Abstract
Resistance to chemotherapy remains a major challenge in the treatment of human glioblastoma (GBM). Glycogen synthase kinase-3β (GSK-3β), a positive regulator of NF-κB–mediated survival and chemoresistance of cancer cells, has been identified as a potential therapeutic target in human GBM. Our objective was to determine the antitumor effect of GSK-3 inhibitor 9-ING-41 in combination with chemotherapy in patient-derived xenograft (PDX) models of human GBM. We utilized chemoresistant PDX models of GBM, GBM6 and GBM12, to study the effect of 9-ING-41 used alone and in combination with chemotherapy on tumor progression and survival. GBM6 and GBM12 were transfected by reporter constructs to enable bioluminescence imaging, which was used to stage animals prior to treatment and to follow intracranial GBM tumor growth. Immunohistochemical staining, apoptosis assay, and immunoblotting were used to assess the expression of GSK-3β and the effects of treatment in these models. We found that 9-ING-41 significantly enhanced 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea (CCNU) antitumor activity in staged orthotopic GBM12 (no response to CCNU) and GBM6 (partial response to CCNU) PDX models, as indicated by a decrease in tumor bioluminescence in mouse brain and a significant increase in overall survival. Treatment with the combination of CCNU and 9-ING-41 resulted in histologically confirmed cures in these studies. Our results demonstrate that the GSK-3 inhibitor 9-ING-41, a clinical candidate currently in Investigational New Drug (IND)-enabling development, significantly enhances the efficacy of CCNU therapy for human GBM and warrants consideration for clinical evaluation in this difficult-to-treat patient population.
Introduction
Glioblastomas (GBMs) are malignant primary brain tumors with a dismal prognosis. The standard of care for newly diagnosed GBM is maximal surgical resection followed by combination adjuvant therapy of temozolomide (TMZ) and radiotherapy.[1], [2] The selection of therapeutics for recurrent GBM varies with few options including administration of TMZ, 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea (CCNU), or bevacizumab.[1], [2] Despite advances in surgical resection and chemoradiotherapy, the median survival of GBM patients remains around 16 months, and a variety of salvage therapies have had little impact on the progression of GBM in the recurrent setting.[1], [2] Thus, GBM remains a challenge for the identification of therapeutic agents that can improve clinical outcomes in a meaningful way.
NF-κB–mediated chemoresistance contributes to tumor progression and recurrence in cancer patients that fail chemotherapy.3 This includes GBM, where the molecular analysis of brain tumor biopsies has identified elevated expression of NF-κB and its target genes compared to normal brain tissue.[4], [5] Constitutive activation of NF-κB has been reported in human GBM tumors and found to be important in promoting tumor invasion and resistance to alkylating agents.[4], [6], [7] Thus, targeting components of NF-κB signaling represents a therapeutic strategy to overcome GBM chemoresistance.
We previously demonstrated that glycogen synthase kinase-3β (GSK-3β) is a positive regulator of NF-κB–mediated survival in cancer cells and that the inhibition of GSK-3β decreases cancer cell survival via suppression of NF-κB–mediated Bcl-2 and XIAP expression in leukemia, pancreatic, and renal cancer cells.[8], [9], [10], [11] Additional studies have credentialed GSK-3β as a therapeutic target in human GBM.[12], [13], [14] These data provide a rationale for evaluating the activity of 9-ING-41, a small molecule inhibitor of GSK-3β, as a novel therapeutic for GBM. Previous studies have demonstrated the antitumor activity and drug-like properties of this compound, including good tolerability at therapeutic doses in tumor-bearing rodents.[15], [16], [17], [18] The two isoforms of GSK-3, α and β, are 98% homologous, and known competitive inhibitors of GSK-3β, including 9-ING-41, inhibit both isoforms.[15], [17], [19] 9-ING-41 is more selective for GSK-3 than for 320 other related kinases as previously described.[15], [17].
In the present study, we evaluated the antitumor effects of 9-ING-41 alone and in combination with the chemotherapeutic agent CCNU in subcutaneous (SC) and orthotopic patient-derived xenograft (PDX) models of GBM. Two PDX models, GBM6 and GBM12, that are radiation and chemotherapy resistant[20], [21] were used for testing 9-ING-41 and CCNU antitumor activity, with results showing regression of established intracranial tumors and histologically confirmed cures, providing a strong rationale for advancing 9-ING-41 into clinical development for treating GBM patients.
Materials and Methods
Reagents
The GSK-3 inhibitor 9-ING-41 was provided by Actuate Therapeutics, Inc. (Fort Worth, TX). All other chemicals were obtained from Sigma. Because 9-ING-41 inhibits both GSK-3 α and β, it will be referred to as a GSK-3 inhibitor.
Immunohistochemical Staining and Immunoblot Analysis
Immunohistochemical staining was performed on paraffin sections of xenograft tumors as previously described.9 For immunoblots, GBM PDX SC tumors were lysed as described previously.9 Tumor lysates (50 μg whole protein extract) were separated by 10% SDS-PAGE, transferred to PVDF membrane, and probed as indicated. The following antibodies (Cell Signaling) were used for immunohistochemical and immunoblot analysis: GSK-3β (cat. 12,456), phospho-glycogen synthase (p-GS) (Ser641) (cat. 3891), glycogen synthase (cat. 3893), and GAPDH (cat. 2118). Bound antibodies were detected as described previously.9
In Vivo Bioluminescence Imaging (BLI)
In vivo BLI was performed with the IVIS Spectrum (Caliper Life Sciences). Mice were anesthetized with isoflurane and imaged 10 minutes after intraperitoneal (i.p.) injection of luciferin (D-luciferin potassium salt, 50 mg/kg, Promega). Beginning 1 week after intracranial GBM cell injection, animals were imaged weekly to stage tumors prior to initiating treatment and to follow therapeutic efficacy.
Glioblastoma Xenografts
Fresh tumor acquisition research protocol was approved by the Northwestern University Institutional Review Board, and all patients provided appropriate informed consent. PCF 373811 and 373742 resected GBM tumor samples were obtained from patients in a deidentified manner. Athymic mice were housed under pathogen-free conditions in accordance with current regulations and standards of the National Institutes of Health. All animal research was approved by Northwestern University Institutional Animal Care and Use Committee. GBM tumor fragments were engrafted subcutaneously in athymic mice, and PDX tumors were propagated for several passages. Human GBM6 and GBM12 PDX tissues were established and maintained as serially passaged SC tumors in athymic mice as previously described.20 Molecular alterations of GBM6 and GBM12 tumors and clinicopathological characteristics have been described previously.20 For the GBM6 and GBM12 PDX models, a small piece of SC GBM PDX tumor was harvested and mechanically dissociated using a tumor dissociator (Miltenyi Biotech). The resultant tumor cell suspension was transduced overnight with a lentiviral vector encoding fluorescent (tdTomato) and bioluminescent (Luc2) genes ex vivo. To establish intracranial PDX tumor models, tdTomato-Luc2–expressing GBM6 and GBM12 SC tumor were dissociated in gentle MACS C Tubes (Miltenyi Biotech). A suspension of GBM cells was prepared and injected intracranially as 100,000 cells into the frontal cortex of the right hemisphere of 6- to 7-week-old athymic mice at a depth of 3 mm as previously described.20 Inoculated mice were evaluated for intracranial tumor growth using IVIS imaging to detect tumor bioluminescence and monitored daily for the development of systemic morbidity or significant neurologic deficit. Treatment was started after tumor growth was confirmed by IVIS imaging. In studies evaluating therapeutic interventions, whole brain was collected from mice after euthanasia, fixed in 10% formalin, and processed to paraffin embedding. For histological detection of GBM tumor in the mouse brain, 5-μm serial coronal sections (50-μm gap between serial sections) were cut through the entire paraffin-embedded brain. These sections were stained with hematoxylin and eosin (H&E) and examined by a trained pathologist for the presence of residual GBM tumor.
