for 24 h followed by immunoprecipitation with anti-Myc and immunoblotting with anti-Flag or anti-Myc. The arrows indicate the mobility of c-Jun, IgG (L), C-term2 and C-term1. The experiment was repeated three times with similar results observed. IP, immunoprecipitate; IB, immunoblot; lys, lysate; IgG (L), the light chain of IgG.
We next asked whether CKIP-1 could associate with c-Jun in vivo. In mammalian cells, CKIP-1 is predominantly localized to the plasma membrane and therefore unable to interact with nuclear c-Jun proteins (Figure 1D). In contrast, C-term1 was partially colocalized with c-Jun in a speckle pattern (Figure 1D). Furthermore, c-Jun was partially co-immunoprecipitated with C-term1 (Figure 1E). As a control, C-term2 (aa 308–409), which was previously shown to be cytoplasmic (Bosc et al, 2000), did not associate with c-Jun in vivo although it possesses all the sequences present in C-term1. These results suggested that CKIP-1 could interact with c-Jun in vitro and in vivo.
C-term1 and C-term2 of CKIP-1 strongly repress AP-1 activity
Considering that c-Jun/AP-1 plays an important role in the regulation of a wide range of cellular processes, including cell proliferation, death, survival and differentiation (Shaulian and Karin, 2001, 2002; Vogt, 2001), we next explored whether the C-term1–c-Jun interaction affects AP-1 activity. COS7 cells, in which high expression of MEKK1 (kinase of kinase of JNK) was observed (Yan et al, 1994), were transfected with an AP-1 promoter-driven reporter gene in the absence or presence of exogenous c-Jun. Overexpression of c-Jun induced a >2.5-fold increase in AP-1 activity. Expression of C-term1 repressed both the endogenous and exogenous AP-1 activity to 15% of c-Jun alone (Figure 2A). Surprisingly, C-term2 also repressed AP-1 activity to a similar extent (Figure 2A), although it was unable to bind to c-Jun directly (Figure 1E). By comparison, either full-length CKIP-1 or other mutants including PH, ΔPH and ΔC-term1 failed to display significant repression. As a control, coexpression of CK2α, which is the catalytic subunit of casein kinase 2 and has previously been shown to repress c-Jun transactivation activity (Lin et al, 1992), displayed a similar weak repression.
Figure 2.
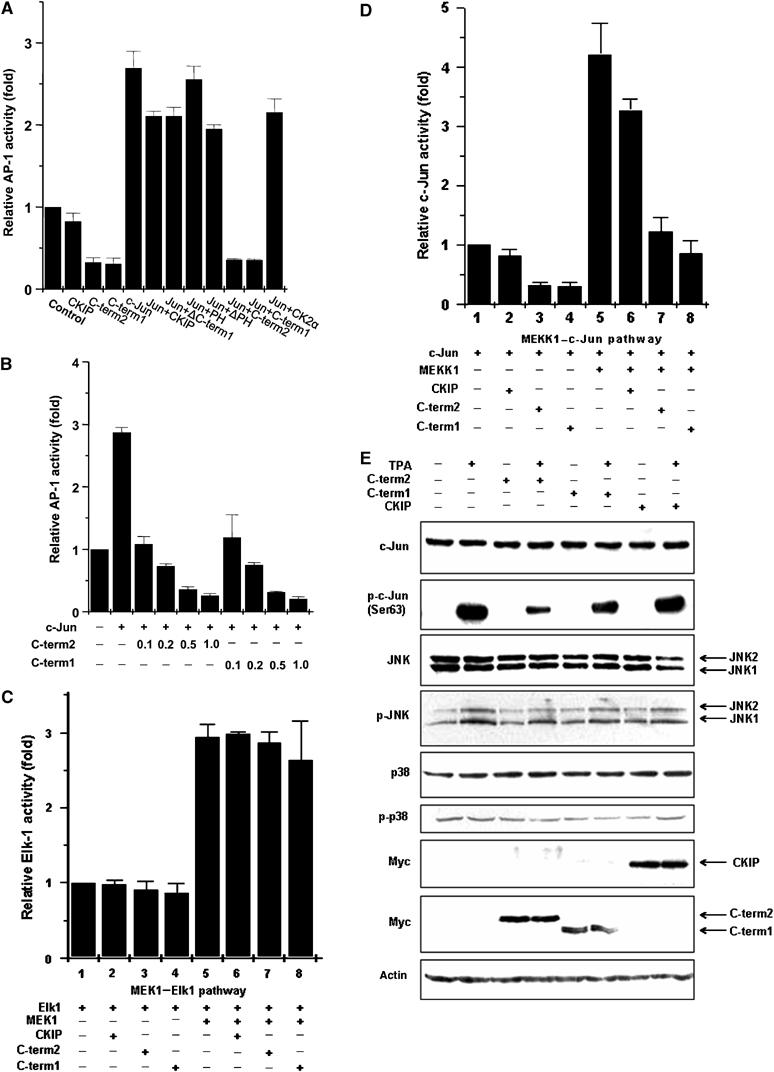
Both C-term1 and C-term2 of CKIP-1 effectively inhibit AP-1 activity. (A) AP-1-driven luciferase reporter activity was assessed in COS-7 cells transfected with or without exogenous c-Jun together with CKIP-1 or the indicated truncates. Reporter activity was assayed as described in Materials and methods and represents the mean±s.d. of three separate experiments. (B) C-term1 and C-term2 inhibited AP-1 activity in a dose-dependent manner (n=3, mean±s.d.). (C) Neither CKIP-1 nor C-term1, C-term2 showed a significant effect on Elk-1 activity, indicating that the effect of CKIP-1 on AP-1 transcription factors is specific. (D) Effect of C-term1 and C-term2 on AP-1 activity with the trans-reporter gene system. COS-7 cells were cotransfected with c-Jun and MEKK1, CKIP-1, C-term2 or C-term1 as indicated. After 24 h, c-Jun activity was assayed by the reporter gene assay as described in Materials and methods (n=3, mean±s.d.). (E) HEK293 cells were transfected with CKIP-1, C-term2 or C-term1. Endogenous expression and phosphorylation levels of c-Jun, JNK and p38 in HEK293 cells in response to the positive stimulus TPA (5 nM, 30 min) were analyzed by immunoblotting with specific antibodies to the proteins indicated on the left. The arrows (right side of figure) indicate the mobility of JNK1, JNK2, CKIP-1, C-term2 and C-term1.
The strong inhibitory effects of C-term1 or C-term2 were dose-dependent (Figure 2B) and specific since they did not affect Elk-1 activation (Figure 2C). It is to be noted that the repression efficiency of 0.1 μg C-term1 or C-term2 was two-fold that of 0.5 μg full-length CKIP-1 (Figure 2A and B). These results were confirmed in the trans-reporter gene system of Figure 2D, in which c-Jun activity was measured as the result of activation by cotransfection of MEKK1. Therefore, C-term1 and C-term2 are efficient negative regulators of AP-1.
We further investigated the mechanisms by which C-term1 and C-term2 repress AP-1 activity. To define the influence of C-term1 and C-term2 on the signaling events necessary for AP-1 activation, we detected the endogenous phosphorylation status and expression levels of relevant signaling components by Western blot analysis. JNKs phosphorylate c-Jun very efficiently, JunD less efficiently, but do not phosphorylate JunB (Kallunki et al, 1996). 12-O-tetradecanoylphorbol-13-acetate (TPA) treatment induced increased phosphorylation of JNK and c-Jun with no detectable changes of their expression levels (Figure 2E). Coexpression of C-term2 led to only a minimal decrease of JNK phosphorylation but a significant decrease of c-Jun serine 63 phosphorylation levels, without an effect on p38 phosphorylation or their expression levels (Figure 2E). We tested whether C-term1 inhibited the DNA-binding activity of c-Jun. Unexpectedly, C-term1 had no effect on the binding of c-Jun to the AP-1 site TRE (data not shown). Collectively, these data suggested that CKIP-1 C-terminal fragments could negatively regulate AP-1 activity via direct binding to c-Jun or indirect regulation of JNK activation.
