Abstract
CpG-DNA and its related synthetic CpG oligodeoxynucleotides (CpG-ODNs) play an important role in immune cell survival. It has been suggested that Akt is one of the CpG-DNA-responsive serine/threonine kinases; however, the target protein of CpG-DNA that leads to Akt activation has not been elucidated. Here, we report that ex vivo stimulation of bone marrow-derived macrophages (BMDMs) from mice lacking the catalytic subunit of DNA-dependent protein kinase (DNA-PKcs) results in defective phosphorylation and activation of Akt by CpG-DNA. Unexpectedly, loss of the Toll-like receptor 9 has a minimal effect on Akt activation in response to CpG-DNA. Further in vitro analysis using purified DNA-PK and recombinant Akt proteins reveals that DNA-PK directly induces phosphorylation and activation of Akt. In addition, in BMDMs, DNA-PKcs associates with Akt upon CpG-DNA stimulation and triggers transient nuclear translocation of Akt. Thus, our findings establish a novel role for DNA-PKcs in CpG-DNA signaling and define a CpG-DNA/DNA-PKcs/Akt pathway.
Keywords: Akt, CpG-DNA, DNA-PKcs, TLR9
Introduction
In addition to stimulating B cells, macrophages and dendritic cells (DCs) to produce cytokines through the Toll-like receptor 9 (TLR9)-dependent pathway (reviewed in Akira and Hemmi, 2003; Wagner, 2004), CpG-DNA and its synthetic CpG oligodeoxynucleotide (CpG-ODN) can also provide survival signals to the immune system (reviewed in Van Uden and Raz, 1999; Yi et al, 1999; Park et al, 2002). It has been shown that CpG-ODN rescues B cells and DCs from spontaneous apoptosis by activating NF-κB and Akt (Yi et al, 1998, 1999; Park et al, 2002).
Mammalian Akt is expressed ubiquitously with three isoforms: Akt1, Akt2 and Akt3 (reviewed in Datta et al, 1999). All three isoforms share a 70–83% homology and contain two distinct functional domains: an N-terminal pleckstrin homology (PH) domain mediating protein–protein and protein–lipid interactions, and a central kinase domain with specificity for serine or threonine residues in substrate proteins (reviewed in Datta et al, 1999; Scheid and Woodgett, 2001). It is well accepted that during the Akt activation process, the PH domain interacts with phosphatidylinositol-3 kinase (PI3K)-generated PI(3,4)-bisphosphate (PIP2) (Franke et al, 1997; Frech et al, 1997) and PI(3,4,5)-trisphosphate (PIP3) (Stephens et al, 1998), facilitating translocation of Akt from the cytosol to plasma membrane (Sable et al, 1998), where PIPs reside. This relocalization of Akt brings it in proximity to regulatory kinases that phosphorylate and activate Akt. However, recent studies reported that interaction of Akt with Tcl1, a 14 kDa protein that is encoded by 14q32.1 or Xq28 regions related to human T-cell prolymphocytic leukemia, induces Akt phosphorylation and activation (Laine et al, 2000; Pekarsky et al, 2000; reviewed in Brazil et al, 2002). These studies suggest that binding of Tcl1 to Akt may mimic the conformational change induced while PIP3 engages the kinase, therefore triggering kinase activation. Two important inducible phosphorylation sites on Akt have been identified: Thr308/309/305 and Ser473/474/471 (Akt1, Akt2 and Akt3 respectively; reviewed in Scheid and Woodgett, 2001). Thr308 (308T) is essential for Akt activation whereas Ser473 (473S) is required for full activation of Akt (reviewed in Datta et al, 1999; Scheid and Woodgett, 2001). Activated Akt subsequently phosphorylates and inactivates several proapoptotic molecules (reviewed in Datta et al, 1999; Scheid and Woodgett, 2001) and phosphorylates the alpha subunit of IκB kinase (IKK), which in turn activates NF-κB (reviewed in Ghosh and Karin, 2002). Thus, Akt is capable of mediating both antiapoptotic and proliferative signals at multiple points of signaling pathways.
DNA-dependent protein kinase (DNA-PK) is a serine/threonine protein kinase and is composed of a large 470 kDa catalytic subunit (DNA-PKcs) and Ku70/80 subunits (reviewed in Smith and Jackson, 1999). Biological and genetic evidence reveals that DNA-PKcs and Ku proteins are essential for repair of double-stranded DNA breaks (DSBs) created by intrinsic and extrinsic events as well as the development of B and T lymphocytes (reviewed in Smith and Jackson, 1999). The molecular mechanism of DNA repair involves interaction of DSBs with Ku proteins, which in turn recruit DNA-PKcs into the complex, leading to DNA-PK activation (reviewed in Smith and Jackson, 1999; Lee and Kim, 2002). However, recent studies have identified that Ku70 is uniquely involved in Bax-related apoptosis (reviewed in Norbury and Zhivotovsky, 2004), suggesting that each DNA-PK subunit can function independently in certain circumstances. Furthermore, DNA-PKcs is involved in activation of IKK and NF-κB in response to CpG-DNA- and DNA-damaging reagents (Chu et al, 2000; Panta et al, 2004). DNA-PKcs is a member of the PI3K-like kinase family. Although available evidence indicates that DNA-PKcs has protein, but not lipid kinase activity (KA; reviewed in Smith and Jackson, 1999), whether DNA-PKcs can target downstream events of the PI3K pathway is largely unknown.
We investigated signal pathways responsive to CpG-DNA that can activate Akt. We have obtained evidence indicating that a pathway leading to Akt activation is dependent on DNA-PKcs, but less likely on TLR9. We demonstrate that DNA-PKcs acts upstream by associating with it and mediating Akt nuclear translocation. We conclude that DNA-PKcs is an important intermediate in the CpG-DNA-triggered Akt signaling pathway.
Results
DNA-PKcs acts upstream to Akt
Recently, a study suggested that CpG-ODN induces phosphorylation of Akt (Park et al, 2002). However, the molecular mechanism of how CpG-ODN activates Akt is not clear. Since we previously showed that CpG-DNA activates DNA-PKcs, which in turn triggers the IKK and NF-κB pathway, we investigated the role of DNA-PKcs in activation of Akt. Initially, we used bone marrow-derived macrophages (BMDMs) from DNA-PKcs-deficient mice (Ouyang et al, 1997; Chu et al, 2000). Wild-type (wt) and DNA-PKcs-deficient BMDMs were treated with CpG-ODN and then KA of three isoforms of Akt (Akt1, Akt2 and Akt3) was determined by a kinase assay. In wt BMDMs, KA of all Akt isoforms was observed as early as 15 min post-CpG-ODN incubation and decayed after 2 h (Figure 1A, upper left panel). In contrast, loss of the DNA-PKcs subunit largely impaired activation of Akts by CpG-ODN (Figure 1A, upper left panel). Quantitative analysis revealed that activation of Akt in DNA-PKcs-deficient BMDMs is significantly lower than that in wt controls (Figure 1A, right panel). We also examined Akt1 kinase activation by the phosphodiester form of CpG-ODN in both wt and DNA-PKcs-deficient cells and found that this form behaved the same way as the phosphorothioate form of CpG-ODN (data not shown). Since TLR9 has been proposed as the receptor for CpG-DNA (reviewed in Wagner, 2004; Ulevitch, 2004), we determined Akt KA in TLR9-deficient BMDMs. Surprisingly, both wt and TLR9-deficient cells were almost equally responsive to CpG-ODN in activating Akts (Figure 1A, upper left panel). As an internal control, we measured IKK KAs in wt and TLR9- and DNA-PKcs-deficient BMDMs. In agreement with previous reports (Chu et al, 2000; Hemmi et al, 2000), IKK KA was largely reduced in either DNA-PKcs- or TLR9-deficient BMDMs (Figure 1A, lower left panel). To confirm our observations, we used BMDMs isolated from SCID mice in which a T to A mutation in the DNA-PKcs gene creates an unstable, truncated protein missing the last 83 amino acids of the kinase domain (Blunt et al, 1996; reviewed in Smith and Jackson, 1999). As shown in Figure 1B, little activation of Akt was observed in SCID BMDMs; however, activation of JNK remained largely unaffected in SCID cells compared to wt controls.
Figure 1.
