Abstract
Glioblastoma (GBM) is a hypervascular neoplasia of the central nervous system with an extremely high rate of mortality. Owing to its hypervascularity, anti-angiogenic therapies (AAT) have been used as an adjuvant to the traditional surgical resection, chemotherapy, and radiation. The benefits of AAT have been transient and the tumors were shown to relapse faster and demonstrated particularly high rates of AAT therapy resistance. Alternative neovascularization mechanisms were shown to be at work in these resilient tumors to counter the AAT therapy insult. Vascular Mimicry (VM) is the uncanny ability of tumor cells to acquire endothelial-like properties and lay down vascular patterned networks reminiscent of host endothelial blood vessels. The VM channels served as an irrigation system for the tumors to meet with the increasing metabolic and nutrient demands of the tumor in the event of the ensuing hypoxia resulting from AAT. In our previous studies, we have demonstrated that AAT accelerates VM in GBM. In this review, we will focus on the origins of VM, visualizing VM in AAT-treated tumors and the development of VM as a resistance mechanism to AAT.
Introduction
Tumor growth and metastasis have been long associated with the processes of angiogenesis and vasculogenesis assuming a pathological form. This dogma was rendered obsolete when a novel non-angiogenic dependent pathway was introduced in 1999 by Maniotis et al. Vascular Mimicry (VM) was described as a completely neoteric blood supply system feeding tumor cells in malignant melanoma, which utilizes the uncanny ability of aggressive melanoma cells to transdifferentiate into stem cell-like state to subsequently assume an endothelial-like phenotype [1]. VM enables the tumors to form matrix-embedded vascular structures containing plasma and blood cells to meet with the increasing nutrient and metabolic demands of the neoplastic tissues. Since the channels are formed without the contribution of pre-existing host endothelial cells, the process is vasculogenic in nature. The ensuing vascular-like structures are not true blood vessels but merely mimic the function of vessels, thereby clearly defining the phenomenon of VM [1], [2], [3], [4]. VM vessels are matrix rich structures rich in laminin, positive for PAS staining and are often found encapsulating nests or lobules of tumor cells as closed loops. The formation of extracellular patterned matrices rich in laminin, proteoglycans, heparan sulfate and collagens IV and VI as a part of their basement membranes visualized by the aforementioned PAS staining has already been established as a crucial histopathological evidence of VM in hypervascular tumors [2], [3], [4], [5], [6], [7], [8]. After the initial discovery of VM as a novel neovascularization mechanism in aggressive melanomas, VM was also reported in many other non-melanoma neoplastic malignancies such as breast [9], ovarian [10], [11], prostate [12], lung [13] and also in glioblastoma (GBM) [14]. However, the phenomenon of VM has been a subject of intense controversy and many studies have in fact questioned the validity of VM [15], [16]. Though the occurrence of VM structures is rare in tumors, the presence of these patterned matrices rich in ECM is positively correlated with the increased risk of metastasis, poor clinical outcome, and bleak survival time after initial diagnosis in patients [7], [17]. In this review, we will describe the possible mechanisms of neovascularization in GBM including VM, antiangiogenic therapy (AAT)-induced VM, different subtypes of VM, visualization of VM, and possible therapy to target VM.
Mechanisms of Neovascularization in Glioblastoma
Tumor blood vessels are radically different from the normal host blood vessels, both morphologically and physiologically. The host endothelial vascular structures are comprised of endothelial cells (ECs) and supporting cells such as pericytes and astrocytes to form an intact highly regulated membranous Blood Brain Barrier (BBB). On the other hand, GBM vessels are tortuous, disorganized, highly permeable, destabilized structures with abnormal endothelial and pericyte coverage [18], [19]. The classical tumor development was attributed, hitherto, to two main mechanisms of vascularization, the classical sprouting angiogenesis, in which the pre-existing ECs proliferate and migrate to form neovessels, and vasculogenesis, in which the cells from the bone marrow are recruited to the tumor sites to contribute to the formation of neovascular structures [20], [21]. Several studies have also reported the role of endothelial progenitor cells (EPCs) in contributing to the tumor vascular endothelium. These EPCs are recruited to the tumor microenvironment (TME) in a VEGF and SDF-1 dependent manner. However, this concept has not been riddled with controversies [22], [23], [24], [25]. As tumors grow in size, they need an increased supply of blood vessels to keep them viable. Avascular tumors that are unable to acquire new blood vessels to meet their metabolic and nutrient demands eventually regress [26], [27], [28]. Co-option of the pre-existing vessels in the neighboring tissue is one of the most critical steps utilized by the tumor to keep itself viable and growing [29]. During the early formative stages of tumor development, angiopoietin-2 (Ang-2) is expressed on the co-opted tumor vasculature, and as the tumors grow in size, there is a widespread increase in the expression of Ang-2 on both the endothelial cells and the tumor cells. The over-expression of Ang-2 causes destabilization of blood vessels caused by the disengagement of pericytes and loss of endothelial barrier integrity to promote tumor hypoxia and necrosis [18], [20], [29]. The initiation of hypoxic conditions in the tumor prompt the translocation of hypoxia inducible factor-1α (HIF-1α) into the nuclei of cells and thereby up-regulating the expression of HIF-1α dependent expression of pro-angiogenic factors such as matrix metalloproteinases (MMP-2 and ‐9), VEGF, stromal cell derived factor-1α (SDF-1α) and inteleukin-8 (IL-8). The combined action of these causes the degradation of ECM scaffold and basement membrane to facilitate the creation of new areas in and around tumors, where neovessels can be established. In addition, these alterations of the ECM scaffolds in the TME bolster the metastatic capability of tumor cells by synergizing the migration and extravasation of tumor cells [30], [31], [32]. VEGF can subsequently up-regulate the expression of VEGFR2 on the ECs and facilitate sprouting angiogenesis and vascular remodeling in conjunction with Ang-2. Many studies have already established the role of Ang-2 in initiation of vessel sprouting and pericyte loss to promote neovessel formation in the tumors [20], [33], [34], [35], [36], [37]. Delta like-4 (Dll4)-Notch-1 signaling and Ephrin B2 regulate the sprouting and branching processes in the endothelial tip cells in response to VEGF. Moreover, greater expression of Dll4 is associated with a high-grade glioma, where it facilitates vascular function by thwarting non-functional angiogenesis in mouse models of glioma and other tumors [38], [39], [40], [41], [42], [43]. Several pro-angiogenic growth factors and chemokines such as VEGF, SDF-1α, platelet-derived growth factor (PDGF), IL8, fibroblast growth factor (FGF2) and angiopoietin-1 create an environment conducive to EC recruitment, proliferation, and migration [18]. Integrin complexes such as αvβ3, αvβ5, and α5β1 are crucial to the tumor angiogenesis [18], [44] with αvβ3 integrin mediating FGF2-dependent angiogenesis and αvβ5 integrin being responsible for VEGF-dependent angiogenesis [45]. The corresponding expression of these integrins on the tumor cells, usually in high-grade gliomas, positively correlates with poor prognosis and an aggressive phenotype [46], [47], [48].
