Abstract
Perivascular epithelioid cell tumors (PEComas) are mesenchymal neoplasms with immunoreactivity for both melanocytic and smooth muscle markers. PEComas occur at multiple sites, and malignant PEComas can undergo metastasis, recurrence and aggressive clinical courses. Although the lung is a common metastatic site of PEComas, they usually appear as multiple nodules but rarely become cystic or cavitary. Here, we describe a female patient whose lungs manifested multiple cystic, cavity-like and nodular metastases 3 years after the resection of uterine tumors tentatively diagnosed as epithelioid smooth muscle tumors with uncertain malignant potential. This patient's subsequent pneumothorax necessitated video-assisted thoracoscopic surgery, and examination of her resected lung specimens eventually led to correcting the diagnosis, i.e., to a PEComa harboring tuberous sclerosis complex 1 (TSC1) loss-of-heterozygosity that originated in the uterus and then metastasized to the lungs. The administration of a gonadotropin-releasing hormone analogue later stabilized her clinical course. To the best of our knowledge, the present case is the first in the literature that associates PEComas with a TSC1 abnormality. Additionally, the pulmonary manifestations, including imaging appearance and pneumothorax, somewhat resembled those of lymphangioleiomyomatosis, a representative disease belonging to the PEComa family. Although PEComas are rare, clinicians, radiologists and pathologists should become aware of this disease entity, especially in the combined clinical setting of multiple cystic, cavity-like, nodular lesions on computed tomography of the chest and a past history of the tumor in the female reproductive system.
Keywords: Cystic lung disease, Loss of heterozygosity, Multiple lung nodules, PEComa, Pneumothorax, Pulmonary metastasis
Abbreviations: CAPUs, clinically aggressive PEComas of the uterine corpus; CT, computed tomography; ESS, endometrial stromal sarcoma; GnRH, gonadotropin-releasing hormone analogue; HPF, high-power fields; LOH, loss of heterozygosity; LAM, lymphangioleiomyomatosis; mTOR, mammalian target of rapamycin; PEComa, perivascular epithelioid cell tumor; PEComa-NOS, PEComa not otherwise specified; α-SMA, α-smooth muscle actin; TFE3, transcription factor E3; TSC, tuberous sclerosis complex
1. Introduction
Perivascular epithelioid cell tumors (PEComas) are the family name of mesenchymal neoplasms composed of characteristic perivascular epithelioid cells, which usually express both melanocytic and smooth muscle markers [1]. PEComas predominate in females, and representative diseases are angiomyolipoma, lymphangioleiomyomatosis (LAM) and clear cell “sugar” tumor of the lung. However, PEComas occurring in such varied anatomic sites as the uterus, uterine cervix, gastrointestinal tract, kidney, liver, breast and lung [2], [3], [4], [5], [6], [7], [8] are collectively termed “PEComa not otherwise specified” (PEComa-NOS). These tumors are so-named, because they fail to meet any criteria for a conventional disease entity defined by histopathological findings [2]. Other than the retroperioneum, the uterus is the site most commonly affected by PEComas [9]. Some kinds of PEComas, especially angiomyolipomas, LAM occurring in the setting of tuberous sclerosis complex (TSC) and sporadic PEComas, frequently have loss of function mutations in the TSC1 or TSC2 gene. Furthermore, a subset of PEComas harbors transcription factor E3 (TFE3) gene fusions, which have been demonstrated in several types of neoplasia like soft-part neoplasia [10].
PEComas may have malignant potential and behave aggressively [11]. Although lungs are a common metastatic site for this tumor, pneumothorax due to lung PEComas has never been reported. The unique presentation of pneumothorax in lungs bearing multiple cystic, cavity-like, nodular lesions on computed tomography (CT) images of the chest reported here was subsequently diagnosed as a metastasis to the lungs of a malignant uterine PEComa.
