Abstract
BACKGROUND:
Acylation-stimulating protein (ASP) is an adipokine synthesized within adipocytes environment due to adipocyte differentiation.
AIM:
The aim of this study was to assess changes in ASP levels in patients with acute myocardial infarction (MI) and to correlate these variations with disease variables.
SUBJECTS AND METHODS:
A total number of 111 patients previously and currently treated with rosuvastatin or atorvastatin presented with acute MI in a Coronary Care Unit, were divided into three groups, Group A: Thirty-nine patients treated with atorvastatin, Group B: Thirty patients treated with rosuvastatin, compared to 42 patients presented with MI not previously treated with statins were enrolled in this study. ASP and troponin-I levels and lipid profile were estimated in each group.
RESULTS:
The effects of atorvastatin and rosuvastatin compared to nonstatins-treated group on the anthropometric and biochemical variables in patients with acute MI showed significant difference in all biochemical and anthropometric parameters P < 0.05. Serum ASP (nmol/l) levels were higher in control patients 57.25 ± 9.15 compared to atorvastatin-treated patients 48.43 ± 7.42 and rosuvastatin-treated patients 49.33 ± 6.52 P = 0.0124.
CONCLUSION:
ASP levels are elevated in patients with acute MI and regarded as surrogate biomarker for acute MI also; therapy with statins leads to significant reduction in ASP levels compared to nonstatins-treated patients that presented with acute MI.
Keywords: Acute myocardial infarction, acylation-stimulating protein, atorvastatin, rosuvastatin
Introduction
Acylation-stimulating protein (ASP) is an adipokine synthesized within adipocytes environment due to adipocyte differentiation; it acts as autocrine molecules on adipocytes to increase glucose transport, fatty acid esterification, and then triglyceride (TG) synthesis.[1] The effects of ASP are mediated by a G-protein coupled receptor called C5 L2 receptor leading to inhibition of hormone sensitive lipase and augmentation of insulin action on adipocyte.[2] Generation of ASP occurs through activation of alternative pathways to complement system, when complement C3 interacts with adipsin during tissue injury C3a will be produced which is quickly cleaved by plasma carboxypeptidase to generate C3adesArg which called ASP due to its noticeable action on TG synthesis in adipocytes.[3] C3 and adipsin are produced from liver and adipose tissue in response to insulin and plasma lipid levels thus; C3 and ASP are positively correlated with dyslipidemia, insulin resistance, obesity, and coronary heart diseases.[4]
ASP stimulates TG storage and glucose transporter 1 and 4 in adipocytes with significant inhibition of adipocyte lipolysis through the activation of adipocyte phosphodiesterase, therefore, ASP serum levels are correlated with postprandial lipemia since it responsible for the reduction of nonesterifies-free fatty acids and TG clearance consequently ASP is highly correlated with TG levels and weakly correlated with low-density lipoprotein (LDL) and apo B levels.[5]
Animal model study by Xia et al. revealed that in ASP-deficient mice; there were a delays in TG clearance which is regulated by ASP administration.[6]
Moreover, ASP resistance leads to high TG and low high-density lipoprotein cholesterol (HDL-c) due to delayed in TG clearance; these changes lead to high TG/HDL-c ratio which contributes into high LDL and consequently induction of dyslipidemia-induced coronary dysfunction.[7]
In addition, C3 and ASP levels are higher in hypertensive patients with and without coronary artery disease (CAD) thus; C3 regarded as an indicator of myocardial infarction (MI) and other ischemic events.[8]
Indeed, immunological changes appear to attend or follow MI and regarded as an independent risk factor regardless of the number of occluded vessels.[9]
Uses of statins therapy in the treatment and prevention of CAD were extensively studied depending on the clinical evidence of statins safety and efficacy.[10] In addition, statins modulate proinflammatory and anti-inflammatory cytokines in experimental MI in rats, atorvastatin significantly reduced interleukin-10 (IL-10), tumor necrosis factor, C-reactive protein, and plasminogen activator inhibitor-1.[11]
Therefore, this study was undertaken to assess changes in ASP levels in patients with acute MI and to correlate these variations with disease variables.
