Abstract
Background
Pregnancy has considerable effects on the pharmacokinetic properties of drugs used to treat uncomplicated Plasmodium falciparum malaria. The role of pharmacogenetic variation on anti-malarial drug disposition and efficacy during pregnancy is not well investigated. The study aimed to examine the effect of pharmacogenetics on lumefantrine (LF) pharmacokinetics and treatment outcome in pregnant women.
Methods
Pregnant women with uncomplicated falciparum malaria were enrolled and treated with artemether-lumefantrine (ALu) at Mkuranga and Kisarawe district hospitals in Coast Region of Tanzania. Day-7 LF plasma concentration and genotyping forCYP2B6 (c.516G>T, c.983T>C), CYP3A4*1B, CYP3A5 (*3, *6, *7) and ABCB1 c.4036A4G were determined. Blood smear for parasite quantification by microscopy, and dried blood spot for parasite screening and genotyping using qPCR and nested PCR were collected at enrolment up to day 28 to differentiate between reinfection from recrudescence. Treatment response was recorded following the WHO protocol.
Results
In total, 92 pregnant women in their second and third trimester were included in the study and 424 samples were screened for presence of P. falciparum. Parasites were detected during the follow up period in 11 (12%) women between day 7 and 28 after treatment and PCR genotyping confirmed recrudescent infection in 7 (63.3%) women. The remaining four (36.4%) pregnant women had reinfection: one on day 14 and three on day 28. The overall PCR-corrected treatment failure rate was 9.0% (95% CI 4.4–17.4). Day 7 LF concentration was not significantly influenced by CYP2B6, CYP3A4*1B and ABCB1 c.4036A>G genotypes. Significant associations between CYP3A5 genotype and day 7 plasma LF concentrations was found, being higher in carriers of CYP3A5 defective variant alleles than CYP3A5*1/*1 genotype. No significant influence of CYP2B6, CYP3A5 and ABCB1 c.4036A>Genotypes on malaria treatment outcome were observed. However, CYP3A4*1B did affect malaria treatment outcome in pregnant women followed up for 28 days (P = 0.018).
Conclusions
Genetic variations in CYP3A4 and CYP3A5may influence LF pharmacokinetics and treatment outcome in pregnant women.
Keywords: Malaria in pregnancy, Day 7 lumefantrine concentration, Recrudescence, Pharmacogenetics
Background
Pregnancy-induced physiological changes alter the pharmacokinetic properties of a number of anti-malarial drugs, usually resulting in lower drug exposures and lower cure rates especially in advanced pregnancy stage compared to non-pregnant population [1, 2]. Pregnancy-associated physiological changes result in lower drug absorption, enhances drug clearance, and increase body fluid volume of distribution [3–5]. Drug exposure depends greatly on the rate of metabolism and differences in activity of metabolizing enzymes can significantly alter the efficacy of drugs. Elevated levels of oestrogens, progesterone, cortisol, and prolactin during pregnancy have been linked to altered expression and metabolic activity of several hepatic cytochrome P450 enzymes. For instance, catalytic activity of CYP3A4, CYP2C9 and CYP2A6 enzymes increases during pregnancy, while CYP2C19 and CYP1A2 enzyme activity decreases [6, 7]. These enzymes are involved in metabolism of several anti-malarial drugs including LF and artemether [8–10], and are genetically polymorphic displaying wide inter-individual variations in enzyme activity [11–16].
Lower drug exposure levels in pregnant women have been reported for artemether/dihydroartemisinin, artesunate/dihydroartemisinin, dihydroartemisinin and lumefantrine (LF) [1, 17–19]. This increases the risks of treatment failure, increase risk of adverse outcomes for the fetus associated with malaria complications, and development of resistance to malaria parasites. A higher treatment failure rate has indeed been observed in pregnant women compared to non-pregnant ones living in the same area [1, 19, 20]. In this case, treatment failure may not be caused by intrinsic parasite resistance but is instead the result of inadequate drug levels due to pregnancy, pharmacogenetic profile of the host or other non-genetic modifiers of the pharmacokinetic parameters.
Genetic variation in drug metabolizing enzymes and transporter proteins might predict plasma exposure and treatment failure and/or emergence of drug resistant pathogens on infectious and non-infectious diseases, such as malaria, HIV and tuberculosis [20–24]. CYP3A responsible for metabolism of artemether and LF is induced by approximately twofold during the third trimester of human pregnancy. Low cure rates (83.5%) have been reported for pregnant women in Thailand receiving ALu [2]. However, pregnant women in Uganda showed an adequate clinical response (98.2%) using similar doses of ALu [25]. The differences in these studies might be explained by differences in host genetics, pharmacokinetics, different resistance patterns of malaria parasites, or higher levels of background immunity among individual pregnant women [25]. Another study reported that the wide difference in CYP3A4*1B allele frequency between the Tanzania and Cambodia populations presents a potential explanation for the lower efficacy of ALu in Cambodia and highlighted the importance of pharmacogenetic considerations in the decision-making process of first-line treatment policies for specific populations [8].
To date, there are limited studies that attempted to examine the role of pharmacogenetics on the pharmacokinetics of anti-malarial drugs and treatment outcomes in pregnant women. The aim of this study is to investigate the effects of pharmacogenetics on day 7 LF plasma concentrations and treatment outcome in pregnant patients treated with ALu in Tanzania. The findings might have implications for treatment policies of not only anti-malarial drugs, in particular the widely used artemisinin-based combinations, but also other drugs metabolized by these enzymes.
Methods
Study design and population
This was a one-arm prospective cohort study that included all pregnant women who gave consent to participate in the study when attending antenatal clinics at Kisarawe and Mkuranga district hospitals, northern Tanzania. Between May 2014 to April 2015, pregnant women attending the antenatal clinics (ANCs) were screened for malaria infection by using malaria rapid diagnostic test (MRDT). Pregnant women with uncomplicated Plasmodium falciparum infection and haemoglobin level of >8 g/d were enrolled. The study received ethics approval from the institutional review board of Muhimbili University of Health and Allied Sciences (MUHAS). Participants were informed about the aim of the study and gave written consent before participating in the study. To ensure confidentiality, women's identification numbers were used when labelling samples and during data entry into confidential report forms (CRF).
Sample size
Considering anticipated population proportion (P) of clinical failures in pregnant women being 18% [19], with 95% confidence level and 10% precision, 92 malaria positive pregnant women were enrolled in this study.