Statistical Analysis
All values are presented as mean ± SE. GBM PDX SC tumor data were analyzed with one-way analysis of variance. To test the difference in survival between various treatment groups of animals bearing intracranial GBM PDX tumors, Kaplan-Meier survival plots were generated, and the log-rank (Mantel-Cox) test was performed. P < .05 was considered significant. Statistical analysis was performed using GraphPad Prism 6.0 software.
Results
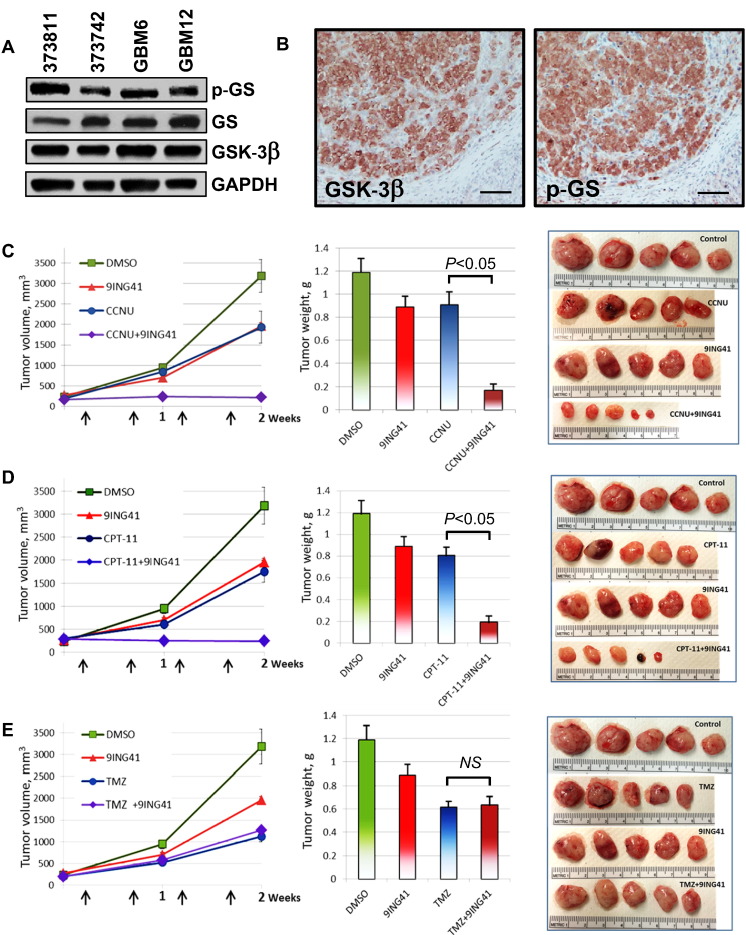
GBM cell line data have been used to credential GSK-3β as a therapeutic target in GBM.[12], [13], [14] Using immunoblotting, we found that GSK-3β is expressed in subcutaneous GBM PDX tumors (Figure 1A). We also detected the expression of p-GS, a direct downstream substrate for GSK-3, indicating that GSK-3 is active in GBM PDX tumors (Figure 1, A and B). Because the inhibition of GSK-3β might overcome NF-κB–mediated chemoresistance in cancer cells, we hypothesized that the combination of GSK-3 inhibitor 9-ING-41 with chemotherapy could potentially lead to GBM tumor regression. We initially used the GBM6 PDX tumor model grown SC to select the most active chemotherapy for combination with 9-ING-41 and to determine the optimal dose and schedule for orthotopic GBM experiments. GBM6 tumors (SC) were staged to approximately 200 mm3 prior to initiating treatment and randomized to four cohorts: control, 9-ING-41, chemotherapy [TMZ, lomustine (CCNU), or irinotecan (CPT-11)], and chemotherapy + 9-ING-41 (Figure 1, C-E). Vehicle or drugs were injected i.p. twice weekly for 2 weeks as indicated by the arrows (Figure 1, C-E). Statistical analysis of PDX tumor weights obtained at the end of the study demonstrated that 9-ING-41 significantly potentiates the antitumor effect of CCNU and CPT-11 in the GBM6 SC model (P < .05) (Figure 1, C and D). However, we did not see any enhancement of antitumor activity when 9-ING-41 was combined with TMZ (Figure 1E). Based on these results, we selected CCNU for further combination studies with 9-ING-41 in orthotopic GBM PDX models.
Figure 1.
Treatment with GSK-3 inhibitor 9-ING-41 enhances the antitumor effect of CCNU and CPT-11 in an SC model of GBM6 PDX tumor. (A) Tumor proteins were extracted from fresh GBM PDX SC tumor tissues as indicated; tumor lysates were separated by SDS-PAGE (50 μg/well) transferred to PVDF membrane and probed with indicated antibodies. GS, glycogen synthase. (B) Serial tissue sections from GBM6 SC tumor were stained for GSK-3β and p-GS. Scale bar = 200 μm. (C-E) GBM6 PDX tumor pieces were engrafted SC to nude mice. Tumors were size matched, and mice were randomized into treatment groups (five mice per group). Vehicle (DMSO), 70 mg/kg 9-ING-41, 1 mg/kg CCNU (C), 5 mg/kg CPT-11 (D), or 1 mg/kg TMZ (E) was injected i.p. at indicated doses as shown by arrows (C-E, left panel). Mean tumor volumes are plotted; bars, SE. Mice were sacrificed 2 weeks after initiation of treatment, and the weight of resected tumors was determined (C-E, middle panel). Bar graphs: mean tumor weight; SE is indicated. Representative pictures of GBM6 PDX SC tumors from each group of animals (C-E, right panel).
Pharmacokinetic studies demonstrated that 9-ING-41 (20 mg/kg) crosses the blood-brain barrier and reaches a brain concentration of 44 ± 5 μM (formulation #1) at 30 minutes following a single intravenous administration with a brain:plasma ratio >6 (Supplemental Table 1), indicating a high degree of uptake in the mouse brain. Because 9-ING-41 showed a GI50 < 5 μM against several GBM cell lines in vitro (data not shown), we expected that brain exposure to 9-ING-41 would be sufficient to significantly inhibit GSK-3 activity in intracranial GBM PDX models.