Subjects and Methods
The study was done in the Department of Clinical Pharmacology and Therapeutic, College of Medicine, Al-Mustansiriyia University, in cooperation with the Department of Cardiology; Al-Yarmouk Teaching Hospital, from October 2015 to February 2016, Baghdad-Iraq. This study was approved by the Clinical Research, Ethical Committee, College of Medicine, Al-Mustansiriyia University. A total number of 111 patients (79 males, 32 females) previously and currently treated with rosuvastatin or atorvastatin presented with acute MI in a Coronary Care Unit (CCU), were divided into three groups, Group A: Thirty-nine patients (29 males + 10 females) treated with atorvastatin 20 mg/day, Group B: Thirty patients (20 males + 10 females) treated with rosuvastatin 20 mg/day, compared to 42 patients presented with MI not previously treated with statins (30 males + 12 females) were enrolled in this study. Each patient was clinically examined by a cardiologist, and the diagnosis was achieved through electrocardiographic (ECG) changes and cardiac troponin-I (cTnI). The inclusion criteria were patients with a recent acute MI admitted into the CCU. The exclusion criteria were patients with valvular heart diseases, malignant diseases, acute infection, inflammatory disorders and blood disorders, advanced renal disease, liver disease, and diabetes mellitus (DM). All enrolled patients gave written informed approval before their participations. The procedures were prepared according to the Declaration of Helsinki.[12]
After the interview, medical history, current drug pharmacotherapy and anthropometric measures were done. Body mass index (BMI) was estimated as kg/m2.[13] Ten milliliters of venous blood were withdrawn at 9 am after an overnight fasting, into a plain tube 5 ml (for routine investigations) and into an EDTA tube 5 ml for ASP and troponin I estimations.
ASP in nmol/L (Human ASP ELISA kit-LS-F4497) and troponin-I in pg/ml (Human cTnI ELISA Kit ab200016) serum levels were determined by ELISA KIT method at 450 nm according to the kit instructions.
Assessment of lipid profile: TG, total cholesterol (TC) and HDL were assessed by specific ELISA kits; from this profile, we can measure the followings:[14]
Atherogenic index (AI) = log (TG/HDL), when TG and HDL measured in mmol/l.
LDL = TC − HDL − TG/5.
very LDL = TG/5.
Cardiac risk ratio (CRR) = TC/HDL.
Statistical analysis
The data were presented as mean ± standard deviation, the unpaired Student's t-test was used for estimations of differences when P < 0.05 regarded significant. All data were analyzed by means of SPSS version 22 SPSS version 22 (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Armonk, NY: IBM Corp). ANOVA was used to detect the significance of differences between treated groups compared to control. Bivariate correlation was used for correlating ASP with other disease variables.
Results
Flowchart and outline of the protocol for patient's selection of acute MI and their enrollments in the study demonstrated that a total of 115 patients with acute MI were enrolled, only four patients were excluded because they were not met the inclusion criteria then; 111 (96.52%) patients were randomized into three groups: 39 patients (35.13%) treated with atorvastatin and 30 patients (20.02%) treated with rosuvastatin compared to 42 (37.83%) patients presented with MI not received statins regarded as controls [Figure 1].
Figure 1.
