Abstract
Background
Family socioeconomic status (SES) is an important source of child health disparities in the U.S. Chronic stress is one way SES may impact children’s physiology with implications for later health inequalities. These processes may work differently across childhood due to differences in exposure and susceptibility to stressors at different ages. We assess associations between family SES and one biomarker of chronic stress exposure—low grade inflammation detected by elevated C-reactive protein (CRP)—and evaluate differences in the associations by child age.
Methods
We used nationally-representative data from the National Health and Nutrition Survey (NHANES) and Tobit regression models to estimate SES associations with CRP and the moderating effects of age for children ages 2–18 years. Our sample was limited to CRP ≤ 10 mg/l to focus on low-grade inflammation (N=13,165).
Results
Children whose parent had less than a high school degree had 35% higher CRP than those with a college-graduate parent; and, poor children had 24% higher CRP than those with high family income, net of controls. When children’s BMI was accounted for, low education and poverty associations were reduced to 19% and 15%, respectively. Child age interactions were negative and significant for both parental education and family income.
Conclusion
This study provides new evidence that SES is associated with low-grade inflammation in children, and that these associations may be particularly strong during early and mid-childhood. Future research should further our understanding of stressors related to low family SES that may lead to immune system dysregulation during childhood.
Keywords: child health, stress, C-reactive protein, poverty, parental education
INTRODUCTION
A large body of literature documents socioeconomic status (SES) disparities in child health.1, 2 Researchers are now challenged with understanding how SES may “get under the skin” to produce these disparities. One potentially important pathway from low SES to poor child health is through exposure to chronic stress and related biological changes.3–6 Biological research suggests that exposure to chronic stress may result in dysregulation of the neuroendocrine and immune systems; and, these systems may be particularly sensitive during childhood.7, 8
In this study we assess how family SES is associated with one biomarker of chronic stress exposure–low-grade inflammation9—in U.S. children. Psychosocial stress may affect inflammation levels by stimulating the production of proinflammatory cytokines, which increase acute phase proteins (such as C-reactive protein, CRP) circulating in the blood.10 This inflammatory response may be further enhanced if repeated activation of the autonomic nervous system and hypothalamic-pituitary-adrenal axis due to chronic stress has resulted in glucocorticoid resistance; so that, immune cells lose their sensitivity to glucocoritcoids that normally would stop the inflammatory process.11 Chronic stress could thus result in long-term circulation of low levels of CRP in the blood, an indicator of low-grade, systemic inflammation. Low-grade inflammation has been associated with depression12, 13 and cardiovascular risk factors in children;14, 15 and, may increase the risk for adult inflammation and chronic disease.16, 17
Our study builds on existing research18 by using nationally-representative data and multivariable regression analysis to assess how two measures of family SES – parental education and family income – are associated with low-grade inflammation (indicated by CRP) in children ages 2–18 years. We consider the mediating role of body mass index (BMI) due to evidence that low family SES is associated with higher BMI in children,19 and that adipose tissue can increase the production of CRP.20 Importantly, we assess whether child age conditions the SES-CRP associations, given past findings that the SES-health gradient differs by child age.21, 22 To date, research on age as a modifier of SES-child health associations has relied on parental reports of child health, with little attention to biomarkers such as CRP.
METHODS
Data
Our data come from the continuous National Health and Nutrition Surveys (NHANES), a nationally-representative dataset on the health and nutrition of children and adults collected by the National Center for Health Statistics (NCHS) every two years. We use physical health exam and interview data across 6 waves spanning 1999–2010. Blood samples were collected from all participants >1 year in waves 2, 3, and 4; from participants >3 years in waves 1, 3 and 6; and from children ages 3–5 and 12+ years in wave 5. In all waves an adult household respondent (a parent or guardian for children under 16 years) was interviewed to gather SES, child illness, and household demographic information. We concatenated the survey waves to obtain stable estimates for children ages 2–18 years. Each wave of NHANES was approved by the NCHS Research Ethics Review Board.
