Abstract
Background
Cathepsin B is a lysosomal cysteine protease involved in apoptosis and oocytes which have lower developmental competence show higher expression of Cathepsin B. Furthermore, expression of Cathepsin B show a decreasing trend from oocyte toward blastocyst stage.
Results
Present study assessed the effect of cathepsin B inhibitor, E-64, on developmental competency and cryo-survival of pre-implantation ovine IVF derived embryos. Cathepsin B inhibitor was added during day 3 to 8 of development. One μM E-64 was defined as the optimal concentration required for improving blastocyst rate. This concentration also reduced DNA fragmentation and BAX as apoptotic markers while increasing total cell number per blastocyst and improving anti-apoptotic marker, the BCL2. We further showed that addition of 1.0 μM of E-64 during day 3 to 8 of development improved re-expansion and hatching rates of blastocysts post vitrification. E-64 also reduced rate of DNA fragmentation and BAX expression and increased total cell number per blastocyst and BCL2 expression post vitrification. However, addition of E-64 post vitrification reduced the hatching rate.
Conclusion
Therefore, it can be concluded that inhibition of cathepsin B in IVC, not only improves quality and quantity of blastocysts but also improves the cryo-survival of in vitro derived blastocysts.
Keywords: Cathepsin B inhibitor (E-64), Ovine, Blastocyst, Apoptosis, Cryosurvival
Background
Assisted reproductive technologies, such as in vitro fertilization (IVF) and intra-cytoplasmic sperm injection (ICSI), not only have transformed treatment of human infertility but also have had significant impact on farm animal reproduction and productivity. Among these techniques, embryos vitrification has progressed to become a useful adjunct technique, allowing storage of excess embryos for future use in human and animal embryo transfer programs. Despite intense research efforts and progress in field of vitrification, significant observable morphological and biochemical alterations are associated with vitrification. These alterations may lead to blastomere death and, eventually loss of embryo viability that are attributed to type and concentration of cryoprotectants [1], freezing protocol [2, 3], species, genotype, developmental stage and type of embryo [4, 5]. In this regard, some researchers have shown that the presence of dead cells is a common physiological finding in mammalian pre-implantation development and the number of dead cells is increased by vitrification [6].
Cell death in embryos has been mainly associated with programmed cell death or apoptosis [7] and is considered as potential cellular response to suboptimal developmental conditions and stress, such as vitrification [8, 9]. Numerous phenotypic phenomena including plasma-membrane blebbing, cell shrinkage, nuclear fragmentation, chromatin compaction, and chromosomal DNA fragmentation are associated with apoptosis [10]. Considerable researches have correlated these phenomena with low potential of embryos to reach blastocyst stage [11]. Therefore, improvement of culture media by different supplements to avoid apoptosis, is indispensable for production of good quality embryos in vitro.
Recent studies have shown that supplementation of culture medium with Rho-associated protein kinase inhibitor (ROCK inhibitor) [12], proteasome inhibitor [13], and antioxidants such as β-mercaptoethanol [14] can enhance and improve survival and quality of embryos produced in vitro.
Cathepsins are lysosomal cysteine proteases that play a crucial role in degradation of intracellular proteins in lysosomes [15]. They are also involved in induction of apoptosis through activating initiator caspases [16]. Indeed, it has been reported that sub-optimal pH and in vitro stressors can lead to secretion of cathepsins from lysosomes [17]. In this regard, cathepsin inhibitor (E-64) supplementation may overcome in vitro induced apoptosis and improve in vitro embryo development.
Inhibition of cathepsins improves in vitro developmental competency of embryos [18–25]. However, to our knowledge there is no report on the effect of cathepsin inhibitors on developmental competency of vitrified embryos. Therefore, aim of the current study was to assess the effect of culture supplementation with E-64, a cathepsin B inhibitor, on developmental competence, apoptosis and cryo-survival of in vitro produced ovine embryos.
Methods
Design of study
Experiment 1
To select the optimal concentration of E-64, on day 3 of culture during exchange of medium, embryos were randomly allocated to different concentrations (0.0, 0.1, 1.0, 10 μM) of E-64. Embryos were cultured in these concentrations of E-64 for up to 6 days. Percentage of morula, and blastocyst formed were determined on day 7–8 in four replicates.
Experiment 2
Following determination of optimal concentration of E-64, expanded blastocysts obtained from in vitro culture of embryos in the presence (IVC+) or absence (IVC−) of 1 μM of E-64 were vitrified and warmed. Vitrified/warmed blastocysts, here referred to as post warming (PW), derived from the after-mentioned groups (IVC+ or IVC−) were then randomly divided and cultured for 24 h in presence (IVC−/PW+, IVC+/ PW+) or absence (IVC+/ PW−, IVC−/PW−) of E-64. Cryo-survival (defined as rate of re-expansion) and hatching rates were determined and compared between groups. Finally, hatched blastocysts derived from each experimental group were used for mRNA analyses (3 replicates and minimum number of examined blastocysts in each replicate was 10), determination of cell number (3 replicates and minimum number of examined blastocysts in each replicate was eight) and assessment of DNA fragmentation by TUNEL assay (7 replicates and minimum number of examined blastocysts in each replicate was sixteen).
Materials
Unless otherwise specified, all chemicals and media were obtained from Sigma Chemical Co. (St. Louis, MO, USA) and Gibco (Grand Island, NY, USA), respectively.
In vitro embryo production
The procedure used for production of ovine embryos was according to Moulavi et al. [26]. Ovaries were obtained from a local abattoir and were transported to the laboratory in saline (15 °C–20 °C) and stored for additional 12 h at 15 C. COCs (cumulus-oocyte complexes) were isolated from 2 to 6 mm antral follicles with the aid of 20-G needles. Subsequently, they were washed with Hepes-supplemented tissue culture medium- 199 (HTCM199) + 10% FBS (fetal bovine serum). COCs with more than three layers of cumulus cells and homogenous cytoplasm were selected. Finally, 10 washed and selected COCs were cultured in 50 μl maturation medium [(MM: TCM199 + 10% FBS with 10 μg/ml FSH (follicle-stimulating hormone),10 μg/ml LH (luteinizing hormone), 1 μg/ml 17-beta estradiol, 0.1 mM cysteamine,10 ng/ml EGF (epidermal growth factor) and 100 ng/ml IGF1(insulin-like growth factor 1)] under mineral oil for 22–24 h at 38.5 °C, 5% CO2, in humidified air.