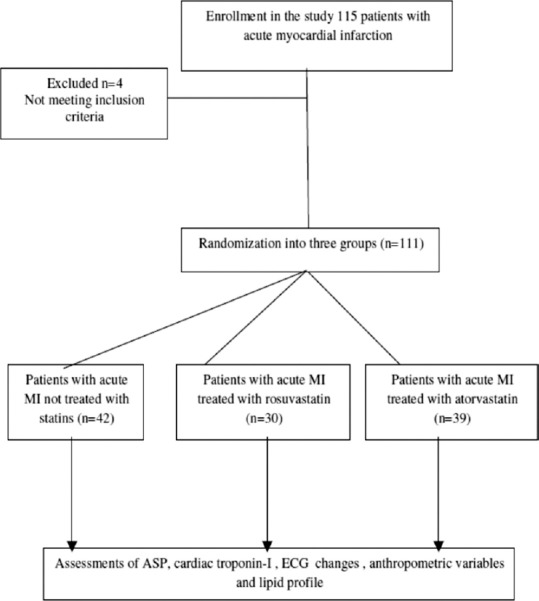
Flow chart and outline of the protocol for patient selection with acute myocardial infarction and their enrollments in the study
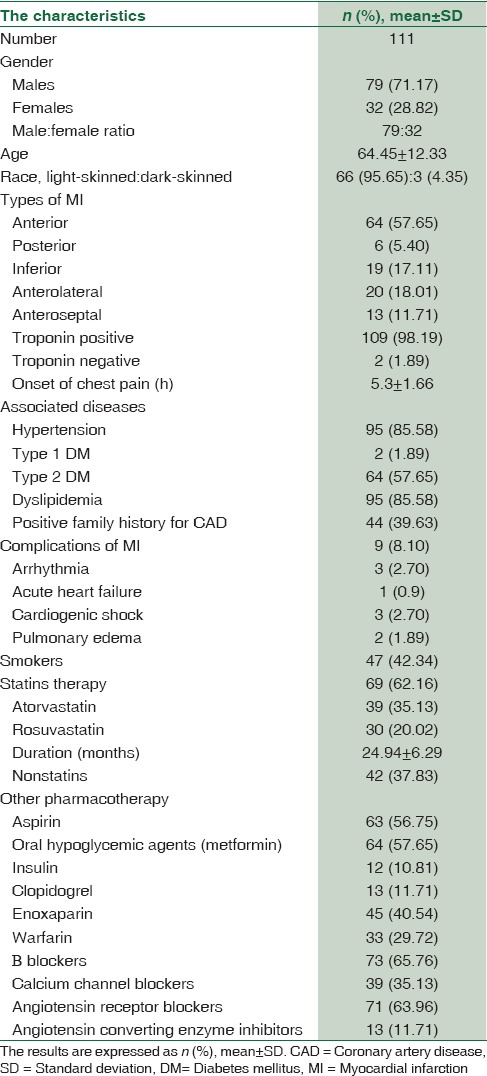
Characteristics of patient presented with acute MI in the present study showed a higher percentage of male enrollments 79 (71.17%) compared to females enrollments 32 (28.82%). Most of recruited patients were of white race 95.65% compared to the dark race 4.35%. Regarding the types of MI in the present study according to the ECG records, anterior 57.65% and anterolateral 18.01% types were most types of presented MI compared to the other types, also 98.19% of them were positive about cardiac serum troponin-I, and only 1.89% patients were troponin-I negative. The associated diseases in the enrolled patients with acute MI were hypertension 85.58%, type 1 DM 1.89%, type 2 DM 57.65%, and dyslipidemia 85.58%. Moreover, 42.34% of the patient were chronic smokers, and 39.63 of patients disclosed a positive family history for CAD. The duration of statins therapy in the present study was 24.94 ± 6.29 months for both atorvastatin and rosuvastatin. Other pharmacotherapy in concomitant with statins was aspirin 56.75%, oral hypoglycemic agents 57.65%, insulin 10.81%, clopidogrel 11.71%, and other agents regarding acute and intensive therapy for management of acute MI, [Table 1].
Table 1.
Demographic characteristics of the patient presented with acute myocardial infarction
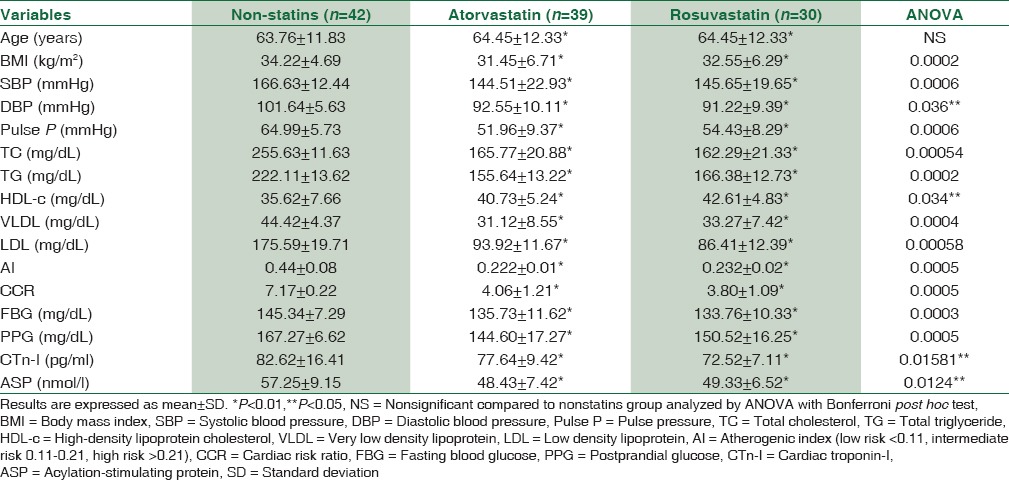
The effects of atorvastatin and rosuvastatin compared to nonstatins treated group on the anthropometric and biochemical variables in patients with acute MI showed significant difference in all biochemical and anthropometric parameters P < 0.05. Serum ASP (nmol/l) levels were higher in control patients 57.25 ± 9.15 compared to atorvastatin-treated patients 48.43 ± 7.42 and rosuvastatin-treated patients 49.33 ± 6.52 P = 0.0124, [Table 2].