Sample
Our sample consists of children ages 2–18 years who provided blood samples, excluding 1% of children with a household respondent who was <17 years older than the child (to ensure that the household respondent was not a sibling or the index child).23 Due to our interest in low-grade inflammation as a response to chronic stress we limited our sample to children with CRP ≤10 mg/l.9, 24 Past research indicates that CRP values >10 mg/l likely reflect acute inflammation in response to an infection or injury and should not be considered in studies of low-grade inflammation.25
CRP was missing for 25% of our sample; however, we found no substantial difference in child or family characteristics between those with and without CRP, with the exception of child age. Children missing CRP were younger than those in our sample (mean of 7 vs. 11 years). This may be due to NHANES age restrictions in some waves and higher refusal rates for blood collection in younger children. In our analyses we apply survey weights to account for the non-response.
Our sample includes children with undetectable CRP levels, indicated in NHANES as <0.1mg/l. These cases are considered censored (non-missing, but unknown true value) and require Tobit regression analysis. Since Tobit models cannot be estimated with imputed data, we dropped cases missing independent variable data (19%), resulting in a final sample of 13,165 children. Median CRP for missing cases was similar to our sample median, 0.4 and 0.3, respectively. The top quarter of the sample had a CRP range of 1.2–9.7 among those missing and 1.0–10 for those not missing independent variable data.
Measures
Our outcome variable is a continuous measure of CRP in mg/l. For regression analyses, we logged CRP (base 10) due to its skewed distribution.
We measured family SES as two categorical variables: parental education and family income-to-poverty ratio (IPR). Household respondents provided their education level as < high school degree, high school graduate, some college or associates degree, and graduated college (reference category in regression models). We refer to this as “parental” education because for 83% of the sample children (those under age 16) a parent/guardian was the household respondent. While we could not confirm relationship of the household respondent for the 16–18 year olds, limiting the sample to children whose household respondent was ≥17 years older than the child ensured that the respondent was likely a parent or guardian. Household respondents over age 60 (possible grandparent) occurred in 4% of the sample cases - the findings were robust to models excluding these cases.
Our second SES measure was family IPR, calculated as total family income divided by the federal poverty line (FPL) for a given family size and year, capped at 5 x FPL by NHANES. We created the following categories: poor (<1 x FPL), low income (1–2 x FPL), moderate income (2.1–3.9 x FPL), and high income (≥4 x FPL) (reference group in regression models).
Statistical Methods
We weighted both the descriptive statistics and regression analyses with individual medical exam weights computed for the 12-year period. Our multivariable analyses utilized Tobit regression of log CRP on family SES. Tobit models are estimated with maximum likelihood and take into account the censored nature of our dependent variable.26
Our first regression model included the family SES variables and all control variables. Child-level controls were: age, illness (recent cold, infection or stomach illness), gender (male=1), and race (black, Hispanic, other race vs. non-Hispanic white). Household respondent (parent) controls included: age, gender, U.S.-born, and marital status (single, cohabiting, separated/divorced, and widowed vs. married). Household size and dummies for NHANES survey waves were also included.
Our second model added child BMI z-scores, calculated using measured height and weight and 2000 CDC growth charts as the reference population. We entered BMI z-scores sequentially to assess changes in our SES associations when controlling for BMI, which along with supplemental analyses, allowed us to evaluate potential mediation of family SES by child BMI. Our final models included interaction terms between child age and the SES variables to test for moderating effects of child age. All analyses were performed using Stata V.13 and significance was assessed through two-tailed z-tests of p<0.05.
RESULTS
Key characteristics of the sample children are shown in Table 1. Median CRP was 0.3 mg/l. Median child age was 10 years and 38% had a recent illness, with colds being the most common. The sample median BMI z-score was 0.4, just over half the sample was male, and 61% of the children were reported as non-Hispanic white.
Table 1.
