Abstract
Objective:
This descriptive and cross-sectional study was undertaken to determine the factors affecting quality of life (QOL) in breast cancer patients.
Methods:
We collected data from 60 patients of carcinoma breast post modified radical mastectomy on radiotherapy in a tertiary care hospital. We included volunteered patients with a signed informed consent and at least 70 Karnofsky Performance Scale points. The data was gathered by interview technique using EORTC QLQ-C30 and QLQ-BR23 (Breast Cancer Module).
Results:
The mean age at presentation was 47.6 years (range 30-75 years).75% patients were of low socio-economic status and 63.3% belonged to rural areas. Younger Women in the age group of 30-39 years had faired worst on physical, social and emotional scores as compared to older women in the age group of 70-79 years. Other factors which affected Quality of Life of patients during treatment were stage of disease at presentation, performance score of the patients, socioeconomic status of disease at follow up.
Conclusion:
Age, Education status, Performance Score, Stage of disease at presentation and status of disease at last follow up are few factors which significantly affects QOL in Carcinoma breast patients though the treatment remains same. Advanced studies on individual quality of life factors affecting cancer would empower physicians for better personal care techniques and patients for easily overcoming the disease.
KEYWORDS: Breast cancer, woman health, quality of life
INTRODUCTION
Breast cancer is the leading cause of death among cancer patients worldwide.[1] More than 100,000 patients are estimated to be newly diagnosed in India which depicts the disease burden. This neoplasm is probably the most feared by women, especially by the negative stigma brought by its diagnosis and due to its psychological effects, which affect the perception of sexuality and their own personal image.
With the improvement in disease-free survival with advanced treatment modalities, question of quality of life (QOL) arises. Most of the health care should be ultimately evaluated in terms of the impact that the therapy has on QOL.[2] Although it is understood that physical problems and those related to health dominate the life of patients with cancer, it is also true that by virtue of its seriousness and life endangering nature, cancer produces concerns in many life spheres.[3]
QOL is a subjective phenomenon and there is no generally agreed definition. Many definitions have been given by different researchers but no explicit definition has received universal acceptance among researchers.[4] Cells and Cherin defined QOL as “Patient's appraisal of and satisfaction with their current level of functioning as compared to what they perceive to be possible or ideal.” Shumaker et al. defined it as “individuals' overall satisfaction with life and their general sense of personal well-being.”[5] While Schipper described it as “A pragmatic day to day, functional representation of a patient's physical, psychological and social response to a disease and its treatment.” World Health Organization defines it as “The condition of life resulting from the combination of the effects of a complete range of factors such as those determining health, happiness including comfort in the physical environment and a satisfying occupation, education, social and intellectual attainments, freedom of actions, justice, and expression.[6]
Many factors affect QOL positively or negatively. Few of them are age, stage of disease at presentation, performance status, and disease status at last follow-up, which we found in our study significantly impacting QOL of patients.[7,8]
We carried out this study to determine the factors affecting the QOL of cancer patients. The research questions were:
Is there any relationship between sociodemographic characteristics of patients and their QOL scores?
Is there any relationship between age of patients and their QOL scores?
Is there any relationship between QOL scores and stage of disease?
Is there any relationship between disease status during follow up and QOL scores?
There are many instruments to measure QOL but EORTC QLQ-C30 is a reliable and valid measure of QOL of cancer patients as tested in multicultural clinical research trials. It is cancer specific, multidimensional appropriate for self-administration and is applicable across a range of cultures. EORTC QLQ-C30 has been translated into 36 languages including Hindi till now.
There are very few Indian studies to the best of our knowledge which have studied the factors affecting the QOL in breast cancer patients using EORTC QLQ-C-30 and EORTC QOL Questionnaire-Breast Cancer (QLQ-BR 23) (Breast Cancer Module). In the light of above, the present study was planned to determine factors affecting QOL in breast cancer patients during active treatment and during follow up.[9,10,11]
METHODS
This study has been carried out on sixty histopathologically proven postmodified radical mastectomy (MRM) patients of carcinoma breast after obtaining an ethical approval by hospital ethical committee in compliance with Helsinki Declaration and informed consent was obtained from all patients involved in this study.
The study included a convenience sample of 60 women in the period between April 2014 and April 2016, with the following inclusion criteria: Age greater or equal to 18 years, diagnosed with breast cancer at any stage of disease, being on radiotherapy after surgery and chemotherapy from the day they reported for adjuvant radiation, and being present 5 days of the week. Regarding clinical data, all patients included in this study had undergone MRM either upfront or after neoadjuvant chemotherapy. After completion of adjuvant chemotherapy the patients received external beam radiotherapy with three-dimensional conformal radiotherapy by tangent pair technique on Linear Accelerator after simulation on Oldelft Simulix HP with computed tomography (CT0 extension).