Treatment, clinical procedures and follow-up
The study participants received six doses of four tablets of ALu (Coartem®; Novartis Pharma AG, Basel, Switzerland) (20 mg artemether and 120 mg lumefantrine) over the course of 3 days at 0, 8, 24, 36, 48, and 60 h. For each patient, general physical examination was performed at enrollment (day 0) and on follow up visits on days 2, 7, 14, 21 and 28. Approximately 50 μl of blood was collected on filter paper (Whatman grade 3) for later PCR analysis. Each filter paper was dried and individually stored in a plastic bag and kept frozen at −80 °C. At enrollment day, 1 ml of whole blood was taken into an EDTA containing vacutainer tube and stored at −80 °C at MUHAS laboratory until further analysis. Additionally, 3 mls of venous blood were drawn from pregnant women in heparinized tubes on day 7 to determine plasma LF concentrations.
CYP3A4, CYP3A5, CYP2B6, and ABCB1 genotyping
Genomic DNA was isolated from peripheral leukocytes in whole blood samples using QIAamp DNA Midi Kit (Qiagen GmbH, Hilden, Germany) according to the manufacturer’s instructions. Genotyping for the common functional variant alleles for CYP2B6*6, CYP2B6*18, CYP3A4*1B, CYP3A5*3, CYP3A5*6, CYP3A5*7 and ABCB1 c.4036A>G (rs3842), which have been reported to be relevant for artemether and LF disposition [26, 27] were done as described previously [12, 22]. In brief genotyping was performed using TaqMan drug metabolism genotyping assay reagents for allelic discrimination (Applied Biosystems Genotyping Assays) with the following ID numbers for each SNP: C__7817765_60 for CYP2B6*6 (c.516G4T, rs3745274), C__60732328_20 for CYP2B6*18 (c.983T4C, rs28399499), C__26201809_30 for CYP3A5*3 (c.6986A4G, rs776746), C__30203950_10 for CYP3A5*6 (g.14690G4A,rs10264272) and C__32287188_10 for CYP3A5*7 (g.27131_27132insT rs41303343) and C__11711730_20) for ABCB1 c.4036A>G (rs3842), and C__11711730_20 for CYP3A4*1B (−392A>G, rs2740574). Genotyping was carried out using Quant Studio 12 K Flex Real-Time PCR system (Life Technologies Holding, Singapore, Singapore). The final volume for each reaction was 10 μl, consisting of TaqMan fast advanced master mix (Applied Biosystems, Waltham, MA, USA), TaqMan 20X drug metabolism genotyping assays mix (Applied Biosystems) and genomic DNA. The PCR profile consisted of an initial step at 60 °C for 30 s, hold stage at 95 °C for 10 min and PCR stage for 40 cycles step 1 with 95 °C for 15 and step 2 with 60 °C for 1 min and after read stage with 60 °C for 30 s.
Parasite detection and genotyping
Dried blood spots on filter papers obtained at enrolment (day 0) and on follow-up days (day 2, 7, 14, 21 and 28) were punched, and one circle 5 mm in diameter was used for DNA extraction using QIAamp DNA blood micro kit (Qiagen GmbH, Hilden, Germany) following the manufacturer’s recommendations. Detection of Plasmodium parasites was performed using a species-specific PCR targeting the ssRNA gene [28]. PCRs were carried out in duplicate in a 25-μl final volume containing 12.5 μl of Universal PCR Master Mix, 5 μl of DNA, forward and reverse primers at various concentrations, and P. falciparum, Plasmodium malariae, Plasmodium vivax and Plasmodium ovale probes at a final concentration of 100 nM. All reactions were run on an ABI Prism 7000 sequence detection system (Applied Biosystems) with the default settings; each sample was initially denatured at 95 °C for 10 min and cycled 40 times, with each cycle consisting of 95 °C for 15 s and 60 °C for 60 s. Each reaction plate included four positive controls for P. falciparum, P. ovale, P. vivax, and P. malariae and a negative control with molecular-grade water in place of DNA.
PCR genotyping to differentiate recurrent P. falciparum infections from reinfections were done according to World Health Organization (WHO) recommendations [29], by characterizing the length polymorphism of the merozoite surface protein 2 gene (msp2) in samples collected at day 0 and on the day recurrent parasitaemia was found. Recrudescence was determined when at least one msp2 allele of the same allelic type and of identical base pair size was found in samples collected on day 0 and on the day of recurrent infection. A reinfection was defined as all alleles were of different length in the sample collected on day 0 and that collected on the day of recurrent infection.
Quantification of LF plasma concentrations
Blood samples for the determination of LF plasma concentrations were collected on day 7 (corresponding to 168 h) following initiation of ALu treatment. Blood samples for quantification of LF levels were centrifuged and plasma was stored at −80 °C until analysis. Plasma LF concentration was analysed using a validated method of high performance liquid chromatography (HPLC) with ultraviolet detection at Sida/MUHAS bioanalytical laboratory in Dar-es-Salaam, Tanzania [30]. The coefficients of variation (CV %) during the analysis of LF were 8.4, 4.7 and 4.5% at 100, 1000, and 8000 ng/ml, respectively. The lower limit of quantification was 50 ng/ml.
Data analysis
LF plasma concentration data were log transformed to achieve normality of data distribution. Median (interquartile range) was used to describe day-7 LF plasma concentrations. Comparison of day-7 median LF plasma concentrations in pregnant women between the different genotypes were carried out using Kruskal–Wallis one-way analysis of variance (ANOVA). X2 test was used to compare the observed and expected allele frequencies according to the Hardy–Weinberg equilibrium. Influence of human genotype on malaria treatment outcome was analysed using Pearson’s Chi square and Fisher’s exact test. Haploview software package (version 4.2) was used to analyse linkage disequilibrium (LD) and haplotype construction. Statistical analyses were performed using Statistical Package for Social Sciences (SPSS) software, version 22.0 (IBM Corporation, Somers, NY, USA). P values <0.05 were considered to be statistically significant.
Malaria treatment outcomes were classified following the WHO protocol [31], as adequate clinical and parasitological response (ACPR), corrected for reinfection using PCR genotyping on day 28 and treatment failure (TF); designated as early treatment failure (ETF), late clinical failure (LCF), or late parasitological failure (LPF). The influence of genetic variations on malaria treatment outcome was evaluated using Cox regression analysis.