Weighted descriptive statistics. Children ages 2–18 years with CRP ≤10mg/l, NHANES 1999–2010. N=13,165.
| Variable | Median | % | Min. | Max. |
|---|---|---|---|---|
| C-Reactive protein (mg/l)1 | 0.3 | 0.1 | 10 | |
| Family Socioeconomic Status | ||||
| Parental Education | ||||
| Less than high school | 20% | 0 | 1 | |
| High school degree | 24% | 0 | 1 | |
| Some college | 30% | 0 | 1 | |
| College graduate | 26% | 0 | 1 | |
| Family income-to-poverty ratio2 | 2.3 | 0 | 5 | |
| Poor (< 1 × FPL) | 21% | 0 | 1 | |
| Low income (1–2 × FPL) | 24% | 0 | 1 | |
| Moderate income (2.1–3.9 × FPL) | 29% | 0 | 1 | |
| High income (≥ 4 × FPL) | 26% | 0 | 1 | |
| Child Characteristics | ||||
| Age in years | 10 | 2 | 18 | |
| Recent head or chest cold | 23% | 0 | 1 | |
| Recent flu, pneumonia, ear infection | 6% | 0 | 1 | |
| Recent stomach or intestinal illness | 9% | 0 | 1 | |
| BMI z-score | 0.4 | −4.9 | 4.6 | |
| Male | 52% | 0 | 1 | |
| Non-Hispanic white | 61% | 0 | 1 | |
| Hispanic | 19% | 0 | 1 | |
| African American | 13% | 0 | 1 | |
| Other race | 7% | 0 | 1 | |
| Household Respondent Characteristics | ||||
| Age | 40 | 20 | 80 | |
| Male | 52% | 0 | 1 | |
| U.S. Born | 81% | 0 | 1 | |
| Married | 70% | 0 | 1 | |
| Cohabiting | 4% | 0 | 1 | |
| Single | 7% | 0 | 1 | |
| Divorced/separated | 17% | 0 | 1 | |
| Widowed | 2% | 0 | 1 | |
| Household size | 4 | 2 | 7 | |
FPL = Federal Poverty Line.
Those missing CRP values did not differ from analytical sample by family socioeconomic status, race/ethnicity or other social characteristics. Missing CRP children did have significantly lower age (median 7 y) and slightly lower BMI z-score (median 0.3) than sample children.
Capped at 5.
The SES measures indicated that 44% of children had a parent with a high school degree or less, 30% had some college or an associate’s degree, and 26% had a bachelor’s degree or higher. Approximately one-fifth of children lived below the poverty line, 24% were low-income (1–2 x FPL), 29% moderate income (2.1–3.9 x FPL), and 26% were in high-income families.
Table 2 provides the regression results (not all controls were shown for brevity). Since our dependent variable is logged, the coefficients are interpreted as percent increase in CRP between the SES category of interest and the reference category. Results from Model 1 indicated strong associations between low education and family income with log CRP. Children whose parent had < high school degree had 35% higher CRP than those with a college-graduate parent, net of control variables. Parent education levels of high school degree and some college were associated with 29% and 15% higher child CRP, respectively, than children with a college-graduate parent. With response to family income, children living in poverty had, on average, 24% higher CRP than those in high-income families. Children in families with low and moderate income were estimated to have 14% and 18% higher CRP, respectively, than those in the highest family income category.
Table 2.
Tobit regression results of family SES associations with log CRP. Children ages 2–18 years with CRP ≤10mg/l, NHANES 1999–2010. N=13,165.