Table 2.
The effect of atorvastatin or rosuvastatin compared to the control (nonstatins) on the anthropometric and biochemical variables in patients with acute myocardial infarction
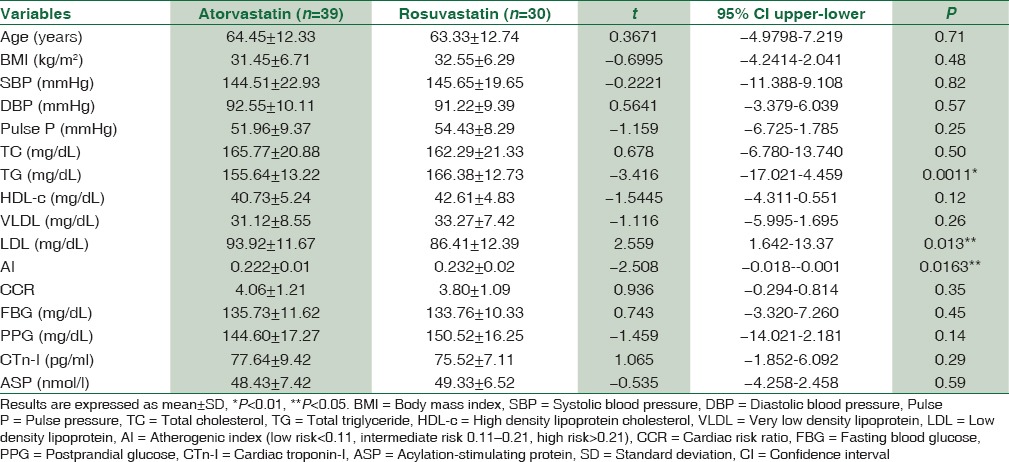
Regarding the differences in effect of atorvastatin or rosuvastatin on the anthropometric and biochemical variables in patients with acute MI, serum TG (mg/dL) levels were low in atorvastatin-treated patients (155.64 ± 13.22) compared to 166.38 ± 12.73 in rosuvastatin-treated patients significantly P = 0.0011, while LDL (mg/dL) levels were significantly low 86.41 ± 12.39 in rosuvastatin-treated compared to 93.92 ± 11.67 in atorvastatin-treated patients P = 0.013. In addition, AI in atorvastatin-treated patients was 0.222 ± 0.01 which is significantly differring from rosuvastatin-treated patients P = 0.0163. There are nonsignificant differences in other biochemical parameters regarding ASP (nmol/l) levels which were 48.43 ± 7.42 in atorvastatin-treated patients and 49.33 ± 6.52 in rosuvastatin-treated patients P < 0.05, [Table 3].
Table 3.