For in vitro fertilization (IVF), 100 μl of fresh sperm from a ram with proven fertility were kept under Tyrode’s albumin lactate pyruvate medium in 5% CO2, 38.5 °C, and humidified air for up to 45 min to allow motile sperm to swim up. After swim up, insemination was carried out by adding 5 × 103 sperm/ matured COCs in fertilization medium containing NaCl 114 mM, KCl 3.15 mM, NaH2PO4, 0.39 mM, Na-lactate 13.3 mM, CaCl2 2 mM, MgCl2 0.5 mM, Na-pyruvate 0.2 mM, Penicillin 50 IU/ml, Streptomycin 50 μg/ml, NaHCO3 25 mM, Heparin 10 μg/ml for 18–24 h at 38.5 °C under 5% CO2 in humidified air overlaid with light mineral oil.
On the next day, to remove the cumulus cells, the presumptive zygotes were vortexed in HTCM199 + FBS for 3 min. Then, they were cultured for 3 days in glucose and serum free modified synthetic oviductal fluid (mSOF) [27]. After the third day, cleaved embryos were transferred to mSOF in the presence of charcoal stripped serum (5%) and glucose (1.5 mM) for 5 days at 39 °C, 6% CO2, 5% O2 in humidified air under oil. Day 0 was defined as the day of fertilization. Therefore cleavage, blastocyst and hatching rates were determined on the day 3, 7 and 8 post embryo cultures.
Vitrification and warming process
Vitrification–warming process was adopted from Martinez et al. [28]. Briefly, blastocysts were washed for 1 min in basic solution [BS: composed of phosphate buffer saline (PBS) and 20% FBS]. Then, blastocysts were equilibrated in equilibration solution (ES: 7.5% EG and7.5% DMSO in BS) for 5 min. Finally, they were exposed to vitrification solution (VS: 15% EG + 15% DMSO + 0.5 M sucrose) for 30 to 50 s and vitrified in minimal amount of vitrification solution on cryotops (Cryologic; CVM™, Fibreplug & Sleeve, Australia), which were quickly plunged into liquid nitrogen (LN2).
For warming, the cryotops were removed from LN2 and immediately tipped to warming solution 1 for 1 min (WS1: 1 M sucrose in BS) that was pre-equilibrated at 38.5 °C. Then, they were transferred for 3 min to warming solution 2 (WS2: 0.5 M sucrose in BS). Finally, blastocysts were allowed to remain in BS for 5 min and eventually co-cultured in mSOF in presence of charcoal stripped serum (5%) and glucose (1.5 mM) for 18-24 h. At this time, the percentages of re-expanded and hatched blastocysts were determined. Then blastocysts were used for evaluation of total cell number and DNA fragmentation.
DNA-fragmentation
In Situ Cell Death Detection Kit (Promega Diagnostic Corporation, Mannheim, Germany), known as TUNEL (TdT-mediated dUTP-digoxigenin nick end labeling), was used for detection of apoptotic cells in blastocysts [10]. Hatched blastocysts were thoroughly washed in PBS + 1 mg/ml polyvinyl alcohol (PVA). Then, they were fixed in humid condition for 1 h at room temperature using 4.0% paraformaldehyde (w/v) in PBS. Following fixations, blastocysts were washed again in PBS/PVA and permeabilized for 30 min at room temperature, in 0.5% (v/v) Triton X-100 and sodium citrate. Next, permeable blastocysts were incubated for 10 min at room temperature in EQ buffer. Subsequently the blastocysts were incubated at 37 °C for 1 h in the dark under humid condition in TUNEL reaction mixture (equilibration buffer, nucleotide mix, and rTdT enzyme). Then, the blastocysts were allowed to remain in Buffer 2X for 15 min at room temperature. Eventually, the blastocysts were counterstained to label all nuclei with propidium iodide (PI) for 15 min, washed extensively in PBS, mounted on microscopic slides and observed under a fluorescence microscope (Olympus, Tokyo, Japan). Total numbers of nuclei were counted by PI. Cells were considered as TUNEL positive if their nuclei showed light green fluorescence against the background of PI (Fig. 2c).
Fig. 2.
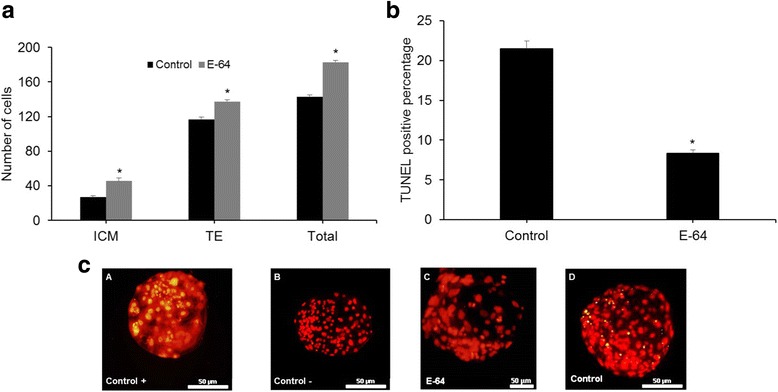
Effect of E-64 (1 μM) during embryo culture medium on a blastocyst total cell number and b percentage of DNA fragmentation assessed by TUNEL assay. Mean values with asterisk denote significant difference at P < 0.05 compared to control. c TUNEL-positive cells that appeared in yellow-green. Scale bar represents 50 μm
Differential staining
Differential staining was carried out according to Moulavi et al. [29]. Briefly, day 8 hatched blastocysts were washed in HTCM199 + 5 mg/ml BSA and permeabilized in 0.5% Triton X-100 in HTCM with 5 mg/mL BSA for 30 s. Then, blastocysts were transferred to 30 μg/ml of propidium iodide in basic medium and incubated for 10–20 s. Eventually, blastocysts were incubated for 15 min to 10 μg/ml Hoechst (H33342) at 4 °C, mounted in a drop of glycerol and observed under fluorescence microscope (Olympus, Tokyo, Japan). Inner cell mass (ICM) and trophectoderm (TE) numbers were distinguished based on their blue and red colors, respectively.
