Abstract
Background:
Hormonal fluctuations during menopause lead to endocrine changes in women, especially in their sex steroid hormone production. Studies have documented the role of estrogen and progesterone (Pg) on autoimmune disorders such as multiple sclerosis, systemic lupus erythematosus, and rheumatoid arthritis. Lichen planus (LP), an autoimmune disorder, seen frequently in perimenopausal women, may also get affected by sex steroid hormones, but no direct relationship has been established yet.
Aim:
The aim of this study is to find the incidence of oral LP (OLP) in perimenopausal women and evaluate the factors associated with it.
Materials and Methods:
This cross-sectional study was conducted over a period of 1 year. All the perimenopausal women (44.69 ± 3.79 years) who came to the dental outpatient department were evaluated for the presence of LP and various factors associated with it. Depression Anxiety Stress Scale-21 questionnaire was used for psychometric evaluation of perimenopausal women.
Results:
According to our study, incidence of LP in postmenopausal women was 10.91%, which is higher than incidence of LP in general population, i.e., 0.5% to 2.0%. Incidence of LP increased with the severity of depression in perimenopausal women (P = 0.000).
Conclusion:
The incidence of OLP is higher in perimenopausal women than in general population and increases significantly with increase in the severity of depression. LP in perimenopausal women can be mediated by declined level of estrogen and Pg directly or indirectly through causing depression that can trigger LP.
KEYWORDS: Autoimmunity, depression, estrogen, lichen planus, perimenopausal women, progesterone
INTRODUCTION
The World Health Organization (WHO) has defined three age stages during the midlife age for women: (1) Menopause is the year of the final physiological menstrual period retrospectively designated as 1 year without flow (unrelated to pregnancy or therapy) in women aged ≥40 years. (2) Premenopause begins at ages 35–39 years; during this stage, decreased fertility and fecundity appear as the first manifestations of ovarian follicle depletion and dysfunction, despite the absence of menstrual changes. (3) Perimenopause includes the period of years immediately before menopause and the 1st year after menopause.[1] Perimenopause is the time of irregular periods until menopause. The perimenopausal age in Indian women is 44.69 ± 3.79 years.[2] During this phase, hormonal fluctuations lead to endocrine changes in women, especially in their sex steroid hormone production. As the monotropic follicle-stimulating hormone rises, which is characteristic of the menopausal transition (MT), estradiol (E2) can also be raised in the early follicular phase of the cycle, especially in the early MT. Early follicular E2 levels are the last biomarker of the transition to irreversible change, with a quick decline beginning 2 years before the final menstrual period and reaching stability 2 years afterward.[3] Progesterone (Pg) (P4) levels become insufficient or absent.[4]
Studies have documented the role of estrogen and Pg on autoimmune disorders such as multiple sclerosis (MS), systemic lupus erythematosus, and rheumatoid arthritis (RA). Lichen planus (LP), a chronic, autoimmune, mucocutaneous, psychosocial disease that usually presents in middle-aged females, may also get affected by sex steroid hormones, but no direct relationship has been established yet.[1] LP is estimated to affect 0.5%–2.0% of the general population, with a prevalence of 2.6% in the Indian population.[5]
Our study was an endeavor to find the incidence of oral LP (OLP) in perimenopausal women and evaluate various factors associated with it such as psychosocial factors that may play a role in the etiology of the disease. We also made an effort to explain the influence of fluctuating sex steroid hormones of perimenopausal women on LP.
MATERIALS AND METHODS
This cross-sectional study was conducted in the Department of Oral Medicine and Radiology. The study protocol was approved by the Institutional Ethical Committee. The duration of the present study was 12 months and included 1576 perimenopausal women (44.69 ± 3.79 years)[2] who reported to our department from January 2016 to December 2016, out of which 172 were clinically diagnosed with OLP. The clinical diagnostic criteria for oral lesions used in this study were as follows: (i) the presence of keratotic, pinhead-sized, white, slightly elevated papules (papular LP), which may be discrete or arranged in reticular (reticular LP) or plaque-like (plaque-like LP) configurations; (ii) atrophic LP, characterized by thinning of the epithelium leading to the appearance of atrophic red areas within the white lesions; (iii) erosive (ulcerative) LP, characterized by areas of well-defined ulceration within the abovementioned lesions; and (iv) bullous LP, characterized by the presence or development of bullous areas within the abovementioned lesions.[5]
Exclusion criteria included the use of hormone replacement therapy, any systemic steroids, immunosuppressive drugs, or nonsteroidal anti-inflammatory drugs within the last 4 weeks and the use of topical medications within the last 2 weeks. Other exclusion criteria included tobacco use, the presence of any known systemic diseases, other dermatologic diseases affecting immune system, and any malignancy. Patients receiving any medication that can cause lichenoid reaction such as antihypertensive drugs and oral hypoglycemics were also excluded from the study.