The following instruments were used: Sociodemographic and clinical form; European Organization for Research and Treatment of Cancer 30-Item QOL Questionnaire (EORTC QLQ-C30), version 3.0, in Hindi; and QLQ-BR23. The EORTC QLQ-C30 and the QLQ-BR23 are questionnaires of QOL related to health, translated and validated in Hindi; the use of both questionnaires is authorized by the EORTC.[12]
Interview technique was used as method to gather data. The questionnaires were filled out during patient's interview when they presented to outpatient Radiotherapy Department AHR and R after obtaining an informed consent.
The principal for scoring these scales is same in all cases. In the present study, all the scores were calculated as per EORTC scoring manual procedures as follows:
The average of the items that contribute to the scale was estimated; this was the raw score
A linear transformation was used to standardize the raw score so that the scores ranges from 0 to 100.
In practical terms, if items II, I2In are included in a scale the procedure is as follows:
Raw score
The raw score was calculated as raw score = RS = (II + I2 + In)/n
Linear transformation
The linear transformation was applied to 0–100 to obtain score S,
Functional scales: S = (1− [RS − 1]/range) × 100
Symptom scale/items: S = ([RS − 1]/range) × 100
Global health status/QOL: S = ([RS − 1]/range) × 100
Range is the difference between the maximum possible value of RS and minimum possible value. The scoring approach was identical for QLQ-BR 23. All mean scores were transformed linearly into a scale from zero to one hundred points, as described above, where zero represents the worst health status and one hundred the best state of health except for the symptom scale, in which the higher score represents more symptoms and the worst QOL.
Statistical analysis has been performed using WINKS SDA 7.0.5 by TexaSoft. Tables containing two groups have been compared with the Student's t-test analysis and table containing more than two groups have been analyzed with one-way analysis of variance. The confidence interval was 95% that is P < 0.05 is indicative of the significant difference between the groups.
The development of the study met national and international standards of ethics in research involving human beings.
RESULTS
The sample comprised sixty women who were on radiotherapy post-MRM for breast cancer. The socioeconomic status of patients was assessed using modified Kuppuswami scale.[13] The mean age at presentation was 47.6 years (range 30–75). Seventy-five percent patients belonged to low socioeconomic status and 63.3% belonged to rural areas. Lump breast was the presenting symptom in all patients. Stage-wise distribution at presentation was stage IIB 33.3%, stage IIIA 45%, and stage III B 21.7%.
Many factors affect QOL positively or negatively. Age, stage of disease at presentation, performance status, and disease status at last follow up are few factors which directly impacts QOL of patients with statistical significance.
Variation with age
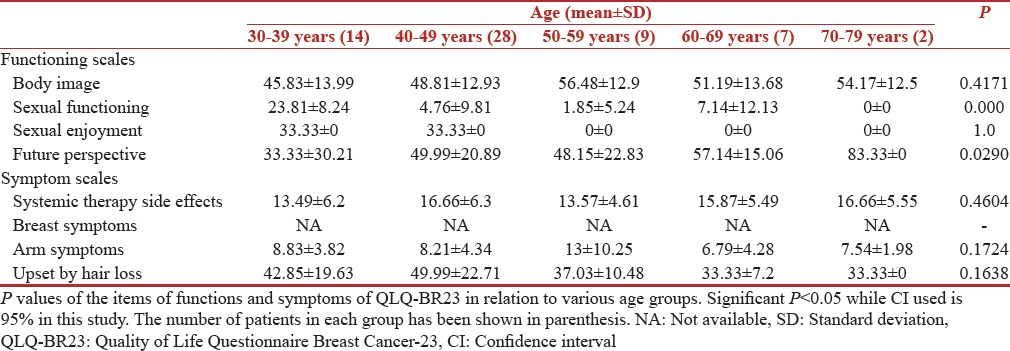
The correlation of QOL functioning scores and symptom scores with age is shown in Tables 1 and 2, respectively. The young breast (30–39 years) cancer survivors showed significantly worse QOL outcomes compared with older age groups on all other functional scales, except for sexual functioning. The mean global health score in the age group of 30–39 years was 57.14 (standard deviation [SD] 18.59) while the score was 66.66 (SD 8.33) in 70–79 years [Table 1]. While as per the QLQBR-23 scale younger age group 30–39 years had a mean score of 42.85 (SD 19.63) for hair loses while this score was only 33.33 (SD 0) in older age group [Table 2]. However, the statistical significance was found only for following functions, physical functioning (P = 0.0003), social functioning (P = 0.0313), financial impact (P = 0.0105), while future perspective had a (P = 0.029).