The differences in effect of atorvastatin or rosuvastatin on the anthropometric and biochemical variables in patients with acute myocardial infarction
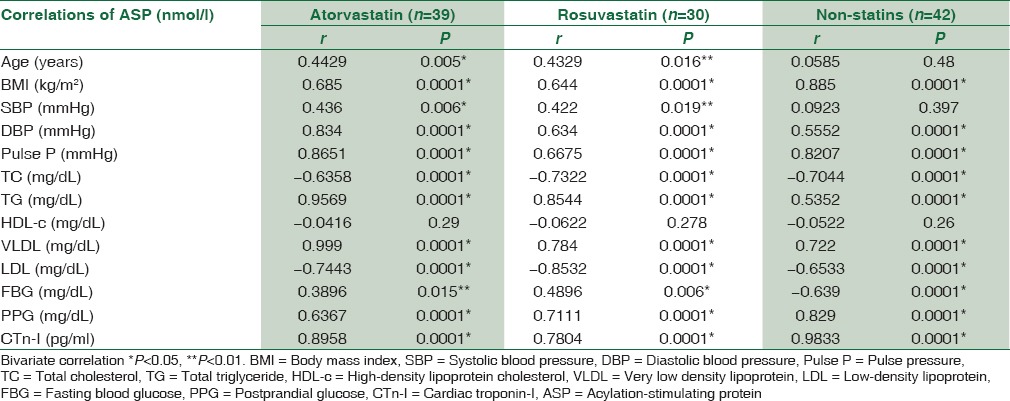
ASP serum levels in atorvastatin and rosuvastatin-treated patients and control patients were positively correlated with all anthropometric and biochemical variables significantly P < 0.01, except for age, systolic blood pressure and fasting blood glucose in atorvastatin-treated patients P < 0.05, but ASP serum levels in both treated and untreated patients were negatively correlated with total serum TG significantly P = 0.0001 and insignificantly with HDL-c P > 0.05 [Table 4].
Table 4.
Correlations of acylation-stimulating protein serum levels with lipid profile and anthropometric variables in patient with acute myocardial infarction treated with and without statins
Therefore, levels of both ASP and cTnI are elevated in patients with acute MI compared to control P < 0.01 [Figure 2].
Figure 2.
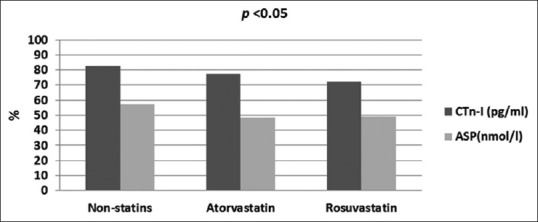
Differential effects of statins on acylation-stimulating protein and cardiac troponin-I in patients with acute myocardial infarction
Discussion
The mechanisms of inflammatory process that implicated for myocardial damage after acute MI are not recognized, but reductions in the levels of complements indicating complement activations and stimulation of ASP during acute MI.[15]
The present study revealed a significant increment in ASP serum levels in patients with acute MI, which in agreement with many previous studies that demonstrated a significant increase in ASP levels in patients with CAD, especially in those with concomitant dyslipidemia, hypertriglyceridemia, and type 2 DM[16,17] since; 57.65% of our patients were of type 2 DM.
Moreover, most of the recruited patients with MI in the present study were associated with hypertension, DM, high BMI, and dyslipidemia which may explain the elevated ASP levels in the current study that supported by Saleh et al.’ study that revealed an associations with high ASP levels and obesity, LDL, TG level, and waist/hip ratio which is the main predictor of ASP level.[18]
Indeed, elevated ASP levels in acute MI strongly predicted by obesity and dyslipidemia due to overproduction of ASP as consequence of ASP resistance, since ASP resistance causing metabolic alterations and lipid changes which contributed to the formation of atherogenic LDL that encourage AI and incidence of acute MI,[19] this may explain the elevated CRR and AI in association with high ASP levels in patients with acute MI of the present study.
In addition, ethnic difference in ASP levels showed by Scantlebury-Manning et al. study that pointed out to the higher levels of ASP in Caucasian due to higher TG fasting levels and ASP resistance compared to the other ethnic population that associated with low fasting TG level and higher ASP sensitivity,[20] these findings are in agreement with finding of the present study since; all of enrolled patients were Caucasian that associated with high ASP levels.