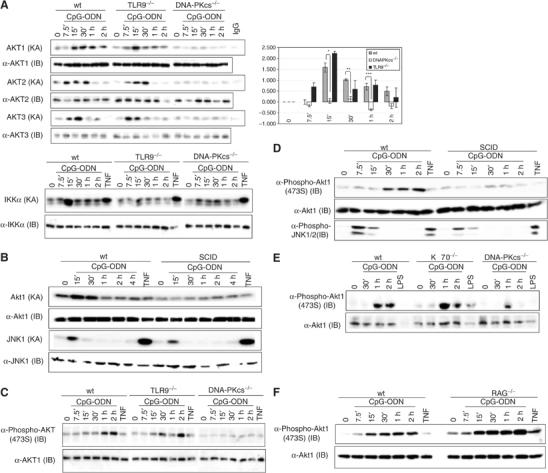
DNA-PKcs is involved in activation and phosphorylation of Akt in BMDMs. (A) Left panel: wt, DNA-PKcs−/− and TLR9−/− BMDMs were treated with CpG-ODN (10 μg/ml), TNF (10 ng/ml) or left untreated. At the indicated durations, cells were lysed by a lysis buffer (200 mM NaCl). A 200 μg portion of cell lysates was used to determine Akt KA by a kinase assay using GSK3α/β as a substrate (upper left panel) or IKK KA using GST-IκBα(1–54) as a substrate (lower left panel). The equal presence of samples was determined by an immunoblotting (IB) analysis using anti-Akt1, anti-Akt2 and anti-Akt3 antibodies. Right panel: quantitative analysis of Akt1 KA (relative fold) in wt, DNA-PKcs-deficient and TLR9-deficient BMDMs was performed using the Sigma Gel software. Data are mean±s.e.m. (n=4). *P<0.018, **P<0.003 and ***P<0.005 indicate statistical significance of Akt KAs in wt versus that in DNA-PKcs-deficient BMDMs treated by CpG-ODN for 7.5, 15, 30, 60 or 120 min. (B) wt and SCID BMDMs were treated with CpG-ODN (10 μg/ml), TNF (20 ng/ml) or left untreated. At the indicated durations, cell lysates were prepared. Akt KA was determined. JNK KA was measured by a kinase assay using GST-ATF2(6–96) as a substrate. The equal presence of proteins was determined by an IB analysis. (C–F) wt, DNA-PKcs−/−, TLR9−/−, SCID, Ku70−/− and Rag1−/− BMDMs were treated with CpG-ODN (10 μg/ml), LPS (3 μg/ml) or TNF (10 ng/ml) for the indicated durations or left untreated. Cell lysates were prepared and phosphorylation of Akt or JNK1/2 was detected using anti-phospho antibodies against Akt (473S) and JNK1/2, respectively.
Next, we determined if activation of Akt by CpG-ODN is due to phosphorylation of Akt on the canonical sites. In line with a previous report (Park et al, 2002), incubation of CpG-ODN with wt BMDMs induced phosphorylation of 473S on Akt (Figure 1C–F). However, much lower levels of phosphorylation of Akt (473S) were observed in either DNA-PKcs-deficient (Figure 1C) or SCID BMDMs (Figure 1D). Interestingly, loss of TLR9 had no evident consequence on Akt phosphorylation (Figure 1C). Since our experimental data showed that CpG-ODN induced weak phosphorylation of Akt on 308(309)T, we decided to immunoprecipitate Akt2 and perform an in vitro phosphorylation assay. Strong phosphorylation of Akt2 on 309T and 474S was observed in wt but not DNA-PKcs-deficient cells (data not shown).
Since DNA-PKcs is important for DNA repair, it is possible that a defect in Akt activation by CpG-ODN is due to genomic instability or a defect in development. To rule out these possibilities, we examined Akt phosphorylation in BMDMs isolated from Ku70- or Rag1-deficient mice, which have a similar phenotype to DNA-PKcs deficiency (Kurimasa et al, 1999; Bassing et al, 2002). As shown in Figure 1E and F, both Ku70- and Rag1-deficient BMDMs were as efficient as wt cells in inducing phosphorylation of Akt by CpG-ODN.
Taken together, our findings demonstrate that DNA-PKcs acts upstream to Akt and is important for both phosphorylation and activation of Akt in BMDMs in response to CpG-ODN.
DNA-PK directly activates Akt in vitro
To further explore the role of DNA-PKcs in activation of Akt, we determined whether commercially available, purified DNA-PK directly activates recombinant Akt purified from Sf9 cells. Freshly prepared, inactive recombinant Akts only displayed very low basal KA. However, incubation of DNA-PK with Akt in the absence of PIP3 induced robust Akt KA measured by GSK3α/β phosphorylation (Figure 2A and B). Although DNA-PK itself induced phosphorylation of GSK3α/β, its activity was considerably lower than that achieved by Akt in the presence of DNA-PK (Figure 2A and B). Intriguingly, DNA-PK together with CpG-ODN resulted in a large increase in Akt2 activity (Figure 2B), whereas DNA-PK itself strongly induced Akt1 and Akt3 activation, suggesting that once sufficient amounts of DNA-PK are present it may activate Akt by direct protein–protein interaction (Figure 2A). As a positive control, we examined activation of Akt by recombinant active 3-phosphoinositide-dependent kinase-1 (PDK1), the physiological kinase for Akt (reviewed in Datta et al, 1999), purified from Sf9 cells. Incubation of inactive Akt1 with active PDK1 in the absence of PIP3 induced little GSK3α/β phosphorylation (Figure 2C). In contrast, incubation of active Akt1, which was preincubated with active PDK1 in the presence of PIP3 and then purified, resulted in tremendous phosphorylation of GSK3α/β (Figure 2C). Thus, these results further confirmed that PIP3 is essential for PDK1 to activate Akt. In addition, we found that coincubation of DNA-PK with Akt augmented phosphorylation of GSK3α/β in a time-dependent manner (Figure 2D). Increased levels of phosphorylated GSK3α/β were accompanied by correspondingly enhanced phosphorylation of Akt (Figure 2D). Finally, we explored contributions of both 308T and 473S phosphorylation to activation of Akt by DNA-PK. Recombinant GST-Akt1 and GST-Akt1 point mutants purified from bacteria were incubated with or without DNA-PK in the presence or absence of CpG-ODN, respectively. As shown in Figure 2E, mutations of T308A, S473A or both T308A and S473A impaired activation of Akt by DNA-PK, suggesting that phosphorylation of both 308T and 473S is important for full activation of Akt by DNA-PK.
Figure 2.