Table 1.
European Organization for Research and Treatment of Cancer 30-Item Quality of Life Questionnaire scores (n=60)
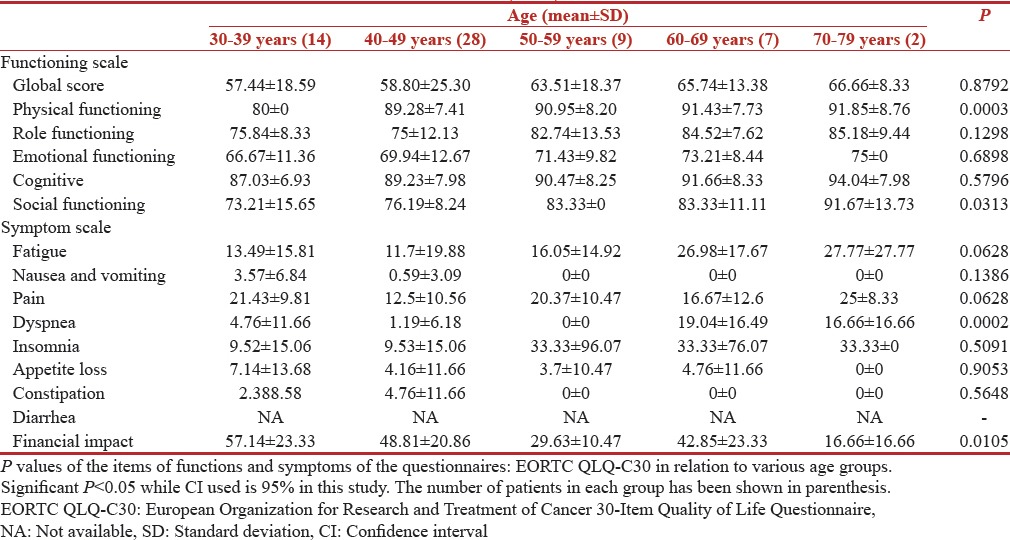
Table 2.
Quality of Life Questionnaire Breast Cancer-23 scores (n=60)
Variation with education
In this study, 63.3% of patients belonged to rural background and about 60% of patients were uneducated. The QOL scores as per EORTC QLQC-30 scale were not significantly different in two groups [Table 3] while as per the QLQ BR-23 scale educated group was more concerned about their future with a mean score of 64.58 (SD 32.21) and uneducated group had a score of 55.85 (SD 28) although not statistically significant [Table 4]. Between educated and uneducated groups we found statistical significance for global health with a (P = 0.0036) and sexual functioning with a (P = 0.0255).
Table 3.
Quality of Life European Organization for Research and Treatment of Cancer scores (n=60)
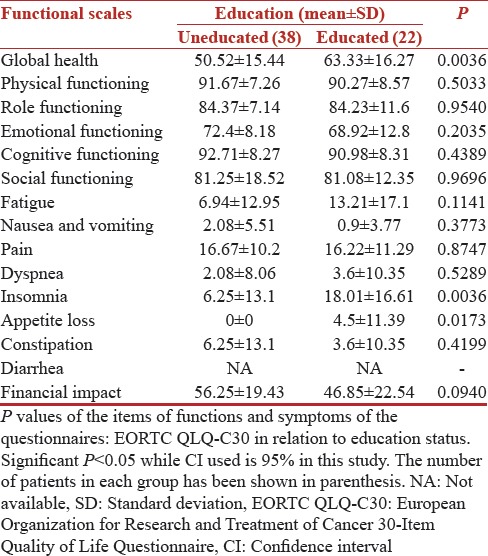
Table 4.
Quality of Life Questionnaire Breast Cancer-23 scores (n=60)

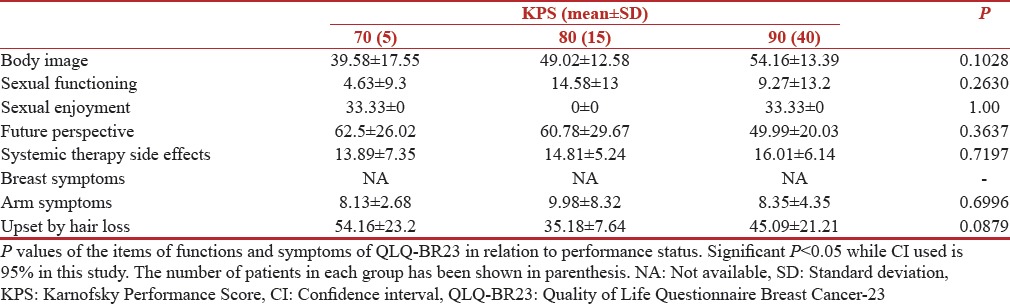
Variation with Karnofsky Performance Score
The correlation of QOL functioning scores and symptom scores with Karnofsky Performance Score (KPS) is shown in Tables 5 and 6, respectively. A better performance status correlated very well with better Functioning scores in our study. Even in breast cancer module (BR-23) patients with higher KPS 90[14] were more confident about their body image with a mean score of 54.16 (SD 13.39) while patients with KPS 70 had a mean score of 39.58 (SD 17.55). The scores for future perspective were 49.99 (SD 20.03) in patients with KPS 90. The functions with significant P values were, fatigue 0.0063 and financial impact 0.0322. The patients with low KPS were more fatigued and had statistically significant financial impact on their QOL.
