Abstract
Hepatocellular carcinoma (HCC) is one of the most lethal cancers worldwide with an overall survival rate of less than 15% in developed countries. Despite attempts at new therapeutic strategies, the majority of patients succumb to this cancer. Buttressed by the highly successful clinical impact in melanoma, immunotherapy is gaining momentum as the next treatment modality for many human cancers. Chimeric antigen receptors (CAR) contain the antigen binding moieties of a monoclonal antibody and the co-stimulatory and signaling domains associated with effector receptor signaling. Bispecific antibodies (BsAb) combine the binding specificities of two different monoclonal antibodies, one activating a receptor on a killer effector cell, while the other engaging a tumor-associated antigen to initiate tumor cytotoxicity. In this review, we survey the HCC targets for which CARs and bispecific antibodies have been generated. The pros and cons of these targets for T-cell and Natural Killer cell based immunotherapy will be discussed.
Keywords: Chimeric Antigen Receptor, Bispecific Antibody, Hepatocellular Carcinoma, Treatment
1. Introduction
Hepatocellular carcinoma (HCC), the most common primary liver cancer, is the fifth most common cancer and the second most common cause of cancer deaths worldwide. HCC developed mostly in livers with chronic inflammation. The main causal factors for the latter are chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) infections. However, other factors such as excessive alcohol consumption, non-alcoholic fatty liver disease, obesity, diabetes, aflatoxin, and smoking also play important roles in the pathogenesis of this neoplasm [12]. Despite advances in treatment, the five-year survival rate of patients with HCC remains poor averaging 5–15% [12]. Although viral vaccines [69] and effective anti-hepatitis drugs [75] in recent years have greatly reduced the incidence and the severity of chronic infection in developed countries, for the rest of the world, these benchmarks will take decades to be realized. In the meantime, finding an effective therapeutic for HCC remains an unmet need.
1.1 Immunobiology of liver and liver cancer
Through the portal blood flow from intestine to heart, bacterial products, toxins and antigens continually challenge the liver parenchyma. To effectively neutralize these environmental threats, the liver contains a rich source of innate immune cell including macrophages, natural killer (NK) cells, NKT cells, and γδT cells. Among these, NK cells are a unique population comprising about 30–50% of liver lymphocytes in healthy individuals and up to 90% in liver malignancies [39]. Compared to the peripheral blood NK cells, liver NK cells are more cytotoxic against HCC. In fact, interleukin-2 (IL2)-stimulated liver NK cells express tumor necrosis factor–related apoptosis-inducing ligand (TRAIL), whose receptor is highly expressed on poorly differentiated HCC [52]. Although this local population of NK cells generally have low CD16 expression [17], a limitation for most natural anti-tumor IgG1 antibodies, a BsAb targeting CD16 could potentially overcome this limitation. Since NK cells are less restricted by the immunosuppressive tumor microenvironment intended for T cells, their activation using bispecific antibodies against HCC is appealing.
The role of Lymphocytes in defense against HCC is well known. In fact, there is a positive correlation between T and NK cell infiltration into the tumor site and higher survival rate in HCC patients [24, 53]. However, immunosuppressive tumor microenvironment undermines lymphocyte function. Myeloid derived suppressor cells [41], mesenchymal stem cells [117], regulatory T cells [108], cancer-associated fibroblasts [3], tumor associated macrophages [30], and programmed cell death protein 1 (PD-1)hi regulatory B cell [111] suppress immune cells and promote HCC progression. Furthermore, overexpression of inhibitory receptors including PD-1 and T cell immunoglobulin and mucin-domain containing-3 (TIM-3) on circulating or tumor-infiltrating T cells was associated with poor clinical outcomes [64, 100]. In addition, PD-1 ligand (PD-L1) or B7-H3 expressed on HCC cells can induce T cell apoptosis or inhibit T cell functions [100, 102]. Hence, modulation of immunosuppressive cells and molecules is an active area of investigation [54, 64, 107].
A Phase I/II clinical trial to evaluate the safety and efficacy of nivolumab, a fully human IgG4 PD-1 blocking monoclonal antibody, was completed on 41 patients with HCC. Patients were treated for up to two years with nivolumab (0.1 – 10 mg/kg intravenously) [31]. Among 39 patients whose response was evaluable, 2 complete responses, 7 partial responses, 18 stable diseases, and 12 progressive diseases were reported. 71% patients experienced drug-related adverse effects (17% grade 3/4) including rash and elevated serum levels of aspartate aminotransferase, alanine aminotransferase, and amylase. Overall survival rate was 72% after six months. In a recent case report, a 75-years old male patient with metastatic HCC unresponsive to sorafenib was treated with pembrolizumab, a humanized IgG4 PD-1 blocking monoclonal antibody. After six cycles of treatment (each cycle with 2 mg/kg every three weeks), the patient’s tumor mass and blood alpha fetoprotein was markedly reduced [107]. In murine models of HCC, it was shown that sorafenib treatment increases intratumoral hypoxia leading to increased expression of stromal cell-derived 1 alpha (SDF1α), PD-1 ligand (PDL1), and accumulation of immunosuppressive cells [20]. Combination of sorafenib, murine PD-1 blocking antibody, and SDF1α receptor inhibitor provided the most potent tumor growth delay [21].
1.2 Antibody-based T cell-dependent immunotherapy
Compelled by the striking recent clinical results of T-cell based therapies, cancer immunotherapy was named as the breakthrough of the year in 2013 [25]. Chimeric antigen receptors (CAR) and bispecific antibodies are two powerful extensions of this approach. CARs were originally developed by fusing the antigen-binding moiety of an antibody to the transmembrane and cytoplasmic domains of CD3ζ [34] T cells equipped with these CARs had in vitro cytotoxicity, but their in vivo persistence and function were suboptimal. Therefore, second and third generation CARs were developed by addition of one or two costimulatory domains (e.g. CD28, 4-1BB, OX40), respectively to the intracellular domain of the first generation CARs (fig. 1) [77]. Bispecific antibodies (BsAb) combine the specificities of two monoclonal antibodies in a single molecule. These bispecific reagents can neutralize the effect of two tumor-associated-antigens. More commonly, they activate effector cells and bring them to engage cancer cells to execute their cytotoxic functions [77]. CAR technology has been tested against the target CD19 (in human cancers such as ALL, CLL), GD2 (neuroblastoma), CD22 (ALL), mesothelin (mesothelioma), and HER2 (sarcoma) [4, 8, 38, 45, 76, 90], and IL13R (glioblastoma) [13] with overwhelming success in CD19(+) leukemia, but only select solid tumors. Several factors might be responsible for the inferior efficacy of CART cells in solid versus hematological malignancies including target antigen heterogeneity, poor trafficking and penetration of therapeutic cells, and the hostile tumor microenvironment (hypoxia, acidosis, nutrient depletion, tumor-derived immunosuppressive molecules, various immunosuppressive cells including tumor-associated macrophages, regulatory T cells, and myeloid-derived suppressor cells) [85]. BsAb was successfully proven for CD19 (ALL), leading to the FDA approval of blinatumomab for Philadelphia chromosome (ph)-negative relapsed/refractory B-cell precursor ALL (BLINCYTOTM) in 2016. BsAb targeting CD33, CD52, HER2, CD20, GD2, GPC3, and CD123 are in the clinical pipeline. It is timely to review preclinical and clinical studies where these T-cell based therapeutic strategies have been applied to HCC. Table 1 summarizes the BsAb and CAR targets of HCC. Table 2 summarizes clinical trials involving anti-HCC BsAb and CARs. Table 3 and 4 summarize the characteristics of BsAbs and CARs used against HCC targets.
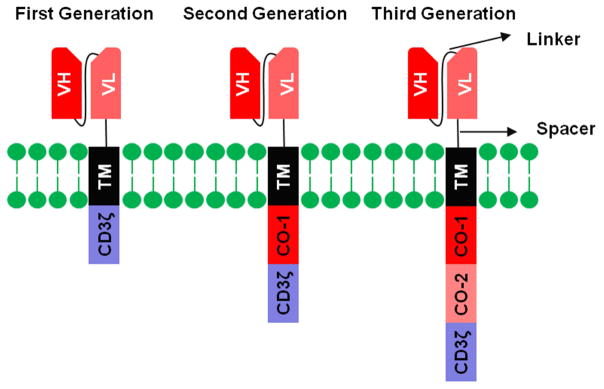
Figure 1.
Structure of chimeric antigen receptors (CAR). CARs are composed of a single-chain fragment variable (scFv, containing the heavy chain variable domain (VH) and the light chain variable domain (VL) of a monoclonal antibody attached together via a flexible linker) linked via a spacer sequence to a transmembrane (TM) domain and to the CD3ζ chain (first-generation CARs). Second- and third-generation CARs additionally contain one or two costimulatory domains, respectively. VH, heavy chain variable domain; VL, light chain variable domain; TM, transmembrane domain; co-1, costimulatory domain 1; co-2, costimulatory domain 2.
Table 1.
Advantages and disadvantages of HCC-associated antigens for CAR and antibody development
| Marker | Advantages | Disadvantages |
|---|---|---|
| GPC3 |
|
|
| Viral antigens |
|
|
| EpCAM | ||
| VEGF-A and osteopontin |
|
|
Table 2.
