Abstract
Background
The epidemiology of pediatric septic arthritis, such as annual hospitalization rates and disparities by age, gender, race/ethnicity, and socioeconomic status, remains unclear.
Methods
We obtained hospital discharge records of patients under the age of 20 years with septic arthritis from the kids inpatient database for 2006, 2009 and 2012. We weighted the records to estimate the number of hospitalizations in the US and calculated the annual rates of hospitalization due to septic arthritis. We used multivariable logistic regression to assess risk factors associated with comorbidities of osteomyelitis and bacteremia/septicemia.
Results
Overall annual hospitalization rates showed a decreasing trend (4.23, 3.64, and 3.28 per 100,000 children in 2006, 2009, and 2012, respectively). Children who were male, white or black, and between the ages of 0–4 years were more likely to have higher hospitalization rates than others. The proportions of hospitalizations were high among children living in lower-income areas. Large joints at the lower limbs were the most frequently affected sites of infection. Infections of large joints and age category of 10–14 years were the factors that were significantly associated with comorbidities of osteomyelitis and bacteremia/septicemia.
Conclusions
We demonstrated a change in the epidemiological patterns of pediatric septic arthritis and identified risk factors associated with comorbidities of osteomyelitis and bacteremia/septicemia.
Keywords: children, septic arthritis, kids inpatient database
Abbreviations: SpA, Septic arthritis; KID, Kids Inpatient Database; AHRQ, the Agency for Healthcare Research and Quality; HCUP, the Healthcare Cost and Utilization Projects; ICD, 9CM, International Classification of Disease, Ninth Revision, Clinical Modification
1. Introduction
Septic arthritis (SpA) of bacterial origin accounts for 6.5% of all childhood arthritis cases.1 The epidemiology of pediatric SpA has continued to change and be influenced by many factors, such as the advent of antibiotics and vaccines, pattern of causative organisms, and sophisticated diagnostic tools, including bone scans and magnetic resonance imaging.2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13
Several studies with varying numbers of pediatric patients with SpA have revealed that SpA is more common among boys8, 9, 11 and younger children.12, 13 The joints of the lower extremities, such as the knee, hip, and ankle, were the most commonly affected sites of infection.5, 9, 13
However, these previous studies were limited because they were conducted within a few hospital catchment areas and, thus, the sample sizes were relatively small.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13 Some of these studies were published between the 1980s and 1990s, furthermore, no large multicenter study or national survey on SpA-associated hospitalizations in children has been conducted. In addition, the potential effects of race/ethnicity and socioeconomic status on SpA remain unclear.
The present study aimed to investigate SpA-associated hospitalizations among children based on patient and hospital characteristics, using a national representative database in the US. In addition, we ascertained factors that were associated with SpA and the comorbidities of osteomyelitis and bacteremia/septicemia.
2. Materials and methods
2.1. Study population
We conducted a serial, cross-sectional analysis among hospitalized patients with SpA under the age of 20 years using the national representative Kids Inpatient Database (KID) for the years 2006, 2009 and 2012. The data are compiled by the Agency for Healthcare Research and Quality (AHRQ).14, 15 KID is a large national all-payer hospital pediatric discharge database. KID is designed to generate robust national estimates of annual pediatric hospitalizations, and to present hospital use, outcome and cost for children aged 20 years or younger.14, 15 The sampling flame of KID was constructed using all US short-term, non-federal, general and specialty hospitals that participated in the Healthcare Cost and Utilization Projects (HCUP). KID had more than 3 million pediatric discharge records per year from 38 states in 2006, and 44 states in 2009 and 2012. Discharge-level weights variables (DISCWT) were provided with KID, and we used them to obtain the national estimates of annual hospitalization rates in the US.