2. Case presentation
A 44-year-old woman was referred to our hospital for the evaluation of lung nodules in May 2015. Her past medical history noted that she had been admitted in May 2012 because of a massive intraabdominal hemorrhage caused by the rupture of a subserosal uterine leiomyoma extending into the broad ligament. Enucleatic myomectomy (6.9 cm in size) was performed, and pathological examination yielded a diagnosis of an epithelioid smooth muscle tumor of uncertain malignant potential. No apparent dissemination was noted. In September 2013, right oophorectomy was performed to resect an endometrial cyst, and monthly treatment of endometriosis was initiated with a gonadotropin-releasing hormone (GnRH) analogue (1.88 mg of leuprorelin acetate) lasting until May 2014. Meanwhile, a small amount of ascites present before the initiation of GnRH treatment increased after its discontinuation, as pelvic magnetic resonance imaging showed.
At this patient's initial presentation to our hospital in May 2015, the results of physical examination were unremarkable, and blood tests revealed no abnormalities with the exception of increased carbohydrate antigen 125 (263 U/ml; normal range, 0–35). However, a computed tomography (CT) scan of her chest depicted bilateral scattered lung nodules, cavity-like lesions with inhomogenously-thickened walls and multiple thin-walled cysts (Fig. 1). Thereafter, her monthly subcutaneous injections of GnRH analogue resumed to regulate the endometriosis and ascites.
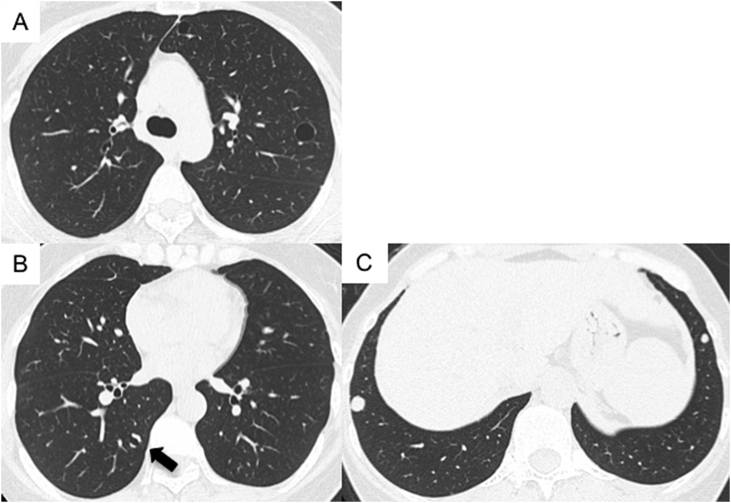
Fig. 1.
Computed tomography (CT) scans at this patient's first visit (in May 2015) showed multiple cysts (A), a cavity-like lesion (B; arrow), and lung nodules (C). The cavity-like lesion had a inhomogenously-thickened wall.
Four months later (September, 2015), she came to the Emergency Department with back pain of 3 days' duration on her right side. After chest radiography revealed a right-side pneumothorax, she was hospitalized and a chest tube was placed. Although the lung was well expanded by continuous suction, air leakage from the chest tube still remained. A chest CT on the 6th hospital day portrayed the new cyst in the S8 area of the right lung (Fig. 2A). Accordingly, video-assisted thoracoscopic surgery was performed on the 10th hospital day. We detected air leakage at the site of this new cyst by conducting a water sealing test; therefore, a partial resection of the right lung surrounding the cyst followed (Fig. 2B). Additionally, partial resection of the solid nodule in the basal region of the right lung was performed. Air leakage disappeared immediately after the resection, and the chest tube was removed on the next day.