Anti-Angiogenic Therapy Resistance in Glioblastomas
Ide et al. in 1939 made the first observation of tumor growth being juxtaposed with formation of new blood vessels in a process known as angiogenesis [49]. Folkman et al. demonstrated that injecting tumor cells into isolated perfused organs did not facilitate tumor growth, but when the same tumor cells were transplanted into syngeneic mice, these tumors grew beyond few millimeters owing to rapid revascularization. This groundbreaking experiment proved beyond doubt that tumors depended on blood supply and neovascularization for growth. Following these experiments was the idea that since tumor growth was angiogenesis-dependent, inhibition of this process could lead to therapeutic benefits. Thus the idea of “anti-angiogenesis” came into existence, both as a concept and in to clinical practice, so much so that tumor angiogenesis has become one of the “hallmarks of cancer” [26], [50], [51]. Owing to the hypervascular nature of the GBM tumors, anti-angiogenic therapies (AAT) including, vatalanib, sunitinib, and cediranib were used to control the abnormal angiogenesis and also to normalize the tumor vasculature [52], [53], [54], [55], [56], [57]. It was believed that since the endothelial cells have comparatively lower genetic instability than tumor cells, a more viable option would be to target the VEGF-VEGFR pathways to counter angiogenesis without imposing drug resistance. Regrettably, the benefits offered by AAT are transitory, and the tumors showed higher rates of relapse and potent refractoriness [58]. Previous studies in our lab have already shown that targeting VEGFR2 using vatalanib (PTK787) significantly increased the tumor size as visualized using DCE-MRI [59]. Vatalanib treatment induced hypoxia and was associated with the increased expression of VEGF, SDF-1α, HIF-1α, FGF-1, FGF-2, ephrin-A1, ephrin-A2, angiopoietin-1, VEGFR2, VEGFR3, and EGFR at the peripheral part of the tumors compared to that of central part of tumors [60]. Cediranib and sunitinib, inhibitors of VEGFR, PDGFR and c-kit receptors, have been associated with limited therapeutic efficacy and offered transient benefits with high toxicity rates despite showing promising results in pre-clinical trials [61], [62], [63], [64], [65]. Similarly, vatalanib did not offer any considerable benefits in clinical trials [64]. In addition, another study has reported that there was an increase in the plasma concentration of FGF-2 in colon cancer and GBM patients following AAT [66]. Vatalanib (PTK787, Novartis Pharmaceuticals Corporation, East Hanover, NJ) is a pan-VEGFR, PDGFR, and c-Kit tyrosine kinase inhibitor (TKI) and its utility in recurrent GBM has been studied both as monotherapy [67] or in combination with temozolomide and lomustine [68]. Vatalanib also significantly increased CD68+ myeloid cells, and CD133+, CD34+, and Tie2+ endothelial cell signatures demonstrated in a recent study from our lab [91]. By preventing the mobilization of BMDCs and interaction of CXCR4-SDF-1 using whole body irradiation and AMD3100, respectively, paradoxical growth of tumor following Vatalanib treatment was controlled [69]. Bevacizumab, a humanized monoclonal antibody against VEGF-165, the predominant isoform of VEGF-A was the first angiogenesis inhibitor that got the approval of the Food and Drug Administration (FDA) in 2004. It also was the first commercially successful anti-angiogenic drug marketed [70]. Bevacizumab in combination with cytotoxic chemotherapy produced some dramatic tumor size reduction as seen on radiographic images with prolonged progression free survival (PFS) and also reduced the need for corticosteroids [71]. However, prolonged use of bevacizumab resulted in deteriorated clinical outcome with patients developing resistance and eventually succumbing to the disease owing to ineffective therapies [71], [72], [73]. The development of resistance to AAT is thought to be due to activation of alternative pathways of neovascularization [21]. One of the mechanisms is VM.
VM in gliomas is associated with extremely poor prognosis and positively correlates with high grades of tumor malignancy and invasiveness [74], [75]. In the light of these evidences, it becomes imperative to understand VM and its molecular signaling mechanisms to develop novel therapeutics to target tumor growth and metastasis.
Alternative Neovascularization Pathways and Vasculogenic Mimicry (VM)
Anti-angiogenic therapies act through different pathways in concert to delay tumor progression and prolong survival in patients. Tumor starvation was achieved by the coordinated mechanisms of action such as the induction of apoptosis of ECs, preventing the migration of EPCs, inhibition of formation of new blood vessels, obliteration of existing microvessels, decrease tumor vascular irrigation and thus impeding the oxygenation and nutrient supply to the tumor [49]. Evolutionary biology has always promoted natural selection of survival by favoring the development or acquisition of novel adaptive dynamics that produce a resilient system. Since AAT works as a selective pressure upon cancer cells, the surviving tumor cells develop adaptive mechanisms that ensure a survival advantage for these cells in the TME. Cancer cells are notorious for being genetically instable and heterogeneous. The hypoxia that ensues following AAT paves way for initiation of alternative pathways of tumor adaptation, activates pro-angiogenic, invasive and metastatic gene signatures in tumors cells and ECs, up-regulates a variety of growth factors, chemokines and cytokines necessary for recruitment of ECs, EPCs, vasculogenic leukocytes, angiogenic myeloid cells to cope with the demands of the compromised microenvironment [93], [94], [95], [96], [97], [98]. In the event of a therapeutic attack with AAT, the tumors resort to various neovascularization mechanisms in addition to the regularly documented angiogenesis and vasculogenesis. VM is one such novel neovascularization mechanism that is slowly gaining attention in the scientific field due to its widely accepted existence across several tumors and validated evidences. Glioblastoma stem-like cells (GSCs) and transdifferentiated tumor cells with endothelial properties are the cellular source of tumor blood vessels.
In the burgeoning tumor, the neoplastic cells are hyper proliferative and their metabolic and nutrient demands grow exponentially. This surpasses the rate of formation of new blood vessels and simulates a scenario of a tumor undergoing AAT. To meet with the increasing demands, the tumor has to resort to a non-endothelial system of nutrient supply and waste disposal. A novel yet interesting phenomenon that encompasses an irrigational system of perfusing tumors is speculated to be VM. VM describes the uncanny ability of tumor cells to transdifferentiate into endothelial-like cells and thereby acquire the capability of forming tube like structures mimicking blood vessels independent of the ECs [1], [76]. These transformed endothelial-like cells secrete matrix proteins such as collagens IV and VI, proteoglycans, heparan sulfate and laminin that aid the formation of tubular networks within the tumors that are anatomically in stark contrast to the regular traditional blood vessels, where the basal lamina is present behind the ECs. These VM like tubular networks have basal lamina towards the lumen and concomitantly have polysaccharides as markers of VM demonstrating strong positive staining for periodic Acid-Schiff (PAS) [2]. The plasticity of tumor cells and their corresponding heterogeneity favors the acquisition of endothelial markers such as CD31, VEGFR2 and VE-Cadherin and as such enables the tumor cells to incorporate into host endothelial tubular structures and form a connection with them [1]. Similar to the contribution of the trophoblasts to the formation of non-endothelial vascular structures in the maternal uterine walls [77], the vascular structures formed by the endothelial-like transformed cells function as a percolative system controlling the oxygen and nutrient supply to meet with the metabolic needs as well as to discharge the cellular secretion. Eventually, the tumor-derived vasculature merges with the endothelial-derived vasculature to form a “mosaic vasculature”, an anatomically hybrid structure engineered by the tumor as a fusion of both the endothelial and non-endothelial cellular sources [2], [78], [79], [80].
Types of Vasculogenic Mimicry (VM)
There are two distinct types of VM reported in literature, the tubular type [81] and the classical patterned matrices type [2], [14]. The tumor cells associated with the VM structures revert to an undifferentiated, embryonic like phenotype and acquire the characteristics of endothelial cells. This conversion of the tumor cells into an embryonic like state empowers the cells to form blood vessel like structures reminiscent of the embryonic vasculogenic structures [1], [82], [83].
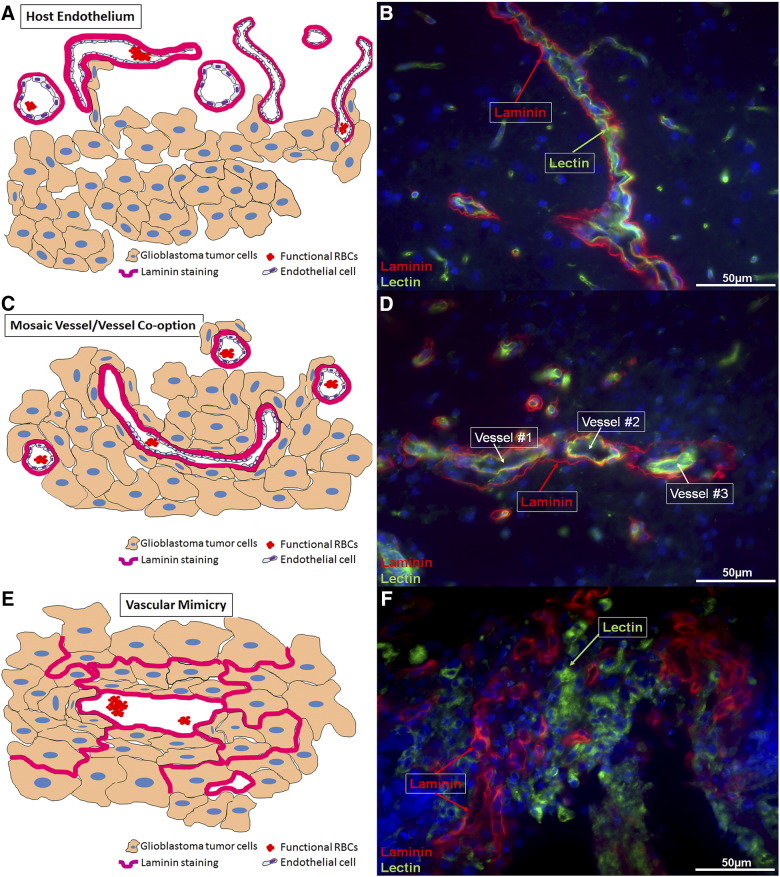
In our previous study [84], we have established histopathological confirmation of the existence of different forms of VM, including mosaic vessels, sustenance of vascular mimicry (as patterned matrices), and VM like structures. Figure 1A depicts a pictographical representation of a host endothelial-cell derived blood vessel. Figure 1B shows the host endothelial vessel outside the tumor area. Upon observation, it is clearly evident that the laminin staining is present on the outer side of the vessel lumen and the lectin staining is present on the inside of the lumen. This characteristic differential localization of laminin (outside) and lectin (inside) is specific to the host endothelium. Figure 1C is a pictographical depiction of tumor vessel co-option and formation of a mosaic vessel at the tumor periphery. Since mosaic vessels are the hybrid structures formed by the tumor and host endothelial cell contribution, the laminin staining (in pictographical depiction) comes from the tumor cells and host endothelial cells. However, the host endothelial lectin staining differentiates the tumor from the host blood vessels. Figure 1D shows tumor encapsulation of the existing host endothelial structures in an attempt to form mosaic vessels. The figure clearly demonstrates the tumor cells attempting to incorporate into the host endothelial vascular structures as represented by the laminin and lectin co-localization. The cartoon in Figure 1E depicts the VM like structures in the tumor center. These structures are laminin positive and are devoid of any host endothelial contribution. Figure 1F shows the VM in the tumor center. Laminin-positive loops characteristic of VM are the most standard hallmarks of this form of neovascularization. These laminin loops are devoid of any lectin staining but are believed to acquire lectin staining once the tumors transdifferentiate into endothelial phenotypes.