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
|---|---|---|---|---|---|---|---|---|
| Variables | ß | 95% CI | ß | 95% CI | ß | 95% CI | ß | 95% CI |
| Family Socioeconomic Status (SES) | ||||||||
| Parent < high school degree1 | 0.35** | (0.21, 0.50) | 0.19** | (0.05, 0.32) | 0.60** | (0.28, 0.93) | 0.18** | (0.05, 0.31) |
| Parent high school degree1 | 0.29** | (0.15, 0.42) | 0.17** | (0.05, 0.30) | 0.50** | (0.16, 0.84) | 0.17** | (0.04, 0.29) |
| Parent some college1 | 0.15* | (0.023, 0.27) | 0.061 | (−0.05, 0.18) | 0.36* | (0.04, 0.68) | 0.056 | (−0.06, 0.17) |
| Poor2 | 0.24** | (0.09, 0.39) | 0.15* | (0.01, 0.28) | 0.14* | (0.01, 0.28) | 0.58** | (0.25, 0.91) |
| Low income2 | 0.14* | (0.002, 0.28) | 0.09 | (−0.04, 0.21) | 0.09 | (−0.04, 0.21) | 0.40* | (0.06, 0.74) |
| Moderate income2 | 0.18** | (0.06, 0.31) | 0.11 | (−0.01, 0.23) | 0.11 | (−0.005, 0.23) | 0.19 | (−0.16, 0.54) |
| Child Age and Health | ||||||||
| Age in years | 0.07** | (0.06, 0.08) | 0.05** | (0.04, 0.06) | 0.08** | (0.06, 0.10) | 0.070** | (0.05, 0.09) |
| Recent cold | 0.58** | (0.49, 0.68) | 0.60** | (0.51, 0.69) | 0.59** | (0.50, 0.68) | 0.59** | (0.50, 0.68) |
| Recent flu, pneumonia, ear infection | 0.35** | (0.19, 0.52) | 0.21** | (0.05, 0.36) | 0.21** | (0.05, 0.36) | 0.20** | (0.05, 0.36) |
| Recent stomach illness | 0.14 | (−0.01, 0.28) | 0.09 | (−0.05, 0.22) | 0.09 | (−0.06, 0.22) | 0.09 | (−0.05, 0.22) |
| BMI z-score | 0.63** | (0.59, 0.66) | 0.63** | (0.59, 0.66) | 0.63** | (0.59, 0.66) | ||
| Child Age * Family SES Interactions | ||||||||
| Age * Parent < high school degree | −0.04** | (−0.06, −0.01) | ||||||
| Age * Parent high school degree | −0.03* | (−0.06, −0.002) | ||||||
| Age * Parent some college | −0.03* | (−0.05, −0.001) | ||||||
| Age * Poor | −0.04** | (−0.06, −0.01) | ||||||
| Age * Low income | −0.03* | (−0.05, −0.001) | ||||||
| Age * Moderate income | −0.01 | (−0.03, − 0.02) | ||||||
p<0.01,
p<0.05. FPL = Federal Poverty Level. Control variables included in models but not shown for brevity: child gender, child race, parent age, parent gender, parent foreign-born, parent marital status, household size, survey wave dummy variables.
Ref: Parent is a college graduate.
Ref: High family income (income-to-poverty ratio ≥ 4 x FPL).
In Model 2, we estimate the associations between children’s BMI z-scores with log CRP and compare the results with Model 1 to assess mediation of family SES by BMI. Results indicated a 63% increase in CRP with each point increase in BMI z-score (Table 2, Model 2). Including BMI z-score reduced the parent education coefficients, with a decrease from 0.35 to 0.19 for < high school degree, 0.29 to 0.17 for high school degree, and a now insignificant coefficient on parent with some college (see Models 1 & 2). The family poverty effect declined from 0.24 to 0.15 when accounting for child BMI, while low- and moderate-income coefficients became small and insignificant. Additional evidence of mediation was found in supplementary multivariate OLS regression models (not shown here), which indicated significant positive associations between the education and income categories and BMI z-scores, net of control variables.
Finally, we assessed the moderating role of child age by including child age × parent education (Model 3) and child age × IPR (Model 4) interaction terms. Results from both models indicated significant negative interaction effects, suggesting larger associations between family SES and log CRP in younger children. To illustrate the impact of the interaction effects, Figure 1 shows the predicted percent differences in CRP for children between low versus high SES categories by child age. Among the youngest children in the sample (age 2), having a parent with < a high school degree was associated with 52% higher CRP than for those with high parental education. Each additional year of age reduced the association by approximately 4%; so that, by age 13, the predicted percent difference in CRP for children with low versus high parent education was reduced to insignificance. Also shown in Figure 1, the youngest children had a predicted 50% higher CRP when living in poverty compared with similar children in high-income families. The poverty effect was reduced to insignificance by age 12.
Figure 1.