Clinical trials involving CARs and bispecific antibodies in HCC
| name | format | Phase | Clinical trial number | Sponsor | Study population | status |
|---|---|---|---|---|---|---|
| ERY974 | CD3×GPC3 IgG4-based | 1 | NCT02748837 | Chugai Pharmaceutical | Patients with GPC3(+)advanced solid tumors | recruiting |
| GPC3-CAR | - | 1 | NCT02932956 | Baylor College of Medicine | Patients with elapsed or refractory GPC3(+)solid tumors | Not yet recruiting |
| GPC3-CAR | - | 1 | NCT02905188 | Baylor College of Medicine | Patients with unresectable, HCC | Not yet recruiting |
| GPC3-CAR | - | 1 | NCT02395250 | RenJi Hospital | Patients with relapsed or refractory GPC3(+)HCC | recruiting |
| GPC3-CAR | - | ½ | NCT02723942 | Fuda Cancer Hospital, Guangzhou | Patients with GPC3(+)HCC without extrahepatic metastasis | recruiting |
| GPC3/mesotheli n/CEA CAR | - | ½ | NCT02959151 | Shanghai GeneChem Co., Ltd | Patients with GPC3(+)HCC | recruiting |
| MUC1 CAR | - | ½ | NCT02587689 | PersonGen BioTherapeutics (Suzhou) Co., Ltd | Patients with MUC1(+) HCC, NSCLC1, pancreatic and breast cancer | recruiting |
| MUC1 CAR | - | 1/2 | NCT02839954 | PersonGen BioTherapeutics (Suzhou) Co., Ltd | Patients with MUC1(+) HCC, NSCLC1, pancreatic, glioma, colorectal, gastric, and breast cancer | recruiting |
Non-small cell lung cancer
Table 3.
Characteristics of bispecific antibodies generated against HCC
| Name/format | MW(kDa) | Affinity (KD) nM | EC50 pM | clone | |||
|---|---|---|---|---|---|---|---|
| Effector (CD3, etc) | Target (GPC3, etc) | ||||||
| Parent clone | Bispecific format | ||||||
| ERY974 (Humanized IgG4 MAb) [42] | 150 | - | - | - | - | - | |
| TriFab [43] | 150 | - | 3.4 | 3.4 | - | GC33 (GPC3) | |
| EpCAM×CD3 BiTE [63] | 54 | - | - | - | 1.42 - 96 | 1H8 (EpCAM) | |
| EpCAM×CD3 BiTE (Solitomab, AMG110, MT110) [113] | 55 | 77 | - | 16–230 | 7.9 | diL2K (CD3) | |
| EpCAM×CD3 (Catumaxomab) [114] | 150 | - | 0.55 | 0.56 | - | Ho-3 (EpCAM), | |
| VEGF×Osteopontin [85] | 200 | - | HuA.4.6.1 (1.55) hu1A12 (9.27) |
HuA.4.6.1 (1.64) hu1A12 (9.43) |
- | HuA.4.6.1 (Bevacizum ab, VEGF), hu1A12 (osteopontin) | |
| (CD3×anti-HCC) DQ-33 [91] | 150 | - | - | - | - | Hepama-6 (anti-HCC) | |
| [92] | CD3×anti- HCC | 100 | - | - | - | - | OKT3 (CD3), L-7- 6 (anti- HCC) |
| CD16×anti- HCC | 100 | - | - | - | - | 3-G-8 (CD16), L- 7-6 (anti- HCC) | |
Table 4.
Characteristics of CARs generated against HCC
| Reference | Hinge | TM | Costimulatory | Signaling | Epitope | Clone | Virus |
|---|---|---|---|---|---|---|---|
| [38, 41] | CD8α | CD8α | CD28, 41BB | CD3ξ | C-terminal region of GPC3 | GC33 | Lenti |
| [39] | IgG1 | CD28 | CD28, 41BB | CD3ξ | C-terminal region of GPC3 | GC33 | Retro |
| [40] | - | - | CD27, CD28, 41BB | CD3ξ | Conformational epitope (N- and C-terminal) of GPC3 | HN3 (Kd 0.6nM) | Lenti |
| [41] | CD8α | CD28 | CD28, 41BB | CD3ξ | C-terminal region of GPC3ξ | GC33 | Lenti |
| [49, 50] | Human IgG1 Fc | CD28 | CD28 | CD3ξ | conformational epitope in the HBs antigen | C8, and 5a19 | Retro |
| [53] | Human IgG Fc | CD28 | CD28 | CD3ξ | HCV/E2 | e137 | Retro |
| [61] | CD8α | CD28 | CD28 | CD3ξ | EGF-like domain I of EpCAM | MOC31 | Lenti |
| [62] | CD8 | CD28 | CD28 | CD3ξ | EGF-like domain I of EpCAM | C215 | Retro |
| [88] | - | - | CD28 | CD3ξ | AFP158–166
peptide presented by HLA-A*02:01 |
ET1402L1 | Lenti |
1.3 Glypican-3 (GPC3)
GPC3 (also known as GTR2-2, intestinal protein OCI-5, and MXR7) is a heparan sulfate proteoglycan expressed as a 70kDa precursor protein. Upon cleavage by furin, GPC3 is divided into an N-terminal 40kDa and a C-terminal 30kDa fragment. The latter, which contains two heparin sulfate chains, is attached to the cell membrane via a glycosylphosphatidylinositol anchor [49].
It has been shown that GPC3 is expressed on the majority of hepatocellular carcinoma (HCC) and hepatoblatsoma cases but not or to a lower degree on normal liver tissue [51, 57, 67, 84]. Interestingly, GPC3 expression could be used as an HCC precancerous marker in cirrhotic livers since it is associated with dysplasia in cirrhotic livers [74]. Furthermore, overexpression of GPC3 in HCC is associated with poor prognostic indicators including poor tumor differentiation, higher TNM score, and tumor invasion into blood vessels [65].
1.3.1 Glypican-3 CAR
The first GPC3 CAR was generated by fusing the scFv of an anti-GPC3 antibody to the CD8α hinge and transmembrane regions followed by the intracellular signaling domain of the CD3ζ. This first-generation CAR does not have the co-stimulatory domains. Therefore a third-generation CAR composed of the anti-GPC3 scFv, CD8α hinge, CD28 transmembrane and intracellular signaling domains, 4-1BB, and CD3ζ was produced. Although human T cells transduced with concentrated lentiviral vectors containing either of the CAR constructs specifically lysed HCC cell lines, the third-generation CAR T cells produced higher levels of interleukin-2 and IFNγ, which was positively correlated with the level of GPC3 expression on target cells. The presence of soluble GPC3 protein, which has been reported in the serum of some HCC patients, only mildly (10%) inhibited the cytotoxicity of CAR T cells. T cells equipped with the third-generation CAR could suppress the growth of established orthotopic xenografts in immunodeficient mice [40].
Another group compared first-, second-, and third-generation anti-GPC3 CARs and found that the second- and third-generation constructs outperformed the first- generation CAR in vivo [68]. The choice of co-stimulatory domain might influence CART cell behavior. Anti-GPC3 CARs containing CD28, 4-1BB or both have been tested against HCC [68]. The in vitro cytotoxicity of T cells equipped with a CAR signaling through CD28 was higher than one signaling through 4-1BB. Both CARs eliminated HCC cells in vitro and in mice. Whereas CD28 induced preferential production of Th2 cytokines (IL-4 and IL-10), 4-1BB induced generation of Th1 cytokines (IFNγ and GM-CSF) as well as superior T cell proliferation in vitro and in vivo. More recently, a fourth generation GPC3- specific CAR was generated containing three co-stimulatory domains (CD27, CD28, and 4-1BB) and the inducible caspase-9 suicide gene in a lentiviral backbone [62]. While this CAR induced efficient lysis of GPC3(+) cell lines in vitro, pre-incubation of targets with kinase inhibitor sorafenib improved CART cell cytotoxicity. However, the effect of sorafenib on CART cells was not reported.
Besides HCC, lung squamous cell carcinoma (SCC) also expresses GPC3. T cells expressing a third generation (CD28, and 4-1BB as co-stimulatory molecules) GPC3-specific CAR were able to infiltrate subcutaneously inoculated lung SCC tumors and significantly reduce their size in xenograft models [66].
1.3.2 Glypican-3 BsAb
Recently an FcγR-silenced IgG4-based humanized bispecific antibody bearing monovalent specificities for CD3 and GPC3 was successfully tested in mouse models of HCC (fig 2A). To reduce the chance of cytokine release, corticosteroids were tested without compromising the anti-tumor effect [47]. A phase I clinical trial to assess the safety and efficacy of the antibody is in progress (NCT02748837).
Figure 2.
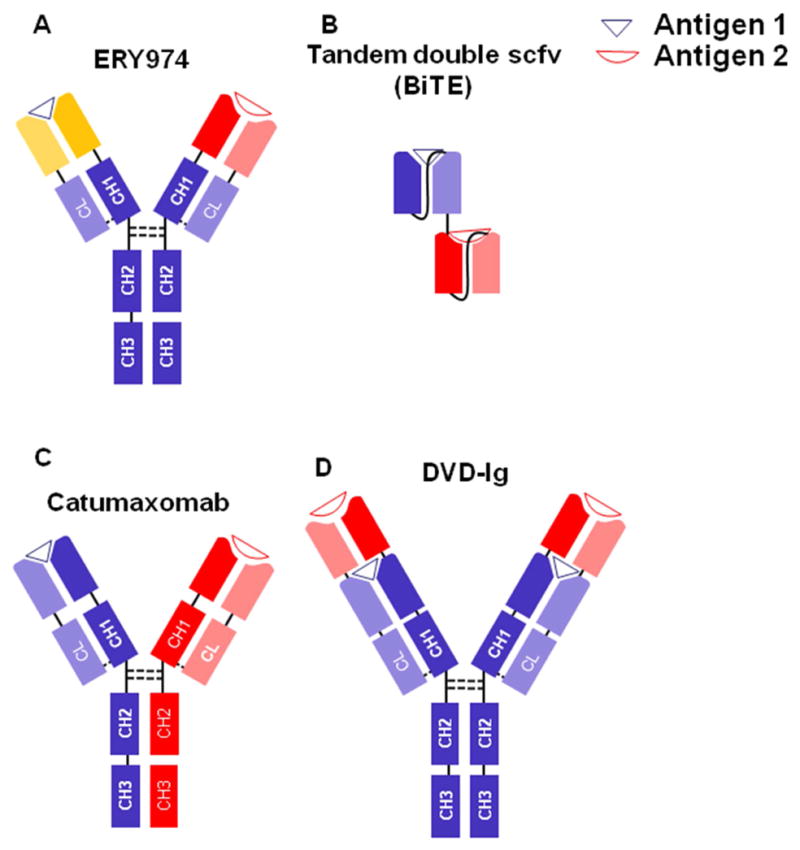
Structure of BsAbs used against HCC. Heavy chains are depicted in dark colors while light chains are in the same but lighter colors. Linkers are shown by continuous lines and disulfide bonds are demonstrated by dotted lines. (A) ERY974 is an anti-GPC3/CD3 Bispecific Antibody. (B) BiTE consists of two scFv linked together via a flexible linker. (C) Catumaxomab is an anti-EpCAM/CD3 Bispecific Antibody. (D) DVD-Ig is formed by connecting the variable domains of an antibody to the variable domains of an IgG. DVD-Ig, Dual-Variable-Domain Immunoglobulin; VH, variable heavy chain; VL, variable light chain; CL, constant light chain; CH1-3, constant heavy chains 1 to 3. BiTE, bispecific T cell engager.
