Abstract
Background
Alzheimer’s disease is a progressive neurodegenerative disease. Although some of the current treatments offer some symptomatic relief, this disease cannot be cured at present. Electroacupuncture may be effective for Alzheimer’s disease for cognitive function, but the evidence for its effectiveness is still limited. The aim of this study is to evaluate the add-on effect of electroacupuncture to donepezil for improving the cognitive function of Alzheimer’s disease.
Methods/design
A total of 334 participants with Alzheimer’s disease will be randomly assigned to either an electroacupuncture combined with donepezil group or a donepezil group with a ratio of 1:1. Participants in the electroacupuncture combined with donepezil group will receive electroacupuncture in addition to donepezil for 12 weeks and will keep taking donepezil for the following 24 weeks. Participants in the control group will take donepezil only. The primary outcome is the change from baseline in the total score of the Alzheimer’s Disease Assessment Scale-cognition at week 12. A follow-up will be conducted 24 weeks after the treatment.
Discussion
We expect to verify the hypothesis that acupuncture in addition to donepezil is better than donepezil in improving the cognitive function of patients with Alzheimer’s disease. This trial has a limitation that participant blinding is impossible.
Trial registration
Clinical Trials.gov: ID: NCT02305836. Registered on 13 November 2014.
Electronic supplementary material
The online version of this article (doi:10.1186/s13063-017-2052-y) contains supplementary material, which is available to authorized users.
Keywords: Electroacupuncture, Donepezil, Alzheimer’s disease, RCT, Study protocol
Background
Alzheimer’s disease (AD) is a neurodegenerative disease characterized by an insidious onset and a progressive impairment of cognitive function [1]. The ongoing decline of cognitive function leads to a high risk of disability which is a significant burden for patients and caregivers around the world. The incidence of AD increases in people over 65 years of age, with a prevalence of 4.8% in this age group and representing approximately 60–80% of dementias [1, 2]. Moreover, the health costs associated with AD, the reduced quality of life, and the increased risk of mortality severely affects the social development and quality of family life of AD patients [3–5].
Although some of the current treatments offer some symptomatic relief, this disease cannot be cured at present [6]. Cholinesterase-inhibitors (CIs) donepezil, rivastigmine, and galantamine are recommended for clinical use according to the guideline of The National Institute for Clinical Excellence (NICE, now National Institute for Health and Clinical Excellence) [7]. The glutamate receptor antagonist memantine has also been approved and recommended by the Food and Drug Administration (FDA) to treat moderate to severe AD [8]. CIs have modest symptomatic but not curative effects [9] and these classes of drugs have considerable adverse reactions [10, 11]. Some studies [12–14] have shown that acupuncture may be effective for improving the cognitive function of AD patients; however, because of the lack of rigorous randomization and adequate sample size, there is still not enough evidence to show the effectiveness of acupuncture. This trial aims to determine the effectiveness of electroacupuncture (EA) as an adjuvant therapy for donepezil in improving the cognitive function of patients with AD.
Methods/Design
This trial is a prospective, randomized controlled, clinical trial to be conducted between June 2017 and December 2019. Three hundred and thirty-four participants with AD will be recruited at Guang’anmen Hospital, Beijing, China via the hospital web or posters. Recruiting and screening will be in the charge of a research assistant, whereas the diagnosis will be made by a neurologist. A 2-week baseline assessment will be conducted. Randomization is performed by the pharmacological assessment center at Guang’anmen Hospital. The participants identified as eligible will be randomized into two groups—EA combined with donepezil and donepezil—at a ratio of 1:1. The random block is set to 4, and random grouping is produced by using the Statistics Analysis System (SAS) software. Random numbers and information about grouping are sealed into opaque envelopes with correlative sequence numbers of entering the trial marked on the outside. Acupuncturists will open the envelopes according to the sequence numbers. The flowchart of the trial is shown in Fig. 1. The total study period of this trial is 38 weeks (Fig. 1), including 2-week baseline assessment, 12-week treatment period and 24-week follow-up period.