Results
Patient characteristics
In total, 1835 pregnant women were screened using MRDT and a total of 92 pregnant women with malaria infection consented and were enrolled in the study. Baseline characteristics are presented in Table 1. The median age of pregnant patients was 23 (range 15–41) years and the majority (54.3%) were multigravida. Most participants were in the second trimester (60.7%). The median parasite density was 2700 (range 400–72,500) parasites/µl.
Table 1.
Baseline characteristics of study participants (n = 92)
| Characteristic | Number of pregnant women | Percentage |
|---|---|---|
| Age (years) | ||
| <18 | 8 | 8.7 |
| 19–25 | 52 | 56.5 |
| >25 | 32 | 34.8 |
| Gravida (n) | ||
| Primigravida | 42 | 45.7 |
| Secundigravida | 17 | 18.5 |
| Multigravida | 33 | 35.9 |
| Trimester (n) | ||
| Second | 66 | 60.7 |
| Third | 26 | 39.3 |
| Parasitaemia (parasites/µl blood) | ||
| <1000 | 14 | 15.5 |
| 1000–10,000 | 51 | 55.0 |
| >10,000 | 27 | 29.5 |
Day 28 malaria treatment outcome in pregnant women
In total, 424 samples were screened for presence of parasites and P. falciparum was present in 11 (12%) patients during the follow up period. Two samples were detected on day 7, four on day 14, one on day 21 and four on day 28 after ALu treatment. PCR based genotyping of msp2 confirmed recrudescent infection in seven women (63.3%), two on day 7, three on day 14, one on day 21 and one on day 28. The remaining four (36.4%) pregnant women had reinfection; one on day 14 and three on day 28. The overall rate of reinfection was 4.3% (95% CI 0.15–8.45).
PCR uncorrected ACPR on day 28 was 88.9% (95% CI 82.06–95.74) and TF rates were 11.1% (95% CI 4.26–17.94). Rate of ACPR was calculated using per-protocol method, where patients were excluded due to lost to follow-up, protocol violations and TF due to reinfection. PCR-corrected ACPR rate as defined by absence of parasitaemia on day 28, irrespective of axillary temperature, in patients who did not previously meet any of the criteria of ETF, LCF or LPF was 91.0% (95% CI 82.62–95.58). The rate of PCR-corrected LPF, as defined by presence of parasitaemia on any day between day 7 and 28 in patients who did not previously meet any of the criteria of ETF or LPF, was 9.0% (95% CI 4.42–17.38). There was no ETF or LCF.
Allele, genotype and haplotype frequency distributions
The overall CYP2B6*6, CYP2B6*18, CYP3A4*1B, CYP3A5*3, CYP3A5*6, CYP3A5*7, ABCB1 c.4036A>G genotype and allele frequencies in Tanzanian pregnant women is presented in Table 2. There was no significant deviation between the observed and expected genotype frequencies from Hardy–Weinberg equilibrium. The variant allele frequency was highest (76.1%) for CYP3A4*1B followed by CYP2B6 c.516G>T (*6, 33.5%). CYP2B6 c.983T>C (*18) had the lowest allele frequency which was 9.3%. Genotype of CYP450 enzymes and ABCB1 transporter was equally distributed among the age, gravida, trimester and PMTCT status of pregnant women (P > 0.05).
Table 2.
Genotype and variant allele frequency distribution among pregnant women with uncomplicated Plasmodium falciparum infection in Tanzania
| Genotype | Frequency |
|---|---|
| N (%) | |
| CYP2B6 c.516G>T (*6) | |
| *1/*1 | 38 (46.3%) |
| *1/*6 | 33 (40.2%) |
| *6/*6 | 11 (13.4%) |
| CYP2B6 c.983T>C (*18) | |
| *1/*1 | 75 (82.4%) |
| *1/*18 | 15 (16.5%) |
| *18/*18 | 1 (1.1%) |
| CYP3A4*1B (−392A>G) | |
| *1/*1 | 3 (3.2%) |
| *1/*1B | 38 (41.3%) |
| *1B/*1B | 51 (55.4%) |
| CYP3A5*3 c.6986A>G | |
| *1/*1 | 51 (56.6%) |
| *1/*3 | 37 (41.1%) |
| *3/*3 | 2 (2.2%) |
| CYP3A5*6 c.14690G>A | |
| *1/*1 | 59 (64.1%) |
| *1/*6 | 28 (30.4%) |
| *6/*6 | 5 (5.4%) |
| CYP3A5*7 27131_27132insT | |
| *1/*1 | 68 (75.6%) |
| *1/*7 | 22 (24.4%) |
| *7/*7 | 0 |
| ABCB1 c.4036AG (rs3842) | |
| AA | 54 (58.7%) |
| AG | 26 (28.3%) |
| GG | 12 (13.4%) |
| Allele | Minor allele | % |
|---|---|---|
| CYP2B6 c.516G>T (*6) | *6 | 33.5 |
| CYP2B6 c.983T>C (*18) | *18 | 9.3 |
| CYP3A4*1B | *1B | 76.1 |
| CYP3A5*3 | *3 | 22.8 |
| CYP3A5*6 | *6 | 20.6 |
| CYP3A5*7 | *7 | 12.2 |
| ABCB1 c.4036AG (rs3842) | G | 27.2 |
Haplotype analysis of CYP3A4*1B (–392A>G), CYP3A5*3 (g.6986A>G), CYP3A5*6 (g.14690G>A), CYP3A5*7 (27131_27132insT) is presented in Fig. 1 and Table 3. There was no linkage between the two CYP2B6 variant alleles (c.516G>T and c.983T>C). Likewise, no LD between the three CYP3A5 SNPs (CYP3A5*3, CYP3A5*6, CYP3A5*7) was found. Instead each SNP was inversely linked to each other (i.e., each SNP is in strong LD with the wild type variant of the other two SNPs). The haplotype frequency of CYP3A5*1, *3, *6 and *7 was 44.5, 22.6, 20.7 and 12.2%, respectively. Interestingly all the three CYP3A5 variant alleles (CYP3A5*3, CYP3A5*6 and *7) occur in high LD with CYP3A4*1B. The major CYP3A haplotype was CYP3A4*1B alone (34.2%) followed by its linkage with CYP3A5*6 (17.6%) and CYP3A5*3 (13.3%) (Table 3).
Fig. 1.