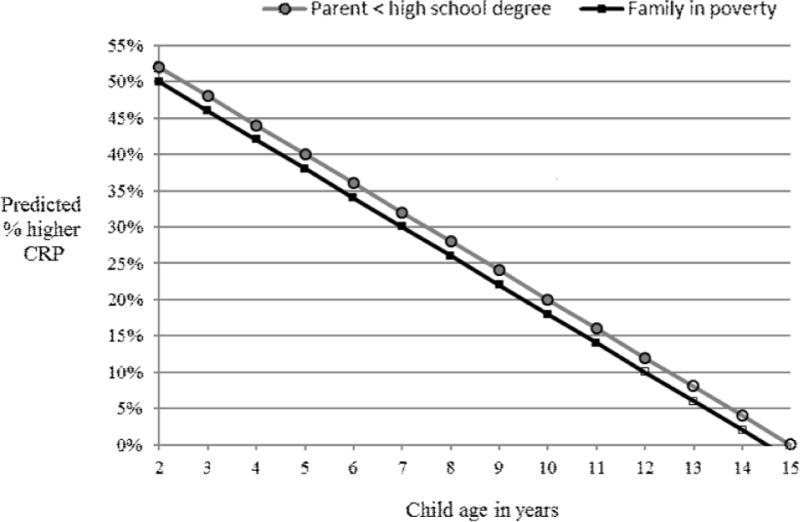
Associations between low family SES and CRP by child age. Calculated based on results from Models 3 & 4.
DISCUSSION
In this study we assessed family SES inequalities in a biological marker of low-grade inflammation, elevated CRP, in a representative sample of U.S. children. Our results indicated that both low parental education and family income were independently associated with higher child CRP net of potential confounders. We found that child BMI partially mediated the family SES associations; however, significant associations between the lower SES categories and CRP remained. Our child age-SES interaction effects, although somewhat small, provided evidence of strong associations between SES and CRP in early childhood even when controlling for BMI.
The strengths of our study include: assessment of two aspects of children’s SES (parental education and family income), the use of regression techniques and inflammation parameters consistent with previous research for comparisons across studies, and the use of national-level data with children spanning the SES spectrum and full childhood age range. Further, our analysis of CRP levels ≤ 10 mg/l and controlling for children’s recent illnesses allowed us to focus on the associations between SES and psychosocial stress-related inflammation. Finally, we provided a more nuanced understanding of SES-CRP associations, with evidence that SES works both through and apart from BMI, and new findings of child age as a moderator of SES-CRP associations.
The main limitation of our study is the lack of longitudinal data to assess how family SES may impact inflammation as children age, and whether the effects are long-term. Cross-sectional data also limit the study to associational rather than causal findings. Further, we were unable to assess SES-related stressors that may explain our findings.
Despite these limitations, this study increases our understanding of SES as an aspect of children’s family contexts that may induce inflammation related to chronic stress exposure. Our income findings corroborate with another national-level study that found significant positive associations between family income and inflammation in children 3–16 years.18 Our results for parental education are consistent with studies of older children in Los Angeles,27 Ohio,28 and 10-year olds in the UK.29 Thus, we add to the growing literature that suggests low SES may be an important risk factor for low-grade inflammation in children.
The finding that children’s BMI mediates the SES effect is also consistent with past studies of income18, 30 and education29 effects on children’s CRP. Findings across studies, including ours, indicate that low SES works to affect CRP, in part, through higher BMI in low-SES children. This may indicate another “stress” pathway, whereby psychosocial stress in low-SES family contexts may produce biological effects that increase body fat.31, 32 However, our finding that the lowest SES categories had significant associations with CRP net of BMI suggests a direct link between low-SES and inflammation. The child age interaction models, which included BMI z-scores, indicated that the associations between SES and low-grade inflammation may be particularly important in early childhood.
Our finding that child age moderated the SES-inflammation relationship is consistent with child health research indicating SES associations with other, non-biological measures of child health are stronger in early childhood.33, 34 Our study contradicts other research that finds family income effects on health to be more pronounced during adolescence.21 It may be that low-grade inflammation, unlike global measures of parent-reported child health, is more sensitive to development stage rather than cumulative effects of children’s social contexts. Since the moderation effect of child age has not been reported in other studies of SES and CRP, this remains to be further studied.