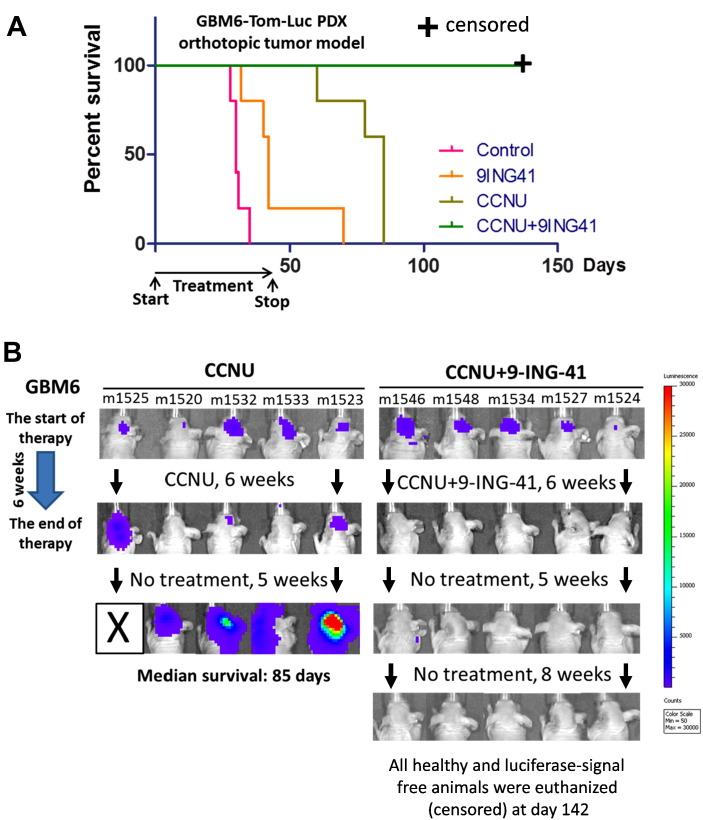
We utilized two orthotopic PDX models of refractory GBM, GBM6 and GBM12,[20], [21] to test whether 9-ING-41 could potentiate the effect of chemotherapy. The rapid growth of intracranial GBM6 and GBM12 tumors in mice leads to rapid development of systemic morbidity including cachexia and neurologic deficit (Figure 2, Figure 4) as well as detection (within 1 week) of a bioluminescent signal after intracranial injection of tdTomato-Luc2–expressing GBM6 and GBM12 tumor cells.
Figure 2.
Treatment with CCNU + 9-ING-41 leads to regression of intracranial GBM6 PDX tumors. (A) Kaplan-Meier survival analysis of treated mice bearing intracranial human GBM6 PDX-Tom-Luc tumors. GBM6-bearing mice were staged and randomized based on BLI. Mice were treated two times a week with vehicle control (DMSO; n = 5), 2 mg/kg CCNU (n = 5), 70 mg/kg 9-ING-41 (n = 5), and CCNU + 9-ING-41 (n = 5) as indicated. The median survival in the vehicle control, 9ING41, and CCNU groups was 30, 42, and 85 days, respectively. All of the 9-ING-41 + CCNU–treated animals were intentionally euthanized (censored) for histological analysis of brain at day 142 despite being healthy and luciferase-signal free. The combination therapy of CCNU and 9-ING-41 significantly prolonged survival of animals as compared to CCNU-treated group (P < .05). (B) Representative IVIS images of GBM6-bearing animals treated as indicated.
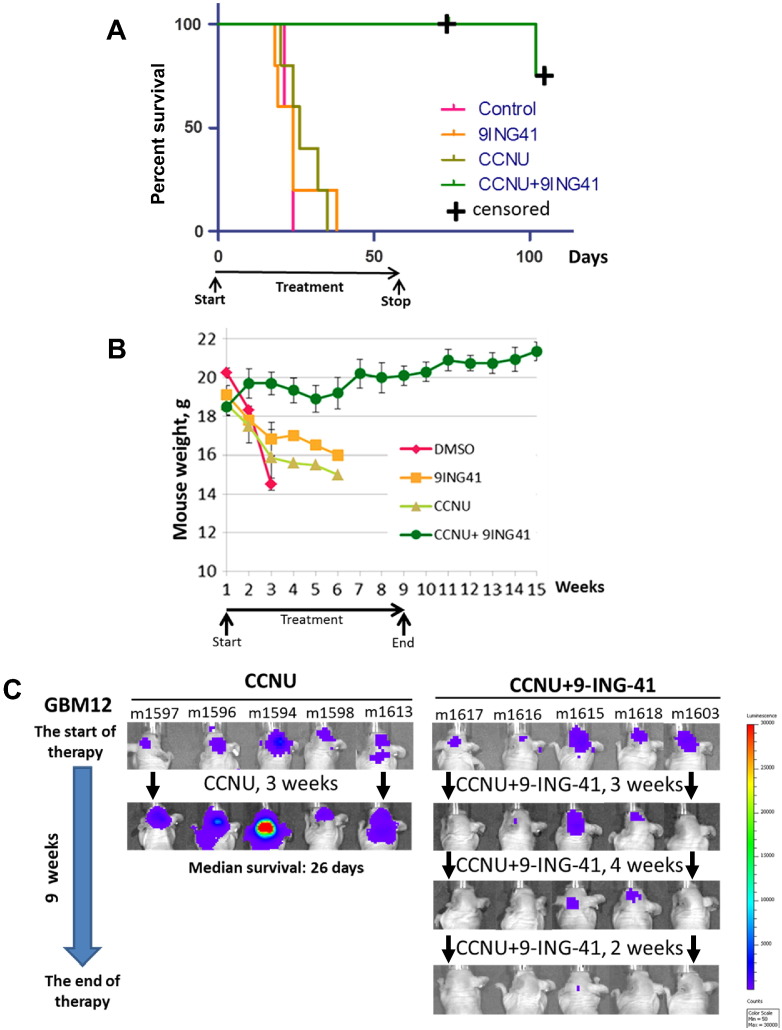
Figure 4.
Treatment with CCNU + 9-ING-41 leads to regression of intracranial GBM12 PDX tumors. (A) Kaplan-Meier survival analysis of treated mice bearing intracranial human GBM12 PDX-Tom-Luc tumors. Mice were staged and randomized based on BLI. Mice were treated two times a week with vehicle control (DMSO; n = 5), 5 mg/kg CCNU (n = 5), 70 mg/kg 9-ING-41 (n = 5), and CCNU + 9-ING-41 (n = 5) as indicated. The median survival in the vehicle control, 9-ING-41, and CCNU groups was 24, 24, and 26 days, respectively. Four of five 9-ING-41 + CCNU–treated animals were intentionally euthanized (censored) for histological analysis of brain at day 74 (m1618) and day 105 (m1603, m1616, m1617) despite being healthy and luciferase-signal free. The combination of CCNU and 9-ING-41 significantly prolonged survival of animals as compared to CCNU-treated group (P < .05). (B) Animal weight was measured weekly. Graph, mean animal weight; bars, SE. (C) Representative IVIS images of GBM12-bearing animals treated as indicated.