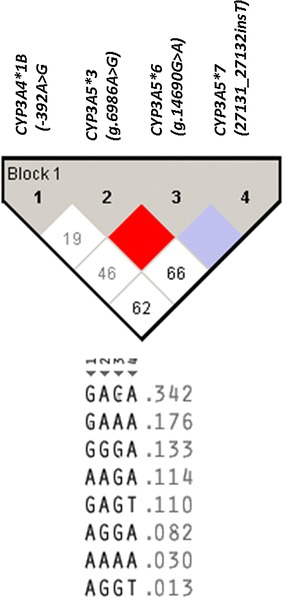
Linkage disequilibrium (LD) plot of CYP3A4*1B (−392A>G), CYP3A5*3 (g.6986A>G), CYP3A5*6 (g.14690G>A), CYP3A5*7 (27131_27132insT) and the observed D′ values (within the diagonal boxes). The pair-wise LD relationship between two SNPs and the respective D′ value is indicated in each square. The color gradient from red to white reveals higher to lower LD (D′ 1–0) values
Table 3.
CYP3A haplotype distribution among pregnant women with uncomplicated Plasmodium falciparum infection in Tanzania
| CYP3Ahaplotype | CYP3A SNP combinations | Frequency (%) | |||
|---|---|---|---|---|---|
|
CYP3A4*1B
−392A>G |
CYP3A5 *3
g.6986A>G |
CYP3A5 *6
g.14690G>A |
CYP3A5 *7
27131_27132insT |
||
| GAGA | *1B | wt | Wt | wt | 34.2 |
| GAAA | *1B | wt | *6 | wt | 17.6 |
| GGA | 1B | *3 | Wt | wt | 13.3 |
| AAGA | wt | wt | Wt | wt | 11.4 |
| GAGT | *1B | wt | Wt | *7 | 11.0 |
| AGGA | wt | *3 | Wt | wt | 8.2 |
| AAAA | wt | wt | *6 | wt | 3.0 |
| AGGT | wt | wt | Wt | *7 | 1.3 |
wt wild type variant allele, SNP single-nucleotide polymorphism
The effect of genotype on day 7 LF concentration in pregnant women
Based on the haplotype analysis result, subjects were grouped according to the numbers of functional CYP2B6*1 and CYP3A5*1 variant alleles; two (*1/*1), one (heterozygous for defective variant alleles) and zero (homozygous for defective variant alleles) to investigate the effect of genotype on day 7 plasma LF concentrations.
The median day 7 plasma LF concentration was 650 with the range of 2936 (124–3059) ng/ml. Comparison of the median plasma LF concentrations between the different genotypes is presented in Table 4. There was no significant effect of CYP2B6, CYP3A4*1B or ABCB1 c.4036AG genotype on day 7 LF plasma concentrations. The median plasma LF concentration was significantly associated with CYP3A5 genotypes (P = 0.039). CYP3A5 defective allele carriers displayed a higher plasma LF concentrations compared to those homozygous for CYP3A5*1/*1 genotypes (P = 0.04). There was significant association between having CYP3A5 genotype and having plasma LF concentration ≥600 ng/ml (Pearson Chi square test, P = 0.01). The proportion of subjects with two functional CYP3A5 genotype (*1/*1) was significantly higher among those with plasma LF conc <600 ng/ml (34.6%) compared to those with ≥600 ng/ml (5.9%). On the other hand proportion of subjects with lacking functional CYP3A5 (homozygous for defective variant alleles) was higher among those with plasma LF conc ≥600 ng/ml (38.2%) compared to those with >600 ng/ml (19.2%).
Table 4.
Comparison of median and inter quartile range (IQR) of day 7 LF median plasma concentration between different genotype groups in pregnant women with uncomplicated Plasmodium falciparum infection using Kruskal–Wallis ANOVA
| Plasma lumefantrine concertation at day 7 | |||
|---|---|---|---|
| n | Median (IQR) | P value | |
| No of CYP2B6*1 allele | |||
| Zero | 17 | 650.0 (285.8–1603.2) | 0.85 |
| One | 37 | 671.6 (280.0–1935.3) | |
| Two | 28 | 672.0 (350.0–2161.8) | |
| CYP3A4*1B | |||
| *1A/*1A | 3 | 294.6 (270.0–319.2) | 0.24 |
| *1A/*1B | 38 | 689.7 (354.1–1935.3) | |
| *1B/*1B | 51 | 644.6 (280.0–2615.0) | |
| No of CYP3A5*1 allele | |||
| Zero | 27 | 716.6 (340.9–2109.4) | 0.039 |
| One | 47 | 689.7 (280.0–3059.1) | |
| Two | 18 | 354.2 (285.8–1603.2) | |
| ABCB1 c.4036AG | |||
| AA | 54 | 585.2 (330.0–2615.0) | 0.28 |
| AG | 26 | 710.7 (282.3–2161.8) | |
| GG | 12 | 1055.1 (285.8–2109.4) | |
No of CYP2B6*1 allele: two, *1/*1; one, heterozygous for *6 or *18; zero, homozygous for *6 or *18 or combination thereof
No of CYP3A5*1 allele: two, *1/*1; one, heterozygous for *3, *6 or *7; zero, homozygous for *3, *6 or *7, combination thereof
The effect of genotype on malaria treatment outcome in pregnant women
The possible influence of genetic variations in CYP2B6, CYP3A5 and ABCB1 on malaria treatment outcome was evaluated using Cox regression analysis. A log rank test was run to determine differences in the cumulative hazard distribution between the different genotypes. There were no associations between malaria treatment outcome and ABCB1 transporter and most of CYP450 genotypes with an exception to CYP3A4 (Table 5).
Table 5.
