Abstract
Background
In recent years, systemic chemotherapy and molecular targeted therapy have become standard first-line treatments for locally advanced or metastatic nonsquamous non-small cell lung cancer (NSCLC). The objective of this survey was to investigate first-line anticancer treatment patterns and gene aberration test status of patients with advanced nonsquamous NSCLC in China.
Methods
Patients included in this study had unresectable Stage IIIB/IV nonsquamous NSCLC and were admitted during August 2015 to March 2016 into one of 12 tertiary hospitals throughout China for first-line anticancer treatment. Patient data (demographics, NSCLC histologic type, Eastern Cooperative Oncology Group [ECOG] Performance Status [PS], gene aberration test and results [if performed], and first-line anticancer treatment regimen) were extracted from medical charts and entered into Medical Record Abstraction Forms (MERAFs), which were collated for analysis.
Results
Overall, 1041 MERAFs were collected and data from 932 MERAFs were included for analysis. Patients with unresectable Stage IIIB/IV nonsquamous NSCLC had a median age of 59 years, 56.4% were male, 58.2% were never smokers, 95.0% had adenocarcinoma, and 92.9% had an ECOG PS ≤1. A total of 665 (71.4%) patients had gene aberration tests; 46.5% (309/665) had epidermal growth factor receptor (EGFR) gene mutations, 11.5% (48/416) had anaplastic lymphoma kinase (ALK) gene fusions, and 0.8% (1/128) had a c-ros oncogene 1 gene fusion. The most common first-line treatment regimen for unresectable Stage IIIB/IV nonsquamous NSCLC was chemotherapy (72.5%, 676/932), followed by tyrosine kinase inhibitors (TKIs; 26.1%, 243/932), and TKIs plus chemotherapy (1.4%, 13/932). Most chemotherapy regimens were platinum-doublet regimens (93.5%, 631/676) and pemetrexed was the most common nonplatinum chemotherapy-backbone agent (70.2%, 443/631) in platinum-doublet regimens. Most EGFR mutation-positive patients (66.3%, 205/309) were treated with EGFR-TKIs.
Conclusions
Findings from our survey of 12 tertiary hospitals throughout China showed an increased rate of gene aberration testing, compared with those rates reported in previous surveys, for patients with advanced nonsquamous NSCLC. In addition, pemetrexed/platinum-doublet chemotherapy was the predominant first-line chemotherapy regimen for this population. Most patients were treated based on their gene aberration test status and results.
Electronic supplementary material
The online version of this article (doi:10.1186/s12885-017-3451-x) contains supplementary material, which is available to authorized users.
Keywords: Chemotherapy, China, Epidermal growth factor receptor, First-line anticancer treatment, Non-small cell lung cancer, Tyrosine kinase inhibitor
Background
Lung cancer is a major public health concern in China, accounting for 21.3% of all new cancer cases and 27.1% of all deaths caused by cancer in 2012 [1]. Approximately 85% of patients presenting with lung cancer have non-small cell lung cancer (NSCLC) [2], with about 70% of these patients diagnosed with locally advanced or metastatic disease [3]. Recommended first-line treatments for these patients are platinum-doublet chemotherapy or molecular targeted therapy, if sensitive gene aberrations are detected [3–6]. Platinum-doublet chemotherapy has been shown to prolong survival and improve quality of life in patients with advanced NSCLC [4], with comparable efficacy among the various regimens [7]. In NSCLC patients with gene aberrations, molecular targeted therapies have been shown to have greater efficacy and lower toxicity than standard chemotherapy, whereas they have limited efficacy in NSCLC patients without gene aberrations [8].
Findings from a 2010 survey of physicians [9] and a retrospective review of hospital outpatient databases from 2004 to 2013 [10] in China indicated that NSCLC patients were mostly treated with platinum-doublet chemotherapy in the first-line setting. In patients with advanced NSCLC, the most commonly used chemotherapy regimen was gemcitabine/carboplatin-doublet chemotherapy [9, 10]. Of those patients treated with first-line epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs), nearly 50% had an unknown or negative EGFR mutation status [10]. Reported rates of EGFR gene mutation testing in China suggest that only 30% of NSCLC patients with adenocarcinoma are tested for gene aberrations [11] despite more than 40% having EGFR mutations [12, 13].