Real time-PCR
Total RNA of blastocyst with the aid of the Micro-RNeasy kit (Qiagen, Canada) was extracted. For reverse transcription, 10 μl of total RNA was used in a final volume of a 20 μl reaction that contained 1 μl of random hexamer, 4 μl RT buffer (10×), 2 μl of dNTP, 1 μl of RNase inhibitor (20 IU), and 1 μl of reverse transcriptase (Fermentas, Canada). Reverse transcription was carried out at 25 °C for 10 min, 42 °C for 1 h and 70 °C for 10 min. Moreover, real Time-PCR was implemented using 1 μl of cDNA (50 ng), 5 μl of the SYBR Green qPCR Master Mix (2X) (Fermentas, Germany) and 1 μl of forward and reverse primers (5 pM) adjusted to a total volume of 10 μl using nuclease-free water. Real time PCR program was 1) 95 °C 4 min, 2) 94 °C 10 s, Ta 30 s, 72 °C 30 s, 40 cycles. To diminish the technical errors, Real Time-PCR was repeated three times. The transcripts abundance of BCL2 and BAX were normalized to beta actin as reference gene (Table 1).
Table 1.
Primer sequences
| Gene symbol | Forward primer (5′-3′) | Reverse primer (5′-3′) | Annealing temp. (°C) |
|---|---|---|---|
| BCL2 | CCTTCTTTGAGTTCGGAG | CCTTCAGAGACAGCCAG | 61 |
| BAX | AGCGAGTGTCTGAAGCG | CCCAGTTGAAGTTGCCGT | 61 |
| β-actin | CCATCGGCAATGAGCGGT | CGTGTTGGCGTAGAGGTC | 59 |
Statistical analysis
Data percentages were modeled to the binomial model of parameters by ArcSin transformation. Blastocysts rates, Real-time reverse transcription PCR data were examined using a one-way ANOVA followed by Tukey’s post hoc tests. For TUNEL staining, and differential staining, t-test was used. The differences were considered significant at P < 0.05. All data were presented as means ± S.E.M. and differences were considered as significant at P < 0.05.
Results
Effect of E-64 treatment on in vitro embryo production
As shown in Fig. 1, in the first experiment to investigate appropriate concentration of E-64, different concentrations (0, 0.1, 1.0, and 10 μM) of E-64 were added to IVC medium on day 3 in which embryos were cultured for 6 days. Addition of 1.0 μM E-64 from day 3 to 8 significantly increased compaction rates compared to control (69.5 ± 2.9% vs. 37.25 ± 2.13%, respectively; P < 0.05). The percentage of embryos that developed to the blastocyst stage in 1.0 μM (24.75 ± 3.1%) was also significantly higher than that of embryos cultured with 0.1, 10 μM and control group (12.75 ± 2.4, 5.25 ± 0.9, 13.75 ± 1.1, respectively; P < 0.05).
Fig. 1.
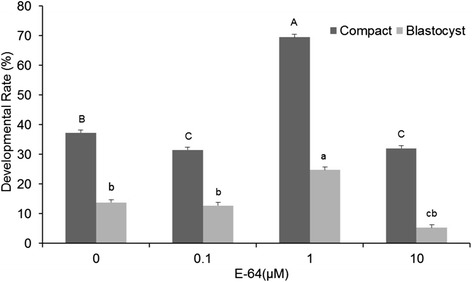
Effect of various concentrations of E-64 on developmental competence of ovine IVF embryos. Values in columns with different letters are significantly different (P < 0.05). Uppercases and lowercases denote significance for compact morula and blastocyst rate, respectively
Hatching rates were 7.5 ± 1.29, 4.75 ± 2.06, 11.5 ± 2.88%, 1.25 ± 0.75 in 0.0, 0.1, 1.0 and 10.0 μM E-64, respectively. Hatching rate was significantly higher in 1.0 μM compared to other groups (P < 0.05).
Moreover, for analysis of quality of hatched blastocysts, differential staining of the blastocysts showed that E-64 supplementation significantly affected total cell number of blastocyst (183 ± 1.6 vs. 143.16 ± 1.6; P < 0.05; Fig. 2a). Also inner cell mass (ICM) and trophectoderm (TE) cell number in presence of 1.0 μM E-64 were significantly higher than the control group (ICM: 26.75 ± 1.84 vs. 45.4 ± 3.93; TE: 116.42 ± 3.11 vs. 137.52 ± 1.67; P < 0.05).
Furthermore, the rate of TUNEL-positive cells of blastocyst derived from the E-64-treated group were significantly lower than the control group (8.34 ± 0.4% vs. 21.4 ± 0.9%, P < 0.05, Fig. 2b).
Effect of E-64 on post-warming survival of vitrified blastocysts
In vitro re-expansion and hatching rates of blastocyst after vitrification/warming are shown in Fig. 3. In this regard, the proportion of survived and also hatched vitrified/warmed blastocysts in the experimental groups (IVC+/PW+, IVC−/PW+, IVC+/PW−, IVC−/PW−) were (33.9 ± 3.8% and 4.9 ± 3.2%), (54.2% ± 3.4 and 15.8 ± 6.1%), (89.7% ± 2.1% and 58.1 ± 1.7%), and (72.1 ± 1.4% and 35.8 ± 2.6%) which were significantly (P < 0.05) different between groups (Fig. 3).