Hospitalization discharge records were obtained from KID in 2006, 2009 and 2012. Hospitalizations with SpA of bacterial origin were identified using International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9CM) code (pyogenic arthritis: 711.0×) in the primary diagnosis field. Hospitalizations due to SpA were subdivided into the affected sites of infection using ICD9-CM codes: shoulder (711.01), elbow (711.02), wrist (711.03), hand (711.04), pelvic region and hip (711.05), knee (711.06), ankle and foot (711.07), and others (711.00 and 71108). Multiple sites infections were defined if the patients had two or more sites of infections or had ICD-9CM code of multiple sites (711.09). Comorbidities of osteomyelitis (730.1×, 730.2×, 730.8×) and bacteremia/septicemia (790.xx)/(038.xx) were also identified.
2.2. Measurements of variables
Patient characteristics consisted of age categories (0–4 years, 5–9 years, 10–14 years, or 15–19 years), gender (male or female), race/ethnicity (black, Hispanic, white, Asian and Pacific Islanders, or others), primary payer information (private, Medicare/Medicaid, self-pay or no insurance/information), and income quartiles for counties of residence for patient’s zip code (very low, low, high or very high: see detail in supplement 1).
Hospital characteristics included hospital location (Northeast, Midwest, South, or West), type of hospital (urban teaching, urban nonteaching, or rural), and bed size (small, medium or large).16, 17, 18 The definitions of bed size are different across the hospital locations. For instance, in Northeast region, hospital bed size was classified small if rural hospital had 1 to 49 beds, urban nonteaching hospital had 1 to 124 beds, or urban teaching hospital had fewer than 250 beds. The bed size was considered medium if rural hospital had 50 to 99 beds, urban nonteaching hospital had 125 to 199 beds, and urban teaching hospital had 250 to 424 beds. A large-size hospital could be defined by ≥100 beds for rural hospital, ≥200 beds for urban nonteaching hospital, and ≥425 beds for urban teaching hospital (see supplement 2).16, 17, 18
2.3. Outcomes
The outcomes of interest were annual hospitalization rates due to SpA of bacterial origin with respect to age, gender, and race/ethnicity. The annual hospitalization rates were calculated: the number of hospitalizations as the numerator and the population of each subgroup as the denominator derived from the US census in 2006, 2009 and 2012.19, 20, 21, 22 We also identified risk factors that were associated with comorbidities of osteomyelitis and bacteremia/septicemia.
2.4. Statistical analysis
The number of SpA-associated hospitalizations and the annual hospitalization rates were calculated using DSCWT. We calculated 95% confidence intervals for the hospitalization rates, assuming a normal approximation to a Poisson distribution. Multivariable logistic regression was used to identify risk factors that were associated with osteomyelitis or bacteremia/septicemia. Data were analyzed using STATA software version 14.1 (StataCorp LP, Texas, USA).
3. Results
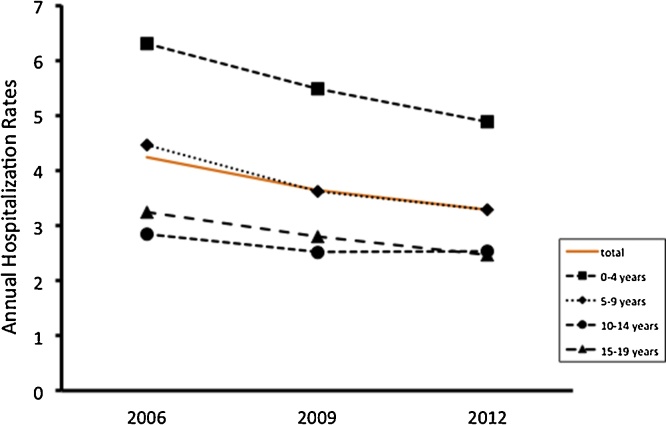
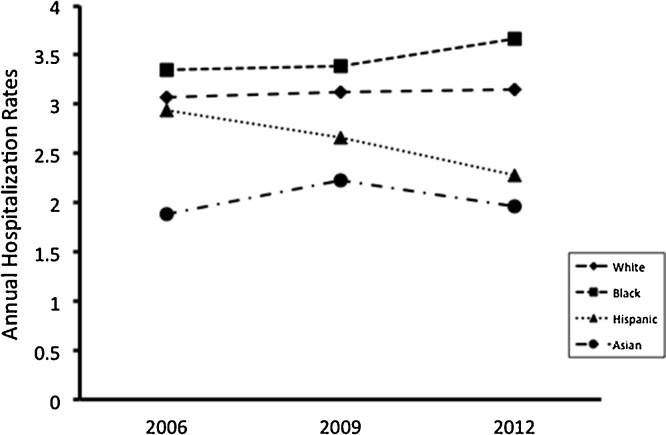
Fig. 1 showed a decreasing trend of annual hospitalization rates due to pediatric SpA with respect to the age categories. The highest hospitalization rates were children aged between 0 and 4 years. Fig. 2 showed the differences of annual hospitalization rates by race/ethnicity. White children had the highest hospitalization rates, whereas Asian children did the lowest rates. The rates among Hispanic children decreased from 2006 to 2012. Male children had higher hospitalization rates than female children (Table 1).