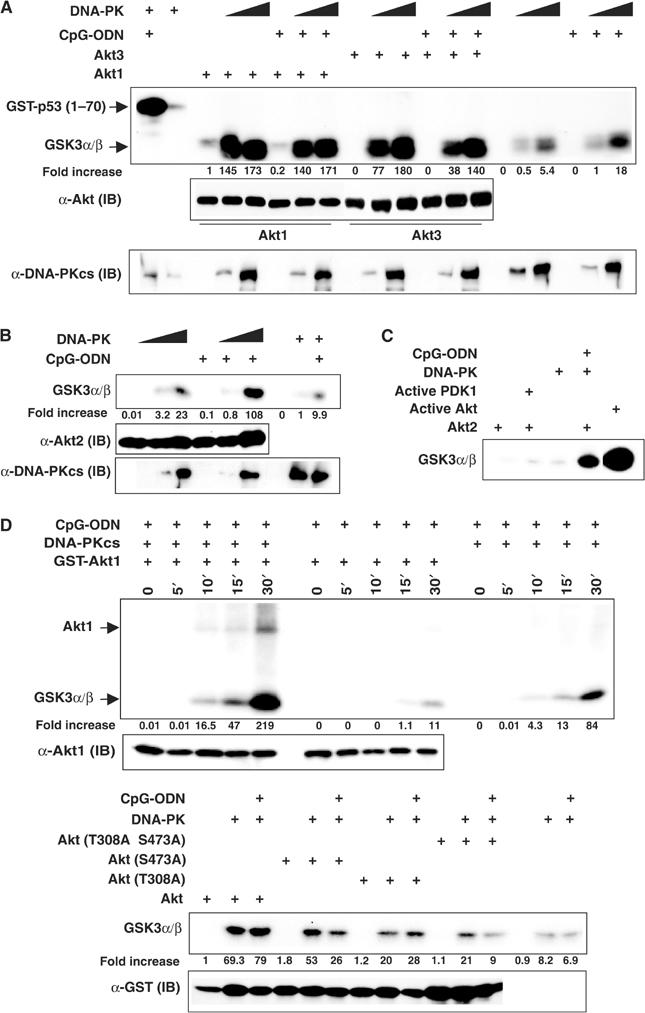
DNA-PK directly activates Akt in vitro. (A, B) Recombinant inactive Akt1 (50 ng), inactive Akt2 (100 ng) and inactive Akt3 (50 ng) purified from Sf9 cells were incubated with purified DNA-PK (10 or 30 U), 0.25 μg of GSK3α/β or 0.4 μg of GST-p53(1–70) and 3.3 μCi of [γ-32P]ATP in the presence or absence of CpG-ODN (2.5 ng/reaction) at 30°C for 30 min. The reactions were stopped by addition of 4 × sample buffers. The samples were boiled, loaded on 10% SDS–PAGE, transferred onto PVDF membranes and visualized via autoradiography followed by an IB analysis using anti-DNA-PKcs (mAb, cocktail), anti-Akt1, anti-Akt2 and anti-Akt3 antibodies. (C) Inactive Akt2 (200 ng) and GSK3α/β were incubated with active PDK1, purified DNA-PK (25 U), purified DNA-PK (25 U) together with CpG-ODN (1.25 ng) or left alone. In all, 20 U of active AKT (phosphorylated by active PDK1 in the presence of PIP3, Upstate, NY, USA) was incubated with GSK3α/β. Reactions were performed in the presence of 3.3 μCi of [γ-32P]ATP at 30°C for 30 min. (D) Activation of Akt1 by DNA-PK is in a time-dependent manner. Inactive GST-Akt1 (50 ng) together with purified DNA-PK (10 U), inactive GST-Akt1 (50 ng) or purified DNA-PK was incubated with 0.25 μg of GSK3α/β and 3.3 μCi of [γ-32P]ATP in the presence of CpG-ODN (2.5 ng/reaction) at 30°C for the indicated time points. The equal presence of Akt was examined by an IB assay using an anti-Akt1 antibody. (E) wt GST-Akt1(301–479, Akt) and GST-Akt1(301–479) mutant (Akt mutants) constructs were generated and expressed in Escherichia coli. Purified recombinant GST fusion proteins (25 ng) were incubated with DNA-PK (25 U), 0.25 μg of GSK3α/β and 3.3 μCi of [γ-32P]ATP in the presence or absence of CpG-ODN (2.5 ng/reaction) at 30°C for 30 min. The equal presence of each sample was determined by an IB assay using an anti-GST antibody. Note: Quantitative analysis of Akt KA (fold increase) in all in vitro kinase assays was performed using the Sigma Gel software.
DNA-PK induces phosphorylation of Akt in vitro
Our results above prompted us to further examine whether DNA-PK directly phosphorylates Akt, using in vitro phosphorylation assays. Incubation of recombinant inactive Akt2 with DNA-PK resulted in strong phosphorylation of Akt on 309T (Akt2, lane 7 versus lane 3) (Figure 3A), which was not significantly enhanced by the presence of CpG-ODN (lanes 11 and 12 versus lanes 7 and 8) (Figure 3A). However, incubation of Akt1 with DNA-PK led to an increase in phosphorylation of Akt1 on 308T (lane 8 versus lane 4) (Figure 3B), which was further intensified in the presence of CpG-ODN (lane 12 versus lanes 8 and 4, lane 11 versus lanes 7 and 3) (Figure 3B).
Figure 3.
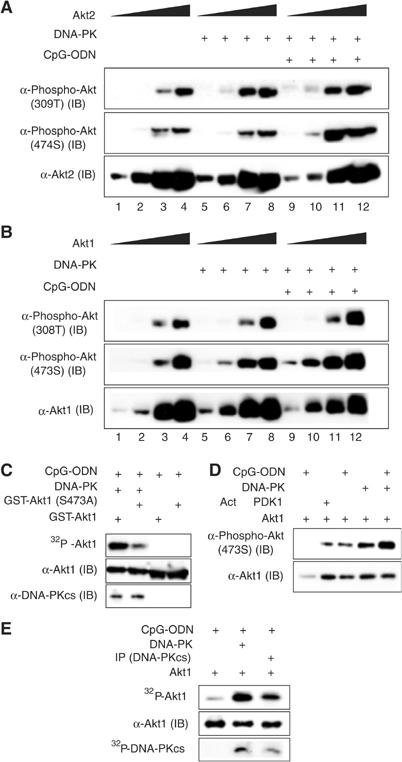
DNA-PK induces phosphorylation of Akt in vitro. (A, B) Inactive Akt1 (12.5, 50, 125 or 250 ng) or inactive Akt2 (50, 125, 250 or 500 ng) was incubated with purified DNA-PK (25 U) in the presence or absence of CpG-ODN at 30°C for 30 min. Phosphorylation of 473(4)S and 308(9)T on Akts was determined by IB assays using anti-phospho-Akt (473S) and anti-phospho-Akt (308T) antibodies, respectively. The equal presence of Akts was determined by IB assays using anti-Akt1 and anti-Akt2 antibodies. (C) GST-Akt1(301–479) (200 ng) or GST-Akt1(301–479, S473A) (GST-Akt1(S473A)) (200 ng) was incubated with CpG-ODN (2.5 ng/reaction) and 5 μCi of [γ-32P]ATP in the presence or absence of purified DNA-PK (25 U) at 30°C for 30 min. The equal presence of GST-Akt or DNA-PKcs was determined by IB assays using anti-Akt1 and anti-DNA-PKcs (cocktail) antibodies, respectively. (D) Active PDK1 does not phosphorylate Akt (473S) in vitro. Inactive Akt1 (100 ng) was incubated with DNA-PK (25 U) or active PKD1 (10 ng) in the presence or absence of CpG-ODN at 30°C for 30 min. Phosphorylation of Akt1 on 473S was determined. (E) Purified DNA-PK (40 U) was incubated with an anti-DNA-PKcs antibody (mAb, cocktail) and 20 μl protein G beads in 200 μl of lysis buffer at 4°C overnight. The beads were washed four times with lysis buffer. Inactive Akt1 (50 ng) together with immunoprecipitated DNA-PKcs (IP (DNA-PKcs)), purified DNA-PK (15 U) or Akt1 alone was incubated with 5 μCi of [γ-32P]ATP and CpG-ODN (2.5 ng/reaction) at 30°C for 30 min. Phosphorylation of Akt1 or DNA-PKcs was visualized by autoradiography and the equal presence of Akt was assessed by an IB assay using an anti-Akt1 antibody.
Unlike 309T, phosphorylation of Akt2 on 474S was not significantly enhanced by DNA-PK alone (lanes 7 and 8 versus lanes 3 and 4) (Figure 3A), but was largely induced in the presence of CpG-ODN (lanes 3, 4 versus lanes 7, 8 and lanes 11, 12) (Figure 3A). Interestingly, incubation of DNA-PK with Akt1 resulted in considerable phosphorylation of 473S (lane 7 versus lane 3), which was further augmented by the presence of CpG-ODN (lane 10 versus lanes 6 and 2, lane 11 versus lanes 7 and 3) (Figure 3B). Indeed, DNA-PK has the ability to phosphorylate Akt on 473S as the substitution of 473S with alanine (S473A) impaired DNA-PK-induced Akt phosphorylation (Figure 3C). As we expected, active PDK1 did not induce phosphorylation of Akt1 on 473S (Figure 3D) (reviewed in Datta et al, 1999; Brazil et al, 2002).
However, commercially available DNA-PK purified from HeLa cells contains Ku proteins and may not be 100% pure. In order to verify that DNA-PKcs mediates phosphorylation of Akt, we immunoprecipitated DNA-PKcs from this purified DNA-PK and then performed an in vitro kinase assay using recombinant Akt1 as a substrate. As shown in Figure 3E, immunoprecipitated DNA-PKcs enhanced Akt phosphorylation. Taken together, our findings demonstrate that DNA-PK induces phosphorylation of Akt on 308T and 473S.
DNA-PKcs associates with Akt in vitro and in vivo
To assess the possible functional interaction between DNA-PKcs and Akt, we first examined interaction of Akt with DNA-PKcs in vitro. As shown in Figure 4A, DNA-PKcs was co-immunoprecipitated with Akt using an anti-Akt antibody, but not control IgG. Next, we immunoprecipitated endogenous DNA-PKcs and analyzed the immune complexes for the presence of Akt using anti-Akt antibodies. Association of DNA-PKcs with Akt was detected in BMDMs as early as 15 min poststimulation with CpG-ODN (Figure 4B, upper panel). As a negative control, treatment with TNF did not induce interaction between DNA-PKcs and Akt in BMDMs (Figure 4B, upper panel). To further verify the association, we incubated immobilized GST-Akt1 beads with CpG-ODN-treated 8226 (a human B-lymphoma cell line) cell lysates and found that DNA-PKcs was co-immunoprecipitated with GST-Akt1, but not with GST-c-Jun (Figure 4C).