Fig. 3.
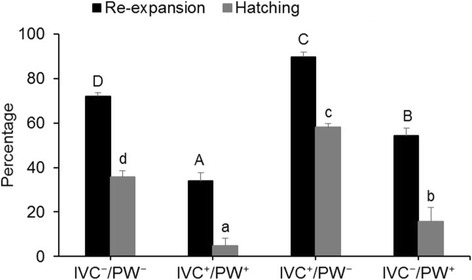
Effect of E-64 supplementation during in vitro culture and/or post warming on re-expansion and hatching rates of blastocyst. Columns with different letters are considered as significant (P < 0.05). Uppercases and lowercases denote significance for re-expansion and hatching rate, respectively. IVC+ means embryos cultured in the presence of 1 μM E-64, while IVC− means embryo cultured in absence of E-64. PW+ and PW− refers to presence or absence of 1 μM E-64 after warming, respectively
Analysis of TCN of cryopreserved blastocysts indicated significant differences between the two groups [IVC+/PW− (147 ± 2) compared to IVC−/PW− (118 ± 1), Fig. 4a]. Also ICM and TE cell number in the IVC+/PW− group was significantly higher than the control group (ICM: 15.45 ± 1.0 vs. 29.76 ± 1.08; TE: 102.41 ± 2.65 vs. 117.28 ± 3.56; P < 0.05).
Fig. 4.
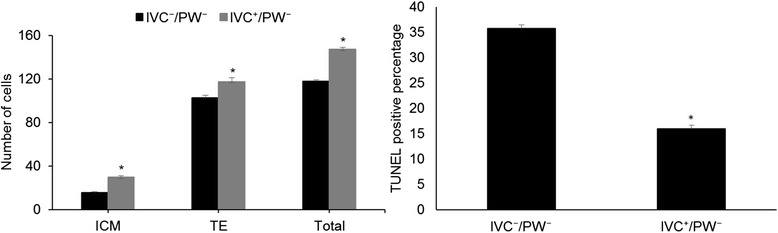
Comparison of a total cell number and b percentage of nuclei with DNA fragmentation in blastocysts derived from IVC+/PW− and IVC−/PW− groups. Mean values with asterisk denote significant difference at P < 0.05
Furthermore, TUNEL assessment of the cryopreserved blastocysts clearly revealed a significant difference in the percentage of TUNEL-positive cells between two groups [IVC+/PW− (15.9 ± 0.6%) compared to IVC−/PW− (35.7 ± 0.6%), Fig. 4b; P < 0.05].
Within E-64 supplemented groups, IVC+/PW− induced the best cryo-protection.
Effect of E-64 on expression of apoptosis-related genes before and after vitrification
Figure 5 shows that the expression of anti-apoptosis-related gene, BCL2 was significantly higher in blastocysts from E-64 treatment than the control (P < 0.05) while the expression of pro-apoptotic gene, BAX was significantly less in blastocysts from E-64 treatment groups than in the control (P < 0.05). Interestingly, after vitrification/warming, expression of BCL2 significantly increased in IVC+/PWˉ compared to IVC−/PW− (P < 0.05). Conversely, expression of BAX in IVC+/PW− was significantly lower than IVC−/PW− (P < 0.05, Fig. 6).
Fig. 5.
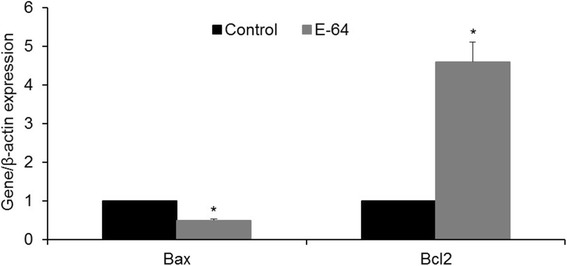
Effect of E-64 (1 μM) during in vitro culture on relative expression of BAX and BCL2, in ovine IVF blastocysts. Asterisks indicate statistically significant differences from control (P < 0.05)
Fig. 6.
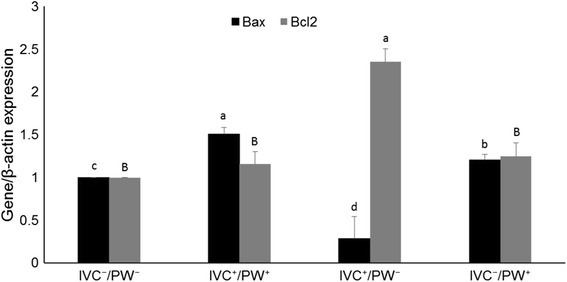
Effect of E-64 on in vitro culture and/or post warming on relative expression of BAX and BCL2, in ovine IVF blastocysts. Values in columns with different letters are significantly different (P < 0.05)
Discussion
Lysosomes are specialized intracellular organelles and play indispensable role in many physiological functions including: endocytosis, phagocytosis, and autophagy [30–32]. In addition, in presence of exogenous stresses such as altered pH and heat shock or suboptimal culture conditions [17], lysosomal cysteine proteases, like cathepsin B, are released from lysosomes. Cathepsins can induce apoptosis directly through initiator caspases or indirectly via release of cytochromes from mitochondria and eventually leads to completion of apoptosis via activation of effector caspases [33].
Increased expression of cathepsin B is inversely correlated with quality of cumulus-oocyte complexes (COCs) in cattle [7]. Furthermore, expression of cathepsin B is higher in poor quality bovine oocytes as compared to good-quality ones [22]. In this regard, Balboula et al. showed that addition of 1 μM E-64 during in vitro maturation (IVM), improves quality of COCs and their developmental competency. These authors also showed that heat shock stress increases the expression of cathepsin B and its inhibition by E-64 reduces injuries due to heat stress [34].