Figure 1.
Schematic of vascular mimicry development driving anti-angiogenic therapy (AAT) resistance in glioblastoma. (A) Host endothelial-dependent blood vessel formation. (B) Host endothelial vessels visualized outside the tumor area. In the host tissue, the Laminin staining is present on the outside and the Lectin staining is present on the inside. (C) Formation of mosaic vessels in the tumor periphery. (D) The formation of a mosaic vessels can be evidenced here. The three host endothelial vessels are being encapsulated by the tumor cells (in red, Laminin). However, the tumors can be seen trying to form a physiological connection with the host endothelial-cell dependent vessels as observed in the vessels #1 and #2. The laminin-positive loops are seen to be attached or incorporated into host endothelial vessels and thus present as a super-imposed yellow colored structures (green from host endothelium and red from the tumor derived laminin). (E) Vascular Mimicry-Tube like vascular structures in the tumor center. Here the laminin based patterned networks are independent of the host endothelial cell contribution. (F) Vascular mimicry like patterned networks can be seen in the tumor center. In the tumor center, the Laminin staining is present independently of the Lectin staining because the tumor has begun the process of VM. The Lectin now depicts the tumor cells acquiring endothelial like properties, reminiscent of the host endothelial cells (Magnification 40×).
AAT-Induced VM in Glioblastomas
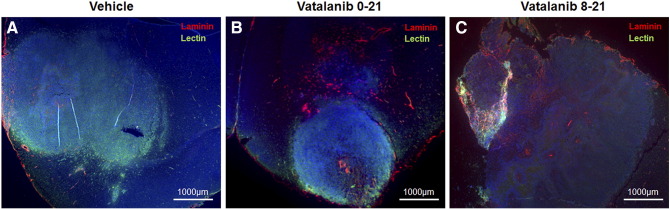
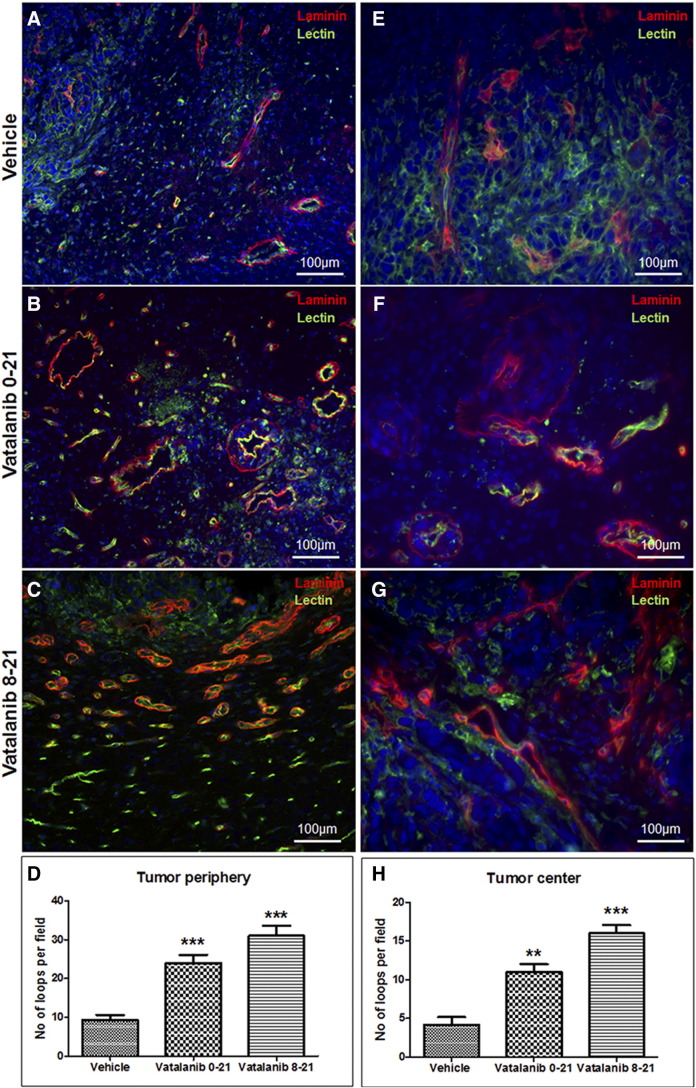
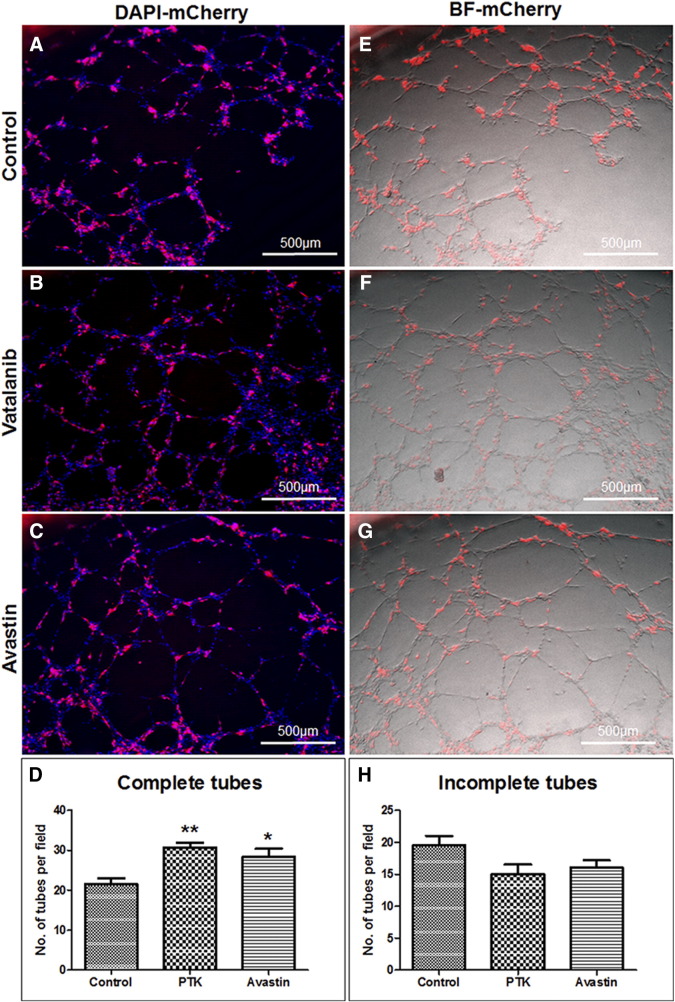
Investigators have reported increased number of VM in therapy resistant tumors. Our recently published works also indicated higher number of VM in glioblastomas following AAT [84]. We noticed accelerated VM both in the center and in the peripheral part of the AAT-treated tumors. Figure 2 shows the example of VM following AAT. Figure 2 represents the tumor areas co-stained with laminin and lectin. The figure shows that the vatalanib-treated tumors have comparatively higher laminin-positive areas than the vehicle-treated group. We have quantified the laminin-positive loop like structures indicative of the VM or mosaic vessels (laminin co-localized with lectin) both at the tumor periphery as in Figure 3, A–D and in the tumor center as in Figure 3, E–H. In our in vitro studies, mCherry U251 GBM cells were co-cultured with HUVEC cells on matrigel. After incubation for 6 h, we found that the GBM cells had incorporated into the endothelial (HUVEC)-dependent vascular tube like networks as shown in Figure 4, A–C, E–G. We analyzed the number of complete tube like networks without any breaks or disintegration and also analyzed the incomplete tube like-structures in the control (PBS) and AAT (vatalanib and avastin (bevacizumab))-treated groups as shown in Figure 4, D and H. We found that the number of complete tube-like networks were significantly higher in AAT-treated wells compared to the control group (Figure 4D). This clearly demonstrates the fact that following AAT, tumors resort to aggressive neovascularization mechanisms to cope with the therapeutic insult and thereby adopt VM as a novel neovascularization mechanism to counter the ensuing hypoxic environment within the tumor.