All the perimenopausal women underwent a psychological evaluation using the Depression Anxiety Stress Scale-21 (DASS-21). Other factors such as residential area of patient, menstrual irregularities, extraoral and intraoral location of lesions, and type of intraoral lesion were evaluated in LP patients.
Patients were also evaluated for the presence of hypertension, diabetes, thyroid disorders, and hepatitis B virus (HBV) and hepatitis C virus (HCV). They were also asked to get liver function test (LFT) done.
Statistical analysis of the data was performed using the Statistical Package for Social Sciences (version 17.0; SPSS, Inc., Chicago, IL, USA). Chi-square test was used for nonparametric values. A probability (P) <0.05 was considered to be significant and P < 0.001 was considered to be highly significant.
RESULTS
A total of 9100 females reported to our department from January 2016 to December 2016, out of which 1576 were perimenopausal women. About 10.91% of the perimenopausal women suffered from OLP.
Psychological state
Incidence of LP significantly increased with the severity of depression in perimenopausal women (P = 0.000) [Table 1].
Table 1.
Incidence of lichen planus significantly increased with the severity of depression in perimenopausal women
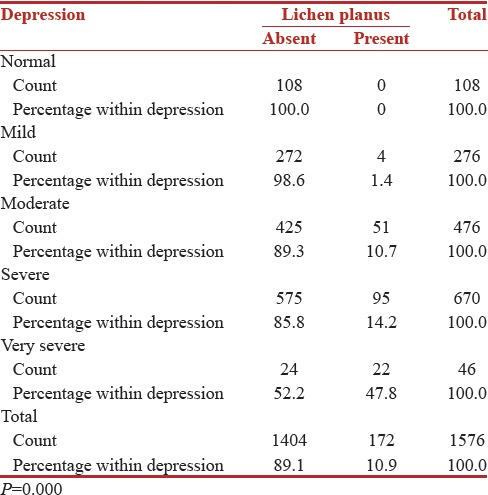
According to the DASS, depression was severe in 55.2% patients, moderate in 29.7%, very severe in 12.8%, and mild in 2.3% OLP patients. These differences were statistically significant (P = 0.000).
Anxiety was severe in 41.3% patients, mild in 26.7%, moderate in 20.9%, and very severe in 11% patients.
There was moderate stress in 69.8% patients, severe in 21.5%, mild in 7.6%, and very severe in 1.2% patients.
Menstrual cycle
About 73.8% of patients reported menstrual irregularities while 26.2% of patients did not notice any significant change in menstrual cycle.
Rural, suburban, urban areas and oral lichen planus cases
Maximum, i.e., 46.5% of patients belonged to urban areas, 34.3% to suburban areas, and minimum, i.e., 19.2% to rural areas.
Extraoral location of lesion in oral lichen planus cases
Of all the perimenopausal women with OLP who reported to our department, skin lesions were present in only 6.4% cases. Nails were affected in 4.1% and lips in 7% patients. About 2.3% of patients had genital lesions. Nearly 0.6% of the patients had lesions on the scalp.
Visual analog scale score in oral lichen planus cases
Pain in the oral cavity associated with the lesions was assessed using visual analog scale and a rating of 8 was given by 34.3% patients, 7 by 27.9%, 9 by 11%, 0 by 5.8%, 3 by 5.2%, 4 by 5.2%, 5 by 5.2%, and 6 by 5.2%.
Intraoral subtypes of lichen planus in oral lichen planus cases
Out of the six subtypes of LP, the most common was reticular LP, found in 70.3% cases. Erosive LP was seen in 57.6% cases, atrophic in 38.4% cases, plaque-like in 16.3% cases, bullae in 6.4%, and papular in 5.8% cases.