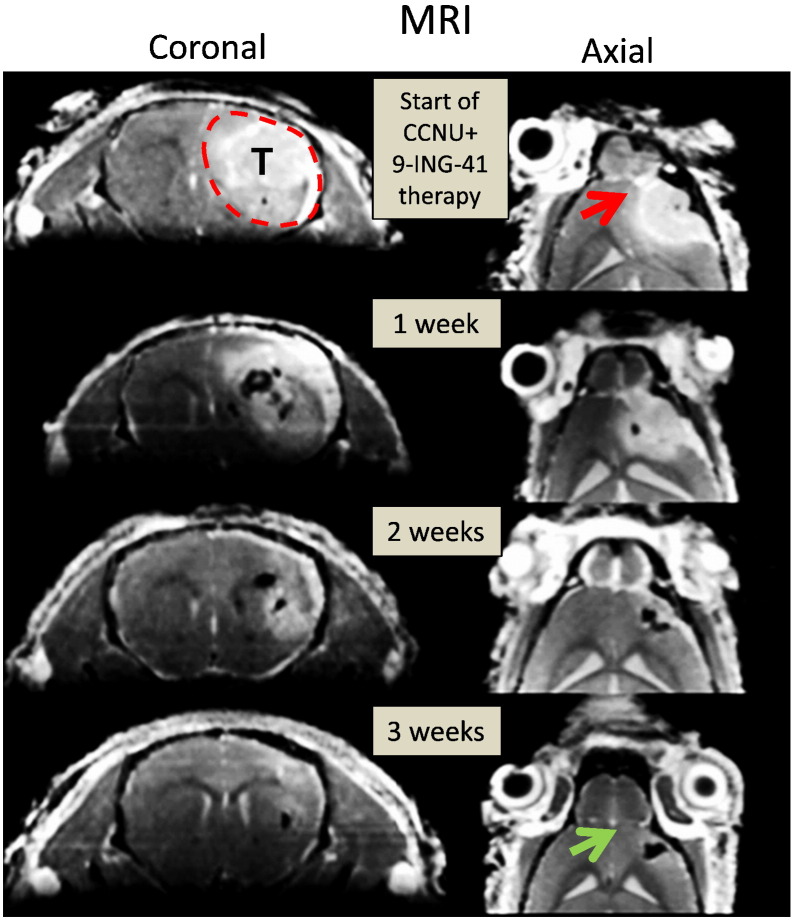
Using the GBM6 orthotopic model, we found that some mice demonstrated intial response to CCNU monotherapy (2 mg/kg, twice a week), but tumors progressed in all animals within a few weeks of ceasing treatment and had to be euthanized (Figure 2). No significant response was observed in GBM6-bearing animals receiving 9-ING-41 monotherapy (Figure 2). However, the combination of CCNU and 9-ING-41 resulted in regression of GBM6 tumors in all animals by the end of the treatment period (6 weeks), with no tumor relapse observed by IVIS imaging after discontinuation of treatment (Figure 2). Concomitant increases in animal survival correlated with the IVIS imaging by showing a significant survival benefit (P < .05) for mice treated with the 9-ING-41 + CCNU combination (Figure 2). All of the 9-ING-41 + CCNU–treated animals were intentionally euthanized for histological analysis of brain at day 142 despite being healthy and luciferase-signal free (Figure 2). The absence of tumor in the CCNU + 9-ING-41–treated animals was confirmed by histological evaluation of serial sections of mouse brain (Supplementary Figure 1). In addition, we found a complete recovery of normal mouse brain structures in CCNU + 9-ING-41–treated animals (Supplementary Figure 1). Using magnetic resonance imaging (MRI), we also demonstrated the regression of advanced intracranial GBM6 tumor (treatment was started in 3 weeks after intracranial injection of GBM6 cells) and the recovery of mouse brain structures in a CCNU + 9-ING-41–treated mouse just 3 weeks after the initiation of the combination treatment (Figure 3).
Figure 3.
Treatment with CCNU + 9-ING-41 resulted in complete regression of intracranial GBM6 PDX tumor and recovery of mouse brain structures. CCNU (2 mg/kg) + 9-ING-41 (70 mg/kg) treatment was started in 3 weeks after intracranial transplantation of GBM6 PDX tumor. Mouse was treated by i.p. injections twice a week for 3 weeks. MR images were taken every week after the initiation of the treatment as indicated. Direct invasion of the tumor and significant deformation of olfactory bulb are indicated by red arrow. Green arrow indicates an absence of the tumor and a complete recovery of olfactory bulb and other brain structures by week 3 of the treatment. T, tumor.
In contrast to the partially responsive GBM6 model, intracranial GBM12 tumors were completely resistant to 9-ING-41 (70 mg/kg, twice a week) and CCNU monotherapy even at higher doses of CCNU (5 mg/kg, twice a week). All mice in control, CCNU, and 9-ING-41 groups quickly became cachectic and moribund with high bioluminescence signal confirming the progression of intracranial GBM12 tumors (Figure 4). However, administration of the CCNU + 9-ING-41 combination led to tumor regressions as demonstrated by BLI and significantly (P < .05) prolonged survival (Figure 4). Four out of five mice in the CCNU + 9-ING-41 cohort thrived with no signal by BLI up to time of euthanasia (Figure 4). Histological analysis of brains from these mice demonstrated an absence of cancer cells and recovery of mouse brain structures by histological evaluation of H&E-stained sections (Supplementary Figure 1). Only one GBM12 mouse (m1615) treated with the CCNU + 9-ING-41 demonstrated tumor relapse and progression (Figure 4C). We found that expression of p-GS, a downstream target of GSK-3, was downregulated in 9-ING-41–treated intracranial GBM12 PDX tumors, indicating target engagement (Supplementary Figure 2A). We also found extensive apoptosis (12% of GBM12 tumor cells were apoptotic) in a satellite cohort of mice treated with CCNU + 9-ING-41 for 2 weeks. In contrast, few apoptotic bodies (<0.2% of cancer cells) were detected in intracranial GBM12 tumors obtained from animals treated with vehicle control, CCNU, or 9-ING-41 monotherapy (Supplementary Figure 2B). These results demonstrate the ability of 9-ING-41 to dramatically enhance the antitumor activity of CCNU leading to regressions of intracranial GBM12 tumors that are resistant to either CCNU or 9-ING-41 monotherapy.