Release of C-term1 and C-term2 from intact CKIP-1 through caspase-3 cleavage during apoptosis
To test whether C-term1 and C-term2 could be produced via proteolysis under physiological or pathological conditions, the changes of CKIP-1 during apoptosis were monitored. We established stable C-terminal Myc-tagged CKIP-1-expressing SK-BR-3 breast cancer cells, in which endogenous CKIP-1 expression was undetectable (data not shown). The cells were treated with tumor necrosis factor (TNF) plus the protein synthesis inhibitor cycloheximide (CHX) or a higher dose of CHX alone. The treatment induced CKIP-1 cleavage. Caspase-3, a major effector caspase in apoptosis, was also cleaved into subunits (Figure 3A). The cleavage could be blocked by addition of the general caspase inhibitor, zVAD-fmk, but not by other protease inhibitors (Figure 3B), indicating the involvement of caspases in the cleavage of CKIP-1.
Figure 3.
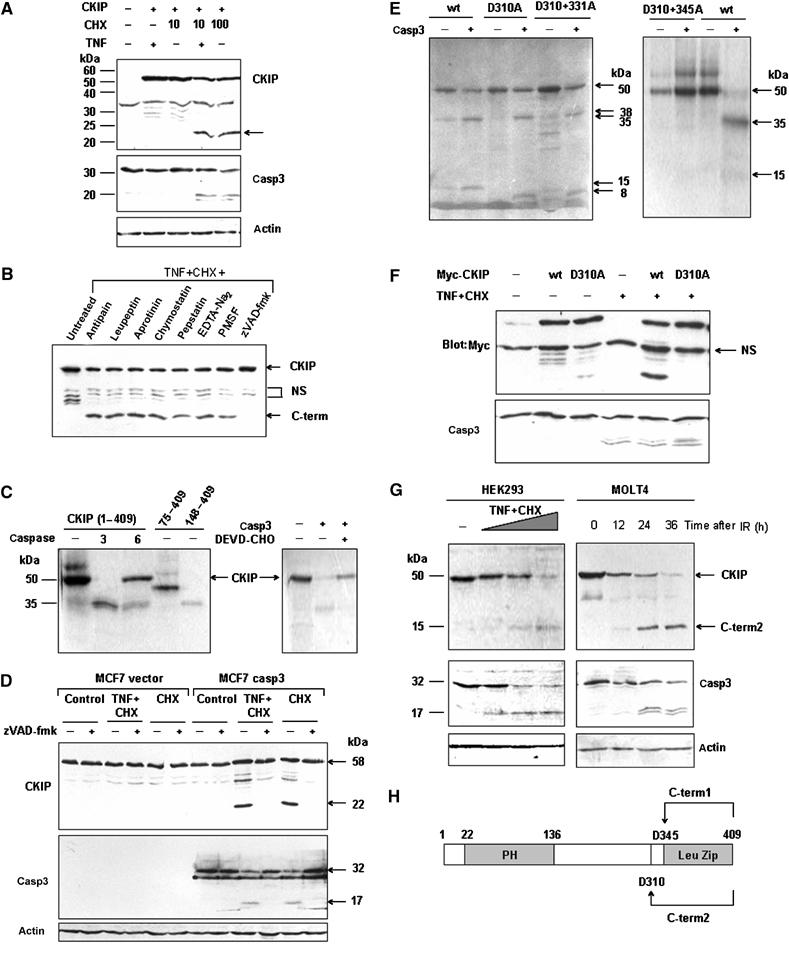
C-term1 and C-term2 can be released through caspase-3-mediated cleavage. (A) A 22 kDa fragment of CKIP-1 could be released in response to TNF plus CHX. SK-BR-3 cells that stably expressed CKIP-1 or not were deprived of serum overnight and treated for 8 h with TNF, CHX or both. Cell lysates were analyzed by immunoblotting. The mobilities of CKIP-1, caspase-3 and actin are indicated. The arrow indicates a band with a mobility similar to that predicted for cleaved C-term2. (B) CKIP-1/SK-BR-3 cells were treated with TNF (20 ng/ml) plus CHX (10 μg/ml) for 8 h. The following protease inhibitors were added, separately, to the medium 4 h before treatment: antipain (100 μM), leupeptin (200 μM), aprotinin (200 μM), chymostatin (200 μM), pepstatin (100 μM), EDTA-Na2 (200 μM), PMSF (200 μM) or zVAD-fmk (50 μM). Cell lysates were blotted with anti-Myc antibody. (C) In vitro-translated CKIP-1 was incubated in the absence of caspase or with purified active caspase-3 (together with caspase-3 inhibitor DEVD-CHO or not, right panel) or caspase-6. Autoradiographs are shown. The truncates 75–409 and 148–409 were also translated and loaded to compare the mobility with the cleaved CKIP-1 fragments. They represent two C-terminal fragments downstream of two potential caspase cleavage sites D74 and D147, which conform to the caspase-3 and caspase-6 consensus sequences DXXD and (V/I/L)EXD, respectively. (D) Caspase-3-deficient MCF-7 cells transfected without or with Flag-caspase-3 were treated with TNF plus CHX (10 μg/ml), or 100 μg/ml CHX in the absence or presence of zVAD-fmk (50 μM). Cell lysates were analyzed for cleavage of CKIP-1 and caspase-3. (E) Wild type or point mutants of CKIP-1 were used in the in vitro caspase-3 cleavage assay as in (C). (F) D310 is the major cleavage site in TNF-induced apoptosis. HEK293 cells were transfected with plasmids as indicated above the lanes and treated with TNF+CHX to induce apoptosis. Cell lysates were analyzed by immunoblotting with anti-Myc or caspase-3 antibody. NS, nonspecific. (G) Endogenous CKIP-1 can be cleaved during apoptosis induced by TNF treatment in HEK293 cells or ionizing radiation (10 Gy) in MOLT-4 cells. HEK293 cells were treated with TNF+CHX as indicated and MOLT4 cells were irradiated (IR, 10 Gy) and cultured for the indicated times. Cell lysates were analyzed by immunoblotting with anti-CKIP-C, caspase-3 and actin antibodies. The arrows indicate full-length CKIP-1 and endogenous cleaved C-term2 fragment. (H) Schematic representation of the caspase-3 cleavage sites in CKIP-1.
The fact that CKIP-1 protein contains 24 aspartic residues led us to evaluate CKIP-1 as a potential caspase substrate. To prove the hypothesis, caspase-3 and caspase-6 were used in in vitro cleavage assays. CKIP-1 was cleaved completely into a 35 kDa fragment after incubation with caspase-3, but less effectively with caspase-6 (Figure 3C). Addition of the caspase-3 inhibitor DEVD-CHO abolished the appearance of the fragment (Figure 3C). Moreover, in caspase-3-deficient MCF-7 cells treated with the same death stimuli, CKIP-1 was not cleaved. Re-expression of Flag-tagged caspase-3 in MCF-7 cells restored the CKIP-1 cleavage (Figure 3D). These data indicate that CKIP-1 is a novel caspase-3 substrate.
We next mapped the caspase-3 cleavage site(s) in CKIP-1. The fact that a fragment of 22 kDa (Figure 3A, arrow) appeared after TNF treatment suggests that the site might lie about 100–120 aa to the C-terminus of CKIP-1. This is in consideration of the fact that CKIP-1 is extended by 51 aa due to the tag. Hence, the 22 kDa fragment (Figure 3A, arrow) encompasses both C-term2 (15 kDa) and the tag (7 kDa). Several potential cleavage sites, D310, D331, D338 and D345, are within the region. Single substitution by D310A altered the size but did not abolish the appearance of cleaved products. It resulted in a slightly larger fragment (38 kDa) (Figure 3E; Supplementary Figure S1), indicating the existence of another cleavage site located C-terminal to D310. Double mutation of D310+345A, but not D310+331A, abolished cleavage of CKIP-1 (Figure 3E); hence, the cleavage sites for caspase-3 are located in positions D310 and D345, which are conserved in human, mouse and rat CKIP-1 (Supplementary Figure S1). Interestingly, C-term2 and C-term1 can mimic the two C-terminal fragments produced by cleavage in vitro (Figure 3H).