Another bispecific antibody named trivalent IgG-Shaped (TriFab) construct was developed by fusing two anti-GPC3 Fab fragments via a flexible linker to one asymmetric third Fab-sized binding domain [79]. The bivalent Fab arms bind GPC3 with similar affinity of the parent IgG antibody. The third domain was designed to bind another antigen or to carry a cytotoxic payload. No in vivo experiment was reported.
1.4 Viral antigens
It is well known that several viruses, called oncoviruses, can induce cancer. Hepatitis B (HBV) and C (HCV) viruses are key HCC risk factors accounting for approximately 80% of HCC cases [32]. These viruses can induce HCC via several mechanisms including insertional mutagenesis (mainly for HBV versus HCV) and accumulation of genetic damage due to chronic inflammation and oxidative stress. Furthermore, direct effects of hepatitis B virus x-protein (HBx) on regulatory non-coding RNAs, as well as its interaction with various signaling pathways such as p53, Wnt, and nuclear factor-κB could also account for HBV carcinogenesis [42, 43, 105].
1.4.1 Viral antigen CAR
A second-generation CAR specific for the S domain of all three envelope proteins (S, M, and L protein, combined as HBsAg) of HBV was generated and tested in immunocompetent HBV transgenic mice. Since HBsAg is expressed on the surface of HBV-replicting cells, it can be targeted by CARs [23]. Adoptively-transferred CAR-transduced murine T cells were able to control HBV infection with only transient liver damage. Besides, the high serum level of circulating HBV antigen did not affect the function of CAR T cells. However, anti-tumor effect of this CAR was not tested [61]. In another study, second-generation CARs (containing CD28 costimulation) specific for HBV S and L antigens enabled T cells to eliminate HBV-infected human hepatocytes and hepatoma cells. CART cells specific for the S antigen (that is expressed at higher levels on infected cells) outperformed those reactive to the L antigen (which is expressed at lower levels on infected cells) in the generation of interferon-γ and cytotoxicity [10].
Other investigators using T cells transduced with a T cell receptor (TCR) specific for the S domain showed that although electroporation of T cells with anti-HBV TCR mRNA could equip nearly 80% of T cells with the transgene, TCR expression was transient and disappeared within 72 hours. In contrast to retrovirally-transduced T cells that were able to completely eliminate the HCC xenografts after a single T cell transfer, multiple injections of RNA-electroporated T cell were necessary to suppress, while not able to eradicate, HCC tumors [58]. Since HBV antigens such as the S antigen are expressed on HCC as well as infected hepatocytes, the risk of collateral damage by CART cells against infected liver could be dose-limiting [14].
HCV E2 glycoprotein (HCV/E2) is the key target for the host immune system during HCV infection and also the most variable HCV protein. In an attempt to control this infection in vitro, a second generation CAR was constructed based on a broadly cross-reactive/cross neutralizing anti HCV/E2 monoclonal antibody. Human T cells retrovirally transduced with this CAR were able to generate anti-viral and proinflammatory cytokines and lyse HCV-infected hepatoma cells [96]. However, whether the CAR-transduced T cells can completely eliminate the HCV infection in vivo or eradicate HCV-associated liver cancers remains an open question.
1.4.2 Viral antigen BsAb
To engage T cells to the site of HBx-expressing HCC, an anti-HBx × anti-CD3 BsAb was generated by hybrid-hybridoma technology where anti-HBx and anti-CD3 hybridoma cell lines are fused together. When administered in combination with in vitro-cultured effector cells, the bispecific reagent induced apoptosis and suppressed the growth of HCC xenografts in immunodeficient mice [70]. Other investigators reported a tetravalent BsAb composed of one anti-CD3 and one anti-CD28 scFv connected to two anti-HBs antigen scFv via the IgG1 Fc-domain [11]. To minimize the chance of FcγIIR (CD16)-mediated antibody dependent cell mediated cytotoxicity (ADCC), the Fc domain was mutated. The BsAb mediated activation of T cells and redirected their cytotoxicity to HBs antigen infected hepatocytes in vitro.
1.5 Epithelial cell adhesion molecule (EpCAM)
Epithelial cell adhesion molecule (EpCAM, CD326) is a 39–42kDa glycoprotein comprised of a large extracellular domain, a transmembrane anchor, and a short cytoplasmic tail. The extracellular sequence contains a thyroglobulin type-1 and an epidermal growth factor-like domain [86]. EpCAM is expressed on the surface of various epithelial cells and plays important roles in cell proliferation, differentiation and migration [86]. EpCAM was reported as a cancer stem cell (CSC) marker in HCC [104, 116]. Expression of this marker was associated with poorly differentiated HCC and poor prognosis [73, 103, 104].
1.5.1 EpCAM CAR
Although not tested in HCC, anti-EpCAM CARs have been assessed in other solid tumors [6, 26, 94]. Transduction of the clinically-relevant NK-92 cells with IL-15 and an anti-EpCAM CAR resulted in proliferation of CART cells in the absence of exogenous cytokines and redirected their cytotoxicity against breast cancer cells that were resistant to unmodified NK cells [94]. In another study, peripheral blood lymphocytes transduced with an anti- EpCAM CAR preferentially lysed EpCAMhi cells in vitro and suppressed the growth of corresponding cells in vivo [26]. Peritoneal carcinomatosis can occur in patients with advanced gastrointestinal or gynecological neoplasms. To target peritoneal carcinomatosis, CD28/41BB-containing third generation anti-EpCAM CART cells were generated using lentiviral vectors or mRNA transfection. Whereas a single intraperitoneal injection of 10 million lentivirally-transduced EpCAM specific CART cells dramatically reduced the signal of established peritoneal ovarian cancer cells, frequent injections of mRNA-electroporated T cells was necessary to slow down tumor growth. Temporary expression of CAR on electroporated T cells might provide some safety though at the expense of efficacy. Furthermore, life threatening side effects after CART cell injection might rapidly occur [82] necessitating swift methods for eliminating T cells including the inducible caspase-9 system [27]. EpCAM-specific CART cells are undergoing clinical testing in nasopharyngeal carcinoma (NCT02915445).
1.5.2 EpCAM BsAb
The first bispecific anti-EpCAM antibody tested against HCC was a bispecific T cell engager (BiTE, fig 2B) comprised of an anti-EpCAM scFv fused to an anti-CD3 scFv via a Gly4Ser linker. While the parent anti-EpCAM monoclonal antibody failed to suppress tumor growth, the co-administration of peripheral blood mononuclear cells (PBMCs) and BiTE was able to suppress the growth of HCC cell lines in vitro and in vivo. Furthermore, it was shown that overexpression of galectin-1 (Gal-1) on target cells inhibited the BiTE-induced cytotoxicity while Gal-1 knock-down broke the tumor resistance to therapy [123]. Solitomab (AMG110, MT110) is a humanized anti-EpCAM×anti-CD3 BiTE. Co-administration of this BiTE and γδ T cells caused a near-complete lysis of HCC and hepatoblatsoma cell lines in vitro [50]. In addition to liver cancers, anti- EpCAM bispecific antibodies have been tested against some other tumors [9, 33, 36, 37, 87, 88, 97, 98]. Ex vivo, incubation of autologous tumor-associated lymphocytes with EpCAM(+) cancer cells in the presence of Solitomab led to activation and proliferation of effector cells and diminished the number of target cells including uterine serous papillary carcinoma [9], and Uterine and ovarian carcinosarcomas [33, 37]. Furthermore, intraperitoneal administration of catumaxomab, an IgG-based antibody with monovalent specificity against EpCAM and CD3 (fig 2C), led to regression of breast cancer-induced liver metastases [87]. In Europe, catumaxomab has been approved for treatment of malignant ascites in patients with EpCAM(+) carcinomas [98]. It is important to note that EpCAM shedding occurs in some cancer patients. Although controversial, soluble EpCAM can interfere with the function of anti-EpCAM bispecific antibodies in vitro [88, 97]. More studies are needed to clarify if EpCAM shedding compromises the anti-tumor effects of CART or BsAb directed at this antigen.
1.6 Angiogenic factors and BsAb
Vascular endothelial growth factor-A (VEGF-A) and osteopontin are two angiogenic factors with different characteristics. Either of these factors can induce the expression of the other and they can function synergistically [91]. Both factors can induce endothelial cell motility that is essential for angiogenesis; however, VEGF chemotaxis is RAC dependent while osteopontin’s effect is independent of RAC activation [91, 101] Whereas osteopontin suppresses lipid raft clustering, VEGF stimulates it [109].
VEGF-A gene is comprised of 8 exons that when alternatively-spliced, generate various isoforms with different characteristics. The most common VEGF-A isoform is a secreted 45kDa heparin-binding homodimeric glycoprotein [35]. It has been reported that VEGF mRNA and protein expression is increased in HCC cells [81, 110]. Furthermore, VEGF overexpression was reported in HCC cases with recurrence [119]. Moreover, it was shown that the expression of VEGF was higher in HCC samples overexpressing the CSC markers than in those samples with a lower CSC profile [119].