Fig. 1.
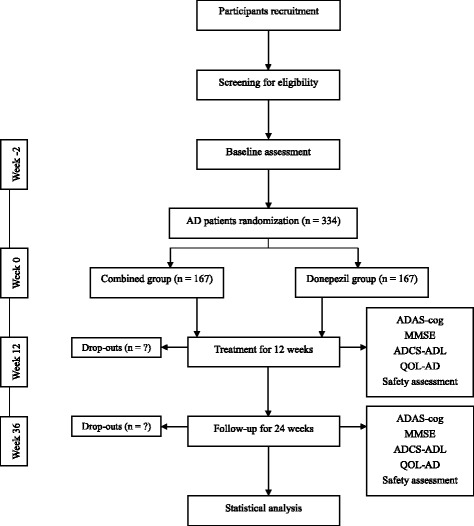
The flowchart of the trial
The trial will be conducted in accordance with the principles of the Declaration of Helsinki, and has been approved by the review boards and ethics committees of the participating hospital (Ethics approval number: 2014EC087).
Participants
Participants meeting all of the following criteria will be included in this trial: (1) the diagnostic criteria of Neurological Communicative Disorders and Stroke and the Alzheimer Disease and Related Disorders Association (NINCDS-ADRDA) [15] or the Operational Criteria for the Diagnosis of Alzheimer’s Disease (OCDAD) [16], (2) aged between 60–85 years, (3) cognitive impairment based on the scores of the Chinese version of the Mini Mental State Examination (MMSE) (an illiteracy group ≤19, a primary school group ≤22, a junior high school and high school group ≤3, and a well-educated group ≤26) [17], (4) absence of depression (via an emotional assessment), (5) magnetic resonance imaging (MRI) confirmation of atrophy of the hippocampus or the medial temporal lobe volume, (6) the Medial Temporal Lobe Atrophy Rating Scale (MTA-scale) score (≥2 for those under 75 years, and ≥3 for those over 75 years).
Participants meeting any one of the following criteria will be excluded from this trial: (1) cognitive impairment caused by other factors (e.g., vascular dementia, dementia with Lewy bodies, frontotemporal dementia, hormone or metabolic abnormalities, hypothyroidism, folic acid or vitamin B12 deficiency, delirium or other mental and emotional disorders (such as schizophrenia and depression)), (2) a serious heart condition, hepatic disease, renal system disease, hematopoietic system disease, or whole-body malnutrition, (3) aphasia, disturbance of consciousness, or failure to cooperate with the related examinations due to physical disability, (4) anticoagulant treatments such as warfarin or heparin, (5) use of pacemakers, or (6) receiving acupuncture or CI treatment in the past 2 weeks.
Intervention
EA combined with donepezil group
The acupoints are GV 16, GV 20, GV 24, and Shang Yintang (up to GV 29 1 cun), as well as bilateral EX-HN 5 and KI 4. The location of the acupoints is based on the The Name and Location of Acupoints drafted in 2006 by the National Standard of the People’s Republic of China (GB/T 12346-2006). The acupuncture protocol is based on reviews of acupuncture for AD [18, 19] published and expert consensus. Acupuncturists who have at least 2 years of practical experience will perform the intervention. Disposable, sterilized needles made of stainless steel (Hwato, Suzhou Medical Appliance Factory, Suzhou, China) and electric stimulators (SDZ-V electroacupuncture apparatus, Suzhou Medical Appliance Factory, Suzhou, China) will be used in the treatment group. After routine disinfection, the GV 16 will be needled while participants are in the sitting position with their head slightly forward and their muscles relaxed. The needle (25 mm in length) will be inserted obliquely and slowly downward toward the mandibular direction to a depth of 12.5 mm. The needle will be immediately removed after “de qi” (de qi, which is very important to therapeutic effect, is a sensation of soreness, heaviness, and numbness when needling [20]) and not retained. Shang Yintang, GV 20, and GV 24 will be needled (40 mm in length) at a 30° angle to a depth of approximately 12.5 mm while participants are in the decubitus position. For bilateral EX-HN 5 and KI 4, the needle (40 mm in length) will be vertically inserted to a depth of 25 mm. The electric stimulator will be applied to GV 20, GV 24, and bilateral EX-HN 5 with a spare-dense wave of 10/50 Hz, 0.5–5.0 mA. The current intensity is set up to the maximum tolerance of patients. There are three sessions per week with each session lasting for 30 min. There will be 12 weeks of treatment with 36 sessions for each participant in total. EA treatment will last for 12 weeks.