Fig 1.
Annual hospitalization rates due to pediatric SpA with respect to the age categories.
Fig. 2.
Annual hospitalization rates by race/ethnicity.
Table 1.
The number of hospitalization and annual hospitalization rate.
| 2012 |
2009 |
2006 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Na | Rateb | 95%CI | N | Rate | 95%CI | N | Rate | 95%CI | |
| Total | 2701 | 3.28 | 3.16−3.41 | 3006 | 3.64 | 3.51−3.77 | 3453 | 4.23 | 4.09−4.38 |
| Age | |||||||||
| 0–4 | 982 | 4.88 | 4.58−5.20 | 1163 | 5.49 | 5.07−5.93 | 1284 | 6.31 | 5.86−6.77 |
| 5–9 | 669 | 3.28 | 3.05−3.52 | 733 | 3.62 | 3.33−3.93 | 878 | 4.47 | 4.15−4.81 |
| 10–14 | 522 | 2.53 | 2.32−2.77 | 501 | 2.52 | 2.28−2.78 | 587 | 2.84 | 2.54−3.16 |
| 15–19 | 522 | 2.46 | 2.20−2.74 | 595 | 2.80 | 2.51−3.12 | 681 | 3.25 | 2.91−3.63 |
| Gender | |||||||||
| Male | 1704 | 4.15 | 4.00−4.29 | 1841 | 4.37 | 4.21−4.52 | 2168 | 5.21 | 5.01−5.39 |
| Female | 996 | 2.47 | 2.33−2.62 | 1132 | 2.80 | 2.64−2.97 | 1240 | 3.11 | 2.91−3.31 |
| Age Group by Gender | |||||||||
| Male | |||||||||
| 0–4 | 551 | 5.36 | 4.87−5.88 | 659 | 6.08 | 5.46−6.73 | 746 | 7.16 | 6.54−7.81 |
| 5–9 | 384 | 3.74 | 3.38−4.12 | 406 | 3.92 | 3.52−4.36 | 507 | 5.05 | 4.57−5.57 |
| 10–14 | 366 | 3.56 | 3.20−3.96 | 332 | 3.26 | 2.87−3.69 | 410 | 3.88 | 3.44−4.36 |
| 15–19 | 401 | 3.90 | 3.48−4.36 | 440 | 4.08 | 3.61−4.59 | 498 | 4.69 | 4.16−5.26 |
| Female | |||||||||
| 0–4 | 431 | 4.38 | 4.02−4.75 | 500 | 4.83 | 4.37−5.30 | 539 | 5.42 | 4.89−5.95 |
| 5–9 | 286 | 2.86 | 2.54−3.20 | 310 | 3.14 | 2.75−3.56 | 346 | 3.61 | 3.17−4.08 |
| 10–14 | 156 | 1.55 | 1.31−1.83 | 169 | 1.74 | 1.47−2.05 | 175 | 1.73 | 1.39−2.14 |
| 15–19 | 121 | 1.17 | 0.95−1.43 | 153 | 1.47 | 1.19−1.79 | 179 | 1.74 | 1.43−2.10 |
| Race/Ethnicity | |||||||||
| White | 1369 | 3.15 | 2.92−3.38 | 1403 | 3.13 | 2.87−3.39 | 1425 | 3.07 | 2.81−3.37 |
| Black | 410 | 3.66 | 3.16−4.18 | 397 | 3.39 | 2.92−3.92 | 397 | 3.35 | 2.76−4.05 |
| Hispanic | 448 | 2.28 | 1.90−2.70 | 499 | 2.66 | 2.22−3.18 | 499 | 2.94 | 2.41−3.56 |
| Asianc | 74 | 1.96 | 1.42−2.69 | 83 | 2.23 | 1.68−2.96 | 64 | 1.88 | 1.35−2.61 |
N: The number of hospitalization per year.
