ABSTRACT
Background: The obesity epidemic has been growing steadily across the whole world, and so far not a single country has been able to reverse it. The cause of obesity is stated by the World Health Organization as an energy imbalance between calories consumed and calories expended. However, growing evidence suggests that the calorie imbalance concept may not be sufficient to manage and reverse the obesity epidemic.
Objective: To discuss the use of the calorie imbalance concept and its elements as a tool for weight management as well as its possible negative consequences and implications for public health, with the aim to point toward the need of an updated concept for causes of obesity. This update should guide public health interventions more efficiently to limit obesity by preventing weight gain or promoting weight loss.
Methods: This is a literature reviews based on a semi-structured approach to determine the material to be examined.
Results: After revisiting general facts about fat generation and accumulation, we propose an updated concept for the causes of obesity including diet composition and hormonal regulation of fat metabolism.
Conclusions: We discuss how this updated concept could benefit the overall efficiency of strategies against obesity, and hypothesize how potential resistance to adopting this new view could be lowered.
KEYWORDS: Obesity, calorie imbalance, obesity cause, hormonal imbalance, insulin, public health
Background
The World Health Organization (WHO) defines obesity as an ‘abnormal or excessive fat accumulation that may impair health’, and states that ‘the fundamental cause of obesity and overweight is an energy imbalance between calories consumed and calories expended’ (n.b. Food energy is commonly expressed as ‘kilocalories [kcal]’ [1]. However, the common use is to name them just ‘calories [cal]’. In this article kcal and cal will be used interchangeably). This concept is deeply rooted in public opinion as the core reason for overweight and obesity. Most public health strategies aiming to tackle obesity are based on this concept, i.e. they aim to decrease caloric consumption, to increase calories expended or ideally a combination of both. However, the concept of energy balance or imbalance may be incomplete (n.b. Because the relevant energy unit is calories, energy balance, calorie balance, calorie imbalance and energy imbalance will be used interchangeably). It may even be argued that this concept could be one of the reasons why public health strategies have been so unsuccessful in reversing the obesity epidemic [2]. The energy balance will be referred to as ‘concept 1’ in this article.
An alternative, although not exclusive, conceptual frame links obesity to the insulin-dependent regulation of fat generation and takes note of the different metabolic pathways to degrade macronutrients, i.e. carbohydrates (CHO), fat and proteins. This article revisits causes for obesity in the light of the latter frame and its concepts, e.g. glycemic index, and will discuss how this frame can overcome some of the weaknesses of concept 1, with the aim to more efficiently guide public health interventions to limit obesity by either preventing weight gain or promoting weight loss. This can be seen as a hormonal-balance concept, and it will here be referred to as concept 2. Strengths and weaknesses of both concepts will be discussed.
Methods
This is a narrative literature review covering evidence about obesity, its causes, control efforts and public health strategies. There are several approaches to study the causes of obesity, and this diversity provokes limitations. Therefore, this review does not claim to analyze the literature comprehensively, nor can we fully exclude potential risks of drawing conclusions on a possibly less-than-perfect basis. However, the chosen approach appears adequate to integrate complex interactions that apparently determine obesity [3].
Calorie imbalance as the suggested cause for obesity
Concept 1 has its roots in two laws of thermodynamics, i.e. the first law known as the ‘conservation law’ and the second law also known as the ‘dissipation law’. The first law states that in a closed system in thermal equilibrium, the form of energy may change but the total is always conserved [4]. This has been interpreted as a caloric balance, i.e. ‘calories in should equal calories out’. Therefore, if you gain weight you are either eating too much or moving too little, and in order to lose weight you would have to ‘eat less and/or move more’. Despite its apparently straightforward logic, this concept is not complete and the second law also needs to be considered. This states that ‘in any irreversible process, the entropy must increase and balance is not expected’ [4]. That means that for digestive processes where food turns into bolus, but bolus cannot be converted back into food, some of the input energy is irreversibly lost during the metabolic process and results in thermogenesis.
Concept 1 to avoid or reduce overweight and obesity
Because concept 1 assumes that a positive energy balance results in fat mass, individuals aiming to prevent weight gain should avoid a positive balance. Of the same tenor, individuals aiming to lose weight should look for a negative balance. Dietary guidelines commonly suggest the calories deficit needs to be in the range of 500–750 kcal per day for an adult to lose weight [5]. This value is based on the ‘3500 kcal rule’, also known as the ‘Wishnofsky rule’ [6], which is still used as the basis for some guidelines, publications and nutrition textbooks [7], despite its inaccuracy and very limited effectiveness [8]. The recommended deficit tacitly assumes ‘that a calorie is a calorie’ independently of its source [4], hence ignoring the second rule of thermodynamics. When the different values of catabolism-induced thermogenesis are considered for each macronutrient [9], ‘a calorie is a calorie’ may no longer hold true, but probably many among the common population and even some nutrition professionals ignore this [7,10]. By consequence, inaccurate assessments and recommendations for weight management may result.
Thus, concept 1 may lead to the misperception that total calorie intake is more important than the source of the calories and nutrient balance. Therefore, an individual trying to lose weight may become vulnerable to malnutrition when focusing only on calories. For example, restricting energy intake to 2000 kcal per day [5], if the resulting diet consists only of industrialized food (n.b. Products made from processed substances, extracted or refined from whole foods… [They are] very durable, palatable, and ready to consume… [They] are typically energy-dense, have a high glycemic load, are low in dietary fibers, micronutrients, and phytochemicals, and are high in unhealthy types of dietary fat, free sugars, and sodium’ [116]. Industrialized food or ultraprocessed food will be used interchangeably), may result in an overload of nutrients correlated with development of obesity and a lack of essential nutrients and micronutrients known to act against obesity [11]. Without discriminating the sources of calories, reducing the calorie intake usually results in a short phase of rapid weight loss, although the loss is not necessarily one of accumulated fat, but rather of fat-free mass [12]. Given that the main problem in obesity is, however, accumulated fat, losing any mass other than fat may be unproductive and not desirable.
Assuming that to respect a negative calorie balance could indeed be useful for individuals to lose weight, food information on energy intake and tools to measure the energy expense should be available, and they are. However, in reality it appears quite difficult for an individual to achieve this balance, because a constant error of only 175 kcal per day would have a considerable impact of up to 3 kg lost or gained within a year [13,14]. The available ways to measure caloric intake and expenditure cannot overcome this margin of error, as will be discussed in the following sub-sections.
Energy intake and front label information
Front labels on food are meant to inform consumers’ choices, including those who may wish to count their calorie intake. However, some industrialized foods have shown differences between the content of energy claimed on their front label and analyses by a third-party, e.g. a health organization [15,16]. Similarly, the caloric content of the dishes in a restaurant menu may be underestimated by either the individuals or the supplier [17,18]. However, it seems more problematic that this information may not be understood correctly, if individuals do not have a certain minimal knowledge of nutrition [19,20]. In addition, when one self-assesses energy intake, under- and over-reporting consumption may occur [21]. Several technological devices have been developed to assist assessment of energy intake to reduce these biases, e.g. chewing count devices, wrist moving devices, or special digital photographic cameras for food. However, these devices may not yield sufficiently valid data [22–25]. Therefore, measurements of the energy balance may not meet the aforementioned narrow margin of error of 175 kcal per day.