Donepezil (Eisai Pharmaceuticals, Shanghai, China) 5 mg will be given once daily before bed-time for the first 4–6 weeks. Based on the treatment effect, the dosage may be increased to 10 mg once daily before bed-time for the next 6–8 weeks. Donepezil will be taken for continuously for 24 weeks.
Donepezil group
Participants will only receive donepezil using the same administration method as with the EA combined with donepezil group and the therapy will last for 24 weeks.
Routine medications and therapies taken by participants with other complex chronic diseases will be recorded in detail in a paper Case Report Form. The intervention will be terminated due to severe adverse events.
Outcome measures
The primary outcome is the change from baseline in the Alzheimer’s Disease Assessment Scale-cognition (ADAS-cog) [21] score measured at week 12. The maximum score of the ADAS-cog is 70 including 15 items of cognitive dysfunction assessment. The higher values indicate higher degree of deficit [22].
The secondary outcomes include the following items: the change from baseline in the ADAS-cog score measured at week 36. The change from baseline in total MMSE score [23], in the Alzheimer’s Disease Cooperative Study-Activity of Daily Living (ADCS-ADL) score, and in the Quality of Life-Alzheimer’s Disease (QOL-AD) score measured at weeks 12 and 36. The MMSE score is commonly used for estimating the severity of cognitive impairment with a maximum score of 30. The questions in this scale included orientation to time, orientation to place, attention and calculation, recall of three words, and visual construction. The ADCS-ADL score contains 19 domains about assessment of basic and operational ability of daily living [24]. The QOL-AD score, which includes physical health, mental health, social and financial assessment, and quality of life domains, is completed by both patients and caregivers [25].
All the adverse events will be recorded and assessed throughout the whole study period. Adverse reactions related to acupuncture may be fainting, hematoma formation, local infection or skin pain. Individuals who develop severe complications will be excluded from continuing in study, and the reasons for their termination will be recorded.
Sample size and statistical analysis
The calculation of sample size is based on the primary outcome which is the change from baseline in the ADAS-cog score. According to previous studies [26], 39% of patients showed an improvement of at least 4 points on the ADAS-cog score after being treated with donepezil. We estimated the rate to be 39% in the donepezil group and 55% in the EA combined with donepezil group. Three hundred and four participants were needed to provide 80% power at a significant level of 5% using analysis of variance. The total sample size required for the study is 334 (167 each group) assuming a 10% loss to follow-up.
Baseline score of the ADAS-cog and age will be used as a covariate when assessing the differences between the EA combined with donepezil group and the donepezil group by an analysis of covariance for the primary and the secondary variables if data fits the normal distribution. Otherwise, a nonparametric test will be used to compare the statistical difference between the two groups. All the data will be blindly analyzed by the statisticians from the Clinical Evaluation Center of Guang’anmen Hospital. SPSS 20.0 (IBM Corp., Armonk, NY, USA) statistical software will be used for the statistical analyses. Subanalysis will be applied to the statistical analysis according to the severity of the condition of participants measured by the ADAS-cog [27]).
Efficiency and safety analysis will be based on all the participants at randomization according to the intention-to-treat (ITT) principle. Missing values assumed missing at random will be handled by the multiple imputation method [28]. Continuous data will be presented by the average and standard error. Two-sided tests will be used for all outcomes. A P value of less than 0.05 is considered to show statistical significance.
For the safety assessments, a description on the adverse events and adverse reactions will be presented in tables. These tables will include the categories, severity, rate of incidence, and correlation with the treatment. Individuals with serious adverse events should stop receiving the intervention and details will be reported in a timely manner.