Figure 4.
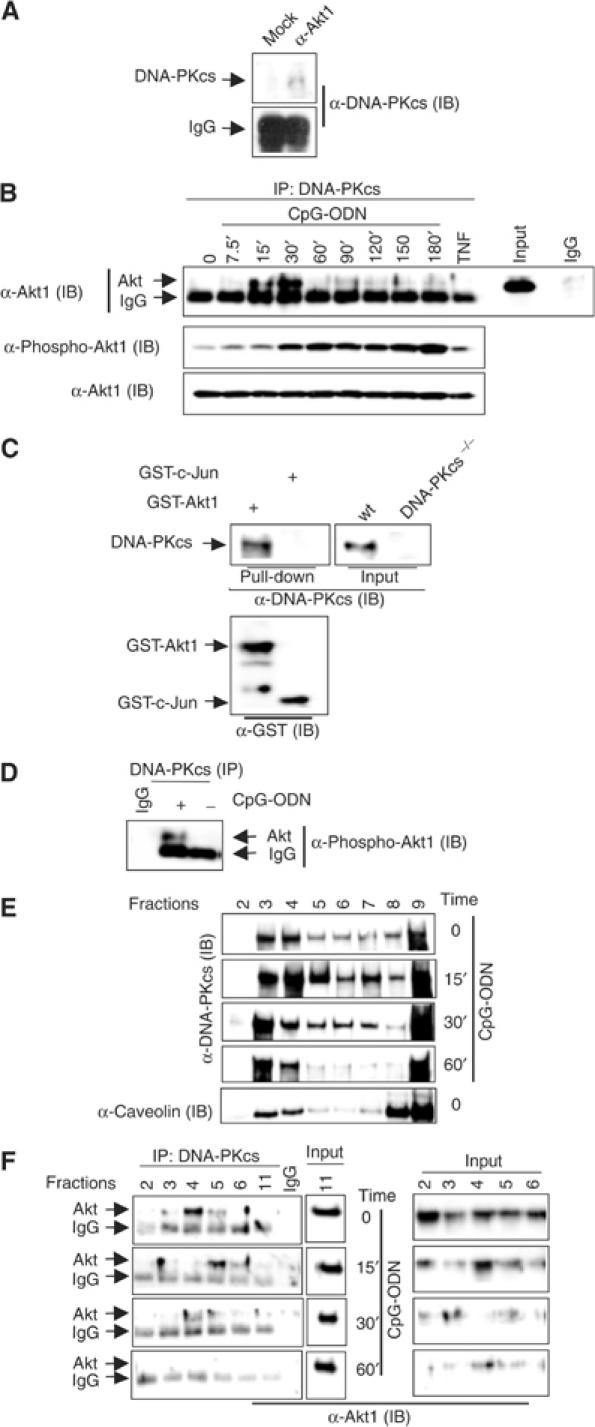
DNA-PKcs interacts with Akt in vitro and in vivo. (A) DNA-PKcs interacts with Akt in vitro. A 50 ng portion of recombinant Akt1, 25 U of purified DNA-PK and 20 μl of protein A beads were incubated with an anti-Akt1 antibody (rabbit polyclonal antibody) or rabbit IgG in 200 μl of lysis buffer (0.2 M NaCl). The protein A beads were washed four times with the same lysis buffer, boiled, loaded on a 7.5% SDS–PAGE, transferred onto a PVDF membrane and then probed by an anti-DNA-PKcs antibody (mAb, cocktail). (B) Upper panel: DNA-PKcs associates with Akt in BMDMs upon CpG-ODN treatment. BMDMs were treated with CpG-ODN (10 μg/ml) for the indicated durations and then lysed. A 200 μg portion of cell lysates was incubated with anti-DNA-PKcs mAb (cocktail) or mouse IgG and 20 μl of protein A/G beads at 4°C overnight. Beads were washed four times with lysis buffer, boiled, loaded onto a 10% SDS–PAGE and transferred onto a PVDF membrane followed by an IB assay using an anti-Akt1 antibody. Lower panel: the same cell lysates were blotted with an anti-phospho-Akt (473S) antibody. (C) 8226 cells were treated with CpG-ODN for 30 min and then lysed. Immobilized GST-Akt1 or GST-c-Jun(1–79) (GST-c-Jun) beads were incubated with 200 μg of lysates at 4°C overnight. The beads were washed with lysis buffer four times, boiled, loaded on 10% SDS–PAGE, transferred onto a PVDF membrane and then probed with anti-DNA-PKcs (mAb, cocktail) or anti-GST antibodies. Note: ‘Input' means that 40 μg of wt and DNA-PKcs−/− BMDM lysates were loaded as controls, thus providing the referred size for DNA-PKcs pulled down from 8226 cell lysates by GST-Akt1. (D) BMDMs were treated with CpG-ODN for 2 h and then DNA-PKcs was immunoprecipitated. The presence of phospho-Akt in the complex was determined by an IB assay using an anti-phospho-Akt (473S) antibody. (E) Localization of DNA-PKcs and Akt in lipid rafts. 8226 cells were treated with CpG-ODN for 0, 15, 30 or 60 min and then lysed in TNEX followed by floating in step sucrose gradient at 4°C. Fractions were collected from (1) to (bottom) (12) of the gradients as described under Materials and methods. Lipid rafts are presented in fractions 2–6, and soluble component is presented in fractions 9–12. Equal volume gradient fractions (20 μl) were separated onto SDS–PAGE and detected by IB with anti-DNA-PKcs (mAb, cocktail), anti-Akt1/2 and anti-caveolin 1 antibodies. (F) DNA-PKcs interacts with Akt in the lipid rafts. A 200 μl portion of each gradient fraction was incubated with anti-DNA-PKcs mAb (cocktail) or mouse IgG and 20 μl of protein A/G beads at 4°C overnight. The beads were washed four times with TNEX, boiled, loaded onto SDS–PAGE, transferred onto a PVDF membrane and blotted with anti-Akt1/2 antibodies.
Next, we examined whether this interaction correlates with Akt activation by CpG-ODN. As shown in Figure 4B (lower panel), phosphorylation of Akt was observed after 15 min incubation with CpG-ODN, gradually increasing and reaching maximal level around 180 min, suggesting that association of Akt with DNA-PKcs results in initial activation of Akt by CpG-ODN. Moreover, phosphorylated Akt was detected in the DNA-PKcs–Akt complex upon CpG-ODN treatment (Figure 4D).
Recently, a study suggested that DNA-PKcs localizes in the lipid rafts (Lucero et al, 2003). We confirmed this finding using 8226 cells and further found that peaks of DNA-PKcs in the lipid rafts were slightly changed after stimulation with CpG-ODN (Figure 4E). As a positive control, caveolin 1 was observed in the lipid rafts (Figure 4E and data not shown). Since a study reported that Akt and its 473S kinase complex reside in the lipid rafts (Hill et al, 2002), we decided to examine if DNA-PKcs interacts with Akt at this special inner cell membrane. Our immunoprecipitation assay results showed that DNA-PKcs associated with Akt in the lipid rafts in unstimulated 8226 cells, and in cells stimulated with CpG-ODN for 15 min (Figure 4F, left panel). Weak association was also observed in cells stimulated for 30 min, but not 60 min (Figure 4F, left panel).