Physical activity and energy expenditure within concept 1
There are also barriers to assessing physical activity. The first one is the lack of standardized definitions of its levels, i.e. moderate, intermediate or vigorous intensity. Every level implies a different energy expenditure that also depends on other factors such as duration, body size and age. Hence, it is difficult to agree on which kind of physical activity should be recommended for effective weight control [26]. Whereas some studies and guidelines recommend vigorous physical activity [27,28], other evidence suggests that low-intensity activities such as walking, yoga, meditation and stretching are equally effective in reducing weight [28–30]. Second, energy expenditure might be assessed through technological tools aiming to enhance accuracy, such as pedometers and accelerometers, which measure walked distances and estimate the energy expenditure based on the weight, age and height of an individual. These measurements, however, have already an error of about 100 kcal/day [13]. Finally, despite the common belief that physical activity could equilibrate an energy imbalance, this is in reality difficult to achieve. For example, adults who eat a sandwich containing around 290 kcal should have to walk for around 90 minutes or almost 5 kilometers [31].
The energy expended in non-exercise activities that are part of neither the basal expenditure nor the digestive process is known as ‘non-exercise activity thermogenesis’ or NEAT [32] and also plays a role in the energy balance. These activities include ‘energy expenditure of occupation, leisure, sitting, standing, walking, talking, toe-tapping, playing guitar, dancing, and shopping’ [32], among others. The multitude of elements involved in NEAT and their variability make it even more challenging to assess them than physical activity, and hence, for them to be considered in the energy balance.
Despite the consensus of the health benefits of physical activity [33–35], independently from its suggested reduction of fat mass [36], there is controversy about whether sedentary behavior is directly involved in obesity development or not [37]. The relation between obesity and lack of physical activity seems not to be strong [38,39], and physical activity appears not necessarily to be a useful tool to ‘increase energy expenditure’ [40–45]. Nevertheless sedentary behaviors and reduction of physical work in jobs have been consistently stated as major factors for the development of obesity in modern societies [46].
Possible consequences of using concept 1
Ethical considerations on obesity and overweight
‘Eat less, move more’ appears as the most feasible solution to overweight and obesity and both possibilities seem to be within people’s reach [47]. The solution gives the impression of being so simple and straightforward that presumably all that are needed are willingness and self-control. Failing to revert obesity or overweight may be interpreted as lack of character, as one study has shown [48]. Concept 1, thus, may lead to blame and even stigmatize people with these conditions, e.g. punishments such as imposing a special tax on people with overweight and obesity using airplanes are already being discussed in both academia and mass media [49,50]. However, it may be ethically doubtful to attribute full responsibility to people with obesity and overweight, when individuals do not have full control over their food availability or accessibility [51,52]. In addition, it is thought that people may become obese through different pathways that possibly are independent of caloric balance, e.g. brain mediation of body fat mass, diminution of muscle mass and strength, as well as gut microbiota [53–55]. Therefore, it would be more ethical to use a ‘cause of obesity’ concept that does not transfer total responsibility to the individual.
Public health strategies based on concept 1
Most anti-obesity programs are based on concept 1 or focus at least on one of its elements [56,57]. Possibly as a result, within certain populations, the bigger the average BMI the higher is the prevalence of dieters or exercisers [58]. However, the obesity epidemic continues to grow worldwide, and under the current trends, the chance to reverse it is virtually zero [59]. If apparently people and governments do what they are supposed to do to tackle the epidemic, but only isolated and not sustained results are achieved [60,61], it may be possible that the symptoms are being treated instead of the roots [62].
The use of concept 1 may have certain advantages, e.g. it is easy to understand, it has a straightforward logic, it creates awareness on food consumption and it can reinforce discipline. However, the disadvantages of the concept may outperform its advantages under the current global circumstances. Continuing to base strategies solely on concept 1 may, therefore, not only be ineffective in terms of public health, but also be an inefficient use of resources.
Food industry and obesity
The participation of the food industry to reverse the obesity epidemic would be crucial [63,64], because it plays a major role in people’s choices and perceptions of food through its products and its marketing [65]. For example, the food industry communicates characteristics of its products through front label information, influencing consumption decisions [65]. However, most of the companies that dominate the global food market seem to focus only on meeting specific growth targets [66], overlooking the impact of their strategies on people’s health and weight, because the calories can be balanced anyway.
Subsidies from government to agricultural production are commonly based on the economic incentive of the volume of pruchase by the food industry, turning out certain raw materials such as sugar cane, cornand palm oil, cheap for the farmers to produce and sell to the food industry. Although such subsidized agricultural production is not a problem by itself, these materials are turned into harmful ingredients once they are ultra-processed by the industry [67], e.g. refined sugar, corn syrup and hydrogenated fats, all of which have proven to be closely related to obesity and to development of obesity-related chronic diseases [67–70]. Under concept 1, the effect of these foods on people’s health may be overlooked because individuals would self-regulate only their calorie balance.
Thus, the food industry has a share of responsibility within the obesity epidemic that may not be evident, because concept 1 transfers the responsibility of regulation to the individuals. By placing prime importance on the calorie balance, therefore, the food industry would always have a way out for not participating actively in a common frontline to tackle the obesity epidemic, which our concept 2 may avoid. The next section will propose an approach which differs from the calorie concept and might lead to more efficient actions aiming to tackle overweight and obesity.
An alternative approach to causes for obesity
Different classes of food influence differently the energy balance. For example, if an individual follows a 2000 kcal diet with 55% of the total kcal from CHO, 30% from fat and 15% of protein, and if thermogenesis is taken into account, the remaining energy would be 1825 kcal and not 2000 kcal. That is because the body would use approximately 175 kcal out of those 2000 kcal only for catabolizing the macronutrients [4]. The energy needed for catabolism differs for every kind of macronutrient during the digestion process [9].
Therefore, it may be also possible that diet composition affects the whole digestive process and, hence, fat production and accumulation [53,71]. Given that obesity is an ‘abnormal or excessive fat accumulation that may impair health’ [1], it would appear appropriate to focus on the details of fat production and accumulation. This view may strengthen the strategies aiming to reverse the obesity epidemic.
Lipogenesis
The production of fat is called lipogenesis. The body uses fat in different ways, but mostly as fuel [72]. Lipogenesis is commonly balanced with fat usage. Obesity is not a rapidly developing condition, but develops only if more fat is being produced than used – hence stored – continuously [73].
Potentially, all the macronutrients could be used by the body to produce fat. However, proteins are normally converted into amino-acids or peptides, and only exceptionally into fat [74,75]. Protein intake may also play an indirect role in lipogenesis through different hormonally controlled digestive processes [76]. Thus, it is normally considered that lipid production and storage mostly originate either from direct intake of fat, or from a process known as de novo lipogenesis, which is the transformation of CHO into fat [77].
Fat metabolism
Fat is normally ingested in the form of triglycerides (TG). Fat needs to be oxidized into free fatty acids (FFA) to be used as energy [78,79]. This can happen through two different pathways, either directly from fat in food or from fat in adipose tissue. Insulin plays a key role to determine which pathway dominates [80]. That means that when insulin is high due to food intake, the breakdown of fat from adipose tissue stops and fat storage starts [73,81]. Conversely, when the insulin level is low, e.g. during a fasting state, the body makes use of the adipose tissue to generate energy [79]. Thus, energy production is regulated between fat storage in the presence of food and fat degradation when fasting.