Figure 2.
Accelerated VM in AAT-treated tumors in orthotopic human GBM mouse model. (B and C) Vatalanib-treated tumors show significantly higher number of laminin-positive areas that are spread over the entire tumor when compared to the vehicle-treated tumors (A). The images have been taken at a magnification of 2.5× to ease the depiction of tumor size in each treated animal of the respective group.
Figure 3.
Mosaic vessels and Vascular Mimicry like-structures in the AAT-treated tumors. Tumor periphery showing laminin loops present in conjunction with the host-endothelial structures as visible with the green lectin staining in vehicle- (A) and vatalanib (B and C)-treated tumors. The mosaic vessels are also present at the tumor periphery, which are evident as the red-laminin and green-lectin superimposed yellow loop like structures. (D) Quantification of laminin-positive loop like-structures in the tumor periphery. In the central areas, Vatalanib-treated (F and G) tumors show significantly higher number of laminin-positive loop like structures indicative of VM, compared to the vehicle-treated tumors (E). These laminin loops are present independently of the host-endothelial structures. These laminin-positive patterned matrices are formed by the tumor cells alone, without any contribution from the host endothelial cells. (H) The quantification of the laminin-positive loops in the tumor center. Images have been taken from four different areas of the tumor. One representative image from one animal in the respective group has been shown here. The laminin-positive loops have been counted using Image J software and the statistical analyses were performed using Student's t test and P <.05 was considered significant.
Figure 4.
Post-treated glioblastoma cells incorporate into host endothelial blood vessels. Panels A–C represent the super-imposition of red fluorescent (mCherry U251 cells) and the DAPI images. (D) Quantification of complete tubes in AAT-treated groups. Panels E–G represent the superimposition of red fluorescent (mCherry U251 cells) image over the bright field (BF) image (HUVEC cells). (H) Quantification of incomplete tubes in AAT-treated groups. mCherry U251 GBM cells and HUVEC cells were seeded in 1:2 ratio, treated with AAT post seeding and incubated for 6 h. One representative image from each treatment group has been shown here. Statistical analyses were performed using Student's t test and P < .05 was considered significant.
A Focus on the Cancer Stem Cells (CSCs) and Glioblastoma Stem Like Cells (GSCs) in VM
It is already known beyond doubt that the cancer cells exhibiting high degree of plasticity acquire endothelial-like phenotypes owing to their multipotent nature. These multipotent cells undergo sequential transformations from being epithelial to mesenchymal to endothelial phenotypes, resembling embryonic stem cells [2]. Though specific criteria for establishing GSCs capable of VM are lacking, CD133 and Nestin have been established as biomarkers common to GSC and VM-initiating stem cells [85], [86]. Anti‐CD133 antibodies can be used to isolate and enrich cancer stem cells (CSCs) or CSCs can also be obtained by the generation of neurospheres in culture conditions using serum-free media containing epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF) [87]. The transdifferentiation of tumor cells into endothelial like cells (termed tumor derived endothelial cells, TDECs), which were CD45-CD31+ CD34+ was proposed as a critical switch in functional phenotype of tumor cells to promote VM in GBM models [88]. Yet another study highlighted the role of glioblastoma stem-like cells of CD133+ CD144+ phenotype that promote the VM in GBM tumors. The role of CD133+ CD144- cells in generating the endothelial progenitors (CD133+ CD144+) in the TME due to the tumor-secreted angiogenic factors was conclusively demonstrated with the help of efficient in vitro and in vivo studies [89]. Previous studies from our lab and others have also reported the endothelial progenitor like characteristics of CD133+ glioma stem like cells or glioma initiating cells [90], [91], [92]. Hypoxia is a critical factor that governs VM and hypoxia induced up-regulation of CD144 (VE-Cadherin) has been cited as the key factor driving VM in GBM [93]. A recent study demonstrated that a subpopulation of melanoma cells expressing the vascular cell adhesion molecule PECAM1 but not VEGFR2 is capable of undergoing VM and forming vascular tube like networks. At the mechanistic level, activating enhancer binding protein-2α (AP-2α) was found to be the crucial transcription factor governing the expression of PECAM-1 [94]. The formation of CSCs in general and GSCs in particular is a process that encompasses two closely related yet different mechanisms of (i) multistep mutagenic insults to the normal stem cells and (ii) sequential de-differentiation of epithelial cells into mesenchymal phenotypes termed epithelial-to-mesenchymal transition (EMT), a process that sets the stage for the acquisition of dedifferentiated phenotypes with mesenchymal features reminiscent of the embryonic stem cells. The generation of GSCs is therefore a bridge that connects VM and EMT, thus explaining why EMT is crucial to the formation of VM in tumors.
Evidence in Clinical Settings, Clinical Implications, and Future Prospects of Therapy in VM
In a meta-analysis of 22 clinical studies which had 3062 patients across 15 different types of cancers enrolled for a study, the 5-year overall survival of the VM+ cancer patients was 31% whereas the overall survival for VM− cancer patients was found to be around 56%. Also, the relative risk of failure of the VM+ patient surviving for five years was calculated to the significantly higher that the VM− cancer patients [95]. Of all the cancer types observed, studied and analyzed to date, VM is reported to be present in less than 50% of the cases and therefore may be indicative or characteristic of a common, aggressive phenotype. The presence of VM was detected in around 22.7% (15 of 66) tumors in osteoblastic-type osteosarcomas [96], 43% (52 of 120) carcinomas analyzed in ovarian tumors [97], 22% (40 of 173) patients with gastric adenocarcinoma and 35% of triple-negative breast cancers (TNBC) as opposed to 17.8% in non-TNBC cases [98]. In yet another study of analyzed ovarian carcinomas, 43% cases were reported to be VM+. These VM+ patients displayed significantly higher pathological grade, histologic type and had overall poor survival [99]. A recent study implicated the role of mTOR in the VM as observed in 26.8% (34 of 127) glioma cases [100]. 20.5% (31 of 151) hepatocellular carcinoma (HCC) cases exhibited evidence of patterned matrix VM and the presence of VM was associated with larger tumors, vascular invasion, high-grade HCC, and late-stage HCC. Also, the VM+ cases had poorer overall survival and disease-free survival compared to the VM− cases [101]. 21.67% (44 of 203) cases were shown to have both VM and endothelium dependent vessel in laryngeal squamous cell carcinoma (LSCC). The presence of VM in these LSCC cases contributed to progression of LSCC by promoting lymph node metastasis and served as an independent indicator of poor prognosis [102].
Future Direction
Since the process of VM involved the transdifferentiation of tumor cells into endothelial-like phenotypes, the genes associated with angiogenesis/vascular signaling (VE-Cadherin, VEGFR1 and 2, EphA2), embryonic signaling (Nodal, Notch, Snail, Slug) and hypoxia-related signaling (HIF-1α, Twist1) pathways have been implicated for VM as well [103]. Hypoxia seems to be one of the crucial inducers of VM [1], [104]. In the light of several experimental evidences coming from in-vitro studies, many studies have shown VM to occur in normoxia as well [105], including the results published from our lab in this manuscript. Several pro-angiogenic molecules such as VEGF and its cognate receptor VEGFR2 have been implicated in VM [106], [107]. In our studies, we have demonstrated that AAT accelerated VM. This led us to further probe into the mechanistic details of VM to search for better therapeutic targets. Studies from our lab have previously shown that HET0016, a selective inhibitor of 20-HETE synthesis, decreases pro-angiogenic factors and inhibits growth of TNBC in mice [108]. 20-HETE regulates the angiogenic functions of EPC in vitro and EPC-mediated angiogenesis in vivo and using a 20-HETE antagonist, 20-HEDGE, the proliferation of EPCs was negated [109]. In our very recent study, we found HET0016 as a novel therapeutic drug capable of inhibiting VM in vivo [84]. In the light of these interesting evidences from our lab, we are excited to look into the prospect of developing HET0016 as a novel therapeutic for VM and study the mechanistic details of the action of HET0016 in inhibiting VM at the molecular level. Since VM serves as an alternative neovascularization mechanism capable of providing vascular irrigation to tumors in the event of an AAT therapy attack, understanding the molecular mechanisms and delineating the process of VM at the cellular level is the need of the hour. Identifying VM in the growing tumors and focusing scientific inquiries into designing novel therapies to counter VM is the current exigency.