Mutant CKIP-1-D310A was resistant to cleavage in TNF-induced apoptosis, proving D310 to be the major site in vivo (Figure 3F). Not that only one C-terminal fragment of 22 kDa consistent with the fragment of 15 kDa in vitro was observed in TNF-induced apoptosis due to the fact that proteolysis of CKIP-1 at D345 requires phosphorylation by CK2 at S346, followed by sequential phosphorylation by glycogen synthase kinase 3 (GSK3) at S342 (unpublished data).
More importantly, endogenous CKIP-1 could also be cleaved in response to serum withdrawal or TNF treatment in HEK293 cells, TNF treatment in HeLa cells and ionizing radiation in MOLT-4 or Jurkat cells (Figure 3G, data not shown). Taken together, these results strongly suggest that both in vivo (at least for the D310 cleavage) and in vitro, CKIP-1 is cleaved by caspase-3 in positions D310 and D345.
The kinetics of CKIP-1 cleavage are correlated with those of AP-1 activity during apoptosis
If CKIP-1 cleavage is important for regulating AP-1 activity during apoptosis, the kinetics of both events should be similar. To determine the time courses of these events, SK-BR-3 stable transfectants induced to undergo apoptosis with TNF plus CHX were harvested at 2 h intervals. Cleavage of CKIP-1 occurred 4 h after treatment, and the amount of the C-term2-like fragment was increased significantly 6 h after treatment (Figure 4A). Significantly, the onset of the decrease of c-Jun S63 phosphorylation at 6 h postinduction coincided with the appearance of caspase-cleaved CKIP-1, whereas moderate changes were seen in the control cells. At 8 h postinduction, the c-Jun S63 phosphorylation level approximated 20% of that at 2 h postinduction in CKIP-1-expressing cells (Figure 4A and C), which is similar to the effect of transiently expressed C-term2 (Figure 2).
Figure 4.
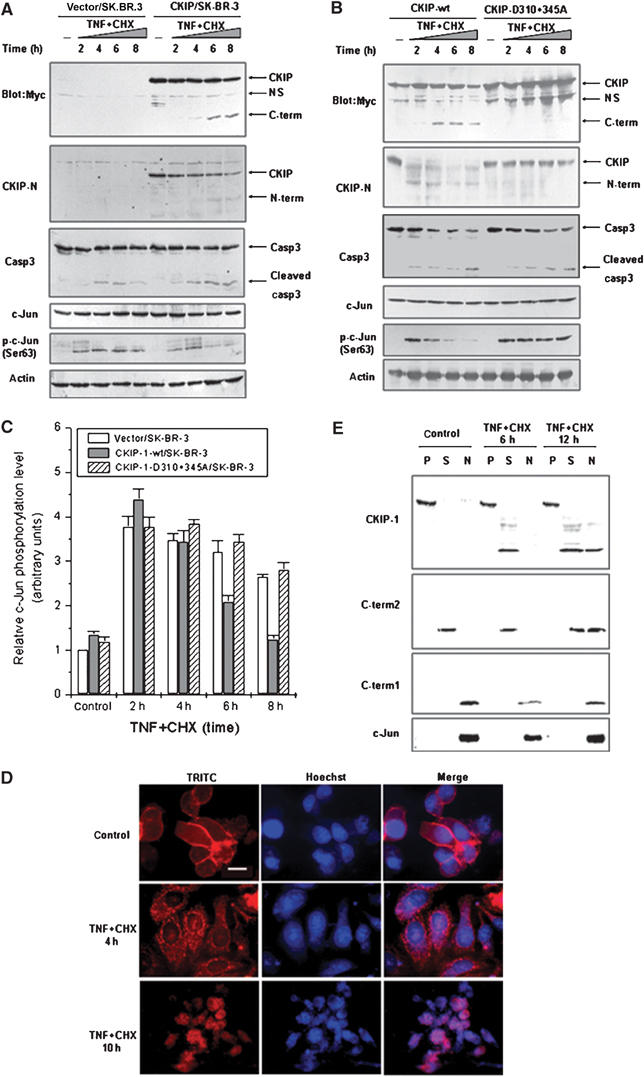
CKIP-1 regulates AP-1 activation via caspase-3-mediated cleavage and translocation during apoptosis. (A, B) CKIP-1/SK-BR-3, D310+345A/SK-BR-3 or control pcDNA3.1/SK-BR-3 stable transfectant cells were seeded in 12-well plates. After serum withdrawal overnight, the cells were treated with TNF (20 ng/ml) plus CHX (10 μg/ml) for the indicated times. The cell lysates were analyzed by immunoblotting. Arrows on the right indicate the mobility of the indicated proteins. NS, nonspecific. (C) Regulation of c-Jun phosphorylation by CKIP-1 and D310+345A during apoptosis. The results of three separate experiments (mean±s.d.) are shown. Immunoblotting with antibodies to phospho-c-Jun(Ser63) was performed as in panels A and B and quantified by densitometry. (D) Translocation of CKIP-1 during apoptosis. CKIP-1/SK-BR-3 cells were treated as in panels A and B. Immunofluorescence analysis was performed with anti-Myc antibodies as described in Materials and methods. Scale bar, 10 μm. (E) P100 (P), S100 (S) and nuclear (N) fractions of CKIP-1/SK-BR-3 and SK-BR-3 cells transiently transfected with Myc-C-term1 or Myc-C-term2 were isolated and subjected to immunoblot analysis with Myc antibody. The distribution of endogenous c-Jun is also shown.
If c-Jun phosphorylation decrease depends upon cleavage of CKIP-1, this alteration should not be observed with the CKIP-1-D310+345A mutant. As expected, CKIP-1-D310+345A was resistant to cleavage and failed to inhibit c-Jun phosphorylation (Figure 4B and C). These data demonstrate that regulation of c-Jun phosphorylation by CKIP-1, like cleavage of CKIP-1 during apoptosis, is dependent on caspase-3.
Different from full-length CKIP-1, C-term2 has been shown to be localized in the cytoplasm (Bosc et al, 2000). We asked whether the localization of CKIP-1 was altered during apoptosis. Stably expressed CKIP-1 was localized to the plasma membrane in SK-BR-3 cells, even if the cells were serum-starved overnight. At 4 h after TNF treatment, CKIP-1 displayed a punctate pattern in the cytoplasm around a well-defined nucleus; most nuclei were still intact. At 10 h post-treatment, CKIP-1 was in the nucleus, and chromatin condensation was apparent (Figure 4D). Furthermore, since C-terminal fragments, but not full-length CKIP-1, inhibit the transcriptional activity of Jun (Figure 2), the cellular localization of C-term1 and C-term2 in response to stress was examined. Through cellular fractionation, P100, S100 and N (nuclear) fractions were obtained and analyzed by Western blot. The efficiency of the fractionation was confirmed by analysis of plasma membrane marker protein Na+/K+-ATPase α1, and nuclear marker proteins lamin A and nucleolin (Supplementary Figure S2). Figure 4E shows that C-term1 and c-Jun were constitutively localized to the N fraction, and C-term2 could partially translocate from the cytoplasm to the nucleus after TNF treatment. Full-length CKIP-1 was retained at the plasma membrane, while the cleaved C-term2-like fragment appeared in the cytoplasm and the nucleus, indicating that cleavage was followed by translocation. The results support the hypothesis that CKIP-1 was cleaved by caspase-3 and translocated from the plasma membrane to the cytoplasm and nucleus, thereby negatively regulating AP-1 activity.