CHO metabolism
Digestible CHO are degraded to their simplest form, i.e. monosaccharides, mainly glucose. That is why such CHO are also called glycemic. Non-digestible CHO, namely fibers, are non-glycemic. Therefore, CHO can be classified according to whether or not they lead to raised glucose levels in the blood. How fast a glycemic CHO leads to a raised glucose concentration in blood is measured by its glycemic index (GI). CHO that are rapidly degraded and hence quickly raise the blood sugar level have a high GI, whereas the slowly degraded ones have a low GI.
Whenever a macronutrient is consumed, there may be an insulin response. However, the highest and longest secretion of insulin is stimulated by CHO with a high GI. Such CHO are common constituents of ultra-processed products. Insulin is a key to gear the metabolic processing of high GI carbohydrates into glucose (for immediate energy) or to build up fat reserves (for future energy needs). The latter pathway may be favored by some monosaccharides, e.g. fructose [82]. In both cases, a high and prolonged release of insulin normally takes place which relates to fat production and accumulation [83]. Whether CHO with high GI are consumed as liquid, e.g. sugary drinks, or solids, e.g. sugary cakes, seems to provoke a different insulin response, hence, different lipogenesis reactions. The liquid forms of CHO appear to be the most obesogenic form of high-GI carbohydrates [84,85]. The responses of insulin to food intake may be also influenced by genetic characteristics [86], suggesting that certain populations could be more vulnerable to overweight and obesity depending on their food availability [86].
Negative cycle: insulin-overweight
Insulin plays a role in the process of lipogenesis and subsequent fat storage. This is also a self-regulated system of energy utilization. A problem may occur, however, if the system loses its self-regulation capacity, e.g. by a constant stimulation and consequent high presence of insulin due to the diet composition, and not necessarily by its overall caloric content. This disturbance of self-regulation may result in ‘more fat in and less fat out’. Additionally, a constantly high presence of insulin is known to provoke insulin and leptin resistance. Insulin resistance turns into a higher risk of developing diabetes type II, as several studies have shown [74,87–93], whereas leptin resistance may impede the body from using the stored fat as a source of energy [94].
We hypothesize that food composition could push an individual into a negative cycle: Under a constantly high presence of insulin, the body may increase its capacity for fat production and storage. As a result, the body grows bigger, and this leads to higher energy demands. If the body constantly stores fat but does not use it as a source for energy, it would not only grow bigger but also become constantly hungry. This would possibly cause a repeated intake of the diet that is at the origin of the imbalance, thus making the body even bigger and hungrier and so on. In this situation, ‘laziness’ and ‘gluttony’, i.e. concept 1, would actually be the symptoms of obesity and overweight, whereas the cause would include a hormonal imbalance, i.e. concept 2. This represents a situation that can be aggravated for populations with genetic predisposition to lipogenesis and fat storage, i.e. high insulin responses [86].
An insight into the insulin reaction provoked by different diet compositions with the same caloric content is shown in Figure 1. The graph was generated using data from the simulator of glucose and insulin levels in the human body, developed by the Illinois Institute of Technology [95]. This simulator works based on a mathematical model considering the physiological, metabolic and chemical reactions of the human body upon carbohydrate intake [96]. The depicted differences in insulin levels provoked by different diet compositions are apparently considerable.
Figure 1.
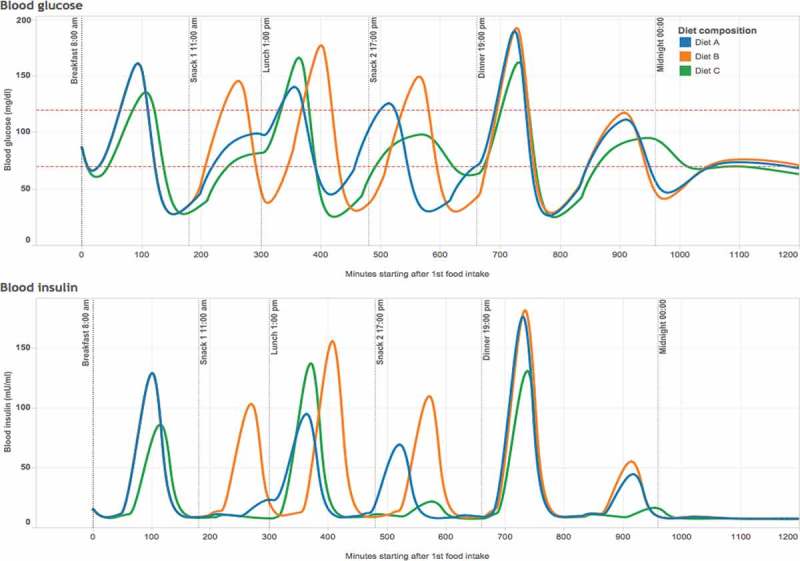
Differences in levels of blood glucose (top) and blood insulin (bottom) in the human body as provoked by diets with identical caloric content, but different compositions of macronutrients.
Notes: Diets were assumed in this model calculation as follows, Diet A (blue): 1670 kcal per day of energy content (7000 kjoules) with a composition of 53% CHO, i.e. 218 grams of CHO; 22% protein; and 25% fat. The CHO content is divided among three equal intakes during the day, i.e. 72.6 g for breakfast, 72.6 g for lunch and 72.6 g for dinner. Diet B (orange): same composition as diet A but adding two snacks, i.e. one apple between breakfast and lunch (34 g of CHO) and one banana between lunch and dinner (30 g of CHO). Diet C (green): the same energy content as in other diets, 1670 kcal per day (7000 kjoules), but with a composition of 38% CHO, i.e. 156 grams of CHO; 32% protein; and 30% fat. The CHO content is again divided into three equal intakes during the day, i.e. 52 g in breakfast, 52 g in lunch and 52 g in dinner. All diets were modeled for a non-diabetic adult of 60 kg of body weight using the web-based simulator of glucose and insulin levels in the human body ‘Glucosim’ developed by the Illinois Institute of Technology [95].
It is worth noting the insulin reaction provoked by fruit consumption (Figure 1). This reaction may be considerably overestimated by the simulator because it does not differentiate between types of CHO. However, fructose as found in fruits may not be comparable in terms of either quality or quantity in reality to that found in industrial products [97]. There is indeed an actual controversy about the role of fruits in overweight and obesity, due to their fructose content and its role in fat production [98]. While some researchers have provided evidence on successful weight loss diets when removing fruits, fruit juices and other sources of fructose [99], others have argued that whole fruits do not cause overweight because their flavonoid and polyphenol content regulates sugar absorption [100]. Nonetheless, regarding the controversy, there is an apparent consensus in that the industrialized form of fructose (commonly sucrose mixed with high-fructose corn syrup) is the one mostly linked to obesity [82,97,101,102].
Macro-level studies may also support the latter assumption. For instance Riera-Chrichton and colleagues [103] analyzed population data from the USA between 1974 and 2006 and from 164 countries between 2001 and 2010. They determined that the strongest association between a specific type of macronutrients and development of obesity was with CHO, although they did not clearly distinguish what kind of CHO and, hence, the association is too broad given the different types of CHO. Similarly, Austin and colleagues [93] analyzed population data from the U.S.A between 1971 and 2006 and found that the increase in obesity prevalence was closely related to a higher consumption of CHO, again without any distinction of the types of CHO. Other studies and meta-analyses have found similar results [61,104,105]. Processed food apparently induces continuous insulin production in the human body, possibly due not only to large amounts of highly glycemic ingredients but also to the resulting diet composition, in terms of macronutrients, from consuming these products [76,83,106–108].