Acknowledgement
The study is supported by the grants from the National Institutes of Health, National Cancer Institute (R01CA160126) and Georgia Cancer Center startup funds. We also thank Dr. Bhagelu Achyut, Dr. Asm Iskander, Dr. Meenu Jain, Mohammad Rashid, Adarsh Shankar and Roxan Ara for their support and help.
References
- 1.Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM, Pe'er J, Trent JM, Meltzer PS, Hendrix MJ. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol. 1999;155(3):739–752. doi: 10.1016/S0002-9440(10)65173-5. [S0002–9440(10)65173–5 [pii]] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Folberg R, Maniotis AJ. Vasculogenic mimicry. APMIS. 2004;112(7–8):508–525. doi: 10.1111/j.1600-0463.2004.apm11207-0810.x. [APMapm11207–0810 [pii]] [DOI] [PubMed] [Google Scholar]
- 3.Vartanian AA. Signaling pathways in tumor vasculogenic mimicry. Biochemistry (Mosc) 2012;77(9):1044–1055. doi: 10.1134/S000629791209012X. [BCM77091258 [pii]] [DOI] [PubMed] [Google Scholar]
- 4.Clemente M, Perez-Alenza MD, Illera JC, Pena L. Histological, immunohistological, and ultrastructural description of vasculogenic mimicry in canine mammary cancer. Vet Pathol. 2010;47(2):265–274. doi: 10.1177/0300985809353167. [DOI] [PubMed] [Google Scholar]
- 5.Chen X, Maniotis AJ, Majumdar D, Pe'er J, Folberg R. Uveal melanoma cell staining for CD34 and assessment of tumor vascularity. Invest Ophthalmol Vis Sci. 2002;43(8):2533–2539. [PubMed] [Google Scholar]
- 6.Clarijs R, Otte-Holler I, Ruiter DJ, de Waal RM. Presence of a fluid-conducting meshwork in xenografted cutaneous and primary human uveal melanoma. Invest Ophthalmol Vis Sci. 2002;43(4):912–918. [PubMed] [Google Scholar]
- 7.Folberg R, Rummelt V, Parys-Van Ginderdeuren R, Hwang T, Woolson RF, Pe'er J, Gruman LM. The prognostic value of tumor blood vessel morphology in primary uveal melanoma. Ophthalmology. 1993;100(9):1389–1398. doi: 10.1016/s0161-6420(93)31470-3. [DOI] [PubMed] [Google Scholar]
- 8.Seftor RE, Seftor EA, Koshikawa N, Meltzer PS, Gardner LM, Bilban M, Stetler-Stevenson WG, Quaranta V, Hendrix MJ. Cooperative interactions of laminin 5 gamma2 chain, matrix metalloproteinase-2, and membrane type-1-matrix/metalloproteinase are required for mimicry of embryonic vasculogenesis by aggressive melanoma. Cancer Res. 2001;61(17):6322–6327. [PubMed] [Google Scholar]
- 9.Shirakawa K, Tsuda H, Heike Y, Kato K, Asada R, Inomata M, Sasaki H, Kasumi F, Yoshimoto M, Iwanaga T. Absence of endothelial cells, central necrosis, and fibrosis are associated with aggressive inflammatory breast cancer. Cancer Res. 2001;61(2):445–451. [PubMed] [Google Scholar]
- 10.Sood AK, Seftor EA, Fletcher MS, Gardner LM, Heidger PM, Buller RE, Seftor RE, Hendrix MJ. Molecular determinants of ovarian cancer plasticity. Am J Pathol. 2001;158(4):1279–1288. doi: 10.1016/S0002-9440(10)64079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sood AK, Fletcher MS, Zahn CM, Gruman LM, Coffin JE, Seftor EA, Hendrix MJ. The clinical significance of tumor cell-lined vasculature in ovarian carcinoma: implications for anti-vasculogenic therapy. Cancer Biol Ther. 2002;1(6):661–664. doi: 10.4161/cbt.316. [DOI] [PubMed] [Google Scholar]
- 12.Sharma N, Seftor RE, Seftor EA, Gruman LM, Heidger PM, Jr., Cohen MB, Lubaroff DM, Hendrix MJ. Prostatic tumor cell plasticity involves cooperative interactions of distinct phenotypic subpopulations: role in vasculogenic mimicry. Prostate. 2002;50(3):189–201. doi: 10.1002/pros.10048. [DOI] [PubMed] [Google Scholar]
- 13.Passalidou E, Trivella M, Singh N, Ferguson M, Hu J, Cesario A, Granone P, Nicholson AG, Goldstraw P, Ratcliffe C. Vascular phenotype in angiogenic and non-angiogenic lung non-small cell carcinomas. Br J Cancer. 2002;86(2):244–249. doi: 10.1038/sj.bjc.6600015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yue WY, Chen ZP. Does vasculogenic mimicry exist in astrocytoma? J Histochem Cytochem. 2005;53(8):997–1002. doi: 10.1369/jhc.4A6521.2005. [DOI] [PubMed] [Google Scholar]
- 15.McDonald DM, Munn L, Jain RK. Vasculogenic mimicry: how convincing, how novel, and how significant? Am J Pathol. 2000;156(2):383–388. doi: 10.1016/S0002-9440(10)64740-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fausto N. Vasculogenic mimicry in tumors. Fact or artifact? Am J Pathol. 2000;156(2):359. doi: 10.1016/S0002-9440(10)64738-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun B, Zhang S, Zhao X, Zhang W, Hao X. Vasculogenic mimicry is associated with poor survival in patients with mesothelial sarcomas and alveolar rhabdomyosarcomas. Int J Oncol. 2004;25(6):1609–1614. [PubMed] [Google Scholar]
- 18.Jain RK, di Tomaso E, Duda DG, Loeffler JS, Sorensen AG, Batchelor TT. Angiogenesis in brain tumours. Nat Rev Neurosci. 2007;8(8):610–622. doi: 10.1038/nrn2175. [nrn2175 [pii]] [DOI] [PubMed] [Google Scholar]
- 19.de Vries NA, Beijnen JH, Boogerd W, van Tellingen O. Blood-brain barrier and chemotherapeutic treatment of brain tumors. Expert Rev Neurother. 2006;6(8):1199–1209. doi: 10.1586/14737175.6.8.1199. [DOI] [PubMed] [Google Scholar]
- 20.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3(6):401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 21.Hardee ME, Zagzag D. Mechanisms of glioma-associated neovascularization. Am J Pathol. 2012;181(4):1126–1141. doi: 10.1016/j.ajpath.2012.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Palma M, Venneri MA, Roca C, Naldini L. Targeting exogenous genes to tumor angiogenesis by transplantation of genetically modified hematopoietic stem cells. Nat Med. 2003;9(6):789–795. doi: 10.1038/nm871. [DOI] [PubMed] [Google Scholar]
- 23.Gothert JR, Gustin SE, van Eekelen JA, Schmidt U, Hall MA, Jane SM, Green AR, Gottgens B, Izon DJ, Begley CG. Genetically tagging endothelial cells in vivo: bone marrow-derived cells do not contribute to tumor endothelium. Blood. 2004;104(6):1769–1777. doi: 10.1182/blood-2003-11-3952. [DOI] [PubMed] [Google Scholar]
- 24.Peters BA, Diaz LA, Polyak K, Meszler L, Romans K, Guinan EC, Antin JH, Myerson D, Hamilton SR, Vogelstein B. Contribution of bone marrow-derived endothelial cells to human tumor vasculature. Nat Med. 2005;11(3):261–262. doi: 10.1038/nm1200. [DOI] [PubMed] [Google Scholar]
- 25.Purhonen S, Palm J, Rossi D, Kaskenpaa N, Rajantie I, Yla-Herttuala S, Alitalo K, Weissman IL, Salven P. Bone marrow-derived circulating endothelial precursors do not contribute to vascular endothelium and are not needed for tumor growth. Proc Natl Acad Sci U S A. 2008;105(18):6620–6625. doi: 10.1073/pnas.0710516105. [0710516105 [pii]] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285(21):1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 27.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86(3):353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 28.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407(6801):242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 29.Holash J, Maisonpierre PC, Compton D, Boland P, Alexander CR, Zagzag D, Yancopoulos GD, Wiegand SJ. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;284(5422):1994–1998. doi: 10.1126/science.284.5422.1994. [DOI] [PubMed] [Google Scholar]