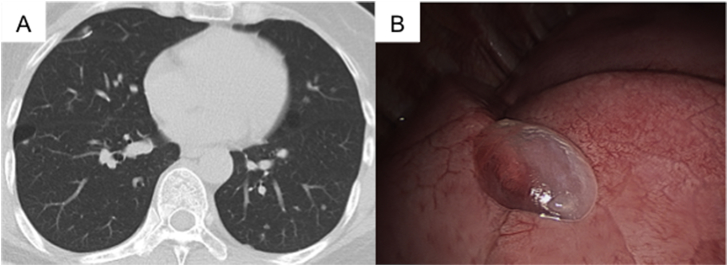
Fig. 2.
(A) CT scan of the chest on the 6th hospital day revealed a new cyst with an inhomogeneous wall thickness in the S8 area of the right lung. (B) Video-assisted thoracoscopic surgery pinpointed the translucent cyst in the S8 area of the right lung.
Pathological examination of the S8 area revealed a bullous cyst with nodular proliferation of tumor cells at the basal region of lung parenchyma (Fig. 3A and B). The bullous cyst wall was composed of thickened visceral pleura and was infiltrated by tumor cells; elastic fibers in the visceral pleura were markedly disrupted (Fig. 3B). A cavity-like lesion was included in the resected specimen and consisted of normal alveolar tissue and nodular proliferation of tumor cells (Fig. 3C). No tumor necrosis, which is generally assumed to lead to the formation of cavitary lesions, was identified within the resected specimen. The nodule at the right lung base was 10 mm in its size of the maximum diameter. This nodule was mainly composed of tumor cells, and epithelial cells covered its surface (Fig. 3D). All tumor cells proliferating in visceral pleura and lung parenchyma were round- or spindle-shaped, bearing clear to eosinophilic cytoplasm, and normochromatic ovoid or polygonal nuclei (Fig. 3D). Although no cytological pleomorphism, necrosis or vascular invasion were apparent, the mitotic rate was about 1/50 high-power fields (HPF). Immunohistochemically, the tumor cells were positive for immunostaining with monoclonal antibody clone HMB45, hence expressing melanoma-associated antigen gp100 (Fig. 3E), and also positive for α-smooth muscle actin (α-SMA) (Fig. 3F). Those cells were also positive for the estrogen and progesterone receptors, desmin and vimentin, but negative for Melan-A and S-100 (data not shown). Based on these findings, we diagnosed the lung lesions as PEComa-NOS.
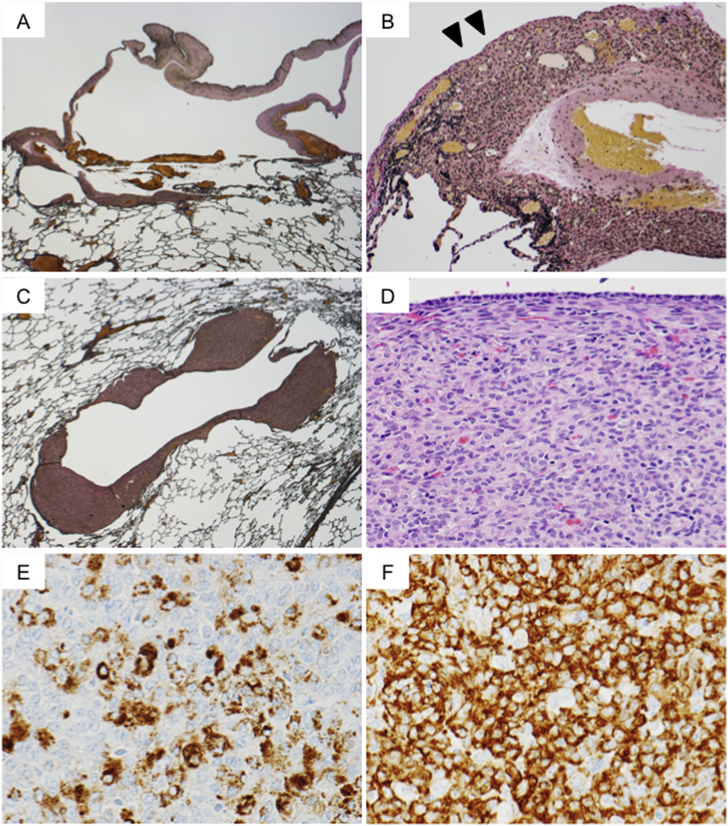
Fig. 3.