It has also been reported that E-64 treatment after IVF followed by IVC for 6 days significantly improved developmental competences and increased number of good quality bovine embryos [23]. On the other hand, Min et al. (2014) reported that treatment during IVC with E-64 (0.1 and 0.5 μM) significantly improves developmental rates without any noticeable effect on cleavage rate [24]. Unlike the former study which assessed role of cathepsin B inhibition during IVM, other research groups showed that addition of E-64 during in vitro culture (IVC) also improves rate and quality of derived blastocysts [7, 22, 24, 34]. Both these studies concluded that addition of E-64 to IVC medium has no effect on early cleavage rates. Based on these result, we assessed the effect of E-64 post maternal embryonic transition on quantity and quality of ovine derived blastocysts. In agreement with previous studies, this study also revealed that among assessed concentrations, 1.0 μM E-64 significantly improves rates of ovine embryo compaction and blastocyst formation. In addition, we showed that rate of apoptosis, assessed by TUNEL, was significantly reduced by treatment with E-64. Since cathepsin B mediates its indirect effect through mitochondria intrinsic pathway, we also assessed the relative expression of pro-apoptotic (BAX) and anti-apoptosis (BCL2) related genes which play an important role in regulating cell death by controlling release of cytochrome-c into cytosol [35]. As depicted in Fig. 5, addition of E-64 increased expression of BCL2 and reduced expression of BAX, indicating that E-64 can limit apoptosis induced by sub-optimal culture conditions.
The second point highlighted in this study was the link between developmental competence and vitrification in ovine embryos. During vitrification, embryo exposure to a highly-concentrated solution of cryo-protectants leads to stress or injuries to membranes, cellular organelles and release of cathepsin B from lysosomes [36–41]. Moreover, the sensitivity of embryos to cryopreservation is closely related to culture conditions [8, 9]. Therefore, in this study, we evaluated the effect of addition of E-64 during day3 to day8 on cryosurvival of derived blastocysts.
In results depicted in Fig. 3, addition of E-64 to culture medium during embryonic development enhances the overall re-expansion and cryo-viability of the blastocysts. However, the difference for rate of blastocyst re-expansion became significant when E-64 was added to IVC before vitrification during day 3 to 7 (90% ± 2% IVC+/PW−) compared to control (IVC−/PW−) or when E-64 was added before and after vitrification (IVC+/PW+). These data are consistent with the interpretation of positive effect of E-64 addition to IVC. It is very likely that addition of E-64 leads to production of more competent embryos with better cryosurvival potential, which was further confirmed by assessment of percentage of apoptotic cells, total cell number and expression of pro- and anti-apoptotic genes. In contrast, the data indicate that addition of E-64 post warming has a negative effect on the rate of re-expansion. The rate of re-expansion is significantly lower when E-64 was used after warming (IVC−/PW+ or IVC+/PW+) compared to its absence before and after vitrification (IVC−/PW−). This observation raises the questions; could cathepsin B have a role in blastocyst re-expansion or is this effect due to toxic effect of high concentration of E-64? Indeed, it is know that permeability of embryos is highly altered through cryopreservation. Therefore, could the optimal concentration be toxic post vitrification, as higher concentration of E-64 (10 μM) reduced the developmental competency. Therefore, further experiment and optimization is needed to define the concentration of E-64 required after vitrification.
The overall improved effect observed by E-64 treatment can be explained by direct and indirect mechanism of action of cathepsin B. It is likely, exposure to cryo-protectant or reactive oxygen species (ROS) produced during cryopreservation, may directly activate Type II class, initiator caspases. Alternatively, cryopreservation may lead to release of cathepsin B from lysosomes and induce mitochondrial membrane degradation, a condition known as permeability transition. This effect leads to the release of pro-apoptotic factors into the cytosol. In this regard, Balboula et al. has shown that heat stress in oocytes leads to a defect in lysosomal membrane permeability which results in lysosomal aggregation and release of cathepsin B into the cytosol [34]. Kim et al. evaluated localization of cathepsin B and cytochrome C in presence of E-64 and showed co-localization of these factors in porcine embryos [25]. In both bovine and porcine embryos, these observations were reversed by treatment with E-64. E-64 decreases both the activity of caspase 3 and its mRNA while decreasing only the activity of cathepsin B. It is also important to note that cathepsin B, on its own, can also induce nuclear apoptosis independent of caspase 3 [42, 43] and ablation of cathepsin B makes cells more resistant to inflammation induced apoptosis [44].
Similarly, rates of hatching were significantly reduced by adding E-64 post warming, while its addition during IVC significantly improved the hatching rate compared to control (IVC−/PW−). It is noteworthy that blastocysts hatch by their intrinsic ability to produce zonalytic factor(s) that have cysteine protease-like activity. In this regard, several proteases are expressed before hatching [45, 46] and they play important role in this process [47–53]. Therefore, it is likely that E-64 inhibits these proteases involved in hatching and this may explain the reduced rate of hatching by presence of E-64 post warming. Indeed, the rate of hatching was also reduced in all concentration of E-64 during IVC compared to control, except at 1 μM concentration. The improved higher hatching rate at 1 μM E-64 is very likely related to intrinsic effect of E-64 to improve the quality of derived blastocysts. The ability of E-64 to reduce hatching rate also indicate that cathepsins are likely to be involved in ovine blastocyst hatching.
Conclusion
In conclusion, results of this study indicated that supplementation of IVC media with 1 μM E-64, an exogenous inhibitors of Cathepsins, improves quality and quantity of blastocyst formation. Furthermore, addition of E-64 during IVC also improve rate of re-expansion and hatching post vitrification. However, addition of 1 μM E-64 to media post vitrification/warming has a negative effect on embryo re-expansion and hatching rates. This effect may be related to toxic concentration of E-64 which is likely to be related to altered membrane permeability post vitrification. Moreover, negative effect of E-64 after warming can be related to incomplete blastocyst hatching because of interference in secretion of zonalytic proteases which requires further investigation.
Acknowledgements
The authors would like to express their gratitude to Royan Institute for their full support.
Funding
This study was funded by a grant from the Royan Institute of IRI (grant no. 94000068).