- 30.Forsyth PA, Wong H, Laing TD, Rewcastle NB, Morris DG, Muzik H, Leco KJ, Johnston RN, Brasher PM, Sutherland G. Gelatinase-A (MMP-2), gelatinase-B (MMP-9) and membrane type matrix metalloproteinase-1 (MT1-MMP) are involved in different aspects of the pathophysiology of malignant gliomas. Br J Cancer. 1999;79(11–12):1828–1835. doi: 10.1038/sj.bjc.6990291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brat DJ, Castellano-Sanchez AA, Hunter SB, Pecot M, Cohen C, Hammond EH, Devi SN, Kaur B, Van Meir EG. Pseudopalisades in glioblastoma are hypoxic, express extracellular matrix proteases, and are formed by an actively migrating cell population. Cancer Res. 2004;64(3):920–927. doi: 10.1158/0008-5472.can-03-2073. [DOI] [PubMed] [Google Scholar]
- 32.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16(9):4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vajkoczy P, Farhadi M, Gaumann A, Heidenreich R, Erber R, Wunder A, Tonn JC, Menger MD, Breier G. Microtumor growth initiates angiogenic sprouting with simultaneous expression of VEGF, VEGF receptor-2, and angiopoietin-2. J Clin Invest. 2002;109(6):777–785. doi: 10.1172/JCI14105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahmad SA, Liu W, Jung YD, Fan F, Wilson M, Reinmuth N, Shaheen RM, Bucana CD, Ellis LM. The effects of angiopoietin-1 and -2 on tumor growth and angiogenesis in human colon cancer. Cancer Res. 2001;61(4):1255–1259. [PubMed] [Google Scholar]
- 35.Hu B, Guo P, Fang Q, Tao HQ, Wang D, Nagane M, Huang HJ, Gunji Y, Nishikawa R, Alitalo K. Angiopoietin-2 induces human glioma invasion through the activation of matrix metalloprotease-2. Proc Natl Acad Sci U S A. 2003;100(15):8904–8909. doi: 10.1073/pnas.1533394100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanaka S, Mori M, Sakamoto Y, Makuuchi M, Sugimachi K, Wands JR. Biologic significance of angiopoietin-2 expression in human hepatocellular carcinoma. J Clin Invest. 1999;103(3):341–345. doi: 10.1172/JCI4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu Q, Stamenkovic I. Angiopoietin-2 is implicated in the regulation of tumor angiogenesis. Am J Pathol. 2001;158(2):563–570. doi: 10.1016/S0002-9440(10)63998-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hellstrom M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, Alva J, Nilsson AK, Karlsson L, Gaiano N. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445(7129):776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 39.Sawamiphak S, Seidel S, Essmann CL, Wilkinson GA, Pitulescu ME, Acker T, Acker-Palmer A. Ephrin-B2 regulates VEGFR2 function in developmental and tumour angiogenesis. Nature. 2010;465(7297):487–491. doi: 10.1038/nature08995. [DOI] [PubMed] [Google Scholar]
- 40.Li JL, Sainson RC, Shi W, Leek R, Harrington LS, Preusser M, Biswas S, Turley H, Heikamp E, Hainfellner JA. Delta-like 4 Notch ligand regulates tumor angiogenesis, improves tumor vascular function, and promotes tumor growth in vivo. Cancer Res. 2007;67(23):11244–11253. doi: 10.1158/0008-5472.CAN-07-0969. [DOI] [PubMed] [Google Scholar]
- 41.Li JL, Sainson RC, Oon CE, Turley H, Leek R, Sheldon H, Bridges E, Shi W, Snell C, Bowden ET. DLL4-Notch signaling mediates tumor resistance to anti-VEGF therapy in vivo. Cancer Res. 2011;71(18):6073–6083. doi: 10.1158/0008-5472.CAN-11-1704. [DOI] [PubMed] [Google Scholar]
- 42.Noguera-Troise I, Daly C, Papadopoulos NJ, Coetzee S, Boland P, Gale NW, Lin HC, Yancopoulos GD, Thurston G. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature. 2006;444(7122):1032–1037. doi: 10.1038/nature05355. [DOI] [PubMed] [Google Scholar]
- 43.Ridgway J, Zhang G, Wu Y, Stawicki S, Liang WC, Chanthery Y, Kowalski J, Watts RJ, Callahan C, Kasman I. Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature. 2006;444(7122):1083–1087. doi: 10.1038/nature05313. [DOI] [PubMed] [Google Scholar]
- 44.Vitolo D, Paradiso P, Uccini S, Ruco LP, Baroni CD. Expression of adhesion molecules and extracellular matrix proteins in glioblastomas: relation to angiogenesis and spread. Histopathology. 1996;28(6):521–528. doi: 10.1046/j.1365-2559.1996.d01-471.x. [DOI] [PubMed] [Google Scholar]
- 45.Friedlander M, Brooks PC, Shaffer RW, Kincaid CM, Varner JA, Cheresh DA. Definition of two angiogenic pathways by distinct alpha v integrins. Science. 1995;270(5241):1500–1502. doi: 10.1126/science.270.5241.1500. [DOI] [PubMed] [Google Scholar]
- 46.Gladson CL, Cheresh DA. Glioblastoma expression of vitronectin and the alpha v beta 3 integrin. Adhesion mechanism for transformed glial cells. J Clin Invest. 1991;88(6):1924–1932. doi: 10.1172/JCI115516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schnell O, Krebs B, Wagner E, Romagna A, Beer AJ, Grau SJ, Thon N, Goetz C, Kretzschmar HA, Tonn JC. Expression of integrin alphavbeta3 in gliomas correlates with tumor grade and is not restricted to tumor vasculature. Brain Pathol. 2008;18(3):378–386. doi: 10.1111/j.1750-3639.2008.00137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.DeLay M, Jahangiri A, Carbonell WS, Hu YL, Tsao S, Tom MW, Paquette J, Tokuyasu TA, Aghi MK. Microarray analysis verifies two distinct phenotypes of glioblastomas resistant to antiangiogenic therapy. Clin Cancer Res. 2012;18(10):2930–2942. doi: 10.1158/1078-0432.CCR-11-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ferrara N. VEGF and Intraocular Neovascularization: From Discovery to Therapy. Transl Vision Sci Technol. 2016;5(2):10. doi: 10.1167/tvst.5.2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Folkman J, Long DM, Jr., Becker FF. Growth and metastasis of tumor in organ culture. Cancer. 1963;16:453–467. doi: 10.1002/1097-0142(196304)16:4<453::aid-cncr2820160407>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 51.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 52.Mittal K, Ebos J, Rini B. Angiogenesis and the tumor microenvironment: vascular endothelial growth factor and beyond. Semin Oncol. 2014;41(2):235–251. doi: 10.1053/j.seminoncol.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 53.Bruno A, Pagani A, Magnani E, Rossi T, Noonan DM, Cantelmo AR, Albini A. Inflammatory angiogenesis and the tumor microenvironment as targets for cancer therapy and prevention. Cancer Treat Res. 2014;159:401–426. doi: 10.1007/978-3-642-38007-5_23. [DOI] [PubMed] [Google Scholar]
- 54.Samples J, Willis M, Klauber-DeMore N. Targeting Angiogenesis and the Tumor Microenvironment. Surg Oncol Clin N Am. 2013;22(4):629–639. doi: 10.1016/j.soc.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saharinen P, Eklund L, Pulkki K, Bono P, Alitalo K. VEGF and angiopoietin signaling in tumor angiogenesis and metastasis. Trends Mol Med. 2011;17(7):347–362. doi: 10.1016/j.molmed.2011.01.015. [S1471-4914(11)00025-6 [pii]] [DOI] [PubMed] [Google Scholar]
- 56.Rahman R, Smith S, Rahman C, Grundy R. Antiangiogenic therapy and mechanisms of tumor resistance in malignant glioma. J Oncol. 2010;2010:251231. doi: 10.1155/2010/251231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dietrich J, Norden AD, Wen PY. Emerging antiangiogenic treatments for gliomas - efficacy and safety issues. Curr Opin Neurol. 2008;21(6):736–744. doi: 10.1097/WCO.0b013e3283131370. [pii] [DOI] [PubMed] [Google Scholar]
- 58.Miller KD, Sweeney CJ, Sledge GW., Jr. Can tumor angiogenesis be inhibited without resistance? EXS. 2005;94:95–112. doi: 10.1007/3-7643-7311-3_7. [DOI] [PubMed] [Google Scholar]