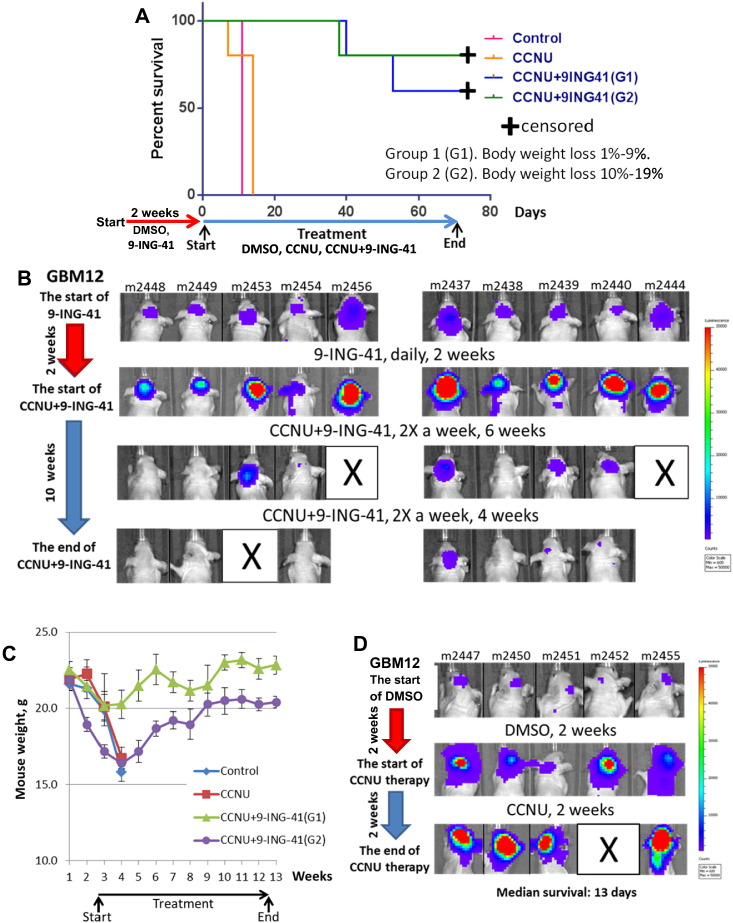
Next, we decided to evaluate whether daily administration of 9-ING-41 would improve the antitumor activity of this drug as a monotherapy given its ability to inhibit GBM12-derived cell line growth in vitro (Supplementary Figure 2C). In vitro analysis also revealed that 9-ING-41 (GI50 = 1.4 μM) is significantly more active than a GSK-3 inhibitor (LY2090314; GI50 > 20 μM) that had previously advanced into early clinical trials (Supplementary Figure 2C). Daily treatments with 9-ING-41 were started after staging tumor growth by BLI (Figure 5). After 2 weeks of 9-ING-41 therapy (40 mg/kg, daily, Mon-Fri), we found progression of GBM12 in all animals in vehicle control (DMSO, n = 10) as well as in 9-ING-41 (n = 10) groups, as indicated by BLI (Figure 5B). All animals in vehicle control and 9-ING-41 groups showed weight loss because of tumor progression (Figure 5C), with survival falling in the range of 10 to 14 days (Figure 4A). We decided to test whether the combination of CCNU + 9-ING-41 could still rescue cachectic animals bearing advanced (3 weeks after intracranial transplantation) and rapidly progressing intracranial GBM12 tumors. CCNU + 9-ING-41–treated mice were stratified based on body weight loss: G1 (1%-9% body weight loss) and G2 (10%-19% body weight loss). Regardless of weight loss, combination treatment was well tolerated (Figure 5C) and caused tumor regression in 7 of 10 mice (Figure 5B). Three mice (m2453, m2456, m2444) with the largest GBM12 tumors at initiation of combination treatment could not be rescued (Figure 5B). Surviving mice from combination treatment thrived during the course of CCNU + 9-ING-41 therapy and remained healthy until euthanized (Figure 5, B and C). The absence of cancer cells and recovery of mouse brain structures in surviving CCNU + 9-ING-41–treated animals (6 of 10 mice) were confirmed by histological evaluation.
Figure 5.
Treatment with CCNU + 9-ING-41 leads to regression of intracranial GBM12 PDX tumors and recovery of cachectic mice. (A) Kaplan-Meier survival analysis of treated mice bearing intracranial human GBM12 PDX-Tom-Luc tumors. Mice were staged and randomized based on IVIS imaging. Mice were treated with vehicle control (DMSO; n = 10) or 40 mg/kg 9-ING-41 (n = 10) daily for 2 weeks as indicated by red arrow. Next, mice were treated two times a week with DMSO (n = 5), 5 mg/kg CCNU (n = 5), and CCNU + 9-ING-41 (n = 10) as indicated by blue arrow. The median survival (from the start of vehicle, CCNU and CCNU + 9-ING-41 treatment) in the vehicle control and CCNU was 11 and 13 days, respectively. Six of ten 9-ING-41 + CCNU–treated animals were intentionally euthanized (censored) for histological analysis of brain at day 66 despite being healthy and luciferase-signal free. The combination of CCNU and 9-ING-41 significantly prolonged survival of cachectic animals as compared to CCNU-treated group (P < .05). (B) Representative IVIS images of GBM12-bearing animals treated i.p. with combination of 5 mg/kg CCNU and 40 mg/kg 9-ING-41 twice a week as indicated. (C) Animal weight was measured weekly. Graph, mean animal weight; bars, SE. (D) Representative IVIS images of GBM12-bearing animals treated i.p. with 5 mg/kg CCNU twice a week.
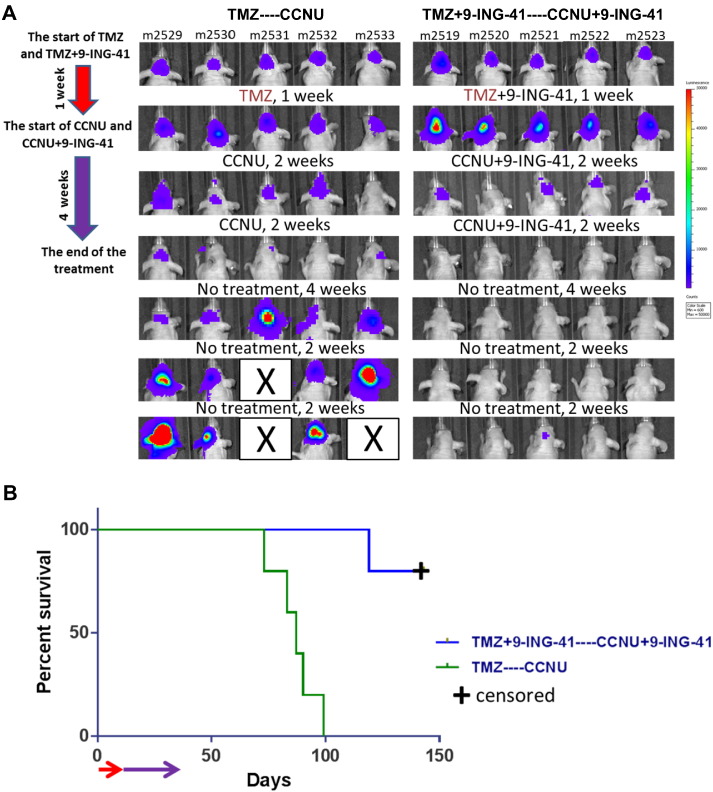
Finally, we evaluated the effect of 9-ING-41 on a sequential course of treatment using first-line (TMZ) and second-line (CCNU) treatment. Treatment with TMZ (n = 5) and TMZ + 9-ING-41 (n = 5 mice) was initiated after staging of intracranial GBM12 tumor growth by BLI after intracranial transplantation of GBM12 cells (Figure 6A). No response was detected by BLI after 1 week of treatment with either TMZ or TMZ + 9-ING-41 (Figure 6A). Although our previous in vivo results showed complete resistance of treatment-naïve intracranial GBM12 tumors to CCNU (Figure 4), we found that CCNU treatment of TMZ-pretreated animals resulted in partial regressions of intracranial GBM12 tumors as shown by BLI (Figure 6A). However, these tumors relapsed and progressed after cessation of CCNU treatment (Figure 6A). In contrast, we found that treament with CCNU + 9-ING-41 of mice pretreated with TMZ + 9-ING-41 resulted in complete regression in four of five animals as shown by BLI (Figure 6A) and confirmed by histological analysis; one of these mice (m2521) exhibited a partial regression of tumor with subsequent recurrence and progression. Also of note, the combination of CCNU + 9-ING-41 in this salvage therapy setting significantly prolonged survival as compared to the CCNU salvage monotherapy group (Figure 6B).