Table 5.
European Organization for Research and Treatment of Cancer 30-Item Quality of Life Questionnaire scores (n=60)
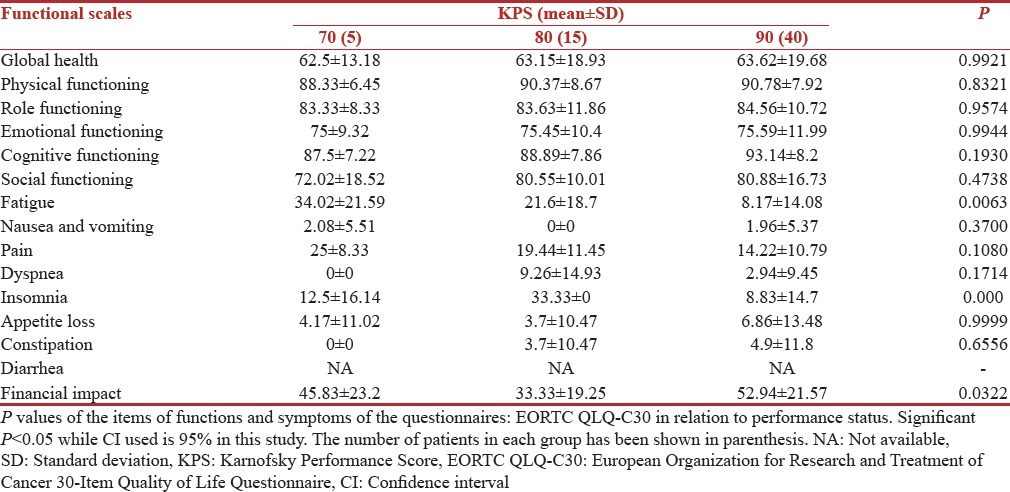
Table 6.
Quality of Life Questionnaire Breast Cancer-23 scores (n=60)
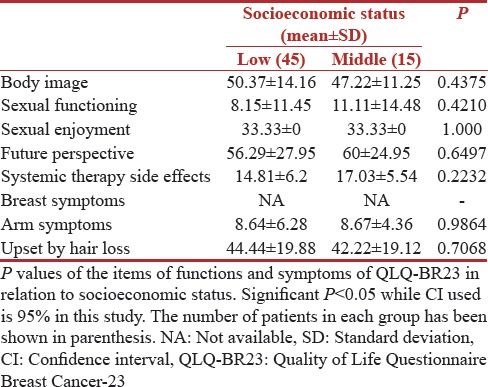
Variation with socioeconomic status
The correlation of QOL functioning scores and symptom scores with socioeconomic status is shown in Tables 7 and 8, respectively. Low socioeconomic status resulted in poor-EORTC functioning scores in all domains while the socioeconomic status did not effect the sexual enjoyment scores of 33.33 (SD 0) in both groups. People belonging to both socioeconomic group were equally worried about their future with a mean score of 56.29 (SD 27.95) and 60 (SD 24.95) in low- and middle-socioeconomic groups. The socioeconomic status was assessed as per the modified Kuppuswami scale, and 75% of patients belonged to a low socioeconomic group in our study. However, we did not find any statistical significance for any of the functions as per the socioeconomic status of the patients.
Table 7.
European Organization for Research and Treatment of Cancer 30-Item Quality of Life Questionnaire scores (n=60)
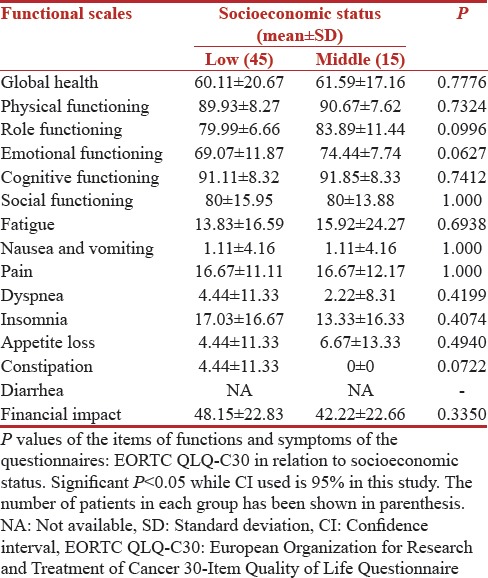
Table 8.