- 59.Ali MM, Kumar S, Shankar A, Varma NR, Iskander AS, Janic B, Chwang WB, Jain R, Babajeni-Feremi A, Borin TF. Effects of tyrosine kinase inhibitors and CXCR4 antagonist on tumor growth and angiogenesis in rat glioma model: MRI and protein analysis study. Transl Oncol. 2013;6(6):660–669. doi: 10.1593/tlo.13559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ali MM, Janic B, Babajani-Feremi A, Varma NR, Iskander AS, Anagli J, Arbab AS. Changes in vascular permeability and expression of different angiogenic factors following anti-angiogenic treatment in rat glioma. PLoS One. 2010;5(1):e8727. doi: 10.1371/journal.pone.0008727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Batchelor TT, Duda DG, di Tomaso E, Ancukiewicz M, Plotkin SR, Gerstner E, Eichler AF, Drappatz J, Hochberg FH, Benner T. Phase II study of cediranib, an oral pan-vascular endothelial growth factor receptor tyrosine kinase inhibitor, in patients with recurrent glioblastoma. J Clin Oncol. 2010;28(17):2817–2823. doi: 10.1200/JCO.2009.26.3988. [JCO.2009.26.3988 [pii]] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kamoun WS, Ley CD, Farrar CT, Duyverman AM, Lahdenranta J, Lacorre DA, Batchelor TT, di Tomaso E, Duda DG, Munn LL. Edema control by cediranib, a vascular endothelial growth factor receptor-targeted kinase inhibitor, prolongs survival despite persistent brain tumor growth in mice. J Clin Oncol. 2009;27(15):2542–2552. doi: 10.1200/JCO.2008.19.9356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Neyns B, Sadones J, Chaskis C, Dujardin M, Everaert H, Lv S, Duerinck J, Tynninen O, Nupponen N, Michotte A. Phase II study of sunitinib malate in patients with recurrent high-grade glioma. J Neuro-Oncol. 2011;103(3):491–501. doi: 10.1007/s11060-010-0402-7. [DOI] [PubMed] [Google Scholar]
- 64.Reardon DA, Egorin MJ, Desjardins A, Vredenburgh JJ, Beumer JH, Lagattuta TF, Gururangan S, Herndon JE, II, Salvado AJ, Friedman HS. Phase I pharmacokinetic study of the vascular endothelial growth factor receptor tyrosine kinase inhibitor vatalanib (PTK787) plus imatinib and hydroxyurea for malignant glioma. Cancer. 2009;115(10):2188–2198. doi: 10.1002/cncr.24213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reardon DA, Vredenburgh JJ, Coan A, Desjardins A, Peters KB, Gururangan S, Sathornsumetee S, Rich JN, Herndon JE, Friedman HS. Phase I study of sunitinib and irinotecan for patients with recurrent malignant glioma. J Neuro-Oncol. 2011;105(3):621–627. doi: 10.1007/s11060-011-0631-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lieu C, Heymach J, Overman M, Tran H, Kopetz S. Beyond VEGF: inhibition of the fibroblast growth factor pathway and antiangiogenesis. Clin Cancer Res. 2011;17(19):6130–6139. doi: 10.1158/1078-0432.CCR-11-0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Conrad C, Friedman H, Reardon D, Provenzale J, Jackson E, Serajuddin H, Laurent D, Chen B, Yung WK. A phase I/II trial of single-agent PTK 787/ZK 222584 (PTK/ZK), a novel, oral angiogenesis inhibitor, in patients with recurrent glioblastoma multiforme (GBM) J Clin Oncol Off J Am Soc Clin Oncol. 2004;22(14_suppl):1512. [Google Scholar]
- 68.Reardon D, Friedman H, Yung WK, Brada M, Conrad C, Provenzale J, Jackson E, Serajuddin H, Chen B, Laurent D. A phase I/II trial of PTK787/ZK 222584 (PTK/ZK), a novel, oral angiogenesis inhibitor, in combination with either temozolomide or lomustine for patients with recurrent glioblastoma multiforme (GBM) J Clin Oncol Off J Am Soc Clin Oncol. 2004;22(14_suppl):1513. [Google Scholar]
- 69.Shaaban S, Alsulami M, Arbab SA, Ara R, Shankar A, Iskander A, Angara K, Jain M, Bagher-Ebadian H, Achyut BR. Targeting Bone Marrow to Potentiate the Anti-Tumor Effect of Tyrosine Kinase Inhibitor in Preclinical Rat Model of Human Glioblastoma. Int J Cancer Res. 2016;12(2):69–81. doi: 10.3923/ijcr.2016.69.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rosen LS. Clinical experience with angiogenesis signaling inhibitors: focus on vascular endothelial growth factor (VEGF) blockers. Cancer Control. 2002;9(2 Suppl.):36–44. doi: 10.1177/107327480200902S05. [DOI] [PubMed] [Google Scholar]
- 71.Norden AD, Young GS, Setayesh K, Muzikansky A, Klufas R, Ross GL, Ciampa AS, Ebbeling LG, Levy B, Drappatz J. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology. 2008;70(10):779–787. doi: 10.1212/01.wnl.0000304121.57857.38. [70/10/779 [pii]] [DOI] [PubMed] [Google Scholar]
- 72.Kreisl TN, Zhang W, Odia Y, Shih JH, Butman JA, Hammoud D, Iwamoto FM, Sul J, Fine HA. A phase II trial of single-agent bevacizumab in patients with recurrent anaplastic glioma. Neuro-Oncology. 2011;13(10):1143–1150. doi: 10.1093/neuonc/nor091. [nor091 [pii]] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Reardon DA, Desjardins A, Peters KB, Gururangan S, Sampson JH, McLendon RE, Herndon JE, II, Bulusu A, Threatt S, Friedman AH. Phase II study of carboplatin, irinotecan, and bevacizumab for bevacizumab naive, recurrent glioblastoma. J Neuro-Oncol. 2012;107(1):155–164. doi: 10.1007/s11060-011-0722-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang SY, Ke YQ, Lu GH, Song ZH, Yu L, Xiao S, Sun XL, Jiang XD, Yang ZL, Hu CC. Vasculogenic mimicry is a prognostic factor for postoperative survival in patients with glioblastoma. J Neuro-Oncol. 2013;112(3):339–345. doi: 10.1007/s11060-013-1077-7. [DOI] [PubMed] [Google Scholar]
- 75.Liu XM, Zhang QP, Mu YG, Zhang XH, Sai K, Pang JC, Ng HK, Chen ZP. Clinical significance of vasculogenic mimicry in human gliomas. J Neuro-Oncol. 2011;105(2):173–179. doi: 10.1007/s11060-011-0578-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Folberg R, Hendrix MJ, Maniotis AJ. Vasculogenic mimicry and tumor angiogenesis. Am J Pathol. 2000;156(2):361–381. doi: 10.1016/S0002-9440(10)64739-6. [S0002–9440(10)64739–6 [pii]] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhou Y, Fisher SJ, Janatpour M, Genbacev O, Dejana E, Wheelock M, Damsky CH. Human cytotrophoblasts adopt a vascular phenotype as they differentiate. A strategy for successful endovascular invasion? J Clin Invest. 1997;99(9):2139–2151. doi: 10.1172/JCI119387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Auguste P, Lemiere S, Larrieu-Lahargue F, Bikfalvi A. Molecular mechanisms of tumor vascularization. Crit Rev Oncol Hematol. 2005;54(1):53–61. doi: 10.1016/j.critrevonc.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 79.Liu J, Huang J, Yao WY, Ben QW, Chen DF, He XY, Li L, Yuan YZ. The origins of vacularization in tumors. Front Biosci. 2012;17:2559–2565. doi: 10.2741/4071. [DOI] [PubMed] [Google Scholar]
- 80.Zhang S, Zhang D, Wang Y, Zhao W, Guo H, Zhao X, Sun B. Morphologic research of microcirculation patterns in human and animal melanoma. Med Oncol. 2006;23(3):403–409. doi: 10.1385/MO:23:3:403. [DOI] [PubMed] [Google Scholar]
- 81.El Hallani S, Boisselier B, Peglion F, Rousseau A, Colin C, Idbaih A, Marie Y, Mokhtari K, Thomas J-L, Eichmann A. A new alternative mechanism in glioblastoma vascularization: tubular vasculogenic mimicry. Brain. 2010;133(4):973–982. doi: 10.1093/brain/awq044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hendrix MJ, Seftor EA, Hess AR, Seftor RE. Vasculogenic mimicry and tumour-cell plasticity: lessons from melanoma. Nat Rev Cancer. 2003;3(6):411–421. doi: 10.1038/nrc1092. [DOI] [PubMed] [Google Scholar]
- 83.Seftor EA, Meltzer PS, Kirschmann DA, Pe'er J, Maniotis AJ, Trent JM, Folberg R, Hendrix MJ. Molecular determinants of human uveal melanoma invasion and metastasis. Clin Exp Metastasis. 2002;19(3):233–246. doi: 10.1023/a:1015591624171. [DOI] [PubMed] [Google Scholar]