Analysis of genetics effect on malaria treatment outcome (risk of recrudescence) in pregnant women
| Genotype | No of patients | No. of recrudescence | Percentage (%) | P value (log-rank test) |
|---|---|---|---|---|
| CYP2B6*6 | 0.676 | |||
| CYP2B6*1/*1 | 35 | 1 | 2.8 | |
| CYP2B6*1/*6 | 32 | 2 | 6.5 | |
| CYP2B6*6/*6 | 11 | 1 | 9.0 | |
| CYP2B6*18 | 0.605 | |||
| CYP2B6*1/*1 | 72 | 5 | 6.9 | |
| CYP2B6*1/*18 | 14 | 2 | 14.2 | |
| CYP2B6*18/*18 | 1 | 0 | 0 | |
| CYP3A4*1B | 0.018 | |||
| CYP3A4*1/*1 | 2 | 1 | 50 | |
| CYP3A4*1/*1B | 37 | 3 | 8.1 | |
| CYP3A4*1B/*1B | 49 | 3 | 6.1 | |
| CYP3A5*3 | 0.087 | |||
| CYP3A5*1/*1 | 48 | 3 | 6.2 | |
| CYP3A5*1/*3 | 36 | 3 | 8.3 | |
| CYP3A5*3/*3 | 2 | 1 | 50 | |
| CYP3A5*6 | 0.645 | |||
| CYP3A5*1/*1 | 57 | 4 | 7.0 | |
| CYP3A5*1/*6 | 26 | 3 | 11.5 | |
| CYP3A5*6/*6 | 5 | 0 | 0 | |
| CYP3A5*7 | 0.134 | |||
| CYP3A5*1/*1 | 66 | 7 | 10.6 | |
| CYP3A5*1/*7 | 20 | 0 | 0 | |
| CYP3A5*7/*7 | – | – | – | |
| ABCB1 rs3842 | 0.462 | |||
| A/A | 12 | 0 | 0 | |
| A/G | 25 | 3 | 12 | |
| G/G | 51 | 4 | 7.8 |
Discussion
In the present study, the effect of pharmacogenetics on LF pharmacokinetics (focusing on day 7 LF plasma concentration, which is a surrogate marker for AUC and malaria cure rate) and treatment outcome in pregnant women was investigated. For this purpose, functional variant alleles in CYP450 (CYP2B6, CYP3A4, CYP3A5) and ABCB1 genes involved in the metabolism of anti-malarial drugs were assessed in pregnant malaria patients treated with ALu. The major finding includes: (i) CYP3A5 genotype has significant influence on plasma LF concentration and (ii) CYP3A4*1B is associated with malaria treatment outcome. A number of studies have speculated that lower artemether and LF concentration in pregnant women has been due to changes in catalytic activities of CYP450 metabolizing enzymes particularly the increased activity of CYP3A4 [19, 20, 32]. This is the first study in pregnant women to investigate the effect of pharmacogenetics on day 7 LF concentrations and malaria treatment outcome after a follow up of 28 days. The observed variant allele frequencies for CYP2B6*6 (c.516G→T), CYP3A4*1B, and CYP3A5*3 are very similar to those previously reported from previous studies conducted in Tanzanian population [8, 12, 22, 23].
Lumefantrine, the long-acting component of the most widely used artemisinin-based combination therapy (ACT) in Africa is metabolized by CYP3A4. Artemether is metabolized by CYP3A4 and CYP3A5. Therefore, information on the role of pharmacogenetics on drug disposition and treatment outcome in pregnant women using ALu is needed. CYP3A5 is mainly expressed in black population and its genotype contributes to variations in the total CYP3A enzyme activity as measured by 4beta-hydroxycholesterol, an endogenous CYP3A marker [13, 15, 33, 34].
There was significantly higher plasma LF concentration in patients carrying CYP3A5 defective alleles than those without. Though not significant, a similar finding was observed in a recent HIV-malaria cohort study in Tanzania [22]. Pregnant women withCYP3A5*1/*1 genotype had significantly higher risk of having LF plasma concentration <600 ng/ml. Plasma LF concentration <600 ng/ml is associated with risk of recurrent parasitaemia in pregnant women [20]. However, there was no significant impact of CYP3A5 genotype on malaria treatment outcome which is similar to previous reports [22]. This may indicate that CYP3A5 plays a minor role compared to CYP3A4 in determining malaria treatment outcome. Indeed, CYP3A4 is the major drug metabolizing enzyme than CYP3A5 for most CYP3A substrate drugs [35].
In this study, there was no influence of CYP3A4*1B on day 7 LF plasma levels. The findings are similar to previous reports from Cambodia and Tanzania [8]. A study conducted in Uganda reported that lower LF day 7 plasma concentrations observed during pregnancy were caused by a decrease of 36.5% in the inter-compartmental clearance in pregnant women [18]. The decrease in clearance can be attributed to changes in body composition (increased plasma volume, water, and fat content) during pregnancy [36] where the distribution of the lipophilic LF through the body might be altered substantially. In another study conducted in Tanzania it was also reported that CYP3A4*1B genotype did not affect day 7 LF plasma levels unless it was induced by a potent CYP3A4 inducer [22].
The study also analysed patients with recrudescence and compared their pharmacogenetic profiles with those with an ACPR. There was a significant association between CYP3A4*1B genotype and treatment outcome in pregnant women. It was observed that majority of pregnant women with CYP3A4*1B/*1B had attained ACPR compared to those with CYP3A4*1/*1. Inconclusive data on alterations of enzyme activity in CYP3A4*1B carriers are reported in the literature. Some investigators have suggested CYP3A4*1B is associated with increased CYP3A4 expression and enhanced drug elimination in carriers of CYP3A4*1B may lead to treatment failure [37]. In contrast, the association of CYP3A4*1B with lower CYP3A4 enzyme activity in Tanzanians is reported previously, where carriers of CYP3A4*1B had a significantly lower enzyme activity than CYP3A4*1 [38]. The finding of significantly lower total CYP3A activity in Tanzanians than whites (Swedes) and Asians (Koreans) despite having high allele frequency ofCYP3A4*1B in Tanzanians (77%) [34] may indicate the association of CYP3A4*1B with low enzyme activity in blacks. CYP3A4*1B variant allele is absent in Asians and occurs at a much lower frequency (2–9%) in whites [39].
Preliminary finding indicates that pharmacogenetic variation in CYP3A4 and CYP3A5 influences the LF plasma exposure and malaria treatment outcome in pregnant women. Since CYP3A is responsible for the metabolism of artemether and LF, interplay between CYP3A4 and CYP3A5 genotypes may determine ACT plasma exposure and treatment outcome. CYP3A4 and CYP3A5 haplotypes are located in the same gene locus, effects initially considered to be due to a CYP3A4 allele might actually be due to a CYP3A5 allele in LD [40]. LD between CYP3A4*1B and CYP3A5*1Ais suggested as possible cause of inter individual variation in CYP3A metabolism [41, 42].