Intraoral location of lesion in oral lichen planus cases
Most common location in the oral cavity was the buccal mucosa which was involved in 89% cases followed by buccal vestibule (61.6%), tongue (16.3%), floor of the mouth (5.2%), and palate (5.2%). Gingival desquamation was present in 68.6% cases.
Hypertension and diabetes
About 16.3% of patients were diagnosed with hypertension and 16.9% with diabetes. Nearly 10.46% of perimenopausal women with OLP were diagnosed with hypertension as well as diabetes.
Thyroid disorder
Nearly 1.2% of patients were diagnosed with hypothyroidism.
Liver function test
In 1.2% of patients, LFT revealed abnormal values.
Hepatitis B virus and hepatitis C virus
None of the patients were positive for HBV, but 1.2% of patients were positive for HCV.
DISCUSSION
According to our study, incidence of LP in postmenopausal women was 10.91%, which was higher than incidence of LP in general population, i.e., 0.5%–2.0%. These results can be justified by understanding the etiopathogenesis of LP and effect of estrogen and Pg which fluctuate and eventually fall down during menopause on the immune system.
Cell-mediated immunity plays an important part in the pathogenesis of OLP. OLP probably results from an immunologically induced degeneration of basal layer and is characterized by cytotoxic CD8+ cell response on modified keratinocyte surface antigen.[6]
Langerhans cells are increased in OLP lesions, and MHC class II expression is upregulated. Langerhans cells probably mediate the MHC class II antigen presentation in OLP. There is MHC class II antigen presentation to CD4+ helper T-cells, followed by keratinocyte apoptosis triggered by CD8+ cytotoxic T-cells.[6]
Sex hormones are known to play a role in the immune response of the body. Estrogens boost the humoral immunity but have a different impact on cell-mediated immunity which actually plays the main part in pathogenesis of OLP. Estrogen has been shown to modulate all subsets of T-cells that include CD4+ (Th1, Th2, Th17, and Tregs) and CD8+ cells. Estrogen promotes the expansion and frequency of Treg cells, which play a crucial role in downregulating immune responses. Protective effects of estrogen in autoimmune conditions such as MS and RA are believed to be due to a combined result of estrogen-mediated Treg expansion and activation.[7]
Androgens and Pg are natural immune suppressors. In vivo and in vitro evidence suggest that Pg can suppress CD4+ T-cell proliferation and Th1/Th17 differentiation and effector functions. In contrast, Pg can enhance Th2 and Treg differentiation.[8]
White et al. found that the cytolytic activity of CD3+ CD8+ T-cells in the uterine lining of women in the secretory phase of the menstrual cycle (Pg + E2 effects) was significantly reduced compared to that in the proliferative phase (E2 dominant), signifying Pg and E2 together suppress cytotoxicity.[9]
As the level of estrogen and Pg fluctuates and finally goes down, so does the protective effect of these hormones and increases the chances of LP.
In our study, incidence of LP significantly increased with the severity of depression in perimenopausal women, so depression appears to play a role in the etiopathogenesis of LP.
It has been documented that perimenopausal women having symptoms of depression are reported to have lower plasma estrone levels than nondepressed perimenopausal women. Studies have elucidated that depressive symptoms were more common in perimenopausal than postmenopausal women. The majority of depressive episodes occurred during the late menopause transition which is characterized by estradiol “withdrawal” relative to either the postmenopause or the early perimenopause suggesting an endocrine trigger related to the perimenopause in the onset of perimenopausal depression.[10]
Existing data suggest that the antidepressant effects of estradiol are mediated by estrogen receptor β and can be reversed by the coadministration of a 5HT1A receptor antagonist. Selective agonists of estrogen receptor β also have anxiolytic effects on behavior tests of anxiety in rodents and decrease the HPA response to stress. Thus, behavioral studies in lower animals confirm that central nervous system function and behaviors relevant to affective adaptation, and stress responsivity is modulated by ovarian steroids.[10]
It is well known that psychological factors trigger and exacerbate LP.[11] There is a bidirectional interaction between the skin and the mind. Psychologically, the skin is an erogenous zone and channel for emotional discharge. Hence, a skin disorder could be considered a manifestation of unexpressed anger or an inner conflict due to external stress.[12]
Depression is characterized by low positivity, loss of self-esteem and incentive, dysphonic mood (e.g., feelings of sadness or worthlessness), and a sense of hopelessness. Such psychological distress can precipitate a dermatological or mucosal disorder, such as LP.[13] This could also explain the correlation of depression score with LP in the present study.