DNA-PKcs is involved in Akt nuclear translocation in response to CpG-ODN
Akt primarily resides in the cytoplasm, but in some cells it is also localized in the nucleus (Ahmed et al, 1993). It has been well established that, in certain circumstances (e.g., insulin- and IGF-stimulated cells as well as Tcl1-transfected cells), Akt translocates into the nucleus (Andjelkovic et al, 1997; Meier et al, 1997; Pekarsky et al, 2000). DNA-PKcs, on the other hand, has been reported to be in both the cytoplasm and nucleus in human cells, but mainly in the nucleus except during the M phase of the cell cycle (Carter et al, 1990; Danska et al, 1996; Koiker et al, 1999; Nilsson et al, 1999). Surprisingly, DNA-PKcs was detected to be primarily within the cytoplasm of mouse embryonic fibroblasts (MEFs) and BMDMs, whereas it is predominantly a nuclear protein in HeLa cells (Figure 5A and B). Thus, we sought to determine whether CpG-ODN treatment affects subcellular localization of both Akt and DNA-PKcs in BMDMs. Since all three isoforms of Akt have some basal activity in quiescent BMDMs, we decided to perform an immunofluorescence assay using an anti-phospho-Akt (308T) antibody, which is able to recognize all isoforms of phospho-Akt (pAkt). Although interaction of DNA-PKcs with Akt identified by an immunoprecipitation assay in untreated BMDMs was undetectable (Figure 4A), colocalization of DNA-PKcs and pAkt was observed (Figure 5B). Nuclear translocation of both pAkt and DNA-PKcs was enhanced after 30 min incubation with CpG-ODN and reached maximal level after 45 min (Figure 5B). In contrast, loss of DNA-PKcs largely impaired nuclear translocation of pAkt in response to CpG-ODN (Figure 5B). We also quantitatively analyzed nuclear intensity of DNA-PKcs and pAkt versus that of whole cells. As shown in Figure 5C and D, DNA-PKcs and pAkt transiently translocated into the nucleus after CpG-ODN treatment. The nuclear translocation of pAkt in response to CpG-ODN was further confirmed by an IB analysis using nuclear fractions of CpG-DNA-treated BMDMs from both wt and DNA-PKcs-deficient mice (data not shown). Taken together, our observations demonstrate that CpG-ODN induces transiently nuclear translocation of both DNA-PKcs and pAkt, and that DNA-PKcs is important for CpG-ODN-induced pAkt nuclear translocation.
Figure 5.
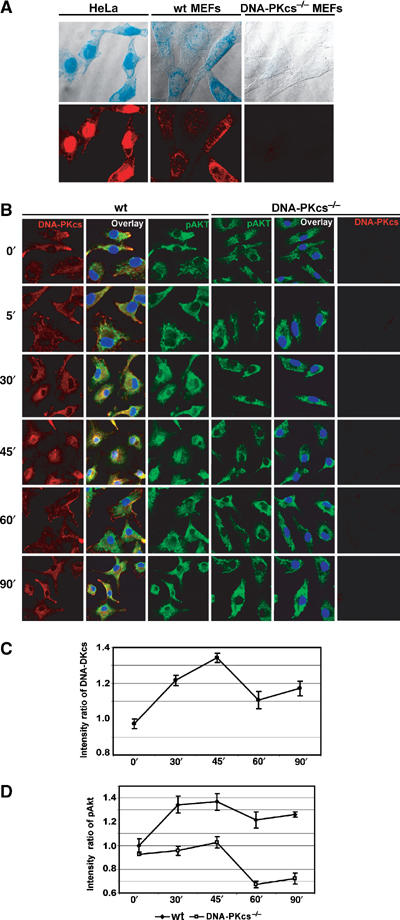
CpG-ODN induces DNA-PKcs and DNA-PKcs-dependent pAkt nuclear translocation. (A) Localization of DNA-PKcs in both HeLa and MEFs. HeLa, wt MEFs and DNAPKcs−/− MEFs were stained with anti-DNA-PKcs (mAb, cocktail) and anti-mouse-rhodamine antibodies. Images were detected by a confocal microscopy. The fluorescent images (lower panels) were superimposed on a bright field image of the respective cells (upper panels). (B) pAkt translocates into the nucleus in a DNA-PKcs-dependent manner following CpG-ODN treatment. wt and DNA-PKcs−/− BMDMs were treated with CpG-ODN (10 μg/ml) as indicated, fixed, permeabilized and immunostained with an anti-DNA-PKcs mAb (cocktail)/rhodamine and an anti-phospho-Akt (308T) antibody/FITC. The nuclear region was identified with DAPI staining. Quantitative analysis of DNA-PKcs (C) and pAkt (D) translocation was performed using the Leica Confocal software: the ratio of mean intensity of DNA-PKcs (C) or pAkt (D) signal in the nuclear region versus the total mean intensity for the respective cell was calculated for at least 10 randomly selected cells from several different fields. The means of all calculated ratios for DNA-PKcs in wt BMDMs (C) or pAkt in wt and DNA-PKcs−/− BMDMs (D) following CpG-ODN treatment were graphed. The nucleus was defined by DAPI staining.
Discussion
Activation of the immune system by CpG-DNA has been shown to be dependent on its ability to induce gene expression. However, now it is recognized that the CpG-driven cell survival through activation of the NF-κB and PI3K/Akt pathways (Yi et al, 1998, 1999; Park et al, 2002) also plays a role in regulating immune response.
Since TLR9 has been known as the receptor for CpG-DNA (reviewed in Ulevitch, 2004; Wagner, 2004), it is expected that TRL9 is required for activation of Akt by CpG-ODN. Intriguingly, neither the presence of TLR9 inhibitor, chloroquine (A-M Dragoi and W-M Chu, unpublished observation), nor loss of TLR9 impairs activation of Akt by CpG-ODN. In contrast, a previous report showed that TLR9 deficiency leads to a defect in activation of IKK by CpG-ODN (Figure 1; Hemmi et al, 2000). The discrepancy regarding the role of TLR9 in activation of the immune system in response to CpG-DNA has been previously reported. For example, TLR9 is not critical for CpG-ODN-mediated crosspresentation of DNA-conjugated antigens, but pivotal for crosspriming of CD8 cells (Heit et al, 2003). TLR9 is not required for activation of human neutrophils, but essential for the function of human plasmacytoid DCs in response to CpG-DNA (Krug et al, 2001a, 2001b; Kerkmann et al, 2003; Trevani et al, 2003). These studies suggest that TLR9 is not the only receptor for CpG-DNA; in the absence of TLR9, other protein factors could be critical for activation of CpG-DNA pathways in certain circumstances. Indeed, our results presented here not only provide an additional line of evidence to support this scenario, but also demonstrate a novel pathway that leads to Akt activation by CpG-DNA.
We have obtained evidence indicating that Akt activation is dependent on DNA-PKcs, which acts upstream to Akt. We showed that phosphorylation and activation of Akt are largely impaired in DNA-PKcs-deficient BMDMs (Figure 1). Moreover, SCID BMDMs cannot mount a normal Akt phosphorylation and activation in response to CpG-ODN. Furthermore, loss of Ku70 and Rag1 did not result in a defect in Akt phosphorylation, excluding roles of DNA instability and developmental failure in the CpG-DNA/Akt pathway. Thus, our findings reveal a link between DNA-PKcs and Akt. This fits nicely with the function of DNA-PKcs and Akt, which are both important for maintaining homeostasis of the immune system (Hill et al, 2002; Rathmell and Thompson, 2002).
Our results further demonstrate that DNA-PK directly triggers Akt activation, which is largely dependent on phosphorylation of Akt on 308T and 473S. Previous work has established that PDK1 is the physiological kinase for 308T on Akt1. However, phosphorylation of Akt1 on 308T by PDK1 requires PIP3. Here, we showed that, in the absence of PIP3, DNA-PK was able to induce phosphorylation of 308(309)T in vitro. As previously shown for Tcl1 (Laine et al, 2000; Pekarsky et al, 2000), phosphorylation of 308(309)T and consequently activation of Akt by DNA-PK could be due to its interaction with Akt and the subsequent conformational change of Akt. Indeed, we identified that Akt interacted with DNA-PKcs in vitro (Figure 4A). Moreover, our results showed that incubation of DNA-PK with inactive Akt also resulted in robust phosphorylation of 473(474)S on Akt. Using GST-Akt1 and GST-Akt1 (S473A) as substrates, we observed that S473A mutation largely impaired phosphorylation of Akt by DNA-PK (Figures 2 and 3C), suggesting that DNA-PK is a kinase for 473S. This scenario is supported by recent evidence showing that DNA-PKcs is involved in phosphorylation of Akt on 473S in response to insulin and pervanadate (Feng et al, 2004). Furthermore, whereas activation of DNA-PK was important for Akt2 activation (Figure 2B), there was no significant difference between DNA-PK and DNA-PK together with CpG-ODN in activation of Akt1 and Akt3. This does not mean that activity of DNA-PK is not important for Akt1 and Akt3 activation. In fact, DNA-PK inhibitor, wortmannin, was able to inhibit activation of Akt1 by DNA-PK in vitro (A-M Dragoi and W-M Chu, unpublished observation). Moreover, phosphorylation of Akt1 on 308T and 473S was further enhanced by DNA-PK in the presence of CpG-ODN (Figure 3A and B). Therefore, it seems that both interaction and DNA-PK KA are important for phosphorylation and activation of Akt in vitro. In addition, genetic evidence reveals that biological consequences of Akt1 and Akt2 deficiency are different, suggesting that Akt1 and Akt2 could be regulated differently. Thus, it is possible that DNA-PK differentially activates Akt1 and Akt2 in certain circumstances.