Previous studies in white population reported that CYP3A4*1B is linked with the functional CYP3A5*1 resulting in high enzyme activity [42, 43]. In contrast our extensive haplotype analysis in Tanzanians (Fig. 1; Table 3) indicates that CYP3A4*1B is linked with CYP3A5 defective variant alleles (*3, *5 and *7) and hence may be associated with low enzyme activity. It is well known that sub-Saharan African population is the most genetically heterogeneous population globally, characterized by extensive population substructure and unique LD pattern compared to non-African populations [44, 45]. Lack of LD between the two CYP2B6 variant alleles (c.516G>T and c.983T>C) and between the three CYP3A5 SNPs (c.6986A4G, g.14690G4A and g.27131_27132 insT) in this study is similar to previously reports from Africa [11, 13, 15, 23].
Limitation of this study includes that the sample size was calculated based on the previous study whereby treatment failure was reported to be 18% which is higher than the failure rate reported in this study. As a result, although the sample size is enough to investigate anti-malarial drug efficacy in the population, the study is under-powered to investigate the impact of CYP3A haplotypes on treatment outcome. However, the study finding highlights the importance of genetic variation in the CYP3A locus for LF pharmacokinetics and treatment outcome in pregnant women. This study also presents haplotype structure of the most common CYP3A functional variant alleles in African population for future genetic association studies.
Conclusions
In general, the study finding indicates association of the low enzyme activity genotype of CYP3A4*5 and CYP3A4*1B with high LF plasma exposure and better malaria treatment outcome, respectively. The effects of pharmacogenetics on LF pharmacokinetics were well characterized in pregnant patients with uncomplicated P. falciparum malaria. The importance of these findings is that in the future genotyping can be used to predict the need for anti-malarial drugs dosage adjustment to pregnant women with CYP3A4*1/*1or CYP3A5*1/*1. The impact of CYP3A haplotypes on the metabolism of anti-malarial drugs in a large sample size cohort needs to be further evaluated.
Authors’ contributions
AARK EA and SSM conceived the study, participated in the study protocol development, coordination and manuscript writing. OMSM, AF, AM, EA and MVH participated in the data analysis as well as manuscript writing. RFM participated in study protocol development, data collection and analysis and in the preparation and writing of the manuscript. All authors participated in reading. All authors read and approved the final manuscript.
Acknowledgements
We are grateful to our fellow malaria team members at MUHAS-Dr. Billy Ngasala, Dr. ElifordNgaimisi and Dr. Edna Gasarasi for providing assistance. We thank Dorisia Nanage for her dedicated technical assistance. We also thank the health care providers and the study participants from Mkuranga and Kisarawe district hospitals for all the co-operation they gave to us throughout the study period.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets used and analysed during the current study is available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The study received ethics approval from the institutional review board of Muhimbili University of Health and Allied Sciences (MUHAS). Participants were informed about the aim of the study and gave written consent before participating in the study. To ensure confidentiality, women`s identification numbers were used when labeling samples and during data entry into confidential report forms (CRF).
Funding
This study was supported by grants from the Swedish International Development Cooperation Agency Programme (SIDA) under MUHAS Reproductive health projects.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ritah F. Mutagonda, Email: rittdavisrida@yahoo.com
Appolinary A. R. Kamuhabwa, Email: enali2012@gmail.com
Omary M. S. Minzi, Email: minziobejayesu@gmail.com
Siriel N. Massawe, Email: snanzia@gmail.com
Muhammad Asghar, Email: asghar.muhammad@ki.se.
Manijeh V. Homann, Email: manijeh.vafa-homann@ki.se
Anna Färnert, Email: anna.farnert@ki.se.
Eleni Aklillu, Email: eleni.aklillu@ki.se.
References
- 1.McGready R, Stepniewska K, Lindegardh N, Ashley EA, La Y, Singhasivanon P, et al. The pharmacokinetics of artemether and lumefantrine in pregnant women with uncomplicated falciparum malaria. Eur J Clin Pharmacol. 2006;62:1021–1031. doi: 10.1007/s00228-006-0199-7. [DOI] [PubMed] [Google Scholar]
- 2.McGready R, Tan SO, Ashley EA, Pimanpanarak M, Viladpai-Nguen J, Phaiphun L, et al. A randomised controlled trial of artemether-lumefantrine versus artesunate for uncomplicated Plasmodium falciparum treatment in pregnancy. PLoS Med. 2008;5:e253. doi: 10.1371/journal.pmed.0050253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pavek P, Ceckova M, Staud F. Variation of drug kinetics in pregnancy. Curr Drug Metab. 2009;10:520–529. doi: 10.2174/138920009788897993. [DOI] [PubMed] [Google Scholar]
- 4.Ward SA, Sevene EJ, Hastings IM, Nosten F, McGready R. Antimalarial drugs and pregnancy: safety, pharmacokinetics, and pharmacovigilance. Lancet Infect Dis. 2007;7:136–144. doi: 10.1016/S1473-3099(07)70025-7. [DOI] [PubMed] [Google Scholar]
- 5.Anderson GD. Pregnancy-induced changes in pharmacokinetics: a mechanistic-based approach. Clin Pharmacokinet. 2005;44:989–1008. doi: 10.2165/00003088-200544100-00001. [DOI] [PubMed] [Google Scholar]
- 6.Isoherranen N, Thummel KE. Drug metabolism and transport during pregnancy: how does drug disposition change during pregnancy and what are the mechanisms that cause such changes? Drug Metab Dispos. 2013;41:256–262. doi: 10.1124/dmd.112.050245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeong H. Altered drug metabolism during pregnancy: hormonal regulation of drug-metabolizing enzymes. Expert Opin Drug Metab Toxicol. 2010;6:689–699. doi: 10.1517/17425251003677755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Staehli Hodel EM, Csajka C, Ariey F, Guidi M, Kabanywanyi AM, Duong S, et al. Effect of single nucleotide polymorphisms in cytochrome P450 isoenzyme and N-acetyltransferase 2 genes on the metabolism of artemisinin-based combination therapies in malaria patients from Cambodia and Tanzania. Antimicrob Agents Chemother. 2013;57:950–958. doi: 10.1128/AAC.01700-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mukonzo JK, Waako P, Ogwal-Okeng J, Gustafsson LL, Aklillu E. Genetic variations in ABCB1 and CYP3A5 as well as sex influence quinine disposition among Ugandans. Ther Drug Monit. 2010;32:346–352. doi: 10.1097/FTD.0b013e3181da79d6. [DOI] [PubMed] [Google Scholar]