Rate: Annual hospitalization rate (per 100,000 children).
Asian: Asian and Pacific Islander.
Patient socioeconomic status and hospital characteristics were summarized in Table 2. Children living in areas of low or very low median household income by patient ZIP Code were more likely to be hospitalized. Approximately ninety percent of patients had primary payer information of private or Medicare/Medicaid. The highest proportions of hospital characteristics were urban teaching hospital with large bed size in West.
Table 2.
Patients’ socioeconomic status (primary payer information and median household income by patients’ ZIP Code) and Hospital characteristics (hospital region, type, and bed size).
| 2012 |
2009 |
2006 |
||||
|---|---|---|---|---|---|---|
| N | Percent | N | Percent | N | Percent | |
| Total | 2701 | 100 | 3006 | 100 | 3453 | 100 |
| Median Household income by patient Zip Code | ||||||
| Very Low | 742 | 27.8 | 861 | 29.2 | 876 | 26.0 |
| Low | 644 | 24.1 | 749 | 25.4 | 886 | 26.3 |
| High | 646 | 24.2 | 728 | 24.7 | 762 | 22.6 |
| Very High | 637 | 23.9 | 610 | 20.7 | 847 | 25.1 |
| Primary Payer Information | ||||||
| Private | 1337 | 49.5 | 1563 | 52.0 | 1850 | 53.6 |
| Medicare/Medicaid | 1137 | 42.1 | 1169 | 38.9 | 1268 | 36.7 |
| Self/No insurance | 227 | 8.4 | 273 | 9.1 | 336 | 9.7 |
| Hospital Region | ||||||
| Northeast | 409 | 15.1 | 425 | 14.2 | 435 | 12.6 |
| Midwest | 561 | 20.8 | 638 | 21.2 | 799 | 23.1 |
| West | 1129 | 41.8 | 1218 | 40.5 | 1468 | 42.5 |
| South | 601 | 22.3 | 724 | 24.1 | 751 | 21.7 |
| Hospital Type | ||||||
| Urban Teaching | 2101 | 77.8 | 1835 | 69.9 | 2257 | 67.3 |
| Urban nonteaching | 465 | 17.2 | 417 | 22.7 | 848 | 25.3 |
| Rural | 135 | 5.0 | 31 | 7.4 | 251 | 7.5 |
| Hospital Size | ||||||
| Large | 1763 | 65.3 | 1553 | 59.2 | 1871 | 55.8 |
| Medium | 667 | 24.7 | 769 | 29.3 | 997 | 29.7 |
| Small | 271 | 10.0 | 303 | 11.5 | 488 | 14.5 |
Table 3 describes the proportions of the affected sites of infection and comorbidities of osteomyelitis or bacteremia/septicemia. The knee and hip/pelvic joints were the most frequently affected sites of infection. Approximately 10%–13% of hospitalized children with SpA had osteomyelitis, and 9%−11% of children with SpA had bacteremia/septicemia.
Table 3.