In summary, macronutrients influence fat production and accumulation either by a direct response provoked solely by intake, e.g. of CHO, or by hormonal regulation of the digestive processes, e.g. of protein and fat. A detrimental diet composition may produce a hormonal imbalance that could turn into a negative loop where individuals accumulate fat, but are not properly nourished which makes them constantly hungry, causing them to eat more from that diet composition. The resulting trap is reinforced by the modern food availability. Diet composition, thus, possibly has a larger influence on obesity and overweight than the calorie balance.
A proposed revision of causes for obesity
We propose to revise the current concept of causes for obesity. Instead of placing prime importance on the energy balance, i.e. concept 1, we suggest to emphasize the effect of food on the metabolism. The guiding principle should be: ‘Overweight and obesity are determined by a hormonal imbalance characterized by a constant and sustained secretion of insulin, which results from particular dietary compositions. These are closely linked to modern food habits.’ In contrast to concept 1, this revision accounts better for recent insights into biochemical and hormonal pathways, is consistent with epidemiological and behavioral evidence, and may allow for more adequate concepts and actions in public health to reverse the obesity epidemic.
In regards of public health strategies, the revised concept 2 would associate the problem predominantly with an imbalance of hormonal processes that are biologically common to all humans, and which normally control a balanced insulin action on fat metabolism. As a result, leading public organisms may focus on tasks and solutions to reduce food-induced insulin overproduction that could be adopted and applied in contexts of their respective influence [109]. Targeting actions toward insulin-related metabolic processes could also lead to more homogeneous evidence to tackle obesity and overweight. This may also increase efficiency within the actions to reverse the obesity epidemic by building on a reliable ground [110]. Because the costs of obesity, including actions against it, are estimated at 2 trillion USD per year on the global gross domestic product (GDP) [111], even a small improvement in efficiency of actions could have a huge impact on savings.
Regarding ethics and stigma, the understanding of concept 2 may distribute responsibilities among the stakeholders, as opposed to concept 1 where corrective and preventive actions are attributed solely to the individual’s will. However, the individual dietary composition depends greatly on the availability and accessibility of food. These, in turn, are the result of regulations, economic incentives and food security, among other factors [112] that are out of individuals’ reach. For example, under concept 1, the minimal responsibility of government would be to ensure food availability and accessibility regardless of its composition, and provide knowledge about concept 1. Hence, the individual would have the option to self-achieve her or his calorie balance. Under the same scenario, the minimal responsibility of the food industry may be to support availability, accessibility and calorie balance knowledge. By contrast, under concept 2, a government’s minimal responsibility would be to ensure the availability and accessibility of such food that does not create havoc in the hormonal regulation of digestive processes along with knowledge of how a diet’s composition may affect individuals’ health. The food industry’s minimal responsibility would be to support the availability and accessibility of that non-harmful food, as well as supporting the knowledge about diet composition. Under this scenario, it would be difficult for the food industry and government to provide food that is marked as harmful by the knowledge that they also provide.
It is clear that consumers would always share in the responsibility about their food intake, but under concept 2, this responsibility may not remain exclusively with them. Clear areas of responsibilities may be drawn and stakeholders could be even made accountable for them. Hence, the revised concept may help to reduce, although not to eliminate, the negative connotations of an individual’s responsibility and the resulting stigmatization.
Guided by our proposed change of perspective, industry may start developing better products by paying attention to specific characteristics of industrial products, i.e. ingredients involved in hormonal imbalance. Moreover, the food industry may change its current defensive approach toward a more participative one. This could mean a significant and positive impact against the obesity epidemic. In addition, because all macronutrients have a role in insulin regulation, the proposed revision of the obesity concept may also have the advantage of not pointing to a single food and thus blaming a single item. This revision might even avoid a possible backfire in obesity prevalence caused by the control strategies, as may have happened when fat was stigmatized as the overweight promoter [88,113,114].
The proposed revision may have some disadvantages too. For example, people may find it harder to understand concept 2 rather than concept 1. Hence, educating people in concept 2 may be challenging. Another possible disadvantage is that because most of the current policies and supportive environment, e.g. front labels, mobile applications, textbooks, and Internet articles, are predominantly based on calories, concept 2 may lack initial support. Thus, an individual following concept 2 could have the impression of following a mistaken concept. However, it is possible that the advantages of adopting concept 2 may outperform its disadvantages in the long run.
Leading to change
In advocating for this revised view on causes of obesity, resistance within institutions and their networks is to be expected. Revisiting the causes of obesity may also be interpreted as a withdrawal of the original concept, because concept 1 is broader than concept 2. However, restating the obesity problem as a hormonal imbalance is not necessarily in conflict with concept 1. There is an overlapping area that can be used to integrate the proposed change of perspective with the prevailing concepts of causes for obesity. In fact, the foods most closely related to concept 2, i.e. simple CHO, are also commonly discouraged by concept 1, although for different reasons. Lucan and DiNicolantonio [55] analyzed certain areas of compatibility between what they call ‘calorie-focused thinking’ and ‘more-nuanced thinking’, namely concept 1 and concept 2. They found certain overlaps as illustrated in Table 1, particularly in encouraging vegetables and discouraging sugary drinks.
Table 1.
Overlaps between calorie-focused thinking and more-nuanced thinking from Lucan and DiNicolantonio [55]. Reprinted with permission.
| Calorie-focused thinking |
|||
|---|---|---|---|
| Encouraged | Discouraged | ||
| More-nuanced thinking | Encouraged | Most vegetables, legumes, whole fresh fruits and unprocessed or sprouted grains; lean meats, poultry, and fish; water and unsweetened tea and coffee | Nuts and nut butters; avocados, olives and olive oil; whole dairy; oily fish |
| Discouraged | 100% fruit juices; enriched breads and pastas; fortified breakfast cereals (e.g. corn flakes, crisp rice); low-fat dairy (including sugary flavored fat-free yoghurts) |
Sodas and other sugar- sweetened beverages; candies; baked sweets; French fries and butter-fried foods, snack chips and other processed items | |
The encouragement to base diets on non-industrialized food, whole plants and certain lean meats, all of which do not overstimulate insulin production, is also compatible with concept 2. Most of the non-obesogenic diets, such as the Mediterranean diet, Paleolithic diet or low-glycemic diet, are, therefore, compatible with concept 2. Katz and Meller compiled an extensive review on this [115] and their findings are shown in Table 2. This could also mean that most of the foregoing obesity research framed as concept 1 would still be relevant after reframing the information into concept 2. Therefore, the resistance to change could be minimized.
Table 2.