To determine if these practices have changed in recent times, we investigated first-line anticancer treatment patterns and gene aberration test status of patients with unresectable Stage IIIB/IV nonsquamous NSCLC treated at one of 12 tertiary hospitals throughout China.
Methods
Study design
This was a survey of medical charts from 12 tertiary hospitals located throughout China (Additional file 1: Table S1). Data were extracted from medical charts of patients discharged from hospital between 1 August 2015 and 15 March 2016.
The protocol was approved by the Research Ethics Committee of the Guangdong General Hospital, Guangzhou, Guangdong, China. Each site obtained its own institutional review board or ethics committee approval before the start of the study. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and Good Clinical Practice, and was supported by the Chinese Thoracic Oncology Group (CTONG study number 1506).
Study population
The medical charts of patients meeting the following criteria were included for review: aged ≥18 years; diagnosis of unresectable Stage IIIB or IV (according to the American Joint Committee on Cancer staging system, 7th edition), nonsquamous NSCLC; no previous systemic anticancer treatment for Stage IIIB or IV disease; and most recent hospitalization was for anticancer treatment.
Study protocol
Data from all patients’ medical charts who met the inclusion criteria were extracted and entered into the Medical Record Abstraction Form (MERAF) by designated hospital staff after patient discharge. Data extracted were demographics, NSCLC histological type, Eastern Cooperative Oncology Group (ECOG) Performance Status (PS), gene aberration test status and results (if performed), and first-line anticancer treatment regimen. Data entry was reviewed on-site by an independent data management organization (Shanghai Centennial Scientific Ltd., Shanghai, China), who assessed accuracy of data entry by checking 20% of all MERAFs collected at one hospital selected at random. Completed MERAFs were collected for analysis.
Data from all collected MERAFs were entered into a database for analysis, with data entered and verified twice to ensure accurate data entry. MERAFs were excluded from analysis if data were missing for gene aberration test status or first-line anticancer treatment regimen and if more than 10% of other data were missing.
Statistical analysis
Given that there are no published data in China to describe the proportion of patients receiving different types of chemotherapy, we assumed the proportion of patients receiving first-line TKI treatment was stable and could be estimated using the EGFR gene mutation rate. The sample size calculation assumed an EGFR gene mutation rate of 30% for East Asian populations [11], data from 897 patients to provide 2-sided 95% confidence intervals (CIs) with a precision of 3%, and exclusion of 5 to 10% of MERAFs because of missing data or other errors. Thus, collection of data from 1000 patients was planned.
Data were summarized with descriptive statistics using frequency and percentages for categorical data, and median, minimum, and maximum for continuous data. Analyses were done using SAS® Version 9.3 (SAS Institute, Cary, NC, USA).
Results
Patient disposition, demographics, and clinical characteristics
A total of 1041 MERAFs were collected. Of these, 109 MERAFs were excluded because they were of patients who were discharged from hospital before 1 August 2015 (study start date, n = 74), had missing data (n = 13), or were duplicates (n = 22). Thus, 932 MERAFs were included for analysis.
Overall, 73.9% of patients were less than 65 years of age, 56.4% were male, 58.2% had never smoked, 95.0% had adenocarcinoma, and 92.9% had an ECOG PS of 0 or 1 (Table 1).
Table 1.