Transdermal estradiol replacement has shown positive results in the effective treatment of depression in perimenopausal women.[14] It is possible that such a therapy may be beneficial in depression-induced OLP as well.
In our study, majority of the patients reported menstrual irregularities which are characteristic of perimenopausal phase.
In our study, maximum patients belonged to urban areas and minimum belonged to rural areas. In OLP, psychogenic factors seem to play an important role, and increased level of depression, anxiety, and stress has been reported in urban areas due to increased stressors and factors such as overcrowded and polluted environment, high levels of violence, and reduced social support which can result in development of more cases of OLP.[15,16]
Of all the perimenopausal women with OLP who reported to our department, skin lesions were present in only 6.4% cases in contrast to 15% that is reported in general population. Similarly, in our study, only 2.3% of patients reported genital lesions in contrast to previously reported 20% in general population.[17] This discrepancy can be due to the fact that patients who reported to our department were concerned mainly with the oral cavity. Either they did not have extraoral lesions at all or they were not prominent enough to be noticed by the patient. Cutaneous and genital involvement of LP can precede, arise concurrently with, or appear after the development of OLP. We, as specialists in oral medicine, should carefully examine the skin of patients with OLP, inquire regarding signs/symptoms of genital lesions, and when relevant, referral to an appropriate specialist should be carried out.
Multiple oral sites involvement and more than one type of OLP were common in our patients. Buccal mucosa was the most common site, and reticular type of OLP was the most common form followed by erosive and atrophic OLP. Maximum patients suffered from oral pain and burning sensation because erosive and atrophic forms were common which are known to be associated with pain.
A rare association between LP, diabetes mellitus, and hypertension was first reported by Grinspan in 1966. Because drug therapy for diabetes mellitus and hypertension is capable of producing lichenoid reactions of the oral mucosa, the question arises as to whether Grinspan's syndrome is an iatrogenically induced syndrome.[18] In our study, patients who were already on antihypertensives or therapy for diabetes were excluded from the study, and 10.46% of perimenopausal women with LP were diagnosed with both hypertension and diabetes which were previously undetected. Hence, in our study, Grinspan's syndrome was present and was not iatrogenically induced.
The concept of possible correlation between thyroid diseases and OLP has emerged from numerous reports of patients who were affected by both OLP and thyroid diseases.[19] In a study conducted by Unnikrishnan et al. in 2013, the overall prevalence of hypothyroidism was 10.95%, of which 7.48% of patients self-reported the condition, whereas 3.47% were previously undetected.[20] In our study, only 1.2% of patients were diagnosed with hypothyroidism and thus did not have any correlation with LP.
Previous studies have revealed a high prevalence of HCV-RNA in patients with LP.[21] In our patients, we found that 1.2% of patients had positive serum anti-HCV or serum HCV-RNA.
CONCLUSION
The incidence of OLP is higher in perimenopausal women than in general population and increases significantly with increase in the severity of depression. LP in perimenopausal women can be mediated by declined level of estrogen and Pg directly or indirectly through causing depression that can trigger LP. Transdermal estradiol replacement has shown positive results in the effective treatment of depression in perimenopausal women. Further studies are required to evaluate the effect of such a therapy in depression-induced OLP as well.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Grover CM, More VP, Singh N, Grover S. Crosstalk between hormones and oral health in the mid-life of women: A comprehensive review. J Int Soc Prev Community Dent. 2014;4(Suppl 1):S5–10. doi: 10.4103/2231-0762.144559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahuja M. Age of menopause and determinants of menopause age: A PAN India survey by IMS. J Midlife Health. 