CKIP-1 forms a feedback loop with caspase-3
Interestingly, caspase-3 was cleaved earlier and more significantly in CKIP-1/SK-BR-3 cells than in the control (Figure 4A), implying that expression of CKIP-1 may increase the sensitivity of the cells to TNF. To confirm this, caspase-3 activation was measured by following cleavage of Ac-DEVD. After TNF treatment, caspase-3 was activated within 2 h in CKIP-1/SK-BR-3 cells but at 4 h in control cells (Figure 5A). In contrast, the D310+345A mutant had little effect, apart from a moderate decrease of caspase-3 activation after 6 h (Figure 5A). Note that, at 8 h, the difference was indistinguishable.
Figure 5.
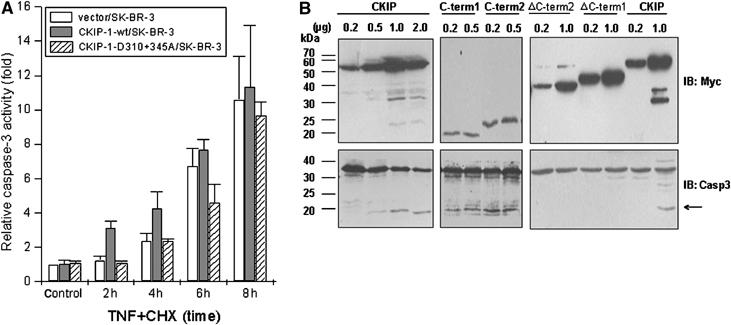
CKIP-1 is an amplifier of caspase-3. (A) SK-BR-3 cells stably transfected with vector alone or with wild-type CKIP-1 or mutant (D310+345A) were treated with TNF+CHX for the indicated times followed by measurements of caspase-3 activity as described in Materials and methods. Expression of CKIP-1 increased the sensitivity to caspase-3 activation. (B) HEK293 cells were transfected with the indicated Myc-tagged constructs for 24 h. Immunoblotting with anti-Myc and caspase-3 antibody was performed. Overexpression of C-term1 or C-term2 induces constitutive caspase-3 activation. The arrow indicates the mobility of cleaved caspase-3.
In addition, transient expression of higher doses of CKIP-1 was sufficient to activate caspase-3 and, in turn, to induce cleavage of CKIP-1 (Figure 5B). Overexpression of C-term1 or C-term2 could constitutively activate caspase-3, whereas deletion of either segment abolished the function, suggesting that the C-terminal fragments mediated both the decreased AP-1 activity and the increased caspase-3 activity. These results indicate that CKIP-1 is not only a substrate but also an amplifier of caspase-3.
CKIP-1 promotes apoptosis dependent on caspase-3
The fact that CKIP-1 is both a negative regulator of c-Jun activity and a positive regulator of caspase-3 activity suggests that CKIP-1 may be a potentiator of apoptosis. SK-BR-3 is one of the HER-2/neu-overexpressing breast cancer cell lines. Overexpression of HER-2/neu correlates with poor survival of breast and ovarian cancer patients and induces resistance to TNF (Zhou et al, 2000). To address the contribution of CKIP-1 to cell death, SK-BR-3 and CKIP-1/SK-BR-3 cells were treated with TNF+CHX. Apoptotic cells displayed profoundly shrunken morphology and chromosome condensation, as judged by Hoechst 33342 staining. CKIP-1/SK-BR-3 cells showed an about 1.5-fold increase in condensation over control cells throughout the 16 h of the treatment (Figure 6A). We also compared the percentage of apoptotic cells as indicated by sub-G1 DNA content through flow cytometry analysis. Stable expression of CKIP-1 alone was unable to induce cell death; however, it significantly enhanced the TNF-induced apoptosis over a time course of 8 h (Figure 6B). The potentiation effect on apoptosis was more significant when C-term2 was overexpressed. In contrast, cells expressing the uncleavable D310+345A mutant were apoptotically indistinguishable from cells that did not express CKIP-1 (Figure 6B). In addition, preincubation with DEVD-CHO inhibited the increased sensitivity to TNF, indicating that CKIP-1 promoted apoptosis in a caspase-3-dependent manner. Furthermore, a clear DNA ladder was observed in CKIP-1/SK-BR-3 cells 12 h after TNF treatment, whereas no DNA ladder or a weak DNA ladder was seen in control cells. The DNA ladder was observed at 24 h in control cells, and this was intensified in CKIP-1/SK-BR-3 cells (Figure 6C). Stable expression of CKIP-1 in NIH3T3 fibroblasts clearly altered their morphology (Supplementary Figure S3). Cells grew more slowly than mock-transfected control cells even in 20% FBS-containing medium (data not shown). Moreover, we utilized confocal microscopy to monitor the localization of transiently expressed CKIP-1 in HepG2 cells. Strikingly, we found that, during TNF-induced apoptosis, CKIP-1 expression led to membrane ruffling and pinocytic vesicle formation (Supplementary Figure S4). The localization of CKIP-1 being tightly correlated with the cell shape shrinkage further supports the notion that CKIP-1 might be implicated in the regulation of cytoskeleton reorganization and collapse.
Figure 6.
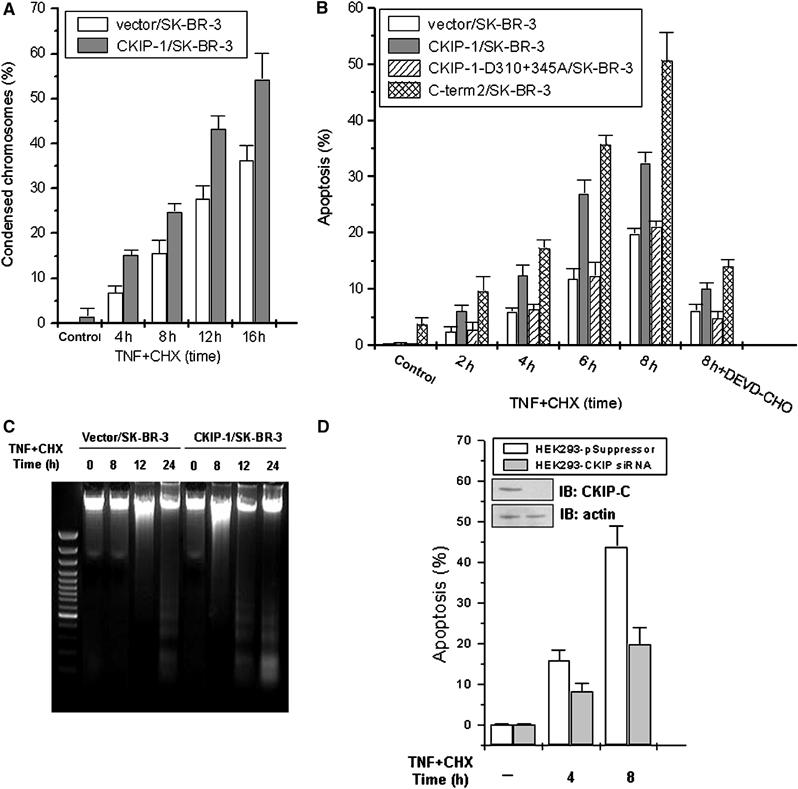
CKIP-1 promotes apoptosis dependent on caspase-3. (A) CKIP-1/SK-BR-3 and pcDNA3.1/SK-BR-3 cells were treated as in Figure 4A and stained with Hoechst 33342 for DNA. The percentages of condensed chromosomes are shown as the ratio between apoptotic fragmented nuclei and the total stained nuclei for 200–250 cells in each field as indicated. At least three independent fields were counted. (B) The apoptosis ratio for the indicated SK-BR-3 stable transfectants was assessed by flow cytometric analysis as described in Materials and methods. (C) DNA fragmentation assay. DNA was extracted and electrophoresed on a 1.5% agarose gel. Lane 1, 100-base-pair standard ladder. (D) siRNA-mediated knockdown of CKIP-1 in HEK293 cells attenuated sensitivity to TNF. Stable transfectants of HEK293 cells expressing CKIP siRNA or vector alone were assayed for apoptosis after TNF+CHX treatment as indicated. The inset shows the expression of CKIP-1 by immunoblotting as compared to actin, revealing the efficiency of the siRNA knockdown. Apoptosis was assessed by flow cytometric analysis.