Quality of Life Questionnaire Breast Cancer-23 scores (n=60)
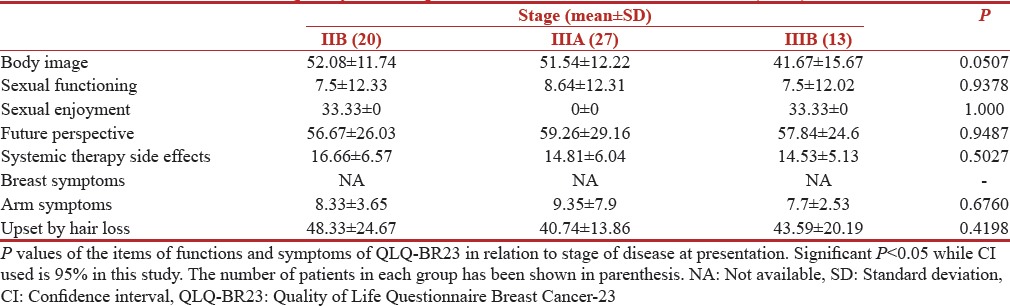
Variation with stage
The correlation of QOL functioning scores and symptom scores with stage is shown in Tables 9 and 10 respectively. The patients with an advanced stage of disease had lowest scores on all functional scales including physical, cognitive, emotional as well as global. Patient with advanced stage of diseases performed worst on symptom scale with a mean score of 34.61 (SD 17.72) and 7.22 (10.7) in stage III B and II B, respectively for fatigue. While financial impact scores were all most same in all stages. Advanced disease stage of the disease led to a highly significant P = 0.0001 for fatigue.
Table 9.
European Organization for Research and Treatment of Cancer 30-Item Quality of Life Questionnaire scores (n=60)
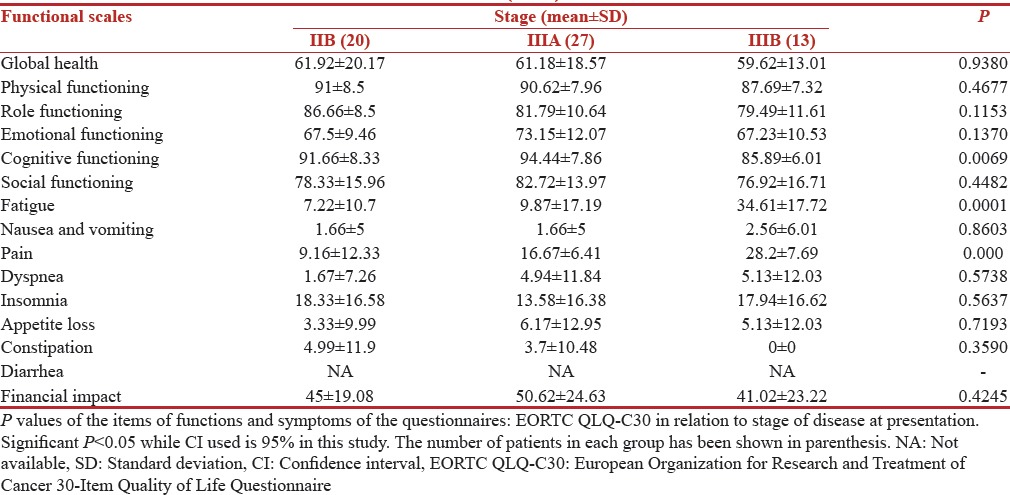
Table 10.
Quality of Life Questionnaire Breast Cancer-23 scores (n=60)
Variation with disease status at last follow-up
The correlation of QOL functioning scores and symptom scores with Disease Status at last follow up is shown in Tables 11 and 12 respectively. Worst scores were reported by patients who had metastatic disease. Patients with metastatic disease at last follow up in our study were really worried about their future with a mean score of 56.41 (SD 28.15) while these scores were 66.67 (SD 19.24) in patients who were disease-free at last follow up. Disease status at last follow up significantly affected the QOL of patients. We found a significant P value for global health 0.0053, role functioning (P = 0.0349), fatigue (P = 0.0049), and pain (P = 0.005), respectively.
Table 11.