Figure 6.
Treatment with CCNU + 9-ING-41 leads to regression of TMZ-resistant intracranial GBM12 tumors. (A) Mice were staged and randomized based on BLI. Mice were treated (red arrow) daily, Monday to Friday, with TMZ (n = 5; 50 mg/kg, oral administration) or TMZ + 9-ING-41 (n = 5; 40 mg/kg of 9-ING-41, i.p.) for 1 week. The treatment was continued (purple arrow) with CCNU (5 mg/kg, i.p., TMZ-treated group) and CCNU + 9-ING-41 (TMZ + 9-ING-41-treated group) twice a week for 4 weeks. (B) Kaplan-Meier survival analysis of GBM12-bearing mice treated as described in panel A. The median survival in the CCNU group was 87 days. Four of five CCNU + 9-ING-41–treated animals were intentionally euthanized (censored) for histological analysis of brain at day 130 despite being healthy and luciferase-signal free. Combination of CCNU and 9-ING-41 significantly prolonged survival of animals as compared to CCNU-treated group (P < .05).
Discussion
The treatment of GBM remains a clinical challenge. Currently, GBM progresses in most patients even after surgical resection and adjuvant chemoradiotherapy.[1], [2] Although a variety of factors contribute to the inability of current treatments to provide meaningful clinical benefit, the observation of inherent and acquired chemoresistance in glioma is an underlying cause of poor prognosis in this disease. Overcoming chemoresistance in GBM has been a goal of new drug development for decades, but attempts to date have been underwhelming with respect to their extent of success. For example, in a recent prospective phase III trial that compared CCNU with enzastaurin in patients with recurrent GBM,22 patients treated with CCNU reported a response rate of 4.3% and a median overall survival of just 7.1 months compared to 6.6. months for enzastaurin.
In general, few single-agent chemotherapies targeting advanced cancer work well as a monotherapy, necessitating the investigation of combination regimens for improving single-agent activities. Consistent with this, we show that monotherapy with 9-ING-41 does not significantly affect GBM PDX tumor progression, with modest response observed in only a small percentage of mice bearing intracranial GBM6 tumors. These results therefore mirror previously published results showing that monotherapy with drugs having activity against GSK-3 is not effective in treating GBM patients.[22], [23] Enzastaurin, which has also been shown to inhibit GSK-3 (IC50 ~ 24 nM) as well as PKCβ,[12], [24] failed to improve GBM patient survival as cited above despite radiographic indication of some antitumor activity.22 These clinical results support our hypothesis that a GSK-3 inhibitor should be combined with chemotherapy in order to enhance GBM response.
Recent studies identified GSK-3β, a positive regulator of NF-κB–mediated survival and chemoresistance of cancer cells, as a therapeutic target in human GBM.[12], [13], [14], [25], [26] Here, we show that the GSK-3 inhibitor 9-ING-41 can potentiate the effect of CCNU leading to sustained complete regression of GBM PDX tumors. We demonstrate that 9-ING-41 significantly increases CCNU antitumor activity in two different orthotopic PDX models: GBM12, which is completely resistant to CCNU, and GBM6, which shows a transient response to CCNU. To the best of our knowledge, this is the first in vivo study showing a high percentage of cures in orthotopic GBM PDX tumor models with distinct chemoresistant phenotypes. In addition to the remarkable, durable remission of intracranial GBM PDX tumors, CCNU + 9-ING-41 combination treatment also led to a complete recovery of mouse brain structures affected by GBM growth, as indicated by histopathological evaluation of serial H&E sections of mouse brain. In addition, MRI analysis showed regression of an advanced intracranial GBM6 tumor with recovery of brain structures in a CCNU + 9-ING-41–treated mouse just 3 weeks after initiation of treatment. Finally, little toxicity was observed at the doses and schedules tested in these studies, and the combination-treated groups thrived and gained weight while on combination treatment, which sharply contrasts with the weight loss observed in other treatment cohorts.
Our GBM results presented here are consistent with previously published data showing regressions of established metastatic breast cancer PDX when administering 9-ING-41 combined with CPT-11.18 It is unlikely that 9-ING-41 will prove to be a pan-chemotherapy enhancer given the lack of activity observed for combining this GSK-3 inhibitor with TMZ. Studies are ongoing to evaluate different chemotherapy combinations with 9-ING-41 in GBM as well as in other cancer PDX models. In addition, molecular studies are under way to identify markers that will allow the development of precision approaches to identifying active 9-ING-41 combinations in the clinic. These studies are expected to identify chemotherapeutics whose antitumor activity is enhanced by 9-ING-41 and, as well, lead to increased mechanistic understanding of why the antitumor activity of specific agents is enhanced by 9-ING-41.
Given the translational potential of 9-ING-41, our study provides a rationale for the clinical translation of 9-ING-41 in combination with CCNU for treating GBM. CCNU is commonly given to a GBM patient at a dose of 100 mg/m2/q6w.1 Using interspecies conversion from man to mouse, we found that the mouse equivalent dose is too toxic for immunodeficient mice. In this study, we decreased the dose and altered the schedule of CCNU administration to reflect previous clinical experience using CCNU dosed more frequently to coincide with the 9-ING-41 schedule (twice weekly) using a dose and schedule that were tolerated by immunodeficient mice. Using this approach, we achieved complete regressions and cures in two distinct chemoresistant orthotopic PDX models of GBM. The dose and schedule of CCNU (4-10 mg/kg/week) used in our animal studies correspond to approximately 12 to 30 mg/m2/week in humans. A CCNU dose of 30 mg/m2/week for 12+ weeks was previously evaluated in a phase I clinical study in patients with advanced cancer and showed minimal toxicity while demonstrating antitumor activity.27 Thus, we present a rationale for administering CCNU using a weekly schedule that we hypothesize will be most amenable to combination treatment with 9-ING-41 in the clinic.