Compatibility between diets (adapted from [115]). Reprinted with permission.
| Low-carbohydrate diet | Low-fat/vegetarian/vegan diet | Low-glycemic | Mediterranean diet | Mixed/balanced diet | Paleolithic | |
|---|---|---|---|---|---|---|
| Emphasis on | Restriction of refined starches and added sugars | Plant foods direct from nature; avoidance of harmful fats | Restriction of starches, added sugars; high fiber intake | Foods direct from nature; mostly plants; healthful oils, especially monounsaturated | Minimization of highly processed, energy-dense foods; emphasis on wholesome foods in moderate quantities | Minimal intake of processed foods. Maximization of natural plant foods and lean meats |
| Compatible elements | Limited refined starches, added sugars, processed foods; limited intake of certain fats; emphasis on whole plant foods, with or without lean meats, fish, poultry, seafood | |||||
| Potential general consistency | Food, not too much, mostly plants. Portion control depending on the quality of foods, because higher quality foods have the tendency to promote satiety with fewer calories. Although neither the low-carbohydrate nor Paleolithic diet need to be ‘mostly plants’, both can be | |||||
Conclusion
We have discussed that the use of calories as energy units and concept 1 may be relevant to some areas of public health, but this concept has apparently not provided an efficient framework against the obesity epidemic. It may even foster stigma, produce negative consequences to unrelated third parties and prevent tackling strategies from being efficient.
Given the big impact of obesity on global GDP, it is a necessity to look for strategies that may increase the cost-efficiency of actions. By revisiting the causes for obesity, especially those stated by the leading health organisms, this article makes the case to view obesity as a consequence of disturbing the auto-regulation of hormones related to fat production and accumulation by diet composition, in particular that produced by industrialized processed food. Among other advantages, this proposed concept may offer an opportunity for the food industry to play a more responsible role in controlling overweight. If we really want to succeed in tackling the obesity epidemic, we must stop treating its symptoms, and start treating its causes instead.
Acknowledgments
SC is thankful to Prof. Christiane Schwieren from the Alfred-Weber Institute (Chair of Behavioral Economics) for academic support. He is also thankful to the German Academic Exchange Services (DAAD) and the Mexican National Council of Science and Technology (CONACYT) for a doctoral scholarship. The authors acknowledge the financial support for publication provided by the Deutsche Forschungsgemeinschaft and Ruprecht-Karls-Universität Heidelberg within the funding program Open Access Publishing as well as of the Institute of Public Health of the Ruprecht-Karls-Universität Heidelberg.
Responsible Editor Stig Wall, Umeå University, Sweden
Funding Statement
SC receives a scholarship from the German Academic Exchange Services (DAAD) and the Mexican National Council of Science and Technology (CONACYT). A grant from the Deutsche Forschungsgemeinschaft and Institute of Public Health of the Ruprecht-Karls-Universität Heidelberg was received for open access publishing.
Author contributions
SC conceived the article and prepared the first draft. AR revised and contributed to the production of the subsequent drafts. Both authors contributed to the drafting of the final version. Both authors have read and approved the final manuscript.
Availability of data and material
The data analysis from Figure 1 is publicly available online at the ‘Tableau Public’ repository: https://public.tableau.com/views/Insulinandglucosemodels/Dashboard1?:embed=y:display_count=yes.
Disclosure statement
No potential conflict of interest was reported by the authors.
Ethics and consent
None.
Paper context
The calorie balance as the cause of obesity is more complex than an intake–expense relation. By revisiting general facts about fat generation and accumulation, we propose to update the perceived causes of obesity including diet composition and recent insights into biochemical and hormonal pathways. The disadvantages of the currently stated cause of obesity may outbalance its advantages. A revision of this cause may promote more efficient actions in public health to reverse the obesity epidemic.
References
- [1].WHO Obesity and overweight [Internet]. WHO. 2014. [cited 2014 October 27]. Available from: http://www.who.int/mediacentre/factsheets/fs311/en/
- [2].Roberto CA, Swinburn B, Hawkes C, et al. Patchy progress on obesity prevention: emerging examples, entrenched barriers, and new thinking. Lancet [Internet]. [cited 2015. February 23];385:2400–12. Available from: http://www.sciencedirect.com/science/article/pii/S014067361461744X [DOI] [PubMed] [Google Scholar]
- [3].Rumrill J, Fitzgerald SM.. Using narrative literature reviews to build a scientific knowledge base. Work. 2001. January 1;16:165–170. [PubMed] [Google Scholar]
- [4].Feinman RD, Fine EJ. “A calorie is a calorie” violates the second law of thermodynamics. Nutr J. 2004;3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults. A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and The Obesity Society. J Am Coll Cardiol [Internet]. 2014. Jul 1 [cited 2016 March 9];63 Available from: http://dx.doi.org/10.1016/j.jacc.2013.11.004 [DOI] [PubMed] [Google Scholar]
- [6].Wishnofsky M. Caloric equivalents of gained or lost weight. Am J Clin Nutr. 1958;6:542–546. [DOI] [PubMed] [Google Scholar]
- [7].Thomas DM, Martin CK, Lettieri S, et al. Can a weight loss of one pound a week be achieved with a 3500-kcal deficit? Commentary on a commonly accepted rule. Int J Obes. 2013;37:1611–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Thomas DM, Gonzalez MC, Pereira AZ, et al. Time to correctly predict the amount of weight loss with dieting. J Acad Nutr Diet. 2014. June;114:857–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Jéquier E. Pathways to obesity. Nature [Internet]. 2002. August 14 [cited 2016 Mar 9];26 Available from: http://www.nature.com/ijo/journal/v26/n2s/abs/0802123a.html [Google Scholar]
- [10].Chaput J-P, Ferraro ZM, Prud’homme D, et al. Widespread misconceptions about obesity. Can Fam Physician. 2014. November 1;60:973–975. [PMC free article] [PubMed] [Google Scholar]
- [11].Mink M, Evans A, Moore CG, et al. Nutritional imbalance endorsed by televised food advertisements. J Am Diet Assoc. 2010. June;110:904–910. [DOI] [PubMed] [Google Scholar]
- [12].Heymsfield SB, Thomas D, Martin CK, et al. Energy content of weight loss: kinetic features during voluntary caloric restriction. Metabolism. 2012;61:937–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hall KD, Heymsfield SB, Kemnitz JW, et al. Energy balance and its components: implications for body weight regulation. Am J Clin Nutr. 2012. April 1;95:989–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hall KD, Sacks G, Chandramohan D, et al. Quantification of the effect of energy imbalance on bodyweight. Lancet. 2011. September 2;378:826–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Metcalfe A, Elliott C. Differences in the nutritional content of baby and toddler foods with front-of-package nutrition claims issued by manufacturers v. governments/health organizations. Public Health Nutr. 2015. January;18:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Baer DJ, Gebauer SK, Novotny JA. Measured energy value of pistachios in the human diet. Br J Nutr. 2012. January;107:120–125. [DOI] [PubMed] [Google Scholar]