Histopathologic and immunohistochemical findings for the lung specimen. (A) The bullous cyst wall was primarily composed of thickened visceral pleura (Elastica van Gieson stain, original magnification ×5). (B) The thickened visceral pleura was infiltrated by proliferating tumor cells. Elastic fibers in the pleura were disrupted by tumor cells (arrowhead) (Elastica van Gieson stain, original magnification ×34). (C) Representative photomicrograph of a cystic or cavity-like lesion. Part of the cyst wall was primarily composed of nodules with proliferated tumor cells. Elastica van Gieson stain, original magnification ×6). (D) The nodules were composed of uniformly-proliferating and round tumor cells with clear to eosinophilic cytoplasm and ovoid nuclei (hematoxylin and eosin stain, original magnification ×100). (E) The tumor cells showed cytoplasmic staining for melanoma-associated antigen gp100 (stained by monoclonal antibody clone HMB45), original magnification ×150) and (F) α-smooth muscle actin (α-SMA), original magnification ×150).
Considering the foregoing evidence, we re-evaluated the uterine specimen that had been diagnosed as an epithelioid smooth muscle tumor of uncertain malignant potential (Fig. 4A). That tumor was approximately 7 cm in diameter and diffusely composed of cells showing a morphology similar to those found in the lung specimen (Fig. 4A); i.e., rounded tumor cells with clear to lightly eosinophilic cytoplasm and normochromatic ovoid or polygonal nuclei. Neither cytological pleomorphism, mitosis nor vascular invasion was found, but cellularity was increased and some areas of the tumor were necrotic. Immunohistochemical examination of the tumor cells demonstrated that tumor cells were positive for gp100 (Fig. 4B) and α-SMA (Fig. 4C).
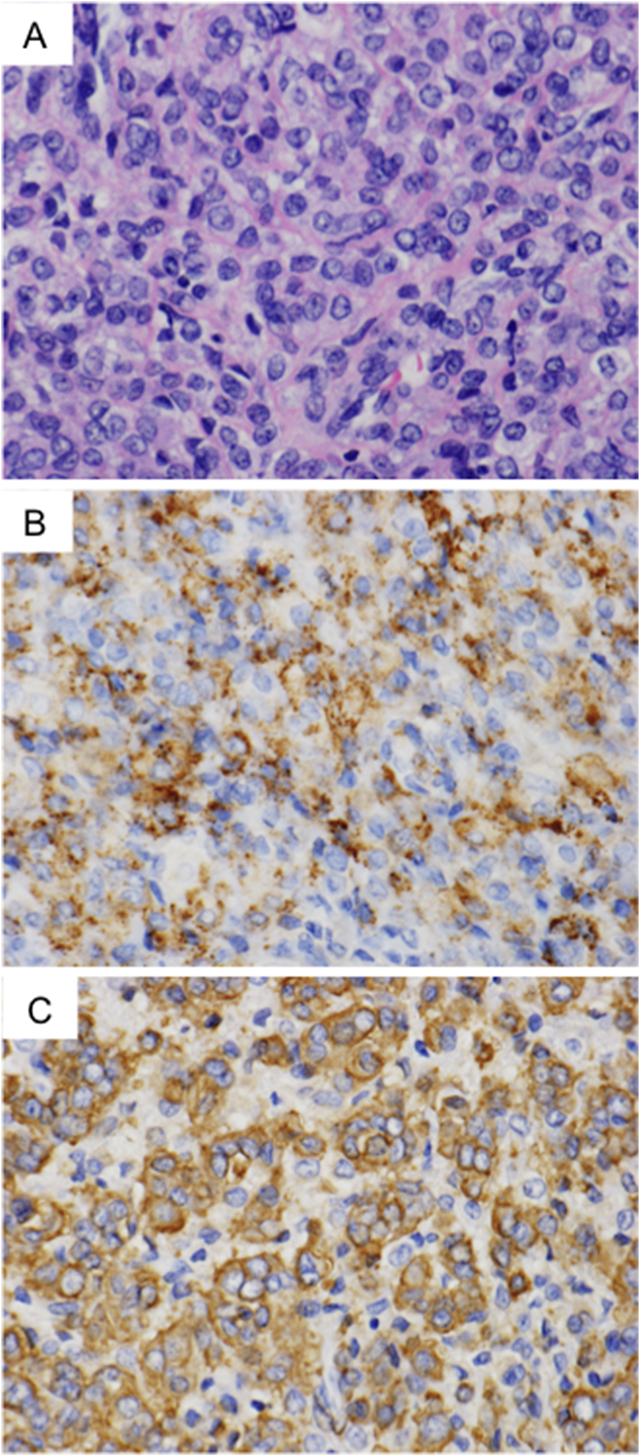
Fig. 4.