The FDA has recently granted 9-ING-41 orphan drug status for the treatment of GBM, and thus, the results of the current study should prove useful for developing 9-ING-41 for the treatment of GBM patients. Further studies are currently under way evaluating 9-ING-41 with additional chemotherapy combinations, and the results to date do not show any indication of 9-ING-41–associated drug combinations as being limited to a particular subtype of GBM. Rather, antitumor activities for one PDX appear to be generalizable to other GBM PDX models. Finally, although this study focuses on 9-ING-41 combinations with chemotherapy, there is emerging evidence suggesting that the inhibition of GSK-3 will also enhance the antitumor activity of both tyrosine kinase and immune checkpoint inhibitors,[28], [29] both of which have shown some therapeutic potential in treating GBM. Going forward, combinations of 9-ING-41 with contemporary therapeutics will also be studied, with results potentially expanding the armamentarium of 9-ING-41 drug combinations.
Conclusions
Here, we show that 9-ING-41, a small molecule inhibitor of GSK-3, significantly enhances the antitumor effect of CCNU in chemoresistant GBM PDX models. Combination therapy with 9-ING-41 and CCNU leads to a complete regression of intracranial GBM PDX tumors, thus credentialing 9-ING-41 as a drug candidate for the treatment of GBM. In addition to the durable remissions of intracranial GBM PDX tumors observed in these studies, CCNU and 9-ING-41 combination treatment also led to a complete recovery of mouse brain structures affected by GBM growth. Thus, the results of the current study provide a rationale for advancing 9-ING-41 into the clinic for the treatment of GBM.
The following are the supplementary data related to this article.
Supplementary Figure 1. Representative images of H&E staining of the brains (coronal section) of animals treated with the indicated drugs. Complete regressions of intracranial GBM6 and GBM12 tumors and recovery of normal mouse brain structures were observed in CCNU + 9-ING-41–treated animals. Scale bar = 200 μm.
Supplementary Figure 2. (A) Representative pictures of GSK-3β and p-GS expression in intracranial GBM12 tumors obtained from DMSO-treated (control) and 9-ING-41–treated animals. Scale bar = 200 μm. (B) TUNEL staining was performed using tissue sections of intracranial GBM12 tumors obtained from animals treated with DMSO (control), CCNU (5 mg/kg), 9-ING-41 (70 mg/kg), and CCNU + 9-ING-41 twice a week for 2 weeks. Scale bar = 200 μm. (C) Relative cell growth was measured by CellTiter 96® AQueous assay (Promega) in GBM12 cancer cell line treated with 0.5 μM, 2 μM, and 10 μM of 9-ING-41 or LY2090314 for 96 hours as indicated.
Supplementarty material.
9-ING-41 crosses mouse blood-brain barrier.
Acknowledgements
We thank Jian-Jun Wei, Jeremy Mathews, Demirkan Gursel, and Stephen Rohan (Pathology Core Facility, Robert H. Lurie Comprehensive Cancer Center, Northwestern University) for providing us clinical samples of GBM. This work was supported by the Northwestern University Pathology Core Facility and a Cancer Center Support Grant (NCI CA060553). We express our gratitude to Mouse Histology and Phenotyping Laboratory and Oleksii Dubrovskyi (Center for Developmental Therapeutics) for technical assistance. We thank MicroConstants (San Diego, CA) for bioanalytical method development and analysis of 9-ING-41 in mouse plasma and brain samples.
Footnotes
Funding: This study was supported by a generous donation from the Baskes family to the Robert H. Lurie Comprehensive Cancer Center of Northwestern University, a pilot grant from the Northwestern University Brain Tumor Institute (A.P.M.), Northwestern Memorial Foundation Dixon Translational Award (A.P.M.), and Cancer Center Support Grant 2 P30 CA060553-19 (A.P.M., A.U.) to the Robert H. Lurie Comprehensive Cancer Center of Northwestern University.
Conflict of Interest: 9-ING-41 has been licensed to Actuate Therapeutics, Inc. Alan Kozikowski, Andrey Ugolkov, Thomas O'Halloran, and Andrew Mazar hold an equity interest in Actuate Therapeutics, Inc. Alan Kozikowski and Irina Gaisina are inventors on the 9-ING-41 patent.
References
- 1.Le Rhun E, Taillibert S, Chamberlain MC. The future of high-grade glioma: where we are and where are we going. Surg Neurol Int. 2015;6(Suppl. 1):S9–S44. doi: 10.4103/2152-7806.151331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Delgado-López PD, Corrales-García EM. Survival in glioblastoma: a review on the impact of treatment modalities. Clin Transl Oncol. 2016;18(11):1062–1071. doi: 10.1007/s12094-016-1497-x. [DOI] [PubMed] [Google Scholar]
- 3.Aggarwal B. Nuclear factor-κB: the enemy within. Cancer Cell. 2004;6(3):203–208. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Raychaudhuri B, Han Y, Lu T, Vogelbaum MA. Aberrant constitutive activation of nuclear factor kappaB in glioblastoma multiforme drives invasive phenotype. J Neurooncol. 2007;85(1):39–47. doi: 10.1007/s11060-007-9390-7. [DOI] [PubMed] [Google Scholar]
- 5.Robe PA, Bentires-Alj M, Bonif M. In vitro and in vivo activity of the nuclear factor-kappaB inhibitor sulfasalazine in human glioblastomas. Clin Cancer Res. 2004;10(16):5595–5603. doi: 10.1158/1078-0432.CCR-03-0392. [DOI] [PubMed] [Google Scholar]
- 6.Brown RE, Law A. Morphoproteomic demonstration of constitutive nuclear factor-kappaB activation in glioblastoma multiforme with genomic correlates and therapeutic implications. Ann Clin Lab Sci. 2006;36(4):421–426. [PubMed] [Google Scholar]
- 7.Bredel M, Bredel C, Juric D. Tumor necrosis factor-alpha–induced protein 3 as a putative regulator of nuclear factor-kappaB–mediated resistance to O6-alkylating agents in human glioblastomas. J Clin Oncol. 2006;24(2):274–287. doi: 10.1200/JCO.2005.02.9405. [DOI] [PubMed] [Google Scholar]
- 8.Ougolkov A, Fernandez-Zapico M, Savoy D, Urrutia R, Billadeau D. Glycogen synthase kinase-3beta participates in nuclear factor kappaB–mediated gene transcription and cell survival in pancreatic cancer cells. Cancer Res. 2005;65(6):2076–2081. doi: 10.1158/0008-5472.CAN-04-3642. [DOI] [PubMed] [Google Scholar]