Sites of infections and Comorbidities of osteomyelitis and bacteremia/septicemia.
| 2012 |
2009 |
2006 |
||||
|---|---|---|---|---|---|---|
| Number | Percent | Number | Percent | Number | Percent | |
| Total | 2701 | 100 | 3006 | 100 | 3453 | 100 |
| Site of infection | ||||||
| Knee | 1126 | 41.7 | 1237 | 41.1 | 1527 | 44.2 |
| Hip/Pelvic | 354 | 31.4 | 982 | 32.7 | 1037 | 30.0 |
| Ankle/Foot | 238 | 8.8 | 265 | 8.8 | 347 | 10.0 |
| Elbow | 189 | 7.0 | 184 | 6.1 | 184 | 5.3 |
| Hand | 113 | 4.2 | 153 | 5.1 | 166 | 4.8 |
| Shoulder | 104 | 3.8 | 109 | 3.6 | 102 | 3.0 |
| Wrist | 47 | 1.7 | 57 | 1.9 | 49 | 1.4 |
| Other | 38 | 1.4 | 31 | 1.0 | 45 | 1.3 |
| Multiple site | 49 | 1.8 | 17 | 0.6 | 25 | 0.7 |
| Comorbidity | ||||||
| Osteomyelitis | 351 | 13.0 | 392 | 13.0 | 364 | 10.6 |
| Bacteremia/Septicemia | 301 | 11.2 | 300 | 10.0 | 318 | 9.2 |
We used multivariable logistic regression to analyze the association between age categories, the affected sites of infection and the presence of osteomyelitis (Table 4). Crude analysis showed that age categories of 10–14 years and 15–19 years, and septic arthritis at hip/pelvic, ankle/foot, elbow and shoulder were significantly associated with osteomyelitis. After adjusting for gender, race/ethnicity, socioeconomic status, and hospital characteristics, the odds ratio of osteomyelitis among children who aged between 10 and 14 years, and who had septic arthritis at hip/pelvic, ankle/foot, elbow, and shoulder, remained statistically significant.
Table 4.
Multivariable logistic regression for the association between age categories, site of infection and the presence of osteomyelitis.
| Crude Analysis |
Adjusted Analysis |
|||||
|---|---|---|---|---|---|---|
| OR | 95%CI | p-value | OR | 95%CI | p-value | |
| Age Category | ||||||
| 0–4 year | – | – | – | – | – | – |
| 5–9 year | 0.91 | 0.74−1.13 | 0.41 | 1.00 | 0.80−1.26 | 0.96 |
| 10–14 year | 1.50 | 1.20−1.87 | <0.001 | 1.68 | 1.31−2.13 | <0.001 |
| 15–19 year | 0.63 | 0.47−0.83 | 0.001 | 0.79 | 0.58−1.06 | 0.12 |
| Site of infection | ||||||
| Knee | – | – | – | – | – | – |
| Hip/Pelvic | 1.51 | 1.22−1.86 | <0.001 | 1.48 | 1.18−1.85 | 0.001 |
| Ankle/Foot | 3.53 | 2.76−4.52 | <0.001 | 3.39 | 2.61−4.39 | <0.001 |
| Elbow | 3.81 | 2.88−5.05 | <0.001 | 3.69 | 2.72−5.01 | <0.001 |
| Hand | 0.79 | 0.43−1.45 | 0.44 | 0.57 | 0.29−1.13 | 0.11 |
| Shoulder | 4.12 | 2.90−5.84 | <0.001 | 3.82 | 2.55−5.73 | <0.001 |
| Wrist | 1.27 | 0.59−2.73 | 0.55 | 1.25 | 0.55−2.82 | 0.59 |
| Other | 2.30 | 1.54−5.09 | 0.001 | 2.73 | 1.50−4.99 | 0.001 |
| Multiple site | 0.93 | 0.44−1.97 | 0.86 | 0.79 | 0.35−1.78 | 0.56 |
Multivariable logistic regression was conducted to ascertain the association between age categories, the affected sites of infection and the presence of bacteremia/septicemia (Table 5). In crude analysis, age category of 10–14 years and septic arthritis at hip/pelvic, ankle/foot, hand or shoulder were significantly related to the presence of bacteremia/septicemia. After adjusting for covariates, the same factors (10–14 years of age, and hip/pelvic, ankle/foot, hand or shoulder arthritis of bacterial origin) were significantly associated with bacteremia/septicemia.