- [17].Urban LE, Dallal GE, Robinson LM, et al. The accuracy of stated energy contents of reduced-energy, commercially prepared foods. J Am Diet Assoc. 2010. January 1;110:116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chandon P, Wansink B. The biasing health halos of fast-food restaurant health claims: lower calorie estimates and higher side-dish consumption intentions. J Consum Res. 2007. October 1;34:301–314. [Google Scholar]
- [19].Miller LMS, Cassady DL, Beckett LA, et al. Misunderstanding of front-of-package nutrition information on US food products. PLoS One. 2015. April 29;10:e0125306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Stern D, Tolentino L. Revisión del etiquetado frontal: análisis de las Guías Diarias de Alimentación (GDA) y su comprensión por estudiantes de nutrición en México. Instituto Nacional De Salud Publica [Internet]. [cited 2015. January 27]. Available from: http://www.infoalimentario.com/web/wp-content/uploads/2012/05/140422_revisionEtiquetado.pdf [Google Scholar]
- [21].Dhurandhar NV, Schoeller D, Brown AW, et al. Energy balance measurement: when something is not better than nothing. Int J Obes. 2015;39:1109–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Martin CK, Correa JB, Han H, et al. Validity of the remote food photography method (RFPM) for estimating energy and nutrient intake in near real-time. Obesity. 2012. April 1;20:891–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Dong Y, Hoover A, Scisco J, et al. A new method for measuring meal intake in humans via automated wrist motion tracking. Appl Psychophysiol Biofeedback. 2012. April 10;37:205–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Fontana JM, Higgins JA, Schuckers SC, et al. Energy intake estimation from counts of chews and swallows. Appetite. 2015;1:14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sazonov ES, Makeyev O, Schuckers S, et al. Automatic detection of swallowing events by acoustical means for applications of monitoring of ingestive behavior. IEEE Trans Biomed Eng. 2010. March;57:626–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Duvivier BMFM, Schaper NC, Bremers MA, et al. Minimal intensity physical activity (standing and walking) of longer duration improves insulin action and plasma lipids more than shorter periods of moderate to vigorous exercise (cycling) in sedentary subjects when energy expenditure is comparable. PLoS One. 2013. February 13;8:e55542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].OMS Recomendaciones mundiales sobre actividad física para la salud [Internet]. WHO. [cited 2015. February 26]. Available from: http://www.who.int/dietphysicalactivity/publications/9789241599979/es/
- [28].Ness AR, Leary SD, Mattocks C, et al. Objectively measured physical activity and fat mass in a large cohort of children. PLoS Med. 2007. March 20;4:e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Manzoni GM, Pagnini F, Gorini A, et al. Can relaxation training reduce emotional eating in women with obesity? An exploratory study with 3 months of follow-up. J Am Diet Assoc. 2009;109:1427–1432. [DOI] [PubMed] [Google Scholar]
- [30].Chaput J-P, Sharma AM. Is physical activity in weight management more about “calories in” than “calories out”? Br J Nutr. 2011. December;106:1768–1769. [DOI] [PubMed] [Google Scholar]
- [31].Swartz JJ, Dowray S, Braxton D, et al. Simplifying healthful choices: a qualitative study of a physical activity based nutrition label format. Nutr J. 2013;12:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Levine JA. Non-exercise activity thermogenesis (neat). Nutr Rev. 2004. July 1;62:S82–97. [DOI] [PubMed] [Google Scholar]
- [33].Reiner M, Niermann C, Jekauc D, et al. Long-term health benefits of physical activity – a systematic review of longitudinal studies. BMC Public Health. 2013;13:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Coen PM, Menshikova EV, Distefano G, et al. Exercise and weight loss improve muscle mitochondrial respiration, lipid partitioning, and insulin sensitivity after gastric bypass surgery. Diabetes. 2015. November 1;64:3737–3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Duncan GE. The “fit but fat” concept revisited: population-based estimates using NHANES. Int J Behav Nutr Phys Act. 2010. May 24;7:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Loprinzi P, Smit E, Lee H, et al. The “Fit but Fat” paradigm addressed using accelerometer-determined physical activity data. North Am J Med Sci. 2014. July;6:295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].PLOS. Type 2 diabetes: insulin resistance may be the result of mitochondrial dysfunction. PLoS Med [Internet]. 2005. Sep;2:E292 [cited 2016 Apr 4]. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1184228/ [Google Scholar]
- [38].Petersen L, Schnohr P, Sorensen TI. Longitudinal study of the long-term relation between physical activity and obesity in adults. Int J Obes Relat Metab Disord [Internet]. 2004;28 Available from: http://dx.doi.org/10.1038/sj.ijo.0802548 [DOI] [PubMed] [Google Scholar]
- [39].Katzmarzyk PT, Craig CL, Gauvin L. Adiposity, physical fitness and incident diabetes: the physical activity longitudinal study. Diabetologia [Internet]. 2007;50 Available from: http://dx.doi.org/10.1007/s00125-006-0554-3 [DOI] [PubMed] [Google Scholar]
- [40].Church TS, Martin CK, Thompson AM, et al. Changes in weight, waist circumference and compensatory responses with different doses of exercise among sedentary, overweight postmenopausal women. PLoS One [Internet]. 2009. February 18 [cited 2016 Jul 8];4 Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2639700/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Shaw KA, Gennat HC, O’Rourke P, et al. Exercise for overweight or obesity In: The Cochrane Collaboration, editor. Cochrane database of systematic reviews [Internet]. Chichester: Wiley; 2006. [cited 2016 Jul 8]. Available from: http://doi.wiley.com/10.1002/14651858.CD003817.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Janssen GM, Graef CJ, Saris WH. Food intake and body composition in novice athletes during a training period to run a marathon. Int J Sports Med. 1989. May;10:S17–21. [DOI] [PubMed] [Google Scholar]
- [43].Westerterp KR. Physical activity and physical activity induced energy expenditure in humans: measurement, determinants, and effects. Front Physiol. 2013;4:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Dhurandhar EJ, Kaiser KA, Dawson JA, et al. Predicting adult weight change in the real world: a systematic review and meta-analysis accounting for compensatory changes in energy intake or expenditure. Int J Obes 2005. 2015. August;39:1181–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Bouchard C, Tremblay A, Després J-P, et al. The response to exercise with constant energy intake in identical twins. Obes Res. 1994;2:400–410. [DOI] [PubMed] [Google Scholar]
- [46].Gortmaker SL, Swinburn BA, Levy D, et al. Changing the future of obesity: science, policy, and action. Lancet. 2011. September 2;378:838–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Guth E. Healthy weight loss. JAMA. 2014;312:974. [DOI] [PubMed] [Google Scholar]