To evaluate whether endogenous CKIP-1 is required for the execution of apoptosis, we abolished its expression by RNAi. As expected, knockdown of CKIP-1 expression by RNAi in HEK293 cells attenuated the sensitivity to apoptotic signaling (Figure 6D), suggesting a physiological role of CKIP-1 in sensitizing cells to death stimuli. Similar results were obtained in HeLa cells (data not shown).
CKIP-1 cleavage modulates apoptosis through AP-1/c-Jun activity
The above results demonstrate that (i) the C-terminus of CKIP-1 interacts with c-Jun and controls AP-1 function and (ii) CKIP-1 is cleaved by caspase-3 and as such serves as a caspase amplifier mechanism for cell death induction. To assess whether the inhibitory effect of CKIP-1 fragments on c-Jun phosphorylation and AP-1 activity also contributes to apoptosis, CKIP-1/SK-BR-3 cells were transiently transfected with c-Jun and treated with TNF at 24 h post-transfection. The percentage of apoptotic cells was measured by flow cytometric analysis. As shown in Figure 7A, coexpression of c-Jun could partially antagonize the proapoptotic effect of wild-type CKIP-1, with no effect of the D310+345A mutant. To clarify whether the proapoptotic effect of CKIP-1 was limited to apoptosis priming by TNF, staurosporine (STS) was used as another potent cell death stimulus. In this case, CKIP-1/SK-BR-3 cells showed similar apoptosis ratio to the control cells (Figure 7B), which was expected since STS does not activate JNK efficiently (data not shown). To confirm the effect of expression of Jun on apoptosis inhibition related to CKIP-1 or TNF, another proapoptotic protein, Bax, was overexpressed together with, or without, c-Jun; cells were then treated with TNF. Figure 7C clearly shows that c-Jun had no effect on Bax-induced apoptosis. These results suggest that CKIP-1 cleavage promotes apoptosis through inhibition of AP-1/c-Jun activity.
Figure 7.
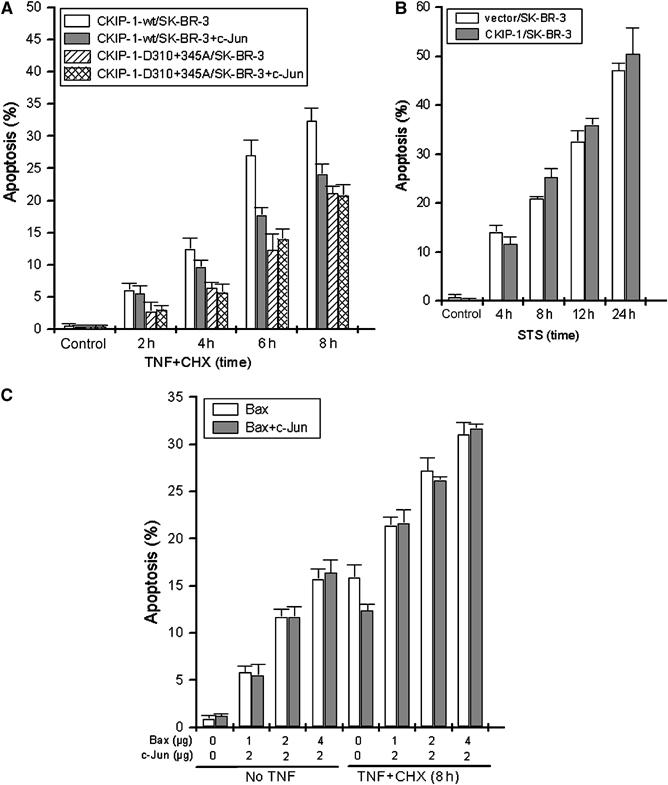
CKIP-1 modulates apoptosis through AP-1/c-Jun inhibition. (A) CKIP-1/SK-BR-3 and CKIP-D310+345A/SK-BR-3 cells were transfected with Flag-c-Jun and apoptosis was induced with TNF+CHX. The apoptosis ratio was assessed by flow cytometric analysis. Overexpression of c-Jun antagonized the proapoptotic effect of CKIP-1, but not that of mutant CKIP-1 resistant to caspase-3. (B) Apoptosis in the SK-BR-3 stable transfectants was induced by STS (0.4 μM) for the indicated times and analyzed by flow cytometry. (C) SK-BR-3 cells were transiently transfected with Myc-Bax with or without cotransfection of Flag-c-Jun. At 24 h post-transfection, the cells were treated with TNF+CHX for 8 h or not. Apoptotic cells were assessed by flow cytometry.
Discussion
Taken together, our data strongly suggest that CKIP-1 promotes apoptosis by acting both upstream and downstream of caspase-3 activation. Similar observations have been made for the dependence receptors DCC, RET and Ptc-1 (Mehlen et al, 1998; Bordeaux et al, 2000; Thibert et al, 2003) and kinases such as Mst (Lee et al, 2001). We established that the 50 kDa CKIP-1 is cleaved to generate a 15 kDa active fragment during apoptosis induced by TNF plus a low dose of CHX, or by a higher dose of CHX alone. This cleavage product is detected within 4 h post-treatment when 85% of the cells are still viable (Figures 4A and 7B), indicating that CKIP-1 proteolysis occurs as an early event during apoptosis. This was also evident in SK-BR-3 cells, which are resistant to TNF. Several lines of evidence suggest that CKIP-1 cleavage is mediated by caspase-3. First, recombinant caspase-3 cleaves CKIP-1 in vitro to generate a fragment that is identical to that observed in vivo (Figure 3). Second, in caspase-3-deficient MCF-7 cells, CKIP-1 cleavage is blocked and restored if ectopic caspase-3 is re-expressed (Figure 3D). Third, mutational analysis indicates that CKIP-1 is cleaved in vitro by caspase-3 at two noncanonical cleavage sites, RIQD310L and SPPD345S (Figure 3E). It is widely accepted that the caspase-3 consensus motif is DXXD (Cohen, 1997). However, noncanonical cleavage sites for caspase-3 have been identified in putative caspase-3 substrates including p21WAF1/Cip1 (SMTD149F) and Akt1 (ECVD462S) (Park et al, 1998; Xu et al, 2002). These findings support the notion that CKIP-1 is a novel plasma membrane-localized target for caspase-3 and plays a role in sensitizing cells to extracellular apoptotic signals such as TNF.
It is important to emphasize that we observed only one C-terminal fragment of 15 kDa (or 22 kDa if the long tag is included) corresponding to the D310-cleaved product in TNF-induced apoptosis. The proteolysis of CKIP-1 at D345 requires phosphorylation by CK2 at S346 followed by sequential phosphorylation by GSK3 at S342 (unpublished data). The motif encompassing D345 is very attractive since the S346ESE motif conforms to the consensus CK2 substrate site SXX(D/E) or SXX(pS/pY) (pS, phosphoserine; pY, phosphotyrosine). The S342PPDS346 motif conforms to the GSK3 site SXXXpS; the best GSK3 substrates appear to have several proline residues between the two serines (Kennelly and Krebs, 1991; Litchfield and Luscher, 1993). A similar dual-kinase mechanism has been found in the Hedgehog signaling effector Cubitus interruptus and β-catenin (Price and Kalderon, 2002; Liu et al, 2002).
The fact that CKIP-1 is broadly expressed indicates that its cleavage is likely to be a feature of apoptosis in many types of cells. In this respect, endogenous CKIP-1 could also be cleaved in response to serum withdrawal or TNF treatment in HEK293 cells, TNF treatment in HeLa cells and in response to ionizing radiation in MOLT-4 or Jurkat cells (Figure 3G; data not shown). These findings suggest that CKIP-1 cleavage may be a general feature of the apoptotic response of diverse cells to a wide variety of stimuli. Intriguingly, CKIP-1 is generally expressed at low levels or short-lived in tumor cell lines and serves as a growth suppressor of tumor cells (unpublished data).