Histopathologic and immunohistochemical findings of uterine tumor. (A) Proliferating cells appeared morphologically similar to cells identified in lung lesions: i.e., rounded cells with clear to eosinophilic cytoplasm and ovoid nuclei (hematoxylin and eosin stain, original magnification ×151). The tumor cells' cytoplasmic staining was positive for gp100. B, original magnification ×151) and α-SMA (C, original magnification ×151).
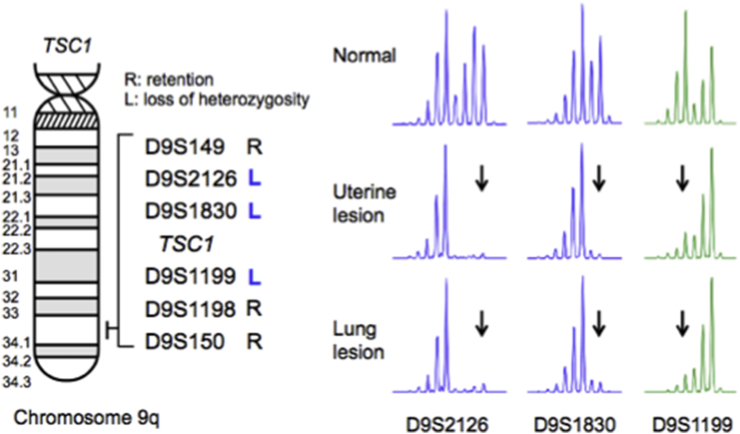
To determine the molecular basis of this PEComa, we tested for loss of heterozygosity (LOH) at the TSC1 and TSC2 loci. We found that tumor cells from the lung as well as uterus harbored only TSC1 LOH with an identical LOH pattern (Fig. 5). No TFE3 gene fusion was observed in the lung's tumor cells by RT-PCR analysis (data not shown). Therefore, we concluded that the PEComa-NOS (uterine PEComa) that had been resected 3 years earlier slowly developed pulmonary metastases and caused the pneumothorax by forming a neoplastic cyst underneath the visceral pleura and eventually disrupting it.
Fig. 5.
Results of loss-of-heterozygosity (LOH) analysis. The scheme shows chromosome 9q with a TSC1-associated region and the distribution of microsatellite markers we examined LOH. Genomic DNA was isolated from normal lung tissue, and tumor cells were microdissected from uterine or lung specimens. The pattern of LOH was identical to that at the microsatellite markers D9S2126, D9S1830 and D9S1199, whereas heterozyosity was retained at the remaining microsatellite markers. Arrows indicate the disappearance of alleles.