- 10.Piedade R, Gil JP. The pharmacogenetics of antimalaria artemisinin combination therapy. Expert Opin Drug Metab Toxicol. 2011;7:1185–1200. doi: 10.1517/17425255.2011.608660. [DOI] [PubMed] [Google Scholar]
- 11.Mirghani RA, Sayi J, Aklillu E, Allqvist A, Jande M, Wennerholm A, et al. CYP3A5 genotype has significant effect on quinine 3-hydroxylation in Tanzanians, who have lower total CYP3A activity than a Swedish population. Pharmacogenet Genom. 2006;16:637–645. doi: 10.1097/01.fpc.0000230411.89973.1b. [DOI] [PubMed] [Google Scholar]
- 12.Mugusi S, Ngaimisi E, Janabi M, Minzi O, Bakari M, Riedel KD, et al. Liver enzyme abnormalities and associated risk factors in HIV patients on Efavirenz-based HAART with or without tuberculosis co-infection in Tanzania. PLoS ONE. 2012;7:e40180. doi: 10.1371/journal.pone.0040180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gebeyehu E, Engidawork E, Bijnsdorp A, Aminy A, Diczfalusy U, Aklillu E. Sex and CYP3A5 genotype influence total CYP3A activity: high CYP3A activity and a unique distribution of CYP3A5 variant alleles in Ethiopians. Pharmacogenom J. 2011;11:130–137. doi: 10.1038/tpj.2010.16. [DOI] [PubMed] [Google Scholar]
- 14.Aklillu E, Djordjevic N, Carrillo JA, Makonnen E, Bertilsson L, Ingelman-Sundberg M. High CYP2A6 enzyme activity as measured by a caffeine test and unique distribution of CYP2A6 variant alleles in Ethiopian population. OMICS. 2014;18:446–453. doi: 10.1089/omi.2013.0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Habtewold A, Amogne W, Makonnen E, Yimer G, Nylen H, Riedel KD, et al. Pharmacogenetic and pharmacokinetic aspects of CYP3A induction by efavirenz in HIV patients. Pharmacogenom J. 2013;13:484–489. doi: 10.1038/tpj.2012.46. [DOI] [PubMed] [Google Scholar]
- 16.Hatta FH, Lundblad M, Ramsjo M, Kang JH, Roh HK, Bertilsson L, et al. Differences in CYP2C9 genotype and enzyme activity between Swedes and Koreans of relevance for personalized medicine: role of ethnicity, genotype, smoking, age, and sex. OMICS. 2015;19:346–353. doi: 10.1089/omi.2015.0022. [DOI] [PubMed] [Google Scholar]
- 17.Tarning J, Rijken MJ, McGready R, Phyo AP, Hanpithakpong W, Day NP, et al. Population pharmacokinetics of dihydroartemisinin and piperaquine in pregnant and nonpregnant women with uncomplicated malaria. Antimicrob Agents Chemother. 2012;56:1997–2007. doi: 10.1128/AAC.05756-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kloprogge F, Piola P, Dhorda M, Muwanga S, Turyakira E, Apinan S, et al. Population pharmacokinetics of lumefantrine in pregnant and nonpregnant women with uncomplicated Plasmodium falciparum malaria in Uganda. CPT Pharmacometr Syst Pharmacol. 2013;2:e83. doi: 10.1038/psp.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mosha D, Guidi M, Mwingira F, Abdulla S, Mercier T, Decosterd LA, et al. Population pharmacokinetics and clinical response for artemether-lumefantrine in pregnant and nonpregnant women with uncomplicated Plasmodium falciparum malaria in Tanzania. Antimicrob Agents Chemother. 2014;58:4583–4592. doi: 10.1128/AAC.02595-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mutagonda RF, Kamuhabwa AA, Minzi OM, Massawe SN, Maganda BA, Aklillu E. Malaria prevalence, severity and treatment outcome in relation to day 7 lumefantrine plasma concentration in pregnant women. Malar J. 2016;15:278. doi: 10.1186/s12936-016-1327-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maganda BA, Ngaimisi E, Kamuhabwa AA, Aklillu E, Minzi OM. The influence of nevirapine and efavirenz-based anti-retroviral therapy on the pharmacokinetics of lumefantrine and anti-malarial dose recommendation in HIV-malaria co-treatment. Malar J. 2015;14:179. doi: 10.1186/s12936-015-0695-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maganda BA, Minzi OM, Ngaimisi E, Kamuhabwa AA, Aklillu E. CYP2B6*6 genotype and high efavirenz plasma concentration but not nevirapine are associated with low lumefantrine plasma exposure and poor treatment response in HIV-malaria-coinfected patients. Pharmacogenom J. 2016;16:88–95. doi: 10.1038/tpj.2015.37. [DOI] [PubMed] [Google Scholar]
- 23.Ngaimisi E, Habtewold A, Minzi O, Makonnen E, Mugusi S, Amogne W, et al. Importance of ethnicity, CYP2B6 and ABCB1 genotype for efavirenz pharmacokinetics and treatment outcomes: a parallel-group prospective cohort study in two sub-Saharan Africa populations. PLoS ONE. 2013;8:e67946. doi: 10.1371/journal.pone.0067946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ngaimisi E, Mugusi S, Minzi O, Sasi P, Riedel K, Suda A, et al. Effect of rifampicin and CYP2B6 genotype on long-term efavirenz autoinduction and plasma exposure in HIV patients with or without tuberculosis. Clin Pharmacol Ther. 2011;90:406–413. doi: 10.1038/clpt.2011.129. [DOI] [PubMed] [Google Scholar]
- 25.Piola P, Nabasumba C, Turyakira E, Dhorda M, Lindegardh N, Nyehangane D, et al. Efficacy and safety of artemether-lumefantrine compared with quinine in pregnant women with uncomplicated Plasmodium falciparum malaria: an open-label, randomised, non-inferiority trial. Lancet Infect Dis. 2010;10:762–769. doi: 10.1016/S1473-3099(10)70202-4. [DOI] [PubMed] [Google Scholar]
- 26.Djimde A, Lefevre G. Understanding the pharmacokinetics of Coartem. Malar J. 2009;8(Suppl 1):S4. doi: 10.1186/1475-2875-8-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.German PI, Aweeka FT. Clinical pharmacology of artemisinin-based combination therapies. Clin Pharmacokinet. 2008;47:91–102. doi: 10.2165/00003088-200847020-00002. [DOI] [PubMed] [Google Scholar]
- 28.Shokoples SE, Ndao M, Kowalewska-Grochowska K, Yanow SK. Multiplexed real-time PCR assay for discrimination of Plasmodium species with improved sensitivity for mixed infections. J Clin Microbiol. 2009;47:975–980. doi: 10.1128/JCM.01858-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.WHO. Methods and techniques for clinical trials on antimalarial drug efficacy: genotyping to identify parasite populations. Geneva: World Health Organization. 2008. http://whqlibdoc.who.int/publications/2008/9789241596305_eng.pdf?ua=1. Accessed 30 Dec 2016.