- 9.Ougolkov A, Fernandez-Zapico M, Bilim V, Smyrk T, Chari S, Billadeau D. Aberrant nuclear accumulation of glycogen synthase kinase-3beta in human pancreatic cancer: association with kinase activity and tumor dedifferentiation. Clin Cancer Res. 2006;12(17):5074–5081. doi: 10.1158/1078-0432.CCR-06-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ougolkov A, Bone N, Fernandez-Zapico M, Kay N, Billadeau D. Inhibition of glycogen synthase kinase-3 activity leads to epigenetic silencing of nuclear factor kappaB target genes and induction of apoptosis in chronic lymphocytic leukemia B cells. Blood. 2007;110(2):735–742. doi: 10.1182/blood-2006-12-060947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bilim V, Ougolkov A, Yuuki K. Glycogen synthase kinase-3: a new therapeutic target in renal cell carcinoma. Br J Cancer. 2009;101(12):2005–2014. doi: 10.1038/sj.bjc.6605437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kotliarova S, Pastorino S, Kovell L. Glycogen synthase kinase-3 inhibition induces glioma cell death through c-MYC, NF-kappaB, and glucose regulation. Cancer Res. 2008;68(16):6643–6651. doi: 10.1158/0008-5472.CAN-08-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyashita K, Kawakami K, Nakada M. Potential therapeutic effect of glycogen synthase kinase 3beta inhibition against human glioblastoma. Clin Cancer Res. 2009;15(3):887–897. doi: 10.1158/1078-0432.CCR-08-0760. [DOI] [PubMed] [Google Scholar]
- 14.Korur S, Huber RM, Sivasankaran B. GSK3beta regulates differentiation and growth arrest in glioblastoma. PLoS One. 2009;4(10):e7443. doi: 10.1371/journal.pone.0007443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaisina I, Gallier F, Ougolkov A. From a natural product lead to the identification of potent and selective benzofuran-3-yl-(indol-3-yl)maleimides as glycogen synthase kinase-3 beta inhibitors that suppress proliferation and survival of pancreatic cancer cells. J Med Chem. 2009;52(7):1853–1863. doi: 10.1021/jm801317h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hilliard T, Gaisina I, Muehlbauer A, Gaisin A, Gallier F, Burdette J. Glycogen synthase kinase 3beta inhibitors induce apoptosis in ovarian cancer cells and inhibit in-vivo tumor growth. Anticancer Drugs. 2011;22(10):978–985. doi: 10.1097/CAD.0b013e32834ac8fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pal K, Cao Y, Gaisina IN. Inhibition of GSK-3 induces differentiation and impaired glucose metabolism in renal cancer. Mol Cancer Ther. 2014;13(2):285–296. doi: 10.1158/1535-7163.MCT-13-0681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ugolkov A, Gaisina I, Zhang JS. GSK-3 inhibition overcomes chemoresistance in human breast cancer. Cancer Lett. 2016;380(2):384–392. doi: 10.1016/j.canlet.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhat R, Xue Y, Berg S. Structural insights and biological effects of glycogen synthase kinase 3–specific inhibitor AR-A014418. J Biol Chem. 2003;278(46):45937–45945. doi: 10.1074/jbc.M306268200. [DOI] [PubMed] [Google Scholar]
- 20.Sarkaria JN, Carlson BL, Schroeder MA. Use of an orthotopic xenograft model for assessing the effect of epidermal growth factor receptor amplification on glioblastoma radiation response. Clin Cancer Res. 2006;12(7 Pt 1):2264–2271. doi: 10.1158/1078-0432.CCR-05-2510. [DOI] [PubMed] [Google Scholar]
- 21.Carlson BL, Grogan PT, Mladek AC. Radiosensitizing effects of temozolomide observed in vivo only in a subset of O6-methylguanine-DNA methyltransferase methylated glioblastoma multiforme xenografts. Int J Radiat Oncol Biol Phys. 2009;75(1):212–219. doi: 10.1016/j.ijrobp.2009.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wick W, Puduvalli VK, Chamberlain MC. Phase III study of enzastaurin compared with lomustine in the treatment of recurrent intracranial glioblastoma. J Clin Oncol. 2010;28(7):1168–1174. doi: 10.1200/JCO.2009.23.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kreisl TN, Kotliarova S, Butman JA. A phase I/II trial of enzastaurin in patients with recurrent high-grade gliomas. Neuro Oncol. 2010;12(2):181–189. doi: 10.1093/neuonc/nop042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim G, Billadeau D. GSK-3β inhibition in pancreatic cancer. In: Lowy AM, Leach SD, Philip PA, editors. Pancreatic Cancer. Springer Science+Business Media, LLC; New York: 2008. pp. 635–646. [Google Scholar]
- 25.Nowicki MO, Dmitrieva N, Stein AM. Lithium inhibits invasion of glioma cells; possible involvement of glycogen synthase kinase-3. Neuro Oncol. 2008;10(5):690–699. doi: 10.1215/15228517-2008-041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams SP, Nowicki MO, Liu F. Indirubins decrease glioma invasion by blocking migratory phenotypes in both the tumor and stromal endothelial cell compartments. Cancer Res. 2011;71(16):5374–5380. doi: 10.1158/0008-5472.CAN-10-3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koller CA, Gorski CC, Benjamin RS, Legha SS, Papadopoulos NE, Plager C. A phase I trial of weekly lomustine in patients with advanced cancer. Cancer. 1994;73(1):236–239. doi: 10.1002/1097-0142(19940101)73:1<236::aid-cncr2820730140>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 28.Thorne CA, Wichaidit C, Coster AD, Posner BA, Wu LF, Altschuler SJ. GSK-3 modulates cellular responses to a broad spectrum of kinase inhibitors. Nat Chem Biol. 2015;11(1):58–63. doi: 10.1038/nchembio.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor A, Harker JA, Chanthong K, Stevenson PG, Zuniga EI, Rudd CE. Glycogen synthase kinase 3 inactivation drives T-bet–mediated downregulation of co-receptor PD-1 to enhance CD8(+) cytolytic T cell responses. Immunity. 2016;44(2):274–286. doi: 10.1016/j.immuni.2016.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Representative images of H&E staining of the brains (coronal section) of animals treated with the indicated drugs. Complete regressions of intracranial GBM6 and GBM12 tumors and recovery of normal mouse brain structures were observed in CCNU + 9-ING-41–treated animals. Scale bar = 200 μm.
Supplementary Figure 2. (A) Representative pictures of GSK-3β and p-GS expression in intracranial GBM12 tumors obtained from DMSO-treated (control) and 9-ING-41–treated animals. Scale bar = 200 μm. (B) TUNEL staining was performed using tissue sections of intracranial GBM12 tumors obtained from animals treated with DMSO (control), CCNU (5 mg/kg), 9-ING-41 (70 mg/kg), and CCNU + 9-ING-41 twice a week for 2 weeks. Scale bar = 200 μm. (C) Relative cell growth was measured by CellTiter 96® AQueous assay (Promega) in GBM12 cancer cell line treated with 0.5 μM, 2 μM, and 10 μM of 9-ING-41 or LY2090314 for 96 hours as indicated.
Supplementarty material.
9-ING-41 crosses mouse blood-brain barrier.