Quality control
All of the investigators participating in this trial will take a training course which lasts for 1 day. The assessment of all the scales will be in the charge of the research assistants who do not know the distribution of groups. Members of an independent Data Monitoring Committee (DMC) will regularly inspect the data and monitor the trial progression before the data statistics. Data management and statistical analysis will be under the control of the statisticians.
Ethics and dissemination
This study has been approved by the Ethics Committee of Guang’anmen Hospital, CACMS (2015EC043). This protocol was written following the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) Checklist (Additional file 1) and figure (Fig. 2). All important protocol modifications will be submitted to the Ethics Committee of Guang’anmen Hospital. All participants and caregivers will be informed about the potential risks and benefits of the study. Informed consent will be obtained for either all participants or, if their capacity to consent is absent, their nearest relatives or their caregivers. All personal information about participants will be prohibited from other use and stored in specific cabinets in order to protect confidentiality before, during, and after the trial. Statistical and monitoring managers will have access to the final trial dataset. For compensation to those who suffer harm from trial participation, we will provide free corresponding therapies and medical consultation. The results of this trial are planned to be disseminated in conferences or publications.
Fig. 2.

Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) figure. MRI Magnetic Resonance Imaging; MTA-scale Medial Temporal Lobe Atrophy Rating Scale; EA electroacupuncture; ADAS-cog Alzheimer’s disease Assessment Scale-cognition; MMSE Mini Mental State Examination; ADCS-ADL Alzheimer's disease cooperative study-activity of daily living; Qol-AD Quality of life-Alzheimer’s disease
Discussion
In recent years, previous studies have shown that acupuncture might be effective in improving the cognitive function of AD patients [29, 30]. The results of this study will contribute to a better understanding that whether EA has additional effects to donepezil for improving the cognitive function of patients with AD.
Although clinical diagnosis is the core criteria for AD patients, preclinical diagnostic criteria, such as bio-marker evidence for AD, are also used in clinical practice and research [17]. Medial temporal lobe atrophy (MTA) as the typical imaging appearance can differentiate AD from ageing with a sensitivity and specificity of 80–85%. The severity of MTA combined with clinical information seems justified to be taken into account when diagnosing AD in clinical practice [31]. Therefore, the included criteria of this trial are based on the degree of atrophy of hippocampal or medial temporal lobe volume (measured by MRI). Moreover, as the MTA-scale scores differentiate between moderate to severe AD patients with a sensitivity of 70–100% and a specificity of 67–96%, according to the severity of the condition of participants, subgroup analysis will be applied in the statistical analysis. Furthermore, donepezil is approved by the FDA to treat the symptoms of mild to moderate AD [24, 25] and studies have shown that it can improve the neuropsychiatric, cognitive, and global functions [32–34]. Accordingly, donepezil was chosen as a positive control in this trial. Additionally, an appraisal of drug evolution for AD has shown that the primary outcomes of trials can be described within cognitive, functional, global change, and severity domains [17]. On the basis of this appraisal, the primary outcome is the ADAS-cog score. The ADAS-cog, assessing the severity of impairment of selective areas of cognition, is a sensitive and reliable neuropsychological test [35]. Moreover, a previous trial [36] which had a similar design also evaluated the change in ADAS-cog score after treatment, but follow-up effectiveness was not assessed. Therefore, a 12-week treatment with a follow-up at 24 weeks is expected to better demonstrate the additional effect of EA to donepezil in treating AD.
However, a limitation of this trial is that participant blinding is not possible due to the two different therapeutic strategies employed for each group. Therefore, to minimize any bias that may result from the two treatments, the process of randomization will be under strict control and the investigators and data analysts are to be blinded. Acupuncture as a complex intervention produced greater response expectancies and placebo effects [37, 38]. However, the expectancy effects of acupuncture cannot be estimated in this trial due to the impaired cognitive function of AD patients.
Trial status
We are currently recruiting participants for this trial.