Availability of data and materials
Data supporting the manuscript’s findings can be found in the manuscript.
Abbreviations
- 6-DMAP
6-dimethyl aminopurine
- BS
Basic medium
- CPA
Cryoprotectant
- DMSO
Dimethyl sulfoxide
- EG
Ethylene glycol
- ES
Equilibration solution
- IVF
In vitro fertilization
- LN2
Liquid nitrogen
- MM
Maturation medium
- PBS
Phosphate-buffered saline
- ROCK
Rho-associated protein kinase
- TCM-199
Tissue culture medium-199
- VS
Vitrification solution
Authors’ contributions
SMH and MHNE perceived and designed the study; MP, SO, ShRV and FS performed the experiments; and MP and MHNE wrote the manuscript. ShRV prepared the draft version of the article for submission. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors read the manuscript thoroughly and consent to submit the manuscript to the journal of BMC Developmental Biology.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
M. Pezhman, Email: melissa.pezhman@gmail.com
S. M. Hosseini, Email: morteza.hosseini.royan@gmail.com
S. Ostadhosseini, Email: somayyehhosseini@gmail.com
Sh. Rouhollahi Varnosfaderani, Email: roholahishiva@yahoo.com
F. Sefid, Email: fatemeh.sefid@yahoo.com
M. H. Nasr-Esfahani, Phone: +983195015680, Email: mh.nasr-esfahani@royaninstitute.org
References
- 1.Watson AJ, Kidder GM. Immunofluorescence assessment of the timing of appearance and cellular distribution of Na/K-ATPase during mouse embryogenesis. Dev Biol. 1988;126:80–90. doi: 10.1016/0012-1606(88)90241-2. [DOI] [PubMed] [Google Scholar]
- 2.Dobrinsky J. Cryopreservation of pig embryos. Journal of reprod fertil. 1996;52:301–312. [PubMed] [Google Scholar]
- 3.Berthelot F, Martinat-Botte F, Locatelli A, Perreau C, Terqui M. Piglets born after vitrification of embryos using the open pulled straw method. Cryobiology. 2000;41:116–124. doi: 10.1006/cryo.2000.2273. [DOI] [PubMed] [Google Scholar]
- 4.Cocero MJ, Sebastian AL, Barragan ML, Picazo RA. Differences on post-thawing survival between ovine morulae and blastocysts cryopreserved with ethylene glycol or glycerol. Cryobiology. 1996;33:502–507. doi: 10.1006/cryo.1996.0053. [DOI] [PubMed] [Google Scholar]
- 5.Leibo S, Martino A, Kobayashi S, Pollard J. Stage-dependent sensitivity of oocytes and embryos to low temperatures. Anim Reprod Sci. 1996;42:45–53. doi: 10.1016/0378-4320(96)01543-6. [DOI] [Google Scholar]
- 6.Bissoyi A, Nayak B, Pramanik K, Sarangi SK. Targeting cryopreservation-induced cell death: a review. Biopreserv and biobank. 2014;12:23–34. doi: 10.1089/bio.2013.0032. [DOI] [PubMed] [Google Scholar]
- 7.Bettegowda A, Patel OV, Lee KB, Park KE, Salem M, Yao J, Ireland JJ, Smith GW. Identification of novel bovine cumulus cell molecular markers predictive of oocyte competence: functional and diagnostic implications. Biol Reprod. 2008;79:301–309. doi: 10.1095/biolreprod.107.067223. [DOI] [PubMed] [Google Scholar]
- 8.Gomez E, Rodriguez A, Munoz M, Caamano JN, Hidalgo CO, Moran E, Facal N, Diez C. Serum free embryo culture medium improves in vitro survival of bovine embryos to vitrification. Theriogenology. 2008;69:1013–1021. doi: 10.1016/j.theriogenology.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 9.Pereira DC, Dode MA, Rumpf R. Evaluation of different culture systems on the in vitro production of bovine embryos. Theriogenology. 2005;63:1131–1141. doi: 10.1016/j.theriogenology.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 10.Kitagawa Y, Suzuki K, Yoneda A, Watanabe T. Effects of oxygen concentration and antioxidants on the in vitro developmental ability, production of reactive oxygen species (ROS), and DNA fragmentation in porcine embryos. Theriogenology. 2004;62:1186–1197. doi: 10.1016/j.theriogenology.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 11.Mateusen B, Van Soom A, Maes DG, Donnay I, Duchateau L, Lequarre A-S. Porcine embryo development and fragmentation and their relation to apoptotic markers: a cinematographic and confocal laser scanning microscopic study. Reproduction. 2005;129:443–452. doi: 10.1530/rep.1.00533. [DOI] [PubMed] [Google Scholar]
- 12.Huang S, Ding C, Mai Q, Xu Y, Zhou C. Inhibition of rho-associated protein kinase increases the ratio of formation of blastocysts from single human blastomeres. Mol Med Rep. 2016;13:2046–2052. doi: 10.3892/mmr.2016.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen K, Li X, Dai X, Wang P, Li S, Xiong Z, Chen P, Liu Q, Shi D. Effects of MG132 on the in vitro development and epigenetic modification of Debao porcine somatic cell nuclear transfer embryos. Theriogenology. 2017;94:48–58. doi: 10.1016/j.theriogenology.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Castillo-Martín M, Bonet S, Morató R, Yeste M. Comparative effects of adding β-mercaptoethanol or L-ascorbic acid to culture or vitrification–warming media on IVF porcine embryos. Reprod Fertil Dev. 2014;26:875–882. doi: 10.1071/RD13116. [DOI] [PubMed] [Google Scholar]