European Organization for Research and Treatment of Cancer 30-Item Quality of Life Questionnaire scores (n=60)
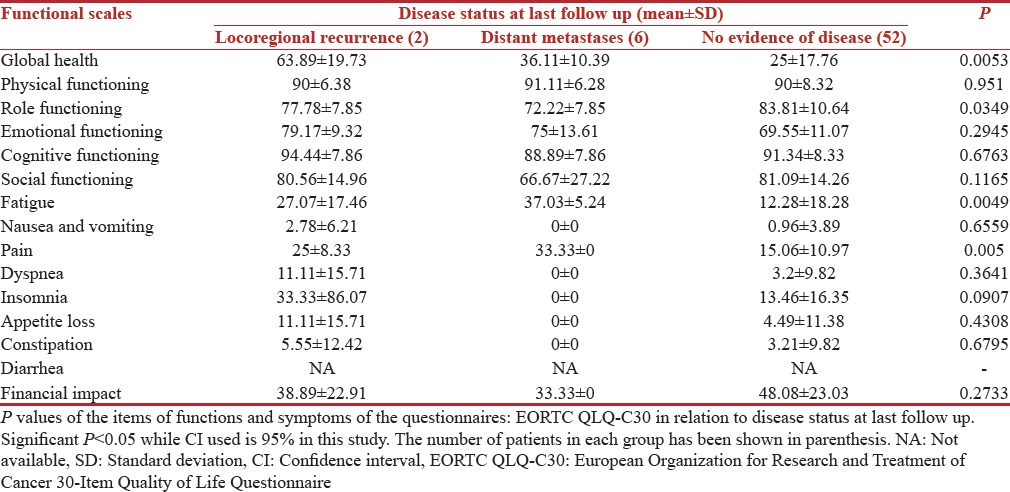
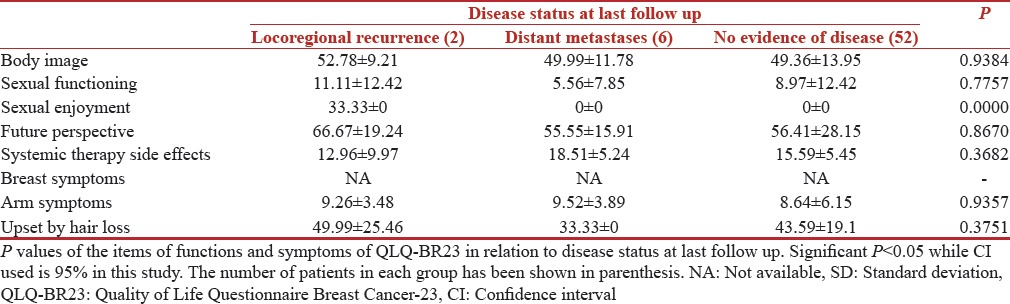
Table 12.
Quality of Life Questionnaire Breast Cancer-23 scores (n=60)
DISCUSSION
The limitations of the results of this study are related to the cross-sectional design that does not allow establishing relations of cause and effect. It is important to study the effect of other factors like socioeconomic status, education levels etc., especially in Indian women, as most of them are from rural background with limited literacy. The patients perception of QOL varies according to the social milieu and cultural beliefs of a country and hence, the international result might not be wholly applicable to our patients. Accordingly, our study complements an Indian perspective.
Age
In this study, the young breast (30–39 years) cancer survivors showed significantly worse QOL outcomes compared with older age groups in relation to physical functioning (P = 0.0003), social functioning (P = 0.0313), and future perspective (P = 0.029). This shows that younger age group felt more social inhibitions while undergoing treatment for carcinoma breast, especially postmastectomy as compared to older age group. Younger patients were more concerned about their future too. Though in a similar study by Bantema-Joppe et al., they found that the development of role, emotional, and cognitive functioning over time of the oldest age group differed from the two younger age groups (role functioning P < 0.001; emotional functioning P = 0.010, and cognitive functioning P < 0.001), with a trend towards better outcomes in the younger group and worse outcomes in the oldest age group.[15]
In a study by Cimprich and Ronis, it was found that long-term survivors of breast cancer who were diagnosed at an older age (<65 years) showed significantly (P < 0.05) worse QOL outcomes in the physical domain, while those who were diagnosed at a younger age (27–44) showed worse QOL in the social domain than other age groups in accordance with our study.[16]
Stage
Patient with advanced stage of diseases performed worst on symptom scale with a worse fatigue score (P = 0.0001) as compared to early stage disease. Financial impact scores were almost same in all stages without any statistical significance. Lack of financial impact can be explained by free of cost treatment being provided to all patients at our center. Oates et al. in their study in oropharyngeal cancer patients also found that deterioration in most domains was most frequent for stage III/IV patients at 3 months (both modules), whereas stage I/II patients experienced this at 6 months (QLQ-C30) and 12 months (H and N35). Their results were similar to our study, indicating advanced stages of disease lead to wore QOL in cancer patients.[17]
Karnofsky Performance Score
The correlation of QOL functioning scores and symptom scores with KPS is shown in Tables 5 and 6 respectively. A better performance status correlated very well with better functioning scores in our study. A poor KPS lead to a statistical significance with P < 0.05 for fatigue. Vergara et al. used subjective global assessment (SGA) score to assess the nutritional status and classified patients as SGA-A, patients having adequate nutrition, SGA-B (moderately nourished), SGA-C (severely malnourished). They observed a different global score across different groups 76.14 ± 15.49 among those who are well nourished (SGA-A), to a score of 61.46 ± 16.77 to those who are moderately malnourished (SGA-B) and a score of 40.47 ± 18.28 to those who are poorly nourished (SGA-C), and this was significant (P < 0.001). We also observed similar findings in our study where patients with a better performance scale had better QOL.[18]