Osteopontin (also known as secreted phosphoprotein 1 (SPP1), early T-lymphocyte activation 1 (ETA-1), and bone sialoprotein 1 (BSP-1)), is an O-glycosylated phosphoprotein that belongs to the Small integrin binding ligand N-linked glycoprotein family. It is expressed by various cell types including lymphocytes, fibroblasts, endothelial and epithelial cells, macrophages, dendritic cells, and neutrophils. Upon binding to its receptors (CD44 and various integrins) on target cells, osteopontin plays various physiological and pathological roles [7]. In HCC, osteopontin is overexpressed in carcinomas with capsular infiltration and osteopontin-positive cells are positioned in the cancer-stroma interface [16, 44]. Furthermore, osteopontin overexpression was associated with vascular invasion, tumor metastasis, resistance to cisplatin chemotherapy, poor prognosis, and was introduced as an early HCC diagnostic marker and an HCC-stem cell marker [15, 29, 59, 71, 99, 122].
In an attempt to target both VEGF and osteopontin, two bispecific antibodies named dual-variable domain immunoglobulin (DVD-Ig) were generated (Fig 2D) in which the binding moieties of an anti-VEGF antibody bevacizumab, and an anti-osteopontin antibody were fused together in two different orders. Results demonstrated that while the antibody with the VEGF-osteopontin orientation retained its parent antibody affinity, the osteopontin-VEGF format suffered a great loss in the VEGF-domain affinity. The former antibody was able to confer therapeutic efficacy against a human HCC cell line in mouse [60].
1.7 TCR mimics
One of the limitations of antibodies and CARs is that they target cell surface antigens; however, the majority of tumor associated antigens are intracellular. These proteins are processed within the cytoplasm and expressed as major histocompatibility (MHC)-peptide complexes on the cell surface. Alpha-fetoprotein (AFP) is a CSC marker on HCC and is associated with poorer prognosis [113, 115]. To target AFP, an scFv specific to the AFP peptide-MHC (HLA-A*02:01) complex was isolated from a human phage library and incorporated into a second-generation CAR containing the CD28 domain [72]. While intratumoral injection of CART cells had a profound and lasting anti-tumor effect, intravenously-injected CART cells were only able to reduce the growth of subcutaneously-injected HCC xenografts in immunodeficient mice. This led the investigators to propose local delivery of CART cells to the site of tumor via direct injection into the tumor or administration via the hepatic artery. Hepatic artery infusion (HAI) of chemotherapy agents is an established clinical technique. Compared to systemic administration, this method can increase local concentration of chemotherapeutic agents, reduce systemic toxicities, and improve treatment success [55]. In fact, HAI of CART cells specific for carcinoembryonic antigen(+) liver metastasis have been tested in a phase I clinical trial [56].
1.8 Other targets
In an attempt to generate bispecific constructs against liver cancer, antibody-producing cells that secrete antibodies against human hepatoma and a CD3-associated determinant, were fused together. Whereas phytohemagglutinin-activated lymphocytes lysed hepatoma cells in the presence of the bispecific molecule, resting peripheral blood lymphocytes failed to do so [19]. In another study, the Fab or Fab’ fragments of anti-CD3 or anti-CD16 antibodies were chemically attached to the Fab or Fab’ fragments of an anti HCC antibody to generate two bispecific antibodies. Lymphokine-activated killer cells (LAK) or pokeweed mitogen-activated LAK cells (PWM-LAK) were used as effector cells. Whereas 55% of the LAK cells after three days of culture expressed CD16, the phenotype of these cells shifted to CD3+ T cells afterwards. This is why the CD16-containing bispecific antibody mediated cytotoxicity only when it was administered with early LAK cells (day three of culture). On the other hand, CD3-containing bispecific molecule was effective whether early or late LAK cells or PWM-LAK cells were used [95]. Despite the well known immunobiology of natural killer cells biology of the human liver, the role of CD16(+) Natural Killer and NKT cells in HCC has not been adequately explored.
2. Summary and future directions
T cells are highly proficient killer showing successful clinical results in various human cancers [1, 2, 78, 89, 92, 93]. In HCC, NK cells comprise 90% of lymphocytes [39]. Redirection of these cells against HCC may hold therapeutic potential. While CART cells should migrate to the tumor site upon administration, BsAb can redirect tumor-residing NK cells against cancer cells and eliminate the homing step.
Targeting CSCs is of paramount importance if a cure for tumors is envisaged. Growing evidence points to the origination of HCC from transformation of liver stem and progenitor cells [48]. Several candidate antigens expressed on CSCs have been discovered including CD13 [46], CD24 [63], CD44 [80], CD56 [18], CD90 [114, 120], CD133 [121], DLK1 [112], EpCAM [104, 106, 116], cytokeratin-19 (CK-19) [5], and OV6 [118]. Besides, it was shown that cells expressing two CSC markers, CD133/EpCAM or CD133/CD44, might be better representatives of CSCs and demonstrate superior tumorigenicity in vitro and in vivo [22, 124].
Parallel to the enhancement in effectiveness and potency of immunotherapeutic agents, side effects might also increase, which could jeopardize or slow down their regulatory approval. Therefore the choice of target is critical especially with CAR-modified effector cells which can persist for a long time in vivo or with bispecific antibodies that can recruit polyclonal effector cells. The presence of tumor cells in a vital organ or the unexpected upregulation of even low levels of the target antigen in normal tissues could cause catastrophic side effects. For example, localization of anti-ERBB2 CAR-modified T cells to the pulmonary tissue due to the low expression of ERBB2 on lung epithelial cells culminated in fatal pulmonary edema in a patient with metastatic colon cancer [83]. The lack of dose-limiting adverse effects in early clinical trials does not always guarantee the absence of life-threatening late complications. To reduce the chance of adverse effects mediated by CAR T cells, incorporation of suicide genes, such as the inducible caspase 9, into the CAR constructs would enable rapid destruction of effector cells when needed [28].
Currently, only few centers hold the necessary technology and expertise for development and implementation of CAR-based therapies. This and also the high cost of CAR and bispecific antibody therapeutics, which in most cases are not supported by the insurance companies, would impede widespread commercialization of these therapies. Development of manufacturing processes and technology transfer for widespread generation of affordable therapeutic agents on the one hand and improvement in the insurance companies’ coverage for treatment costs on the other hands will enable more patients to benefit from these powerful anti cancer remedies.
Highlights
Hepatocellular carcinoma (HCC), the second most common cause of cancer deaths worldwide.
Chimeric antigen receptors (CAR) contain the antigen binding domain of an antibody to redirect effector cells to cancer cells.
Bispecific antibodies (BsAb) redirect effector cells toward cancer cells for their killing.
HCC targets for which CARs and bispecific antibodies have been generated will be discussed in this paper.
Acknowledgments
Funding
This work was supported in part by Funds from Enid A. Haupt Endowed Chair, Kids Walk for Kids with Cancer NYC, Katie Find a Cure Foundation, the Robert Steel Foundation and NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Contributors
NKC designed the concept, SSH and NKC wrote and edited the manuscript.
Conflict of interest
MKSCC and NK Cheung have financial interest in Y-mabs Therapeutics Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chimeric Antigen Receptor-Modified T Cells in Chronic Lymphoid Leukemia; Chimeric Antigen Receptor-Modified T Cells for Acute Lymphoid Leukemia; Chimeric Antigen Receptor T Cells for Sustained Remissions in Leukemia. The New England journal of medicine. 2016;374:998. doi: 10.1056/NEJMx160005. [DOI] [PubMed] [Google Scholar]