Since the sizes of the pulmonary cysts and nodules remained unchanged after the initiation of treatment with GnRH analogue, except for the nodule that underwent cystic change and triggered the pneumothorax, we continued GnRH therapy without additional medication after establishing the diagnosis. Presently, approximately 24 months have passed in which no new pulmonary metastasis or enlargement of the existing pulmonary lesions have been identified.
3. Discussion
To the best of our knowledge, the patient described here is the first documented as developing a pneumothorax caused by cystic, cavity-like, nodular pulmonary metastases from a PEComa-NOS (uterine PEComa). Although several reports have appeared in which imaging [12] or clinical features [13] of uterine PEComas and pulmonary metastasis were cited, those accounts were limited to the imaging scans of pulmonary metastasis or discrete nodular lesions. No narrative citing such patients who developed a pneumothorax is available in the literature. Acosta AM and Adely BP recently reviewed 16 reports of clinically aggressive PEComas of the uterine corpus (CAPUs) comprising a total of 20 patients: half of them had lung metastases, all had radiologically evident nodules, but none had the complication of a pneumothorax [13]. Interestingly, but not an exception, one publication pertained to a patient whose acute presentation of a PEComa and a spontaneous uterine rupture resembled but did not duplicate our patient's situation [14]. Intraperitoneal dissemination due to microscopic or macroscopic rupture of the serosa overlying a tumor could occur in CAPUs. Although no macroscopic implants were reported at the operation on our patient's endometrial ovarian cyst, possibly microscopic intraperitoneal implants might have caused her ascites rather than the endometriosis.
The pulmonary manifestations of CAPUs require clear differentiation from LAM, leiomyosarcoma and low-grade endometrial stromal sarcoma (ESS) [13]. We previously described a patient with ESS who showed cystic, nodular and cavitary lung metastases to the lungs and also the complication of a pneumothorax induced by tumor-cell infiltration of the visceral pleura [15]. Accordingly, our first diagnosis for the present patient was a suspicion of low-grade ESS based on the clinical presentation and imaging appearances similar to those we previously treated [15]. However, the current patient had no true cavity formation due to central ischemic necrosis within a metastatic tumor nodule, a finding frequently seen in malignant neoplasms. Indeed, histopathology demonstrated that the “cavity-like” object on her CT image was a focal proliferation of tumor cells along the pre-existing bullous cyst wall. This histopathologic feature also resembled that found in LAM, a representative disease of PEComa family, but this focal proliferation was more prominent than in LAM, eventually mimicking cavitary lesions on a CT image. In this context, the pulmonary manifestation of present patient seems to be a sort of “intermediate phenotype” between that of LAM and those of an ordinary pulmonary metastasis from CAPUs and other neoplasms.
The uterine PEComa of the present case was considered malignant from not only a clinical but also a pathological viewpoint. Folpe et al. reviewed 26 patients with PEComas, and then proposed to classify them into benign, uncertain malignant potential and malignant categories [11]. They suggested that malignancy was predicted by the presence of two of the following findings: tumor size >5 cm, infiltrative growth pattern, high nuclear grade and cellularity, mitotic activity >1/50 HPF, necrosis and vascular invasion. Recurrence and/or metastasis in their report were strongly associated with tumor size >8 cm, mitotic activity >1/50 HPF and necrosis. Based on their criteria, we classified the uterine PEComa in the present patient as malignant in terms of its size, increased cellularity, necrosis and metastases to the lungs.