Socioeconomic status
Socioeconomic status could not lead to any statistical significance for any of the functions in both groups. This can be explained by free of cost treatment being provided to all our patients at our institute. In a study by Montazeri et al. QOL was measured at two points in time (baseline and 3 months after initial treatment) using three standard instruments; the Nottingham Health Profile, the European Organization for Research and Cancer Treatment QOL Questionnaire (EORTC QLQ-C30) and its lung cancer supplement (QLQ-LC13). Out of129 lung cancer patients 82 patients were complete (at baseline and follow-up). Nearly 57% of patients were of lower socioeconomic status and they had more health problems, less functioning, and more symptoms as compared to affluent patients.[19] Klein et al. in a similar study in 246 patients of carcinoma prostate found that lower socioeconomic status measured y income, education and occupational status is significantly associated with lower QOL 6 months after treatment.[20]
Disease status at last follow up
Siddiqi et al. found in their study that patients experiencing recurrence of disease suffered greater decrements in health-related QOL, particularly in symptoms and physical functioning, than did patients with metastatic disease or primary, nonmetastatic disease.[21] While in our study, we observed that patients with metastatic disease at last follow up that is 2 years after starting the study had worst QOL with significant P < 0.05 for global health, role functioning, fatigue, and pain, respectively.
Education status
In this study, we observed significant P = 0.0036 and 0.0255 for global health and sexual functioning respectively in relation to education levels of a patient. While in the studies by various authors such as Dehkordi et al. and Heydarnejad et al. done in patients undergoing chemotherapy no significance was found for demographic variables such as age, education, marital status, income, etc.[22,23]
This study has helped us in identifying the factors which affect the QOL in these patients even when they are being subjected to exactly similar treatment. These factors are age, education status, performance score, stage of disease at presentation and disease status at last follow up. Sometimes these factors, especially like age and KPS even guide us in deciding the treatment approach.
CONCLUSION
This study demonstrates the strength of the relationship between clinical and sociodemographical factors and breast cancer patients' QOL. Psychological and financial support for women experiencing breast cancer diagnosis may improve QOL. This study fills a gap in the literature related to QOL in Indian women suffering from carcinoma breast. Several recommendations for future research are needed to explore the impact of symptom distress on QOL with a larger sample size.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We thank all the patient for allowing us to publish the study and use their inputs.