Since DNA-PK directly activates Akt via phosphorylation and protein–protein interaction in vitro, we postulated that DNA-PKcs physiologically interacts with Akt in vivo, leading to Akt activation. We obtained evidence that DNA-PKcs associates with Akt in BMDMs in a CpG-DNA-dependent manner. This association is functional as the interaction of Akt with DNA-PKcs correlated with Akt phosphorylation in response to CpG-ODN and phosphorylated Akt was identified in the Akt/DNA-PKcs immune complex. Interestingly, our confocal results showed that some pAkt colocalized with DNA-PKcs close to the membrane in unstimulated BMDMs. This colocalization of pAkt may bring it in proximity to DNA-PKcs that further enhances its phosphorylation and activation in response to CpG-DNA. Alternatively, the colocalization may suggest that pAkt loosely contacts DNA-PKcs in quiescent BMDMs in an association that is too weak to be detectable by a conventional immunoprecipitation assay. However, association of Akt and DNA-PKcs in the lipid rafts was observed in quiescent human B-lymphoma cells (Figure 4F).
Finally, our results demonstrate that CpG-DNA induces transient nuclear translocation of both pAkt and DNA-PKcs and that the pAkt nuclear translocation is DNA-PKcs-dependent. DNA-PKcs was originally identified as a nuclear protein in human cells and later was found to be in the cytosol and the lipid rafts (Carter et al, 1990; Danska et al, 1996; Koiker et al, 1999; Nilsson et al, 1999; reviewed in Smith and Jackson, 1999; Lucero et al, 2003). Surprisingly, DNA-PKcs primarily resides in the cytoplasm of MEFs and mouse BMDMs (Figure 5B). To verify this observation, we used different anti-DNA-PKcs antibodies to examine the distribution of DNA-PKcs in HeLa, wt and DNA-PKcs-deficient cells. As we expected, DNA-PKcs was mainly localized in the nucleus of HeLa cells and was not detected in DNA-PKcs-deficient BMDMs and MEFs (Figure 5A and B). Thus, the nuclear distribution of DNA-PKcs in mouse cells is different from that in human cells. Coincidentally, the majority of pAkt was detected in the cytoplasm. It has been suggested that stimuli, such as, insulin and IGF, induce transient nuclear translocation of Akt, which does not have an inherent nuclear localization sequence, in human embryonic kidney cells (293) or MEFs (Andjelkovic et al, 1997; Meier et al, 1997). This translocation was independent of Akt KA and the PH domain of Akt (Andjelkovic et al, 1997). A recent study indicated that in MEFs overexpressing Akt and Tcl1, Akt1 constitutively localized in the nucleus (Pekarsky et al, 2000). Since Akt1 interacts with Tcl1, it is possible that interaction between Akt and Tcl1 is responsible for Akt nuclear translocation (Pekarsky et al, 2000). However, since Tcl1 is only expressed in certain lymphocytes, additional molecules responsible for Akt nuclear translocation may exist. Here, we identified that CpG-DNA induces transient nuclear translocation of DNA-PKcs, which parallels that of pAkt in BMDMs (Figure 5B). This change is DNA-PKcs-dependent as loss of the DNA-PKcs largely impaired CpG-ODN-induced pAkt nuclear translocation. Thus, it is possible that DNA-PKcs interacts with and further activates Akt upon CpG-DNA stimulation, leading to the assembly of a complex that promotes the nuclear transport of pAkt.
The physiological outcome of the DNA-PKcs-dependent Akt activity is expected to occur through phosphorylation of Akt-specific targets, which are present in the lipid rafts, the cytosol and the nucleus. One of them is the IKK. It has been suggested that Akt is upstream to IKKα and NF-κB (reviewed in Ghosh and Karin, 2002). Moreover, our previous study and another suggested that DNA-PKcs is also involved in activation of IKK and NF-κB (Chu et al, 2000; Panta et al, 2004). Thus, it is possible that DNA-PKcs activates Akt, which in turn activates IKK and NF-κB. Since both Akt and NF-κB are important for cell survival, our results may help explain some clinical effects of CpG-DNA and DNA-PK inhibitors. For insistence, it has been suggested that the CpG-ODN-induced enhancement of the efficacy of monoclonal antibody (mAb) therapy for chronic lymphocytic leukemia (CLL) was due, at least in part, to the CpG-ODN's antiapoptotic effect on B cells (Warren and Weiner, 2002). This antiapoptotic effect may be caused by activation of Akt and NF-κB by DNA-PKcs. Furthermore, we observed that in human B lymphoma (8226) cells, Akt has high basal activity (data not shown) and associates with DNA-PKcs in the lipid rafts (Figure 4F). The former is in line with observations that Akt activity is associated with cancer development and anticancer drug resistance (reviewed in Hanada et al, 2004); the latter may provide insight into how Akt is activated in cancerous cells. Indeed, it has been reported that tumor cells resistant to anticancer drugs show increases in both DNA-PK expression and activity, and that the use of DNA-PK inhibitor wortmannin is likely to improve the effectiveness of anticancer drug, suggesting that DNA-PK may play an important role in the development of multi-drug resistance (MDR) (Christodoulopoud et al, 1998; Muller et al, 1998, 2000; Um et al, 2003). Since DNA-PKcs interacts with and activates Akt, it is very possible that both DNA-PK and Akt activities contribute to cancerous cell survival. Thus, selective inhibition of DNA-PK or Akt activation may be beneficial for anticancer therapy.
In conclusion, this work identifies Akt as a target of DNA-PKcs, but not a downstream relay molecule of TLR9 in response to CpG-ODN. DNA-PKcs associates with Akt, specifically inducing both phosphorylation and activation of Akt as well as promoting transient nuclear translocation of pAkt. Thus, DNA-PKcs not only plays a role in activation of IKK and NF-κB but also acts upstream to Akt upon CpG-DNA stimulation.
Materials and methods
Mice and bone marrow-derived macrophages
DNA-PKcs−/−, Ku70−/−, TLR9−/− and wt mice on C57/B6/129 genetic background were bred as previously reported (Ouyang et al, 1997; Kurimasa et al, 1999; Hemmi et al, 2000). Rag1−/−, SCID mice and their controls were purchased from the Jackson laboratory. BMDMs were prepared and cultured as described (Chu et al, 2000).
CpG-ODNs, proteins and antibodies
Endotoxin-free CpG-ODN (1018, phosphorothioate) was purchased from Trilinker Biotechnology, CA, USA. Purified DNA-PK was from Promega Biotechnology, MI, USA. Recombinant Akts (Akt1, Akt2 and Akt3) and PDK1 were from Upstate Biotechnology, NY, USA. Anti-phospho antibodies against Akt, JNK1/2 and GSK3α/β substrate were from Cell Signaling, MA, USA. Anti-DNA-PKcs mAb (cocktail) was purchased from Neomarker Biotechnology, CA, USA. All regular antibodies were purchased from Santa Cruz Biotechnology, CA, USA.
Kinase assays and immunoblotting assays
After 7 days, BMDMs were trypsinized, cultured in DMEM supplemented with 5% FBS overnight and then starved for 4 h. After starvation, BMDMs were treated with endotoxin-free CpG-ODN (10 μg/ml) (Chu et al, 2000), LPS (Sigma, MO, USA; 3 μg/ml) or TNF (Biosource, CA, USA; 20 ng/ml) for indicated durations or left untreated. Cell lysates were prepared and Akt kinase complexes were immunoprecipitated with anti-Akt1, anti-Akt2 or anti-Akt3 antibodies. The KAs were determined by a kinase assay using GSK3α/β as a substrate followed by IB analysis by normalizing equal loading. IKK and JNK kinase assays were performed as previously described (Chu et al, 1999, 2000). Phosphorylation of Akts and JNK1/2 was detected by IB with antibodies against phospho-Akt473S and -JNK1/2, respectively.
The in vitro kinase assay was performed according to Chu et al (2000) with modification. Briefly, purified DNA-PK or recombinant active PDK1 was incubated with various amounts of recombinant Akts freshly purified from baculovirus–insect system or GST-Akts from bacteria, 0.25 μg of GSK3α/β and 3.3 μCi of [γ-32P]ATP (Amersham, IL, USA) in the presence or absence of CpG-ODN (2.5 ng/reaction) in a 20 μl of reaction buffer at 30°C for 30 min. Reactions were stopped by the addition of 4 × loading buffers. Samples were boiled, loaded on 10% SDS–PAGE, transferred onto a PVDF membrane and visualized by autoradiography followed by probing the same hot membranes with anti-DNA-PKcs or anti-Akts antibodies.