- 2.Adusumilli PS, Cherkassky L, Villena-Vargas J, Colovos C, Servais E, Plotkin J, Jones DR, Sadelain M. Regional delivery of mesothelin-targeted CAR T cell therapy generates potent and long-lasting CD4-dependent tumor immunity. Science translational medicine. 2014;6:261ra151. doi: 10.1126/scitranslmed.3010162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Affo S, Yu L, Schwabe RF. The Role of Cancer-Associated Fibroblasts and Fibrosis in Liver Cancer. Annual review of pathology. 2016 doi: 10.1146/annurev-pathol-052016-100322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmed N, Brawley VS, Hegde M, Robertson C, Ghazi A, Gerken C, Liu E, Dakhova O, Ashoori A, Corder A, Gray T, Wu MF, Liu H, Hicks J, Rainusso N, Dotti G, Mei Z, Grilley B, Gee A, Rooney CM, Brenner MK, Heslop HE, Wels WS, Wang LL, Anderson P, Gottschalk S. Human Epidermal Growth Factor Receptor 2 (HER2) -Specific Chimeric Antigen Receptor-Modified T Cells for the Immunotherapy of HER2-Positive Sarcoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33:1688–1696. doi: 10.1200/JCO.2014.58.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ali N, Allam H, May R, Sureban SM, Bronze MS, Bader T, Umar S, Anant S, Houchen CW. Hepatitis C virus-induced cancer stem cell-like signatures in cell culture and murine tumor xenografts. Journal of virology. 2011;85:12292–12303. doi: 10.1128/JVI.05920-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ang WX, Li Z, Chi Z, Du SH, Chen C, Tay JC, Toh HC, Connolly JE, Xu XH, Wang S. Intraperitoneal immunotherapy with T cells stably and transiently expressing anti-EpCAM CAR in xenograft models of peritoneal carcinomatosis. Oncotarget. 2017 doi: 10.18632/oncotarget.14592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bandopadhyay M, Bulbule A, Butti R, Chakraborty G, Ghorpade P, Ghosh P, Gorain M, Kale S, Kumar D, Kumar S, Totakura KVS, Roy G, Sharma P, Shetti D, Soundararajan G, Thorat D, Tomar D, Nalukurthi R, Raja R, Mishra R, Yadav AS, Kundu GC. Osteopontin as a therapeutic target for cancer. Expert Opin Ther Tar. 2014;18:883–895. doi: 10.1517/14728222.2014.925447. [DOI] [PubMed] [Google Scholar]
- 8.Beatty GL, Haas AR, Maus MV, Torigian DA, Soulen MC, Plesa G, Chew A, Zhao Y, Levine BL, Albelda SM, Kalos M, June CH. Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce anti-tumor activity in solid malignancies. Cancer immunology research. 2014;2:112–120. doi: 10.1158/2326-6066.CIR-13-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bellone S, Black J, English DP, Schwab CL, Lopez S, Cocco E, Bonazzoli E, Predolini F, Ferrari F, Ratner E, Silasi DA, Azodi M, Schwartz PE, Santin AD. Solitomab, an EpCAM/CD3 bispecific antibody construct (BiTE), is highly active against primary uterine serous papillary carcinoma cell lines in vitro. American journal of obstetrics and gynecology. 2016;214:99 e91–98. doi: 10.1016/j.ajog.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bohne F, Chmielewski M, Ebert G, Wiegmann K, Kurschner T, Schulze A, Urban S, Kronke M, Abken H, Protzer U. T cells redirected against hepatitis B virus surface proteins eliminate infected hepatocytes. Gastroenterology. 2008;134:239–247. doi: 10.1053/j.gastro.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Bohne F, Hasreiter J, Quitt O, Meyer C, Dhamodaran A, Alvarez L, Bruss V, Moldenhauer G, Momburg F, Protzer U. Bispecific Antibody Constructs Mediate Immunotherapeutic Retargeting of Effector Cells Towards HBV Infected Target Cells. Zeitschrift für Gastroenterologie. 2015;53:A5_10. [Google Scholar]
- 12.Bosetti C, Turati F, La Vecchia C. Hepatocellular carcinoma epidemiology. Best practice & research Clinical gastroenterology. 2014;28:753–770. doi: 10.1016/j.bpg.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Brown CE, Alizadeh D, Starr R, Weng L, Wagner JR, Naranjo A, Ostberg JR, Blanchard MS, Kilpatrick J, Simpson J, Kurien A, Priceman SJ, Wang X, Harshbarger TL, D'Apuzzo M, Ressler JA, Jensen MC, Barish ME, Chen M, Portnow J, Forman SJ, Badie B. Regression of Glioblastoma after Chimeric Antigen Receptor T-Cell Therapy. The New England journal of medicine. 2016;375:2561–2569. doi: 10.1056/NEJMoa1610497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buschow SI, Sprengers D, Woltman AM. To target or not to target viral antigens in HBV related HCC? J Hepatol. 2015;62:1449–1450. doi: 10.1016/j.jhep.2015.01.043. [DOI] [PubMed] [Google Scholar]
- 15.Cao L, Fan X, Jing W, Liang Y, Chen R, Liu Y, Zhu M, Jia R, Wang H, Zhang X, Zhang Y, Zhou X, Zhao J, Guo Y. Osteopontin promotes a cancer stem cell-like phenotype in hepatocellular carcinoma cells via an integrin-NF-kappaB-HIF-1alpha pathway. Oncotarget. 2015;6:6627–6640. doi: 10.18632/oncotarget.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cao L, Fan XY, Jing W, Liang YC, Chen R, Liu YY, Zhu MH, Jia RJ, Wang H, Zhang XH, Zhang YY, Zhou XY, Zhao J, Guo YJ. Osteopontin promotes a cancer stem cell-like phenotype in hepatocellular carcinoma cells via an integrin-NF-kappa B-HIF-1 alpha pathway. Oncotarget. 2015;6:6627–6640. doi: 10.18632/oncotarget.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carrega P, Bonaccorsi I, Di Carlo E, Morandi B, Paul P, Rizzello V, Cipollone G, Navarra G, Mingari MC, Moretta L, Ferlazzo G. CD56(bright)perforin(low) noncytotoxic human NK cells are abundant in both healthy and neoplastic solid tissues and recirculate to secondary lymphoid organs via afferent lymph. Journal of immunology. 2014;192:3805–3815. doi: 10.4049/jimmunol.1301889. [DOI] [PubMed] [Google Scholar]
- 18.Chan AW, Tong JH, Chan SL, Lai PB, To KF. Expression of stemness markers (CD133 and EpCAM) in prognostication of hepatocellular carcinoma. Histopathology. 2014;64:935–950. doi: 10.1111/his.12342. [DOI] [PubMed] [Google Scholar]
- 19.Chen Q, Sun P, Prigione I, Xie H, Ferrini S. Targeting of "T" lymphocytes against human hepatoma cells by a bispecific monoclonal antibody: role of different lymphocyte subsets. Tumori. 1992;78:79–86. doi: 10.1177/030089169207800204. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y, Huang Y, Reiberger T, Duyverman AM, Huang P, Samuel R, Hiddingh L, Roberge S, Koppel C, Lauwers GY, Zhu AX, Jain RK, Duda DG. Differential effects of sorafenib on liver versus tumor fibrosis mediated by stromal-derived factor 1 alpha/C-X-C receptor type 4 axis and myeloid differentiation antigen-positive myeloid cell infiltration in mice. Hepatology. 2014;59:1435–1447. doi: 10.1002/hep.26790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Y, Ramjiawan RR, Reiberger T, Ng MR, Hato T, Huang Y, Ochiai H, Kitahara S, Unan EC, Reddy TP, Fan C, Huang P, Bardeesy N, Zhu AX, Jain RK, Duda DG. CXCR4 inhibition in tumor microenvironment facilitates anti-programmed death receptor-1 immunotherapy in sorafenib-treated hepatocellular carcinoma in mice. Hepatology. 2015;61:1591–1602. doi: 10.1002/hep.27665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Y, Yu DK, Zhang H, He HW, Zhang CX, Zhao WL, Shao RG. CD133(+)EpCAM(+) Phenotype Possesses More Characteristics of Tumor Initiating Cells in Hepatocellular Carcinoma Huh7 Cells. Int J Biol Sci. 2012;8:992–1004. doi: 10.7150/ijbs.4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chu CM, Liaw YF. Membrane staining for hepatitis B surface antigen on hepatocytes: a sensitive and specific marker of active viral replication in hepatitis B. Journal of clinical pathology. 1995;48:470– 473. doi: 10.1136/jcp.48.5.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chuang WL, Liu HW, Chang WY. Natural killer cell activity in patients with hepatocellular carcinoma relative to early development and tumor invasion. Cancer. 1990;65:926–930. doi: 10.1002/1097-0142(19900215)65:4<926::aid-cncr2820650418>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 25.Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science. 2013;342:1432–1433. doi: 10.1126/science.342.6165.1432. [DOI] [PubMed] [Google Scholar]
- 26.Deng Z, Wu Y, Ma W, Zhang S, Zhang YQ. Adoptive T-cell therapy of prostate cancer targeting the cancer stem cell antigen EpCAM. BMC immunology. 2015;16:1. doi: 10.1186/s12865-014-0064-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di Stasi A, Tey SK, Dotti G, Fujita Y, Kennedy-Nasser A, Martinez C, Straathof K, Liu E, Durett AG, Grilley B, Liu H, Cruz CR, Savoldo B, Gee AP, Schindler J, Krance RA, Heslop HE, Spencer DM, Rooney CM, Brenner MK. Inducible apoptosis as a safety switch for adoptive cell therapy. The New England journal of medicine. 2011;365:1673–1683. doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Di Stasi A, Tey SK, Dotti G, Fujita Y, Kennedy-Nasser A, Martinez C, Straathof K, Liu E, Durett AG, Grilley B, Liu H, Cruz CR, Savoldo B, Gee AP, Schindler J, Krance RA, Heslop HE, Spencer DM, Rooney CM, Brenner MK. Inducible Apoptosis as a Safety Switch for Adoptive Cell Therapy. New Engl J Med. 2011;365:1673–1683. doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ding K, Fan L, Chen S, Wang Y, Yu H, Sun Y, Yu J, Wang L, Liu X, Liu Y. Overexpression of osteopontin promotes resistance to cisplatin treatment in HCC. Oncology reports. 2015;34:3297–3303. doi: 10.3892/or.2015.4306. [DOI] [PubMed] [Google Scholar]
- 30.Ding T, Xu J, Wang F, Shi M, Zhang Y, Li SP, Zheng L. High tumor-infiltrating macrophage density predicts poor prognosis in patients with primary hepatocellular carcinoma after resection. Human pathology. 2009;40:381–389. doi: 10.1016/j.humpath.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 31.El-Khoueiry AB, Melero I, Crocenzi TS, Welling TH, Yau TC, Yeo W, Chopra A, Grosso J, Lang L, Anderson J. Phase I/II safety and antitumor activity of nivolumab in patients with advanced hepatocellular carcinoma (HCC): CA209-040. ASCO Annual Meeting Proceedings; 2015; p. LBA101. [Google Scholar]