Demographics and clinical characteristics of patients with unresectable Stage IIIB/IV nonsquamous non-small cell lung cancer
| Characteristic, n (%) | All patients |
|---|---|
| N = 932 | |
| Age, years | |
| Median (min., max.) | 59 (23, 80) |
| < 65 | 689 (73.9) |
| ≥ 65 | 243 (26.1) |
| Sex | |
| Male | 526 (56.4) |
| Female | 406 (43.6) |
| Residence area | |
| Rural | 420 (45.1) |
| Urban | 512 (54.9) |
| Smoking status | |
| Current Smoker | 157 (16.8) |
| Former Smoker | 233 (25.0) |
| Never Smoker | 542 (58.2) |
| Histologic subtype | |
| Adenocarcinoma | 885 (95.0) |
| Large Cell Carcinoma | 14 (1.5) |
| Other | 33 (3.5) |
| ECOG PS | |
| 0 | 291 (31.2) |
| 1 | 575 (61.7) |
| 2 | 55 (5.9) |
| 3 | 11 (1.2) |
ECOG Eastern Cooperative Oncology Group, max. maximum, min. minimum, PS Performance Status
Gene aberration test status and results
Overall, 665 (71.4%) patients had gene aberration tests. Gene aberration test rates were 71.4% (665/932) for EGFR gene mutations, 44.7% (416/932) for anaplastic lymphoma kinase (ALK) gene fusions, and 13.7% (128/932) for c-ros oncogene 1 (ROS1) gene fusions. Demographics and clinical characteristics of patients who did and did not have an EGFR gene mutation test were similar (Additional file 2: Table S2).
Gene aberration rates were 46.5% (309/665) for EGFR gene mutations, 11.5% (48/416) for ALK gene fusions, 0.8% (1/128) for ROS1 gene fusions (Fig. 1). Three patients had a co-existing EGFR gene mutation and an ALK gene fusion. EGFR gene mutation rates according to histological subtype were 47.6% (305/641) for adenocarcinoma, 22.2% (2/9) for large cell carcinoma, and 13.3% (2/15) for other histological types. Compared with the overall study population, a numerically higher proportion of EGFR mutation-positive patients were female (43.6%, 406/932 vs 56.6%, 175/309, respectively) and had never smoked (58.2%, 542/932 vs 70.2%, 217/309, respectively) (Table 1 and Additional file 2: Table S2).
Fig. 1.
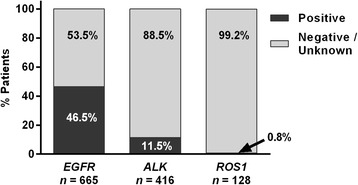
Gene aberration rates of patients with unresectable Stage IIIB/IV nonsquamous non-small cell lung cancer. Patients tested for gene aberrations were classified as positive (activating mutations in exons 18-21), wild type, or unknown (findings inconclusive) for epidermal growth factor receptor (EGFR) gene mutations, and positive or negative for anaplastic lymphoma kinase (ALK) and c-ros oncogene 1 (ROS1) gene fusions
First-line anticancer treatment regimens
The predominant first-line anticancer treatment regimen for patients with unresectable Stage IIIB/IV nonsquamous NSCLC was chemotherapy (72.5%, 676/932) followed by TKIs (26.1%, 243/932) and TKIs plus chemotherapy (1.4%, 13/932).
Chemotherapy
Most chemotherapy regimens were platinum-doublet regimens (93.3%, 631/676). Platinum-doublet chemotherapy regimens consisted primarily of cisplatin (65.0%) as the platinum agent and pemetrexed (70.2%) as the nonplatinum chemotherapy-backbone agent (Fig. 2).
Fig. 2.