Acknowledgements
All authors of this manuscript would like to give our sincere thanks to the editors of Edanz Group China who have helped us to modify the language.
Funding
This study is funded by Guang’anmen Hospital, China Academy of Chinese Medical Sciences. The financial organization has no rights to influence the design, conduction, data statistics and analyses or paper writing of this study.
Availability of data and materials
As the trial is still in recruitment, there are no supporting data available yet.
Authors’ contributions
ZL contributed to the conception and design of this trial and to the revisions of the manuscript. WP and JZ contributed to the conception and design of this trial and to manuscript writing. JZ contributed to data collection and manuscript writing. MX, QF, and BL contributed to data collection and analysis. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
All authors have consented to publication of this article.
Ethics approval and consent to participate
This trial has been approved by the Ethics Committee of Guang’anmen Hospital (Ethics approval number: 2014EC087). After screening, informed consent will be given to participants and they will have a discussion with the researchers about the procedure, treatment, and possible risks and benefits of the trial. Participants are voluntarily participating and have the right to discontinue the trial and they will sign the informed consent before randomization.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- AD
Alzheimer’s disease
- ADAS-cog
Alzheimer’s Disease Assessment Scale-cognition
- ADCS-ADL
Alzheimer’s Disease Cooperative Study-Activities of Daily Living
- CIs
Cholinesterase inhibitors
- DMC
Data Monitoring Committee
- EA
Electroacupuncture
- FDA
Food and Drug Administration
- ITT
Intention-to-treat
- MMSE
Mini Mental State Examination
- MRI
Magnetic resonance imaging
- MTA-scale
Medial Temporal Lobe Atrophy Rating Scale
- NICE
National Institute for Health and Clinical Excellence
- NINCDS-ADRDA
Neurological Communicative Disorders and Stroke and the Alzheimer Disease and Related Disorders Association
- OCDAD
Operational Criteria for the Diagnosis of Alzheimer’s disease
- QOL-AD
Quality of Life-Alzheimer’s Disease
- SAS
Statistics Analysis System
Additional file
SPIRIT Checklist. (DOCX 21 kb)
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1186/s13063-017-2052-y) contains supplementary material, which is available to authorized users.
Contributor Information
Weina Peng, Email: wnpeng@hotmail.com.
Jing Zhou, Email: zjinbj@sina.com.
Min Xu, Email: doctor_xumin@126.com.
Qing Feng, Email: 469914929@qq.com.
Lulu Bin, Email: ellen_bin@sina.com.
Zhishun Liu, Phone: 86-010-88001124, Email: liuzhishun@aliyun.com.
References
- 1.Prince M, Brycea R, Albanesea E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 2013;9:63–75. doi: 10.1016/j.jalz.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Zhang ZX, Zahner GE, Roman GC, Liu J, Hong Z, Qu QM, et al. Dementia subtypes in China: prevalence in Beijing, Xian, Shanghai, and Chengdu. Arch Neurol. 2005;62:447–53. doi: 10.1001/archneur.62.3.447. [DOI] [PubMed] [Google Scholar]
- 3.Alzheimer’s Association 2011 Alzheimer’s disease facts and figures. Alzheimers Dement. 2011;7:208–44. doi: 10.1016/j.jalz.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, et al. International global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366:2112–7. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wimo A, Winblad B, Jonsson L. The worldwide societal costs of dementia: estimate for 2009. Alzheimers Dement. 2010;6:98–103. doi: 10.1016/j.jalz.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Mischoulon D, Raab MF. The role of folate in depression and dementia. Clin Psychiatry. 2007;68(suppl10):28–33. [PubMed] [Google Scholar]