Caspase cleavage of CKIP-1 probably represents an initiating step for CKIP-1-accelerated apoptosis by allowing the release of C-terminal fragments of CKIP-1, which then redistribute and effectively repress AP-1. The latter step may also contribute to apoptosis, since both JNK/JunD and JNK/c-Jun can mediate cell survival signaling (Shaulian and Karin, 2002; Lamb et al, 2003). CKIP-1 expression in SK-BR-3 cells shortened the duration of c-Jun phosphorylation (partially representing JNK activity) and promoted apoptosis (Figures 4 and 6), providing evidence for the antiapoptotic role of JNK/AP-1 pathway at least in SK-BR-3 breast cancer cells. This is consistent with some recent reports (Lamb et al, 2003; Yu et al, 2004) and in contrast to others (Tournier et al, 2000; Deng et al, 2003; Sakon et al, 2003). We noted that in SK-BR-3 cells, TNF-induced JNK activation was sustained for at least 8 h (Figure 4A and B), whereas in MEFs it lasts only 1 h (Sakon et al, 2003). This may be caused by different cellular contexts. It also suggests that JNK is mediating an antiapoptotic signal in SK-BR-3 cells and that c-Jun phosphorylation plays an important role in regulating cell death. Our model is consistent with a study using c-jun−/− cells, which demonstrated that c-Jun protects fibroblasts against UV, depending on its phosphorylation on Ser63 and Ser73 (Wisdom et al, 1999). However, it disagrees with the work of Behrens et al (1999), who showed that fibroblasts in which the c-jun gene was replaced by a mutant c-jun allele with Ser63 and Ser73 mutated to Ala displayed stress-induced apoptotic defects. Overexpressing c-Jun in cells is likely to have distinct effects from modulating the levels of c-Jun phosphorylation. This is particularly important since C-term2 does not affect the level of expression of c-Jun.
We established direct links between caspase cleavage, C-term1/2 release and AP-1 inhibition. On the one hand, CKIP-1 represses AP-1 activity and promotes apoptosis in a caspase-3-dependent manner; on the other hand, CKIP-1 promotes apoptosis via inhibition of AP-1 activity. When caspase-3 mediated CKIP-1 cleavage was blocked by the D310+345A mutations, both the c-Jun phosphorylation level and the apoptosis ratio were indistinguishable in CKIP-1-expressing and control cells (Figures 4B and 6B). This mechanism is distinct from known c-Jun-interacting proteins (Chinenov and Kerppola, 2001), all of which are localized to the cytoplasm or nucleus and function in their intact forms. To the best of our knowledge, CKIP-1 is the first PH domain-containing protein or plasma membrane-associated protein that efficiently regulates nuclear AP-1 activity. Recently, it was found that the Notch-1 intracellular domain (NIC) represses AP-1-mediated transactivation following cleavage by a furin-like enzyme and TACE (a membrane-bound metalloprotease) and nuclear translocation of NIC (Chu et al, 2002). This pattern of cleavage-mediated control of AP-1 is similar to CKIP-1.
CKIP-1 C-term1 can interact directly with c-Jun (and JunD) both in vitro and in vivo. The leucine zipper of c-Jun mediates the interaction with C-term1. This interaction is specific since other members of the AP-1 family were unable to interact with C-term1 although they also contain a conserved leucine zipper domain. C-term1 itself contains a putative leucine zipper motif (aa 352–380), which has been widely found in c-Jun-interacting bZIP transcription factors. Like these bZIP proteins, the leucine zipper motif of CKIP-1 also mediates interaction with CKIP-1 itself in the yeast two-hybrid system (Figure 1B; Olsten et al, 2004), and homodimerization and trimerization in vivo (unpublished data).
Using phospho-specific antibodies to different phosphorylated forms of c-Jun, Morton et al (2003) demonstrated that JNK is the MAPK required for stress-induced phosphorylation of c-Jun on Ser63 and Ser73. Consistent with this study, we propose a model in which C-term2 inhibits c-Jun phosphorylation by preventing the binding of c-Jun to JNK. This mechanism has already been implicated for Jip-1-dependent regulation of the JNK pathway (Whitmarsh and Davis, 1999). Furthermore, the regulatory function of CKIP-1 in AP-1 activity is activated upon caspase-3-mediated cleavage and, in turn, CKIP-1 functions as part of a feedback loop that contributes to amplifying the apoptotic response. Although the mechanisms by which CKIP-1 might influence caspase-3 activity and apoptosis are unclear, the ability of CKIP-1 to regulate AP-1 activity suggests that one of them may involve the JNK/AP-1 pathway. Coexpression of c-Jun inhibits the proapoptotic effect of CKIP-1 in TNF-primed apoptosis, but had no effect on the cell death induced by Bax expression or STS (Figure 7). However, we cannot rule out other possibilities. More recently, we observed that CKIP-1 overexpression significantly induced Akt/PKB kinase cleavage at the C-terminal tail (position D462), which is also mediated by caspase-3 (unpublished data). Considering that Akt/PKB kinase mediates a major cell survival pathway, the negative regulation of Akt/PKB by CKIP-1 may be one of the consequences of the amplified caspase-3 activation by CKIP-1. Further studies will be required to determine the precise role of the JNK/AP-1 pathway and the Akt/PKB pathway in CKIP-1-promoted apoptosis.
Materials and methods
Plasmid constructs, antibodies and reagents
Plasmids of CKIP-1, c-Jun and others including deletion and point mutants were constructed by PCR or recombinant PCR, followed by subcloning into various vectors. For detailed information, see Supplementary data. Rabbit antiserum CKIP-1-N was raised against the N-terminus of CKIP-1 (aa 1–136) containing the PH domain. Rabbit antiserum CKIP-1-C against aa 308–409 of CKIP-1 was a kind gift from Dr David W Litchfield. Anti-Myc antibody and the caspase inhibitors, zVAD-fmk and DEVD-CHO, were purchased from Clontech. Anti-Flag M2 antibody, CHX, TPA, leupeptin, antipain, aprotinin, chymostatin, pepstatin and phenylmethylsulfonyl fluoride (PMSF) were from Sigma. TNF-α was purchased from Chemicon. Antibodies to c-Jun (H-79), phospho-c-Jun-Ser63 (KM-1), JNK, phospho-JNK, p38, phospho-p38, caspase-3 (H-277), β-actin, Lamin A (C-20), nucleolin (MS-3) and Na+/K+-ATPase α1 (N-15) were obtained from Santa Cruz.
Yeast two-hybrid and GST pull-down assays
Cotransformation of pGBKT7 and pGADT7 carrying various cDNAs into the yeast strain Y190 and the liquid o-nitrophenyl β-D-galactoside assay were performed according to the manufacturer's instructions (Clontech). The experiments were performed three times independently (mean±s.d.). GST-c-Jun proteins were expressed in Escherichia coli BL21 (DE3) and purified with glutathione Sepharose 4B (Pharmacia). In vitro GST pull-down was performed as previously described (Matsuzawa and Reed, 2001).
Immunoprecipitation, immunoblotting and cell fractionation
For general cell lysis and co-immunoprecipitation of CKIP-1-C-term1 and c-Jun, cell lysates were prepared in lysis buffer (100 mM Tris–HCl (pH 7.5), 300 mM NaCl, 2% (v/v) Tween 20, 0.4% NP-40, 20% glycerol) supplemented with protease inhibitor cocktail (Roche) and phosphatase inhibitors (10 mM NaF and 1 mM Na3VO4). Immunoprecipitations were performed using anti-Myc or anti-Flag antibodies and protein A/G-agarose (Santa Cruz) at 4°C. The lysates and immunoprecipitates were detected using the indicated primary antibodies and then the appropriate secondary antibody, followed by detection with SuperSignal chemiluminescence kit (Pierce). Cell fractionation was performed as described previously (Safi et al, 2004).