- 15.Barrett AJ, Kirschke H. Cathepsin B, cathepsin H, and cathepsin L. Methods Enzymol. 1981;80:535–561. doi: 10.1016/S0076-6879(81)80043-2. [DOI] [PubMed] [Google Scholar]
- 16.Vancompernolle K, Van Herreweghe F, Pynaert G, Van de Craen M, De Vos K, Totty N, Sterling A, Fiers W, Vandenabeele P, Grooten J. Atractyloside-induced release of cathepsin B, a protease with caspase-processing activity. FEBS Lett. 1998;438:150–158. doi: 10.1016/S0014-5793(98)01275-7. [DOI] [PubMed] [Google Scholar]
- 17.Calkins CC, Sloane BF. Mammalian cysteine protease inhibitors: biochemical properties and possible roles in tumor progression. Biol Chem Hoppe Seyler. 1995;376:71–80. [PubMed] [Google Scholar]
- 18.Tsukamoto S, Taichi HA, Yamamoto A, Ayako WA, ISHIDA Y, NISHIKAWA T, MINAMI N, KOKUBO T. Functional analysis of lysosomes during mouse preimplantation embryo development. J Reprod Dev. 2013;59:33–39. doi: 10.1262/jrd.2012-096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aboelenain M, Balboula AZ, Kawahara M, Montaser AE, Zaabel SM, Kim SW, Nagano M, Takahashi M. Pyridoxine supplementation during oocyte maturation improves the development and quality of bovine preimplantation embryos. Theriogenology. 2017;15:127–133. doi: 10.1016/j.theriogenology.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 20.Son HH, Min SH, Yeon JY, Kim JW, Park SY, Lee YH, Jeong PS, Koo DB. Cathepsin B inhibitor, E-64, affects preimplantation development, apoptosis and oxidative stress in pig embryos. Reprod dev biol. 2013;37:175–183. doi: 10.12749/RDB.2013.37.4.175. [DOI] [Google Scholar]
- 21.Seshagiri PB, Lalitha HS, Mishra A, Sireesha GV. Embryo-endometrial proteases during early mammalian development. Indian J Exp Biol. 2003;41:756–763. [PubMed] [Google Scholar]
- 22.Balboula AZ, Yamanaka K, Sakatani M, Hegab A, Zaabel S, Takahashi M. Cathepsin B activity is related to the quality of bovine cumulus oocyte complexes and its inhibition can improve their developmental competence. Mol Reprod Dev. 2010;77:439–448. doi: 10.1002/mrd.21164. [DOI] [PubMed] [Google Scholar]
- 23.Balboula AZ, Yamanaka K, Sakatani M, Hegab A, Zaabel S, Takahashi M. Intracellular cathepsin B activity is inversely correlated with the quality and developmental competence of bovine preimplantation embryos. Mol Reprod Dev. 2010;77:1031–1039. doi: 10.1002/mrd.21250. [DOI] [PubMed] [Google Scholar]
- 24.Min SH, Song BS, Yeon JY, Kim JW, Bae JH, Park SY, Lee YH, Chang KT, Koo DB. A cathepsin B inhibitor, E-64, improves the preimplantation development of bovine somatic cell nuclear transfer embryos. J reprod dev. 2014;60:21–27. doi: 10.1262/jrd.2013-074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim SH, Zhao MH, Liang S, Cui XS, Kim NH. Inhibition of cathepsin B activity reduces apoptosis by preventing cytochrome c release from mitochondria in porcine parthenotes. J reprod dev. 2015;61:261–268. doi: 10.1262/jrd.2015-019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moulavi F, Hosseini SM, Hajian M, Forouzanfar M, Abedi P, Ostadhosseini S, Asgari V, Nasr-Esfahani MH. Nuclear transfer technique affects mRNA abundance, developmental competence and cell fate of the reconstituted sheep oocytes. Reproduction. 2013;145:345–355. doi: 10.1530/REP-12-0318. [DOI] [PubMed] [Google Scholar]
- 27.Asgari V, Hosseini SM, Forouzanfar M, Hajian M, Nasr-Esfahani MH. Vitrification of in vitro produced bovine embryos: effect of embryonic block and developmental kinetics. Cryobiology. 2012;65:278–283. doi: 10.1016/j.cryobiol.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Martinez AG, Valcarcel A, MA d l H, de Matos DG, Furnus C, Brogliatti G. Vitrification of in vitro produced bovine embryos: in vitro and in vivo evaluations. Anim Reprod Sci. 2002;73:11–21. doi: 10.1016/S0378-4320(02)00121-5. [DOI] [PubMed] [Google Scholar]
- 29.Moulavi F, Hosseini SM, Ashtiani SK, Shahverdi A, Nasr-Esfahani MH. Can Vero cell co-culture improve in-vitro maturation of bovine oocytes? Reprod BioMed Online. 2006;13:404–411. doi: 10.1016/S1472-6483(10)61446-0. [DOI] [PubMed] [Google Scholar]
- 30.Eskelinen EL, Tanaka Y, Saftig P. At the acidic edge: emerging functions for lysosomal membrane proteins. Trends Cell Biol. 2003;13:137–145. doi: 10.1016/S0962-8924(03)00005-9. [DOI] [PubMed] [Google Scholar]
- 31.Reiser J, Adair B, Reinheckel T. Specialized roles for cysteine cathepsins in health and disease. J Clin Invest. 2010;120:3421–3431. doi: 10.1172/JCI42918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saftig P, Klumperman J. Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function. Nat rev Mol Cell biol. 2009;10:623–635. doi: 10.1038/nrm2745. [DOI] [PubMed] [Google Scholar]
- 33.Nishikawa H, Ozaki Y, Nakanishi T, Blomgren K, Tada T, Arakawa A, Suzumori K. The role of cathepsin B and cystatin C in the mechanisms of invasion by ovarian cancer. Gynecol Oncol. 2004;92:881–886. doi: 10.1016/j.ygyno.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 34.Balboula AZ, K-i Y, Sakatani M, Kawahara M, Hegab A, Zaabel S, Takahashi M. Cathepsin B activity has a crucial role in the developmental competence of bovine cumulus–oocyte complexes exposed to heat shock during in vitro maturation. Reproduction. 2013;146:407–417. doi: 10.1530/REP-13-0179. [DOI] [PubMed] [Google Scholar]