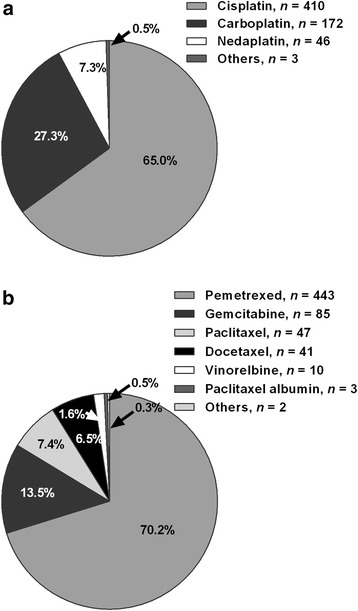
Doublet chemotherapy regimens of patients with unresectable Stage IIIB/IV nonsquamous non-small cell lung cancer, n = 631. a Platinum agents. b Nonplatinum chemotherapy-backbone agents
Other chemotherapy regimens were triplet regimens (platinum-doublet chemotherapy plus bevacizumab; 3.7%, 25/676) and singlet regimens (3.0%, 20/676). Triplet regimens consisted of cisplatin (48.0%, 12/25), carboplatin (32.0%, 8/25), or other platinum agents (20.0%, 5/25) and pemetrexed (60.0%, 15/25), paclitaxel (28.0%, 7/25), gemicitibine (8.0%, 2/25), or docetaxel (4.0%, 1/25) as nonplatinum chemotherapy-backbone agents. Patients treated with triplet regimens were mostly <65 years (80.0%) with an ECOG PS ≤1 (100%) (Additional file 3: Table S3). Singlet regimens consisted of pemetrexed (40.0%, 8/20), docetaxel (30.0%, 6/20), gemcitabine (20.0%, 4/20), or other nonplatinum chemotherapy agents (10.0%, 2/20). Patients treated with singlet regimens were more likely to be ≥65 years (50.0%) and have an ECOG PS ≥2 (30%) (Additional file 3: Table S3).
Tyrosine kinase inhibitors
In total, 243 patients with unresectable Stage IIIB/IV nonsquamous NSCLC treated with TKIs. Of these patients, 223 (91.8%) were treated with EGFR-TKIs (gefitinib, erlotinib, icotinib, epitinib, or allitinib), of which 205 (91.9%) were EGFR mutation positive. Fewer patients (7.8%, 19/243) were treated with the ALK-TKIs, crizotinib or ceritinib, and 1 (0.4%) patient was treated with the vascular endothelial growth factor receptor (VEGFR)-TKI, apatinib.
Tyrosine kinase inhibitors plus chemotherapy
There were 13 patients treated with TKIs plus chemotherapy; 9 patients received EGFR-TKIs plus chemotherapy and 4 patients received ALK-TKIs plus chemotherapy.
First-line treatment according to gene aberration test status
Most patients with unresectable Stage IIIB/IV nonsquamous NSCLC were treated according to their gene aberration test status (Table 2). A large proportion of patients with EGFR gene mutations were treated with EGFR-TKIs (67.0%) and nearly all patients with a negative or unknown gene aberration status were treated with chemotherapy (96.5%). Patients with ALK gene fusions were treated with either chemotherapy (56.3%) or ALK-TKIs (35.4%). Three patients with ALK gene fusions treated with EGFR-TKIs had co-existing EGFR gene mutations.
Table 2.
First-line anticancer treatment according to gene aberration test status
| Treatment, n (%) | Gene aberration | ||||
|---|---|---|---|---|---|
| N = 932 | |||||
| Positive | Negative | Unknown | |||
| EGFRa | ALKb | ROS1 | n = 307 | n = 267 | |
| n = 309c | n = 48 | n = 1 | |||
| TKI, n = 243 | 207 (67.0) | 20 (41.7)d | 1 | 6 (2.0) | 9 (3.4) |
| EGFR,e n = 223 | 205 (66.3) | 3 (6.25)d | 0 | 6 (2.0) | 9 (3.4) |
| ALK,f n = 19 | 1 (0.3) | 17 (35.4) | 1 | 0 | 0 |
| Chemotherapy,g n = 676 | 95 (30.7) | 27 (56.3) | 0 | 296 (96.4) | 258 (96.6) |
| TKI + Chemotherapy, n = 13 | 7 (2.3) | 1 (2.1) | 0 | 5 (1.6) | 0 |
ALK anaplastic lymphoma kinase, EGFR epidermal growth factor receptor, ROS1 c-ros oncogene 1, TKI tyrosine kinase inhibitor, VEGFR vascular endothelial growth factor receptor
a EGFR gene mutation positive test included all activating mutations in exons 18-21
b ALK tests were determined by fluorescence in situ hybridization, immunohistochemistry, or next-generation sequencing
cOne patient with an EGFR gene mutation was treated with the VEGFR-TKI apatinib
dThree patients with ALK gene fusions had coexisting EGFR gene mutations and were treated with EGFR-TKIs
eEGFR-TKIs were gefitinib, erlotinib, icotinib, epitinib, and allitinib
fALK-TKIs were crizotinib and ceritinib
gChemotherapy included singlet, platinum-doublet, and platinum-doublet plus bevacizumab (triplet) regimens
Discussion
In this survey, we investigated first-line anticancer treatment patterns and gene aberration test status of patients with unresectable Stage IIIB/IV nonsquamous NSCLC at 12 tertiary hospitals throughout China. More than two thirds of patients had gene aberration testing and 46.5% of those tested had EGFR gene mutations. The predominant first-line treatment regimen for unresectable Stage IIIB/IV nonsquamous NSCLC was pemetrexed/platinum-doublet chemotherapy. Most patients (66.3%) with EGFR gene mutations were treated with first-line EGFR-TKIs. These findings provide an updated and broad overview of the treatment of unresectable Stage IIIB/IV nonsquamous NSCLC in China.