The genetic background of the PEComa of this patient was associated with TSC1 LOH; presumably, this is the first PEComa documented in which a tumorigenic TSC1 abnormality seems to be involved. A comprehensive genetic analysis of 38 PEComas revealed the dichotomy of genetic abnormalities, either TSC2 mutations or TFE3 rearrangement; no TSC1 mutations were identified [16]. Although each protein product encoded by the TSC1 and TSC2 genes forms a heterodimer and exerts inhibitory control on the downstream mechanistic target of rapamycin (mTOR) pathway, it is well-known that clinical pictures of patients with TSC have the milder TSC1 disease than the more severe TSC2 counterpart [17]. Although a histopathologic diagnosis of malignant PEComa was established in our patient, her clinical course may prove to be optimistic because of its stabilization by GnRH therapy so far. No new pulmonary lesions have been found as of this writing. We speculate that this mild phenotype would have something to do with TSC1-driven tumorigenesis, similar to the situation in patients with TSC. Currently, no therapeutic strategy for PEComas has been established. However, since several studies have shown clinical benefits from mTOR inhibitors in PEComa recipients, such sirolimus, everolimus or temsirolimus [18], this option would be well worth attempting as treatment, if needed.
4. Conclusion
PEComa, especially PEComa-NOS (uterine PEComa), is a rare disease, therefore clinicians and pathologists have difficulty in its diagnosis. Yet, awareness of PEComas should be considered in the differential diagnosis for female patients with cystic, cavity-like, nodular pulmonary lesions and a history of a neoplasm in the reproductive organs.
Funding
None.
Conflicts of interest
None.
Disclosures
None.
Acknowledgment
We thank Ms. Phyllis Minick for her excellent proofreading and editing in English.
Contributor Information
Shouichi Okamoto, Email: sho-okamoto@juntendo.ac.jp.
Moegi Komura, Email: komuramoegi@gmail.com.
Yasuhisa Terao, Email: yterao@juntendo.ac.jp.
Aiko Kurisaki-Arakawa, Email: akurisa@juntendo.ac.jp.
Takuo Hayashi, Email: tkhyz@juntendo.ac.jp.
Tsuyoshi Saito, Email: tysaitou@juntendo.ac.jp.
Shinsaku Togo, Email: shinsaku@juntendo.ac.jp.
Akira Shiokawa, Email: shiokawa@med.showa-u.ac.jp.
Keiko Mitani, Email: m-kei@juntendo.ac.jp.
Etsuko Kobayashi, Email: ekobaya@juntendo.ac.jp.
Toshio Kumasaka, Email: t_kumasaka@emobile.ne.jp.
Kazuhisa Takahashi, Email: kztakaha@juntendo.ac.jp.
Kuniaki Seyama, Email: kseyama@juntendo.ac.jp.
References
- 1.Thway K., Fisher C. PEComa: morphology and genetics of a complex tumor family. Ann. Diagn. Pathol. 2015;19(5):359–368. doi: 10.1016/j.anndiagpath.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Fadare O., Parkash V., Yilmaz Y., Mariappan M.R., Ma L. Perivascular epithelioid cell tumor (PEComa) of the uterine cervix associated with intraabdominal “PEComatosis”: a clinicopathological study with comparative genomic hybridization analysis. World J. Surg. Oncol. 2004;19(2):35. doi: 10.1186/1477-7819-2-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conlon N., Soslow R.A., Murali R. Perivascular epithelioid tumours (PEComas) of the gynaecological tract. J. Clin. Pathol. 2015;68(6):418–426. doi: 10.1136/jclinpath-2015-202945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanazawa A., Fujii S., Godai T.I., Ishibe A., Oshima T. Perivascular epithelioid cell tumor of the rectum: report of a case and review of the literature. World J. Surg. Oncol. 2014;13(12):12. doi: 10.1186/1477-7819-12-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi H., Cao Q., Li H., Zhen T., Lai Y. Malignant perivascular epithelioid cell tumor of the kidney with rare pulmonary and ileum metastases. Int. J. Clin. Exp. Pathol. 2014;15;7(9):6357–6363. [PMC free article] [PubMed] [Google Scholar]