Regarding statins pharmacotherapy, in statins-treated patients with acute MI showed a significant low ASP levels compared to the non-statins treated patient, both rosuvastatin and atorvastatin decreases ASP levels indirectly through modulation of complement which is regarded as precursor of ASP, since C3 play a role in initiations of CAD and cardiovascular complications.[21] Furthermore, CAD is associated with higher fasting and postprandial C3 due to elevated TG levels which upregulate C3 that encourage premature microvascular and macrovascular complications through C3 itself or ASP which is the end product of C3 activations.[22]
In addition, Halkes et al., study demonstrated that simvastatin therapy in patients with CAD significantly lowers C3 levels and then indirectly ASP serum levels[23] that consistent with findings of the present study, but unfortunately serum C3 levels were not measured in the present study. In addition, terminal complement complex, ASP, and other inflammatory biomarkers that involved in pathogenesis of CAD appears to be a direct stimulant of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA), thus HMG-CoA inhibitors such as cerivastatin will decrease ASP levels and other inflammatory mediator like IL-6[24] which may explain the statins effect on ASP levels on CAD in the current study.
Both atorvastatin and rosuvastatin in the present study showed comparable effects with significant differences in LDL, TG, and AI, since rosuvastatin is more potent and more efficacious than atorvastatin as supported by Qian et al., who disclosed a superior effect of rosuvastatin to atorvastatin effect on CAD,[25] but there was insignificant differences between them regarding the ASP lowering effect, both decreases C3 and subsequently; ASP serum levels in patients with dyslipidemia and CAD.[26,27]
Regarding other treatment for patients presented with acute MI, metformin drug was used in 57.65% of our patients due to type 2 DM, thus reduction in ASP levels might be due to metformin effects as observed by Oktenli et al., study that revealed a significant reduction in ASP levels in women patients with polycystic ovary syndrome following 3 months therapies with metformin;[28] however, the selected effect of metformin on ASP levels was not estimated at the present study since; we have a previous study regarding the cardioprotective effect of metformin in patients presented with acute MI that previously treated with metformin.[29]
ASP levels were negatively correlated with LDL cholesterol (LDL-c), HDL-c, and TC in both statins- and nonstatins-treated patients with acute MI, and positively correlated with other lipid profile and anthropometric variables, these findings are correlated with Jiang et al., findings that revealed a significant association and correlation between high serum ASP levels and total TG and BMI with significant negative correlation with LDL-c, HDL-c, and TC in diabetic obese patients regarding Han and Hui populations.[30]
Furthermore, ASP levels were positively correlated with cTnI in patients with acute MI regardless of statins therapy, since ASP levels are positively correlated with severity of CAD and cTnI indicating that ASP is implicated in pathogenesis of CAD due to metabolic alterations in lipid profile.[31]
There are numerous limitations of the current study, relative small sample size, determination of ASP receptors in coronary vessels, and HMG-CoA activity in response to the ASP levels are the main points that limit the present study. On the other hand, this study may be to our knowledge, the first novel study regarding the effect of statins on ASP levels in CAD.
Conclusion
In summary, this study shows that ASP levels are elevated in patients with acute MI and regarded as surrogate biomarker for acute MI also; therapy with statins lead to significant reduction in ASP levels compared to non-statins treated patients that presented with acute MI.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to thank Professor Dr. Sadiq Alhamash Head of Almustansiriyia University for his great supports.