In China, testing for EGFR gene mutations is recommended before treating advanced NSCLC [6] and is considered essential for patients with adenocarcinoma given the high rate of EGFR gene mutations in East Asian patients [8, 14, 15]. In our survey, 71.4% of patients with unresectable Stage IIIB/IV nonsquamous NSCLC were tested for EGFR gene mutations, a rate higher than those reported previously [9, 11]. A 2010 survey of physicians at general hospitals, chest hospitals, and comprehensive cancer centers located in 12 major cities throughout China found only 9.6% of patients with advanced NSCLC (squamous and nonsquamous histology) were tested for EGFR gene mutations [9]. Similarly, a 2011 retrospective online survey of patient records found that China had the lowest rate of EGFR gene mutation testing of the 6 Asian Pacific countries assessed, with 18.3% of all NSCLC patients and 30.3% of NSCLC patients with adenocarcinoma histology tested [11]. The improved EGFR gene mutation test rate in our survey suggests changes in clinical practice since 2010–11, possibly due to increased coverage of testing technology, improved tissue sample collection, and reduced cost. In addition, there may have been less reliance on patient characteristics associated with EGFR positive mutations that prompt testing because similar proportions of never smokers versus previous/current smokers (72.5%, 393/542, vs 69.7%, 272/390, respectively) and females versus males (73.9%, 300/406, vs 69.4%, 365/526) had an EGFR gene mutation test.
The EGFR gene mutation rate detected in our study for all patients (46.5%) and for patients with adenocarcinoma histological subtype (47.6%) were similar to those reported previously for Chinese patients with NSCLC of adenocarcinoma histology (40.3–64.5%) [14]. In a subset analysis of Chinese patients participating in the PIONEER study, a prospective molecular epidemiology study of EGFR gene mutations in Asian patients newly diagnosed with advanced NSCLC of adenocarcinoma histology, 50.2% (95% CI: 46.6–53.8%) of patients were EGFR mutation positive [12]. In addition, characteristics of EGFR mutation-positive patients in our survey were consistent with those associated with higher EGFR gene mutation rates (eg, female, never smoker) [16]. The rate of ALK gene fusions in our study (11.5%) was slightly higher than those reported previously for Chinese patients with adenocarcinomas (5.1–10%) [14]. Most patients tested for ALK gene fusions in our study were EGFR wild type, which may have influenced the proportion of patients testing positive for an ALK gene fusion because the occurrence of coexisting EGFR mutations and ALK gene fusions is rare [17]. The rate of ROS1 gene fusions (0.8%) was similar to those reported previously (1–2%) in Chinese patients with NSCLC [14].