- 6.Ameurtesse H., Chbani L., Bennani A., Toughrai I., Beggui N. Primary perivascular epithelioid cell tumor of the liver: new case report and literature review. Diagn. Pathol. 2014;17(9):149. doi: 10.1186/1746-1596-9-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Govender D., Sabaratnam R.M., Essa A.S. Clear cell 'sugar' tumor of the breast: another extrapulmonary site and review of the literature. Am. J. Surg. Pathol. 2002;26(5):670–675. doi: 10.1097/00000478-200205000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Olivencia-Yurvati A.H., Rodriguez A.E. Clear cell “sugar” tumor of the lung: benign or malignant? Int. Surg. 2015;100(5):924–926. doi: 10.9738/INTSURG-D-14-00021.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fadare O. Perivascular epithelioid cell tumor (PEComa) of the uterus: an outcome-based clinicopathologic analysis of 41 reported cases. 2008;15(2):63–75. doi: 10.1097/PAP.0b013e31816613b0. [DOI] [PubMed] [Google Scholar]
- 10.Argani P., Aulmann S., Illei P.B., Netto G.J., Ro J. A distinctive subset of PEComas harbors TFE3 gene fusions. Am. J. Surg. Pathol. 2010;34(10):1395–1406. doi: 10.1097/PAS.0b013e3181f17ac0. [DOI] [PubMed] [Google Scholar]
- 11.Folpe A.L., Mentzel T., Lehr H.A., Fisher C., Balzer B.L. Perivascular epithelioid cell neoplasms of soft tissue and gynecologic origin: a clinicopathologic study of 26 cases and review of the literature. Am. J. Surg. Pathol. 2005;29(12):1558–1575. doi: 10.1097/01.pas.0000173232.22117.37. [DOI] [PubMed] [Google Scholar]
- 12.Tirumani S.H., Shinagare A.B., Hargreaves J., Jagannathan J.P., Hornick J.L. Imaging features of primary and metastatic malignant perivascular epithelioid cell tumors. AJR Am. J. Roentgenol. 2014;202(2):252–258. doi: 10.2214/AJR.13.10909. [DOI] [PubMed] [Google Scholar]
- 13.Acosta A.M., Adley B.P. Predicting the behavior of perivascular epithelioid cell tumors of the uterine corpus. Arch. Pathol. Lab. Med. 2017 Mar;141(3):463–469. doi: 10.5858/arpa.2016-0092-RS. [DOI] [PubMed] [Google Scholar]
- 14.Bosincu L., Rocca P.C., Martignoni G., Nogales F.F., Longa L. Perivascular epithelioid cell (PEC) tumors of the uterus: a clinicopathologic study of two cases with aggressive features. Mod. Pathol. 2005;18(10):1336–1342. doi: 10.1038/modpathol.3800433. [DOI] [PubMed] [Google Scholar]
- 15.Murakami A., Hayashi T., Terao Y., Mori T., Kumasaka T. Cystic, nodular and cavitary metastases to the lungs in a patient with endometrial stromal sarcoma of the uterus. Intern Med. 2014;53(9):1001–1005. doi: 10.2169/internalmedicine.53.1946. [DOI] [PubMed] [Google Scholar]
- 16.Agaram N.P., Sung Y.S., Zhang L., Chen C.L., Chen H.W. Dichotomy of genetic abnormalities in PEComas with therapeutic implications. Am. J. Surg. Pathol. 2015;39(6):813–825. doi: 10.1097/PAS.0000000000000389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dabora S.L., Jozwiak S., Franz D.N., Roberts P.S., Nieto A. Mutational analysis in a cohort of 224 tuberous sclerosis patients indicates increased severity of TSC2, compared with TSC1, disease in multiple organs. Am. J. Hum. Genet. 2001;68(1):64–80. doi: 10.1086/316951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dickson M.A., Schwartz G.K., Antonescu C.R., Kwiatkowski D.J., Malinowska I.A. Extrarenal perivascular epithelioid cell tumors (PEComas) respond to mTOR inhibition: clinical and molecular correlates. Int. J. Cancer. 2013;132(7):1711–1717. doi: 10.1002/ijc.27800. [DOI] [PMC free article] [PubMed] [Google Scholar]