- 7.Kaduszkiewicz H, Zimmermann T, Beck-Bornholdt HP, van den Bussche H. Cholinesterase inhibitors for patients with Alzheimer’s disease: systematic review of randomised clinical trials. BMJ. 2005;6:321–7. doi: 10.1136/bmj.331.7512.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seow D, Gauthier S. Pharmacotherapy of Alzheimer disease. Can J Psychiatry. 2007;52:620–9. doi: 10.1177/070674370705201003. [DOI] [PubMed] [Google Scholar]
- 9.Atri A, Shaugnessy LW, Locascio JJ, Growdon JH. Long-term course and effectiveness of combination therapy in Alzheimer’s disease. Alzheimer Dis Assoc Disord. 2008;22:209–10. doi: 10.1097/WAD.0b013e31816653bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansen R, Gartlehner G, Webb PA. Efficacy and safety of donepezil, galantamine, and rivastigmine for the treatment of Alzheimer’s disease: a systematic review and meta-analysis. Clin Interv Aging. 2008;3:211–55. [PMC free article] [PubMed] [Google Scholar]
- 11.Tampi RR, Dyck CH. Memantine: efficacy and safety in mild-to-severe Alzheimer’s disease. Neuropsychiatr Dis Treat. 2007;3:245–58. doi: 10.2147/nedt.2007.3.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin CR, Zhang JX, Guo JK, et al. The research of impact of electroacupuncture by using method of “Tiao Shen Yi Zhi” on patients with Alzheimer’s disease. Chin J Gerontol. 2014;34:2225–6. [Google Scholar]
- 13.Zhao LG, Ma L, Li YJ, et al. The clinical research of effect on acupuncturing at GV 20 and GV14 for treating Alzheimer’s disease. JCAM. 2007;23:42–3. [Google Scholar]
- 14.Hu QC, Sun ZY, Meng Y, et al. The effect of acupuncture for treating patients with Alzheimer’s disease by using the method of “Yi Qi Tiao Xue, Fu Ben Pei Yuan”. Shan Xi J Tradit Chin Med. 2001;31:343–4. [Google Scholar]
- 15.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–9. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tian JZ, Shi J, Wei MQ, Wang YY. A Chinese version of clinical criteria for the diagnosis of Alzheimer’s disease. Chin J Front Med Sci. 2012;4:1–6. [Google Scholar]
- 17.Black SE, Doody R, Li H, McRae T, Jambor KM, Xu Y, et al. Donepezil preserves cognition and global function in patients with severe Alzheimer disease. Neurology. 2007;69:459–69. doi: 10.1212/01.wnl.0000266627.96040.5a. [DOI] [PubMed] [Google Scholar]
- 18.Tan MJ, Ma Y, Li Q, Zhang LC. Bibliometric analysis of literature on acupuncture and moxibustion in the treatment of Alzheimer’s disease. J Clin Acupunct Moxibustion. 2012;28:46–8. [Google Scholar]
- 19.Huang ZN, Huang Y, Lu YJ, Wu MX, Xiao HL. Research on the regular pattern of selecting acupoints when treating Alzheimer’s disease with acupuncture. Chin J Basic Med Tradit Chin Med. 2010;16:805–7. [Google Scholar]
- 20.Zhou K, Fang J, Wang X, et al. Characterization of de qi with electroacupuncture at acupoints with different properties. J Altern Complement Med. 2011;17:1007–13. doi: 10.1089/acm.2010.0652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. Am J Psychiatry. 1984;141:1356–64. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Lin C, Zhang L, Cui Y, Gu Y, Guo J, et al. Cognitive improvement during treatment for mild Alzheimer’s disease with a Chinese herbal formula: a randomized controlled trial. PLoS ONE. 10(6):e0130353. doi:10.1371/journal.pone.0130353. [DOI] [PMC free article] [PubMed]
- 23.Feldman H, Gauthier S, Hecker J, Vellas B, Subbiah P, Whalen E. A 24-week, randomized, double-blind study of donepezil in moderate to severe Alzheimer’s disease. Neurology. 2001;57:613–20. doi: 10.1212/WNL.57.4.613. [DOI] [PubMed] [Google Scholar]