In addition to increasing our understanding of inequalities in inflammation during childhood, our findings have potentially important implications for adult health. Low-grade inflammation has been shown to increase the risk of cardiovascular and other diseases in adulthood.35–38 A growing body of literature suggests that this process of disease onset due to chronic low-grade inflammation may begin during childhood.15, 39 Thus, it will be important to further explore low SES during childhood, and related stressors, as potential precursors to adult inflammation.
More research is needed in this area, with particular attention to how biosocial processes related to inflammation (and other biomarkers) unfold over time during childhood and continue into adulthood. The use of biomarkers in child health research allows for the detection of physiological changes that may not be perceptible to parents, uncovering inequalities in children’s biological systems that may lay the foundation for later health disparities. The collection of longitudinal family, social and economic data, along with inflammation and other stress-related biomarkers, will be important for future research to improve our understanding of social contexts, psychosocial stress and health across the life course.
Research Report.
What is already known on this subject:
Chronic stress exposure can increase low-grade inflammation as measured by CRP
Low family socioeconomic status (SES) is one potential source of chronic stress exposure that may increase inflammation in low-SES children.
What this study adds:
Low parent education and family income are associated with higher inflammatory marker concentrations in children.
Much of the SES associations are mediated by children’s body mass index (BMI).
Net of BMI and recent illness, the lowest SES categories are significantly associated with low-grade inflammation in children, with particularly strong effects in young children.
Acknowledgments
This research was funded in part by a K01 grant awarded to Schmeer (1 K01 HD 077063 - 01 A1) through the Eunice Kennedy Shriver National Institute for Child Health and Human Development (NICHD) of the National Institutes of Health (NIH). Support for this project also was provided through a seed grant awarded to Schmeer by the Ohio State University Institute for Population Research through a grant from the Eunice Kennedy Shriver NICHD of NIH, P2CHD058484. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver NICHD or the NIH.
Funding: K01 grant awarded to Schmeer (1 K01 HD 077063 - 01 A1); seed grant awarded to Schmeer by the Ohio State University Institute for Population Research through a grant from the Eunice Kennedy Shriver NICHD of NIH, P2CHD058484.
Footnotes
Competing Interests
None
Contributor Information
Kammi K. Schmeer, Department of Sociology, Ohio State University, Columbus, OH, USA.
Aimee Yoon, Department of Sociology, Ohio State University, Columbus, OH, USA.
References
- 1.Adler NE, Newman K. Socioeconomic disparities in health: Pathways and policies. Health Affairs. 2002;21:60–76. doi: 10.1377/hlthaff.21.2.60. [DOI] [PubMed] [Google Scholar]
- 2.Wickham S, Anwar E, Barr B, et al. Poverty and child health in the UK: using evidence for action. Archives of Disease in Childhood. 2016 doi: 10.1136/archdischild-2014-306746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shonkoff JP, Boyce W, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: Building a new framework for health promotion and disease prevention. JAMA. 2009;301:2252–59. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- 4.Brunner E. Socioeconomic determinants of health - Stress and the biology of inequality. British Medical Journal. 1997;314:1472–76. doi: 10.1136/bmj.314.7092.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adler NE, Snibbe AC. The role of psychosocial processes in explaining the gradient between socioeconomic status and health. Current Directions in Psychological Science. 2003;12:119. [Google Scholar]