Fluorescence analysis of CKIP-1
For immunofluorescence detection (Figure 4D), pcDNA3.1-Myc-CKIP-1/SK-BR-3 cells were fixed with 4% paraformaldehyde and permeabilized in 0.2% Triton X-100 (PBS). CKIP-1 proteins were stained with anti-Myc antibody followed by TRITC-conjugated secondary antibody. The nuclei were stained with Hoechst 33342 (Sigma) and images were visualized with an Olympus IX70 fluorescence microscope and captured with a cooled charge-coupled device color digital camera (Diagnostic). For colocalization of CKIP-1-C-term1 and c-Jun (Figure 1D), GFP-c-Jun together with RFP-C-term1 or RFP-CKIP-1 was cotransfected into COS7 cells and fluorescence was visualized 24 h later.
Reporter gene assay
COS7 cells were seeded into a 24-well plate and transfected with 10 ng pRL-CMV (Renilla control luciferase, Promega) and 0.1 μg pAP-1-luc (firefly luciferase, Stratagene) reporter gene plasmids and constructs encoding various deletion mutants of CKIP-1 (0.5 μg), together with c-Jun plasmid (0.1 μg) or not. After 24 h, luciferase activity was measured and normalized to the cotransfected Renilla luciferase activity (mean±s.d., n=3). In some cases (Figure 2B), various amounts of C-term1 or C-term2 plasmids (0.1–1.0 μg) were cotransfected with c-Jun (0.1 μg) and the luciferase activity was measured. To detect the specificity of CKIP-1 on AP-1 activity (Figure 2C), pAP-1-luc was substituted by pFA-Elk1 (0.1 μg) in the absence or presence of MEK1 (0.1 μg). The plasmids of the trans-reporter gene system of MEKK1-c-Jun and MEK1-Elk1 pathways were a gift from Dr Xiaoming Yang.
In vitro translation and caspase cleavage assay
Wild-type CKIP-1 or various mutants of CKIP-1 were transcribed and translated using the T7 TNT system (Promega) in the presence of [35S]methionine (Pharmacia). Purified active human recombinant caspase-3 and caspase-6 were purchased from Pharmingen. Caspase-mediated cleavage assays were performed as described previously (Mehlen et al, 1998).
Apoptosis analysis
CKIP-1/SK-BR-3 or control cells were deprived of serum overnight and treated with TNF (20 ng/ml) plus CHX (10 μg/ml) or a higher dose of CHX (100 μg/ml) alone for the indicated times to induce apoptosis. The percentage of apoptotic cells was quantified by analysis of sub-G1 DNA. Briefly, cells were harvested by trypsinization and washed with PBS, followed by centrifugation at 2000 r.p.m. for 10 min. The cell pellet was resuspended in 70% ethanol, and stored at −20°C for 24 h. Cells were washed and incubated in PBS containing 50 μg/ml RNase A (Roche) for 30 min at 37°C. Cells were then stained with 50 μg/ml propidium iodide (Sigma). DNA content was analyzed by flow cytometry using a cell sorter (FACSCalibur) and CellQuest software (Beckton Dickinson). Caspase-3 activity was assessed using the ApoAlert Caspase-3 Activity Assay System, which uses the Ac-DEVD-AFC substrate (Clontech), according to the manufacturer's instructions. Caspase-3 activation is shown as the ratio between the caspase activity of the sample and that measured in pcDNA3.1-transfected cells without TNF treatment. DNA fragmentation assays were performed as described previously (Cheung et al, 2003).
RNA interference
A total of 10 siRNA sequences derived from the human CKIP-1 RNA sequence were designed to contain a sense strand of 18–19 nucleotide sequences of CKIP-1 followed by a short spacer (GAGTACTG), the reverse complement of the sense strand, and five thymidines as an RNA polymerase III transcriptional stop signal. These sequences were cloned into the pSuppressor vector (Imgenex) following the manufacturer's instructions and confirmed by sequencing. The pSuppressor-CKIP-1-siRNA plasmids were transfected into HEK293 or HeLa cells and then CKIP-1 expression was assayed by immunoblotting. Of the sequences tested, the two most efficient sequences were selected for stable transfection. Paired forward and reverse oligonucleotides were (i) 5′-tcgaGTTGACGCCCACAGAGAAAgagtactgT TTCTCTGTGGGCGTCAACttttt-3′; 5′-ctagaaaaaGTTGACGCCCACAGAGAAAcagt actcTTTCTCTGTGGGCGTCAAC-3′ and (ii) 5′-tcgaGGAACCAACCTCTTGTGCTgagtactgA GCACAAGAGGTTGGTTCttttt-3′; 5′-ctagaaaaaGAACCAACCTCTTGTG CTcagtactcAGCACAAGAGGTTGGTTCC-3′. The forward and reverse oligonucleotides included an XhoI and an XbaI cleavage site, respectively. Stable HEK293-CKIP-1 siRNA cells and HEK293-pSuppressor cells were constructed. G418 resistant clones were assayed with immunoblotting. Both the above two paired siRNAs were used in the experiments, and similar results were obtained.
Statistical analysis
Statistical evaluation was carried out using Student's t-test.
Supplementary Material
Supplementary Data
Acknowledgments
We thank Mr Bo Dong for the flow cytometry analysis, Dr Lisheng Wang for the caspase-3 antibody, Dr David W Litchfield for the CKIP-1-C antiserum, Dr Wen Yue for the pSuppressor plasmid and Drs Xiaodong Wang, Yi Rao, Kunliang Guan, John JM Bergeron, Bai Lu, Lin Mei and Yong Li for critical reading of the manuscript. This work was partially supported by Chinese National Natural Science Foundation Projects (30321003, 30100090), the Chinese State Key Program in Basic Research (2001CB510204, G1999053903, G1998051012) and the Chinese National High-tech Program (2004AA221100).
References
- Baisden JM, Qian Y, Zot HM, Flynn DC (2001) The actin filament-associated protein AFAP-110 is an adaptor protein that modulates changes in actin filament integrity. Oncogene 20: 6435–6447 [DOI] [PubMed] [Google Scholar]
- Behrens A, Sibilia M, Wagner EF (1999) Amino-terminal phosphorylation of c-Jun regulates stress-induced apoptosis and cellular proliferation. Nat Genet 21: 326–329 [DOI] [PubMed] [Google Scholar]
- Brivanlou AH, Darnell JE Jr (2002) Signal transduction and the control of gene expression. Science 295: 813–818 [DOI] [PubMed] [Google Scholar]
- Bordeaux MC, Forcet C, Granger L, Corset V, Bidaud C, Billaud M, Bredesen DE, Edery P, Mehlen P (2000) The RET proto-oncogene induces apoptosis: a novel mechanism for Hirschsprung disease. EMBO J 19: 4056–4063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosc DG, Graham KC, Saulnier RB, Zhang C, Prober D, Gietz RD, Litchfield DW (2000) Identification and characterization of CKIP-1, a novel pleckstrin homology domain-containing protein that interacts with protein kinase CK2. J Biol Chem 275: 14295–14306 [DOI] [PubMed] [Google Scholar]
- Cheung WL, Ajiro K, Samejima K, Kloc M, Cheung P, Mizzen CA, Beeser A, Etkin LD, Chernoff J, Earnshaw WC, Allis CD (2003) Apoptotic phosphorylation of histone H2B is mediated by mammalian sterile twenty kinase. Cell 113: 507–517 [DOI] [PubMed] [Google Scholar]
- Chevray PM, Nathans D (1992) Protein interaction cloning in yeast: identification of mammalian proteins that react with the leucine zipper of Jun. Proc Natl Acad Sci USA 89: 5789–5793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinenov Y, Kerppola TK (2001) Close encounters of many kinds: Fos–Jun interactions that mediate transcription regulatory specificity. Oncogene 20: 2438–2452 [DOI] [PubMed] [Google Scholar]