- [48].Balko R, Brownell K, Nestle M. America’s obesity crisis: areyou responsible for your own weight? Time [Internet]. [cited 2016. April 22]. Available from: http://content.time.com/time/magazine/article/0,9171,994398,00.html [Google Scholar]
- [49].Bhatta BP. Pay-as-you-weigh pricing of an air ticket: economics and major issues for discussions and investigations. J Revenue Pricing Manag. 2013. March;12:103–119. [Google Scholar]
- [50].Durston J. Airline “fat tax”: should heavy passengers pay more? CNN Travel [Internet]. 2013 [cited 2016. April 22]. Available from: http://travel.cnn.com/airline-fat-tax-should-heavy-passengers-pay-more-619046/
- [51].Nielsen MEJ, Andersen MM. Should we hold the obese responsible? Camb Q Healthc Ethics. 2014. October;23:443–451. [DOI] [PubMed] [Google Scholar]
- [52].Apparicio P, Cloutier M-S, Shearmur R. The case of Montréal’s missing food deserts: evaluation of accessibility to food supermarkets. Int J Health Geogr. 2007. February 12;6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Schéle E, Grahnemo L, Anesten F, et al. Regulation of body fat mass by the gut microbiota: possible mediation by the brain. Peptides. 2016. March;77:54–59. [DOI] [PubMed] [Google Scholar]
- [54].Stenholm S, Harris TB, Rantanen T, et al. Sarcopenic obesity – definition, etiology and consequences. Curr Opin Clin Nutr Metab Care. 2008. November;11:693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Lucan SC, DiNicolantonio JJ. How calorie-focused thinking about obesity and related diseases may mislead and harm public health. An alternative. Public Health Nutr. 2015. March;18:571–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Cismaru M, Lavack AM. Social marketing campaigns aimed at preventing and controlling obesity: a review and recommendations. Int Rev Public Non Profit Mark. 2007;4:9–30. . [Google Scholar]
- [57].Vallgårda S. Governing obesity policies from England, France, Germany and Scotland. Soc Sci Med. 2015. December;147:317–323. [DOI] [PubMed] [Google Scholar]
- [58].Montani J-P, Schutz Y, Dulloo AG. Dieting and weight cycling as risk factors for cardiometabolic diseases: who is really at risk? Obes Rev. 2015. February;1:7–18. [DOI] [PubMed] [Google Scholar]
- [59].NCD Risk Factor Collaboration. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet. 2016. April;387:1377–1396. DOI: 10.1016/S0140-6736(16)30054-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Dietz WH, Baur LA, Hall K, et al. Management of obesity: improvement of health-care training and systems for prevention and care. Lancet [Internet]. [cited 2015. April 14];385:2521–2533. Available from: http://www.sciencedirect.com/science/article/pii/S0140673614617487 [DOI] [PubMed] [Google Scholar]
- [61].Cohen E, Cragg M, deFonseka J, et al. Statistical review of US macronutrient consumption data, 1965–2011: Americans have been following dietary guidelines, coincident with the rise in obesity. Nutrition. 2015. May;31:727–732. [DOI] [PubMed] [Google Scholar]
- [62].Taubes G. Why we get fat: and what to do about it. Reprint ed New York: Anchor Publishers; 2010. 288 p. [Google Scholar]
- [63].Swinburn B, Kraak V, Rutter H, et al. Strengthening of accountability systems to create healthy food environments and reduce global obesity. Lancet [Internet]. [cited 2015. April 18];385:2534–2545. Available from: http://www.sciencedirect.com/science/article/pii/S0140673614617475 [DOI] [PubMed] [Google Scholar]
- [64].Swinburn BA, Sacks G, Hall KD, et al. The global obesity pandemic: shaped by global drivers and local environments. Lancet. 2011. September 2;378:804–814. [DOI] [PubMed] [Google Scholar]
- [65].Chandon P, Wansink B. Is food marketing making us fat? A multi-disciplinary review [Internet]. Report No.: ID 1854370. Rochester (NY): Social Science Research Network; 2011. May [cited 2016 Sep 1]. Available from: http://papers.ssrn.com/abstract=1854370 [Google Scholar]
- [66].Ludwig DS, Nestle M. Can the food industry play a constructive role in the obesity epidemic? JAMA. 2008. October 15;300:1808–1811. [DOI] [PubMed] [Google Scholar]
- [67].Monteiro CA, Moubarac J-C, Cannon G, et al. Ultra-processed products are becoming dominant in the global food system. Obes Rev. 2013. November;1:21–28. [DOI] [PubMed] [Google Scholar]
- [68].Bellisari A. Evolutionary origins of obesity. Obes Rev. 2008. March 1;9:165–180. [DOI] [PubMed] [Google Scholar]
- [69].Ilich JZ, Kelly OJ, Kim Y, et al. Low-grade chronic inflammation perpetuated by modern diet as a promoter of obesity and osteoporosis. Arch Ind Hyg Toxicol. 2014;65:139–148. [DOI] [PubMed] [Google Scholar]
- [70].Campos VC, Tappy L. Physiological handling of dietary fructose-containing sugars: implications for health. Int J Obes. 2016. March;40(S1):S6–11. [DOI] [PubMed] [Google Scholar]
- [71].Filippo CD, Cavalieri D, Paola MD, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci. 2010. August 17;107:14691–14696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Petrovic N, Shabalina IG, de Jong J, et al. Adipose tissues: the brown, the white and the brite. Proc Physiol Soc [Internet]. 2015. [cited 2016 April 18];34 Available from: http://www.physoc.org/proceedings/abstract/Proc%20Physiol%20Soc%2034SA028 [Google Scholar]
- [73].Schutz Y. Concept of fat balance in human obesity revisited with particular reference to de novo lipogenesis. Int J Obes. 2004;28:S3–11. [DOI] [PubMed] [Google Scholar]
- [74].Henry Gadaga T, Burgess A. Macronutrients, water, and energy In: Savage King F, Burgess A, Quinn VJ, et al, editors. Nutrition for developing countries [Internet]. 1st ed. Oxford: Oxford University Press; 2015. [cited 2016 May 17]. p. 11–28. Available from: http://www.oxfordmedicine.com/view/10.1093/med/9780199685226.001.0001/med-9780199685226-chapter-3 [Google Scholar]
- [75].Sarmadi BH, Ismail A. Antioxidative peptides from food proteins: a review. Peptides. 2010. October;31:1949–1956. [DOI] [PubMed] [Google Scholar]
- [76].Boden G, Cheung P, Salehi S, et al. Insulin regulates the unfolded protein response in human adipose tissue. Diabetes. 2014. March 1;63:912–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Minehira K, Vega N, Vidal H, et al. Effect of carbohydrate overfeeding on whole body macronutrient metabolism and expression of lipogenic enzymes in adipose tissue of lean and overweight humans. Int J Obes. 2004. August 10;28:1291–1298. [DOI] [PubMed] [Google Scholar]
- [78].Kahlhöfer J, Karschin J, Silberhorn-Bühler H, et al. Effect of low-glycemic-sugar-sweetened beverages on glucose metabolism and macronutrient oxidation in healthy men. Int J Obes [Internet]. 2016. Feb 12 [cited 2016 April 7];40:990–997. Available from: http://www.nature.com.ubproxy.ub.uni-heidelberg.de/ijo/journal/vaop/ncurrent/full/ijo201625a.html [DOI] [PubMed] [Google Scholar]
- [79].Karpe F, Dickmann JR, Frayn KN. Fatty acids, obesity, and insulin resistance: time for a reevaluation. Diabetes. 2011. Oct 1;60:2441–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Kersten S. Physiological regulation of lipoprotein lipase. Biochim Biophys Acta BBA Mol Cell Biol Lipids. 2014. July;1841:919–933. [DOI] [PubMed] [Google Scholar]
- [81].Newsholme P, Keane K, Gaudel C, et al. (Dys) regulation of insulin secretion by macronutrients In: Islam MS, editor. Islets of langerhans [Internet]. 1st ed. Dordrecht: Springer; 2015. [cited 2016 Apr 18]. p. 129–156. Available from: http://link.springer.com/referenceworkentry/10.1007/978-94-007-6686-0_4 [Google Scholar]