- 35.Strasser A, O'Connor L, Dixit VM. Apoptosis signaling. Annu Rev Biochem. 2000;69:217–245. doi: 10.1146/annurev.biochem.69.1.217. [DOI] [PubMed] [Google Scholar]
- 36.Heng BC, Ye CP, Liu H, Toh WS, Rufaihah AJ, Yang Z, Bay BH, Ge Z, Ouyang HW, Lee EH, Cao T. Loss of viability during freeze-thaw of intact and adherent human embryonic stem cells with conventional slow-cooling protocols is predominantly due to apoptosis rather than cellular necrosis. J Biomed Sci. 2006;1:433–445. doi: 10.1007/s11373-005-9051-9. [DOI] [PubMed] [Google Scholar]
- 37.Naitana S, Ledda S, Loi P, Leoni G, Bogliolo L, Dattena M, Cappai P. Polyvinyl alcohol as a defined substitute for serum in vitrification and warming solutions to cryopreserve ovine embryos at different stages of development. Anim Reprod Sci. 1997;48:247–256. doi: 10.1016/S0378-4320(97)00043-2. [DOI] [PubMed] [Google Scholar]
- 38.Overstrom E, Duby R, Dobrinsky J, Robl J, Baguisi A, Lonergan P, Duffy P, Walsh J, Roche J, Boland M. Cytoskeletal damage in vitrified or frozen bovine embryos. Theriogenology. 1993;39:276. doi: 10.1016/0093-691X(93)90131-N. [DOI] [Google Scholar]
- 39.Zhang T, Rawson DM, Tosti L, Carnevali O. Cathepsin activities and membrane integrity of zebrafish (Danio rerio) oocytes after freezing to− 196° C using controlled slow cooling. Cryobiology. 2008;56:138–143. doi: 10.1016/j.cryobiol.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 40.Persidsky MD, Ellett MH. Lysosomes and cell cryoinjury. Cryobiology. 1971;8:345–349. doi: 10.1016/0011-2240(71)90129-5. [DOI] [PubMed] [Google Scholar]
- 41.Liang S, Yuan B, Kwon JW, Ahn M, Cui XS, Bang JK, Kim NH. Effect of antifreeze glycoprotein 8 supplementation during vitrification on the developmental competence of bovine oocytes. Theriogenology. 2016;86:485–494. doi: 10.1016/j.theriogenology.2016.01.032. [DOI] [PubMed] [Google Scholar]
- 42.Antunes F, Cadenas E, Brunk UT. Apoptosis induced by exposure to a low steady-state concentration of H2O2 is a consequence of lysosomal rupture. Biochem J. 2001;356:549–555. doi: 10.1042/bj3560549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ishisaka R, Utsumi T, Yabuki M, Kanno T, Furuno T, Inoue M, Utsumi K. Activation of caspase-3-like protease by digitonin-treated lysosomes. FEBS Lett. 1998;435:233–236. doi: 10.1016/S0014-5793(98)01080-1. [DOI] [PubMed] [Google Scholar]
- 44.Foghsgaard L, Wissing D, Mauch D, Lademann U, Bastholm L, Boes M, Elling F, Leist M, Jaattela M. Cathepsin B acts as a dominant execution protease in tumor cell apoptosis induced by tumor necrosis factor. J Cell Biol. 2001;153:999–1010. doi: 10.1083/jcb.153.5.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koblinski JE, Ahram M, Sloane BF. Unraveling the role of proteases in cancer. Clin Chem acta. 2000;291:113–135. doi: 10.1016/S0009-8981(99)00224-7. [DOI] [PubMed] [Google Scholar]
- 46.Sireesha GV, Mason RW, Hassanein M, Tonack S, Navarrete Santos A, Fischer B, Seshagiri PB. Role of cathepsins in blastocyst hatching in the golden hamster. Mol Human reprod. 2008;14:337–346. doi: 10.1093/molehr/gan026. [DOI] [PubMed] [Google Scholar]
- 47.Massip A, Mulnard J. Time-lapse cinematographic analysis of hatching of normal and frozen-thawed cow blastocysts. J Reprod Fertil. 1980;58:475–478. doi: 10.1530/jrf.0.0580475. [DOI] [PubMed] [Google Scholar]
- 48.Mishra A, Seshagiri PB. Evidence for the involvement of a species-specific embryonic protease in zona escape of hamster blastocysts. Mol Hum Reprod. 2000;6:1005–1012. doi: 10.1093/molehr/6.11.1005. [DOI] [PubMed] [Google Scholar]
- 49.O'Sullivan CM, Rancourt SL, Liu SY, Rancourt DE. A novel murine tryptase involved in blastocyst hatching and outgrowth. Reproduction. 2001;122:61–71. doi: 10.1530/rep.0.1220061. [DOI] [PubMed] [Google Scholar]
- 50.Salamonsen LA, Nie G. Proteases at the endometrial–trophoblast interface: their role in implantation. Rev Endocr Metab Disord. 2002;3:133–143. doi: 10.1023/A:1015407012559. [DOI] [PubMed] [Google Scholar]
- 51.Sathananthan H, Menezes J, Gunasheela S. Mechanics of human blastocyst hatching in vitro. Reprod BioMed Online. 2003;7:228–234. doi: 10.1016/S1472-6483(10)61757-9. [DOI] [PubMed] [Google Scholar]
- 52.Tang L, Rancourt DE. Murine implantation serine proteinases 1 and 2: structure, function and evolution. Gene. 2005;364:30–36. doi: 10.1016/j.gene.2005.07.041. [DOI] [PubMed] [Google Scholar]
- 53.Vu TK, Liu RW, Haaksma CJ, Tomasek JJ, Howard EW. Identification and cloning of the membrane-associated serine protease, hepsin, from mouse preimplantation embryos. J Biol Chem. 1997;272:31315–31320. doi: 10.1074/jbc.272.50.31315. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting the manuscript’s findings can be found in the manuscript.