- Chu J, Jeffries S, Norton JE, Capobianco AJ, Bresnick EH (2002) Repression of activator protein-1-mediated transcriptional activation by the Notch-1 intracellular domain. J Biol Chem 277: 7587–7597 [DOI] [PubMed] [Google Scholar]
- Cohen GM (1997) Caspases: the executioners of apoptosis. Biochem J 326: 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y, Ren X, Yang L, Lin Y, Wu X (2003) A JNK-dependent pathway is required for TNFα-induced apoptosis. Cell 115: 61–70 [DOI] [PubMed] [Google Scholar]
- Kallunki T, Deng T, Hibi M, Karin M (1996) c-Jun can recruit JNK to phosphorylate dimerization partners via specific docking interactions. Cell 87: 929–939 [DOI] [PubMed] [Google Scholar]
- Karin M, Liu ZG, Zandi E (1997) AP-1 function and regulation. Curr Opin Cell Biol 9: 240–246 [DOI] [PubMed] [Google Scholar]
- Kennelly PJ, Krebs EG (1991) Consensus sequences as substrate specificity determinants for protein kinases and protein phosphatases. J Biol Chem 266: 15555–15558 [PubMed] [Google Scholar]
- Kim S, Lee SH, Park D (2001) Leucine zipper-mediated homodimerization of the p21-activated kinase-interacting factor, βPix. Implication for a role in cytoskeletal reorganization. J Biol Chem 276: 10581–10584 [DOI] [PubMed] [Google Scholar]
- Lamb JA, Ventura J, Hess P, Flavell RA, Davis RJ (2003) JunD mediates survival signaling by the JNK signal transduction pathway. Mol Cell 11: 1479–1489 [DOI] [PubMed] [Google Scholar]
- Lee KK, Ohyama T, Yajima N, Tsubuki S, Yonehara S (2001) MST, a physiological caspase substrate, highly sensitizes apoptosis both upstream and downstream of caspase activation. J Biol Chem 276: 19276–19285 [DOI] [PubMed] [Google Scholar]
- Lin A, Frost J, Deng T, Smeal T, al-Alawi N, Kikkawa U, Hunter T, Brenner D, Karin M (1992) Casein kinase II is a negative regulator of c-Jun DNA binding and AP-1 activity. Cell 70: 777–789 [DOI] [PubMed] [Google Scholar]
- Litchfield DW, Luscher B (1993) Casein kinase II in signal transduction and cell cycle regulation. Mol Cell Biochem 127/128: 187–199 [DOI] [PubMed] [Google Scholar]
- Liu C, Li Y, Semenov M, Han C, Baeg GH, Zhang Z, Lin X, He X (2002) Control of β-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 108: 837–847 [DOI] [PubMed] [Google Scholar]
- Matsuzawa S, Reed JC (2001) Siah-1, SIP, and Ebi collaborate in a novel pathway for β-catenin degradation linked to p53 responses. Mol Cell 7: 915–926 [DOI] [PubMed] [Google Scholar]
- Mehlen P, Rabizadeh S, Snipas SJ, Assa-Munt N, Salvesen GS, Bredesen DE (1998) The DCC gene product induces apoptosis by a mechanism requiring receptor proteolysis. Nature 395: 801–804 [DOI] [PubMed] [Google Scholar]
- Mitsuuchi Y, Johnson SW, Sonoda G, Tanno S, Golemis EA, Testa JR (1999) Identification of a chromosome 3p14.3–21.1 gene, APPL, an adaptor molecule that interacts with the oncoprotein-serine/threonine kinase AKT2. Oncogene 18: 4891–4898 [DOI] [PubMed] [Google Scholar]
- Morton S, Davis RJ, McLaren A, Cohen P (2003) A reinvestigation of the multisite phosphorylation of the transcription factor c-Jun. EMBO J 22: 3876–3886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nateri AS, Riera-Sans L, Costa CD, Behrens A (2004) The ubiquitin ligase SCFFbw7 antagonizes apoptotic JNK signaling. Science 303: 1374–1378 [DOI] [PubMed] [Google Scholar]
- Olsten ME, Canton DA, Zhang C, Walton PA, Litchfield DW (2004) The pleckstrin homology domain of CKIP-1 is required for interactions and recruitment of protein kinase CK2 to the plasma membrane. J Biol Chem 279: 42114–42127 [DOI] [PubMed] [Google Scholar]
- Park JA, Kim KW, Kim SI, Lee SK (1998) Caspase 3 specifically cleaves p21WAF1/CIP1 in the earlier stage of apoptosis in SK-HEP-1 human hepatoma cells. Eur J Biochem 257: 242–248 [DOI] [PubMed] [Google Scholar]
- Price MA, Kalderon D (2002) Proteolysis of the Hedgehog signaling effector Cubitus interruptus requires phosphorylation by glycogen synthase kinase 3 and casein kinase 1. Cell 108: 823–835 [DOI] [PubMed] [Google Scholar]
- Romero F, Fischer S (1996) Structure and function of vav. Cell Signal 8: 545–553 [DOI] [PubMed] [Google Scholar]
- Safi A, Vandromme M, Caussanel S, Valdacci L, Baas D, Vidal M, Brun G, Schaeffer L, Goillot E (2004) Role for the pleckstrin homology domain-containing protein CKIP-1 in phosphatidylinositol 3-kinase-regulated muscle differentiation. Mol Cell Biol 24: 1245–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakon S, Xue X, Takekawa M, Sasazuki T, Okazaki T, Kojima Y, Piao JH, Yagita H, Okumura K, Doi T, Nakano H (2003) NF-κB inhibits TNF-induced accumulation of ROS that mediate prolonged MAPK activation and necrotic cell death. EMBO J 22: 3898–3909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaulian E, Karin M (2001) AP-1 in cell proliferation and survival. Oncogene 20: 2390–2400 [DOI] [PubMed] [Google Scholar]
- Shaulian E, Karin M (2002) AP-1 as a regulator of cell life and death. Nat Cell Biol 4: E131–E136 [DOI] [PubMed] [Google Scholar]
- Thibert C, Teillet MA, Lapointe F, Mazelin L, Douarin NM, Mehlen P (2003) Inhibition of neuroepithelial Patched-induced apoptosis by Sonic hedgehog. Science 301: 843–846 [DOI] [PubMed] [Google Scholar]
- Tournier C, Hess P, Yang DD, Xu J, Turner TK, Nimnual A, Bar-Sagi D, Jones SN, Flavell RA, Davis RJ (2000) Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science 288: 870–874 [DOI] [PubMed] [Google Scholar]
- Vogt PK (2001) Jun, the oncoprotein. Oncogene 20: 2365–2377 [DOI] [PubMed] [Google Scholar]
- Wertz IE, O'Rourke KM, Zhang Z, Dornan D, Arnott D, Deshaies RJ, Dixit VM (2004) Human De-etiolated-1 regulates c-Jun by assembling a CUL4A ubiquitin ligase. Science 303: 1371–1374 [DOI] [PubMed] [Google Scholar]
- Whitmarsh AJ, Davis RJ (1999) Signal transduction by MAP kinases: regulation by phosphorylation-dependent switches. Sci STKE 1: PE1. [DOI] [PubMed] [Google Scholar]
- Wisdom R, Johnson RS, Moore C (1999) c-Jun regulates cell cycle progression and apoptosis by distinct mechanisms. EMBO J 18: 188–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Liu D, Songyang Z (2002) The role of Asp-462 in regulating Akt activity. J Biol Chem 277: 35561–35566 [DOI] [PubMed] [Google Scholar]
- Yan M, Dai T, Deak JC, Kyriakis JM, Zon LI, Woodgett JR, Templeton DJ (1994) Activation of stress-activated protein kinase by MEKK1 phosphorylation of its activator SEK1. Nature 372: 798–800 [DOI] [PubMed] [Google Scholar]
- Yu C, Minemoto Y, Zhang J, Liu J, Tang F, Bui TN, Xiang J, Lin A (2004) JNK suppresses apoptosis via phosphorylation of the proapoptotic Bcl-2 family protein Bad. Mol Cell 13: 329–340 [DOI] [PubMed] [Google Scholar]
- Zhou BP, Hu MC, Miller SA, Yu Z, Xia W, Lin SY, Hung MC (2000) HER-2/neu blocks tumor necrosis factor-induced apoptosis via the Akt/NF-κB pathway. J Biol Chem 275: 8027–8031 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data