- 6.Adler NE, Rehkopf DH. US disparities in health: Descriptions, causes, and mechanisms. Annual Review of Public Health. 2008;29:235–52. doi: 10.1146/annurev.publhealth.29.020907.090852. [DOI] [PubMed] [Google Scholar]
- 7.Pervanidou P, Chrousos GP. Metabolic consequences of stress during childhood and adolescence. Metab-Clin Exp. 2012;61:611–19. doi: 10.1016/j.metabol.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Bauer AM, Boyce WT. Prophecies of childhood: How children’s social environments and biological propensities affect the health of populations. Int J Behav Med. 2004;11:164–75. doi: 10.1207/s15327558ijbm1103_5. [DOI] [PubMed] [Google Scholar]
- 9.McDade TW. Measuring immune function: markers of cell-mediated immunity and inflammation in dried blood spots. Measuring Stress in Humans: A Practical Guide for the Field. 2007;49:181–207. [Google Scholar]
- 10.Black PH. Stress and the inflammatory response: A review of neurogenic inflammation. Brain Behavior and Immunity. 2002;16:622–53. doi: 10.1016/s0889-1591(02)00021-1. [DOI] [PubMed] [Google Scholar]
- 11.Cohen S, Janicki-Deverts D, Doyle WJ, et al. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:5995–99. doi: 10.1073/pnas.1118355109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller GE, Cole SW. Clustering of Depression and Inflammation in Adolescents Previously Exposed to Childhood Adversity. Biological Psychiatry. 2012;72:34–40. doi: 10.1016/j.biopsych.2012.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim JW, Szigethy EM, Melhem NM, et al. Inflammatory Markers and the Pathogenesis of Pediatric Depression and Suicide: A Systematic Review of the Literature. Journal of Clinical Psychiatry. 2014;75:1242–53. doi: 10.4088/JCP.13r08898. [DOI] [PubMed] [Google Scholar]
- 14.Cook DG, Mendall MA, Whincup PH, et al. C-reactive protein concentration in children: relationship to adiposity and other cardiovascular risk factors. Atherosclerosis. 2000;149:139–50. doi: 10.1016/s0021-9150(99)00312-3. [DOI] [PubMed] [Google Scholar]
- 15.Slopen N, Koenen KC, Kubzansky LD. Childhood adversity and immune and inflammatory biomarkers associated with cardiovascular risk in youth: A systematic review. Brain Behavior and Immunity. 2012;26:239–50. doi: 10.1016/j.bbi.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Fagundes CP, Glaser R, Kiecolt-Glaser JK. Stressful early life experiences and immune dysregulation across the lifespan. Brain Behavior and Immunity. 2013;27:8–12. doi: 10.1016/j.bbi.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller GE, Chen E, Parker KJ. Psychological Stress in Childhood and Susceptibility to the Chronic Diseases of Aging: Moving Toward a Model of Behavioral and Biological Mechanisms. Psychological Bulletin. 2011;137:959–97. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dowd JB, Zajacova A, Aiello AE. Predictors of Inflammation in U.S. Children Aged 3–16 Years. American Journal of Preventive Medicine. 2010;39:314–20. doi: 10.1016/j.amepre.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang YF, Zhang Q. Are American children and adolescents of low socioeconomic status at increased risk of obesity? Changes in the association between overweight and family income between 1971 and 2002. American Journal of Clinical Nutrition. 2006;84:707–16. doi: 10.1093/ajcn/84.4.707. [DOI] [PubMed] [Google Scholar]
- 20.Ford ES. C-reactive protein concentration and cardiovascular disease risk factors in children - Findings from the National Health and Nutrition Examination Survey 1999–2000. Circulation. 2003;108:1053–58. doi: 10.1161/01.CIR.0000080913.81393.B8. [DOI] [PubMed] [Google Scholar]
- 21.Case A, Lubotsky D, Paxson C. Economic status and health in childhood: The origins of the gradient. American Economic Review. 2002;92:1308–34. doi: 10.1257/000282802762024520. [DOI] [PubMed] [Google Scholar]
- 22.Chen E, Martin AD, Matthews KA. Socioeconomic status and health: Do gradients differ within childhood and adolescence? Social Science & Medicine. 2006;62:2161–70. doi: 10.1016/j.socscimed.2005.08.054. [DOI] [PubMed] [Google Scholar]