REFERENCES
- 1.Agarwal G, Pradeep PV, Aggarwal V, Yip CH, Cheung PS. Spectrum of breast cancer in Asian women. World J Surg. 2007;31:1031–40. doi: 10.1007/s00268-005-0585-9. [DOI] [PubMed] [Google Scholar]
- 2.Sharma SK, Phaterpekar SJ. New Delhi: 1992. Assessment of Quality of Life (QOL) in Clinical Research. Word Congress on Social Psychiatry (9-13 November, 1992). Symposium on Quality of Life in Relation to Health and Illness: Socio Cultural Issues (QOL) pp. 1–9. [Google Scholar]
- 3.Channabasavanna SM, Chaturvedi SK, Chandra P. New Delhi: 1992. Cancer and Well Being. World Congress on Social Psychiatry (9-13 November, 1992). Symposium on Quality of Life in Relation to Health and Illness: Socio Cultural Issues (QOL) pp. 1–9. [Google Scholar]
- 4.Calman KC. In: Definitions and dimensions of quality of life. The Quality of Life of Cancer Patients. Aaronson NK, Beckmann JH, Bernheim JL, editors. New York: Raven Press; 1987. pp. 1–10. [Google Scholar]
- 5.Shumaker SA, Anderson RT, Czaikowski SM. In: Psychological tests and scales. Quality of Life Assessment in Clinical Trials. Spikiller B, editor. New York: Raven Press; 1999. pp. 95–113. [Google Scholar]
- 6.Pal H, Singhal A, Aruna T, Jena R. Quality of life in breast cancer patients. Hosp Today. 2002;7:35–7. [Google Scholar]
- 7.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European organization for research and treatment of cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–76. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 8.Burgess C, Cornelius V, Love S, Graham J, Richards M, Ramirez A. Depression and anxiety in women with early breast cancer: Five year observational cohort study. BMJ. 2005;330:702. doi: 10.1136/bmj.38343.670868.D3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shapiro SL, Lopez AM, Schwartz GE, Bootzin R, Figueredo AJ, Braden CJ, et al. Quality of life and breast cancer: Relationship to psychosocial variables. J Clin Psychol. 2001;57:501–19. doi: 10.1002/jclp.1026. [DOI] [PubMed] [Google Scholar]
- 10.Eom CS, Shin DW, Kim SY, Yang HK, Jo HS, Kweon SS, et al. Impact of perceived social support on the mental health and health-related quality of life in cancer patients: Results from a nationwide, multicenter survey in South Korea. Psychooncology. 2013;22:1283–90. doi: 10.1002/pon.3133. [DOI] [PubMed] [Google Scholar]
- 11.Miller RC, Atherton PJ, Kabat BF, Fredericksen MB, Geno DM, Deschamps C, et al. Marital status and quality of life in patients with esophageal cancer or Barrett's esophagus: The mayo clinic esophageal adenocarcinoma and Barrett's esophagus registry study. Dig Dis Sci. 2010;55:2860–8. doi: 10.1007/s10620-009-1100-1. [DOI] [PubMed] [Google Scholar]
- 12.Fayers P, Aaronson N, Bjordal K, Sullivan M. EORTC QLQ-C30 Scoring Manual. Belgium: EORTC Quality of Life Study Group; 1995. [Google Scholar]
- 13.Bairwa M, Rajput M, Sachdeva S. Modified kuppuswamy's socioeconomic scale: Social researcher should include updated income criteria, 2012. Indian J Community Med. 2013;38:185–6. doi: 10.4103/0970-0218.116358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Toole DM, Golden AM. Evaluating cancer patients for rehabilitation potential. West J Med. 1991;155:384–7. [PMC free article] [PubMed] [Google Scholar]
- 15.Bantema-Joppe EJ, de Bock GH, Woltman-van Iersel M, Busz DM, Ranchor AV, Langendijk JA, et al. The impact of age on changes in quality of life among breast cancer survivors treated with breast-conserving surgery and radiotherapy. Br J Cancer. 2015;112:636–43. doi: 10.1038/bjc.2014.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cimprich B, Ronis DL. Attention and symptom distress in women with and without breast cancer. Nurs Res. 2001;50:86–94. doi: 10.1097/00006199-200103000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Oates J, Davies S, Roydhouse JK, Fethney J, White K. The effect of cancer stage and treatment modality on quality of life in oropharyngeal cancer. Laryngoscope. 2014;124:151–8. doi: 10.1002/lary.24136. [DOI] [PubMed] [Google Scholar]
- 18.Vergara N, Montoya JE, Luna HG, Amparo JR, Luna CL. Quality of life and nutritional status among cancer patients on chemotherapy. Oman Med J. 2003;28:270–4. doi: 10.5001/omj.2013.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montazeri A, Hole DJ, Milroy R, McEwen J, Gillis CR. Quality of life in lung cancer patients: Does socioeconomic status matter? Health Qual Life Outcomes. 2003;1:19. doi: 10.1186/1477-7525-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klein J, Hofreuter-Gätgens K, Lüdecke D, Fisch M, Graefen M, von dem Knesebeck O. Socioeconomic status and health-related quality of life among patients with prostate cancer 6 months after radical prostatectomy: A longitudinal analysis. BMJ Open. 2016;6:e010968. doi: 10.1136/bmjopen-2015-010968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siddiqi A, Given CW, Given B, Sikorskii A. Quality of life among patients with primary, metastatic and recurrent cancer. Eur J Cancer Care (Engl) 2009;18:84–96. doi: 10.1111/j.1365-2354.2008.01021.x. [DOI] [PubMed] [Google Scholar]
- 22.Dehkordi A, Heydarnejad MS, Fatehi D. Quality of life in cancer patients undergoing chemotherapy. Oman Med J. 2009;24:204–7. doi: 10.5001/omj.2009.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heydarnejad MS, Hassanpour DA, Solati DK. Factors affecting quality of life in cancer patients undergoing chemotherapy. Afr Health Sci. 2011;11:266–70. [PMC free article] [PubMed] [Google Scholar]