- 32.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264–1273. e1261. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.English DP, Bellone S, Schwab CL, Roque DM, Lopez S, Bortolomai I, Cocco E, Bonazzoli E, Chatterjee S, Ratner E, Silasi DA, Azodi M, Schwartz PE, Rutherford TJ, Santin AD. Solitomab, an epithelial cell adhesion molecule/CD3 bispecific antibody (BiTE), is highly active against primary chemo-therapy-resistant ovarian cancer cell lines in vitro and fresh tumor cells ex vivo. Cancer. 2015;121:403–412. doi: 10.1002/cncr.29062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:720–724. doi: 10.1073/pnas.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferrara N. Vascular endothelial growth factor: Basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 36.Ferrari F, Bellone S, Black J, Schwab CL, Lopez S, Cocco E, Bonazzoli E, Predolini F, Menderes G, Litkouhi B, Ratner E, Silasi DA, Azodi M, Schwartz PE, Santin AD. Solitomab, an EpCAM/CD3 bispecific antibody construct (BiTE (R)), is highly active against primary uterine and ovarian carcinosarcoma cell lines in vitro. J Exp Clin Canc Res. 2015;34 doi: 10.1186/s13046-015-0241-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferrari F, Bellone S, Black J, Schwab CL, Lopez S, Cocco E, Bonazzoli E, Predolini F, Menderes G, Litkouhi B, Ratner E, Silasi DA, Azodi M, Schwartz PE, Santin AD. Solitomab, an EpCAM/CD3 bispecific antibody construct (BiTE(R)), is highly active against primary uterine and ovarian carcinosarcoma cell lines in vitro. Journal of experimental & clinical cancer research : CR. 2015;34:123. doi: 10.1186/s13046-015-0241-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fry TJ, Stetler-Stevenson M, Shah NN, Yuan CM, Yates B, Delbrook C, Zhang L, Lee DW, Stroncek D, Mackall CL. Clinical Activity and Persistence of Anti-CD22 Chimeric Antigen Receptor in Children and Young Adults with Relapsed/Refractory Acute Lymphoblastic Leukemia (ALL) Blood. 2015;126:1324–1324. [Google Scholar]
- 39.Gao B, Jeong WI, Tian Z. Liver: An organ with predominant innate immunity. Hepatology. 2008;47:729–736. doi: 10.1002/hep.22034. [DOI] [PubMed] [Google Scholar]
- 40.Gao H, Li K, Tu H, Pan X, Jiang H, Shi B, Kong J, Wang H, Yang S, Gu J, Li Z. Development of T cells redirected to glypican-3 for the treatment of hepatocellular carcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014;20:6418–6428. doi: 10.1158/1078-0432.CCR-14-1170. [DOI] [PubMed] [Google Scholar]
- 41.Gao XH, Tian L, Wu J, Ma XL, Zhang CY, Zhou Y, Sun YF, Hu B, Qiu SJ, Zhou J, Fan J, Guo W, Yang XR. Circulating CD14+ HLA-DR-/low myeloid-derived suppressor cells predicted early recurrence of hepatocellular carcinoma after surgery. Hepatology research : the official journal of the Japan Society of Hepatology. 2016 doi: 10.1111/hepr.12831. [DOI] [PubMed] [Google Scholar]
- 42.Geng M, Xin X, Bi LQ, Zhou LT, Liu XH. Molecular mechanism of hepatitis B virus X protein function in hepatocarcinogenesis. World journal of gastroenterology. 2015;21:10732–10738. doi: 10.3748/wjg.v21.i38.10732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goossens N, Hoshida Y. Hepatitis C virus-induced hepatocellular carcinoma. Clinical and molecular hepatology. 2015;21:105–114. doi: 10.3350/cmh.2015.21.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gotoh M, Sakamoto M, Kanetaka K, Chuuma M, Hirohashi S. Overexpression of osteopontin in hepatocellular carcinoma. Pathol Int. 2002;52:19–24. doi: 10.1046/j.1440-1827.2002.01316.x. [DOI] [PubMed] [Google Scholar]
- 45.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF, Milone MC, Levine BL, June CH. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. The New England journal of medicine. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haraguchi N, Ishii H, Mimori K, Tanaka F, Ohkuma M, Kim HM, Akita H, Takiuchi D, Hatano H, Nagano H, Barnard GF, Doki Y, Mori M. CD13 is a therapeutic target in human liver cancer stem cells. J Clin Invest. 2010;120:3326–3339. doi: 10.1172/JCI42550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hashimoto K, Perera A, Ogita Y, Nakamura M, Ishiguro T, Sano Y, Kinoshita Y. A phase I dose escalation and cohort expansion study of T-cell redirecting bispecific antibody against Glypican 3 in patients with advanced solid tumors. ASCO Annual Meeting, J Clin Oncol; 2016. abstr TPS2592. [Google Scholar]
- 48.He AR, Smith DC, Mishra L. Targeting cancer stem cells in hepatocellular carcinoma. Gastrointestinal Cancer: Targets & Therapy. 2015:5. [Google Scholar]
- 49.Ho M, Kim H. Glypican-3: a new target for cancer immunotherapy. European journal of cancer. 2011;47:333–338. doi: 10.1016/j.ejca.2010.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoh A, Dewerth A, Vogt F, Wenz J, Baeuerle PA, Warmann SW, Fuchs J, Armeanu-Ebinger S. The activity of gamma delta T cells against paediatric liver tumour cells and spheroids in cell culture. Liver Int. 2013;33:127–136. doi: 10.1111/liv.12011. [DOI] [PubMed] [Google Scholar]
- 51.Hsu HC, Cheng W, Lai PL. Cloning and expression of a developmentally regulated transcript MXR7 in hepatocellular carcinoma: Biological significance and temporospatial distribution. Cancer Res. 1997;57:5179–5184. [PubMed] [Google Scholar]
- 52.Ishiyama K, Ohdan H, Ohira M, Mitsuta H, Arihiro K, Asahara T. Difference in cytotoxicity against hepatocellular carcinoma between liver and periphery natural killer cells in humans. Hepatology. 2006;43:362–372. doi: 10.1002/hep.21035. [DOI] [PubMed] [Google Scholar]
- 53.Jochems C, Schlom J. Tumor-infiltrating immune cells and prognosis: the potential link between conventional cancer therapy and immunity. Experimental biology and medicine. 2011;236:567–579. doi: 10.1258/ebm.2011.011007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kalathil SG, Lugade AA, Miller A, Iyer R, Thanavala Y. PD-1+ and Foxp3+ T cell reduction correlates with survival of HCC patients after sorafenib therapy. JCI insight. 2016:1. doi: 10.1172/jci.insight.86182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kanat O, Gewirtz A, Kemeny N. What is the potential role of hepatic arterial infusion chemotherapy in the current armamentorium against colorectal cancer. Journal of gastrointestinal oncology. 2012;3:130–138. doi: 10.3978/j.issn.2078-6891.2011.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Katz SC, Burga RA, McCormack E, Wang LJ, Mooring W, Point GR, Khare PD, Thorn M, Ma Q, Stainken BF, Assanah EO, Davies R, Espat NJ, Junghans RP. Phase I Hepatic Immunotherapy for Metastases Study of Intra-Arterial Chimeric Antigen Receptor-Modified T-cell Therapy for CEA+ Liver Metastases. Clinical cancer research : an official journal of the American Association for Cancer Research. 2015;21:3149–3159. doi: 10.1158/1078-0432.CCR-14-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kinoshita Y, Tanaka S, Souzaki R, Miyoshi K, Kohashi K, Oda Y, Nakatsura T, Taguchi T. Glypican 3 Expression in Pediatric Malignant Solid Tumors. Eur J Pediatr Surg. 2015;25:138–143. doi: 10.1055/s-0034-1393961. [DOI] [PubMed] [Google Scholar]
- 58.Koh S, Shimasaki N, Suwanarusk R, Ho ZZ, Chia A, Banu N, Howland SW, Ong ASM, Gehring AJ, Stauss H, Renia L, Sallberg M, Campana D, Bertoletti A. A Practical Approach to Immunotherapy of Hepatocellular Carcinoma Using T Cells Redirected Against Hepatitis B Virus. Mol Ther-Nucl Acids. 2013:2. doi: 10.1038/mtna.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Korita PV, Wakai T, Shirai Y, Matsuda Y, Sakata J, Cui X, Ajioka Y, Hatakeyama K. Overexpression of osteopontin independently correlates with vascular invasion and poor prognosis in patients with hepatocellular carcinoma. Human pathology. 2008;39:1777–1783. doi: 10.1016/j.humpath.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 60.Kou G, Shi J, Chen L, Zhang D, Hou S, Zhao L, Fang C, Zheng L, Zhang X, Liang P, Zhang X, Li B, Guo Y. A bispecific antibody effectively inhibits tumor growth and metastasis by simultaneous blocking vascular endothelial growth factor A and osteopontin. Cancer letters. 2010;299:130–136. doi: 10.1016/j.canlet.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 61.Krebs K, Bottinger N, Huang LR, Chmielewski M, Arzberger S, Gasteiger G, Jager C, Schmitt E, Bohne F, Aichler M, Uckert W, Abken H, Heikenwalder M, Knolle P, Protzer U. T Cells Expressing a Chimeric Antigen Receptor That Binds Hepatitis B Virus Envelope Proteins Control Virus Replication in Mice. Gastroenterology. 2013;145:456–465. doi: 10.1053/j.gastro.2013.04.047. [DOI] [PubMed] [Google Scholar]
- 62.Le Trinh T, Wu Q, Chang LJ, Ho M, Liu C. Abstract 2316: GPC3-specific chimeric antigen receptor T cell in combination with Sorafenib as a novel therapeutic treatment for hepatocellular carcinoma. Cancer Res. 2016;76:2316–2316. [Google Scholar]
- 63.Lee TKW, Castilho A, Cheung VCH, Tang KH, Ma S, Irene OLN. CD24(+) Liver Tumor-Initiating Cells Drive Self-Renewal and Tumor Initiation through STAT3-Mediated NANOG Regulation. Cell Stem Cell. 2011;9:50–63. doi: 10.1016/j.stem.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 64.Li H, Wu K, Tao K, Chen L, Zheng Q, Lu X, Liu J, Shi L, Liu C, Wang G, Zou W. Tim-3/galectin-9 signaling pathway mediates T-cell dysfunction and predicts poor prognosis in patients with hepatitis B virus-associated hepatocellular carcinoma. Hepatology. 2012;56:1342–1351. doi: 10.1002/hep.25777. [DOI] [PubMed] [Google Scholar]