References
- 1.Rezvani R, Gupta A, Smith J, Poursharifi P, Marceau P, Pérusse L, et al. Cross-sectional associations of acylation stimulating protein (ASP) and adipose tissue gene expression with estradiol and progesterone in pre- and postmenopausal women. Clin Endocrinol (Oxf) 2014;81:736–45. doi: 10.1111/cen.12540. [DOI] [PubMed] [Google Scholar]
- 2.Liu Y, Gupta P, Lapointe M, Yotsapon T, Sarat S, Cianflone K. Acylation stimulating protein, complement C3 and lipid metabolism in ketosis-prone diabetic subjects. PLoS One. 2014;9:e109237. doi: 10.1371/journal.pone.0109237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Théroux P, Martel C. Complement activity and pharmacological inhibition in cardiovascular disease. Can J Cardiol. 2006;22(Suppl B):18B–24B. doi: 10.1016/s0828-282x(06)70982-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kildsgaard J, Zsigmond E, Chan L, Wetsel RA. A critical evaluation of the putative role of C3adesArg (ASP) in lipid metabolism and hyperapobetalipoproteinemia. Mol Immunol. 1999;36:869–76. doi: 10.1016/s0161-5890(99)00108-x. [DOI] [PubMed] [Google Scholar]
- 5.Magnusson B, Asp L, Boström P, Ruiz M, Stillemark-Billton P, Lindén D, et al. Adipocyte differentiation-related protein promotes fatty acid storage in cytosolic triglycerides and inhibits secretion of very low-density lipoproteins. Arterioscler Thromb Vasc Biol. 2006;26:1566–71. doi: 10.1161/01.ATV.0000223345.11820.da. [DOI] [PubMed] [Google Scholar]
- 6.Xia Z, Sniderman AD, Cianflone K. Acylation-stimulating protein (ASP) deficiency induces obesity resistance and increased energy expenditure in ob/ob mice. J Biol Chem. 2002;277:45874–9. doi: 10.1074/jbc.M207281200. [DOI] [PubMed] [Google Scholar]
- 7.Sivakumar K, Bari MF, Adaikalakoteswari A, Guller S, Weickert MO, Randeva HS, et al. Elevated fetal adipsin/acylation-stimulating protein (ASP) in obese pregnancy: Novel placental secretion via Hofbauer cells. J Clin Endocrinol Metab. 2013;98:4113–22. doi: 10.1210/jc.2012-4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cianflone K, Zhang XJ, Genest J, Jr, Sniderman A. Plasma acylation-stimulating protein in coronary artery disease. Arterioscler Thromb Vasc Biol. 1997;17:1239–44. [PubMed] [Google Scholar]
- 9.Al-Ahmad RS, Mahafzah AM, Al-Mousa EN. Immunological changes in acute myocardial infarction. Saudi Med J. 2004;25:923–8. [PubMed] [Google Scholar]
- 10.Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, et al. Efficacy and safety of cholesterol lowering treatment: Prospective metaanalysis of data from 90,056 participantsin 14 randomised trials of statins. Lancet. 2005;366:1267–78. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 11.Tawfik MK, Ghattas MH, Abo-Elmatty DM, Abdel-Aziz NA. Atorvastatin restores the balance between pro-inflammatory and anti-inflammatory mediators in rats with acute myocardial infarction. Eur Rev Med Pharmacol Sci. 2010;14:499–506. [PubMed] [Google Scholar]
- 12.General Assembly of the World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. J Am Coll Dent. 2014;81:14–8. [PubMed] [Google Scholar]
- 13.Reinders I, Murphy RA, Martin KR, Brouwer IA, Visser M, White DK, et al. Body mass index trajectories in relation to change in lean mass and physical function: The health, aging and body composition study. J Am Geriatr Soc. 2015;63:1615–21. doi: 10.1111/jgs.13524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aditya GP, Bari MA. Lipid profile in post-acute coronary syndrome setting: Lipidic risk assessment in young people beyond LDL-C. Mymensingh Med J. 2015;24:697–703. [PubMed] [Google Scholar]
- 15.Latet SC, Hoymans VY, Van Herck PL, Vrints CJ. The cellular immune system in the post-myocardial infarction repair process. Int J Cardiol. 2015;179:240–7. doi: 10.1016/j.ijcard.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 16.Cianflone K, Xia Z, Chen LY. Critical review of acylation-stimulating protein physiology in humans and rodents. Biochim Biophys Acta. 2003;1609:127–43. doi: 10.1016/s0005-2736(02)00686-7. [DOI] [PubMed] [Google Scholar]