- 23.Case A, Paxson C, Vogl T. Socioeconomic status and health in childhood: A comment on Chen, Martin and Matthews, “Socioeconomic status and health: Do gradients differ within childhood and adolescence?” (62:9, 2006, 2161–2170) Social Science & Medicine. 2007;64:757–61. doi: 10.1016/j.socscimed.2006.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDade TW. Early environments and the ecology of inflammation. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:17281–88. doi: 10.1073/pnas.1202244109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Warnberg J, Nova E, Romeo J, et al. Lifestyle-related determinants of inflammation in adolescence. British Journal of Nutrition. 2007;98:S116–S20. doi: 10.1017/S0007114507839614. [DOI] [PubMed] [Google Scholar]
- 26.Long SJ. Regression models for categorical and limited dependent variables. Advanced quantitative techniques in the social sciences. 1997:7. [Google Scholar]
- 27.Chiang JJ, Bower JE, Almeida DM, et al. Socioeconomic Status, Daily Affective and Social Experiences, and Inflammation During Adolescence. Psychosomatic Medicine. 2015;77:256–66. doi: 10.1097/PSY.0000000000000160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pietras SA, Goodman E. Socioeconomic Status Gradients in Inflammation in Adolescence. Psychosomatic Medicine. 2013;75:442–48. doi: 10.1097/PSY.0b013e31828b871a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Howe LD, Galobardes B, Sattar N, et al. Are there socioeconomic inequalities in cardiovascular risk factors in childhood, and are they mediated by adiposity? Findings from a prospective cohort study. International Journal of Obesity. 2010;34:1149–59. doi: 10.1038/ijo.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murasko JE. Male-female differences in the association between socioeconomic status and atherosclerotic risk in adolescents. Social Science & Medicine. 2008;67:1889–97. doi: 10.1016/j.socscimed.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 31.Wilson SM, Sato AF. Stress and Paediatric Obesity: What We Know and Where To Go. Stress and Health. 2014;30:91–102. doi: 10.1002/smi.2501. [DOI] [PubMed] [Google Scholar]
- 32.Stenhammar C, Olsson GM, Bahmanyar S, et al. Family stress and BMI in young children. Acta Paediatrica. 2010;99:1205–12. doi: 10.1111/j.1651-2227.2010.01776.x. [DOI] [PubMed] [Google Scholar]
- 33.West P, Sweeting H. Evidence on equalisation in health in youth from the West of Scotland. Social Science & Medicine. 2004;59:13–27. doi: 10.1016/j.socscimed.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 34.West P. Health inequalities in the early years: is there equalisation in youth? Soc Sci Med. 1997;44:833–58. doi: 10.1016/s0277-9536(96)00188-8. [DOI] [PubMed] [Google Scholar]
- 35.Buckley DI, Fu RW, Freeman M, et al. C-Reactive Protein as a Risk Factor for Coronary Heart Disease: A Systematic Review and Meta-analyses for the US Preventive Services Task Force. Annals of Internal Medicine. 2009;151:483–W161. doi: 10.7326/0003-4819-151-7-200910060-00009. [DOI] [PubMed] [Google Scholar]
- 36.Fahdi IE, Gaddam V, Garza L, et al. Inflammation, infection, and atherosclerosis. Brain Behavior and Immunity. 2003;17:238–44. doi: 10.1016/s0889-1591(03)00052-7. [DOI] [PubMed] [Google Scholar]
- 37.Karakas M, Koenig W. CRP in Cardiovascular Disease. Herz. 2009;34:607–13. doi: 10.1007/s00059-009-3305-7. [DOI] [PubMed] [Google Scholar]
- 38.Ridker PM. C-Reactive Protein and the Prediction of Cardiovascular Events Among Those at Intermediate Risk: Moving an Inflammatory Hypothesis Toward Consensus. Journal of the American College of Cardiology. 2007;49:2129–38. doi: 10.1016/j.jacc.2007.02.052. [DOI] [PubMed] [Google Scholar]
- 39.Juonala M, Viikari JSA, Ronnemaa T, et al. Childhood C-reactive protein in predicting CRP and carotid intima-media thickness in adulthood - The Cardiovascular Risk in Young Finns Study. Arteriosclerosis Thrombosis and Vascular Biology. 2006;26:1883–88. doi: 10.1161/01.ATV.0000228818.11968.7a. [DOI] [PubMed] [Google Scholar]