Table 5.
Multivariable logistic regression for the association between age categories, site of infection and the presence of bacteremia/septicemia.
| Crude Analysis |
Adjusted Analysis |
|||||
|---|---|---|---|---|---|---|
| OR | 95%CI | p-value | OR | 95%CI | p-value | |
| Age Category | ||||||
| 0–4 year | – | – | – | – | – | – |
| 5–9 year | 1.16 | 0.93−1.43 | 0.19 | 1.30 | 1.03−1.65 | 0.029 |
| 10–14 year | 1.99 | 1.59−2.48 | <0.001 | 2.16 | 1.70−2.73 | <0.001 |
| 15–19 year | 0.89 | 0.65−1.21 | 0.44 | 0.98 | 0.70−1.38 | 0.925 |
| Site of infection | ||||||
| Knee | – | – | – | – | – | – |
| Hip/Pelvic | 2.72 | 2.24−3.32 | <0.001 | 2.68 | 2.17−3.30 | 0.001 |
| Ankle/Foot | 1.83 | 1.34−2.49 | <0.001 | 1.71 | 1.22−2.39 | 0.002 |
| Elbow | 1.15 | 0.71−1.85 | 0.569 | 1.04 | 0.62−1.74 | 0.877 |
| Hand | 0.11 | 0.03−0.43 | 0.002 | 0.10 | 0.03−0.43 | 0.002 |
| Shoulder | 4.07 | 2.81−5.89 | <0.001 | 4.08 | 2.76−6.03 | <0.001 |
| Wrist | 1.41 | 0.68−2.94 | 0.36 | 1.25 | 0.57−2.73 | 0.58 |
| Other | 2.13 | 1.04−4.36 | 0.038 | 2.14 | 1.03−4.56 | 0.04 |
| Multiple site | 1.80 | 0.88−3.68 | 0.109 | 1.42 | 0.64−3.14 | 0.38 |
4. Discussion
We investigated the annual hospitalization rates due to pediatric SpA and identified the risk factors that were associated with comorbidities of osteomyelitis and bacteremia/septicemia based on data from the national representative KID for 2006, 2009, and 2012. Annual hospitalization rates showed decreasing trends. Children who were male, young, white or black, or lived in lower-income areas had higher annual hospitalization rates than others. The most commonly affected sites of infection were large joints at the lower limbs. Age category of 10–14 years and infection of the large joints except for knee and wrist were significantly associated with comorbidity of osteomyelitis. Age categories of 5–9 years and 10–14 years and infection of the hip/pelvic, ankle/foot, hand, and shoulder joints were significantly related to comorbidity of bacteremia/septicemia.
Several studies have investigated the frequency of SpA; however, the results have been inconsistent.3, 4, 5, 23 Retrospective studies in the US reported that the annual incidence rates of SpA were estimated to be 1.07 cases per 100,000 children between 2002 and 20043 and in 1982.4 However, our estimates of the annual rates ranged from 4.23 to 3.26 cases per 100,000 children, showing a decreasing trend, and the rates were much higher than those previously reported. The previous studies were conducted with relatively small sample sizes in small areas, and they may have underestimated the rates that should have been observed.
Most studies supported that SpA is more common in boys than in girls,8, 9, 11 and that SpA commonly affects younger children.12, 13 Our study results demonstrated that patients between the age of 0–4 years and males had higher rates of SpA, which was consistent with the results of the previous studies.
Few studies have examined the effect of race/ethnicity and socioeconomic status on the incidence or hospitalization rates of SpA. We revealed that white and black children had higher rates than Hispanic or Asian/Pacific Islander children. Furthermore, children living in lower-income areas were more likely to suffer from SpA than those living in higher-income areas. Thus, race/ethnicity and socioeconomic status may also play important roles in the incidence and hospitalization rates of SpA.