- 24.Philip S, Laura VDP. Atrophy of medial temporal lobes on MRI in “probable” Alzheimer’s disease and normal ageing: diagnostic value and neuropsychological correlates. J Neurol Neurosurg Psychiatry. 2012;83:1038–40. doi: 10.1136/jnnp-2012-302562. [DOI] [PubMed] [Google Scholar]
- 25.Zec RF, Burkett NR. Non-pharmacological and pharmacological treatment of the cognitive and behavioral symptoms of Alzheimer’s disease. NeuroRehabilitation. 2008;23:425–83. [PubMed] [Google Scholar]
- 26.Matthews HP, Korbey J, Wilkinson DG, Rowden J. Donepezil in Alzheimer’s disease: eighteen month results from Southampton Memory Clinic. Int J Geriatr Psychiatry. 2000;5:713–20. doi: 10.1002/1099-1166(200008)15:8<713::AID-GPS187>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 27.Yu X, Wang LH, Li SR, Chen YF, Li DH, He J. Discriminatory potential of the Chinese version of ADAS-Cog for mild versus moderate Alzheimer disease. Chin Ment Health J. 2005;19:31–3. [Google Scholar]
- 28.Joseph LS. Multiple imputation: a Primer. Stat Methods Med Res. 1999;8:3–15. doi: 10.1177/096228029900800102. [DOI] [PubMed] [Google Scholar]
- 29.Peng J, Luo L, Xu L, Chen X. Therapeutic efficacy observation on electroacupuncture for Alzheimer’s disease. J Acupunct Tuina Sci. 2015;13:171–4. doi: 10.1007/s11726-015-0844-8. [DOI] [Google Scholar]
- 30.Liu ZB, Niu WM, Yang XH, Niu XM. The clinical research of impact on cognitive function of patients with Alzheimer’s disease by using “Xiu Sanzhen”. Shan Xi J Tradit Chin Med. 2008;29:11–2. [Google Scholar]
- 31.Narayanan L, Murray AD. What can imaging tell us about cognitive impairment and dementia. World J Radiol. 2016;8:240–54. doi: 10.4329/wjr.v8.i3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carrasco MM, Agüera L, Gil P, Moríñigo A, Leon T. Safety and effectiveness of donepezil on behavioral symptoms in patients with Alzheimer disease. Alzheimer Dis Assoc Disord. 2011;25:333–40. doi: 10.1097/WAD.0b013e318212ab7a. [DOI] [PubMed] [Google Scholar]
- 33.Schwam E, Xu Y. Cognition and function in Alzheimer’s disease: identifying the transitions from moderate to severe disease. Dement Geriatr Cogn Disord. 2010;29:309–16. doi: 10.1159/000269837. [DOI] [PubMed] [Google Scholar]
- 34.Schneider LS, Mangialasche F, Andreasen N, Feldman H, Giacobini E, Jones R, et al. Clinical trials and late-stage drug development for Alzheimer’s disease: an appraisal from 1984 to 2014. J Intern Med. 2014;275:251–83. doi: 10.1111/joim.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rogers SL, Friedhoff LT. Long-term efficacy and safety of donepezil in the treatment of Alzheimer’s disease: an interim analysis of the results of a US multicentre open label extension study. Eur Neuropsychopharmacol. 1998;8:67–75. doi: 10.1016/S0924-977X(97)00079-5. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Qin WG, Yu CD. Clinical observation on effect of cranial suture acupuncture combined with donepezil hydrochloride tablets for Alzheimer’s disease. WJAM. 2014;24:19–24. [Google Scholar]
- 37.Kaptchuk TJ, Goldman P, Stone DA, Stason WB. Do medical devices have enhanced placebo effects. J Clin Epidemiol. 2000;53:786–92. doi: 10.1016/S0895-4356(00)00206-7. [DOI] [PubMed] [Google Scholar]
- 38.Bishop FL, Yardley L, Prescott P, Cooper C, Little P, Lewith GT. Psychological covariates of longitudinal changes in back-related disability in patients undergoing acupuncture. Clin J Pain. 2015;31:254–64. doi: 10.1097/AJP.0000000000000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
As the trial is still in recruitment, there are no supporting data available yet.