Platinum-doublet chemotherapy is recommended for treatment of unresectable, advanced NSCLC [6]. Pemetrexed/platinum-doublet chemotherapy was the predominant treatment regimen for unresectable Stage IIIB/IV nonsquamous NSCLC in our survey. In a previous survey of Chinese physicians [9], gemcitabine/platinum-doublet chemotherapy was the predominant regimen for all advanced NSCLC patients (27.4%) and those with adenocarcinoma histology (32.0%). Although a greater proportion of patients with adenocarcinoma were treated with pemetrexed (16.1% vs non-adenocarcinoma 6%, respectively), the prevalence of gemcitabine was attributed to its favorable benefit/toxicity profile, low cost, reimbursement, and low incidence of alopecia [9]. The preference for pemetrexed/platinum-doublet chemotherapy in our survey may be result of changes in physician opinion regarding first-line treatment of unresectable Stage IIIB/IV nonsquamous NSCLC due to increasing evidence of improved overall survival, better tolerability, and fewer toxicities with pemetrexed-doublet regimens than other doublet regimens [18–21] and approval of pemetrexed for first-line treatment of nonsquamous NSCLC in combination with cisplatin by the China Food and Drug Administration in 2014.
Molecular targeted therapy drugs are recommended as first-line treatment options for advanced NSCLC if sensitive EGFR gene mutations or ALK gene fusions are detected [3–6], because of their higher efficacy and lower toxicity than standard chemotherapy in these patients [8, 22]. In our survey, a large proportion of EGFR mutation-positive patients were treated with first-line EGFR-TKIs (66.3%); the remaining EGFR mutation-positive patients were treated with chemotherapy (30.7%), EGFR-TKIs plus chemotherapy (2.3%), or other TKIs (0.6%). The reason why more than 30% of EGFR mutation-positive patients received chemotherapy only as first-line treatment requires further analysis. Encouragingly, most (91.9%) patients treated with first-line EGFR-TKIs were EGFR mutation positive, a proportion higher than that previously reported in a retrospective review of an outpatient oncology database (2004–13) in China (53.5%) [10].
We acknowledge the following limitations of our survey. Our findings from tertiary hospitals may not reflect the situation for those patients being treated at primary or secondary hospitals throughout China. The standard of lung cancer care in China ranges from practices similar to those in Western countries to basic care because China’s large population, expansive geography, and variable socioeconomic status of patients may affect access to diagnostic tests and quality oncology services and treatment [10, 23, 24]. In addition, patients refusing treatment and outpatients were excluded from our survey, which may have introduced bias into our findings. In a retrospective review of an outpatient oncology databases in China [10], 19.1% of patients refused treatment at diagnosis because of poverty, financial insecurity, or lack of medical insurance.
Conclusion
Our findings from 12 tertiary hospitals located in different geographic areas throughout China provide the most up-to-date overview of treatment patterns and gene aberration test status of patients with unresectable Stage IIIB/IV nonsquamous NSCLC. The rate of gene aberration testing was increased, compared with those rates reported in previous surveys [9, 11], for patients with unresectable Stage IIIB/IV nonsquamous NSCLC. In addition, pemetrexed/platinum-doublet chemotherapy was the predominant first-line regimen for this population and most patients were treated according to their gene aberration test status.
Additional files
Tertiary hospitals participating in the study. (DOCX 15 kb)
Demographics and clinical characteristics of patients with unresectable Stage IIIB/IV nonsquamous non-small cell lung cancer (NSCLC) according to epithelial growth factor receptor (EGFR) gene mutation test status and results. (DOCX 18 kb)
Demographics and clinical characteristics of patients with unresectable Stage IIIB/IV nonsquamous non-small cell lung cancer (NSCLC) according to chemotherapy regimen. (DOCX 16 kb)
Acknowledgements
The authors would like to thank all study participants.
This study was supported by the Chinese Thoracic Oncology Group (CTONG), a national collaborative clinical research group of 33 member hospitals.
Data collection and analysis was provided by Shanghai Centennial Scientific Ltd., and was funded by Lilly Suzhou Pharmaceutical Co., China. Medical writing assistance was provided by Julie Monk, PhD, CMPP and Mark Snape, MB BS, CMPP of ProScribe – Envision Pharma Group, and was funded by Lilly Suzhou Pharmaceutical Co., China. ProScribe’s services complied with international guidelines for Good Publication Practice (GPP3).
Funding
This study was sponsored by Lilly Suzhou Pharmaceutical Co., China. Lilly Suzhou Pharmaceutical Co., China was involved in the study design, interpretation of the data, and preparation of the manuscript.