Osteomyelitis frequently accompanies SpA, and the frequency of SpA with osteomyelitis has been reported to be 12%–26%.5, 9 Our study showed a similar frequency of SpA with osteomyelitis (10.6%–13%). A few studies showed that the frequency of bacteremia/septiceimia among children with SpA was 33%—46%; however our results showed a much lower frequency (10%) than that, which may be due to preceding use of antibiotics and potential miscoding of ICD-9CM of bacteremia/septicemia.
Patients with infections of large joints and in the age category of 10–14 years had higher odds of osteomyelitis and bacteremia/septicemia. Possible reasons for these results may include the amount of blood supply to the large joints, the growth of long bones during puberty, or trauma and extra articular infection before the onset of SpA.
There are some limitations to our study. The number of hospitalizations and SpA may have been underestimated because of possible misclassification and miscoding of ICD-9CM. The detailed clinical information, such as patient symptoms, laboratory data and prognosis, were unknown. Patients with SpA may have been hospitalized repeatedly during the same episode, and we could not report the recurrence rates or the intervals of the recurrence.
The strength of this study was the use of a national representative pediatric inpatient database. We were able to calculate more robust estimates of the characteristics of SpA than the previous studies were able do. To the best of our knowledge, this is the first report demonstrating the characteristics of pediatric SpA at a national level.
In conclusion, we obtained results that were different from those of the previous studies and may be more representative of the national picture of pediatric SpA with respect to age, gender, race/ethnicity and socioeconomic status. Age and sites of infection were the factors that played important roles in the development of the comorbidities of osteomyelitis and bacteremia/septicemia.
Declaration of funding sources
The authors have no research or project support, including internal funding.
Conflict of interest
The authors have no conflict of interest relevant to this article to disclose.
Acknowledgements
None.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jor.2017.06.004.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Kunnamo I., Kallio P., Pelkonen P., Hovi T. Clinical signs and laboratory tests in the differential diagnosis of arthritis in children. Am J Dis Child. 1987;141:34–40. doi: 10.1001/archpedi.1987.04460010034018. [DOI] [PubMed] [Google Scholar]
- 2.Goergens E.D., McEvoy A., Watson M., Barrett I.R. Acute osteomyelitis and septic arthritis in children. J Paediatr Child Health. 2005;41:59–62. doi: 10.1111/j.1440-1754.2005.00538.x. [DOI] [PubMed] [Google Scholar]
- 3.Gafur O.A., Copley L.A., Hollmig S.T. The impact of the current epidemiology of pediatric musculoskeletal infection on evaluation and treatment guidelines. J Pediatr Orthop. 2008;28:777–785. doi: 10.1097/BPO.0b013e318186eb4b. [DOI] [PubMed] [Google Scholar]
- 4.Jackson M.A., Nelson J.D. Etiology and medical management of acute suppurative bone and joint infection in pediatric patients. J Pediatr Orthop. 1982;2:313–323. [PubMed] [Google Scholar]
- 5.Welkon C.J., Long S.S., Fisher M.C., Alburger P.D. Pyogenic arthritis in infants and children: a review of 95 cases. Pediatr Infect Dis. 1986;5:669–676. doi: 10.1097/00006454-198611000-00014. [DOI] [PubMed] [Google Scholar]
- 6.Arnold S.R., Elias D., Buckingham S.C. Changing patterns of acute hematogenous osteomyelitis and septic arthritis: emergence of community-associated methicillin-resistant Staphylococcus aureus. J Pediatr Orthop. 2006;26:703–708. doi: 10.1097/01.bpo.0000242431.91489.b4. [DOI] [PubMed] [Google Scholar]