The in vitro phosphorylation assays were performed as previously described (Chu et al, 2000). Briefly, 200 ng of recombinant Akt1 or GST-Akts was incubated with 10 μCi of [γ-32P]ATP, 20 μM ATP and purified DNA-PK or immunoprecipitated DNA-PKcs at 30°C for 45 min followed by autoradiography. Using a similar strategy, in vitro phosphorylation assays using recombinant Akts as substrates in the absence of [γ-32P]ATP were performed and transferred membranes were probed with anti-phospho-Akt (473S) or anti-phospho-Akt (308T) antibodies and detected by ECL (Amersham, IL, USA).
Immunoprecipitation and lipid rafts
BMDMs were treated with CpG-ODN (10 μg/ml) for the indicated durations and then lysed in a lysis buffer (160 mM NaCl, 20 mM Tris–HCl, pH 7.4, 0.1% Triton X-100, 10% glycerol, 1 mM EDTA, 20 mM β-glycerol phosphate, 0.2 mM Na3VO4 and protease inhibitor cocktails (Roche Diagnostics, IN, USA)). Endogenous DNA-PKcs was immunoprecipitated by overnight incubation with anti-DNA-PKcs (mAb, cocktails or polyclonal anti-DNA-PKcs antibody; 2 μg/mg of lysates) and 20 μl of protein A/G Sepharose (beads) (Amersham, IL, USA). Immune complexes were washed four to five times with lysis buffer, boiled and subjected to 10% SDS–PAGE.
Lipid rafts were prepared as described (Lucero et al, 2003) with modification. Briefly, 8226 cells were washed with cold PBS, and cell pellet was homogenized in TNEX (50 mM Tris–HCl, pH 7.4, 150 mM NaCl, 5 mM EDTA, 1% Triton X-100, 2 mM Na3VO4 and protease inhibitor cocktails) and incubated for 30 min on ice. Extracts were brought to 40% sucrose (Sigma, MO, USA) and overlaid with 2 volumes of 30% sucrose in TNEX and a top layer of 1 volume of 5% sucrose in TNEX. Samples were spun overnight at 4°C in an SW55.1 rotor at 45 000 r.p.m. Fractions of 400 μl were collected from the top of the gradient. A 20 μl portion of lipid raft fractions was used to examine the presence of DNA-PKcs, Akt and caveolin 1 using anti-DNA-PKcs, anti-Akt and anti-caveolin 1 antibodies, respectively. Immunoprecipitation of DNA-PKcs in lipid fractions was performed and the DNA-PKcs immune complex was washed four times with TNEX.
Confocal microscopy
BMDMs were seeded in culture slides at 7.5 × 104 per chamber and were serum deprived 4 h prior to treatment. Following treatment, the cells were fixed with 3% paraformaldehyde, permeabilized with 0.2% Triton X-100 and proteins were detected with anti-DNA-PKcs (cocktail), anti-phospho-Akt (308T), anti-mouse-rhodamine and anti-rabbit-FITC (Biosource Biotechnology, CA, USA) antibodies. DAPI (Santa Cruz Biotechnology, CA, USA) staining defined the nucleus. We used an inverted Leica TCS SP2 AOBS confocal microscope (Leica, Germany) with the × 63 oil immersion objective. The fluorophores were sequentially excited with the 408, 488, and 543 nm laser lines to prevent crossexcitation. Images were analyzed with the Leica Confocal software.
Acknowledgments
We are grateful to Dr Shizuo Akira for providing TLR9−/− mice. We thank Drs J Marshal, E Raz and Z Wang for reading this manuscript and Drs F Alt, M Birmbaum and L Cantley for critical discussion. This work was mainly supported by a grant from NIH to WMC (R01 AI054128-01) and supported by COBRA to WMC.
References
- Ahmed NN, Franke TF, Bellacosa A, Datta K, Gonzalez-Portal ME, Taguchi T, Testa JR, Tsichlis PN (1993) The proteins encoded by c-akt and v-akt differ in post-translational modification, subcellular localization and oncogenic potential. Oncogene 8: 1957–1963 [PubMed] [Google Scholar]
- Akira S, Hemmi H (2003) Recognition of pathogen-associated molecular patterns by TLR family. Immunol Lett 85: 85–95 [DOI] [PubMed] [Google Scholar]
- Andjelkovic M, Alessi DR, Meier R, Fernandez A, Lamb NJ, Frech M, Cron P, Cohen P, Lucocq JM, Hemmings BA (1997) Role of translocation in the activation and function of protein kinase B. J Biol Chem 272: 31515–31524 [DOI] [PubMed] [Google Scholar]
- Bassing CH, Swat W, Alt F (2002) The mechanism and regulation of chromosomal V(D)J recombination. Cell 109: s45–s55 [DOI] [PubMed] [Google Scholar]
- Blunt T, Gell D, Fox M, Taccioli GE, Lehmann AR, Jackson SP, Jeggo PA (1996) Identification of a nonsense mutation in the carboxyl-terminal region of DNA-dependent protein kinase catalytic subunit in the scid mouse. Proc Natl Acad Sci USA 93: 10285–10290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazil DP, Park J, Hemmings BA (2002) PKB binding proteins: getting in on the Akt. Cell 111: 293–303 [DOI] [PubMed] [Google Scholar]
- Carter T, Vancurova I, Sun I, Lou W, DeLeon S (1990) A DNA-activated protein kinase from HeLa cell nuclei. Mol Cell Biol 10: 6460–6471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christodoulopoud G, Muller C, Shalles B, Kazmi R, Panasci L (1998) Potentiation of Chlorambucil cytotoxicity in B cell chronic lymphocytic leukemia by inhibition of DNA-dependent protein kinase activity using wortmannin. Cancer Res 58: 1789–1792 [PubMed] [Google Scholar]
- Chu WM, Gong X, Li ZW, Takabayashi K, Ouyang HH, Chen Y, Lois A, Chen DJ, Li GC, Karin M, Raz E (2000) DNA-PKcs is required for activation of innate immunity by immunostimulatory DNA. Cell 103: 909–918 [DOI] [PubMed] [Google Scholar]
- Chu WM, Ostertag D, Li ZW, Chang L, Chen Y, Hu Y, Williams B, Perrault J, Karin M (1999) JNK2 and IKKbeta are required for activating the innate response to viral infection. Immunity 11: 721–731 [DOI] [PubMed] [Google Scholar]
- Danska JS, Holland DP, Mariathasan S, Williams KM, Guidos CJ (1996) Biochemical and genetic defects in the DNA-dependent protein kinase in murine scid lymphocytes. Mol Cell Biol 16: 5507–5517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta SR, Brunet A, Greenberg ME (1999) Cellular survival: a play in three Akts. Genes Dev 13: 2905–2927 [DOI] [PubMed] [Google Scholar]
- Feng J, Park J, Cron P, Hess D, Hemmings BA (2004) Identification of a PKB/Akt hydrophobic motif Ser-473 kinase as DNA-dependent protein kinase. J Biol Chem 279: 41189–41196 [DOI] [PubMed] [Google Scholar]
- Franke TF, Kaplan DR, Cantley LC, Toker A (1997) Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science 275: 665–668 [DOI] [PubMed] [Google Scholar]
- Frech M, Andjelkovic M, Ingley E, Reddy KK, Falck JR, Hemmings BA (1997) High affinity binding of inositol phosphates and phosphoinositides to the pleckstrin homology domain of RAC/protein kinase B and their influence on kinase activity. J Biol Chem 272: 8474–8481 [DOI] [PubMed] [Google Scholar]
- Ghosh F, Karin M (2002) Missing pieces in the NF-κB puzzle. Cell 109: s81–s96 [DOI] [PubMed] [Google Scholar]
- Hanada M, Feng J, Hemmings BA (2004) Structure, regulation and function of PKB/AKT—a major therapeutic target. Biochim Biophys Acta 1697: 3–16 [DOI] [PubMed] [Google Scholar]
- Heit A, Maurer T, Hochrein H, Bauer S, Huster KM, Busch DH, Wagner H (2003) Cutting edge: Toll-like receptor 9 expression is not required for CpG DNA-aided cross-presentation of DNA-conjugated antigens but essential for cross-priming of CD8T cells. J Immunol 170: 2802–2805 [DOI] [PubMed] [Google Scholar]
- Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S (2000) A Toll-like receptor recognizes bacterial DNA. Nature 408: 740–745 [DOI] [PubMed] [Google Scholar]
- Hill MM, Feng J, Hemmings BA (2002) Identification of a plasma membrane Raft-associated PKB Ser473 kinase activity that is distinct from ILK and PDK1. Curr Biol 12: 1251–1255 [DOI] [PubMed] [Google Scholar]
- Kerkmann M, Rothenfusser S, Hornung V, Towarowski A, Wagner M, Sarris A, Giese T, Endres S, Hartmann G (2003) Activation with CpG-A and CpG-B oligonucleotides reveals two distinct regulatory pathways of type I IFN synthesis in human plasmacytoid dendritic cells. J Immunol 170: 4465–4474 [DOI] [PubMed] [Google Scholar]
- Krug A, Rothenfusser S, Hornung V, Jahrsdörfer B, Blackwell S, Ballas ZK, Endres S, Krieg AM, Hartmann G (2001a) Identification of CpG oligonucleotide sequences with high induction of IFN in plasmacytoid dendritic cells. Eur J Immunol 31: 2154. [DOI] [PubMed] [Google Scholar]
- Krug A, Towarowski S, Britsch S, Rothenfusser S, Hornung V, Bals R, Giese T, Engelmann H, Endres S, Krieg AM, Hartmann G (2001b) Toll-like receptor expression reveals CpG DNA as a unique microbial stimulus for plasmacytoid dendritic cells which synergizes with CD40 ligand to induce high amounts of IL-12. Eur J Immunol 31: 3026. [DOI] [PubMed] [Google Scholar]
- Koiker M, Awaji T, Kataoka M, Tsujimoto G, Kartasova T, Koike A, Shiomi T (1999) Differential subcellular localization of DNA- dependent protein kinase components Ku and DNA-PKcs during mitosis. J Cell Sci 112: 4031–4039 [DOI] [PubMed] [Google Scholar]
- Kurimasa A, Ouyang H, Dong L-J, Wang S, Li X, Cordon-Cardo C, Chen D, Li GC (1999) Catalytic subunit of DNA-dependent protein kinase: impact on lymphocyte development and tumorigenesis. Proc Natl Acad Sci USA 96: 1403–1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine J, Kunstle G, Obata T, Sha M, Noguchi M (2000) The protooncogene TCL1 is an Akt kinase coactivator. Mol Cell 8: 395–407 [DOI] [PubMed] [Google Scholar]
- Lee SH, Kim CH (2002) DNA-dependent protein kinase complex: a multifunctional protein in DNA repair and damage checkpoint. Mol Cell 13: 159–166 [PubMed] [Google Scholar]
- Lucero H, Gae D, Taccioli GE (2003) Novel localization of the DNA-PK complex in lipid rafts. J Biol Chem 278: 22136–22143 [DOI] [PubMed] [Google Scholar]
- Meier R, Alessi DR, Cron P, Andjelkovic M, Hemmings BA (1997) Mitogenic activation, phosphorylation, and nuclear translocation of protein kinase Bbeta. J Biol Chem 272: 30491–30497 [DOI] [PubMed] [Google Scholar]
- Muller C, Christodoulopoud G, Shalles B, Panasci L (1998) DNA-dependent kinase activity correlates with clinical and in vitro sensitivity of chronic lymphocytic leukemia lymphocytes to nitrogen mustards. Blood 92: 2213–2219 [PubMed] [Google Scholar]
- Muller C, Calsou P, Shalles B (2000) The activity of the DAN-PK complex is determinant in the cellular response to nitrogen mustards. Biochemie 82: 25–28 [DOI] [PubMed] [Google Scholar]
- Nilsson A, Sirzen F, Lewensohn R, Wang N, Skog S (1999) Cell cycle-dependent regulation of the DNA-dependent protein kinase. Cell Prolif 32: 239–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norbury CJ, Zhivotovsky B (2004) DNA damage-induced apoptosis. Oncogene 23: 2797–2808 [DOI] [PubMed] [Google Scholar]
- Ouyang H, Nussenzweig A, Kurimasa A, Soares VC, Li X, Cordon-Cardo C, Li W, Cheong N, Nussenzweig M, Iliakis G, Li GC (1997) Ku70 is required for DAN repair, but not for T cell antigen receptor gene recombination in vivo. J Exp Med 186: 921–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panta GR, Kaur S, Cavin LG, Cortes ML, Mercurio F, Lothstein L, Sweatman TW, Israel M, Arsura M (2004) ATM and the catalytic subunit of DNA-dependent protein kinase activate NF-kappaB through a common MEK/extracellular signal-regulated kinase/p90(rsk) signaling pathway in response to distinct forms of DNA damage. Mol Cell Biol 24: 1823–1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y, Lee SW, Sung YC (2002) Cutting edge: CpG DNA inhibits dendritic cell apoptosis by up-regulating cellular inhibitor of apoptosis proteins through the phosphatidylinositide-3′-OH kinase pathway. J Immunol 168: 5–8 [DOI] [PubMed] [Google Scholar]
- Pekarsky Y, Koval A, Hallas C, Bichi R, Tresini M, Malstrom S, Russo G, Tsichlis P, Croce CM (2000) Tcl1 enhances Akt kinase activity and mediates its nuclear translocation. Proc Natl Acad Sci USA 97: 3028–3033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathmell JC, Thompson CB (2002) Pathways of apoptosis in lymphocyte development, homeostasis, and disease. Cell 109: s97–s107 [DOI] [PubMed] [Google Scholar]
- Sable CL, Filippa N, Filloux C, Hemmings BA, Van Obberghen E (1998) Involvement of the pleckstrin homology domain in the insulin-stimulated activation of protein kinase B. J Biol Chem 273: 29600–29606 [DOI] [PubMed] [Google Scholar]
- Scheid MP, Woodgett JR (2001) PKB/AKT: functional insights from genetic models. Nat Rev Mol Cell Biol 2: 760–768 [DOI] [PubMed] [Google Scholar]
- Smith GC, Jackson SP (1999) The DNA-dependent protein kinase. Genes Dev 13: 916–934 [DOI] [PubMed] [Google Scholar]
- Stephens L, Anderson K, Stokoe D, Erdjument-Bromage H, Painter GF, Holmes AB, Gaffney PR, Reese CB, McCormick F, Tempst P, Coadwell J, Hawkins PT (1998) Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-trisphosphate-dependent activation of protein kinase B. Science 279: 710–714 [DOI] [PubMed] [Google Scholar]
- Trevani AS, Chorny A, Salamone G, Vermeulen M, Gamberale R, Schettini J, Raiden S, Geffner J (2003) Bacterial DNA activates human neutrophils by a CpG-independent pathway. Eur J Immunol 33: 3164–3174 [DOI] [PubMed] [Google Scholar]
- Ulevitch RJ (2004) Therapeutics targeting the innate immune system. Nat Rev Immunol 4: 512–520 [DOI] [PubMed] [Google Scholar]
- Um JH, Kang CD, Hwang BW, Ha MY, Hur JG, Kim DW, Chung BS, Kim SH (2003) Involvement of DNA-dependent protein kinase in regulation of the mitochondria heat shock proteins. Leukemia Res 27: 509–516 [DOI] [PubMed] [Google Scholar]
- Van Uden J, Raz E (1999) Immunostimulatory DNA and applications to allergic disease. J Allergy Clin Immunol 104: 902–910 [DOI] [PubMed] [Google Scholar]
- Wagner H (2004) The immunobiology of the TLR9 subfamily. Trends Immunol 25: 381–386 [DOI] [PubMed] [Google Scholar]
- Warren TL, Weiner GJ (2002) CpG oligodeoxynucleotides and monoclonal antibody therapy of lymphoma. In Microbial DNA and Host Immunity, Raz E (ed) pp 329–337. Totowa, NJ: Human Press Inc. [Google Scholar]
- Yi AK, Chang M, Peckham DW, Krieg AM, Ashman RF (1998) CpG oligodeoxyribonucleotides rescue mature spleen B cells from spontaneous apoptosis and promote cell cycle entry. J Immunol 160: 5898. [PubMed] [Google Scholar]
- Yi AK, Peckham DW, Ashman RF, Krieg AM (1999) CpG DNA rescues B cells from apoptosis by activating NFkappaB and preventing mitochondrial membrane potential disruption via a chloroquine-sensitive pathway. Int Immunol 12: 2015–2024 [DOI] [PubMed] [Google Scholar]