- 17.Zheng YY, Xie X, Ma YT, Yang YN, Fu ZY, Li XM, et al. A novel polymorphism (901G > a) of C5L2 gene is associated with coronary artery disease in Chinese Han and Uyghur population. Lipids Health Dis. 2013;12:139. doi: 10.1186/1476-511X-12-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saleh J, Wahab RA, Farhan H, Al-Amri I, Cianflone K. Plasma levels of acylation-stimulating protein are strongly predicted by waist/hip ratio and correlate with decreased LDL size in men. ISRN Obes 2013. 2013:342802. doi: 10.1155/2013/342802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saleh J, Cianflone K, Chaudhary T, Al-Riyami H, Al-Abri AR, Bayoumi R. Increased plasma acylation-stimulating protein correlates with hyperlipidemia at late gestation. Obesity (Silver Spring) 2007;15:646–52. doi: 10.1038/oby.2007.575. [DOI] [PubMed] [Google Scholar]
- 20.Scantlebury-Manning T, Bower J, Cianflone K, Barakat H. Racial difference in acylation stimulating protein (ASP) correlates to triglyceride in non-obese and obese African American and Caucasian women. Nutr Metab (Lond) 2009;6:18. doi: 10.1186/1743-7075-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Onat A, Can G, Rezvani R, Cianflone K. Complement C3 and cleavage products in cardiometabolic risk. Clin Chim Acta. 2011;412:1171–9. doi: 10.1016/j.cca.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 22.Cui G, Li Z, Li R, Huang J, Wang H, Zhang L, et al. A functional variant in APOA5/A4/C3/A1 gene cluster contributes to elevated triglycerides and severity of CAD by interfering with microRNA 3201 binding efficiency. J Am Coll Cardiol. 2014;64:267–77. doi: 10.1016/j.jacc.2014.03.050. [DOI] [PubMed] [Google Scholar]
- 23.Halkes CJ, van Dijk H, de Jaegere PP, Plokker HW, van Der Helm Y, Erkelens DW, et al. Postprandial increase of complement component 3 in normolipidemic patients with coronary artery disease: Effects of expanded-dose simvastatin. Arterioscler Thromb Vasc Biol. 2001;21:1526–30. doi: 10.1161/hq0901.095276. [DOI] [PubMed] [Google Scholar]
- 24.Viedt C, Shen W, Fei J, Kamimura M, Hänsch GM, Katus HA, et al. HMG-CoA reductase inhibition reduces the proinflammatory activation of human vascular smooth muscle cells by the terminal complement factor C5b-9. Basic Res Cardiol. 2003;98:353–61. doi: 10.1007/s00395-003-0437-4. [DOI] [PubMed] [Google Scholar]
- 25.Qian C, Wei B, Ding J, Wu H, Cai X, Li B, et al. Meta-analysis comparing the effects of rosuvastatin versus atorvastatin on regression of coronary atherosclerotic plaques. Am J Cardiol. 2015;116:1521–6. doi: 10.1016/j.amjcard.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 26.Timár O, Szekanecz Z, Kerekes G, Végh J, Oláh AV, Nagy G, et al. Rosuvastatin improves impaired endothelial function, lowers high sensitivity CRP, complement and immuncomplex production in patients with systemic sclerosis – A prospective case-series study. Arthritis Res Ther. 2013;15:R105. doi: 10.1186/ar4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muscari A, Bastagi L, Poggiopollini G, Tomassetti V, Massarelli G, Boni P, et al. Short term effect of atorvastatin and vitamin E on serum levels of C3, a sensitive marker of the risk of myocardial infarction in men. Cardiovasc Drugs Ther. 2001;15:453–8. doi: 10.1023/a:1013314227857. [DOI] [PubMed] [Google Scholar]
- 28.Oktenli C, Ozgurtas T, Dede M, Sanisoglu YS, Yenen MC, Yesilova Z, et al. Metformin decreases circulating acylation-stimulating protein levels in polycystic ovary syndrome. Gynecol Endocrinol. 2007;23:710–5. doi: 10.1080/09513590701666571. [DOI] [PubMed] [Google Scholar]
- 29.Alkuraishy HM, Al-Gareeb AI. New insights into the role of metformin effects on serum omentin-1 levels in acute myocardial infarction: Cross-sectional study. Emerg Med Int 2015. 2015:283021. doi: 10.1155/2015/283021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang H, Liu X, Wang D, Guo F, Liu J, Liang X, et al. Association of acylation-stimulating protein and receptor gene polymorphisms with coronary heart disease in Han and Hui populations. Int J Clin Exp Med. 2015;8:18779–85. [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang H, Guo M, Dong L, Cao C, Wang D, Liang X, et al. Levels of acylation stimulating protein and the complement component 3 precursor are associated with the occurrence and development of coronary heart disease. Exp Ther Med. 2014;8:1861–6. doi: 10.3892/etm.2014.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]