- 7.Ryan M.J., Kavanagh R., Wall P.G., Hazleman B.L. Bacterial joint infections in England and Wales: analysis of bacterial isolates over a four year period. Br J Rhumatol. 1997;36:370–373. doi: 10.1093/rheumatology/36.3.370. [DOI] [PubMed] [Google Scholar]
- 8.Nelson J.D., Koontz W.C. Septic arthritis in infants and children: a review of 117 cases. Pediatrics. 1966;38:966–971. [PubMed] [Google Scholar]
- 9.Speiser J.C., Moore T.L., Osborn T.G., Weiss T.D., Zuckner J. Changing trends in pediatric septic arthritis. Semin Arthritis Rheum. 1985;15:132–138. doi: 10.1016/0049-0172(85)90031-9. [DOI] [PubMed] [Google Scholar]
- 10.Young T.P., Maas L., Thorp A.W., Brown L. Etiology of septic arthritis in children: an update for the new millennium. Am J Emerg Med. 2011;29:899–902. doi: 10.1016/j.ajem.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 11.Wilson N.L., Di Paola M. Acute septic arthritis in infancy and childhood: 10 years’ experience. J Bone Joint Surg Br. 1986;68:584–587. doi: 10.1302/0301-620X.68B4.3733835. [DOI] [PubMed] [Google Scholar]
- 12.Bonhoeffer J., Haeberie B., Schaad U.B., Heininger U. Diagnosis of acute haematogenous osteomyelitis and septic arthritis: 20 years experience at the University of Children’s Hospital Basel. Swiss Med Wkly. 2001;131:575–581. [PubMed] [Google Scholar]
- 13.Fink C.W., Nelson J.D. Septic arthritis and osteomyelitis in children. Clin Rheum Dis. 1986;12:423–435. [PubMed] [Google Scholar]
- 14.Witt WP, Weiss AJ, Elixhauser A. Overview of Hospital Stays for Children in the United states, 2012: Statistical Brief #187. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet]. Rockville (MD): Agency for Healthcare Policy and Research (US); 2006 Feb- 2014 Dec. [PubMed]
- 15.Healthcare Cost and Utilization Project Agency for Healthcare Research and Quality; 2017. HCUP Kids’ Inpatient Database Comparison Report. [Available at: https://www.hcup-us.ahrq.gov/kidoverview.jsp. (Accessed 15 March 2016)] [Google Scholar]
- 16.2012. Introduction to the HCUP Kids’ Inpatient Database (KID) [Avaliable at: https://www.hcup-us.ahrq.gov/db/nation/kid/kid_2012_introduction.jsp. (Accessed 20 March 2016)] [Google Scholar]
- 17.2009. Introduction to the HCUP Kids’ Inpatient Database (KID) [Avaliable at: https://www.hcup-us.ahrq.gov/db/nation/kid/kid_2009_introduction.jsp. (Accessed 20 March 2016)] [Google Scholar]
- 18.2006. Introduction to the HCUP Kids’ Inpatient Database (KID) [Avaliable at: https://www.hcup-us.ahrq.gov/db/nation/kid/kid_2006_introduction.jsp. (Accessed 20 March 2016)] [Google Scholar]
- 19.2012. Age and Sex Composition in the United States-Census.gov. [Available at https://www.census.gov/population/age/data/2012comp.html. (Accessed 10 March 2016)] [Google Scholar]
- 20.2009. Age and Sex Composition in the United States-Census.gov. [Available at https://www.census.gov/population/age/data/2009comp.html. (Accessed 10 March 2016)] [Google Scholar]
- 21.2006. Age and Sex Composition in the United States-Census.gov. [Available at https://www.census.gov/population/age/data/2006comp.html. (Accessed 10 March 2016)] [Google Scholar]
- 22.2017. Racial and Ethnic Composition of the Child Population. [Available at http://www.childtrends.org/?indicators=racial-and-ethnic-composition-of-the-child-population. (Accessed 10 March 2016)] [Google Scholar]
- 23.Christiansen P., Frederiksen B., Glazowski J. Epidemiologic, bacteriologic, and long-term follow-up data of children with acute hematogenous osteomyelitis and septic arthritis: 1 ten-year review. J Pediatr Orthop B. 1999;8:302–305. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.