- 65.Li J, Gao JZ, Du JL, Wei LX. Prognostic and clinicopathological significance of glypican-3 overexpression in hepatocellular carcinoma: a meta-analysis. World journal of gastroenterology. 2014;20:6336–6344. doi: 10.3748/wjg.v20.i20.6336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li K, Pan X, Bi Y, Xu W, Chen C, Gao H, Shi B, Jiang H, Yang S, Jiang L, Li Z. Adoptive immunotherapy using T lymphocytes redirected to glypican-3 for the treatment of lung squamous cell carcinoma. Oncotarget. 2016;7:2496–2507. doi: 10.18632/oncotarget.6595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li W, Guo L, Rathi P, Marinova E, Gao X, Wu MF, Liu H, Dotti G, Gottschalk S, Metelitsa L, Heczey A. Redirecting T cells to Glypican-3 with 4-1BB.zeta CAR results in Th-1 polarization and potent anti-tumor activity. Human gene therapy. 2016 doi: 10.1089/hum.2016.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li W, Guo L, Rathi P, Marinova E, Gao X, Wu MF, Liu H, Dotti G, Gottschalk S, Metelitsa LS, Heczey A. Redirecting T Cells to Glypican-3 with 4-1BB Zeta Chimeric Antigen Receptors Results in Th1 Polarization and Potent Antitumor Activity. Human gene therapy. 2016 doi: 10.1089/hum.2016.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, Zhang Y, Liu J, Gong X, Chen Y, Wang F, Zheng H, Wang F, Guo J, Jia Z, Ma J, Wang H, Luo H, Li L, Jin S, Hadler SC, Wang Y. Reprint of: Epidemiological serosurvey of Hepatitis B in China--declining HBV prevalence due to Hepatitis B vaccination. Vaccine. 2013;31(Suppl 9):J21–28. doi: 10.1016/j.vaccine.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 70.Liao Y, Tang Z, Liu K, Ye S, Li J, Huang Z, Wang D, Segal D. Preparation and application of anti- HBx/anti-CD3 bispecific monoclonal antibody (BsAb) retargeting effector cells for lysis of human hepatoma xenografts in nude mice. Oncology reports. 1996;3:637–644. doi: 10.3892/or.3.4.637. [DOI] [PubMed] [Google Scholar]
- 71.Lin F, Li YY, Cao J, Fan SF, Wen JF, Zhu GH, Du H, Liang YX. Overexpression of osteopontin in hepatocellular carcinoma and its relationships with metastasis, invasion of tumor cells. Mol Biol Rep. 2011;38:5205–5210. doi: 10.1007/s11033-010-0671-4. [DOI] [PubMed] [Google Scholar]
- 72.Liu H, Xu Y, Xiang J, Long L, Green S, Yang Z, Zimdahl B, Lu J, Cheng N, Horan LH, Liu B, Yan S, Wang P, Diaz J, Jin L, Nakano Y, Morales JF, Zhang P, Liu LX, Staley BK, Priceman SJ, Brown CE, Forman SJ, Chan VW, Liu C. Targeting Alpha-Fetoprotein (AFP)-MHC Complex with CAR T-Cell Therapy for Liver Cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2016 doi: 10.1158/1078-0432.CCR-16-1203. [DOI] [PubMed] [Google Scholar]
- 73.Liu R, Shen Y, Nan K, Mi B, Wu T, Guo J, Li M, Lv Y, Guo H. Association Between Expression of Cancer Stem Cell Markers and Poor Differentiation of Hepatocellular Carcinoma: A Meta-Analysis (PRISMA) Medicine. 2015;94:e1306. doi: 10.1097/MD.0000000000001306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu X, Wang SK, Zhang K, Zhang H, Pan Q, Liu Z, Pan H, Xue L, Yen Y, Chu PG. Expression of glypican 3 enriches hepatocellular carcinoma development-related genes and associates with carcinogenesis in cirrhotic livers. Carcinogenesis. 2015;36:232–242. doi: 10.1093/carcin/bgu245. [DOI] [PubMed] [Google Scholar]
- 75.Lok AS, McMahon BJ, Brown RS, Jr, Wong JB, Ahmed AT, Farah W, Almasri J, Alahdab F, Benkhadra K, Mouchli MA, Singh S, Mohamed EA, Abu Dabrh AM, Prokop LJ, Wang Z, Murad MH, Mohammed K. Antiviral therapy for chronic hepatitis B viral infection in adults: A systematic review and meta-analysis. Hepatology. 2016;63:284–306. doi: 10.1002/hep.28280. [DOI] [PubMed] [Google Scholar]
- 76.Louis CU, Savoldo B, Dotti G, Pule M, Yvon E, Myers GD, Rossig C, Russell HV, Diouf O, Liu E, Liu H, Wu MF, Gee AP, Mei Z, Rooney CM, Heslop HE, Brenner MK. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood. 2011;118:6050–6056. doi: 10.1182/blood-2011-05-354449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mackall CL, Merchant MS, Fry TJ. Immune-based therapies for childhood cancer. Nature reviews Clinical oncology. 2014;11:693–703. doi: 10.1038/nrclinonc.2014.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, Mahnke YD, Melenhorst JJ, Rheingold SR, Shen A, Teachey DT, Levine BL, June CH, Porter DL, Grupp SA. Chimeric antigen receptor T cells for sustained remissions in leukemia. The New England journal of medicine. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mayer K, Baumann AL, Grote M, Seeber S, Kettenberger H, Breuer S, Killian T, Schafer W, Brinkmann U. TriFabs--Trivalent IgG-Shaped Bispecific Antibody Derivatives: Design, Generation, Characterization and Application for Targeted Payload Delivery. International journal of molecular sciences. 2015;16:27497–27507. doi: 10.3390/ijms161126037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mima K, Okabe H, Ishimoto T, Hayashi H, Nakagawa S, Kuroki H, Watanabe M, Beppu T, Tamada M, Nagano O, Saya H, Baba H. CD44s Regulates the TGF-beta-Mediated Mesenchymal Phenotype and Is Associated with Poor Prognosis in Patients with Hepatocellular Carcinoma. Cancer Res. 2012;72:3414–3423. doi: 10.1158/0008-5472.CAN-12-0299. [DOI] [PubMed] [Google Scholar]
- 81.Miura H, Miyazaki T, Kuroda M, Oka T, Machinami R, Kodama T, Shibuya M, Makuuchi M, Yazaki Y, Ohnishi S. Increased expression of vascular endothelial growth factor in human hepatocellular carcinoma. J Hepatol. 1997;27:854–861. doi: 10.1016/s0168-8278(97)80323-6. [DOI] [PubMed] [Google Scholar]
- 82.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18:843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case Report of a Serious Adverse Event Following the Administration of T Cells Transduced With a Chimeric Antigen Receptor Recognizing ERBB2. Mol Ther. 2010;18:843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nakatsura T, Yoshitake Y, Senju S, Monji M, Komori H, Motomura Y, Hosaka S, Beppu T, Ishiko T, Kamohara H, Ashihara H, Katagiri T, Furukawa Y, Fujiyama S, Ogawa M, Nakamura Y, Nishimura Y. Glypican-3, overexpressed specifically in human hepatocellular carcinoma, is a novel tumor marker. Biochem Bioph Res Co. 2003;306:16–25. doi: 10.1016/s0006-291x(03)00908-2. [DOI] [PubMed] [Google Scholar]
- 85.Newick K, O'Brien S, Moon E, Albelda SM. CAR T Cell Therapy for Solid Tumors. Annu Rev Med. 2017;68:139–152. doi: 10.1146/annurev-med-062315-120245. [DOI] [PubMed] [Google Scholar]
- 86.Patriarca C, Macchi RM, Marschner AK, Mellstedt H. Epithelial cell adhesion molecule expression (CD326) in cancer: A short review. Cancer Treat Rev. 2012;38:68–75. doi: 10.1016/j.ctrv.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 87.Petrelli F, Borgonovo K, Lonati V, Elia S, Barni S. Regression of liver metastases after treatment with intraperitoneal catumaxomab for malignant ascites due to breast cancer. Target Oncol. 2013;8:291–294. doi: 10.1007/s11523-012-0240-y. [DOI] [PubMed] [Google Scholar]
- 88.Petsch S, Gires O, Ruttinger D, Denzel S, Lippold S, Baeuerle PA, Wolf A. Concentrations of EpCAM ectodomain as found in sera of cancer patients do not significantly impact redirected lysis and Tcell activation by EpCAM/CD3–bispecific BiTE antibody MT110. Mabs-Austin. 2011;3:31–37. doi: 10.4161/mabs.3.1.14193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Porter DL, Hwang WT, Frey NV, Lacey SF, Shaw PA, Loren AW, Bagg A, Marcucci KT, Shen A, Gonzalez V, Ambrose D, Grupp SA, Chew A, Zheng Z, Milone MC, Levine BL, Melenhorst JJ, June CH. Chimeric antigen receptor T-cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Science translational medicine. 2015;7:303ra139. doi: 10.1126/scitranslmed.aac5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. The New England journal of medicine. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ramchandani D, Weber GF. Interactions between osteopontin and vascular endothelial growth factor: Implications for cancer. Biochimica et biophysica acta. 2015;1855:202–222. doi: 10.1016/j.bbcan.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 92.Robbins PF, Morgan RA, Feldman SA, Yang JC, Sherry RM, Dudley ME, Wunderlich JR, Nahvi AV, Helman LJ, Mackall CL, Kammula US, Hughes MS, Restifo NP, Raffeld M, Lee CC, Levy CL, Li YF, El-Gamil M, Schwarz SL, Laurencot C, Rosenberg SA. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:917–924. doi: 10.1200/JCO.2010.32.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rosenberg SA, Yannelli JR, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS, Parkinson DR, Seipp CA, Einhorn JH, White DE. Treatment of patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and interleukin 2. Journal of the National Cancer Institute. 1994;86:1159–1166. doi: 10.1093/jnci/86.15.1159. [DOI] [PubMed] [Google Scholar]