- 84.Angara K, Rashid MH, Shankar A, Ara R, Iskander A, Borin TF, Jain M, Achyut BR, Arbab AS. Vascular mimicry in glioblastoma following anti-angiogenic and anti-20-HETE therapies. Histol Histopathol. 2016:11856. doi: 10.14670/HH-11-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Scully S, Francescone R, Faibish M, Bentley B, Taylor SL, Oh D, Schapiro R, Moral L, Yan W, Shao R. Transdifferentiation of glioblastoma stem-like cells into mural cells drives vasculogenic mimicry in glioblastomas. J Neurosci. 2012;32(37):12950–12960. doi: 10.1523/JNEUROSCI.2017-12.2012. [32/37/12950 [pii]] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, Fiocco R, Foroni C, Dimeco F, Vescovi A. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64(19):7011–7021. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 87.Ricci-Vitiani L, Pallini R, Biffoni M, Todaro M, Invernici G, Cenci T, Maira G, Parati EA, Stassi G, Larocca LM. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature. 2010;468(7325):824–828. doi: 10.1038/nature09557. [DOI] [PubMed] [Google Scholar]
- 88.Soda Y, Marumoto T, Friedmann-Morvinski D, Soda M, Liu F, Michiue H, Pastorino S, Yang M, Hoffman RM, Kesari S. Transdifferentiation of glioblastoma cells into vascular endothelial cells. Proc Natl Acad Sci U S A. 2011;108(11):4274–4280. doi: 10.1073/pnas.1016030108. [1016030108 [pii]] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang R, Chadalavada K, Wilshire J, Kowalik U, Hovinga KE, Geber A, Fligelman B, Leversha M, Brennan C, Tabar V. Glioblastoma stem-like cells give rise to tumour endothelium. Nature. 2010;468(7325):829–833. doi: 10.1038/nature09624. [DOI] [PubMed] [Google Scholar]
- 90.Janic B, Guo AM, Iskander AS, Varma NR, Scicli AG, Arbab AS. Human cord blood-derived AC133+ progenitor cells preserve endothelial progenitor characteristics after long term in vitro expansion. PLoS One. 2010;5(2):e9173. doi: 10.1371/journal.pone.0009173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.He H, Niu CS, Li MW. Correlation between glioblastoma stem-like cells and tumor vascularization. Oncol Rep. 2012;27(1):45–50. doi: 10.3892/or.2011.1484. [DOI] [PubMed] [Google Scholar]
- 92.Chiao MT, Yang YC, Cheng WY, Shen CC, Ko JL. CD133+ glioblastoma stem-like cells induce vascular mimicry in vivo. Curr Neurovasc Res. 2011;8(3):210–219. doi: 10.2174/156720211796558023. [DOI] [PubMed] [Google Scholar]
- 93.Mao XG, Xue XY, Wang L, Zhang X, Yan M, Tu YY, Lin W, Jiang XF, Ren HG, Zhang W. CDH5 is specifically activated in glioblastoma stemlike cells and contributes to vasculogenic mimicry induced by hypoxia. Neuro-Oncology. 2013;15(7):865–879. doi: 10.1093/neuonc/not029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dunleavey JM, Xiao L, Thompson J, Kim MM, Shields JM, Shelton SE, Irvin DM, Brings VE, Ollila DW, Brekken RA. Vascular channels formed by subpopulations of PECAM1+ melanoma cells. Nat Commun. 2014;5:5200. doi: 10.1038/ncomms6200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cao Z, Bao M, Miele L, Sarkar FH, Wang Z, Zhou Q. Tumour vasculogenic mimicry is associated with poor prognosis of human cancer patients: a systemic review and meta-analysis. Eur J Cancer. 2013;49(18):3914–3923. doi: 10.1016/j.ejca.2013.07.148. [DOI] [PubMed] [Google Scholar]
- 96.Ren K, Yao N, Wang G, Tian L, Ma J, Shi X, Zhang L, Zhang J, Zhou X, Zhou G. Vasculogenic mimicry: a new prognostic sign of human osteosarcoma. Hum Pathol. 2014;45(10):2120–2129. doi: 10.1016/j.humpath.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 97.Liang J, Yang B, Cao Q, Wu X. Association of Vasculogenic Mimicry Formation and CD133 Expression with Poor Prognosis in Ovarian Cancer. Gynecol Obstet Investig. 2016;81(6):529–536. doi: 10.1159/000445747. [DOI] [PubMed] [Google Scholar]
- 98.Li M, Gu Y, Zhang Z, Zhang S, Zhang D, Saleem AF, Zhao X, Sun B. Vasculogenic mimicry: a new prognostic sign of gastric adenocarcinoma. Pathol Oncol Res. 2010;16(2):259–266. doi: 10.1007/s12253-009-9220-7. [DOI] [PubMed] [Google Scholar]
- 99.Gao Y, Zhao XL, Gu Q, Wang JY, Zhang SW, Zhang DF, Wang XH, Zhao N, Gao YT, Sun BC. Correlation of vasculogenic mimicry with clinicopathologic features and prognosis of ovarian carcinoma. Zhonghua Bing Li Xue Za Zhi. 2009;38(9):585–589. [PubMed] [Google Scholar]
- 100.Huang M, Ke Y, Sun X, Yu L, Yang Z, Zhang Y, Du M, Wang J, Liu X, Huang S. Mammalian target of rapamycin signaling is involved in the vasculogenic mimicry of glioma via hypoxia-inducible factor-1alpha. Oncol Rep. 2014;32(5):1973–1980. doi: 10.3892/or.2014.3454. [DOI] [PubMed] [Google Scholar]
- 101.Liu WB, Xu GL, Jia WD, Li JS, Ma JL, Chen K, Wang ZH, Ge YS, Ren WH, Yu JH. Prognostic significance and mechanisms of patterned matrix vasculogenic mimicry in hepatocellular carcinoma. Med Oncol. 2011;28(Suppl. 1):S228–S238. doi: 10.1007/s12032-010-9706-x. [DOI] [PubMed] [Google Scholar]
- 102.Wang W, Lin P, Han C, Cai W, Zhao X, Sun B. Vasculogenic mimicry contributes to lymph node metastasis of laryngeal squamous cell carcinoma. J Exp Clin Cancer Res. 2010;29:60. doi: 10.1186/1756-9966-29-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kirschmann DA, Seftor EA, Hardy KM, Seftor RE, Hendrix MJ. Molecular pathways: vasculogenic mimicry in tumor cells: diagnostic and therapeutic implications. Clin Cancer Res. 2012;18(10):2726–2732. doi: 10.1158/1078-0432.CCR-11-3237. [1078–0432.CCR-11-3237 [pii]] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Arbab AS, Jain M, Achyut BR. Vascular Mimicry: The Next Big Glioblastoma Target. Biochem Physiol. 2015;4(3) doi: 10.4172/2168-9652.1000e140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Du J, Sun B, Zhao X, Gu Q, Dong X, Mo J, Sun T, Wang J, Sun R, Liu Y. Hypoxia promotes vasculogenic mimicry formation by inducing epithelial-mesenchymal transition in ovarian carcinoma. Gynecol Oncol. 2014;133(3):575–583. doi: 10.1016/j.ygyno.2014.02.034. [DOI] [PubMed] [Google Scholar]
- 106.Francescone R, Scully S, Bentley B, Yan W, Taylor SL, Oh D, Moral L, Shao R. Glioblastoma-derived tumor cells induce vasculogenic mimicry through Flk-1 protein activation. J Biol Chem. 2012;287(29):24821–24831. doi: 10.1074/jbc.M111.334540. [M111.334540 [pii]] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yao X, Ping Y, Liu Y, Chen K, Yoshimura T, Liu M, Gong W, Chen C, Niu Q, Guo D. Vascular endothelial growth factor receptor 2 (VEGFR-2) plays a key role in vasculogenic mimicry formation, neovascularization and tumor initiation by Glioma stem-like cells. PLoS One. 2013;8(3):e57188. doi: 10.1371/journal.pone.0057188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Borin TF, Zuccari DA, Jardim-Perassi BV, Ferreira LC, Iskander AS, Varma NR, Shankar A, Guo AM, Scicli G, Arbab AS. HET0016, a Selective Inhibitor of 20-HETE Synthesis, Decreases Pro-Angiogenic Factors and Inhibits Growth of Triple Negative Breast Cancer in Mice. PLoS One. 2014;9(12):e116247. doi: 10.1371/journal.pone.0116247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen L, Ackerman R, Saleh M, Gotlinger KH, Kessler M, Mendelowitz LG, Falck JR, Arbab AS, Scicli AG, Schwartzman ML. 20-HETE Regulates the Angiogenic Functions of Human Endothelial Progenitor Cells and Contributes to Angiogenesis In Vivo. J Pharmacol Exp Ther. 2014;348(3):442–451. doi: 10.1124/jpet.113.210120. [DOI] [PMC free article] [PubMed] [Google Scholar]