- 30.Minzi OM, Ngaimisi E, Shewiyo DH, Sasi P, Ignace AI. Interlaboratory development and cross validation of a chromatographic method for determination of lumefantrine in human plasma-a proficient capacity assessment of bioanalytical laboratories in East Africa. J Anal Bioanal Tech. 2012;3:131. [Google Scholar]
- 31.WHO. Methods for surveillance of antimalarial drug efficacy. Geneva: World Health Organization. 2009. http://whqlibdoc.who.int/publications/2009/9789241597531_eng.pdf. Accessed 1 Jan 2017.
- 32.Tarning J, Kloprogge F, Dhorda M, Jullien V, Nosten F, White NJ, et al. Pharmacokinetic properties of artemether, dihydroartemisinin, lumefantrine, and quinine in pregnant women with uncomplicated Plasmodium falciparum malaria in Uganda. Antimicrob Agents Chemother. 2013;57:5096–5103. doi: 10.1128/AAC.00683-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ngaimisi E, Minzi O, Mugusi S, Sasi P, Riedel KD, Suda A, et al. Pharmacokinetic and pharmacogenomic modelling of the CYP3A activity marker 4beta-hydroxycholesterol during efavirenz treatment and efavirenz/rifampicin co-treatment. J Antimicrob Chemother. 2014;69:3311–3319. doi: 10.1093/jac/dku286. [DOI] [PubMed] [Google Scholar]
- 34.Diczfalusy U, Miura J, Roh HK, Mirghani RA, Sayi J, Larsson H, et al. 4Beta-hydroxycholesterol is a new endogenous CYP3A marker: relationship to CYP3A5 genotype, quinine 3-hydroxylation and sex in Koreans, Swedes and Tanzanians. Pharmacogenet Genom. 2008;18:201–208. doi: 10.1097/FPC.0b013e3282f50ee9. [DOI] [PubMed] [Google Scholar]
- 35.Williams JA, Ring BJ, Cantrell VE, Jones DR, Eckstein J, Ruterbories K, et al. Comparative metabolic capabilities of CYP3A4, CYP3A5, and CYP3A7. Drug Metab Dispos. 2002;30:883–891. doi: 10.1124/dmd.30.8.883. [DOI] [PubMed] [Google Scholar]
- 36.Dawes M, Chowienczyk PJ. Drugs in pregnancy. Pharmacokinetics in pregnancy. Best Pract Res Clin Obstet Gynaecol. 2001;15:819–826. doi: 10.1053/beog.2001.0231. [DOI] [PubMed] [Google Scholar]
- 37.Amirimani B, Ning B, Deitz AC, Weber BL, Kadlubar FF, Rebbeck TR. Increased transcriptional activity of the CYP3A4*1B promoter variant. Environ Mol Mutagen. 2003;42:299–305. doi: 10.1002/em.10199. [DOI] [PubMed] [Google Scholar]
- 38.Rodriguez-Antona C, Sayi JG, Gustafsson LL, Bertilsson L, Ingelman-Sundberg M. Phenotype-genotype variability in the human CYP3A locus as assessed by the probe drug quinine and analyses of variant CYP3A4 alleles. Biochem Biophys Res Commun. 2005;338:299–305. doi: 10.1016/j.bbrc.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 39.Lee JS, Cheong HS, Kim LH, Kim JO, Seo DW, Kim YH, et al. Screening of genetic polymorphisms of CYP3A4 and CYP3A5 genes. Korean J Physiol Pharmacol. 2013;17:479–484. doi: 10.4196/kjpp.2013.17.6.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fukushima-Uesaka H, Saito Y, Watanabe H, Shiseki K, Saeki M, Nakamura T, et al. Haplotypes of CYP3A4 and their close linkage with CYP3A5 haplotypes in a Japanese population. Hum Mutat. 2004;23:100. doi: 10.1002/humu.9210. [DOI] [PubMed] [Google Scholar]
- 41.Lamba JK, Lin YS, Schuetz EG, Thummel KE. Genetic contribution to variable human CYP3A-mediated metabolism. Adv Drug Deliv Rev. 2002;54:1271–1294. doi: 10.1016/S0169-409X(02)00066-2. [DOI] [PubMed] [Google Scholar]
- 42.Wojnowski L, Hustert E, Klein K, Goldammer M, Haberl M, Kirchheiner J, et al. Re: modification of clinical presentation of prostate tumors by a novel genetic variant in CYP3A4. J Natl Cancer Inst. 2002;94:630–631. doi: 10.1093/jnci/94.8.630. [DOI] [PubMed] [Google Scholar]
- 43.Dally H, Edler L, Jager B, Schmezer P, Spiegelhalder B, Dienemann H, et al. The CYP3A4*1B allele increases risk for small cell lung cancer: effect of gender and smoking dose. Pharmacogenetics. 2003;13:607–618. doi: 10.1097/00008571-200310000-00004. [DOI] [PubMed] [Google Scholar]
- 44.Aklillu E, Habtewold A, Ngaimisi E, Yimer G, Mugusi S, Amogne W, et al. SLCO1B1 gene variations among Tanzanians, Ethiopians, and Europeans: relevance for African and worldwide precision medicine. OMICS. 2016;20:538–545. doi: 10.1089/omi.2016.0119. [DOI] [PubMed] [Google Scholar]
- 45.Campbell MC, Tishkoff SA. African genetic diversity: implications for human demographic history, modern human origins, and complex disease mapping. Annu Rev Genom Hum Genet. 2008;9:403–433. doi: 10.1146/annurev.genom.9.081307.164258. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analysed during the current study is available from the corresponding author on reasonable request.