- [82].Lustig RH. Fructose: metabolic, hedonic, and societal parallels with ethanol. J Am Diet Assoc. 2010. September;110:1307–1321. [DOI] [PubMed] [Google Scholar]
- [83].Aller EEJG, Larsen TM, Claus H, et al. Weight loss maintenance in overweight subjects on ad libitum diets with high or low protein content and glycemic index: the DIOGENES trial 12-month results. Int J Obes 2005. 2014;38:1511–1517. [DOI] [PubMed] [Google Scholar]
- [84].DiMeglio DP, Mattes RD. Liquid versus solid carbohydrate: effects on food intake and body weight. Int J Obes. 2000. June 21;24:794–800. [DOI] [PubMed] [Google Scholar]
- [85].Ludwig DS, Peterson KE, Gortmaker SL. Relation between consumption of sugar-sweetened drinks and childhood obesity: a prospective, observational analysis. Lancet. 2001. February 17;357:505–508. [DOI] [PubMed] [Google Scholar]
- [86].Hron BM, Ebbeling CB, Feldman HA, et al. Relationship of insulin dynamics to body composition and resting energy expenditure following weight loss. Obesity. 2015. November 1;23:2216–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Savage DB, Petersen KF, Shulman GI. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev. 2007. April 1;87:507–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Harcombe Z, Baker JS, Davies B. Food for thought: have we been giving the wrong dietary advice? Food Nutr Sci. 2013;04:240–244. [Google Scholar]
- [89].Crist C. Insulin resistance due to obesity. Master Sci Nurs MSN Stud Scholarsh [Internet]. 2015. July 1 Available from: http://digitalcommons.otterbein.edu/stu_msn/72
- [90].Jung UJ, Choi M-S. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci. 2014. April 11;15:6184–6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Deng Y, Zhang Y, Zheng S, et al. Postprandial glucose, insulin and incretin responses to different carbohydrate tolerance tests. J Diabetes. 2015. November 1;7:820–829. [DOI] [PubMed] [Google Scholar]
- [92].Samuel VT, Shulman GI. The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux – ProQuest [Internet]. J Clin Investig. 2016;126:12–22. [cited 2016. May 17]. Available from: http://search.proquest.com/openview/0abddaec3da58e8c532056e605654ec0/1?pq-origsite=gscholar&cbl=42166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Austin GL, Ogden LG, Hill JO. Trends in carbohydrate, fat, and protein intakes and association with energy intake in normal-weight, overweight, and obese individuals: 1971–2006. Am J Clin Nutr. 2011. April 1;93:836–843. [DOI] [PubMed] [Google Scholar]
- [94].Feijóo-Bandín S, Portolés M, Roselló-Lletí E, et al. 20 years of leptin: role of leptin in cardiomyocyte physiology and physiopathology. Life Sci. 2015. November 1;140:10–18. [DOI] [PubMed] [Google Scholar]
- [95].Agar BU, Eren M, Cinar A. Glucosim: educational software for virtual experiments with patients with type 1 diabetes. Paper presented at: 2005 IEEE Engineering in Medicine and Biology 27th Annual Conference; 2005. Sep 1–4; Shangai. p. 845–848. [DOI] [PubMed] [Google Scholar]
- [96].Oruklu M, Agar Baris U, Ceylan Erzen F, et al. GlucoSim: a web-based educational simulation package for glucose and insulin levels in the human body [Internet]. 2008 [cited 2016 Apr 25]. Avaiable from: http://simulator.iit.edu/web/glucosim/index.html [Google Scholar]
- [97].Lustig RH. Fructose: it’s “alcohol without the buzz”. Adv Nutr Int Rev J. 2013. March 1;4:226–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].van Buul VJ, Tappy L, Brouns FJPH. Misconceptions about fructose-containing sugars and their role in the obesity epidemic. Nutr Res Rev. 2014. June;27:119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Lustig RH, Mulligan K, Noworolski SM, et al. Isocaloric fructose restriction and metabolic improvement in children with obesity and metabolic syndrome. Obesity. 2016. February 1;24:453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Williamson G. Possible effects of dietary polyphenols on sugar absorption and digestion. Mol Nutr Food Res. 2013. January 1;57:48–57. [DOI] [PubMed] [Google Scholar]
- [101].Herman MA, Samuel VT. The sweet path to metabolic demise: fructose and lipid synthesis. Trends Endocrinol Metab [Internet]. 2016. Jul 4 [cited 2016 August 9];0 Available from: /trends/endocrinology-metabolism/abstract/S1043-2760(16)30066-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Jastreboff AM, Sinha R, Arora J, et al. Altered brain response to drinking glucose and fructose in obese adolescents. Diabetes. 2016;28:db151216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Riera-Crichton D, Tefft N. Macronutrients and obesity: revisiting the calories in, calories out framework. Econ Hum Biol. 2014. July;14:33–49. [DOI] [PubMed] [Google Scholar]
- [104].Fogelholm M, Anderssen S, Gunnarsdottir I, et al. Dietary macronutrients and food consumption as determinants of long-term weight change in adult populations: a systematic literature review. Food Nutr Res [Internet]. 2012. Aug 13 [cited 2016 May 11];56 Available from: http://www.foodandnutritionresearch.net/index.php/fnr/article/view/19103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Johnson RJ, Segal MS, Sautin Y, et al. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am J Clin Nutr. 2007. October 1;86:899–906. [DOI] [PubMed] [Google Scholar]
- [106].Louzada MLDC, Baraldi LG, Steele EM, et al. Consumption of ultra-processed foods and obesity in Brazilian adolescents and adults. Prev Med. 2015;81:9–15. [DOI] [PubMed] [Google Scholar]
- [107].Schulte EM, Avena NM, Gearhardt AN. Which foods may be addictive? The roles of processing, fat content, and glycemic load. PLoS One. 2015. February 18;10:e0117959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Zheng H, Lenard NR, Shin AC, et al. Appetite control and energy balance regulation in the modern world: reward-driven brain overrides repletion signals. Int J Obes. 2009;33:S8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Mumford MD, Zaccaro SJ, Harding FD, et al. Leadership skills for a changing world: solving complex social problems. Leadersh Q. 2000;11:11–35. [Google Scholar]
- [110].Stupak/Leitner Handbook of public quality management, Illustrated ed. New York: CRC Press; 2001. p. 481–776. [Google Scholar]
- [111].Dobbs R, Sawers C, Thompson F, et al. How the world could better fight obesity. McKinsey & Company [Internet]. [cited 2016. May 12]. Available from: http://www.mckinsey.com/industries/healthcare-systems-and-services/our-insights/how-the-world-could-better-fight-obesity
- [112].Caspi CE, Sorensen G, Subramanian SV, et al. The local food environment and diet: a systematic review. Health Place. 2012. September;18:1172–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Wansink B, Chandon P. Can “low-fat” nutrition labels lead to obesity? J Mark Res. 2006. November 1;43:605–617. [Google Scholar]
- [114].Ravnskov U, DiNicolantonio JJ, Harcombe Z, et al. The questionable benefits of exchanging saturated fat with polyunsaturated fat. Mayo Clin Proc. 2014. April;89:451–453. [DOI] [PubMed] [Google Scholar]
- [115].Katz DL, Meller S. Can we say what diet is best for health? Annu Rev Public Health. 2014;35:83–103. [DOI] [PubMed] [Google Scholar]
- [116].Moodie R, Stuckler D, Monteiro C, et al. Profits and pandemics: prevention of harmful effects of tobacco, alcohol, and ultra-processed food and drink industries. Lancet. 2013. March 1;381:670–679. [DOI] [PubMed] [Google Scholar]