Availability of data and materials
The datasets during and/or analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
- ALK
Anaplastic lymphoma kinase
- CI
Confidence interval
- CTONG
Chinese Thoracic Oncology Group
- ECOG
Eastern Cooperative Oncology Group
- EGFR
Epidermal growth factor receptor
- max.
Maximum
- MERAF
Medical Record Abstraction Form
- min.
Minimum
- NSCLC
Non-small cell lung cancer
- PS
Performance Status
- ROS1
c-ros oncogene 1
- TKI
Tyrosine kinase inhibitor
- VEGFR
Vascular endothelial growth factor receptor
Authors’ contributions
QS, LLY, PHZ, Y-LW, and QZ were involved in the study conception and design. All authors, except QS, LLY, and PHZ, were involved in the acquisition of data. QS, LLY, PHZ, and Y-LW were involved in the data analyses. All authors participated in the interpretation of the study results, and in the drafting, critical revision, and approval of the final version of the manuscript.
Ethics approval and consent to participate
The protocol was approved by the Research Ethics Committee of the Guangdong General Hospital, Guangzhou, Guangdong, China and the study was supported by the Chinese Thoracic Oncology Group (CTONG study number 1506). Each site obtained its own institutional review board or ethics committee approval before the start of the study.
Consent for publication
Not applicable.
Competing interests
QS, LLY, and PHZ are employees of Lilly Suzhou Pharmaceutical Co., China. YLW has received honoraria from F. Hoffmann-La Roche, Eli Lilly, AstraZeneca, and Pfizer. All other authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1186/s12885-017-3451-x) contains supplementary material, which is available to authorized users.
References
- 1.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012 v1.0, Cancer incidence and mortality worldwide: IARC CancerBase no. 11. International Agency for Research on Cancer. 2013. [Google Scholar]
- 2.Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83(5):584–594. doi: 10.1016/S0025-6196(11)60735-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Besse B, Adjei A, Baas P, Meldgaard P, Nicolson M, Paz-Ares L, et al. 2nd ESMO consensus conference on lung cancer: non-small-cell lung cancer first-line/second and further lines of treatment in advanced disease. Ann Oncol. 2014;25(8):1475–1484. doi: 10.1093/annonc/mdu123. [DOI] [PubMed] [Google Scholar]
- 4.Reck M, Popat S, Reinmuth N, De Ruysscher D, Kerr KM, Peters S. Metastatic non-small-cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl 3):27–39. doi: 10.1093/annonc/mdu199. [DOI] [PubMed] [Google Scholar]
- 5.Socinski MA, Evans T, Gettinger S, Hensing TA, VanDam SL, Ireland B, et al. Treatment of stage IV non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(Suppl 5):e341S–e368S. doi: 10.1378/chest.12-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhi X-Y, Yu J-M, Shi Y-K. Chinese guidelines on the diagnosis and treatment of primary lung cancer (2015 version) Cancer. 2016;121:3165–3181. doi: 10.1002/cncr.29550. [DOI] [PubMed] [Google Scholar]
- 7.Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346(2):92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 8.Ke EE, Zhou Q, Wu YL. Emerging paradigms in targeted treatments for Asian patients with NSCLC. Expert Opin Pharmacother. 2015;16(8):1167–1176. doi: 10.1517/14656566.2015.1040391. [DOI] [PubMed] [Google Scholar]
- 9.Xue C, Hu Z, Jiang W, Zhao Y, Xu F, Huang Y, et al. National survey of the medical treatment status for non-small cell lung cancer (NSCLC) in China. Lung Cancer. 2012;77(2):371–375. doi: 10.1016/j.lungcan.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 10.Yang LL, Zhang XC, Yang XN, Yang JJ, Wang Z, Chen HJ, et al. Lung cancer treatment disparities in China: a question in need of an answer. Oncologist. 2014;19(10):1084–1090. doi: 10.1634/theoncologist.2014-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yatabe Y, Kerr KM, Utomo A, Rajadurai P, Tran VK, Du X, et al. EGFR mutation testing practices within the Asia Pacific region: results of a multicenter diagnostic survey. J Thorac Oncol. 