2016;7:126–31. doi: 10.4103/0976-7800.191012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santoro N, Randolph JF., Jr Reproductive hormones and the menopause transition. Obstet Gynecol Clin North Am. 2011;38:455–66. doi: 10.1016/j.ogc.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prior JC. Progesterone for symptomatic perimenopause treatment - Progesterone politics, physiology and potential for perimenopause. Facts Views Vis Obgyn. 2011;3:109–20. [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta A, Mohan RP, Gupta S, Malik SS, Goel S, Kamarthi N. Roles of serum uric acid, prolactin levels, and psychosocial factors in oral lichen planus. J Oral Sci. 2017;59:139–46. doi: 10.2334/josnusd.16-0219. [DOI] [PubMed] [Google Scholar]
- 6.Ismail SB, Kumar SK, Zain RB. Oral lichen planus and lichenoid reactions: Etiopathogenesis, diagnosis, management and malignant transformation. J Oral Sci. 2007;49:89–106. doi: 10.2334/josnusd.49.89. [DOI] [PubMed] [Google Scholar]
- 7.Khan D, Ansar Ahmed S. The immune system is a natural target for estrogen action: Opposing effects of estrogen in two prototypical autoimmune diseases. Front Immunol. 2016;6:635. doi: 10.3389/fimmu.2015.00635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hughes GC. Progesterone and autoimmune disease. Autoimmun Rev. 2012;11:A502–14. doi: 10.1016/j.autrev.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.White HD, Crassi KM, Givan AL, Stern JE, Gonzalez JL, Memoli VA, et al. CD3+ CD8+ CTL activity within the human female reproductive tract: Influence of stage of the menstrual cycle and menopause. J Immunol. 1997;158:3017–27. [PubMed] [Google Scholar]
- 10.Schmidt PJ, Rubinow DR. Sex hormones and mood in the perimenopause. Ann N Y Acad Sci. 2009;1179:70–85. doi: 10.1111/j.1749-6632.2009.04982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manolache L, Seceleanu-Petrescu D, Benea V. Lichen planus patients and stressful events. J Eur Acad Dermatol Venereol. 2008;22:437–41. doi: 10.1111/j.1468-3083.2007.02458.x. [DOI] [PubMed] [Google Scholar]
- 12.Tawil M, Sediki N, Hassan H. Psychobiological aspects of patients with lichen planus. Curr Psychiatry. 2009;16:370–80. [Google Scholar]
- 13.Kalkur C, Sattur AP, Guttal KS. Role of depression, anxiety and stress in patients with oral lichen planus: A pilot study. Indian J Dermatol. 2015;60:445–9. doi: 10.4103/0019-5154.159625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soares CN, Almeida OP, Joffe H, Cohen LS. Efficacy of estradiol for the treatment of depressive disorders in perimenopausal women: A double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry. 2001;58:529–34. doi: 10.1001/archpsyc.58.6.529. [DOI] [PubMed] [Google Scholar]
- 15.Sandhu SV, Sandhu JS, Bansal H, Dua V. Oral lichen planus and stress: An appraisal. Contemp Clin Dent. 2014;5:352–6. doi: 10.4103/0976-237X.137946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Srivastava K. Urbanization and mental health. Ind Psychiatry J. 2009;18:75–6. doi: 10.4103/0972-6748.64028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Munde AD, Karle RR, Wankhede PK, Shaikh SS, Kulkurni M. Demographic and clinical profile of oral lichen planus: A retrospective study. Contemp Clin Dent. 2013;4:181–5. doi: 10.4103/0976-237X.114873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lamey PJ, Gibson J, Barclay SC, Miller S. Grinspan's syndrome: A drug-induced phenomenon? Oral Surg Oral Med Oral Pathol. 1990;70:184–5. doi: 10.1016/0030-4220(90)90116-a. [DOI] [PubMed] [Google Scholar]
- 19.Lavaee F, Majd M. Evaluation of the association between oral lichen planus and hypothyroidism: A retrospective comparative study. J Dent (Shiraz) 2016;17:38–42. [PMC free article] [PubMed] [Google Scholar]
- 20.Unnikrishnan AG, Kalra S, Sahay RK, Bantwal G, John M, Tewari N. Prevalence of hypothyroidism in adults: An epidemiological study in eight cities of India. Indian J Endocrinol Metab. 2013;17:647–52. doi: 10.4103/2230-8210.113755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sànchez-Pérez J, De Castro M, Buezo GF, Fernandez-Herrera J, Borque MJ, García-Díez A. Lichen planus and hepatitis C virus: Prevalence and clinical presentation of patients with lichen planus and hepatitis C virus infection. Br J Dermatol. 1996;134:715–9. doi: 10.1111/j.1365-2133.1996.tb06977.x. [DOI] [PubMed] [Google Scholar]