- 94.Sahm C, Schonfeld K, Wels WS. Expression of IL-15 in NK cells results in rapid enrichment and selective cytotoxicity of gene-modified effectors that carry a tumor-specific antigen receptor. Cancer immunology immunotherapy : CII. 2012;61:1451–1461. doi: 10.1007/s00262-012-1212-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Saijyo S, Kudo T, Katayose Y, Saeki H, Chiba N, Suzuki M, Tominaga T, Matsuno S. A new in vitro model of specific targeting therapy of cancer: retargeting of PWM-LAK cells with bispecific antibodies greatly enhances cytotoxicity to hepatocellular carcinoma. The Tohoku journal of experimental medicine. 1996;178:113–127. doi: 10.1620/tjem.178.113. [DOI] [PubMed] [Google Scholar]
- 96.Sautto GA, Wisskirchen K, Clementi N, Castelli M, Diotti RA, Graf J, Clementi M, Burioni R, Protzer U, Mancini N. Chimeric antigen receptor (CAR)-engineered T cells redirected against hepatitis C virus (HCV) E2 glycoprotein. Gut. 2016;65:512–523. doi: 10.1136/gutjnl-2014-308316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Seeber A, Martowicz A, Spizzo G, Buratti T, Obrist P, Fong D, Gastl G, Untergasser G. Soluble EpCAM levels in ascites correlate with positive cytology and neutralize catumaxomab activity in vitro. Bmc Cancer. 2015:15. doi: 10.1186/s12885-015-1371-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Seimetz D. Novel monoclonal antibodies for cancer treatment: the trifunctional antibody catumaxomab (removab) Journal of Cancer. 2011;2:309–316. doi: 10.7150/jca.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shang SF, Plymoth A, Ge SK, Feng ZD, Rosen HR, Sangrajrang S, Hainaut P, Marrero JA, Beretta L. Identification of osteopontin as a novel marker for early hepatocellular carcinoma. Hepatology. 2012;55:483–490. doi: 10.1002/hep.24703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shi F, Shi M, Zeng Z, Qi RZ, Liu ZW, Zhang JY, Yang YP, Tien P, Wang FS. PD-1 and PD-L1 upregulation promotes CD8(+) T-cell apoptosis and postoperative recurrence in hepatocellular carcinoma patients. Int J Cancer. 2011;128:887–896. doi: 10.1002/ijc.25397. [DOI] [PubMed] [Google Scholar]
- 101.Soga N, Connolly JO, Chellaiah M, Kawamura J, Hruska KA. Rac regulates vascular endothelial growth factor stimulated motility. Cell communication & adhesion. 2001;8:1–13. doi: 10.3109/15419060109080703. [DOI] [PubMed] [Google Scholar]
- 102.Sun TW, Gao Q, Qiu SJ, Zhou J, Wang XY, Yi Y, Shi JY, Xu YF, Shi YH, Song K, Xiao YS, Fan J. B7-H3 is expressed in human hepatocellular carcinoma and is associated with tumor aggressiveness and postoperative recurrence. Cancer immunology, immunotherapy : CII. 2012;61:2171–2182. doi: 10.1007/s00262-012-1278-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sun YF, Xu Y, Yang XR, Guo W, Zhang X, Qiu SJ, Shi RY, Hu B, Zhou J, Fan J. Circulating stem cell-like epithelial cell adhesion molecule-positive tumor cells indicate poor prognosis of hepatocellular carcinoma after curative resection. Hepatology. 2013;57:1458–1468. doi: 10.1002/hep.26151. [DOI] [PubMed] [Google Scholar]
- 104.Sung JJ, Noh SJ, Bae JS, Park HS, Jang KY, Chung MJ, Moon WS. Immunohistochemical Expression and Clinical Significance of Suggested Stem Cell Markers in Hepatocellular Carcinoma. Journal of pathology and translational medicine. 2015 doi: 10.4132/jptm.2015.10.09. [DOI] [PMC free article] [PubMed]
- 105.Tarocchi M, Polvani S, Marroncini G, Galli A. Molecular mechanism of hepatitis B virus-induced hepatocarcinogenesis. World journal of gastroenterology. 2014;20:11630–11640. doi: 10.3748/wjg.v20.i33.11630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Terris B, Cavard C, Perret C. EpCAM, a new marker for cancer stem cells in hepatocellular carcinoma. J Hepatol. 2010;52:280–281. doi: 10.1016/j.jhep.2009.10.026. [DOI] [PubMed] [Google Scholar]
- 107.Truong P, Rahal A, Kallail KJ. Metastatic Hepatocellular Carcinoma Responsive to Pembrolizumab. Cureus. 2016;8:e631. doi: 10.7759/cureus.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tu JF, Ding YH, Ying XH, Wu FZ, Zhou XM, Zhang DK, Zou H, Ji JS. Regulatory T cells, especially ICOS+ FOXP3+ regulatory T cells, are increased in the hepatocellular carcinoma microenvironment and predict reduced survival. Scientific reports. 2016;6:35056. doi: 10.1038/srep35056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vannini N, Roch A, Naveiras O, Griffa A, Kobel S, Lutolf MP. Identification of in vitro HSC fate regulators by differential lipid raft clustering. Cell cycle. 2012;11:1535–1543. doi: 10.4161/cc.19900. [DOI] [PubMed] [Google Scholar]
- 110.von Marschall Z, Cramer T, Hocker M, Finkenzeller G, Wiedenmann B, Rosewicz S. Dual mechanism of vascular endothelial growth factor upregulation by hypoxia in human hepatocellular carcinoma. Gut. 2001;48:87–96. doi: 10.1136/gut.48.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xiao X, Lao XM, Chen MM, Liu RX, Wei Y, Ouyang FZ, Chen DP, Zhao XY, Zhao Q, Li XF, Liu CL, Zheng L, Kuang DM. PD-1hi Identifies a Novel Regulatory B-cell Population in Human Hepatoma That Promotes Disease Progression. Cancer discovery. 2016;6:546–559. doi: 10.1158/2159-8290.CD-15-1408. [DOI] [PubMed] [Google Scholar]
- 112.Xu X, Liu RF, Zhang X, Huang LY, Chen F, Fei QL, Han ZG. DLK1 as a Potential Target against Cancer Stem/Progenitor Cells of Hepatocellular Carcinoma. Mol Cancer Ther. 2012;11:629–638. doi: 10.1158/1535-7163.MCT-11-0531. [DOI] [PubMed] [Google Scholar]
- 113.Yamashita T, Forgues M, Wang W, Kim JW, Ye Q, Jia H, Budhu A, Zanetti KA, Chen Y, Qin LX, Tang ZY, Wang XW. EpCAM and alpha-fetoprotein expression defines novel prognostic subtypes of hepatocellular carcinoma. Cancer Res. 2008;68:1451–1461. doi: 10.1158/0008-5472.CAN-07-6013. [DOI] [PubMed] [Google Scholar]
- 114.Yamashita T, Honda M, Nakamoto Y, Baba M, Nio K, Hara Y, Zeng SS, Hayashi T, Kondo M, Takatori H, Yamashita T, Mizukoshi E, Ikeda H, Zen Y, Takamura H, Wang XW, Kaneko S. Discrete nature of EpCAM+ and CD90+cancer stem cells in human hepatocellular carcinoma. Hepatology. 2013;57:1484–1497. doi: 10.1002/hep.26168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yamashita T, Ji J, Budhu A, Forgues M, Yang W, Wang HY, Jia H, Ye Q, Qin LX, Wauthier E, Reid LM, Minato H, Honda M, Kaneko S, Tang ZY, Wang XW. EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology. 2009;136:1012–1024. doi: 10.1053/j.gastro.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yamashita T, Ji JF, Budhu A, Forgues M, Yang W, Wang HY, Jia HL, Ye QH, Qin LX, Wauthier E, Reid LM, Minato H, Honda M, Kaneko S, Tang ZY, Wang XW. EpCAM-Positive Hepatocellular Carcinoma Cells Are Tumor-Initiating Cells With Stem/Progenitor Cell Features. Gastroenterology. 2009;136:1012–1024. doi: 10.1053/j.gastro.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yan XL, Jia YL, Chen L, Zeng Q, Zhou JN, Fu CJ, Chen HX, Yuan HF, Li ZW, Shi L, Xu YC, Wang JX, Zhang XM, He LJ, Zhai C, Yue W, Pei XT. Hepatocellular carcinoma-associated mesenchymal stem cells promote hepatocarcinoma progression: role of the S100A4-miR155-SOCS1- MMP9 axis. Hepatology. 2013;57:2274–2286. doi: 10.1002/hep.26257. [DOI] [PubMed] [Google Scholar]
- 118.Yang W, Wang C, Lin Y, Liu Q, Yu LX, Tang L, Yan HX, Fu J, Chen Y, Zhang HL, Tang L, Zheng LY, He YQ, Li YQ, Wu FQ, Zou SS, Li Z, Wu MC, Feng GS, Wang HY. OV6(+) tumor-initiating cells contribute to tumor progression and invasion in human hepatocellular carcinoma. J Hepatol. 2012;57:613–620. doi: 10.1016/j.jhep.2012.04.024. [DOI] [PubMed] [Google Scholar]
- 119.Yang XR, Xu Y, Yu B, Zhou J, Qiu SJ, Shi GM, Zhang BH, Wu WZ, Shi YH, Wu B, Yang GH, Ji Y, Fan J. High expression levels of putative hepatic stem/progenitor cell biomarkers related to tumour angiogenesis and poor prognosis of hepatocellular carcinoma. Gut. 2010;59:953–962. doi: 10.1136/gut.2008.176271. [DOI] [PubMed] [Google Scholar]
- 120.Yang ZF, Ho DW, Ng MN, Lau CK, Yu WC, Ngai P, Chu PWK, Lam CT, Poon RTP, Fan ST. Significance of CD90(+) cancer stem cells in human liver cancer. Cancer Cell. 2008;13:153–166. doi: 10.1016/j.ccr.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 121.Yin SY, Li JJ, Hu C, Chen XH, Yao M, Yan MX, Jiang GP, Ge C, Xie HY, Wan DF, Yang SL, Zheng SS, Gu JR. CD133 positive hepatocellular carcinoma cells possess high capacity for tumorigenicity. Int J Cancer. 2007;120:1444–1450. doi: 10.1002/ijc.22476. [DOI] [PubMed] [Google Scholar]
- 122.Ying X, Zhao Y, Wang JL, Zhou X, Zhao J, He CC, Guo XJ, Jin GH, Wang LJ, Zhu Q, Han SX. Serum anti-osteopontin autoantibody as a novel diagnostic and prognostic biomarker in patients with hepatocellular carcinoma. Oncology reports. 2014;32:1550–1556. doi: 10.3892/or.2014.3367. [DOI] [PubMed] [Google Scholar]
- 123.Zhang P, Shi B, Gao H, Jiang H, Kong J, Yan J, Pan X, Li K, Zhang P, Yao M, Yang S, Gu J, Wang H, Li Z. An EpCAM/CD3 bispecific antibody efficiently eliminates hepatocellular carcinoma cells with limited galectin-1 expression. Cancer immunology, immunotherapy : CII. 2014;63:121–132. doi: 10.1007/s00262-013-1497-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhu Z, Hao XF, Yan MX, Yao M, Ge C, Gu JR, Li JJ. Cancer stem/progenitor cells are highly enriched in CD133(+)CD44(+) population in hepatocellular carcinoma. Int J Cancer. 2010;126:2067– 2078. doi: 10.1002/ijc.24868. [DOI] [PubMed] [Google Scholar]