2015;10(3):438–445. doi: 10.1097/JTO.0000000000000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi Y, Li J, Zhang S, Wang M, Yang S, Li N, et al. Molecular epidemiology of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology - mainland China subset analysis of the PIONEER study. PLoS One. 2015;10(11):e0143515. doi: 10.1371/journal.pone.0143515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gou L-Y, Wu Y-L. Prevalence of driver mutations in non-small-cell lung cancers in the People’s Republic of China. Lung Cancer Targets Ther. 2014;5:1–9. doi: 10.2147/LCTT.S40817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou C. Lung cancer molecular epidemiology in China: recent trends. Transl Lung Cancer Res. 2014;3(5):270–279. doi: 10.3978/j.issn.2218-6751.2014.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi Y, Au JS, Thongprasert S, Srinivasan S, Tsai CM, Khoa MT, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER) J Thorac Oncol. 2014;9(2):154–162. doi: 10.1097/JTO.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang S, Wang Z. EGFR mutations in patients with non-small cell lung cancer from mainland China and their relationships with clinicopathological features: a meta-analysis. Int J Clin Exp Med. 2014;7(8):1967–1978. [PMC free article] [PubMed] [Google Scholar]
- 17.Lou NN, Zhang XC, Chen HJ, Zhou Q, Yan LX, Xie Z, et al. Clinical outcomes of advanced non-small-cell lung cancer patients with EGFR mutation, ALK rearrangement and EGFR/ALK co-alterations. Oncotarget. 2016;7(40):65185–65195. doi: 10.18632/oncotarget.11218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26(21):3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 19.Xiao HQ, Tian RH, Zhang ZH, Du KQ, Ni YM. Efficacy of pemetrexed plus platinum doublet chemotherapy as first-line treatment for advanced nonsquamous non-small-cell-lung cancer: a systematic review and meta-analysis. Onco Targets Ther. 2016;9:1471–1476. doi: 10.2147/OTT.S112358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu YL, Lu S, Cheng Y, Zhou C, Wang M, Qin S, et al. Efficacy and safety of pemetrexed/cisplatin versus gemcitabine/cisplatin as first-line treatment in Chinese patients with advanced nonsquamous non-small cell lung cancer. Lung Cancer. 2014;85(3):401–407. doi: 10.1016/j.lungcan.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Li M, Zhang Q, Fu P, Li P, Peng A, Zhang G, et al. Pemetrexed plus platinum as the first-line treatment option for advanced non-small cell lung cancer: a meta-analysis of randomized controlled trials. PLoS One. 2012;7(5):e37229. doi: 10.1371/journal.pone.0037229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi Y, Sun Y, Ding C, Wang Z, Wang C, Wang Z, et al. China experts consensus on icotinib for non-small cell lung cancer treatment (2015 version) Ann Transl Med. 2015;3(18):260. doi: 10.3978/j.issn.2305-5839.2015.10.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goss PE, Strasser-Weippl K, Lee-Bychkovsky BL, Fan L, Li J, Chavarri-Guerra Y, et al. Challenges to effective cancer control in China, India, and Russia. Lancet Oncol. 2014;15(5):489–538. doi: 10.1016/S1470-2045(14)70029-4. [DOI] [PubMed] [Google Scholar]
- 24.American Cancer Society . Global cancer facts & figures. 3. Atlanta: American Cancer Society; 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tertiary hospitals participating in the study. (DOCX 15 kb)
Demographics and clinical characteristics of patients with unresectable Stage IIIB/IV nonsquamous non-small cell lung cancer (NSCLC) according to epithelial growth factor receptor (EGFR) gene mutation test status and results. (DOCX 18 kb)
Demographics and clinical characteristics of patients with unresectable Stage IIIB/IV nonsquamous non-small cell lung cancer (NSCLC) according to chemotherapy regimen. (DOCX 16 kb)
Data Availability Statement
The datasets during and/or analysed during the current study available from the corresponding author on reasonable request.


